Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold(I)-catalyzed cycloisomerization of alcohol or amine tethered-vinylidenecyclopropanes providing access to morpholine, piperazine or oxazepane derivatives: a carbene versus non-carbene process†
Jun-Sheng
Wei
a,
Song
Yang
a,
Yin
Wei
 b,
Sima
Shamsaddinimotlagh
c,
Hossein
Tavakol
*c and
Min
Shi
b,
Sima
Shamsaddinimotlagh
c,
Hossein
Tavakol
*c and
Min
Shi
 *ab
*ab
aKey Laboratory for Advanced Materials and Institute of Fine Chemicals, School of Chemistry & Molecular Engineering, East China University of Science and Technology, 130 Mei Long Road, Shanghai 200237, People's Republic of China
bState Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, University of Chinese Academy of Sciences, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: mshi@sioc.ac.cn
cDepartment of Chemistry, Isfahan University of Technology, Isfahan 84156-83111, Iran. E-mail: h_tavakol@cc.iut.ac.ir
First published on 27th February 2023
Abstract
A gold(I)-catalyzed intramolecular cyclization of alcohol or amine tethered-vinylidenecyclopropanes via a carbene or non-carbene process was developed to afford functionalized morpholines, piperazines and oxazepanes in good yields with a broad scope and excellent functional group tolerance under mild conditions. The steric bulkiness or chain length can modulate the reaction pathway. Substrates with a less sterically hindered group located at the allene moiety afford morpholines or piperazines containing an alkylidenecyclopropane via the non-carbene process, while sterically bulky ones give morpholines or piperazines containing a cyclobutene unit through the carbene process. Moreover, extending the carbon chain length of vinylidenecyclopropane enables the formation of seven-membered oxazepane via the carbene process. The synthetic utility of this protocol was also highlighted by its gram-scale synthesis and various transformations of the cyclization products.
Morpholine, piperazine and oxazepane derivatives have gained extensive attention due to their positive physicochemical, metabolic and biological properties.1 They are biologically active nitrogen-containing heterocycles involved in a variety of natural products and pharmaceuticals, such as the antibiotic drug finafloxacin,2 antianginal drug trimetazidine,3 antidepressant agents reboxetine4 and befuraline,5 dopamine D4 receptor ligands,6 noradrenaline reuptake inhibitors7 and so on (Fig. 1). Although numerous methodologies have been established for the synthesis of these useful scaffolds,8 the construction of functionalized morpholine, piperazine or oxazepane derivatives still remains challenging.
 | ||
| Fig. 1 Selected biologically active compounds containing morpholine, piperazine and 1,4-oxazepane skeletons. | ||
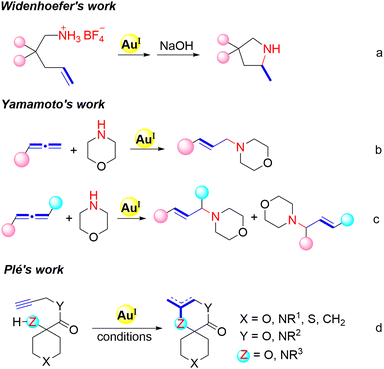
Recently, gold(I)-catalyzed nucleophilic additions to C–C multiple bonds as a popular research branch of gold chemistry have made significant progress.9 Over the past two decades, considerable advancements have been made in gold(I)-catalyzed addition of X–H (X = O, N, C) bonds to unsaturated carbon–carbon bonds of olefins,10 alkynes11 and allenes.12 For instance, Widenhoefer et al.10a reported the first example of gold(I)-catalyzed intramolecular hydroamination of unactivated alkenes, efficiently affording various tetrahydropyrrole and hexahydropiperidine skeletons (Scheme 1a). Later, Yamamoto et al.12a reported the gold(I)-catalyzed intermolecular hydroamination between allenes and morpholine. The appropriate steric environment around gold catalysts plays a pivotal role in carrying out such aliphatic hydroamination. In addition, different from monosubstituted allenes (Scheme 1b), 1,3-disubstituted allenes usually gave a regioisomeric mixture and its ratio also depends on the steric effects (Scheme 1c). Moreover, an analogous cyclization reaction of alkynes has also been developed by Plé's group recently,11a giving N- and O-spirocycles in good to excellent yields (Scheme 1d).
In the meantime, it should be mentioned that catalytic transformations involving gold carbenes as key intermediates are also considered as some of the most important aspects of homogeneous gold catalysis.13 To enrich the chemistry of gold carbene, a gold(I)-catalyzed cycloisomerization or allyl transfer of vinylidenecyclopropane-ene derivatives via controllable carbene or non-carbene processes has been explored in our laboratory,14 providing a new synthetic route for the construction of O-bearing heterocyclic scaffolds containing a fused tricyclic system and fused five- and six-membered ring systems. Substituents adjacent to the oxygen atom on the aryl groups could switch the reaction mode (Scheme 2a).
 | ||
| Scheme 2 Our previous work of gold(I)-catalyzed cycloisomerization of vinylidenecyclopropane-enes via a carbene or non-carbene process and this work. | ||
Therefore, vinylidenecyclopropanes (VDCPs) can be realized as excellent candidates for gold(I)-catalyzed cycloisomerization due to their multiple reaction sites.14,15 During our ongoing efforts on gold-catalyzed cycloisomerization of VDCPs, we subsequently designed and synthesized a series of VDCPs tethered with an alcohol or amine moiety to explore their reaction outcomes in gold(I)-catalyzed intramolecular cyclization (Scheme 2b, this work). Interestingly, we found that the cyclization could proceed through a carbene or a non-carbene process as well depending on the substrate's steric effect or the connected carbon chain length, affording various morpholine, piperazine or oxazepane derivatives in good yields under mild conditions. Herein, we wish to report the details in this context.
At the beginning of our investigation, VDCP 1a was selected as the model substrate to optimize the reaction conditions of this gold(I)-catalyzed intramolecular cyclization. As shown in Table 1, a series of gold catalysts have been screened using AgNTf2 as a silver salt additive and a pre-prepared JohnPhosAu(MeCN)SbF6 complex upon heating in chlorobenzene at 90 °C (Table 1, entries 1–8), and it was found that the product 2a was obtained in poor to moderate yields with complete consumption of substrate 1a, and XphosAuCl was identified as the best gold(I) catalyst in this reaction, affording 2a in 43% yield. Lowering the reaction temperature to 50 °C gave 2a in 60% yield (Table 1, entry 9) and then, we found that by carrying out the reaction at room temperature and prolonging the reaction time, the yield of 2a could be improved to 76% (Table 1, entry 10). The examination of the solvent effect with various commonly used solvents revealed that THF was the best solvent, giving 2a in 95% NMR yield along with 87% isolated yield (Table 1, entries 11–16). During the screening of solvents, it is surmised that the use of acetonitrile as the solvent resulted in essentially complete recovery of the starting material without observing the formation of the product presumably due to its coordination with the monovalent gold(I) catalyst (Table 1, entry 12). Notably, compared to the in situ generation of the cationic gold catalyst, using cationic gold catalyst XPhosAuNTf2 slightly reduced the yield of 2a (Table 1, entry 17). Furthermore, the effect of silver salts on the reaction outcome was explored, identifying that AgNTf2 led to a better result than other silver additives such as AgBF4, AgOTf, and AgSbF6 (Table 1, entries 18–20). No conversion was observed under the same conditions when the silver salt additive was replaced with NaBArF (Table 1, entry 21). As a consequence, alkylidenecyclopropane functionalized morpholine derivative 2a was obtained in 87% isolated yield with excellent regioselectivity using 5 mol% XPhosAuCl as the catalyst and 5 mol% AgNTf2 as the silver salt additive in THF at room temperature under an ambient atmosphere, which serve as the optimal conditions for the production of 2a. The control experiments illustrated that silver salts such as AgNTf2, Brønsted acid HNTf2, and XPhosAuCl did not catalyze the reaction at all, indicating that this cyclization is indeed catalyzed by the gold(I) complex (see S8 in the ESI†). The crystal structure of 2a was unequivocally determined by X-ray diffraction as shown in Table 1, and the ORTEP drawing and its CIF data are presented in the ESI.†
| Entrya | Catalyst | Time (h) | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a The reaction scale is 0.1 mmol of 1a in untreated solvent (0.04 M). b Yield was determined from 1H NMR spectroscopic data using 1,3,5-trimethoxybenzene as an internal standard. c Isolated yield. | |||||
| 1 | IPrAuCl, AgNTf2 | 12 | PhCl | 90 | Trace |
| 2 | (4-CF3Ph)3PAuCl, AgNT2 | 12 | PhCl | 90 | Trace |
| 3 | iPr3PAuCl, AgNTf2 | 12 | PhCl | 90 | Trace |
| 4 | JohnPhosAuCl, AgNTf2 | 12 | PhCl | 90 | 11 |
| 5 | PPh3AuCl, AgNTf2 | 12 | PhCl | 90 | 35 |
| 6 | t Bu3PAuCl, AgNTf2 | 12 | PhCl | 90 | 12 |
| 7 | JohnPhosAu(MeCN)SbF6 | 12 | PhCl | 90 | 38 |
| 8 | XphosAuCl, AgNTf2 | 12 | PhCl | 90 | 43 |
| 9 | XphosAuCl, AgNTf2 | 12 | PhCl | 50 | 60 |
| 10 | XphosAuCl, AgNTf2 | 16 | PhCl | rt | 76 |
| 11 | XphosAuCl, AgNTf2 | 16 | 1,4-Dioxane | rt | 54 |
| 12 | XphosAuCl, AgNTf2 | 16 | CH3CN | rt | Trace |
| 13 | XphosAuCl, AgNTf2 | 16 | DCE | rt | 40 |
| 14 | XphosAuCl, AgNTf2 | 16 | THF | rt | 95 (87)c |
| 15 | XphosAuCl, AgNTf2 | 16 | DCM | rt | 46 |
| 16 | XphosAuCl, AgNTf2 | 16 | Toluene | rt | 68 |
| 17 | XphosAuNTf2 | 16 | THF | rt | 82 |
| 18 | XphosAuCl, AgBF4 | 16 | THF | rt | 50 |
| 19 | XphosAuCl, AgOTf | 16 | THF | rt | 83 |
| 20 | XphosAuCl, AgSbF6 | 16 | THF | rt | 86 |
| 21 | XphosAuCl, NaBArF | 16 | THF | rt | Trace |
Next, we synthesized a variety of alcohol or amine tethered vinylidenecyclopropanes with different R groups located at allene moieties to explore the generality of this cyclization under the optimal conditions and the results are shown in Scheme 3. For alcohol tethered VDCPs 1b–1g, in which the R group was a linear alkyl substituent, an alkyl chain with a terminal protected aldehyde group, a homoallyl group or a benzyl group, the reaction was tolerated, affording the corresponding products 2b–2g in 58–87% yields along with a trace amount of inseparable cyclized compound 2′ derived from a gold–carbene induced cyclization. Remarkably, in the case of VDCP 1h bearing a cyclopropane at the allene moiety, the desired product 2h was formed in 74% yield as a single isomer perhaps due to the less steric hindrance of the cyclopropyl ring. Besides N-Ts protected VCDPs, N-phenylsulfonyl protected substrate 1i and N-Bs protected substrate 1j also performed very well, affording the desired products 2i and 2j in 98% and 87% yields, respectively. Substrates 1k and 1l connected by an oxygen atom or a carbon atom were also compatible, giving the corresponding products 2k and 2l in good yields ranging from 90% to 99% under the standard conditions. We were delighted to find that by replacing primary alcohol with a secondary alcohol, the substrate 1m delivered the desired product 2m in 92% yield as a single diastereoisomer and its stereochemical configuration was determined by 1H NMR NOESY spectroscopic analysis. Moreover, amine tethered vinylidenecyclopropanes 1n and 1o gave the desired functionalized piperazines 2n and 2o as single isomers in 77% and 85% yields, respectively, illustrating that this reaction has excellent functional group tolerance. Interestingly, the use of VDCP 1p as the substrate, which had a cyclobutane at the allene moiety, resulted in an inseparable mixture of 2p and 2p′, which contains a cyclobutene moiety, in an 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio as determined by 1H NMR analysis presumably due to its steric effect. Notably, when replacing the benzylamine unit with a tosylated amine group such as VDCP 1z, no corresponding product could be obtained, perhaps due to its weaker nucleophilicity. In addition, aryl-substituted VDCPs 1aa–1ac were not suitable substrates for this gold-catalyzed transformation, presumably because the aryl substituents on the allene moiety make them difficult to polarize by cationic gold species in our reaction system. Substrate 1ad also failed to give the corresponding products under the standard conditions.
5 ratio as determined by 1H NMR analysis presumably due to its steric effect. Notably, when replacing the benzylamine unit with a tosylated amine group such as VDCP 1z, no corresponding product could be obtained, perhaps due to its weaker nucleophilicity. In addition, aryl-substituted VDCPs 1aa–1ac were not suitable substrates for this gold-catalyzed transformation, presumably because the aryl substituents on the allene moiety make them difficult to polarize by cationic gold species in our reaction system. Substrate 1ad also failed to give the corresponding products under the standard conditions.
In order to gain a better understanding of the reaction processes, several sterically bulky R groups were introduced into the substrates, and we found that all of them could give the cyclobutene containing cyclized products 2q′–2t′ as major products in 56–86% yields (Scheme 4). The crystal structure of 2q′ was unambiguously determined by X-ray diffraction. The ORTEP drawing is given in Scheme 4 and its CIF data are also summarized in the ESI.† Particularly, different from VDCP 1p, cyclobutyl containing amine tethered vinylidenecyclopropane 1v furnished the desired product 2v′ as a sole product in 63% yield via a gold carbene process, indicating that the tethered nucleophile could also significantly affect the reaction pathway. To our surprise, further investigations revealed that our reaction system enables the formation of seven-membered 1,4-oxazepanes 2w′–2y′ in 67–88% yields as a sole product through a gold carbene process, indicating that the connected alkyl chain length of the substrate can modulate the reaction process.
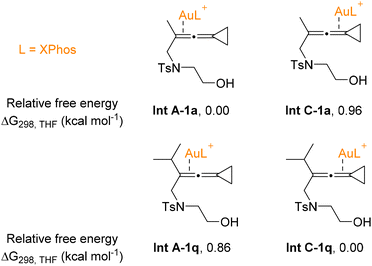
In order to elucidate the impact of substrate steric bulkiness on the selectivity of reaction pathways, substrates 1a and 1q were selected to investigate the energy differences of several gold-VDCP intermediates since the A-value16 of the methyl group is 1.8 kcal mol−1 and that of the isopropyl group is above 3.3 kcal mol−1.16d The relative free energies of several key intermediates are shown in Fig. 2 (for details, see S135 in the ESI†). For substrate 1a, the gold catalyst activates the allene double-bond away from the cyclopropyl unit, which is favored by 0.96 kcal mol−1, suggesting that the non-carbene process is a dominant process for groups with less steric hindrance. For substrate 1q, the gold species activates the allene double-bond connected to the cyclopropyl unit, which is favored by 0.86 kcal mol−1, suggesting that the carbene process becomes a dominant process for groups with more steric hindrance. These results are in good agreement with our experimental findings (Schemes 3 and 4).
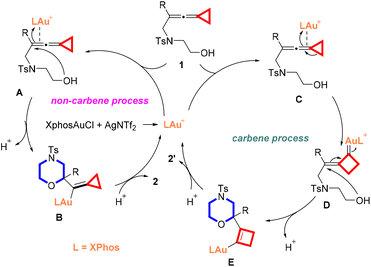
On the basis of the previous literature12e,14,17 and our above results, a plausible mechanism for the present cyclization reaction is depicted in Scheme 5. Under the conditions of gold(I) catalysis, substrate 1 can undergo two different reaction pathways (carbene and non-carbene processes) depending on the steric hindrance of the R groups or the connected alkyl chain length. As for the non-carbene process, the π-activation of the double bond in the allene moiety with the in situ generated gold(I) complex takes place to furnish intermediate A, which triggers a 6-exo-trig cyclization through intramolecular nucleophilic addition by the hydroxyl group onto the electrophilic carbon center to afford the alkenyl gold intermediate B. The protodeauration of intermediate B produces the corresponding morpholine derivative 2 along with the regeneration of the Au(I) catalytic species for the catalytic cycle. In this pathway, the sterically less hindered R groups in substrate 1 may result in a lower transition state energy barrier of 6-exo cyclization, giving the products 2 as the major ones. However, the carbene and non-carbene pathways are competing cyclization processes in these reactions. Sterically more bulky groups at substrate 1's allene moiety may delay the direct 6-exo cyclization since the transition energy barrier is quite high and the carbene process becomes the main cyclization process, affording products 2′ as the major ones. The same reason can be applied for the prolonged alkyl chain since the direct cyclization will become a slow process and the carbene process will dominate the reaction pathway. Thus, coordination of the gold(I) complex with vinylidenecyclopropane 1 bearing a sterically bulky group gives intermediate C, which initiates a ring expansion to afford the gold carbenoid intermediate D. The intramolecular nucleophilic attack of the hydroxyl group onto the electrophilic carbon center in intermediate D and release of a proton furnish intermediate E, which undergoes protodeauration to produce the cyclized product 2′ and regenerates the gold catalyst. More comprehensive investigations on the mechanistic manipulation of carbene and non-carbene processes and the related density functional theory (DFT) calculations will be published in due course.
To illustrate the synthetic utility of the obtained product, a gram-scale reaction of 1a, with 2.15 g (7.0 mmol), was performed and the reaction proceeded smoothly, delivering 1.8 g of 2a in 83% yield (Scheme 6). Then, a variety of transformations of 2a were conducted under different conditions (Scheme 6). Initially, treatment of 2a with HCl aqueous solution (4.0 M in dioxane) gave a chlorine-substituted vinylcyclopropane tethered alcohol, which could be further transformed to compound 3 in 78% overall yield in the presence of p-nitrobenzoyl chloride, and its structure has been clarified by X-ray diffraction as shown in Scheme 6. Its CIF data are also presented in the ESI.† Next, iodine-substituted vinylcyclopropane tethered alcohol 4 was formed in 67% yield upon treating 2a with 1.0 equiv. of BF3·Et2O and 1.5 equiv. of Bu4NI in DCM at room temperature. Considering that product 2a contained an alkylidenecyclopropane subunit, treatment of 2a with Pd(OAc)2 and PhI(OAc)2 afforded the corresponding diacetoxylated product 5 in 42% yield through a palladium-catalyzed ring-opening reaction according to our previous work.18 Furthermore, epoxidation of 2a and 2q′ with m-CPBA in DCM successfully gave the desired epoxide 6 in 68% yield as a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture and the epoxide 7 in 94% yield as a single diastereomer (Scheme 6).
1 diastereomeric mixture and the epoxide 7 in 94% yield as a single diastereomer (Scheme 6).
Asymmetric variants of VDCPs 1a and 1q were also reacted with the chiral gold catalyst (R)-DTBM-SegPhosAuCl combined with AgNTf2 in DCM at room temperature (for details, see S8 in the ESI†). As shown in Scheme 7, we found that treatment of 1a with 5 mol% (R)-DTBM-SegPhosAuCl and 10 mol% AgNTf2 afforded 2a in 78% yield along with 55% ee value. For substrate 1q, adding 5 mol% (R)-DTBM-SegPhosAuCl and 5 mol% AgNTf2 gave the desired product 2q′ in 88% yield along with 72% ee value.
In conclusion, we have developed an efficient and novel synthetic protocol for the construction of morpholine, piperazine and oxazepane derivatives bearing an alkylidenecyclopropane or a cyclobutene moiety in good yields under mild conditions through a gold(I)-catalyzed intramolecular cycloisomerization of alcohol and amine tethered vinylidenecyclopropanes via a carbene or non-carbene process with a broad substrate scope. The steric bulkiness of the substituent at the allene moiety of vinylidenecyclopropanes and the chain length played key roles in the reaction pathway. In general, sterically bulky substituents and the prolonged alkyl chain prefer the gold(I)-catalyzed cyclization via a carbene process. In addition, a variety of transformations of the obtained product 2 have been performed to demonstrate their synthetic utility. Further investigations on the mechanistic paradigm of this gold(I)-catalyzed cyclization and the preparation of biologically active heterocycles are underway in our laboratory.
Author contributions
Shi, M. and Tavakol, H. directed the project and revised the manuscript. Wei, J. S., Shamsaddinimotlagh, S. and Yang, S. wrote the manuscript and carried out the reactions. Wei, Y. checked the spectroscopic data and revised the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful for the financial support from the National Key R & D Program of China (2022YFC2303100), the National Natural Science Foundation of China (21372250, 21121062, 21302203, 20732008, 21772037, 21772226, 21861132014, 91956115, and 22171078), and the Fundamental Research Funds for the Central Universities 222201717003.Notes and references
- (a) F. Arshad, M. F. Khan, W. Akhtar, M. M. Alam, L. M. Nainwal, S. K. Kaushik, M. Akhter, S. Parvez, S. M. Hasan and M. Shaquiquzzaman, Revealing Quinquennial Anticancer Journey of Morpholine: A SAR Based Review, Eur. J. Med. Chem., 2019, 167, 324–356 CrossRef CAS PubMed; (b) V. Hahn, A. Mikolasch, K. Wende, H. Bartrow, U. Lindequist and F. Schauer, Synthesis of Model Morpholine Derivatives with Biological Activities by Laccase-Catalysed Reactions, Biotechnol. Appl. Biochem., 2009, 54, 187–195 CrossRef CAS PubMed; (c) A. P. Kourounakis, D. Xanthopoulos and A. Tzara, Morpholine as a Privileged Structure: A Review on the Medicinal Chemistry and Pharmacological Activity of Morpholine Containing Bioactive Molecules, Med. Res. Rev., 2020, 40, 709–752 CrossRef CAS PubMed; (d) E. Lenci, L. Calugi and A. Trabocchi, Occurrence of Morpholine in Central Nervous System Drug Discovery, ACS Chem. Neurosci., 2021, 12, 378–390 CrossRef CAS PubMed; (e) M. Taha and A. Wadood, Synthesis and Molecular Docking Study of Piperazine Derivatives as Potent Urease Inhibitors, Bioorg. Chem., 2018, 78, 411–417 CrossRef CAS PubMed; (f) E. Vitaku, D. T. Smith and J. T. Njardarson, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- S. Lemaire, F. Van Bambeke and P. M. Tulkens, Activity of Finafloxacin, a Novel Fluoroquinolone with Increased Activity at Acid PH, towards Extracellular and Intracellular Staphylococcus Aureus, Listeria Monocytogenes and Legionella Pneumophila, Int. J. Antimicrob. Agents, 2011, 38, 52–59 CrossRef CAS PubMed.
- T. Kalai, M. Khan, M. Balog, V. K. Kutala, P. Kuppusamy and K. Hideg, Structure–Activity Studies on the Protection of Trimetazidine Derivatives Modified with Nitroxides and their Precursors from Myocardial Ischemia-Reperfusion Injury, Bioorg. Med. Chem., 2006, 14, 5510–5516 CrossRef CAS PubMed.
- H. R. Arias, N. B. Fedorov, L. C. Benson, P. M. Lippiello, G. J. Gatto, D. Feuerbach and M. O. Ortells, Functional and Structural Interaction of (−)-Reboxetine with the Human α4β2 Nicotinic Acetylcholine Receptor, J. Pharmacol. Exp. Ther., 2013, 344, 113–123 CrossRef CAS PubMed.
- D. Dauzonne, J. M. Gillardin, F. Lepage, R. Pointet, S. Risse, G. Lamotte and P. Demerseman, Synthesis and Some CNS Activities of New Benzofuranylacryloylpiperazines, Eur. J. Med. Chem., 1995, 30, 53–59 CrossRef CAS.
- K. Audouze, E. O. Nielsen and D. Peters, New Series of Morpholine and 1,4-Oxazepane Derivatives as Dopamine D4 Receptor Ligands: Synthesis and 3D-QSAR Model, J. Med. Chem., 2004, 47, 3089–3104 CrossRef CAS PubMed.
- T. Yukawa, Y. Nakada, N. Sakauchi, T. Kamei, M. Yamada, Y. Ohba, I. Fujimori, H. Ueno, M. Takiguchi, M. Kuno, I. Kamo, H. Nakagawa, Y. Fujioka, T. Igari, Y. Ishichi and T. Tsukamoto, Design, Synthesis, and Biological Evaluation of a Novel Series of Peripheral-Selective Noradrenaline Reuptake Inhibitors – Part 3, Bioorg. Med. Chem., 2016, 24, 3716–3726 CrossRef CAS PubMed.
- (a) S. J. Gharpure, D. Anuradha, J. V. K. Prasad and P. Srinivasa Rao, Stereoselective Synthesis of cis-2,6-Disubstituted Morpholines and 1,4-Oxathianes by Intramolecular Reductive Etherification of 1,5-Diketones, Eur. J. Org. Chem., 2015, 86–90 CrossRef CAS; (b) J. V. Matlock, T. D. Svejstrup, P. Songara, S. Overington, E. M. McGarrigle and V. K. Aggarwal, Synthesis of 6- and 7-Membered N-Heterocycles Using α-Phenylvinylsulfonium Salts, Org. Lett., 2015, 17, 5044–5047 CrossRef CAS PubMed; (c) J. Kishore Vandavasi, W.-P. Hu, G. Chandru Senadi, H.-T. Chen, H.-Y. Chen, K.-C. Hsieh and J.-J. Wang, Aryl λ3-Iodane-Mediated 6-exo-trig Cyclization to Synthesize Highly Substituted Chiral Morpholines, Adv. Synth. Catal., 2015, 357, 2788–2794 CrossRef CAS; (d) B. Ritzen, S. Hoekman, E. D. Verdasco, F. L. van Delft and F. P. Rutjes, Enantioselective Chemoenzymatic Synthesis of cis- and trans-2,5-Disubstituted Morpholines, J. Org. Chem., 2010, 75, 3461–3464 CrossRef CAS PubMed; (e) P. Maity and B. Konig, Synthesis and Structure of 1,4-Dipiperazino Benzenes: Chiral Terphenyl-type Peptide Helix Mimetics, Org. Lett., 2008, 10, 1473–1476 CrossRef CAS PubMed; (f) K. H. Rohde, H. A. Michaels and A. Nefzi, Synthesis and Antitubercular Activity of 1,2,4-Trisubstitued Piperazines, Bioorg. Med. Chem. Lett., 2016, 26, 2206–2209 CrossRef CAS PubMed; (g) G. J. Mercer and M. S. Sigman, Diastereoselective Synthesis of Piperazines by Manganese-Mediated Reductive Cyclization, Org. Lett., 2003, 5, 1591–1594 CrossRef CAS PubMed; (h) F. Hafeez, A. Mansha, A. F. Zahoor, K. G. Ali, S. G. Khan and S. A. R. Naqvi, Facile Green Approach towards the Synthesis of Some Phenyl Piperazine Based Dithiocarbamates as Potent Hemolytic and Thrombolytic Agents, Pak. J. Pharm. Sci., 2021, 34, 1885–1890 CAS; (i) M. Taha and A. Wadood, Synthesis and Molecular Docking Study of Piperazine Derivatives as Potent Urease Inhibitors, Bioorg. Chem., 2018, 78, 411–417 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi, Gold-Catalyzed Organic Reactions, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed; (b) N. D. Shapiro and F. D. Toste, A Reactivity-Driven Approach to the Discovery and Development of Gold-Catalyzed Organic Reactions, Synlett, 2010, 675–691, DOI:10.1055/s-0029-1219369; (c) A. S. K. Hashmi, Gold-Catalyzed Organic Reactions, in Inventing Reactions, ed. L. J. Gooßen, 2013, vol. 44, pp. 143–164 Search PubMed; (d) H. Ohno, Gold-Catalyzed Cascade Reactions of Alkynes for Construction of Polycyclic Compounds, Isr. J. Chem., 2013, 53, 869–882 CrossRef CAS; (e) C. Shu, L. Li, T.-D. Tan, D.-Q. Yuan and L.-W. Ye, Ring Strain Strategy for the Control of Regioselectivity. Gold-Catalyzed Anti-Markovnikov Cycloisomerization Initiated Tandem Reactions of Alkynes, Sci. Bull., 2017, 62, 352–357 CrossRef CAS PubMed; (f) H. Xiong, H. Wang, L. He, Y. Zhang and Z. Zeng, Application of Gold Catalyst in Nucleophilic Addition of Allene, Chin. J. Org. Chem., 2011, 31, 466–479 CAS.
- (a) C. F. Bender and R. A. Widenhoefer, Gold(I)-Catalyzed Intramolecular Hydroamination of Unactivated C-C Bonds with Alkyl Ammonium Salt, Chem. Commun., 2008, 2741–2743, 10.1039/b804081h; (b) J. L. Zhang, C. G. Yang and C. He, Gold(I)-Catalyzed Intra- and Intermolecular Hydroamination of Unactivated Olefin, J. Am. Chem. Soc., 2006, 128, 1798–1799 CrossRef CAS PubMed; (c) X.-Y. Liu, C.-H. Li and C.-M. Che, Phosphine Gold(I)-Catalyzed Hydroamination of Alkenes under Thermal and Microwave-Assisted Conditions, Org. Lett., 2006, 8, 2707–2710 CrossRef CAS PubMed; (d) R. L. LaLonde, W. E. Brenzovich, Jr., D. Benitez, E. Tkatchouk, K. Kelley, W. A. Goddard, III and F. D. Toste, Alkylgold Complexes by the Intramolecular Aminoauration of Unactivated Alkenes, Chem. Sci., 2010, 1, 226–233 RSC; (e) S. Zhu, L. Ye, W. Wu and H. Jiang, N-Heterocyclic Carbene–Gold(I)-Catalyzed Carboheterofunctionalization of Alkenes with Arylboronic Acids, Tetrahedron, 2013, 69, 10375–10383 CrossRef CAS; (f) A. S. K. Hashmi, L. Schwarz, J. H. Choi and T. M. Frost, A New Gold-Catalyzed C–C Bond Formation, Angew. Chem., Int. Ed., 2000, 39, 2285–2288 CrossRef CAS; (g) A. Aponick, C.-Y. Li and B. Biannic, Au-Catalyzed Cyclization of Monoallylic Diols, Org. Lett., 2008, 10, 669–671 CrossRef CAS PubMed.
- (a) K. E. Soklou, H. Marzag, J. P. Bouillon, M. Marchivie, S. Routier and K. Plé, Gold(I)-Catalyzed Intramolecular Hydroamination and Hydroalkoxylation of Alkynes: Access to Original Heterospirocycles, Org. Lett., 2020, 22, 5973–5977 CrossRef CAS PubMed; (b) M. C. Blanco Jaimes, F. Rominger, M. M. Pereira, R. M. Carrilho, S. A. Carabineiro and A. S. K. Hashmi, Highly Active Phosphite Gold(I) Catalysts for Intramolecular Hydroalkoxylation, Enyne Cyclization and Furanyne Cyclization, Chem. Commun., 2014, 50, 4937–4940 RSC; (c) Z.-Y. Han, H. Xiao, X.-H. Chen and L.-Z. Gong, Consecutive Intramolecular Hydroamination/Asymmetric Transfer Hydrogenation under Relay Catalysis of an Achiral Gold Complex/Chiral Brønsted Acid Binary System, J. Am. Chem. Soc., 2009, 131, 9182–9183 CrossRef CAS PubMed; (d) T. Enomoto, A.-L. Girard, Y. Yasui and Y. Takemoto, Gold(I)-Catalyzed Tandem Reactions Initiated by Hydroamination of Alkynyl Carbamates: Application to the Synthesis of Nitidine, J. Org. Chem., 2009, 74, 9158–9164 CrossRef CAS PubMed; (e) H. Chiba, S. Oishi, N. Fujii and H. Ohno, Total Synthesis of (−)-Quinocarcin by Gold(I)-Catalyzed Regioselective Hydroamination, Angew. Chem., Int. Ed., 2012, 51, 9169–9172 CrossRef CAS PubMed; (f) X. Zeng, G. D. Frey, S. Kousar and G. Bertrand, A Cationic Gold(I) Complex as a General Catalyst for the Intermolecular Hydroamination of Alkynes: Application to the One-Pot Synthesis of Allenes from Two Alkynes and a Sacrificial Amine, Chem. – Eur. J., 2009, 15, 3056–3060 CrossRef CAS PubMed; (g) V. Belting and N. Krause, Gold-Catalyzed Tandem Cycloisomerization–Hydroalkoxylation of Homopropargylic Alcohols, Org. Lett., 2006, 8, 4489–4492 CrossRef CAS PubMed; (h) R. Gauthier, M. Mamone and J.-F. Paquin, Gold-Catalyzed Hydrofluorination of Internal Alkynes Using Aqueous HF, Org. Lett., 2019, 21, 9024–9027 CrossRef CAS PubMed; (i) A. Yamaguchi, S. Inuki, Y. Tokimizu, S. Oishi and H. Ohno, Gold(I)-Catalyzed Cascade Cyclization of Anilines with Diynes: Controllable Formation of Eight-Membered Ring-Fused Indoles and Propellane-Type Indolines, J. Org. Chem., 2020, 85, 2543–2559 CrossRef CAS PubMed; (j) D. P. Zimin, D. V. Dar'in, V. A. Rassadin and V. Y. Kukushkin, Gold-Catalyzed Hydrohydrazidation of Terminal Alkynes, Org. Lett., 2018, 20, 4880–4884 CrossRef CAS PubMed; (k) J. A. Goodwin and A. Aponick, Regioselectivity in the Au-Catalyzed Hydration and Hydroalkoxylation of Alkynes, Chem. Commun., 2015, 51, 8730–8741 RSC.
- (a) Y. Yamamoto and N. Nishina, Gold-Catalyzed Intermolecular Hydroamination of Allenes: First Example of the Use of an Aliphatic Amine in Hydroamination, Synlett, 2007, 1767–1770 CrossRef; (b) D. Eom, D. Kang and P. H. Lee, Synthesis of 2-Alkyl- and Aryl-3-Ethoxycarbonyl-2,5-Dihydrofurans through Gold-Catalyzed Intramolecular Hydroalkoxylation, J. Org. Chem., 2010, 75, 7447–7450 CrossRef CAS PubMed; (c) R. E. M. Brooner, T. J. Brown, M. A. Chee and R. A. Widenhoefer, Effect of Substitution, Ring Size, and Counterion on the Intermediates Generated in the Gold-Catalyzed Intramolecular Hydroalkoxylation of Allenes, Organometallics, 2016, 35, 2014–2021 CrossRef CAS; (d) Menggenbateer, M. Narsireddy, G. Ferrara, N. Nishina, T. Jin and Y. Yamamoto, Gold-Catalyzed Regiospecific Intermolecular Hydrothiolation of Allenes, Tetrahedron Lett., 2010, 51, 4627–4629 CrossRef CAS; (e) N. Nishina and Y. Yamamoto, Gold-Catalyzed Hydrofunctionalization of Allenes with Nitrogen and Oxygen Nucleophiles and its Mechanistic Insight, Tetrahedron, 2009, 65, 1799–1808 CrossRef CAS; (f) Z. Zhang, C. Liu, R. E. Kinder, X. Han, H. Qian and R. A. Widenhoefer, Highly Active Au(I) Catalyst for the Intramolecular exo-Hydrofunctionalization of Allenes with Carbon, Nitrogen, and Oxygen Nucleophiles, J. Am. Chem. Soc., 2006, 128, 9066–9073 CrossRef CAS PubMed; (g) C. Zhang, S. Q. Zhang, H. J. Cai and D. M. Cui, Gold-Catalyzed Intermolecular Hydroamination of Allenes with Sulfonamides, Beilstein J. Org. Chem., 2013, 9, 1045–1050 CrossRef CAS PubMed; (h) Z. Wang, C. Nicolini, C. Hervieu, Y.-F. Wong, G. Zanoni and L. Zhang, Remote Cooperative Group Strategy Enables Ligands for Accelerative Asymmetric Gold Catalysis, J. Am. Chem. Soc., 2017, 139, 16064–16067 CrossRef CAS PubMed.
- (a) T. Wang and A. S. K. Hashmi, 1,2-Migrations onto Gold Carbene Centers, Chem. Rev., 2021, 121, 8948–8978 CrossRef CAS PubMed; (b) L. W. Ye, X. Q. Zhu, R. L. Sahani, Y. Xu, P. C. Qian and R. S. Liu, Nitrene Transfer and Carbene Transfer in Gold Catalysis, Chem. Rev., 2021, 121, 9039–9112 CrossRef CAS PubMed; (c) G.-Q. Gao, G. Ma, X.-L. Jiang, Q. Liu, C.-L. Fan, D.-C. Lv, H. Su, G.-X. Ru and W.-B. Shen, Gold-Catalyzed Cycloadditions of Allenes via Metal Carbenes, Org. Biomol. Chem., 2022, 20, 5035–5044 RSC; (d) R. J. Harris and R. A. Widenhoefer, Gold Carbenes, Gold-Stabilized Carbocations, and Cationic Intermediates Relevant to Gold-Catalysed Enyne Cycloaddition, Chem. Soc. Rev., 2016, 45, 4533–4551 RSC; (e) L. Zhang, A Non-Diazo Approach to α-Oxo Gold Carbenes via Gold-Catalyzed Alkyne Oxidation, Acc. Chem. Res., 2014, 47, 877–888 CrossRef CAS PubMed.
- D. Y. Li, Y. Wei, I. Marek, X. Y. Tang and M. Shi, Gold(I)-Catalyzed Cycloisomerization of Vinylidenecyclopropane-enes via Carbene or non-Carbene Processes, Chem. Sci., 2015, 6, 5519–5525 RSC.
- (a) W. Li, W. Yuan, S. Pindi, M. Shi and G. Li, Au/Ag-Catalyzed Intramolecular Ring-Opening of Vinylidenecyclopropanes (VDCPs): An Easy Access to Functional Tetrahydropyrans, Org. Lett., 2010, 12, 920–923 CrossRef CAS PubMed; (b) B.-L. Lu, Y. Wei and M. Shi, Gold(I) and Brønsted Acid Catalyzed Intramolecular Rearrangements of Vinylidenecyclopropanes, Chem. – Eur. J., 2010, 16, 10975–10979 CrossRef CAS PubMed; (c) B.-L. Lu and M. Shi, Gold(I)-Catalyzed Tandem Oxidative Ring-Opening/C-C Bond Cleavage Reactions of Vinylidenecyclopropanes with Secondary Amines Under an Oxygen Atmosphere, Chem. – Eur. J., 2011, 17, 9070–9075 CrossRef CAS PubMed; (d) W. Yuan, X. Tang, Y. Wei and M. Shi, Gold-Catalyzed Cycloisomerization of Yne-Vinylidenecyclopropanes: A Three-Carbon Synthon for [3+2] Cycloadditions, Chem. – Eur. J., 2014, 20, 3198–3204 CrossRef CAS PubMed; (e) L. Wu and M. Shi, Yb(OTf)3- or Au(I)-Catalyzed Domino Intramolecular Hydroamination and Ring-Opening of Sulfonamide-Substituted 1,1-Vinylidenecyclopropanediesters, Chem. – Eur. J., 2011, 17, 13160–13165 CrossRef CAS PubMed; (f) W. Zang, Y. Wei and M. Shi, Gold(I) Catalyzed Cascade Cyclization: Intramolecular Two-Fold Nucleophilic Addition to Vinylidenecyclopropanes (VDCPs), Org. Chem. Front., 2018, 5, 197–202 RSC; (g) W. Yuan, X. Dong, Y. Wei and M. Shi, An Efficient Method for the Synthesis of Alkylidenecyclobutanones by Gold-Catalyzed Oxidative Ring Enlargement of Vinylidenecyclopropanes, Chem. – Eur. J., 2012, 18, 10501–10505 CrossRef CAS PubMed; (h) D.-Y. Li, Y. Wei and M. Shi, Gold(I)-Catalyzed Intramolecular Carbon-Oxygen Bond Cleavage Reaction via Gold Carbenes Derived from Vinylidenecyclopropanes, Adv. Synth. Catal., 2016, 358, 3002–3009 CrossRef CAS; (i) D.-Y. Li, W. Fang, Y. Wei and M. Shi, C(sp3)–H Functionalizations Promoted by the Gold Carbene Generated from Vinylidenecyclopropanes, Chem. – Eur. J., 2016, 22, 18080–18084 CrossRef CAS PubMed.
- (a) E. Solel, M. Ruth and P. R. Schreiner, London Dispersion Helps Refine Steric A-Values: The Halogens, J. Org. Chem., 2021, 86, 7701–7713 CrossRef CAS PubMed; (b) F. R. Jensen, C. H. Bushwell and B. H. Beck, Conformational Preferences in Monosubstituted Cyclohexanes Determined by Nuclear Magnetic Resonance Spectroscopy, J. Am. Chem. Soc., 1969, 91, 344–351 CrossRef CAS; (c) C. H. Marzabadi, J. E. Anderson, J. Gonzalez-Outeirino, P. R. J. Gaffney, C. G. H. White, D. A. Tocher and L. J. Todaro, Why Are Silyl Ethers Conformationally Different from Alkyl Ethers? Chair-Chair Conformational Equilibria in Silyloxycyclohexanes and Their Dependence on the Substituents on Silicon. The Wider Roles of Eclipsing, of 1,3-Repulsive Steric Interactions, and of Attractive Steric Interactions, J. Am. Chem. Soc., 2003, 125, 15163–15173 CrossRef CAS PubMed; (d) S. Winstein and N. J. Holness, Neighboring Carbon and Hydrogen. XIX. t-Butylcyclohexyl Derivatives. Quantitative Conformational Analysis, J. Am. Chem. Soc., 1955, 77, 5562–5578 CrossRef CAS.
- W. Yang and A. S. K. Hashmi, Mechanistic Insights into the Gold Chemistry of Allenes, Chem. Soc. Rev., 2014, 43, 2941–2955 RSC.
- M. Jiang and M. Shi, Palladium-Catalyzed Diacetoxylation of Methylenecyclopropanes via C(sp3)–C(sp3) Bond Breaking, Organometallics, 2009, 28, 5600–5602 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data of new compounds. CCDC 1537103, 2178224, and 2178226. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo00085k |
| This journal is © the Partner Organisations 2023 |