Copper-catalyzed enantioselective fluoroalkenylation of cyclic imino esters†
Zhiwei
Chen
ac,
Xiang
Huang
bc,
Jian
Liao
 abc and
Min
Wang
abc and
Min
Wang
 *ac
*ac
aNatural Product Research Center, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China. E-mail: wangmin@cib.ac.cn
bChengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 16th November 2022
Abstract
A copper-catalyzed enantioselective fluoroalkenylation of cyclic imino esters was developed. Under mild conditions, fluoroalkenylated products of cyclic imino esters were delivered in good yields with a broad substrate scope. A full-substituted carbon center along with a tetrasubstituted fluoroalkene was forged with good stereoselectivity. Late-stage functionalization of pharmaceuticals was also illustrated through this protocol with high enantioselectivities.
Introduction
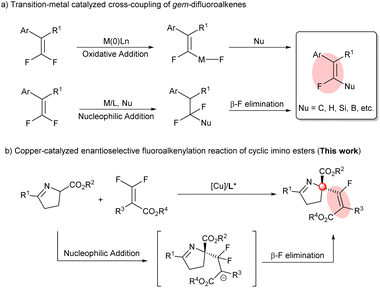
Fluorine, which is of small size with high electronegativity, as expected, possesses some unique properties. More specifically, the incorporation of fluorine or fluorine-containing structural motifs into organic molecules could dramatically influence physical and chemical properties and bioactivity, in particular, solubility, lipophilicity and metabolic stability, compared to their non-fluorinated counterparts.1 Among numerous fluorine-containing molecules, monofluoroalkenes are of particular importance: (a) they are frequently found in pharmaceuticals and bioactive molecules (Scheme 1a);2 (b) they can serve as ideal amide bond mimics with enhanced peptidase stability and a stable conformation in drug discovery (Scheme 1b);3 and (c) they have been used as powerful fluorinated synthons in the field of synthetic organic chemistry in recent years. Therefore, great efforts have been devoted to developing efficient approaches for their synthesis.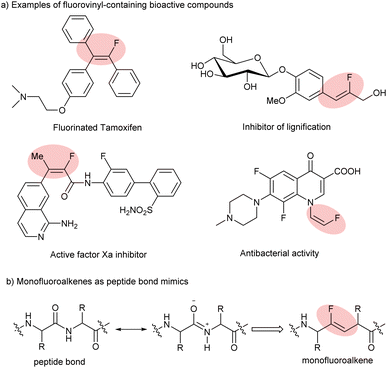 | ||
| Scheme 1 Examples of fluorovinyl-containing bioactive compounds and their application in drug discovery. | ||
Among the strategies developed for the synthesis of monofluoroalkenes, transition-metal catalyzed (Cu,4 Ni,5 Pd,6 Rh7 and Co8) cross-coupling of gem-difluoroalkenes has been proven to be an efficient and prevalent method, where C, H, Si and B reagents (quinolones,7bN-pyrimidinylindoles,8 alkyl halides,5b arylboronic acids,4f,5a protons,4b,6e Et3SiBpin,4d (Bpin)2,4a,cetc.) have been established as reactive coupling nucleophiles to construct structurally diverse monofluoroalkenes (Scheme 2a). Mechanistically, according to the difference of the C–F bond cleavage, these reactions generally entail an oxidative addition of a transition metal to the C–F bond or β-F elimination after nucleophilic addition, where the cleavage of the C–F bond via β-F elimination under mild conditions was more feasible4,7,8 than through the oxidative addition of a C–F bond (120–129 kcal mol−1 for olefinic C–F bonds;9 a high temperature was always necessary5a,6). On the other hand, introduction of monofluoroalkenyl groups to construct chiral C-center-fluoroalkenyl bonds has been rather rare to date. Kong10 first realized the enantioselective Ni-catalyzed monofluoroalkenylation of aryl bromides with gem-difluoroalkenes. Very recently, our group11 reported the enantioselective Cu-catalyzed nucleophilic addition of fluorinated reagents with gem-difluoroalkenes. Nevertheless, employing distinctive partners and developing a novel and efficient method to acquire structurally diverse monofluoroalkenes containing a chiral center through a β-F elimination process is still challenging and highly desirable.
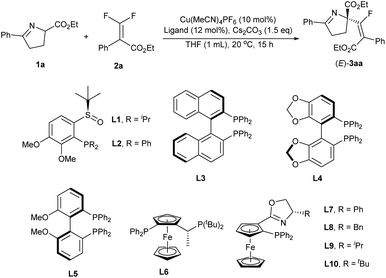
Recently, our group12 has achieved the copper-catalyzed enantioselective electrophilic sulfenylation of cyclic imino esters. In this context, the chiral copper/Phosferrox complex could coordinate with the cyclic imino esters, a kind of versatile synthetic intermediate for the preparation of natural and biologically active compounds, to afford an enolate intermediate that could engage in the asymmetric sulfenylation reaction. We envisaged that in an enantioselective copper catalyzed system, nucleophilic addition of a chiral Cu-coordinated enolate (from cyclic imino esters with the assistance of a base) to fluorinated electrophiles (gem-difluoroalkenes) could be accomplished, followed by β-F elimination to afford the desired products. Herein, we reported the copper-catalyzed enantioselective fluoroalkenylation of cyclic imino esters with difluoroacrylates (Scheme 2b). Following the present protocol, a quaternary carbon stereocenter along with tetrasubstituted monofluoroalkenes was forged with a good enantioselectivity and E/Z ratio.
Results and discussion
We initiated our study with cyclic imino ester 1a and difluoroacrylate 2a as model substrates to evaluate the feasibility of this transformation. Under the reaction conditions (Cu(MeCN)4PF6 (10 mol%) as the copper source and Cs2CO3 (1.5 equiv.) as the base), chiral ligands were first investigated (Table 1, for details, see the ESI†). Most of the chiral ligands applied here could exhibit good reactivity (78–99%) with an (E)-isomer as the major product for the fluoro-olefin, but the enantiocontrol varied (8–98% ee). Specifically, for the SOPs from our group,13P-dialkyl-SOP (L1) with an electron-rich phosphine moiety (iPr) provided better results than P-diaryl-SOP (L2) (Table 1, entries 1 and 2, 31% vs. 8%), which indicated that a more electron-rich ligand is beneficial for enantioselectivity. Subsequently, a variety of commercially available ligands were carefully screened. Biaryl-type ligands, such as (R)-BINAP (L3), (R)-SEGPHOS (L4) and (R)-BIPHEP (L5), were all tolerated in the reaction, resulting in good yields but poor enantioselectivities (Table 1, entries 3–5). Fortunately, chiral ligands with a ferrocene skeleton, which usually showed excellent enantiocontrol in copper catalysis,14 were found beneficial for the stereo-outcome, and the enantioselectivities were greatly improved (Table 1, entries 6–10). We next evaluated various Phosferrox ligands with different moieties and modulated the substituents on the oxazole ring. L9 with an electron-rich and steric resistant moiety (iPr) on the oxazole ring provided the best results (Table 1, entry 9, 95% yield, E/Z = 12.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 98% ee). Thus, after the systematic evaluation of other reaction parameters (for details, see the ESI†), 3aa could be obtained in 78% isolated yield with a satisfactory E-selectivity (E/Z = 12.9/1) and excellent enantioselectivity (98%), using a combination of cyclic imino esters 1a (1.0 equiv.), difluoroacrylate 2a (1.5 equiv.), Cu(MeCN)4PF6 (10 mol%), L9 (12 mol%) and Cs2CO3 (1.5 equiv.) in tetrahydrofuran (THF) at 20 °C for 15 h (Table 1, entry 11). The absolute configuration of the product was assigned as (R)-configuration by X-ray crystallography of 3ka.
1, 98% ee). Thus, after the systematic evaluation of other reaction parameters (for details, see the ESI†), 3aa could be obtained in 78% isolated yield with a satisfactory E-selectivity (E/Z = 12.9/1) and excellent enantioselectivity (98%), using a combination of cyclic imino esters 1a (1.0 equiv.), difluoroacrylate 2a (1.5 equiv.), Cu(MeCN)4PF6 (10 mol%), L9 (12 mol%) and Cs2CO3 (1.5 equiv.) in tetrahydrofuran (THF) at 20 °C for 15 h (Table 1, entry 11). The absolute configuration of the product was assigned as (R)-configuration by X-ray crystallography of 3ka.
| Entry | Ligand | Yieldb (E/Z) | ee (%)c (E) |
|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.12 mmol), Cs2CO3 (0.15 mmol), Cu(MeCN)4PF6 (10 mol%) and ligand (12 mol%) in 1.0 mL of THF at 20 °C for 15 h. b Yields and the E/Z ratio were determined by 1H NMR using 1,1,2,2-tetrachloroethane as an internal standard. c The ee values were determined by chiral HPLC analysis. d Isolated yield (E isomer). e 2a (0.15 mmol) was used. | |||
| 1 | L1 | 88% (6.3/1) | 31 |
| 2 | L2 | 88% (7.8/1) | 8 |
| 3 | L3 | 99% (2.4/1) | 42 |
| 4 | L4 | 92% (4.4/1) | 36 |
| 5 | L5 | 91% (5.1/1) | 12 |
| 6 | L6 | 88% (6.3/1) | 83 |
| 7 | L7 | 91% (3.8/1) | 98 |
| 8 | L8 | 99% (13.1/1) | 95 |
| 9 | L9 | 95% (66%)d (12.6/1) | 98 |
| 10 | L10 | 95% (7.6/1) | 98 |
| 11e | L9 | 78%d (12.9/1) | 98 |
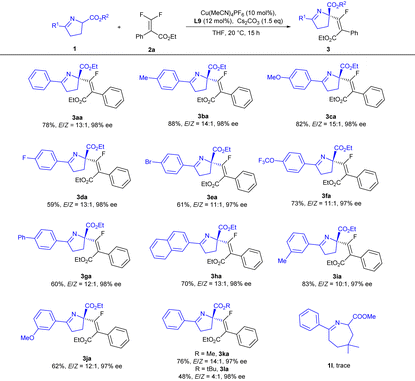
With the optimal conditions in hand, we next explored the substrate scope of cyclic imino esters 1 (Table 2). A variety of functional groups, such as alkyl (Me), halogen (F and Br), ether (OMe and OCF3) and aryl groups (Ph), could be installed at the para- and meta-positions of the aryl ring with little effect on the enantiocontrol, and the corresponding products could be obtained in good yields (59–89%) with excellent enantioselectivities (97–98% ee) and high E/Z-selectivities (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 15
1 → 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for E-isomers) (3aa–3ja). The effect of the ester groups (3ka and 3la) was also investigated, and E-selectivity was found to decrease with the increase of bulkiness of the ester group; when a tert-butyl ester was employed, the E/Z-ratio was reduced to 4
1 for E-isomers) (3aa–3ja). The effect of the ester groups (3ka and 3la) was also investigated, and E-selectivity was found to decrease with the increase of bulkiness of the ester group; when a tert-butyl ester was employed, the E/Z-ratio was reduced to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (3la). When 7-membered cyclic imino ester 1l was introduced, no positive results were achieved.
1 (3la). When 7-membered cyclic imino ester 1l was introduced, no positive results were achieved.
| a Reaction conditions: 1 (0.1 mmol), 2a (0.15 mmol), Cs2CO3 (0.15 mmol), Cu(MeCN)4PF6 (10 mol%) and L9 (12 mol%) in 1.0 mL of THF at 20 °C for 15 h. The E/Z ratio was determined by crude 1H NMR. The ee values were determined by chiral HPLC analysis. Isolated yields of E-isomers. |
|---|
|
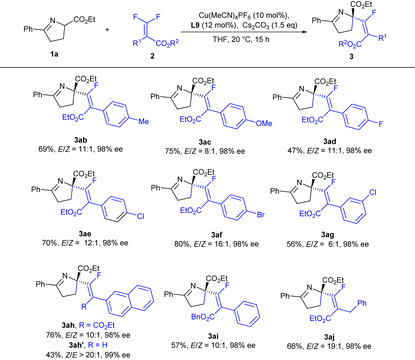
Next, we turned our attention to study the scope of difluoroacrylates 2 (Table 3). When different functional groups (Me, OMe and halogen) were installed at the para-position, the reaction could proceed smoothly to deliver the corresponding products in good yields with excellent enantioselectivities and no remarkable electronic effect was observed on the enantiocontrol (3ab–3af), while a lower reactivity and decreased E/Z-selectivity (3ag, 56%, E/Z = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were observed for a meta-substituted difluoroacrylate, which was probably caused by a steric effect (3aevs.3ag). 2-Naphthyl difluoroacrylate was capable of providing the corresponding product in good yield with excellent enantioselectivity (3ah). The ester group played an important role in reactivity (3ahvs.3ah′), but had no detrimental effect on stereocontrol (3ah′ and 3ai). When benzyl difluoroacrylate was applied, the E/Z ratio could be improved to an excellent level (3aj).
1) were observed for a meta-substituted difluoroacrylate, which was probably caused by a steric effect (3aevs.3ag). 2-Naphthyl difluoroacrylate was capable of providing the corresponding product in good yield with excellent enantioselectivity (3ah). The ester group played an important role in reactivity (3ahvs.3ah′), but had no detrimental effect on stereocontrol (3ah′ and 3ai). When benzyl difluoroacrylate was applied, the E/Z ratio could be improved to an excellent level (3aj).
| a Reaction conditions: 1a (0.1 mmol), 2 (0.15 mmol), Cs2CO3 (0.15 mmol), Cu(MeCN)4PF6 (10 mol%) and L9 (12 mol%) in 1.0 mL of THF at 20 °C for 15 h. The E/Z ratio was determined by crude 1H NMR. The ee values were determined by chiral HPLC analysis. Isolated yields of E-isomers. |
|---|
|
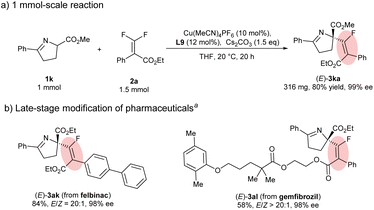
Finally, to further demonstrate the utility of the developed protocol, a scale-up experiment and late-stage functionalization of pharmaceuticals were carried out (Scheme 3). First, this protocol can be readily scaled up to 1 mmol of 1a with no loss in reactivity and stereocontrol (Scheme 3a). gem-Difluoroalkenes derived from felbinac (NSAIDs) and gemfibrozil (cholesterol-lowering agents) were applied to the transformation under the standard conditions. Pleasingly, the corresponding products could be obtained in satisfactory yields with excellent enantioselectivities (Scheme 3b).
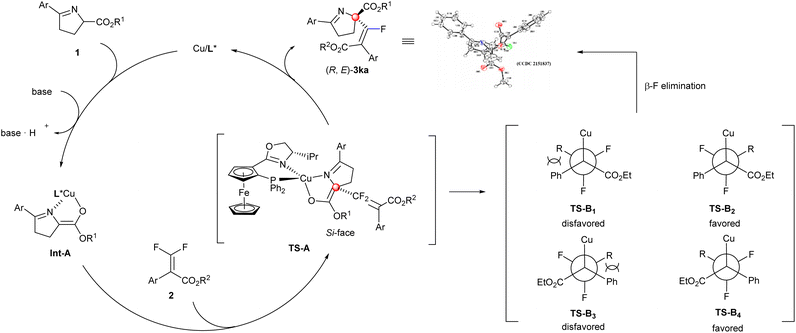
Based on the previous work4c,12,14b and the X-ray structure of (E)-3ka, a possible mechanism was proposed to illustrate the current stereo-outcome (Scheme 4). After the deprotonation of cyclic imino ester with the assistance of Cs2CO3, the Phosferrox ligand and imino ester could coordinate with the central Cu(I) atom to generate the chiral Cu-coordinated enolate Int-A. Due to the steric hindrance between the Ph group (from cyclic imino esters 1) and the isopropyl group (from the oxazoline ring of the chiral ligand) (TS-A), the Re-face of cyclic imino esters was shielded, thus the Si-face predominantly underwent a nucleophilic addition, and then β-F elimination occurred in accordance with the steric repulsion between the cyclic imino ester moiety and the phenyl group (from 2) to give more thermodynamically stable products ((R, E)-3ka).
Conclusions
In summary, a highly enantioselective copper-catalyzed fluoroalkenylation of cyclic imino esters using iPr-Phosferrox as the chiral ligand under mild conditions was developed. Using this protocol, nucleophilic addition and β-F elimination occurred smoothly and tetrasubstituted monofluoroalkenes bearing a chiral cyclic imino ester were afforded in good yields with high E/Z-selectivities and excellent enantioselectivities. In addition, the application was demonstrated by the late-stage functionalization of pharmaceuticals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported financially by the Youth Innovation Promotion Association CAS (2022375), the National Natural Science Foundation of China (No. 21871251 and 22171258) and the Biological Resources Programme, Chinese Academy of Sciences (KFJ-BRP-008).References
- J. Wang, M. S. Rosello, J. L. Aceñ, C. Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011), Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- X. X. Zhang and S. Cao, Recent advances in the synthesis and CAF functionalization of gem-difluoroalkenes, Tetrahedron Lett., 2017, 58, 375–392 CrossRef CAS.
- (a) P. Van der Veken, K. Senten, I. Kertesz, I. De Meester, A. M. Lambeir, M. B. Maes, S. Scharpe, A. Haemers and K. Augustyns, Fluoro-Olefins as Peptidomimetic Inhibitors of Dipeptidyl Eptidases, J. Med. Chem., 2005, 48, 1768–1780 CrossRef CAS PubMed; (b) S. D. Edmondson, L. Wei, J. Xu, J. Shang, S. Xu, J. Pang, A. Chaudhary, D. C. Dean, H. He, B. Leiting, K. A. Lyons, R. A. Patel, S. B. Patel, G. Scapin, J. K. Wu, M. G. Beconi, N. A. Thornberry and A. E. Weber, Fluoroolefins as Amide Bond Mimics in Dipeptidyl Peptidase IV Inhibitors, Bioorg. Med. Chem. Lett., 2008, 18, 2409–2413 CrossRef CAS PubMed.
- (a) H. Sakaguchi, Y. Uetake, M. Ohashi, T. Niwa, S. Ogoshi and T. Hosoya, Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes en Route to Diverse Fluoroalkenes, J. Am. Chem. Soc., 2017, 139, 12855–12862 CrossRef CAS PubMed; (b) J. F. Hu, X. W. Han, Y. Yuan and Z. Z. Shi, Stereoselective Synthesis of Z Fluoroalkenes through Copper Catalyzed Hydrodefluorination of gem-Difluoroalkenes with Water, Angew. Chem., Int. Ed., 2017, 56, 13342–13346 CrossRef CAS; (c) J. Zhang, W. P. Dai, Q. Y. Liu and S. Cao, Cu-Catalyzed Stereoselective Borylation of gem-Difluoroalkenes with B2pin2, Org. Lett., 2017, 19, 3283–3286 CrossRef CAS PubMed; (d) D. H. Tan, E. Lin, W.-W. Ji, Y. F. Zeng, W.-X. Fan, Q. Li, H. Gao and H. G. Wang, Copper-Catalyzed Stereoselective Defluorinative Borylation and Silylation of gem-Difluoroalkenes, Adv. Synth. Catal., 2018, 360, 1032–1037 CrossRef CAS; (e) S. S. Yan, D. S. Wu, J. H. Ye, L. Gong, X. Zeng, C. K. Ran, Y. Y. Gui, J. Li and D. G. Yu, Copper-Catalyzed Carboxylation of C−F Bonds with CO2, ACS Catal., 2019, 9, 987–6992 Search PubMed; (f) Y. Wang, X. Qi, Q. Ma, P. Liu and G. C. Tsui, Stereoselective Palladium-Catalyzed Base-Free Suzuki-Miyaura Cross-Coupling of Tetrasubstituted gem-Difluoroalkenes: An Experimental and Computational Study, ACS Catal., 2021, 11, 4799–4809 CrossRef CAS.
- (a) Y. Xiong, T. Huang, X. F. Ji, J. J. Wu and S. Cao, Nickel-Ctalyzed Suzuki–Miyaura Type Cross Coupling Reactions of (2,2-Difluorovinyl)benzene Derivatives with Arylboronic Acids, Org. Biomol. Chem., 2015, 13, 7389–7392 RSC; (b) X. Lu, Y. Wang, B. Zhang, J. J. Pi, X. X. Wang, T. J. Gong, B. Xiao and Y. Fu, Nickel-Catalyzed Defluorinative Reductive Cross-Coupling of gem-Difluoroalkenes with Unactivated Secondary and Tertiary Alkyl Halides, J. Am. Chem. Soc., 2017, 139, 12632–12637 CrossRef CAS.
- (a) J. Xu, E. A. Ahmed, B. Xiao, Q. Q. Lu, Y. L. Wang, C. C. Yu and Y. Fu, Pd-Catalyzed Regioselective Activation of gem-Difluorinated Cyclopropanes: A Highly Efficient Approach to 2-Fluorinated Allylic Scaffolds, Angew. Chem., Int. Ed., 2015, 54, 8231–8235 CrossRef CAS; (b) M. A. Ahmed, M. Y. Suliman, T. J. Gong and Y. Fu, Palladium-Catalyzed Stereoselective Defluorination Arylation/Alkenylation/Alkylation of gem-Difluorinated Cyclopropanes, Org. Lett., 2019, 21, 5645–5649 CrossRef; (c) L. Y. Lv and C. J. Li, Palladium-Catalyzed Defluorinative Alkylation of gem-Difluorocyclopropanes: Switching Regioselectivity via Simple Hydrazones, Angew. Chem., Int. Ed., 2021, 60, 13098–13104 CrossRef CAS; (d) Q. Ma, Y. H. Wang and G. C. Tsui, Stereoselective Palladium Catalyzed C-F Bond Alkynylation of Tetrasubstituted gem-Difluoroalkenes, Angew. Chem., Int. Ed., 2020, 59, 11293–11297 CrossRef CAS; (e) Q. Ma, C. Liu and G. C. Tsui, Palladium-Catalyzed Stereoselective Hydrodefluorination of Tetrasubstituted gem-Difluoroalkenes, Org. Lett., 2020, 22, 5193–5197 CrossRef CAS; (f) M. Li, Y. H. Wang and G. C. Tsui, Palladium-Catalyzed Stereoselective C−F Bond Vinylation and Allylation of Tetrasubstituted gem-Difluoroalkenes via Stille Coupling: Synthesis of Monofluorinated 1,3- and 1,4-Dienes, Org. Lett., 2021, 23, 8072–8076 CrossRef CAS; (g) Y. H. Wang, Q. Ma and G. C. Tsui, Stereoselective Synthesis of Difluorinated 1,3-Dienes via Palladium-Catalyzed C−F Bond Activation of Tetrasubstituted gem-Difluoroalkenes, Org. Lett., 2021, 23, 5241–5245 CrossRef CAS PubMed.
- (a) P. P. Tian, C. Feng and T. P. Loh, Rhodium-Catalysed C(sp2)–C(sp2) Bond Formation via C–H/C–F Activation, Nat. Commun., 2015, 6, 7472 CrossRef PubMed; (b) L. H. Kong, B. X. Liu, X. K. Zhou, F. Wang and X. W. Li, Rhodium(III)-Catalyzed Regio- and Stereoselective Benzylic α-Fluoroalkenylation with gem-Difluorostyrenes, Chem. Commun., 2017, 53, 10326–10329 RSC.
- L. H. Kong, X. K. Zhou and X. W. Li, Cobalt(III)-Catalyzed Regio- and Stereoselective α-Fluoroalkenylation of Arenes with gem-Difluorostyrenes, Org. Lett., 2016, 18, 6320–6323 CrossRef CAS PubMed.
- J. Vela, J. M. Smith, Y. Yu, N. A. Ketterer, C. J. Flaschenriem, R. J. Lachicotte and P. L. Holland, Synthesis and Reactivity of Low-Coordinate Iron(II) Fluoride Complexes and Their Use in the Catalytic Hydrodefluorination of Fluorocarbons, J. Am. Chem. Soc., 2005, 127, 7857–7870 CrossRef CAS PubMed.
- T. Ma, Y. Chen, Y.-X. Li, Y.-Y. Ping and W.-Q. Kong, Nickel-Catalyzed Enantioselective Reductive Aryl Fluoroalkenylation of Alkenes, ACS Catal., 2019, 9, 9127–9133 CrossRef CAS.
- H. X. Lin, W. Jiao, Z. W. Chen, J. Han, D. M. Fang, M. Wang and J. Liao, Enantioselective Cu-Catalyzed Nucleophilic Addition of Fluorinated Reagents: C−C Bond Formation for the Synthesis of Chiral Vicinal Difluorides, Org. Lett., 2022, 24, 2197–2202 CrossRef CAS.
- Z. W. Chen, H. X. Lin, J. Han, D. M. Fang, M. Wang and J. Liao, Enantioselective Copper-Catalyzed Electrophilic Sulfenylation of Cyclic Imino Esters, Org. Lett., 2021, 23, 9146–9150 CrossRef CAS PubMed.
- (a) T. Jia, P. Cao, B. Wang, Y. Z. Lou, X. M. Yin, M. Wang and J. Liao, A Cu/Pd Cooperative Catalysis for Enantioselective Allylboration of Alkenes, J. Am. Chem. Soc., 2015, 137, 13760–13763 CrossRef CAS; (b) Y. Z. Lou, P. Cao, T. Jia, Y. L. Zhang, M. Wang and J. Liao, Copper-Catalyzed Enantioselective 1,6-Boration of para-Quinone Methides and Efficient Transformation of gem-Diarylmethine Boronates to Triarylmethanes, Angew. Chem., Int. Ed., 2015, 54, 12134–12138 CrossRef CAS PubMed; (c) B. Chen, P. Cao, X. M. Yin, Y. Liao, L. Y. Jiang, J. L. Ye, M. Wang and J. Liao, Modular Synthesis of Enantioenriched 1,1,2-Triarylethanes by an Enantioselective Arylboration and Cross-Coupling Sequence, ACS Catal., 2017, 7, 2425–2429 CrossRef CAS; (d) B. Wang, X. H. Wang, X. M. Yin, W. Z. Yu, Y. Liao, J. L. Ye, M. Wang and J. Liao, Cu-Catalyzed SN2′ Substitution of Propargylic Phosphates with Vinylarene-Derived Chiral Nucleophiles: Synthesis of Chiral Allenes, Org. Lett., 2019, 21, 3913–3917 CrossRef CAS PubMed.
- (a) R. He, X. Huo, L. Zhao, F. Wang, L. Jiang, J. Liao and W. B. Zhang, Stereodivergent Pd/Cu Catalysis for the Dynamic Kinetic Asymmetric Transformation of Racemic Unsymmetrical 1,3-Disubstituted Allyl Acetates, J. Am. Chem. Soc., 2020, 142, 8097–8103 CrossRef CAS; (b) M. H. Zhu, Q. L. Zhang and W. W. Zi, Diastereodivergent Synthesis of β-Amino Alcohols by Dual-Metal Catalyzed Coupling of Alkoxyallenes with Aldimine Esters, Angew. Chem., Int. Ed., 2021, 60, 6545–6552 CrossRef CAS; (c) J. Zhang, X. Huo, J. Xiao, L. Zhao, S. M. Ma and W. B. Zhang, Enantio- and Diastereodivergent Construction of 1,3-Nonadjacent Stereocenters Bearing Axial and Central Chirality through Synergistic Pd/Cu Catalysis, J. Am. Chem. Soc., 2021, 143, 12622–12632 CrossRef CAS PubMed; (d) L. Z. Peng, Z. Z. He, X. H. Xu and C. Guo, Cooperative Ni/Cu-Catalyzed Asymmetric Propargylic Alkylation of Aldimine Esters, Angew. Chem., Int. Ed., 2020, 59, 14270–14274 CrossRef CAS; (e) L. Z. Peng, H. Y. Wang and C. Guo, Copper-Catalyzed Enantioselective Difluoromethylation of Amino Acids via Difluorocarbene, J. Am. Chem. Soc., 2021, 143, 6376–6381 CrossRef CAS PubMed; (f) A. Koizumi, M. Kimura, Y. Arai, Y. Tokoro and S. Fukuzawa, Copper- and Silver-Catalyzed Diastereo- and Enantioselective Conjugate Addition Reaction of 1-Pyrroline Esters to Nitroalkenes: Diastereoselectivity Switch by Chiral Metal Complexes, J. Org. Chem., 2015, 80, 10883–10891 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2151837. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qo01141g |
| This journal is © the Partner Organisations 2023 |