Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rhodium-catalyzed regioselective C–H activation/Lossen rearrangement/annulation for the green synthesis of trisubstituted 2-pyridones†
Yidi
Li‡
ae,
Huiying
Xu‡
 c,
Lin
Huang‡
bd,
Zhi
Zhou
c,
Lin
Huang‡
bd,
Zhi
Zhou
 *c,
Zhenhao
Tang
b,
Haifang
Meng
b,
Wei
Zhang
b,
Wei
Yi
*c,
Zhenhao
Tang
b,
Haifang
Meng
b,
Wei
Zhang
b,
Wei
Yi
 *c and
Xiaowei
Wu
*c and
Xiaowei
Wu
 *abde
*abde
aDrug Discovery and Development Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China. E-mail: wuxiaowei@simm.ac.cn
bZhongshan Institute for Drug Discovery, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Zhongshan 528400, China
cGuangzhou Municipal and Guangdong Provincial Key Laboratory of Protein Modification and Degradation & Molecular Target and Clinical Pharmacology, State Key Laboratory of Respiratory Disease, School of Pharmaceutical Sciences & the Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong 511436, China. E-mail: zhouzhi@gzhmu.edu.cn; yiwei@gzhmu.edu.cn
dSchool of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, China
eUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, China
First published on 18th May 2023
Abstract
Multisubstituted 2-pyridones are prevalent in pharmaceuticals and bioactive molecules. We herein report an efficient and regioselective approach for the synthesis of trisubstituted 2-pyridone derivatives by a rhodium-catalyzed C–H activation/Lossen rearrangement/cyclization cascade reaction between acrylamides and propargyl alcohols. The desirable features of this protocol include a reusable catalytic system, high regioselectivity, uncommon Lossen rearrangement, good functional group tolerance, operation at room temperature, simple purification by filtration in most cases, and scale-up synthesis with as low as 1 mol% catalyst loading. Additionally, deuterium labeling and KIE assays were performed to investigate the reaction mechanism. The vital effect of the hydroxyl group on propargyl alcohols in determining the regioselectivity was demonstrated by control experiments and DFT calculations. In addition, Mulliken atomic charge analysis of the key intermediates was also carried out to probe the origin of the observed preference for the Lossen rearrangement.
Introduction
2-Pyridone is a prevalent scaffold in organic compounds and bioactive molecules.1 As a class of six-membered aza-heterocycles, the 2-pyridone ring possesses a nitrogen heteroatom and a carbonyl group which can act as a hydrogen bond donor/acceptor in medicinal chemistry. In this regard, 2-pyridone usually serves as a bioisostere for pyridine, amide, and N/O-containing heterocycles. Owing to their unique structures, 2-pyridones have been utilized as effective ligands for C–H functionalization as well as kinase hinge binding motifs.2,3 In addition, the use of a 2-pyridone moiety as bioisosteres commonly has evident influences on the solubility, lipophilicity, and metabolic stability of bioactive compounds. As shown in Fig. 1, 2-pyridone derivatives exhibit a variety of pharmacological activities, such as antifungal, antiepileptic, anticancer, antifibrotic, cardiotonic, and anti-HIV activities.3b,4 Considering the prevalence and importance of the 2-pyridone scaffold, it is appealing to develop efficient methods for the synthesis of 2-pyridones.Over the past decades, as a direct and step-economical strategy to construct heterocycles, C–H activation reactions by transition-metal catalysis have received much attention.5 In this respect, transition-metal catalyzed C–H activation/annulation of benzamides or acrylamides with alkynes has become an efficient method for the preparation of isoquinolones or pyridones (Scheme 1a).6–8 In recent years, propargyl alcohols have been frequently utilized in reactions because of their versatile reactivities.9 Additionally, regioselectivity and chemoselectivity are usually controlled by both the hydroxyl group on propargyl alcohols and the directing groups (DGs) in these C–H activation reactions. For example, a ruthenium-catalyzed C–H activation/[4 + 1] annulation of benzamides and propargyl alcohols was pioneered by Liu and coworkers, in which only one carbon of propargyl alcohols was involved in the cyclization (Scheme 1b).9b Very recently, by employing the pivaloyl group to replace the ethyl group of directing groups, we reported a green and efficient rhodium-catalyzed C–H activation/annulation of N-(pivaloyloxy)benzamides and propargyl alcohols for the synthesis of isoquinolones (Scheme 1c).10
Given the prevalence and importance of 2-pyridones, we wish to develop an efficient and novel method to prepare novel multi-substituted 2-pyridone derivatives. Owing to the presence of a hydroxyl group on propargyl alcohols, unique chemoselectivity and regioselectivity are usually achieved. Nevertheless, propargyl alcohols are frequently coupled with aromatic substrates in C–H activation reactions. The C–H activation/annulation of alkenyl substrates and propargyl alcohols for the synthesis of multi-substituted 2-pyridones remains elusive. Inspired by previous works, we speculated whether propargyl alcohols can facilitate some novel transformations when reacted with alkenyl substrates. Thus, we herein report a green and efficient rhodium-catalyzed C–H activation/Lossen rearrangement of acrylamides and propargyl alcohols for the synthesis of novel 2-pyridone derivatives at ambient temperature (Scheme 1d).
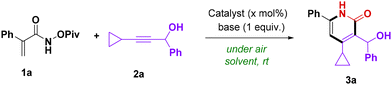
Results and discussion
Optimization studies towards the synthesis of 4-cyclopropyl-3-(hydroxy(phenyl)methyl)-6-phenylpyridin-2(1H)-one 3a are shown in Table 1. In order to develop a green chemical reaction, we first focused on the use of ethyl acetate as the solvent. Then, various transition-metal catalysts were screened in the presence of KOAc at room temperature. No reaction of 2-phenyl-N-(pivaloyloxy)acrylamide 1a with 3-cyclopropyl-1-phenylprop-2-yn-1-ol 2a was promoted by 4 mol% of MnBr(CO)5, [Cp*IrCl2]2, Cp*Co(CO)I2, and [RuCl2(p-cym)]2, while the desired product 3a was obtained in 64% isolated yield when [Cp*RhCl2]2 was used (Table 1, entries 1–5). To our delight, most parts of the product could be isolated by simple filtration, which avoided the use of large amounts of organic solvents for purification. Different solvents were also investigated. Unfortunately, none was superior to our initial choice, EA (entries 6–9). Additive screening revealed that NaOAc and NaHCO3 were the best bases in terms of total isolated yields (entries 10–13). However, the product was obtained in 67% filtration yield in the presence of NaOAc while 58% filtration yield was obtained when NaHCO3 was added. The yield was decreased when KOAc and 2.5 mol% [Cp*RhCl2]2 were used, when compared to entry 5 (entry 14). Similarly, when the base was changed to NaOAc, the total yield also declined to 59% by using 2.5 mol% [Cp*RhCl2]2 (entry 15). The above-mentioned reactions were conducted for 12 hours. Subsequently, the reaction time was decreased to 6 hours. Surprisingly, the yield of 3a improved slightly compared with that obtained in 12 hours (entry 16).| Entry | Catalyst | Additive | Solvent | Yieldb,c (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), catalyst (4 mol%), additive (0.2 mmol), under air, solvent (1.0 mL), room temperature, 12 h. b Total isolated yield. c Isolated yield by filtration is shown in parenthesis. d [Cp*RhCl2]2 (2.5 mol%). e Reaction time was 6.0 h. | ||||
| 1 | MnBr(CO)5 | KOAc | EA | 0 |
| 2 | [Cp*IrCl2]2 | KOAc | EA | 0 |
| 3 | Cp*Co(CO)I2 | KOAc | EA | 0 |
| 4 | [Ru(p-cym)Cl2]2 | KOAc | EA | 0 |
| 5 | [Cp*RhCl2]2 | KOAc | EA | 64 (59) |
| 6 | [Cp*RhCl2]2 | KOAc | EtOH | 22 (16) |
| 7 | [Cp*RhCl2]2 | KOAc | TFE | 40 |
| 8 | [Cp*RhCl2]2 | KOAc | DCM | 44 |
| 9 | [Cp*RhCl2]2 | KOAc | Acetone | 44 (36) |
| 10 | [Cp*RhCl2]2 | NaOAc | EA | 69 (67) |
| 11 | [Cp*RhCl2]2 | Na2CO3 | EA | 58 (47) |
| 12 | [Cp*RhCl2]2 | NaHCO3 | EA | 69 (58) |
| 13 | [Cp*RhCl2]2 | CsOAc | EA | 44 (37) |
| 14d | [Cp*RhCl2]2 | KOAc | EA | 57 (35) |
| 15d | [Cp*RhCl2]2 | NaOAc | EA | 59 (51) |
| 16e | [Cp*RhCl2]2 | NaOAc | EA | 73 (70) |
The optimal reaction conditions were then applied to various substrates, as summarized in Scheme 2. Acrylamides reacted well with different propargyl alcohols. When the cyclopropyl group was placed at R2, both electron-donating (Me and OMe) and electron-withdrawing (F, Cl, Br, I, CN, NO2 and CF3) substituents were compatible, with the isolated yields ranging from 39% to 75% (3b–3k). The structure of 3c was confirmed by X-ray crystallographic analysis (CCDC number 2213771 for 3c, see the ESI† for more details). In addition, alkynols containing cyclohexyl, N-Boc protected piperidyl, and a series of heteroaromatic rings such as thienyl, naphthyl and furyl rings also provided the corresponding products smoothly (3m–3q). It is also noteworthy that the corresponding products showed excellent results when using tertiary alcohols, especially toward those naphthenic groups whose yields even surpassed that of the template reaction (3r–3u). This suggested that the reaction could overcome the issue of steric effect. We next investigated phenyl, methyl and n-butyl at R2 and the reactions also took place smoothly with yields of 42%, 78% and 31%, respectively (3v–3x). Besides, the structure of 3w was also confirmed by X-ray crystallographic analysis (CCDC number 2234440 for 3w, see the ESI† for more details). Subsequently, the introduction of other groups at R1 was examined. Obviously, several substituents with different electron perturbations were tolerated and the yields were moderate (3y–3zb). In addition, it was difficult to obtain a few products (3b, 3j, 3n, 3w, 3x, and 3y) just by simple filtration probably due to their good solubility in ethyl acetate.
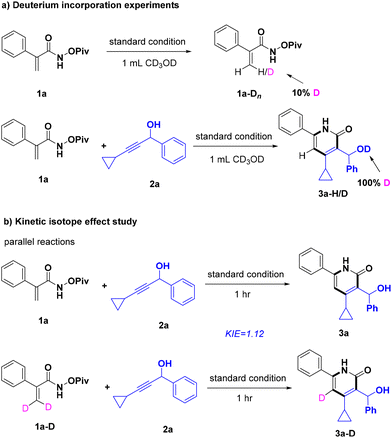
To probe the reaction mechanism, deuterium labeling and KIE assays were carried out (Scheme 3). In the presence of 1a, NaOAc, [Cp*RhCl2]2, and 1.0 mL methanol-d4, about 10% deuterium incorporation occurred at the olefinic bond of acrylamide 1a. The result indicated that the cleavage of the C(sp2)–H bond could be reversible. Approximately 100% deuteration occurred at the hydroxyl group of 3a when 2a was added, and no obvious deuteration was observed at other positions. In addition, two parallel reactions were performed to give a KIE value of 1.12 (Scheme 3b), suggesting that the step of C–H activation was not the rate-determining step.
Besides, to explore the synthetic utility of the methodology, several scale-up experiments were performed with lower catalyst loading. As shown in Scheme 4a, product 3a was obtained in an ideal isolated yield (57%) with 2.5 mol% rhodium catalyst. Then, when 1.0 mol% rhodium catalyst was used, the corresponding product 3a was afforded in 60% yield, respectively. Inspired by these results, we further explored another substrate 2p, and the reaction afforded product 3p in 79% yield. To explore the effect of the hydroxyl group on propargyl alcohols in determining the regioselectivity, two control experiments were conducted (Scheme 4b). The hydroxyl group of 2a was removed to prepare 2a-1 and 2a-2. The annulation of 1a and 2a-1 gave two rearrangement products 4a and 4a′ at the same time with comparable yields. Similarly, the reaction between 1a and 3-cyclopropyl-1-phenylprop-2-yn-1-one 2a-2 also afforded two regioisomers 5a and 5a′ in low yields. The structures of these compounds were determined by using NOESY spectra. A relatively higher regioselectivity could be detected for 2a-2 in comparison with 2a-1; we proposed that the observed regioselectivity might be due to the electron-withdrawing character of the carbonyl moiety. These results indicated that the hydroxyl group plays a vital role in controlling the regioselectivity. Significantly, the recycling experiments of the catalytic system were carried out four times with desirable isolated yields under the standard conditions (Scheme 4c).
Having established the Rh(III)-catalyzed sequential C–H activation/Lossen rearrangement/[4 + 2] annulation cascade of acrylamides with propargyl alcohols, we were next interested to clarify the deep origin of the unconventional regio-/chemoselectivity by detailed DFT calculations. As shown in Scheme 5a, the five-membered rhodacycle INT-0 was rationally selected as the starting point, which coordinated with propargyl alcohol 2a followed by a regioselective migratory alkyne insertion. The calculated results revealed that an additional hydrogen bond affinity between the hydroxyl group and the DG was involved in TS-1 (ΔG‡ = 3.6 kcal mol−1) to give INT-2 with a free energy of −25.2 kcal mol−1, while a relatively higher energy barrier was involved in the converse regioselectivity viaTS-1i (ΔG‡ = 4.8 kcal mol−1). Further IGMH analysis showed obvious hydrogen bond and van der Waals force interactions in TS-1, while only van der Waals force interaction was observed in TS-1i (Scheme 5b). Subsequent coordination change viaTS-2 (ΔG‡ = −14.5 kcal mol−1) afforded INT-3, from which different reaction paths were calculated. The concerted Lossen rearrangement/N–O bond cleavage from INT-3 occurred viaTS-3 with an energy barrier of 7.0 kcal mol−1 (from INT-3 to TS-3) to furnish the isocyanate intermediate INT-4 with an obvious exothermic process. Alternatively, the classic C–N bond reductive elimination viaTS-3ii (ΔG‡ = −0.2 kcal mol−1) involved a higher energy barrier of 23.0 kcal mol−1 (from INT-3 to TS-3ii), which was in line with the experimental result that no 3-phenylpyridin-2(1H)-one framework was formed. Moreover, the Rh(III)–Rh(V)–Rh(III) reaction pathway involving an oxidative addition process from INT-3 was also ruled out owing to the relatively higher free energies of TS-4i/TS-4ii. Taken together, the DFT calculations illustrated a hydrogen bond assisted regioselective alkyne insertion/Lossen rearrangement/intramolecular [4 + 2] cyclization reaction pathway for the developed protocol. In addition, further Mulliken atomic charge analysis of the key intermediates was also carried out to probe the origin of the observed preference for Lossen rearrangement rather than other reaction manifolds (see the ESI† for details). The results suggested that the C2 atom in INT-3 occupied a relatively more positive charge in comparison with the similar benzamide substrate (0.0346 vs. 0.0216). Thus, it can be inferred that there was an inclination to undergo a nucleophilic attack from the N atom, ultimately leading to the Lossen rearrangement process.
On the basis of the above mechanistic studies and literature precedents, we proposed a plausible catalytic cycle for the developed transformation (Scheme 6). Initially, the active Cp*Rh(OAc)2 species was generated by anion ligand exchange in the presence of NaOAc, which coordinated with the acrylamide substrate and participated in the alkenylic C–H bond activation to afford intermediate A. Subsequent regioselective alkyne insertion led to the formation of intermediate B, in which the hydrogen bond affinity played a crucial role in determining the regioselectivity. Furthermore, the Lossen rearrangement process occurred smoothly to give the isocyanate intermediate C, which underwent an intramolecular [4 + 2] cyclization to deliver the 2-pyridone skeleton D. Finally, the protonolysis of D with the assistance of HOAc released the desired product 3a along with the regeneration of the Rh(III) catalyst.
Conclusions
In summary, a green and efficient rhodium(III)-catalyzed C–H activation/Lossen rearrangement of acrylamides and propargyl alcohols for the synthesis of novel 2-pyridone derivatives at ambient temperature was developed. This protocol features a reusable catalytic system, high regioselectivity, uncommon Lossen rearrangement, good functional group tolerance, metal oxidant-free process, operation at room temperature, simple purification by filtration in most cases, scale-up synthesis, and air compatibility. Additionally, deuterium labeling and KIE assays were performed to investigate the reaction mechanism. The vital effect of the hydroxyl group on propargyl alcohols in determining the regioselectivity was also demonstrated by control experiments and DFT calculations. In addition, Mulliken atomic charge analysis of the key intermediates was also carried out to probe the origin of the observed preference for Lossen rearrangement.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Shanghai Pujiang Program (21PJ1415800), Basic and Applied Basic Research Foundation of Guangdong Province (2021A1515110468), Natural Science Foundation of Guangdong Province (2019A1515010935), High-level New R&D Institute (2019B090904008), High-level Innovative Research Institute (2021B0909050003), and NSFC (21877020, 22007020) is gratefully acknowledged.Notes and references
- (a) M. Hagimori, T. Temma, N. Mizuyama, T. Uto, Y. Yamaguchi, Y. Tominaga, T. Mukai and H. Saji, A high-affinity fluorescent Zn2+ sensor improved by the suppression of pyridine-pyridone tautomerism and its application in living cells, Sens. Actuators, B, 2015, 213, 45–52 CrossRef CAS; (b) G. Li, H. M. Duong, Z. Zhang, J. Xiao, L. Liu, Y. Zhao, H. Zhang, F. Huo, S. Li, J. Ma, F. Wudl and Q. Zhang, Approaching a stable, green twisted heteroacene through “clean reaction” strategy, Chem. Commun., 2012, 48, 5974–5976 RSC; (c) S. Hibi, K. Ueno, S. Nagato, K. Kawano, K. Ito, Y. Norimine, O. Takenaka, T. Hanada and M. Yonaga, Discovery of 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (perampanel): a novel, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonist, J. Med. Chem., 2012, 55, 10584–10600 CrossRef CAS PubMed; (d) M. Bialer and H. S. White, Key factors in the discovery and development of new antiepileptic drugs, Nat. Rev. Drug Discovery, 2010, 9, 68–82 CrossRef CAS PubMed; (e) K. Ishiuchi, T. Kubota, H. Ishiyama, S. Hayashi, T. Shibata and J. Kobayashi, Lyconadins C and F, new Lycopodium alkaloids from Lycopodium complanatum, Tetrahedron Lett., 2011, 52, 289–292 CrossRef CAS; (f) M. Torres, S. Gil and M. Parra, New Synthetic Methods to 2-Pyridone Rings, Curr. Org. Chem., 2005, 9, 1757–1779 CrossRef CAS; (g) I. M. Lagoja, Pyrimidine as Constituent of Natural Biologically Active Compounds, Chem. Biodiversity, 2005, 2, 1–50 CrossRef CAS PubMed; (h) P. Hajek, H. McRobbie and K. Myers, Thorax, 2013, 68, 1037–1042 CrossRef PubMed.
- (a) P. Wang, P. Verma, G. Xia, J. Shi, J. Qiao, S. Tao, P. T. W. Cheng, M. A. Poss, M. E. Farmer, K.-S. Yeung and J.-Q. Yu, Ligand-accelerated non-directed C–H functionalization of arenes, Nature, 2017, 551, 489–493 CrossRef CAS PubMed; (b) Y.-Q. Chen, Z. Wang, Y. Wu, S. R. Wisniewski, J. Qiao, W. R. Ewing, M. D. Eastgate and J.-Q. Yu, Overcoming the Limitations of γ- and δ-C–H Arylation of Amines through Ligand Development, J. Am. Chem. Soc., 2018, 140, 17884–17894 CrossRef CAS PubMed; (c) L.-Y. Liu, K.-S. Yeung and J.-Q. Yu, Ligand-Promoted Non-Directed C–H Cyanation of Arenes, Chem. – Eur. J., 2019, 25, 2199–2202 CrossRef CAS PubMed; (d) X.-Y. Chen, Y. Wu, J. Zhou, P. Wang and J.-Q. Yu, Synthesis of β-Arylethenesulfonyl Fluoride via Pd-Catalyzed Nondirected C-H Alkenylation, Org. Lett., 2019, 21, 1426–1429 CrossRef CAS PubMed; (e) P. Wang, G.-C. Li, P. Jain, M. E. Farmer, J. He, P.-X. Shen and J.-Q. Yu, Ligand-Promoted meta-C-H Amination and Alkynylation, J. Am. Chem. Soc., 2016, 138, 14092–14099 CrossRef CAS PubMed; (f) P. Wang, M. E. Farmer, X. Huo, P. Jain, P. Shen, M. Ishoey, J. E. Bradner, S. R. Wisniewski, M. D. Eastgate and J.-Q. Yu, Ligand-Promoted Meta-C–H Arylation of Anilines, Phenols, and Heterocycles, J. Am. Chem. Soc., 2016, 138, 9269–9276 CrossRef CAS PubMed; (g) R.-Y. Zhu, Z.-Q. Li, H. S. Park, C. H. Senanayake and J.-Q. Yu, Ligand-Enabled γ-C(sp3)–H Activation of Ketones, J. Am. Chem. Soc., 2018, 140, 3564–3568 CrossRef CAS PubMed.
- (a) T. Siu, E. S. Kozina, J. Jung, C. Rosenstein, A. Mathur, M. D. Altman, G. Chan, L. Xu, E. Bachman, J. R. Mo, M. Bouthillette, T. Rush, C. J. Dinsmore, C. G. Marshall and J. R. Young, The discovery of tricyclic pyridone JAK2 inhibitors. Part 1: Hit to lead, Bioorg. Med. Chem. Lett., 2010, 20, 7421–7425 CrossRef CAS PubMed; (b) V. Simov, S. V. Deshmukh, C. J. Dinsmore, F. Elwood, R. B. Fernandez, Y. Garcia, C. Gibeau, H. Gunaydin, J. Jung, J. D. Katz, B. Kraybill, B. Lapointe, S. B. Patel, T. Siu, H. Su and J. R. Young, Structure-based design and development of (benz)imidazole pyridones as JAK1-selective kinase inhibitors, Bioorg. Med. Chem. Lett., 2016, 26, 1803–1808 CrossRef CAS PubMed; (c) J. Bach, P. Eastwood, J. Gonzalez, E. Gómez, J. A. Alonso, S. Fonquerna, E. Lozoya, A. Orellana, M. Maldonado, E. Calaf, J. Albertí, J. Pérez, A. Andrés, N. Prats, C. Carreño, E. Calama, J. D. Alba, M. Calbet, M. Miralpeix and I. Ramis, Identification of 2-Imidazopyridine and 2-Aminopyridone Purinones as Potent Pan-Janus Kinase (JAK) Inhibitors for the Inhaled Treatment of Respiratory Diseases, J. Med. Chem., 2019, 62, 9045–9060 CrossRef CAS PubMed; (d) H. S. Eidam, J. Russell, K. Raha, M. DeMartino, D. Qin, H. A. Guan, Z. Zhang, G. Zhen, H. Yu, C. Wu, Y. Pan, G. Joberty, N. Zinn, S. Laquerre, S. Robinson, A. White, A. Giddings, E. Mohammadi, B. G.-V. Meerveld, A. Oliff, S. Kumar and M. Cheung, Discovery of a First-in-Class Gut-Restricted RET Kinase Inhibitor as a Clinical Candidate for the Treatment of IBS, ACS Med. Chem. Lett., 2018, 9, 623–628 CrossRef PubMed; (e) A. J. Massey, S. Stokes, H. Browne, N. Foloppe, A. Fiumana, S. Scrace, M. Fallowfield, S. Bedford, P. Webb, L. Baker, M. Christie, M. J. Drysdale and M. Wood, Identification of novel, in vivo active Chk1 inhibitors utilizing structure guided drug design, Oncotarget, 2015, 6, 35797–35812 CrossRef PubMed; (f) M. C. Bryan, D. J. Burdick, B. K. Chan, Y. Chen, S. Clausen, J. Dotson, C. Eigenbrot, R. Elliott, E. J. Hanan, R. Heald, P. Jackson, H. La, M. Lainchbury, S. Malek, S. E. Mann, H. E. Purkey, G. Schaefer, S. Schmidt, E. Seward, S. Sideris, S. Wang, I. Yen, C. Yu and T. P. Heffron, Pyridones as Highly Selective, Noncovalent Inhibitors of T790M Double Mutants of EGFR, ACS Med. Chem. Lett., 2016, 7, 100–104 CrossRef CAS PubMed.
- (a) M. Niewerth, D. Kunze, M. Seibold, M. Schaller, H. C. Korting and B. Hube, Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors, Antimicrob. Agents Chemother., 2003, 47, 1805–1817 CrossRef CAS PubMed; (b) T. Chen, S.-H. Dai, Z.-Q. Jiang, P. Luo, X.-F. Jiang, Z. Fei, S.-B. Gui and Y.-L. Qi, The AMPAR Antagonist Perampanel Attenuates Traumatic Brain Injury Through Anti-Oxidative and Anti-Inflammatory Activity, Cell. Mol. Neurobiol., 2017, 37, 43–52 CrossRef CAS PubMed; (c) H. Nakazato, H. Oku, S. Yamane, Y. Tsuruta and R. Suzuki, A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level, Eur. J. Pharmacol., 2002, 20, 177–185 CrossRef PubMed; (d) Z. A. Kalme, R. A. Zhalubovskis, A. Shmidlers, J. Celmins and G. Duburs, Synthesis of 6-Bromomethyl-Substituted D Derivatives of Pyridin-2(1H)-Ones and Their Reaction with Nucleophiles, Chem. Heterocycl. Compd., 2004, 40, 862–868 CrossRef CAS; (e) M.-T. Lai, M. Feng, J.-P. Falgueyret, P. Tawa, M. Witmer, D. DiStefano, Y. Li, J. Burch, N. Sachs, M. Lu, E. Cauchon, L.-C. Campeau, J. Grobler, Y. Yan, Y. Ducharme, B. Côté, E. Asante-Appiah, D. J. Hazuda and M. D. Miller, In vitro characterization of MK-1439, a novel HIV-1 nonnucleoside reverse transcriptase inhibitor, Antimicrob. Agents Chemother., 2014, 58, 1652–1663 CrossRef PubMed.
- Selected recent reviews: (a) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, 3d Transition Metals for C–H Activation, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed; (b) D. J. Abrams, P. A. Provencher and E. J. Sorensen, Recent applications of C-H functionalization in complex natural product synthesis, Chem. Soc. Rev., 2018, 47, 8925–8967 RSC; (c) C. Sambiagio, D. Schönbauer, R. Blieck, T. D. Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. W. Delord, T. Besset, B. U. W. Maes and M. Schnürch, A comprehensive overview of directing groups applied in metal-catalysed C-H functionalisation chemistry, Chem. Soc. Rev., 2018, 47, 6603–6743 RSC; (d) Y. Yang, J. Lan and J. You, Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes, Chem. Rev., 2017, 117, 8787–8863 CrossRef CAS PubMed; (e) T. Gensch, M. N. Hopkinson, F. Glorius and J. W. Delord, Mild metal-catalyzed C-H activation: examples and concepts, Chem. Soc. Rev., 2016, 45, 2900–2936 RSC; (f) T. Yamada, Y. Shibata, S. Kawauchi, S. Yoshizaki and K. Tanaka, Formal Lossen Rearrangement/[3+2] Annulation Cascade Catalyzed by a Modified Cyclopentadienyl RhIII Complex, Chem. – Eur. J., 2018, 24, 5723–5727 CrossRef CAS PubMed; (g) C.-Q. Wang, Y. Zhang and C. Feng, Fluorine Effects on Group Migration via a Rhodium(V) Nitrenoid Intermediate, Angew. Chem., Int. Ed., 2017, 56, 14918–14922 CrossRef CAS PubMed; (h) T. Yamada, Y. Shibata and K. Tanaka, Formal Lossen Rearrangement/Alkenylation or Annulation Cascade of Heterole Carboxamides with Alkynes Catalyzed by CpRhIII Complexes with Pendant Amides, Chem. – Eur. J., 2019, 25, 16022–16031 CrossRef CAS PubMed; (i) M. Bian, H. Mawjuda, H. Gao, H. Xu, Z. Zhou and W. Yi, Lossen Rearrangement vs C–N Reductive Elimination Enabled by Rh(III)-Catalyzed C–H Activation/Selective Lactone Ring-Opening: Chemodivergent Synthesis of Quinolinones and Dihydroisoquinolinones, Org. Lett., 2020, 22, 9677–9682 CrossRef CAS PubMed.
- (a) S. Mochida, N. Umeda, K. Hirano, T. Satoh and M. Miura, Rhodium-catalyzed Oxidative Coupling/Cyclization of Benzamides with Alkynes via C–H Bond Cleavage, Chem. Lett., 2010, 39, 744–746 CrossRef CAS; (b) T. K. Hyster and T. Rovis, Rhodium-Catalyzed Oxidative Cycloaddition of Benzamides and Alkynes via C–H/N–H Activation, J. Am. Chem. Soc., 2010, 132, 10565–10569 CrossRef CAS PubMed; (c) G. Song, D. Chen, C. Pan, R. H. Crabtree and X. Li, Rh-Catalyzed Oxidative Coupling between Primary and Secondary Benzamides and Alkynes: Synthesis of Polycyclic Amides, J. Org. Chem., 2010, 75, 7487–7490 CrossRef CAS PubMed; (d) N. Guimond, C. Gouliaras and K. Fagnou, Rhodium(III)-catalyzed isoquinolone synthesis: the N-O bond as a handle for C-N bond formation and catalyst turnover, J. Am. Chem. Soc., 2010, 132, 6908–6909 CrossRef CAS PubMed; (e) N. Guimond, S. I. Gorelsky and K. Fagnou, Rhodium(III)-catalyzed heterocycle synthesis using an internal oxidant: improved reactivity and mechanistic studies, J. Am. Chem. Soc., 2011, 133, 6449–6457 CrossRef CAS PubMed.
- (a) Y. Su, M. Zhao, K. Han, G. Song and X. Li, Synthesis of 2-pyridones and iminoesters via Rh(III)-catalyzed oxidative coupling between acrylamides and alkynes, Org. Lett., 2010, 12, 5462–5465 CrossRef CAS PubMed; (b) T. K. Hyster and T. Rovis, An Improved Catalyst Architecture for Rhodium(III) Catalyzed C-H Activation and its Application to Pyridone Synthesis, Chem. Sci., 2011, 2, 1606–1610 RSC; (c) X. Xu, Y. Liu and C.-M. Park, Rhodium(III)-catalyzed intramolecular annulation through C-H activation: total synthesis of (±)-antofine, (±)-septicine, (±)-tylophorine, and rosettacin, Angew. Chem., Int. Ed., 2012, 51, 9372–9376 CrossRef CAS PubMed; (d) N. Quiñones, A. Seoane, R. G. Fandiño, J. L. Mascareñas and M. Gulías, Rhodium(III)-catalyzed intramolecular annulations involving amide-directed C–H activations: synthetic scope and mechanistic studies, Chem. Sci., 2013, 4, 2874–2879 RSC; (e) J. P. Krieger, D. Lesuisse, G. Ricci, M. A. Perrin, C. Meyer and J. Cossy, Rhodium(III)-Catalyzed C-H Activation/Heterocyclization as a Macrocyclization Strategy. Synthesis of Macrocyclic Pyridones, Org. Lett., 2017, 19, 2706–2709 CrossRef CAS PubMed; (f) L. Song, G. Tian, J. van der Eycken and E. V. Van der Eycken, Intramolecular cascade annulation triggered by rhodium(III)-catalyzed sequential C(sp2)-H activation and C(sp3)-H amination, Beilstein J. Org. Chem., 2019, 15, 571–576 CrossRef CAS PubMed.
- (a) L. Ackermann, A. V. Lygin and N. Hofmann, Ruthenium-catalyzed oxidative synthesis of 2-pyridones through C-H/N-H bond functionalizations, Org. Lett., 2011, 13, 3278–3281 CrossRef CAS PubMed; (b) R. N. P. Tulichala, M. Shankar and K. C. K. Swamy, Ruthenium-Catalyzed Oxidative Annulation and Hydroarylation of Chromene-3-carboxamides with Alkynes via Double C-H Functionalization, J. Org. Chem., 2017, 82, 5068–5079 CrossRef CAS PubMed; (c) D. N. Garad and S. B. Mhaske, Ru-Catalyzed Regioselective Cascade Annulation of Acrylamides with 2-Alkynoates for the Synthesis of Various 6-Oxo Nicotinic Acid Esters, J. Org. Chem., 2019, 84, 1863–1870 CrossRef CAS PubMed; (d) L. Grigorjeva and O. Daugulis, Cobalt-catalyzed, aminoquinoline-directed C(sp2)-H bond alkenylation by alkynes, Angew. Chem., Int. Ed., 2014, 53, 10209–10212 CrossRef CAS PubMed; (e) A. Lerchen, T. Knecht, M. Koy, C. G. Daniliuc and F. Glorius, A General Cp*CoIII-Catalyzed Intramolecular C–H Activation Approach for the Efficient Total Syntheses of Aromathecin, Protoberberine, and Tylophora Alkaloids, Chem. – Eur. J., 2017, 23, 12149–12152 CrossRef CAS PubMed; (f) Y. Yu, L. Huang, W. Wu and H. Jiang, Palladium-catalyzed oxidative annulation of acrylic acid and amide with alkynes: a practical route to synthesize α-pyrones and pyridines, Org. Lett., 2014, 16, 2146–2149 CrossRef CAS PubMed; (g) T. Matsubara, L. Ilies and E. Nakamura, Oxidative C-H Activation Approach to Pyridone and Isoquinolone through an Iron-Catalyzed Coupling of Amides with Alkynes, Chem. – Asian J., 2016, 11, 380–384 CrossRef CAS PubMed; (h) B. Ma, P. Wu, X. Wang, Z. Wang, H.-X. Lin and H.-X. Dai, Efficient synthesis of Spirooxindole Pyrrolones by a Rhodium(III)-Catalyzed C-H Activation/Carbene Insertion/Lossen Rearrangement Sequence, Angew. Chem., Int. Ed., 2019, 58, 13335–13339 CrossRef CAS PubMed; (i) J.-F. Tan, C. T. Bormann, K. Severin and N. Cramer, Alkynyl Triazenes as Fluoroalkyne Surrogates: Regioselective Access to 4-Fluoro-2-pyridones by a Rh(III)-Catalyzed C–H Activation–Lossen Rearrangement–Wallach Reaction, ACS Catal., 2020, 10, 3790–3796 CrossRef CAS.
- (a) F. Wang, Z. Qi, J. Sun, X. Zhang and X. Li, Rh(III)-catalyzed coupling of benzamides with propargyl alcohols via hydroarylation-lactonization, Org. Lett., 2013, 15, 6290–6293 CrossRef CAS PubMed; (b) X. Wu, B. Wang, S. Zhou, Y. Zhou and H. Liu, Ruthenium-Catalyzed Redox-Neutral [4 + 1] Annulation of Benzamides and Propargyl Alcohols via C–H Bond Activation, ACS Catal., 2017, 4, 2494–2499 CrossRef; (c) W. Yi, W. Chen, F.-X. Liu, Y. Zhong, D. Wu, Z. Zhou and H. Gao, Rh(III)-Catalyzed and Solvent-Controlled Chemoselective Synthesis of Chalcone and Benzofuran Frameworks via Synergistic Dual Directing Groups Enabled Regioselective C–H Functionalization: A Combined Experimental and Computational Study, ACS Catal., 2018, 8, 9508–9519 CrossRef CAS; (d) W. Gong, Z. Zhou, J. Shi, B. Wu, B. Huang and W. Yi, Catalyst-Controlled [3 + 2] and [4 + 2] Annulations of Oximes with Propargyl Alcohols: Divergent Access to Indenamines and Isoquinolines, Org. Lett., 2018, 20, 182–185 CrossRef CAS PubMed; (e) X. Wu, B. Wang, Y. Zhou and H. Liu, Propargyl Alcohols as One-Carbon Synthons: Redox-Neutral Rhodium(III)-Catalyzed C-H Bond Activation for the Synthesis of Isoindolinones Bearing a Quaternary Carbon, Org. Lett., 2017, 19, 1294–1297 CrossRef CAS PubMed; (f) W. Chen, F.-X. Liu, W. Gong, Z. Zhou, H. Gao, J. Shi, B. Wu and W. Yi, Hydroxyl Group-Prompted and Iridium(III)-Catalyzed Regioselective C–H Annulation of N-phenoxyacetamides with Propargyl Alcohols, Adv. Synth. Catal., 2018, 360, 2470–2475 CrossRef CAS; (g) X. Wu and H. Ji, Rhodium-Catalyzed [4 + 1] Cyclization via C-H Activation for the Synthesis of Divergent Heterocycles Bearing a Quaternary Carbon, J. Org. Chem., 2018, 83, 4650–4656 CrossRef CAS PubMed; (h) W. Zhou, Y.-L. Mei, B. Li, Z.-Y. Guan and Q.-H. Deng, Synthesis of β-Alkyl 2-Hydroxychalcones by Rhodium-Catalyzed Coupling of N-Phenoxyacetamides and Nonterminal Propargyl Alcohols, Org. Lett., 2018, 20, 5808–5812 CrossRef CAS PubMed; (i) X. Yan, R. Ye, H. Sun, J. Zhong, H. Xiang and X. Zhou, Synthesis of 2-Arylindoles by Rhodium-Catalyzed/Copper-Mediated Annulative Coupling of N-Aryl-2-aminopyridines and Propargyl Alcohols via Selective C-H/C-C Activation, Org. Lett., 2019, 21, 7455–7479 CrossRef CAS PubMed; (j) X. Hu, X. Chen, Y. Zhu, Y. Deng, H. Zeng, H. Jiang and W. Zeng, Rh(III)-Catalyzed Carboamination of Propargyl Cycloalkanols with Arylamines via Csp2-H/Csp3-Csp3 Activation, Org. Lett., 2017, 19, 3474–3477 CrossRef CAS PubMed; (k) J.-L. Pan, C. Liu, C. Chen, T.-Q. Liu, M. Wang, Z. Sun and S.-Y. Zhang, Dual Directing-Groups-Assisted Redox-Neutral Annulation and Ring Opening of N-Aryloxyacetamides with 1-Alkynylcyclobutanols via Rhodium(III)-Catalyzed C-H/C-C Activations, Org. Lett., 2019, 21, 2823–2827 CrossRef CAS PubMed; (l) A. Anukumar, M. Tamizmani and M. Jeganmohan, Ruthenium(II)-Catalyzed Regioselective-Controlled Allenylation/Cyclization of Benzimides with Propargyl Alcohols, J. Org. Chem., 2018, 83, 8567–8580 CrossRef CAS PubMed; (m) P. Sihag and M. Jeganmohan, Regioselective Synthesis of Isocoumarins via Iridium(III)-Catalyzed Oxidative Cyclization of Aromatic Acids with Propargyl Alcohols, J. Org. Chem., 2019, 84, 2699–2712 CrossRef CAS PubMed; (n) Y. Xu, M. Shen, X. Zhang and X. Fan, Selective Synthesis of Pyrazolo[1,2-a]pyrazolones and 2-Acylindoles via Rh(III)-Catalyzed Tunable Redox-Neutral Coupling of 1-Phenylpyrazolidinones with Alkynyl Cyclobutanols, Org. Lett., 2020, 22, 4697–4702 CrossRef CAS PubMed; (o) L. Zhang, J. Chen, X. Chen, X. Zheng, J. Zhou, T. Zhong, Z. Chen, Y.-F. Yang, X. Jiang, Y.-B. She and C. Yu, Rh(III)-catalyzed, hydrazine-directed C-H functionalization with 1-alkynylcyclobutanols: a new strategy for 1H-indazoles, Chem. Commun., 2020, 56, 7415–7418 RSC; (p) X. Song, B. N. D. Doan, X. Zhang, R. Lee and X. Fan, Complementary C-H Functionalization Mode of Benzoylacetonitriles: Computer-Augmented Study of a Regio- and Stereoselective Synthesis of Functionalized Benzofulvenes, Org. Lett., 2020, 22, 46–51 CrossRef CAS PubMed; (q) Y. Xu, B. Li, X. Zhang and X. Fan, One-Pot Synthesis of Fused N,O-Heterocycles through Rh(III)-Catalyzed Cascade Reactions of Aromatic/Vinylic N-Alkoxyamides with 4-Hydroxy-2-alkynoates, Adv. Synth. Catal., 2018, 360, 2613–2620 CrossRef CAS; (r) L. Zhang, Y. Xu, X. Zhang, X. Zhang and X. Fan, Synthesis of pyrazolone fused benzodiazepines via Rh(III)-catalyzed [4 + 3] annulation of 1-phenylpyrazolidinones with propargyl alcohols, Org. Chem. Front., 2020, 7, 2284–2290 RSC; (s) M. Sen, P. Dahiya, J. R. Premkumar and B. Sundararaju, Dehydrative Cp*Co(III)-Catalyzed C-H Bond Allenylation, Org. Lett., 2017, 19, 3699–3702 CrossRef CAS PubMed; (t) W. Kong, L. H. Finger, A. M. Messinis, R. Kuniyil, J. C. A. Oliveira and L. Ackermann, Flow Rhodaelectro-Catalyzed Alkyne Annulations by Versatile C-H Activation: Mechanistic Support for Rhodium(III/IV), J. Am. Chem. Soc., 2019, 141, 17198–17206 CrossRef CAS PubMed; (u) X. Wu, P. Li, Y. Lu, J. Qiao, J. Zhao, X. Jia, H. Ni, L. Kong, X. Zhang and F. Zhao, Rhodium-Catalyzed Cascade Reactions of Indoles with 4-Hydroxy-2-Alkynoates for the Synthesis of Indole-Fused Polyheterocycles, Adv. Synth. Catal., 2020, 362, 2953–2960 CrossRef CAS; (v) F. Zhao, Z. Zhou, Y. Lu, J. Qiao, X. Zhang, X. Gong, S. Liu, S. Lin, X. Wu and W. Yi, Chemo-, Regio-, and Stereoselective Assembly of Polysubstituted Furan-2(5H)-ones Enabled by Rh(III)-Catalyzed Domino C-H Alkenylation/Directing Group Migration/Lactonization: A Combined Experimental and Computational Study, ACS Catal., 2021, 11, 13921–13934 CrossRef CAS; (w) Y. Xu, F. Wang, S. Yu and X. Li, Rhodium(III)-catalyzed selective access to isoindolinones via formal [4+1] annulation of arylamides and propargyl alcohols, Chin. J. Catal., 2017, 38, 1390–1398 CrossRef CAS.
- H. Meng, H. Xu, Z. Zhou, Z. Tang, Y. Li, Y. Zhou, W. Yi and X. Wu, Recyclable rhodium-catalyzed C-H activation/[4+2] annulation with unconventional regioselectivity at ambient temperature: experimental development and mechanistic insight, Green Chem., 2022, 24, 7012–7021 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2213771 (3c) and 2234440 (3w). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo00469d |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |