Versatile access to nitrogen-rich π-extended indolocarbazoles via a Pictet–Spengler approach†
Robin
Heckershoff
a,
Lukas
Eberle
a,
Nick
Richert
a,
Christian
Delavier
a,
Michael
Bruckschlegel
a,
Moritz R.
Schäfer
 a,
Petra
Krämer
a,
Frank
Rominger
a,
Matthias
Rudolph
a and
A. Stephen K.
Hashmi
a,
Petra
Krämer
a,
Frank
Rominger
a,
Matthias
Rudolph
a and
A. Stephen K.
Hashmi
 *ab
*ab
aOrganisch-Chemisches Institut (OCI), Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: hashmi@hashmi.de
bChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
First published on 19th October 2022
Abstract
The Pictet–Spengler reaction was applied to synthesize benzobispyrrolo[3,2-c]quinolines as new scaffolds for organic electronics. The addressed compounds are nitrogen-enriched analogues of π-extended indolocarbazoles formally accessed by the isosteric replacement of CH moieties by nitrogen atoms. The straightforward and robust methodology allows easy access to these heptacycles with the possibility of late-stage functionalization. 17 new compounds were synthesized, fully characterized and their properties were investigated by X-ray crystallography, photophysical measurements and computational methods. The study evaluated the effect of different substituents on the observed photophysical and electronical properties. Comparison with π-extended indolocarbazoles showed that the introduction of two nitrogens to the molecule core results in a significant decrease of the HOMO and LUMO energies and an increase of the optical and HOMO–LUMO gaps.
Introduction
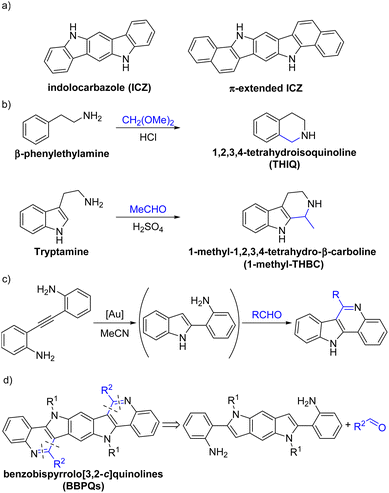
Indolocarbazoles (ICZs) and their π-extended derivatives (Scheme 1a) are valuable compounds in the field of organic functional materials1,2 used in many applications such as organic field-effect transistors (OFETs),3–6 organic light emitting diodes (OLEDs)7–9 and organic photovoltaics (OPVs).10–14 Compared to pentacene, these electron-rich molecules have low lying HOMO (highest occupied molecular orbital) energies in combination with a relatively large band gap, which results in a high stability even in the presence of oxygen. Additionally, their electron donor capabilities make them especially suitable for p-type semiconductors.15,16 To modify the opto-electronical properties of ICZs, several strategies were established. Besides the introduction of substituents,17,18N-arylation19,20 and the elongation of the π-system,21,22 oxidation of the molecules was applied.23,24 Another useful method, which is routinely applied for other polycyclic aromatic hydrocarbons, is the substitution of carbon units by heteroatoms at the molecule core. Especially the isosteric replacement of CH moieties by nitrogen atoms is used to alter molecule properties as frequently seen with azaacenes25–27 and other compounds.28–31 The influence of these replacements can be quite substantial. For example, the introduction of nitrogen atoms can lower the HOMO–LUMO (lowest unoccupied molecular orbital) energies in a fashion that enables to transform a typical electron donor into an acceptor, which allows the application as n-type semiconductor.32–34 For ICZs, this kind of nitrogen introduction is quite rare and for π-extended ICZs only one special example was found.35–39In order to achieve a fast and modular approach to nitrogen-rich π-extended ICZs, we envisioned that the classical Pictet–Spengler reaction (PSR) might be a promising candidate for this endeavor. Already known for more than a century,40 the PSR evolved into one of the most important, efficient and variable synthetic methods for the construction of privileged alkaloid scaffolds such as tetrahydro-isoquinolines (THIQs), tetrahydro-β-carbolines (THBCs) and polyheterocyclic frameworks (Scheme 1b).41–44 Although most of the PSR applications result in partly saturated N-heteropolycycles, there are some examples for syntheses of fully aromatic products.45–49 Rossi et al. reported on a gold-catalyzed one-pot synthesis of 11H-indolo[3,2-c]quinolines via PSR of 2-(1H-indol-2-yl)anilines, which were produced in situ by the hydroamination of diamino alkyne precursors (Scheme 1c).50 By extending this methodology to a bidirectional approach, we aimed for accessing nitrogen-rich π-extended ICZs in the form of benzobispyrrolo[3,2-c]quinolines (BBPQ) by a twofold PSR of 2-aminophenyl-substituted benzodipyrroles (Scheme 1d). This strategy would also allow a late-stage functionalization of the core system to adjust the compound's properties.
Results and discussion
Reaction discovery and optimization
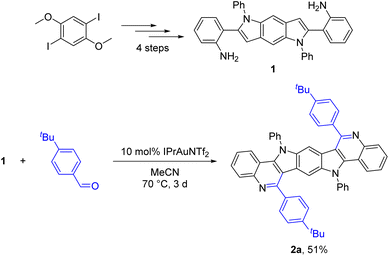
Until recently, appropriate starting materials necessary for the bidirectional PSR were difficult or impossible to synthesize. Fortunately, we lately developed a versatile gold-catalyzed51–59 method for the building of benzodipyrroles and therein showed the example of bis(2-aminophenyl)-substituted benzodipyrrole 1 (Scheme 2).60 This substrate is accessible starting from 1,4-diiodo-2,5-dimethoxybenzene in a straightforward four step synthesis and should be suitable for the synthesis of BBPQs via the PSR. As a first attempt in synthesizing an example of a BBPQ, we started with similar reaction conditions as used within the preceding, monodirectional work. 1 and five equivalents of 4-(tert-butyl)benzaldehyde with 10 mol% IPrAuNTf2 (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) as catalyst were stirred in MeCN at 70 °C. Despite low solubility of 1 in MeCN, the substrate was completely consumed after three days and TLC showed the formation of a new product, which precipitated from the mixture. Filtration and washings with MeOH and pentane were sufficient to obtain an analytically pure sample in 51% yield, which, according to the HR-MS and NMR spectra, strongly suggested being the desired BBPQ 2a. Specimens suitable for single crystal X-ray analysis were obtained and the solid-state molecular structure undoubtedly confirmed the right connectivity of the product (see structural discussion, Fig. 1). In the crude reaction mixture no hints of unsaturated intermediates could be detected, assuming a spontaneous and complete oxidation to the fully aromatic system under the ambient conditions. As a next step, a small screening was conducted to improve the reaction outcome (see ESI† for more details). A better solubility of the substrate was achieved by changing the solvent to 1,2-dichloroethane (DCE), which also allowed slightly higher reaction temperatures. Although a small surplus of the aldehyde (2.1 eq.) was sufficient for a full conversion of the substrate, we decided to utilize three equivalents of these inexpensive reactants in the subsequent reactions to compensate for possible decompositions (e.g. oxidation to carboxylic acids). For the classic PSR, typically mineral acids were applied and therefore, we considered that the usage of a gold catalyst in the preceding work was mainly necessary for the hydroamination forming the indole moiety. Comparing different gold catalysts (IPrAuNTf2, PPh3AuNTf2 and AuCl3) with p-toluenesulfonic acid monohydrate (PTSA·H2O) and trifluoroacetic acid (TFA) showed a clear advantage for the two Brønsted acids, resulting in a completion of the reaction in less than 8 h and isolated yields of up to 79%.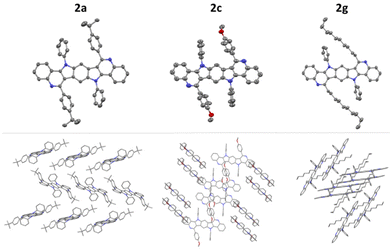 | ||
| Fig. 1 Solid-state molecular structures (top) and packing motifs (bottom) of 2a, 2c and 2g. The thermal ellipsoids are set at a 50% probability level and hydrogen atoms are omitted for clarity. | ||
Scope with regard to the synthesis of N-phenyl BBPQs
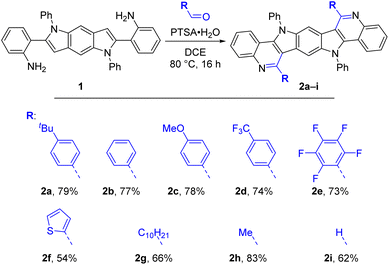
With these optimized conditions at hand, we challenged our reaction protocol using a series of aldehydes including aromatic, heteroaromatic and aliphatic representatives as well as paraformaldehyde (Scheme 3). By treatment with 10 mol% PTSA·H2O in DCE at 80 °C all examples showed full conversion after less than 16 h reaction time. Only small amounts of impurities were detected for most substrates implying very high crude yields for the products. In favor of using the simple workup procedure consisting of filtration and washing of the compounds, naturally, the isolated yields were lower (54–83%) and varying due to different solubilities of the products, which were very low for the arylated derivatives 2b/c/d/f. NMR analytics were challenging for these compounds, necessitating measurements in hot deuterated 1,1,2,2-tetrachloroethane and sometimes resulting in too weak signal intensity to determine quaternary carbon peaks. Unfortunately, the poor solubilities also prevented cyclic voltammetry measurements.Synthesis of N-PMB and N-unsubstituted BBPQs
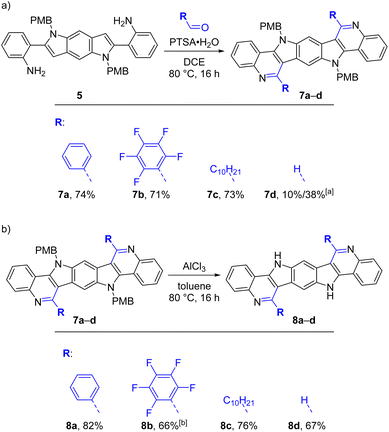
As a next step, we explored the possibility to synthesize BBPQ derivatives without a phenyl substituent on the pyrrole nitrogen. Besides the aim to obtain molecules with altered properties and enabling further derivatizations on this position, N-unsubstituted ICZs showed better performances in some OFET applications especially for vacuum deposition methods.61,62Since there is no feasible method for the removal of the N-phenyl group from the pyrrole moiety, a new synthetic strategy was necessary to obtain N-unsubstituted BBPQs. Several methods for the synthesis of an N-unsubstituted analogue of precursor 1 were attempted. Scheme 4a shows our successful approach starting with p-methoxybenzyl (PMB)-protected diaminodibromobenzene 3, which was converted into the PMB-protected benzodipyrrole 5 in a similar manner as it was previously done with precursor 1. A Sonogashira cross-coupling with 2-ethynylaniline to dialkyne compound 4, which already partly cyclized and direct treatment with IPrAuNTf2 resulted in 5 in 77% yield over two steps. Possible isomers of 5, where the hydroamination occurred on the other amine functionality, were not detected. Other beforehand tested attempts with different starting materials failed. Unselective cyclization results were observed with Boc instead of PMB as protecting group (non-separable mixture of compounds with presumably N-Boc- and NH2-hydroaminations) and unprotected diaminodihalogene compounds were either not accessible (for the diiodo substrate) or showed very low reactivity and stability issues (for the dibromo substrate).
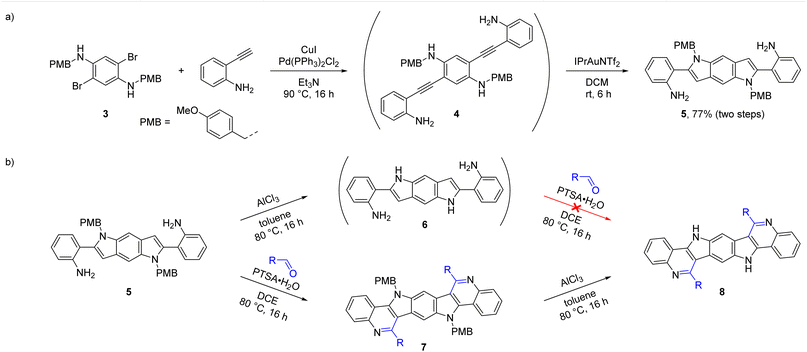 | ||
| Scheme 4 (a) Two step synthesis of 5via Sonogashira cross-coupling and gold-catalyzed cyclization. (b) Two sequences of deprotection and PSR with reversed order forming BBPQs 8. | ||
With 5 two reaction sequences with a reversed order are imaginable to achieve N-unsubstituted BBPQs, either starting with a PMB-deprotection followed by PSR or vice versa (Scheme 4b). PMB (and benzyl) substituents are common protecting groups for indoles and other amino compounds and many convenient deprotection protocols are known.63–72 To our surprise, almost all tested methods did not work for our substrate. Established protocols using of H2/Pd, KOtBu/O2, NaH, DDQ or acids showed no effect or led to decomposition of the substrate. Luckily, treatment with AlCl3 in toluene at 80 °C resulted in full conversion of the starting material, but the reaction was quite unselective and we were not able to fully isolate the desired deprotected compound 6 and its formation could be only assumed by characteristic signals in the HR-MS and 1H NMR spectra. After treatment of the crude product mixture with different aldehydes and PTSA·H2O, we were unable to detect the sought-after products 8 or the substrate in the reaction mixtures, which may indicate that 6 is unstable under the reaction conditions and not suitable for this approach.
To our delight, the second reaction sequence was more successful. The PSR of 5 with four different aldehydes resulted in the formation of the corresponding PMB-protected BBPQs 7a–d (Scheme 5). While 7a–c, bearing phenyl, perfluorophenyl and decyl substituent, gave good isolated yields (71–74%), 7d, which was produced with paraformaldehyde as the aldehyde source, resulted in only 10% yield. In HR-MS spectra of the crude product mixture additional species with seemingly extra methylene units were detected, indicating the introduction of oligomerized formaldehyde to the product (removable by recrystallization of the product). By using N,N-dimethylformamide dimethyl acetal (DMF-DMA) as a formaldehyde source, the isolated yield could be raised to 38%, but other heavier species were still present in the mother liquors. For the following deprotection all the above mentioned deprotection methods besides using AlCl3 were unsuccessful as well. While 7a, 7c and 7d could be deprotected with AlCl3 in toluene at 80 °C in 16 h, 7b – bearing the electron-withdrawing perfluorophenyl substituents – showed a lower reactivity and more equivalents of AlCl3 together with a 110 °C reaction temperature were necessary for completion in the same time. The purifications of the N-unsubstituted BBQPs 8a–d were more challenging. The side products, aluminum hydroxide species as well as organic impurities (mainly benzyl trapping products), needed to be removed and necessitated different combinations of chromatographic and washing procedures depending on the solubilities of the products (generally low in organic solvents besides in DMSO and partly MeOH; see ESI† for more details). Nonetheless, the yields of the final compounds were quite satisfactory (66–82%) and all products were fully characterized.
Structural discussion
For 2a, 2c and 2g, specimens suited for single crystal X-ray analysis were obtained by vapor diffusion of MeOH into concentrated solutions of the compounds in CHCl3. Fig. 1 shows the solid-state molecular structure and packing motifs. All three molecules are each located on crystallographic inversion centers. 2a has a notable twisted backbone, where the angle between the central and outermost benzene ring amounts to 11.9° and the N-phenyl and tert-butylphenyl substituents are rotated out-of-plane by 60.6° and 53.2°, respectively. The molecules build one-dimensional staircases, but the π-systems are orientated perpendicular to the staircase direction with no overlap. 2c is less twisted (5.2°) and the N-phenyl and methoxyphenyl substituents are rotated out-of-plane by 81.1° and 66.3°, respectively. π–π Interactions are negligible due to non-overlapping core structures or large distances of the layers (9.23 Å). For 2g, the five central rings of the molecule are relatively flat and the outermost rings are slightly bent by 6.2°. The N-phenyl substituents are twisted by 76.6° and the alkyl chains are orientated out-of-plane by approximately 42°. 2g crystallizes as isolated dimer pairs exhibiting π–π stacking with a distance of 3.76 Å between the molecular backbones. The dimer pairs interact with the surrounding dimers via CH⋯π short contacts.Photophysical properties
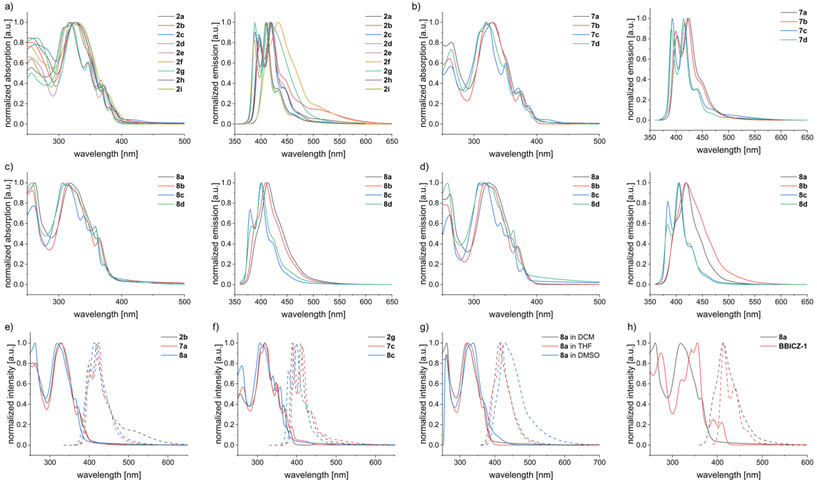
BBPQs 2a–i, 7a–d and 8a–d were studied by UV-Vis and fluorescence spectroscopy in DCM, 8a–d as well in THF and 8a additional in DMSO (Table 1 and Fig. 2). The different substituents of 2a–i have only minor influence on the absorption spectra. The global maxima are located at 317–328 nm, the maxima of the longest absorption wavelength (λmax,abs) at 380–391 nm and the from the absorption onset calculated optical gaps (Eg(opt)) result in values of 3.06–3.15 eV. The arylated derivatives 2a–f are slightly red-shifted compared to 2g–i due to their larger π-system. Compounds 7a–d with a N-PMB instead of the N-phenyl substituent show similar absorption spectra and the measured values lie in the same range as with 2a–i. 8a–d, without any substituent on the pyrrole nitrogen, exhibit a small blue shift of the spectra with λmax,abs of 364–373 nm and Eg(opt) of 3.20–3.25 eV. A stronger influence of the substituents is observed for the fluorescence spectra. 2a–i have the maxima of their shortest emission wavelength (λmax,em) at 387–418 nm, corresponding to Stokes shifts of 336–1668 cm−1, with notable hypsochromic shift for 2g–i and bathochromic shift for thiophene derivative 2f. Again, 7a–d show no major differences while 8a–d give slightly blue shifted emission spectra. Fluorescence quantum yields (QY) are in the medium range of 15–30% for the arylated compounds 2a–e and 7a/b with exception of thiophene derivative 2f (5%) and generally higher values for the alkylated compounds (highest QY of 46% for 2g). QY show similar tendencies for 8a–d but are lower compared to the N-substituted counterparts (9–24%).| Compound |
λ
max,abs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [nm] a [nm] |
λ
max,em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [nm] b [nm] |
Stokes shift [cm−1] | QYc [%] | λ onset,abs [nm] |
E
g(opt)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d [eV] d [eV] |
HOMOe [eV] | LUMOe [eV] |
E
g(calc)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f [eV] f [eV] |
|---|---|---|---|---|---|---|---|---|---|
| a Maximum of the longest absorption wavelength. b Maximum of the shortest emission wavelength. c Fluorescence quantum yield. d Optical gap estimated from λonset,abs: Eg(opt) = 1239.8/λonset,abs. e HOMO/LUMO energies computed by DFT, see ESI.† f HOMO–LUMO gap. g In THF. h In DMSO. i Data from an earlier publication (see also Fig. 4).62 | |||||||||
| 2a | 388 | 398 | 648 | 19 | 403 | 3.08 | −5.41 | −1.62 | 3.79 |
| 2b | 387 | 397 | 651 | 15 | 403 | 3.08 | −5.44 | −1.64 | 3.80 |
| 2c | 386 | 396 | 654 | 22 | 403 | 3.08 | −5.38 | −1.57 | 3.81 |
| 2d | 389 | 416 | 1668 | 17 | 405 | 3.06 | −5.66 | −1.89 | 3.77 |
| 2e | 385 | 396 | 722 | 30 | 399 | 3.11 | −5.79 | −1.94 | 3.85 |
| 2f | 391 | 418 | 1652 | 5 | 405 | 3.06 | −5.48 | −1.71 | 3.77 |
| 2g | 383 | 388 | 336 | 46 | 394 | 3.15 | −5.42 | −1.58 | 3.84 |
| 2h | 381 | 389 | 540 | 37 | 395 | 3.14 | −5.44 | −1.60 | 3.84 |
| 2i | 380 | 387 | 476 | 39 | 393 | 3.15 | −5.50 | −1.66 | 3.84 |
| 7a | 389 | 401 | 769 | 15 | 405 | 3.06 | — | — | — |
| 7b | 389 | 399 | 644 | 24 | 402 | 3.08 | — | — | — |
| 7c | 387 | 392 | 330 | 43 | 399 | 3.11 | — | — | — |
| 7d | 385 | 390 | 333 | 17 | 400 | 3.10 | — | — | — |
| 8a | 364/369g/370h | 395/402g/407h | 2156/2590g/2457h | 10/15g/7h | 388/393g/398h | 3.20/3.15g/3.12h | −5.62 | −1.74 | 3.88 |
| 8b | 364/369g | 393/402g | 2027/2225g | 18/22g | 382/387g | 3.25/3.20g | −5.99 | −2.08 | 3.91 |
| 8c | 373/378g | 379/384g | 424/413g | 24/52g | 385/388g | 3.22/3.20g | −5.59 | −1.69 | 3.90 |
| 8d | 370/374g | 382/384g | 849/696g | 9/30g | 384/387g | 3.23/3.20g | −5.69 | −1.78 | 3.91 |
| BBICZ-1 | 404g | 414g | 598g | 37g | 415g | 2.99g | — | — | — |
| BBICZ-2 | 405g | 413g | 478g | 36g | 420g | 2.95g | — | — | — |
| BBICZ-3 | 397g | 403g | 375g | 15g | 414g | 2.99g | −5.12 | −1.51 | 3.61 |
Changing to THF as solvent results in a small red-shift (2–5 nm) in the absorption spectra of 8a–d and a slightly lower Eg(opt) (by 0.02–0.05 eV). A similar influence of the solvent change is seen in the emission spectra, which are red-shifted by 2–13 nm. QY measurements gave higher values in THF than in DCM with slightly higher QY for 8a (15%) and 8b (22%) and significant higher QY for 8c (52%) and 8d (30%). 8a was also measured in DMSO, which changes the absorption spectra marginally, but initiates a stronger red-shift of the emission spectra (λmax,em = 407 nm) compared to THF and the QY is lowered to 7%.
HOMO–LUMO energies for 2a–i and 8a–d were determined by DFT calculations using Orca 5.0.1.73,74 The structures were optimized using the B3LYP/G functional with the def2/TZVP basis set (Table 1; see ESI† for more details).75–78 For 2a–i the HOMO energies are located between −5.79 and −5.38 eV and for 8a–d between −5.99 and −5.59 eV. Between compounds with different substituents mostly very small differences of less than 0.1 eV are present, which tendencies are in good agreement with the corresponding Hammett parameters.79 Only the strongly electron-withdrawing groups of 2d, 2e and 8b have a more significant influence on the HOMO energies. The LUMO energies differ similarly (values between −1.94 and −1.57 eV), resulting in only slightly differing HOMO–LUMO gaps (Eg(calc)) of 3.77–3.88 eV (2a–i) and 3.88–3.91 eV (8a–d). This follows the same trend as seen with the optical gap. The values for Eg(calc) are higher than for Eg(opt) (about 0.7 eV), which is expected due to the different nature of these energy gaps.80
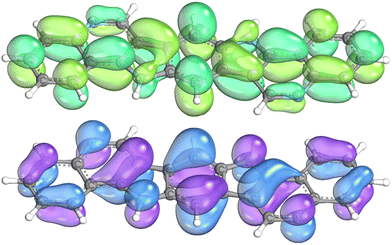
Fig. 3 shows a visualization of the frontier molecular orbitals of 8d. Here, it can be seen that at the positions adjacent to the pyridine nitrogen atom the HOMO has a node and in the LUMO the density is only marginal. This explains the relative little influence of the substituents on the electronic properties of the compounds.
To see the impact of the isosteric replacements of CH moieties by nitrogen on the photophysical properties (in THF), we compared 8a/c/d to structurally related benzo[a]benzo[6,7]-indolo[2,3-h]carbazoles (BBICZ-1/2/3; Fig. 4), which we described in an earlier publication.62 The introduction of two nitrogens to the molecule core results in different relative intensities of the absorption peaks and in a blue shift of the UV-Vis spectra of about 25–35 nm for 8a/c/d in comparison to BBICZ-1/2/3 and therefore a smaller Eg(opt) by 0.1–0.2 eV. The emission spectra are blue-shifted as well for the nitrogen-rich molecules by 10–30 nm. The phenyl derivative 8a has a lower QY than its nitrogen-poorer analogue BBICZ-1 (15% to 37%), whereas the alkyl and N-unsubstituted derivatives 8c/d have higher QY than BBICZ-2/3 (52% to 36%/30% to 15%). The calculated HOMO–LUMO energies for BBICZ-3 amount to −5.12/−1.51 eV, which is significantly higher than the energies of the corresponding nitrogen-rich analogue 8d. In this case, the isosteric replacements of two CH moieties by nitrogen lowers the HOMO by 0.57 eV and the LUMO by 0.27 eV, which enlarges the Eg(calc) by 0.30 eV.
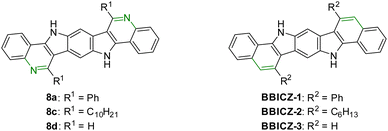 | ||
| Fig. 4 BBPQs 8a/c/d and structurally related BBICZ-1/2/3,62 which are used to compare the difference in the photophysical properties caused by isosteric replacement of CH moieties by nitrogen. | ||
Conclusions
In summary, we successfully established a highly efficient synthesis of nitrogen-rich π-extended indolocarbazoles via the Pictet–Spengler reaction. The isosteric replacements of two CH moieties by nitrogen atoms have a significant influence on the photophysical and electronical properties. In comparison to π-extended indolocarbazoles, the optical and HOMO–LUMO gaps are raised and the HOMO and LUMO are lowered significantly.This promising new methodology will allow access to many new compounds with altered properties, which will impact the field of organic functional materials. We are already working on an extension of the scope and have first successful attempts in the introduction of more nitrogen atoms and other heteroatoms to the framework as well as synthesizing isomeric structures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. H. is grateful for funding by the Deutsche Forschungsgemeinschaft (DFG; SFB 1249 – N-Heteropolyzyklen als Funktionsmaterialien). The authors acknowledge support by the state of Baden-Württemberg through bwHPC and DFG through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster).References
- Y. Wu, Y. Li, S. Gardner and B. S. Ong, Indolo[3,2-b]carbazole-Based Thin-Film Transistors with High Mobility and Stability, J. Am. Chem. Soc., 2005, 127, 614–618 CrossRef CAS PubMed.
- M. Vlasselaer and W. Dehaen, Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials, Molecules, 2016, 21, 785 CrossRef PubMed.
- Y. Li, Y. Wu, S. Gardner and B. S. Ong, Novel Peripherally Substituted Indolo[3,2-b]carbazoles for High-Mobility Organic Thin-Film Transistors, Adv. Mater., 2005, 17, 849–853 CrossRef CAS.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics, Chem. Rev., 2012, 112, 2208–2267 CrossRef CAS PubMed.
- S. H. Nam, P. J. Jeon, S. W. Min, Y. T. Lee, E. Y. Park and S. Im, Highly Sensitive Non-Classical Strain Gauge Using Organic Heptazole Thin-Film Transistor Circuit on a Flexible Substrate, Adv. Funct. Mater., 2014, 24, 4413–4419 CrossRef CAS.
- P. J. Jeon, K. Lee, E. Y. Park, S. Im and H. Bae, Ultrasensitive low power-consuming strain sensor based on complementary inverter composed of organic p- and n-channels, Org. Electron., 2016, 32, 208–212 CrossRef CAS.
- H.-P. Zhao, X.-T. Tao, F.-Z. Wang, Y. Ren, X.-Q. Sun, J.-X. Yang, Y.-X. Yan, D.-C. Zou, X. Zhao and M.-H. Jiang, Structure and electronic properties of triphenylamine-substituted indolo[3,2-b]carbazole derivatives as hole-transporting materials for organic light-emitting diodes, Chem. Phys. Lett., 2007, 439, 132–137 CrossRef CAS.
- H.-C. Ting, Y.-M. Chen, H.-W. You, W.-Y. Hung, S.-H. Lin, A. Chaskar, S.-H. Chou, Y. Chi, R.-H. Liu and K.-T. Wong, Indolo[3,2-b]carbazole/benzimidazole hybrid bipolar host materials for highly efficient red, yellow, and green phosphorescent organic light emitting diodes, J. Mater. Chem., 2012, 22, 8399–8407 RSC.
- H. Shi, J. Dai, X. Wu, L. Shi, J. Yuan, L. Fang, Y. Miao, X. Du, H. Wang and C. Dong, A novel dimesitylboron-substituted indolo[3,2-b]carbazole derivative: Synthesis, electrochemical, photoluminescent and electroluminescent properties, Org. Electron., 2013, 14, 868–874 CrossRef CAS.
- J. Lu, F. Liang, N. Drolet, J. Ding, Y. Tao and R. Movileanu, Crystalline low band-gap alternating indolocarbazole and benzothiadiazole-cored oligothiophene copolymer for organic solar cell applications, Chem. Commun., 2008, 5315–5317 RSC.
- I. Lim, E.-K. Kim, S. A. Patil, D. Y. Ahn, W. Lee, N. K. Shrestha, J. K. Lee, W. K. Seok, C.-G. Cho and S.-H. Han, Indolocarbazole based small molecules: an efficient hole transporting material for perovskite solar cells, RSC Adv., 2015, 5, 55321–55327 RSC.
- J. Tong, P. Guo, H. Zhang, J. Li, P. Zhang, C. Yang, D. Chen and Y. Xia, Synthesis of modified benzothiadiazole-thiophene-cored acceptor and carbazole/indolocarbazole alternating conjugated polymers and their photovoltaic applications, Polym. Bull., 2015, 72, 565–581 CrossRef CAS.
- B. Cai, X. Yang, X. Jiang, Z. Yu, A. Hagfeldt and L. Sun, Boosting the power conversion efficiency of perovskite solar cells to 17.7% with an indolo[3,2-b]carbazole dopant-free hole transporting material by improving its spatial configuration, J. Mater. Chem. A, 2019, 7, 14835–14841 RSC.
- S. Suman, A. Siddiqui, M. L. Keshtov, G. D. Sharma and S. P. Singh, New indolo carbazole-based non-fullerene n-type semiconductors for organic solar cell applications, J. Mater. Chem. C, 2019, 7, 543–552 RSC.
- P.-L. T. Boudreault, S. Wakim, N. Blouin, M. Simard, C. Tessier, Y. Tao and M. Leclerc, Synthesis, Characterization, and Application of Indolo[3,2-b]carbazole Semiconductors, J. Am. Chem. Soc., 2007, 129, 9125–9136 CrossRef CAS PubMed.
- I. Kaur, W. Jia, R. P. Kopreski, S. Selvarasah, M. R. Dokmeci, C. Pramanik, N. E. McGruer and G. P. Miller, Substituent Effects in Pentacenes: Gaining Control over HOMO-LUMO Gaps and Photooxidative Resistances, J. Am. Chem. Soc., 2008, 130, 16274–16286 CrossRef CAS PubMed.
- P.-L. T. Boudreault, S. Wakim, M. L. Tang, Y. Tao, Z. Bao and M. Leclerc, New indolo[3,2-b]carbazole derivatives for field-effect transistor applications, J. Mater. Chem., 2009, 19, 2921 RSC.
- G. Zhao, H. Dong, H. Zhao, L. Jiang, X. Zhang, J. Tan, Q. Meng and W. Hu, Substitution effect on molecular packing and transistor performance of indolo[3,2-b]carbazole derivatives, J. Mater. Chem., 2012, 22, 4409–4417 RSC.
- M. Kirkus, J. Simokaitiene, J. V. Grazulevicius and V. Jankauskas, Phenyl-, carbazolyl- and fluorenyl-substituted derivatives of indolo[3,2-b]carbazole as hole-transporting glass forming materials, Synth. Met., 2010, 160, 750–755 CrossRef CAS.
- J. Simokaitiene, E. Stanislovaityte, J. V. Grazulevicius, V. Jankauskas, R. Gu, W. Dehaen, Y.-C. Hung and C.-P. Hsu, Synthesis and Properties of Methoxyphenyl-Substituted Derivatives of Indolo[3,2-b]carbazole, J. Org. Chem., 2012, 77, 4924–4931 CrossRef CAS PubMed.
- K. S. Park, S. M. Salunkhe, I. Lim, C.-G. Cho, S.-H. Han and M. M. Sung, High-Performance Air-Stable Single-Crystal Organic Nanowires Based on a New Indolocarbazole Derivative for Field-Effect Transistors, Adv. Mater., 2013, 25, 3351–3356 CrossRef CAS PubMed.
- C. M. Hendrich, V. D. Hannibal, L. Eberle, L. E. Hertwig, U. Zschieschang, F. Rominger, M. Rudolph, H. Klauk and A. S. K. Hashmi, Gold–Catalyzed Synthesis of π–Extended Carbazole–Based Systems and their Application as Organic Semiconductors, Adv. Synth. Catal., 2021, 363, 1401–1407 CrossRef CAS.
- R. Gu, K. Robeyns, L. van Meervelt, S. Toppet and W. Dehaen, Facile synthesis of novel indolo[3,2-b]carbazole derivatives and a chromogenic-sensing 5,12-dihydroindolo[3,2-b]carbazole, Org. Biomol. Chem., 2008, 6, 2484–2487 RSC.
- M. Zhao, B. Zhang and Q. Miao, Revisiting Indolo[3,2-b]carbazole: Synthesis, Structures, Properties, and Applications, Angew. Chem., Int. Ed., 2020, 59, 9678–9683 CrossRef CAS.
- Q. Miao, T.-Q. Nguyen, T. Someya, G. B. Blanchet and C. Nuckolls, Synthesis, Assembly, and Thin Film Transistors of Dihydrodiazapentacene: An Isostructural Motif for Pentacene, J. Am. Chem. Soc., 2003, 125, 10284–10287 CrossRef CAS PubMed.
- U. H. F. Bunz, N-heteroacenes, Chem. – Eur. J., 2009, 15, 6780–6789 CrossRef CAS PubMed.
- Q. Miao, N-Heteropentacenes and N-Heteropentacenequinones: From Molecules to Semiconductors, Synlett, 2012, 326–336 CrossRef CAS.
- T. Riehm, G. de Paoli, A. E. Konradsson, L. de Cola, H. Wadepohl and L. H. Gade, Tetraazaperopyrenes: A New Class of Multifunctional Chromophores, Chem. – Eur. J., 2007, 13, 7317–7329 CrossRef CAS PubMed.
- Q. Tang, Z. Liang, J. Liu, J. Xu and Q. Miao, N-heteroquinones: quadruple weak hydrogen bonds and n-channel transistors, Chem. Commun., 2010, 46, 2977–2979 RSC.
- X.-K. Chen, J.-F. Guo, L.-Y. Zou, A.-M. Ren and J.-X. Fan, A Promising Approach to Obtain Excellent n-Type Organic Field-Effect Transistors: Introducing Pyrazine Ring, J. Phys. Chem. C, 2011, 115, 21416–21428 CrossRef CAS.
- T. Wesp, T. Bruckhoff, J. Petry, H. Wadepohl and L. H. Gade, Towards Nitrogen-Rich N-Heteropolycycles: Synthesis of Octaazaperopyrenes (OAPP), Chem. – Eur. J., 2022, 28, e202200129 CAS.
- M. Winkler and K. N. Houk, Nitrogen-Rich Oligoacenes: Candidates for n-Channel Organic Semiconductors, J. Am. Chem. Soc., 2007, 129, 1805–1815 CrossRef CAS PubMed.
- C. Zhao, H. Ge, S. Yin and W. Wang, Theoretical investigation on the crystal structures and electron transport properties of several nitrogen-rich pentacene derivatives, J. Mol. Model., 2014, 20, 2158 CrossRef PubMed.
- Q. Miao, Ten Years of N-Heteropentacenes as Semiconductors for Organic Thin-Film Transistors, Adv. Mater., 2014, 26, 5541–5549 CrossRef CAS PubMed.
- M. L. Trudell, S. L. Lifer, Y. C. Tan, M. J. Martin, L. Deng, P. Skolnick and J. M. Cook, Synthesis of Substituted 7,12-Dihydropyrido[3,2-b:5,4-b’]diindoIes: Rigid Planar Benzodiazepine Receptor Ligands with Inverse Agonist/Antagonist Properties, J. Med. Chem., 1990, 33, 2412–2420 CrossRef CAS.
- C. B. de Koning, J. P. Michael and A. L. Rousseau, A versatile and convenient method for the synthesis of substituted benzo[a]carbazoles and pyrido[2,3-a]carbazoles, J. Chem. Soc., Perkin Trans. 1, 2000, 1705–1713 RSC.
- Q. Zhang, C. Shi, H. R. Zhang and K. K. Wang, Synthesis of 6H-Indolo[2,3-b,][1,6]naphthyridines and Related Compounds as the 5-Aza Analogues of Ellipticine Alkaloids, J. Org. Chem., 2000, 65, 7977–7983 CrossRef CAS PubMed.
- P. Gómez, S. Georgakopoulos, J. P. Cerón, I. da Silva, M. Más-Montoya, J. Pérez, A. Tárraga and D. Curiel, Hydrogen-bonded azaphenacene: a strategy for the organization of π-conjugated materials, J. Mater. Chem. C, 2018, 6, 3968–3975 RSC.
- J. Qian, Z. Lin, Z. Wang, Z. Peng, L. Wu, P. Lu and Y. Wang, Copper-Carbene-Triggered Electrophilic Cyclization of o-Hydroxyarylenaminones with 3-Diazoindolin-2-imines: Synthesis of 3-Indolyl-4H-chromen-4-ones and Pyrido[2,3-b:6,5-b′]diindoles, J. Org. Chem., 2019, 84, 6395–6404 CrossRef CAS PubMed.
- A. Pictet and T. Spengler, Über die Bildung von Isochinolin–derivaten durch Einwirkung von Methylal auf Phenyl–äthylamin, Phenyl–alanin und Tyrosin, Ber. Dtsch. Chem. Ges., 1911, 44, 2030–2036 CrossRef CAS.
- E. D. Cox and J. M. Cook, The Pictet-Spengler Condensation: A New Direction for an Old Reaction, Chem. Rev., 1995, 95, 1797–1842 CrossRef CAS.
- J. Stöckigt, A. P. Antonchick, F. Wu and H. Waldmann, The Pictet–Spengler Reaction in Nature and in Organic Chemistry, Angew. Chem., Int. Ed., 2011, 50, 8538–8564 CrossRef.
- C. Ingallina, I. D'Acquarica, G. Delle Monache, F. Ghirga, D. Quaglio, P. Ghirga, S. Berardozzi, V. Markovic and B. Botta, The Pictet-Spengler Reaction Still on Stage, Curr. Pharm. Des., 2016, 22, 1808–1850 CrossRef CAS PubMed.
- A. Calcaterra, L. Mangiardi, G. Delle Monache, D. Quaglio, S. Balducci, S. Berardozzi, A. Iazzetti, R. Franzini, B. Botta and F. Ghirga, The Pictet-Spengler Reaction Updates Its Habits, Molecules, 2020, 25, 414 CrossRef PubMed.
- J. K. Augustine, A. Bombrun, P. Alagarsamy and A. Jothi, Selective synthesis of 5,6-dihydrophenanthridines, 5,6-dihydrobenzo[c,][1,8]naphthyridines and their fully aromatized analogues via the Pictet–Spengler reaction mediated by peptide coupling agent propylphosphonic anhydride (T3P), Tetrahedron Lett., 2012, 53, 6280–6287 CrossRef CAS.
- S. Guo, J. Wang, X. Fan, X. Zhang and D. Guo, Synthesis of Pyrazolo[1,5c]quinazoline Derivatives through Copper-Catalyzed Tandem Reaction of 5(2-Bromoaryl)1Hpyrazoles with Carbonyl Compounds and Aqueous Ammonia, J. Org. Chem., 2013, 78, 3262–3270 CrossRef CAS.
- S. Guo, L. Tao, W. Zhang, X. Zhang and X. Fan, Regioselective Synthesis of Indolo[1,2c]quinazolines and 11HIndolo[3,2c]quinolines via Copper-Catalyzed Cascade Reactions of 2(2-Bromoaryl)1Hindoles with Aldehydes and Aqueous Ammonia, J. Org. Chem., 2015, 80, 10955–10964 CrossRef CAS.
- B. Li, S. Guo, J. Zhang, X. Zhang and X. Fan, Selective Access to 3Cyano1Hindoles, 9HPyrimido[4,5b]indoles, or 9HPyrido[2,3b]indoles through Copper-Catalyzed One-Pot Multicomponent Cascade Reactions, J. Org. Chem., 2015, 80, 5444–5456 CrossRef CAS PubMed.
- X. Qin, G. Ding, Z. Luo, Z. Wang, S. Zhang, H. Li and F. Gao, A mild acid-free one-pot reaction for synthesis of new phenanthridine dyes, Dyes Pigm., 2016, 134, 613–617 CrossRef CAS.
- G. Abbiati, A. Arcadi, M. Chiarini, F. Marinelli, E. Pietropaolo and E. Rossi, An alternative one-pot gold-catalyzed approach to the assembly of 11H-indolo3,2-cquinolines, Org. Biomol. Chem., 2012, 10, 7801–7808 RSC.
- A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, A New Gold-Catalyzed C–C Bond Formation, Angew. Chem., Int. Ed., 2000, 39, 2285–2288 CrossRef CAS PubMed.
- A. S. K. Hashmi, T. M. Frost and J. W. Bats, Highly Selective Gold-Catalyzed Arene Synthesis, J. Am. Chem. Soc., 2000, 122, 11553–11554 CrossRef CAS.
- A. S. K. Hashmi and G. J. Hutchings, Gold catalysis, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed.
- A. Corma, A. Leyva-Pérez and M. J. Sabater, Gold-catalyzed carbon-heteroatom bond-forming reactions, Chem. Rev., 2011, 111, 1657–1712 CrossRef CAS PubMed.
- J. Xie, C. Pan, A. Abdukader and C. Zhu, Gold-catalyzed C(sp3)-H bond functionalization, Chem. Soc. Rev., 2014, 43, 5245–5256 RSC.
- R. J. Harris and R. A. Widenhoefer, Gold carbenes, gold-stabilized carbocations, and cationic intermediates relevant to gold-catalysed enyne cycloaddition, Chem. Soc. Rev., 2016, 45, 4533–4551 RSC.
- C. M. Hendrich, K. Sekine, T. Koshikawa, K. Tanaka and A. S. K. Hashmi, Homogeneous and Heterogeneous Gold Catalysis for Materials Science, Chem. Rev., 2021, 121, 9113–9163 CrossRef CAS PubMed.
- R. Heckershoff, S. Maier, T. Wurm, P. Biegger, K. Brödner, P. Krämer, M. T. Hoffmann, L. Eberle, J. Stein, F. Rominger, M. Rudolph, J. Freudenberg, A. Dreuw, A. S. K. Hashmi and U. H. F. Bunz, Cyclopentannulated Dihydrotetraazapentacenes, Chem. – Eur. J., 2022, 28, e202104203 CAS.
- R. Heckershoff, G. May, J. Däumer, L. Eberle, P. Krämer, F. Rominger, M. Rudolph, F. F. Mulks and A. S. K. Hashmi, Entropy–Induced Selectivity Switch in Gold Catalysis: Fast Access to Indolo[1,2-a]quinolines, Chem. – Eur. J., 2022, e202201816 CAS.
- R. Heckershoff, T. Schnitzer, T. Diederich, L. Eberle, P. Krämer, F. Rominger, M. Rudolph and A. S. K. Hashmi, Efficient Synthesis of Dipyrrolobenzenes and Dipyrrolopyrazines via Bidirectional Gold Catalysis: a Combined Synthetic and Photophysical Study, J. Am. Chem. Soc., 2022, 144, 8306–8316 CrossRef CAS PubMed.
- H. Zhao, L. Jiang, H. Dong, H. Li, W. Hu and B. S. Ong, Influence of Intermolecular N-H⋯p Interactions on Molecular Packing and Field-Effect Performance of Organic Semiconductors, ChemPhysChem, 2009, 10, 2345–2348 CrossRef CAS PubMed.
- C. M. Hendrich, L. M. Bongartz, M. T. Hoffmann, U. Zschieschang, J. W. Borchert, D. Sauter, P. Krämer, F. Rominger, F. F. Mulks, M. Rudolph, A. Dreuw, H. Klauk and A. S. K. Hashmi, Gold Catalysis Meets Materials Science – A New Approach to π–Extended Indolocarbazoles, Adv. Synth. Catal., 2021, 363, 549–557 CrossRef.
- T. Watanabe, A. Kobyashi, M. Nishiura, H. Takahashi, T. Usui, I. Kamiyama, N. Mochizuki, K. Noritake, Y. Yokoyama and Y. Murakami, Synthetic Studies on Indoles and Related Compounds. XXVI. The Debenzylation of Protected Indole Nitrogen with Aluminum Chloride., Chem. Pharm. Bull., 1991, 39, 1152–1156 CrossRef CAS.
- H. Suzuki, A. Tsukuda, M. Kondo, M. Aizawa, Y. Senoo, M. Nakajima, T. Watanabe, Y. Yokoyama and Y. Murakami, Unexpected Debenzylation of N-Benzylindoles with Lithium Base. A New Method of N-Debenzylation, Tetrahedron Lett., 1995, 36, 1671–1672 CrossRef CAS.
- A. A. Haddach, A. Kelleman and M. V. Deaton-Rewolinski, An efficient method for the N-debenzylation of aromatic heterocycles, Tetrahedron Lett., 2002, 43, 399–402 CrossRef CAS.
- P. Gao, Y. Liu, L. Zhang, P.-F. Xu, S. Wang, Y. Lu, M. He and H. Zhai, Total Synthesis of Indole Alkaloid (±)-Subincanadine F via SmI2-Mediated Ring Opening and Bridge-Forming Mannich Reaction, J. Org. Chem., 2006, 71, 9495–9498 CrossRef CAS PubMed.
- A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, Domino Carbocationic Rearrangement of α-[Bis(methylthio)methylene]alkyl-2-(3/2-indolyl) Cyclopropyl Ketonescyclopropyl ketones, J. Org. Chem., 2007, 72, 1388–1394 CrossRef CAS PubMed.
- C. Willemann, R. Grünert, P. J. Bednarski and R. Troschütz, Synthesis and cytotoxic activity of 5,6-heteroaromatically annulated pyridine-2,4-diamines, Bioorg. Med. Chem., 2009, 17, 4406–4419 CrossRef CAS PubMed.
- S.-Y. Chou and H. J. Tsai, Regioselective Synthesis of 3-(1H-indol-3-yl)-5-(1H-indole-3-carbonyl)-4-hydroxyfuroic Acids: Route to Hydroxyfuroic Acid-Based Insulin Receptor Activators, Drug Dev. Res., 2011, 72, 247–258 CrossRef CAS.
- J. Letessier and H. Detert, First Synthesis of Benzopyridoiodolium Salts and Twofold Buchwald-Hartwig Amination for the Total Synthesis of Quindoline, Synthesis, 2012, 290–296 CAS.
- N. Ramkumar, M. Raghavendra and R. Nagarajan, Friedel–Crafts Cyclodehydration Approach toward the Synthesis of Ellipti-cine and 9-Methoxyellipticine, Synlett, 2014, 2791–2793 CAS.
- Y. Li, D. Zhang-Negrerie, Y. Du and K. Zhao, A convenient synthesis of indoloquinolinones via 3-arylation of indole-2-carboxamides and PIDA-mediated C–N bond formation, Tetrahedron, 2015, 71, 2927–2935 CrossRef CAS.
- F. Neese, The ORCA program system, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Software update: the ORCA program system, version 4.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- A. D. Becke, Density–functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- C. Hansch, A. Leo and R. W. Taft, A survey of Hammett substituent constants and resonance and field parameters, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- J.-L. Bredas, Mind the gap!, Mater. Horiz., 2014, 1, 17–19 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data, spectra and computational details. CCDC 2205008–2205010. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qo01459a |
| This journal is © the Partner Organisations 2023 |