Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vapochromic films of π-conjugated polymers based on coordination and desorption at hypervalent tin(IV)-fused azobenzene compounds†
Masayuki
Gon
 ,
Yusuke
Morisaki
,
Kazuya
Tanimura
,
Kazuo
Tanaka
,
Yusuke
Morisaki
,
Kazuya
Tanimura
,
Kazuo
Tanaka
 * and
Yoshiki
Chujo
* and
Yoshiki
Chujo
Department of Polymer Chemistry, Graduate School of Engineering, Kyoto University Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: tanaka@poly.synchem.kyoto-u.ac.jp
First published on 1st February 2023
Abstract
We report the synthesis and vapochromic behaviors of film materials consisting of hypervalent tin-containing π-conjugated polymers. We prepared copolymers with brominated tin-fused azobenzenes and modified fluorene having tetraethylene glycol as a side chain. The synthesized polymers showed good film-formability and high affinity with coordinating solvent molecules such as dimethyl sulfoxide (DMSO). In particular, we discovered distinct color changes from blue to purple when exposed to DMSO vapor. It was revealed that color changes should originate from reversible alteration of the coordination-number between five and six of hypervalent tin(IV) in the azobenzene compounds involved in the main-chain conjugation. Moreover, we also observed that binding constants between tin and coordinating solvents could be influenced by two substitutions on the tin atom and subsequently modulated responsivity of vapochromism in films by altering the type of substituent. Furthermore, the color-change behaviors can be estimated by quantum calculations with density functional theory. We demonstrate not only that hypervalent tin can work as a switching unit for modulating the electronic structures of π-conjugated polymers triggered by solvent coordination but also that vapochromic behaviors in films can be predicted by estimating the affinity between hypervalent tin and solvent molecules with theoretical calculations.
Introduction
Molecular detection without expensive instruments is a fundamental technology for constructing facile environmental assessments and daily health checking systems. To meet the demands for manufacturing practical sensors, organic materials are a versatile platform because of flexibility in material design which enables us to realize selective and sensitive detection for the targets by adjusting molecular structures.1–3 In particular, π-conjugated polymers have attracted attention because of various advantages such as not only superior light-absorption and emission properties4 but also good film-formability and printability in addition to designability.5,6 Owing to such advantages, so far, various chemical sensors based on π-conjugated polymers have been developed.7–10Vapochromism is known as the phenomenon in which color changes can be reversibly induced by vapor absorption and subsequently desorption.11–13 In the absence of a direct connection between a chromophore and a target, vapochromic behaviors can be obtained. In the case of some kinds of crystalline samples, crystal–crystal transition can be induced by vapor fuming with specific solvents, followed by chromic behaviors.14–23 Alterations of intramolecular structures and/or intermolecular distributions of chromophores including π-conjugated systems or transition metals should be responsible for optical changes in these materials. Optical changes can be recently realized in films consisting of π-conjugated polymers.24,25 By vapor fuming, annealing processes should proceed. As a result, apparent and emission color changes that originated from morphology alterations of π-conjugated polymers can be observed. In these materials, dissolving into solvents and film-formation should be required for restoring the initial state.
If a connection can be formed between chromophores and the targets, sensitivity and selectivity can be improved. Indeed, in the case of metal complexes with organic ligands, chromic behaviors are also inducible by the direct coordination of solvent molecules to the metal centers of complexes with good sensitivity.26–32 As a practical application, complex-doped polymer films have been also prepared.33–38 Selectivity can be tuned by choosing the type of metal center that can coordinate with the target. However, vapochromism from film materials with main-chain-type π-conjugated polymers consisting of metal complexes could be hardly accomplished. Although organoboron complex-containing polymers can exhibit optical changes by reacting with fluoride anions, reversible responses were principally impossible due to the extremely high stability of the B–F bond.39–43 Furthermore, anion sensors can work only in solution.44 Besides anions, the partially reversible sensors for neutral donor molecules such as pyridine in solution were also prepared.45 Although the vapochromic film was developed with the interaction between poly(9-borafluorene) and NH3 vapor,46 the number of examples is limited. Therefore, the development of vapochromic film materials composed of main-chain-type π-conjugated polymers consisting of metal complexes is a next goal not only for demonstrating a new modulation method of electronic properties of highly expanded π-conjugated systems but also for obtaining property-adjustable film sensors in which various properties can be tuned according to the preprogrammed design.
Recently, we proposed the design concept of heteroatom-containing polymers by employing “element-blocks”, which are a minimum functional unit containing heteroatoms, to obtain novel functions originating from loaded elements.47–49 Based on this concept, we have developed main-chain-type π-conjugated molecules and polymers with boron-fused azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) or azomethine (C
N) or azomethine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) groups.25,50–57 They showed various functions such as film-state visible to NIR emission,25,51–54,57 stimuli-responsiveness,25 aggregation-induced emission (AIE),53,54,57,58 and crystallized-induced emission enhancement (CIEE).51,52,59–63 Hypervalent compounds are a class of molecules in which over eight electrons are formally assigned around a valence shell of a main group element beyond the limit of the Lewis octet rule.64–66 More recently, we reported that a series of hypervalent tin-fused azobenzene (TAz) compounds with five-coordinated distorted trigonal bipyramidal geometries.67,68 They exhibited absorption and emission bands from the orange to the NIR region despite the fact that the small π-conjugated systems are involved in these molecules.69 Some hypervalent compounds can change the coordination numbers of their atom centers upon the interaction between the Lewis acidic atom centers and Lewis basic substrates.70–73 In our case, hypsochromic shifts of the spectra can be induced by solvent coordination to the tin center with the coordination-number change from five to six.67 In the crystalline state, vapochromic behaviors were observed by encapsulation of dimethyl sulfoxide (DMSO) vapor with crystal–crystal transition. However, it is still difficult to apply the vapochromism of these compounds to practical sensing materials due to fragility of crystal powders. Moreover, crystal–crystal transitions followed by collapse of regular structures limited the reversibility of chromic behaviors.
N) groups.25,50–57 They showed various functions such as film-state visible to NIR emission,25,51–54,57 stimuli-responsiveness,25 aggregation-induced emission (AIE),53,54,57,58 and crystallized-induced emission enhancement (CIEE).51,52,59–63 Hypervalent compounds are a class of molecules in which over eight electrons are formally assigned around a valence shell of a main group element beyond the limit of the Lewis octet rule.64–66 More recently, we reported that a series of hypervalent tin-fused azobenzene (TAz) compounds with five-coordinated distorted trigonal bipyramidal geometries.67,68 They exhibited absorption and emission bands from the orange to the NIR region despite the fact that the small π-conjugated systems are involved in these molecules.69 Some hypervalent compounds can change the coordination numbers of their atom centers upon the interaction between the Lewis acidic atom centers and Lewis basic substrates.70–73 In our case, hypsochromic shifts of the spectra can be induced by solvent coordination to the tin center with the coordination-number change from five to six.67 In the crystalline state, vapochromic behaviors were observed by encapsulation of dimethyl sulfoxide (DMSO) vapor with crystal–crystal transition. However, it is still difficult to apply the vapochromism of these compounds to practical sensing materials due to fragility of crystal powders. Moreover, crystal–crystal transitions followed by collapse of regular structures limited the reversibility of chromic behaviors.
Herein, we show the synthesis and unique properties of π-conjugated copolymers with TAz and fluorene moieties which can be a film-type vapochromic sensor. Two types of the TAz derivatives having methyl or phenyl groups at tin were prepared. In the fluorene units, tetraethylene glycol (TEG) side chains were introduced for improving the affinity of the hydrophobic π-conjugated polymers to highly polar coordinating solvents. Accordingly, the distinct color change from blue to purple was observed in films when exposed to DMSO vapor. We demonstrate here that molecular coordination at the hypervalent element in polymers can be a trigger for drastically changing the electronic structure of main-chain π-conjugation. Moreover, we were able to explain chromic behaviors with theoretical calculations. In particular, we proved that binding constants of solvent molecules can be estimated by the length of dative bonds between tin and coordinated atoms. We also showed that vapochromic behaviors are correlated with the affinity which can be evaluated with calculations.
Results and discussion
Synthesis and characterization
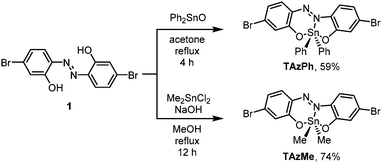
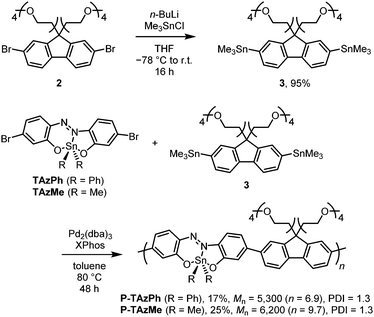
Scheme 1 shows the synthesis of the TAz compounds. The tridentate ligand of azobenzene (1) was prepared according to the literature.57 Dehydration condensation was performed with 1 and diphenyltin(IV) oxide in acetone under reflux conditions to afford TAzPh in 59% isolated yield. TAzMe was obtained through the reaction with 1 and dimethyltin(IV) dichloride in the presence of sodium hydroxide in 74% isolated yield. The bromine groups in these TAz compounds were intended to be used for polymerization.Next, we synthesized π-conjugated copolymers including the TAz moieties (Scheme 2). The modified fluorene compound having two TEG groups as side chains was designed as a comonomer to improve solubility in polar solvents, such as dimethyl sulfoxide (DMSO), dimethyl formamide (DMF), methanol (MeOH) and acetonitrile (MeCN), which have the potential to coordinate to the tin center. Trimethylstannyl groups were introduced through lithiation with 2 and subsequently addition of trimethyltin chloride, and the comonomer 3 was obtained in 95% isolated yield. Polymerization for preparing P-TAzPh and P-TAzMe was conducted via the Migita–Kosugi–Stille cross coupling reaction74,75 with TAzPh or TAzMe and 3 in the presence of Pd2(dba)3 (dba = dibenzylideneacetone) and XPhos (2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl), respectively. The products were purified and fractionated by high performance liquid chromatography (HPLC). The molecular weights were determined using gel permeation chromatography (GPC) with polystyrene standards. Consequently, we isolated P-TAzPh (17%, Mn = 5,200 (n = 6.9), PDI = 1.3, PDI: poly dispersity index) and P-TAzMe (25%, Mn = 6,200 (n = 9.7), PDI = 1.3). All products were characterized by 1H, 13C and 119Sn NMR spectroscopy, high-resolution mass spectrometry (HRMS) and elemental analyses (see the ESI†). These characterization data enabled us to investigate the properties of the TAz derivatives.
Optical properties in diluted solution
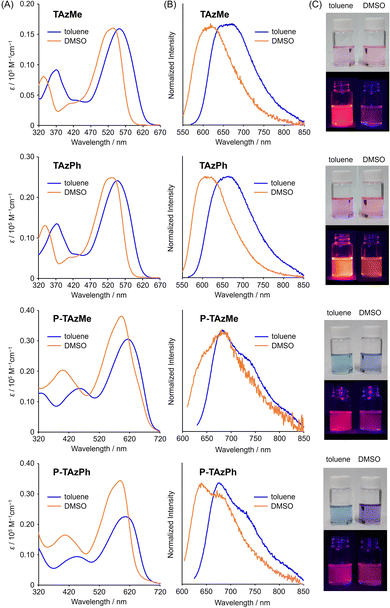
First, we investigated the fundamental optical properties of the TAz monomers and polymers by UV–vis absorption and photoluminescence (PL) measurements in toluene (1.0 × 10−5 M) (Fig. 1 and Table 1). TAzPh and TAzMe showed absorption bands at around 550 nm and emission with the peaks around 670 nm. These long absorption and emission bands were unique characteristics of the TAz derivatives originating from distorted trigonal bipyramidal geometry consisting of a three center–four electron (3c-4e) bond at apical positions and a sp2 hybrid orbital at equatorial positions.67 Both spectra showed bathochromic shifts after copolymerization with the fluorene units. The peak tops of absorption and emission bands reached 600 and 680 nm, respectively. In addition, the band shapes of the emission spectra were sharpened, and the absolute PL quantum yields (ΦPLs) were improved. The enhancement of rigidity by the expansion of π-conjugation through the polymer main-chain could be responsible for emission improvement. The TAzMe derivatives had more efficient emission than TAzPh ones in the deep red region, implying that small alkyl chains on the tin center might contribute to the construction of planar π-conjugation system through polymer main-chains especially in the excited state.| Solvent | λ abs/nm | λ PL /nm | Φ PL /% | |
|---|---|---|---|---|
| a In mixed solvent, toluene/DMSO = 1/99 v/v. b Excited at λabs. c Absolute PL quantum yield. | ||||
| TAzMe | Toluene | 552 | 671 | 18.0 |
| DMSO a | 534 | 618 | 1.3 | |
| TAzPh | Toluene | 547 | 665 | 15.6 |
| DMSO a | 533 | 605 | 3.7 | |
| P-TAzMe | Toluene | 614 | 682 | 24.4 |
| DMSO a | 593 | 680 | 1.0 | |
| P-TAzPh | Toluene | 606 | 677 | 19.2 |
| DMSO a | 583 | 640 | 2.2 | |
We previously reported that solvent coordination to the tin center induced the coordination-number change from five to six followed by hypsochromic shifts of the absorption and emission bands.67 To examine the optical properties of the TAz derivatives in the polymer main-chain, we compared the solvent effects on the optical properties of monomers and polymers and estimated the coordination-number change (Fig. 1 and Fig. S1, ESI,†Table 1). In the case of non-coordinating solvents such as toluene and chloroform (CHCl3), spectrum shifts were hardly observed, while clear hypsochromic shifts were observed in coordinating solvents such as DMSO, DMF, MeOH and MeCN. Since similar changes in optical properties were induced both in monomers and polymers, we concluded that the solvent coordination was able to proceed at the hypervalent tin compounds in the polymer main-chains. Moreover, the degree of the peak shift, which represents strength of binding, was significantly dependent on the type of solvents. Fig. 2 shows relationships with wavelengths of maximum absorption by increasing the ratio of the coordinating solvents from 0 to 20 vol% in toluene. Full titration data from 0 to 99 vol% are shown in Fig. S2–S7 and Tables S1–S4 (ESI†). Accordingly, the solvent coordination to the tin center was stronger in the following order: DMSO > DMF > MeOH > MeCN. Additionally, it was shown that the TAzPh derivatives showed larger shifts than TAzMe ones judging from width of the hypsochromic shifts. To get more insight into the coordination, we estimated a binding constant (K) with a curve fitting method based on 1 to 1 coordination according to our previous work (Table 2 and Fig. S8, ESI†).67 It was shown that the K values were larger in the following order; DMSO > DMF ≫ (MeOH and MeCN, too weak to obtain reliable values). Furthermore, the K value of TAzPh was larger than that of TAzMe. This should be because that a methyl group has stronger electron-donating ability than a phenyl group and it reduces the Lewis acidity of tin. In addition, lower ΦPL values were observed from the stronger-coordinated compounds (Fig. S6 and S7, ESI†). Structural distortion followed by enhancement of nonradiative decay should be caused by the solvent coordination.67 It should be noted that the polymers showed similar solvent effects. In other words, solvent coordination to the TAz moieties should occur in the polymers. In P-TAzMe, a complete hypsochromic shift in the PL spectrum was not observed in toluene/DMSO = 1/99 v/v (Fig. 1B). This indicates that the solvent coordination can be controlled by the substituents on the tin center.
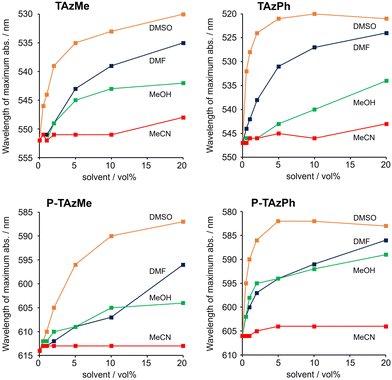 | ||
| Fig. 2 Titration data of TAzMe, TAzPh, P-TAzMe and P-TAzPh. Hypsochromic shifts of wavelengths of maximum absorption depending on solvent vol% in toluene (1.0 × 10−5 M for monomers and 1.0 × 10−5 M per repeating unit for polymers). Full spectrum data are shown in the ESI.† | ||
| K DMSO/M−1 | K DMF/M−1 | |
|---|---|---|
| a Determined in toluene at room temperature (25 °C). | ||
| TAzMe | 2.7 | 0.59 |
| TAzPh | 11 | 1.3 |
Optical properties in polymer thin films
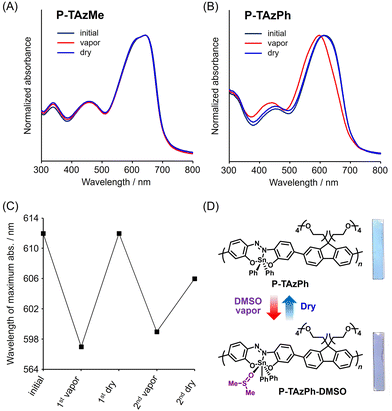
Next, we explored vapochromism in polymer films. We prepared thin films of P-TAzPh and P-TAzMe on quartz substrates (0.9 cm × 5.0 cm) using a spin-coating method (1000 rpm, 30 s, 100 μL of CHCl3 solution (2 mg/300 μL)) and subsequently dried in vacuo for 12 h. We evaluated color changes and especially focused on their reversibility with the films by monitoring UV–vis absorption measurements with the exposure to DMSO, DMF, MeOH and CH3CN vapors for 30 min and following dry with a hair dryer for 30 min at room temperature (Fig. 3 and Fig. S9, S10, ESI†). Accordingly, the most drastic color change and the recovery were found in the combination of P-TAzPh and DMSO vapor (Fig. 3B and D), and almost all other pairs were inactive (Fig. 3A and Fig. S10, ESI†). It should be noted that P-TAzPh showed repeatability of at least two cycles (Fig. 3C). If color changes are induced by morphology alteration and/or degradation of hypervalent tin compounds, it should be impossible to observe reversible behaviors. Thus, it can be said that coordination and desorption of solvent vapor at the hypervalent tin should be responsible for the vapochromic properties in the polymer film. Since the vapochromic behaviors should be dependent on the magnitude of the binding constant, it is likely that the pair of TAzPh and DMSO showed the largest degree of optical changes.67 Crystal–crystal transition was essential for presenting color changes with small molecules according to the previous work.67 It should be emphasized that we succeeded in realizing vapochromism with polymer films which are easily applied to stimuli-responsive materials manufactured by a printing method. It should be noted that reversible color change was also detectable using DMF vapor, which was not observed from the crystalline sample in which it was dissolved by DMF vapor.67 Interestingly, the P-TAzMe was inactive by exposure to DMSO vapor despite the fact that KDMSO of TAzMe is larger than KDMF of TAzPh. It is implied that bulky substituents around tin might make cavities which are capable of capturing solvent molecules. As a result, P-TAzPh can show higher sensitivity toward vapor fuming. In contrast to absorption spectra, the changes in PL spectra were hardly observed upon exposure to various solvent vapors regardless of non-coordinating or coordinating ones. (Fig. S11, ESI†). It might be because of intra- and intermolecular energy migration from DMSO coordinated sites to luminescent sites in film. By combining these results of absorption and emission behaviors in film, it is supported that the color change in absorption should be caused not by the solvent-vapor annealing process but by the solvent coordination to the tin center. Basically, strong concentration quenching occurred (ΦPL = 3.4% for P-TAzMe and ΦPL = 0.3% for P-TAzPh), and the value was almost similar before and after DMSO addition (ΦPL = 3.2% for P-TAzMe and ΦPL = 0.3% for P-TAzPh with DMSO vapor). It was hard to observe the luminescence behavior by the naked eye because the emission band reached the NIR area.Theoretical calculations
We carried out quantum calculations with density functional theory (DFT) and time-dependent (TD)-DFT to simulate optical behaviors of the TAz derivatives with DMSO coordination. M-TAzMe and M-TAzPh which have fluorene units at both sides of TAzMe and TAzPh, respectively, were used as model compounds of the polymers for saving calculation costs. Accordingly, TAzMe exhibited a slightly longer absorption band (509 nm) than TAzPh (501 nm), and that behavior was qualitatively in good agreement with experimental data (Fig. S12B, ESI†). It was observed that the methyl groups can have an electron-donating ability because the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) energy levels of TAzMe were elevated compared to those of TAzPh (Fig. S12A, ESI†). Their DMSO adducts, TAzMe-DMSO and TAzPh-DMSO, showed larger energy gaps (475 and 469 nm, respectively) due to the larger degree of elevation of the energy levels of the LUMO than the HOMO (Fig. S12, ESI†) with keeping molecular orbital (MO) shapes (Fig. S13, ESI†). Our previous research suggested that the hypsochromic shift and different effects on MO energy levels can be caused by coordination-number change of the hypervalent compound from five to six.67 Concretely, elevation of LUMO and HOMO energy levels should be induced by the coordination of the oxygen lone pair in DMSO, which weakened the Sn–N coordination and enhanced electron-donating ability of oxygens at the apical positions of the ligand in the 3c-4e bond.The hypsochromic shift induced by DMSO coordination was also effective in M-TAzMe and M-TAzPh (Fig. 4 and Fig. S12, ESI†). Similarly to the monomers, the shapes of MOs were preserved before and after DMSO coordination. These results are of significance for presenting distinct chromic behaviors because slight shape changes of MOs can secure large oscillator strength (f) associated with the value of a molar extinction coefficient (Fig. S14, ESI†). We previously reported that the binding constants of TAzPh derivatives can be estimated by the equation of d = −0.0049 × (In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KDMSO) + 2.3564 (d: Sn–O (DMSO) bond length (Å) from an optimized structure).67 According to this equation, we can estimate the binding constants as KDMSO = 11.6 for TAzPh and 2.8 for M-TAzPh (Fig. S15, ESI†). The former value was well matched to the experimental data in this study (KDMSO = 11 for TAzPh). Although M-TAzPh was not synthesized and the K value of P-TAzPh was difficult to experimentally estimate due to weak affinity, we can assume that the affinity to DMSO of M-TAzPh should be weaker than that of TAzPh. These calculation results support that DMSO coordination can induce hypsochromic color changes in the polymer thin film.
KDMSO) + 2.3564 (d: Sn–O (DMSO) bond length (Å) from an optimized structure).67 According to this equation, we can estimate the binding constants as KDMSO = 11.6 for TAzPh and 2.8 for M-TAzPh (Fig. S15, ESI†). The former value was well matched to the experimental data in this study (KDMSO = 11 for TAzPh). Although M-TAzPh was not synthesized and the K value of P-TAzPh was difficult to experimentally estimate due to weak affinity, we can assume that the affinity to DMSO of M-TAzPh should be weaker than that of TAzPh. These calculation results support that DMSO coordination can induce hypsochromic color changes in the polymer thin film.
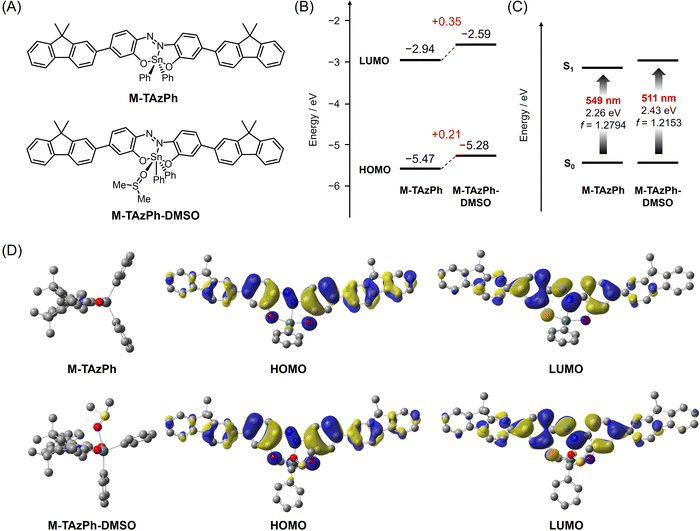 | ||
| Fig. 4 Calculation results of M-TAzPh and M-TAzPh-DMSO as a model compound of P-TAzPh and its DMSO adduct, respectively, with DFT. (A) Chemical structures. (B) Calculated HOMO and LUMO energy levels, and (C) calculated S0 → S1 transition bands and oscillator strengths (f). (D) Side view of optimized structures, and selected Kohn–Sham orbitals (isovalue = 0.02). Calculation details are shown in the ESI.† | ||
Conclusions
π-Conjugated copolymers composed of TAz and fluorene moieties with TEG side chains were synthesized. The substituents at the tin center play a critical role in solvent coordination. Indeed, phenyl groups had larger binding constants than methyl groups. As a result, we achieved observing clear vapochromism in thin films of the copolymer including TAzPh by fuming DMSO vapor, which was the pair having the largest binding constant. The hypsochromic shifts induced by solvent coordination both in solution and film were attributed to the coordination-number change of tin from five to six. This fact clearly indicates that the hypervalent tin atom can work as a switch module for modulating the electronic properties of π-conjugated polymers by employing the azobenzene ligand. Furthermore, the spectrum shift was supported by theoretical calculation. In particular, the binding constant was well predicted by the Sn–O (DMSO) bond length. This means that the binding constant and subsequently sensitivity in vapochromic behaviors in films could be estimated with the theoretical calculations on the basis of our estimation protocols described here. The studies are expected to be used for developing stimuli-responsive materials with inherent element properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Fujimori Science and Technology Foundation (for M. G.), and a Grant-in-Aid for Early-Career Scientists (for M. G.) (JP20K15334), for Scientific Research (B) (for M. G.) (JP22H02130), for Scientific Research (B) (for K. T.) (21H02001), for Exploratory Research (for K. T.) (JP21K19002), and for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No. 2401)” (JP24102013).References
- R. M. Crooks and A. J. Ricco, New Organic Materials Suitable for Use in Chemical Sensor Arrays, Acc. Chem. Res., 1998, 31, 219–227 CrossRef CAS.
- W. Al Zoubi and N. D. Al Mohanna, Membrane sensors based on Schiff bases as chelating ionophores – A review, Spectrochim. Acta, Part A, 2014, 132, 854–870 CrossRef CAS PubMed.
- D. James, S. M. Scott, Z. Ali and W. T. O’Hare, Chemical Sensors for Electronic Nose Systems, Microchim. Acta, 2005, 149, 1–17 CrossRef CAS.
- P. Kumar, A. Ghosh and D. A. Jose, Chemical Sensors for Water Detection in Organic Solvents and their Applications, ChemistrySelect, 2021, 6, 820–842 CrossRef CAS.
- B. Li, S. Santhanam, L. Schultz, M. Jeffries-EL, M. C. Iovu, G. Sauvé, J. Cooper, R. Zhang, J. C. Revelli, A. G. Kusne, J. L. Snyder, T. Kowalewski, L. E. Weiss, R. D. McCullough, G. K. Fedder and D. N. Lambeth, Inkjet printed chemical sensor array based on polythiophene conductive polymers, Sens. Actuators, B, 2007, 123, 651–660 CrossRef CAS.
- D. Khim, G.-S. Ryu, W.-T. Park, H. Kim, M. Lee and Y.-Y. Noh, Precisely Controlled Ultrathin Conjugated Polymer Films for Large Area Transparent Transistors and Highly Sensitive Chemical Sensors, Adv. Mater., 2016, 28, 2752–2759 CrossRef CAS PubMed.
- P. Minei and A. Pucci, Fluorescent vapochromism in synthetic polymers, Polym. Int., 2016, 65, 609–620 CrossRef CAS.
- S. W. Thomas, G. D. Joly and T. M. Swager, Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS PubMed.
- J.-S. Yang and T. M. Swager, Fluorescent Porous Polymer Films as TNT Chemosensors:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Electronic and Structural Effects, J. Am. Chem. Soc., 1998, 120, 11864–11873 CrossRef CAS.
Electronic and Structural Effects, J. Am. Chem. Soc., 1998, 120, 11864–11873 CrossRef CAS. - S. W. Thomas III, J. P. Amara, R. E. Bjork and T. M. Swager, Amplifying fluorescent polymer sensors for the explosives taggant 2,3-dimethyl-2,3-dinitrobutane (DMNB), Chem. Commun., 2005, 4572–4574 RSC.
- O. S. Wenger, Vapochromism in Organometallic and Coordination Complexes: Chemical Sensors for Volatile Organic Compounds, Chem. Rev., 2013, 113, 3686–3733 CrossRef CAS PubMed.
- X. Zhang, B. Li, Z.-H. Chen and Z.-N. Chen, Luminescence vapochromism in solid materials based on metal complexes for detection of volatile organic compounds (VOCs), J. Mater. Chem., 2012, 22, 11427–11441 RSC.
- E. Li, K. Jie, M. Liu, X. Sheng, W. Zhu and F. Huang, Vapochromic crystals: understanding vapochromism from the perspective of crystal engineering, Chem. Soc. Rev., 2020, 49, 1517–1544 RSC.
- A. Kobayashi, Y. Fukuzawa and H.-C. Vapor-Controlled, Linkage Isomerization of a Vapochromic Bis(thiocyanato)platinum(II) Complex: New External Stimuli To Control Isomerization Behavior. Chang and M. Kato, Inorg. Chem., 2012, 51, 7508–7519 CrossRef CAS PubMed.
- D. Saito, T. Galica, E. Nishibori, M. Yoshida, A. Kobayashi and M. Kato, Reversible and Stepwise Single-Crystal-to-Single-Crystal Transformation of a Platinum(II) Complex with Vapochromic Luminescence, Chem. – Eur. J., 2022, 28, e202200703 CAS.
- S. Yokoyama, H. Asahara and N. Nishiwaki, Vapochromic Properties of Diethenylpyrrole with Naphthyl Tethers Induced by Formation of a Distorted Structure in the Solid State, Cryst. Growth Des., 2020, 20, 1383–1387 CrossRef CAS.
- S. Ito, M. Yaegashi, K. Tanaka and Y. Chujo, Reversible Vapochromic Luminescence Accompanied by Planar Half-Chair Conformational Change of a Propeller-Shaped Boron β-Diketiminate Complex, Chem. – Eur. J., 2021, 27, 9194 CrossRef CAS.
- S. Ito, A. Hirose, M. Yamaguchi, K. Tanaka and Y. Chujo, Size-discrimination of volatile organic compounds utilizing gallium diiminate by luminescent chromism of crystallization-induced emission via encapsulation-triggered crystal–crystal transition, J. Mater. Chem. C, 2016, 4, 5564–5571 RSC.
- H. Sasaki, H. Imoto, T. Kitao, T. Uemura, T. Yumura and K. Naka, Fluorinated porous molecular crystals: vapor-triggered on–off switching of luminescence and porosity, Chem. Commun., 2019, 55, 6487–6490 RSC.
- G. Xia, S. Shen, X.-M. Hu, Z. Jiang, K. Xu, M. Wang and H. Wang, Controlling Crystal Structures and Multiple Thermo- and Vapochromic Behaviors of Benzimidazole-Based Squaraine Dyes by Molecular Design and Solvent Adjustment, Chem. – Eur. J., 2018, 24, 13205–13212 CrossRef CAS PubMed.
- A. Sakon, A. Sekine and H. Uekusa, Powder Structure Analysis of Vapochromic Quinolone Antibacterial Agent Crystals, Cryst. Growth Des., 2016, 16, 4635–4645 CrossRef CAS.
- H. Naito, Y. Morisaki and Y. Chujo, o-Carborane-Based Anthracene: A Variety of Emission Behaviors, Angew. Chem., Int. Ed., 2015, 54, 5084–5087 CrossRef CAS PubMed.
- T. Panda, D. K. Maiti and M. K. Panda, Inkless Writing and Self-Erasing Security Feature of (Z)-1,2-Diarylacrylonitrile-Based Materials: A Confidential Data Communication, ACS Appl. Mater. Interfaces, 2018, 10, 29100–29106 CrossRef CAS PubMed.
- S. Ito, M. Fukuyama, K. Tanaka and Y. Chujo, Effects of Regioregularity of π-Conjugated Polymers Composed of Boron β-Diketiminate on Their Stimuli-Responsive Luminescence, Macromol. Chem. Phys., 2022, 223, 2100504 CrossRef CAS.
- S. Ohtani, N. Yamada, M. Gon, K. Tanaka and Y. Chujo, The effect of alkyl chain lengths on the red-to-near-infrared emission of boron-fused azomethine conjugated polymers and their film-state stimuli-responsivities, Polym. Chem., 2021, 12, 2752–2759 RSC.
- I. P. Oliveri, G. Malandrino, S. Mirabella and S. Di Bella, Vapochromic and chemiresistive characteristics of a nanostructured molecular material composed of a zinc(ii)-salophen complex, Dalton Trans., 2018, 47, 15977–15982 RSC.
- Y. Soltani, S. J. Adams, J. Börger, L. C. Wilkins, P. D. Newman, S. J. A. Pope and R. L. Melen, Synthesis and photophysical properties of imine borane adducts towards vapochromic materials, Dalton Trans., 2018, 47, 12656–12660 RSC.
- J. S. Ovens and D. B. Leznoff, Raman Detected Sensing of Volatile Organic Compounds by Vapochromic Cu[AuX2(CN)2]2 (X = Cl, Br) Coordination Polymer Materials, Chem. Mater., 2015, 27, 1465–1478 CrossRef CAS.
- B. Orwat, M. J. Oh, M. Zaranek, M. Kubicki, R. Januszewski and I. Kownacki, Microwave-Accelerated C,N-Cyclometalation as a Route to Chloro-Bridged Iridium(III) Binuclear Precursors of Phosphorescent Materials: Optimization, Synthesis, and Studies of the Iridium(III) Dimer Behavior in Coordinating Solvents, Inorg. Chem., 2020, 59, 9163–9176 CrossRef CAS PubMed.
- H. Ohara, T. Ogawa, M. Yoshida, A. Kobayashi and M. Kato, Reversible luminescent colour changes of mononuclear copper(i) complexes based on ligand exchange reactions by N-heteroaromatic vapours, Dalton Trans., 2017, 46, 3755–3760 RSC.
- E. J. Fernández, J. M. López-de-Luzuriaga, M. Monge, M. Montiel, M. E. Olmos, J. Pérez, A. Laguna, F. Mendizabal, A. A. Mohamed and J. P. Fackler, A Detailed Study of the Vapochromic Behavior of {Tl[Au(C6Cl5)2]}n, Inorg. Chem., 2004, 43, 3573–3581 CrossRef PubMed.
- Y. Wu, X. Zhang, L.-J. Xu, M. Yang and Z.-N. Chen, Luminescent Vapochromism Due to a Change of the Ligand Field in a One-Dimensional Manganese(II) Coordination Polymer, Inorg. Chem., 2018, 57, 9175–9181 CrossRef CAS PubMed.
- G. Iasilli, F. Martini, P. Minei, G. Ruggeri and A. Pucci, Vapochromic features of new luminogens based on julolidine-containing styrene copolymers, Faraday Discuss., 2017, 196, 113–129 RSC.
- M. Ahmad, I. Platonova, A. Battisti, P. Minei, G. Brancato and A. Pucci, Highly selective vapochromic fluorescence of polycarbonate films Doped with an ICT-Based solvatochromic probe, J. Polym. Sci., Part B: Polym. Phys., 2017, 55, 1171–1180 CrossRef CAS.
- I. Platonova, A. Branchi, M. Lessi, G. Ruggeri, F. Bellina and A. Pucci, Zn(II)-bisthienylethynylbipyridine complex: Preparation, characterization and vapochromic behaviour in polymer films, Dyes Pigm., 2014, 110, 249–255 CrossRef CAS.
- P. Minei, M. Koenig, A. Battisti, M. Ahmad, V. Barone, T. Torres, D. M. Guldi, G. Brancato, G. Bottari and A. Pucci, Reversible vapochromic response of polymer films doped with a highly emissive molecular rotor, J. Mater. Chem. C, 2014, 2, 9224–9232 RSC.
- J. R. Kumpfer, S. D. Taylor, W. B. Connick and S. J. Rowan, Vapochromic and mechanochromic films from square-planar platinum complexes in polymethacrylates, J. Mater. Chem., 2012, 22, 14196–14204 RSC.
- J. W. Grate, L. K. Moore, D. E. Janzen, D. J. Veltkamp, S. Kaganove, S. M. Drew and K. R. Mann, Steplike Response Behavior of a New Vapochromic Platinum Complex Observed with Simultaneous Acoustic Wave Sensor and Optical Reflectance Measurements, Chem. Mater., 2002, 14, 1058–1066 CrossRef CAS.
- H. Helten, Doping the Backbone of π-Conjugated Polymers with Tricoordinate Boron: Synthetic Strategies and Emerging Applications, Chem. – Asian J., 2019, 14, 919–935 CrossRef CAS PubMed.
- M. Miyata and Y. Chujo, π-Conjugated Organoboron Polymer as an Anion Sensor, Polym. J., 2002, 34, 967–969 CrossRef CAS.
- H. Li and F. Jäkle, Universal Scaffold for Fluorescent Conjugated Organoborane Polymers, Angew. Chem., Int. Ed., 2009, 48, 2313–2316 CrossRef CAS PubMed.
- K. Hu, Z. Zhang, J. Burke and Y. Qin, Boron “Doped” Polyacetylenes, J. Am. Chem. Soc., 2017, 139, 11004–11007 CrossRef CAS PubMed.
- A. Lik, S. Jenthra, L. Fritze, L. Müller, K.-N. Truong and H. Helten, From Monodisperse Thienyl- and Furylborane Oligomers to Polymers: Modulating the Optical Properties through the Hetarene Ratio, Chem. – Eur. J., 2018, 24, 11961–11972 CrossRef CAS PubMed.
- Y. Adachi, F. Arai, M. Sakabe and J. Ohshita, Effect of the conjugation pathway on the electronic structures of p–π* conjugated polymers with fused borepin units, Polym. Chem., 2021, 12, 3471–3477 RSC.
- A. Sundararaman, M. Victor, R. Varughese and F. Jäkle, A Family of Main-Chain Polymeric Lewis Acids:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Synthesis and Fluorescent Sensing Properties of Boron-Modified Polythiophenes, J. Am. Chem. Soc., 2005, 127, 13748–13749 CrossRef CAS PubMed.
Synthesis and Fluorescent Sensing Properties of Boron-Modified Polythiophenes, J. Am. Chem. Soc., 2005, 127, 13748–13749 CrossRef CAS PubMed. - I. A. Adams and P. A. Rupar, A Poly(9-Borafluorene) Homopolymer: An Electron-Deficient Polyfluorene with “Turn-On” Fluorescence Sensing of NH3 Vapor, Macromol. Rapid Commun., 2015, 36, 1336–1340 CrossRef CAS PubMed.
- Y. Chujo and K. Tanaka, New Polymeric Materials Based on Element-Blocks, Bull. Chem. Soc. Jpn., 2015, 88, 633–643 CrossRef CAS.
- M. Gon, K. Tanaka and Y. Chujo, Recent progress in the development of advanced element-block materials, Polym. J., 2018, 50, 109–126 CrossRef CAS.
- K. Tanaka and Y. Chujo, Modulation of the solid-state luminescent properties of conjugated polymers by changing the connecting points of flexible boron element blocks, Polym. J., 2020, 52, 555–566 CrossRef CAS.
- M. Gon, K. Tanaka and Y. Chujo, Discovery of Functional Luminescence Properties Based on Flexible and Bendable Boron-Fused Azomethine/Azobenzene Complexes with O,N,O-Type Tridentate Ligands, Chem. Rec., 2021, 21, 1358–1373 CAS.
- S. Ohtani, M. Nakamura, M. Gon, K. Tanaka and Y. Chujo, Synthesis of fully-fused bisboron azomethine complexes and their conjugated polymers with solid-state near-infrared emission, Chem. Commun., 2020, 56, 6575–6578 RSC.
- S. Ohtani, M. Gon, K. Tanaka and Y. Chujo, Construction of the Luminescent Donor–Acceptor Conjugated Systems Based on Boron-Fused Azomethine Acceptor, Macromolecules, 2019, 52, 3387–3393 CrossRef CAS.
- M. Gon, J. Wakabayashi, M. Nakamura, K. Tanaka and Y. Chujo, Controlling Energy Gaps of π-Conjugated Polymers by Multi-Fluorinated Boron-Fused Azobenzene Acceptors for Highly Efficient Near-Infrared Emission, Chem. – Asian J., 2021, 16, 696–703 CrossRef CAS PubMed.
- M. Gon, J. Wakabayashi, M. Nakamura, K. Tanaka and Y. Chujo, Preparation of Near-Infrared Emissive π-Conjugated Polymer Films Based on Boron-Fused Azobenzene Complexes with Perpendicularly Protruded Aryl Substituents, Macromol. Rapid Commun., 2021, 42, 2000566 CrossRef CAS PubMed.
- J. Wakabayashi, M. Gon, K. Tanaka and Y. Chujo, Near-Infrared Absorptive and Emissive Poly(p-phenylene vinylene) Derivative Containing Azobenzene–Boron Complexes, Macromolecules, 2020, 53, 4524–4532 CrossRef CAS.
- M. Gon, J. Wakabayashi, K. Tanaka and Y. Chujo, Unique Substitution Effect at 5,5′-Positions of Fused Azobenzene–Boron Complexes with a N = N π-Conjugated System, Chem. – Asian J., 2019, 14, 1837–1843 CrossRef CAS PubMed.
- M. Gon, K. Tanaka and Y. Chujo, A Highly Efficient Near-Infrared-Emissive Copolymer with a N = N Double-Bond π-Conjugated System Based on a Fused Azobenzene–Boron Complex, Angew. Chem., Int. Ed., 2018, 57, 6546–6551 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC.
- S. Ohtani, Y. Takeda, M. Gon, K. Tanaka and Y. Chujo, Facile strategy for obtaining luminescent polymorphs based on the chirality of a boron-fused azomethine complex, Chem. Commun., 2020, 56, 15305–15308 RSC.
- S. Ohtani, M. Gon, K. Tanaka and Y. Chujo, The Design Strategy for an Aggregation- and Crystallization-Induced Emission-Active Molecule Based on the Introduction of Skeletal Distortion by Boron Complexation with a Tridentate Ligand, Crystals, 2020, 10, 615 CrossRef CAS.
- S. Ohtani, M. Gon, K. Tanaka and Y. Chujo, A Flexible, Fused, Azomethine–Boron Complex: Thermochromic Luminescence and Thermosalient Behavior in Structural Transitions between Crystalline Polymorphs, Chem. – Eur. J., 2017, 23, 11827–11833 CrossRef CAS PubMed.
- M. Nakamura, M. Gon, S. Natsuda, Y. Tamai, H. Ohkita, K. Tanaka and Y. Chujo, Development of NIR emissive fully-fused bisboron complexes with π-conjugated systems including multiple azo groups, Dalton Trans., 2022, 51, 74–84 RSC.
- Y. Dong, J. W. Y. Lam, A. Qin, Z. Li, J. Sun, H. H.-Y. Sung, I. D. Williams and B. Z. Tang, Switching the light emission of (4-biphenylyl)phenyldibenzofulvene by morphological modulation: crystallization-induced emission enhancement, Chem. Commun., 2007, 40–42 RSC.
- J. I. Musher, The Chemistry of Hypervalent Molecules, Angew. Chem., Int. Ed. Engl., 1969, 8, 54–68 CrossRef CAS.
- R. Gillespie and B. Silvi, The octet rule and hypervalence: two misunderstood concepts, Coord. Chem. Rev., 2002, 233–234, 53–62 CrossRef CAS.
- K.-y Akiba, Studies on hypervalent compounds and synthetic work using heteroaromatic cations, Heteroatom Chem., 2011, 22, 207–274 CrossRef CAS.
- M. Gon, K. Tanaka and Y. Chujo, Vapochromic Luminescent π-Conjugated Systems with Reversible Coordination-Number Control of Hypervalent Tin(IV)-Fused Azobenzene Complexes, Chem. – Eur. J., 2021, 27, 7561–7571 CrossRef CAS PubMed.
- M. Gon, K. Tanimura, M. Yaegashi, K. Tanaka and Y. Chujo, PPV-type π-conjugated polymers based on hypervalent tin(IV)-fused azobenzene complexes showing near-infrared absorption and emission, Polym. J., 2021, 53, 1241–1249 CrossRef CAS.
- K. E. Bessler, J. A. dos Santos, V. M. Deflon, S. de Souza Lemos and E. Niquet, Organotin Dyes: Synthesis and Structural Characterization of Dibutyltin and Dimethyltin Complexes with 2,2′-Dihydroxyazobenzene, Z. Anorg. Allg. Chem., 2004, 630, 742–745 CrossRef CAS.
- C. Chuit, R. J. P. Corriu, C. Reye and J. C. Young, Reactivity of penta- and hexacoordinate silicon compounds and their role as reaction intermediates, Chem. Rev., 1993, 93, 1371–1448 CrossRef CAS.
- S. Yamaguchi, S. Akiyama and K. Tamao, Photophysical Properties Changes Caused by Hypercoordination of Organosilicon Compounds:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) From Trianthrylfluorosilane to Trianthryldifluorosilicate, J. Am. Chem. Soc., 2000, 122, 6793–6794 CrossRef CAS.
From Trianthrylfluorosilane to Trianthryldifluorosilicate, J. Am. Chem. Soc., 2000, 122, 6793–6794 CrossRef CAS. - N. Singh, K. Kumar, N. Srivastav, R. Singh, V. Kaur, J. P. Jasinski and R. J. Butcher, Exploration of fluorescent organotin compounds of α-amino acid Schiff bases for the detection of organophosphorous chemical warfare agents: quantification of diethylchlorophosphate, New J. Chem., 2018, 42, 8756–8764 RSC.
- M. H. Chisholm, E. E. Delbridge and J. C. Gallucci, Modeling the catalyst resting state in aryl tin(iv) polymerizations of lactide and estimating the relative rates of transamidation, transesterification and chain transfer, New J. Chem., 2004, 28, 145–152 RSC.
- M. Kosugi, K. Sasazawa, Y. Shimizu and T. Migita, REACTIONS OF ALLYLTIN COMPOUNDS III. ALLYLATION OF AROMATIC HALIDES WITH ALLYLTRIBUTYLTIN IN THE PRESENCE OF TETRAKIS(TRIPHENYLPHOSPHINE)PALLADIUM(O), Chem. Lett., 1977, 301–302 CrossRef CAS.
- D. Milstein and J. K. Stille, A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium, J. Am. Chem. Soc., 1978, 100, 3636–3638 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01295b |
| This journal is © the Partner Organisations 2023 |