Ligand engineering of luminescent AuAg nanoclusters for targeted mitochondrial and brain imaging†
Xinxin
Pan‡
a,
Zhongxiang
Zuo‡
a,
Ziping
Wang
b,
Ge
Yang
a,
Haiguang
Zhu
 a,
Yang
Li
a,
Yang
Li
 c and
Xun
Yuan
c and
Xun
Yuan
 *a
*a
aSchool of Materials Science and Engineering, Qingdao University of Science and Technology (QUST), 53 Zhengzhou Rd., Shibei District, Qingdao 266042, P. R. China. E-mail: yuanxun@qust.edu.cn
bWeifang University of Science and Technology, Shandong Peninsula Engineering Research Center of Comprehensive Brine Utilization, Weifang 262700, P. R. China
cJiangsu Key Laboratory of Micro and Nano Heat Fluid Flow Technology and Energy Application, School of Physical Science and Technology, Suzhou University of Science and Technology, Suzhou 215009, P. R. China
First published on 30th January 2023
Abstract
Developing luminescent probes for targeted subcellular mitochondrial and in vivo brain imaging is meaningful but very challenging. Herein we report the design of a novel luminescent AuAg nanocluster (NC)-based probe with aggregation-induced emission (AIE) for targeted mitochondrial and brain imaging based on ligand engineering via conjugating (4-carboxybutyl)triphenylphophonium bromide (TPP) on the surface. Upon conjugating TPP with a mitochondria-targeting function, the as-designed AuAg NCs@TPP probe exhibits several merits such as ultrasmall size (<3 nm), strong luminescence with 610 nm emission, long luminescence lifetime (7.193 μs), amphiphilic surface chemistry, and excellent stability, which make it suitable for both targeted mitochondrial imaging and in vivo brain imaging of living mice. In addition, the AuAg NCs@TPP probe has also been demonstrated to have low cytotoxicity and good in vivo biocompatibility, which are helpful for biomedical applications. This study provides a “killing two birds with one stone” strategy for fabricating metal NC-based probes for both mitochondrial and brain imaging, which may stimulate more research activities in the design and biomedical applications of ultrasmall metal NCs.
Introduction
Highly luminescent coinage metal nanoclusters (MNCs) have been recognized as a new class of luminescent probes for bioimaging1–4 and biosensing,5–9 which are greatly benefited from their ultrasmall size (≤3 nm),10–13 rich yet engineerable surface chemistry,14–16 tunable photoluminescence (PL),17–23 good photostability,24,25 and low biotoxicity.26–28 For example, the ultrasmall hydrodynamic size of MNCs that is below the kidney filtration threshold (<6 nm) grants them excellent renal clearance,29,30 which is not possible with large sized probes (e.g., Au nanoparticles). In the past few years, researchers have put tremendous effort on the design of various MNC-based luminescent probes,31–34 such as PL-tunable ones from the visible to near infrared region in the first and second windows (NIR-I and NIR-II),3,31 those engineered with targeted functions for specific cells/tissues/organs,35 and those holding theranostic attributes.36 Such a plenty of MNC-based luminescent probes substantially promoted the realization of their broad applications ranging from cell imaging,37 disease theranostics,38 to in vivo metabolism assessment,39 which indeed addressed many challenging issues in the biomedical field. However, there are still several pending problems in bioimaging: (1) while many MNCs have been demonstrated to be capable in luminescence imaging of cells/tissues/organs, the advancement in subcellular organelle imaging is very slow, which is due to the lack of appropriate MNCs with targeted functions for specific subcellular organelles (e.g., mitochondria and lysosomes); (2) luminescence imaging of the brain is very challenging because it is difficult for luminescent probes to approach the brain due to the protection of the blood brain barrier (BBB).40 Considering the critical importance of both subcellular organelles for cellular functions and the brain in the control of cognitive and motor functions of whole intelligent organisms, there is therefore a pressing demand in the design of highly luminescent MNCs as adequate luminescent probes for targeted subcellular organelle and brain imaging.By deciphering the structure of cells, it has been determined that mitochondria play a key role in cellular energy metabolism, and their dysfunction could severely affect one's health with the occurrence of many diseases.41 Hence, achieving mitochondrial imaging is beneficial to the fundamental understanding of the mitochondrial behaviour as well as the real-time assessment of body functions and disease signals.42 To realize this, delicate surface engineering of MNCs by conjugating specific molecules with targeted functions may be a feasible way.35 Specifically, molecules with a triphenylphosphine entity could be selected for the surface engineering of MNCs since their delocalized lipophilic properties allow them to move easily across the phospholipid bilayers of cells and then achieve mitochondrial targeting. In addition to mitochondrial imaging, the engineering of MNCs with triphenylphosphine-containing molecules may endow MNCs with amphiphilic surfaces but without remarkable alternation in hydrodynamic size, which is considered as an efficient means to penetrate the BBB, concurrently making the brain imaging possible. However, there are several concerns arising in this aspect. For example, it is unknown whether the surface engineering approach will quench the PL of MNCs or not. How the biotoxicity of MNCs changes before and after surface engineering? Can the engineered MNCs really achieve both subcellular mitochondrial and brain imaging? How about the in vivo stability of the engineered MNCs?
Herein we report the design of a AuAg NC-based probe with aggregation-induced emission (AIE) for targeted mitochondrial and in vivo brain imaging based on chemical conjugation of highly luminescent glutathione (GSH)-protected AuAg NCs with (4-carboxybutyl)triphenylphophonium bromide (TPP) via a simple 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS) cross-linking strategy (Scheme 1). Upon conjugating TPP with mitochondria-targeting function, the as-developed AuAg NCs@TPP probe exhibits an enhanced PL of 1.9 times with a longer PL lifetime (from 6.13 μs to 7.19 μs) due to the rigid structure and electron-rich attributes of TPP. More importantly, the combination of hydrophobic TPP and hydrophilic GSH on the NC surface renders the AuAg NCs amphiphilic, which allows AuAg NCs to easily pass through phospholipid bilayers and accumulate in highly negatively charged mitochondria of living cells, achieving targeted imaging of subcellular mitochondria. In addition, leveraging the ultrasmall size and amphiphilic properties, the AuAg NCs@TPP probe can also realize the brain imaging of living mice. Of note, both in vitro and in vivo experiments reveal that the newly developed AuAg NC-based probe has excellent biocompatibility, ensuring its safe bioapplication. This study is of broad interest because it not only provides a “killing two birds with one stone” strategy to realize both mitochondrial and brain imaging, but also sheds light on the design of NC-based luminescent probes with customized functions for various biomedical applications.
 | ||
| Scheme 1 Schematic illustration of the synthesis of highly luminescent AuAg NCs@TPP through chemically conjugating TPP on the surface of AuAg NCs via the EDC/NHS strategy. | ||
Experimental
Synthesis of AIE-featured GSH-protected AuAg NCs
The AIE-featured GSH-protected AuAg NCs were prepared according to a previously reported method.43 Briefly, aqueous solutions of GSH (50 mM, 3 mL), HAuCl4 (20 mM, 4.5 mL) and AgNO3 (20 mM, 0.5 mL) were added into a flask containing 42 mL of ultrapure water with stirring, and then heated to 85 °C with gentle stirring (600 rpm) for 3 hours. After that, the sample solution of AIE-featured GSH-protected AuAg NCs was collected and purified by using an ultrafiltration cell (Model 8010, Millipore Corporation, USA) with a semipermeable membrane of 3000 Da molecular weight cutoff (MWCO). The obtained pellet of AuAg NCs was redissolved in 50 mL of ultrapure water for further use.Synthesis of AIE-featured AuAg NCs@TPP.
In a typical synthesis, an aqueous solution of NaOH (1 M) was added to the purified solution of GSH-protected AuAg NCs to adjust the pH = 8. In the meanwhile, 0.335 g of EDC and 0.1 g of NHS were separately introduced into the TPP solution (50 mM, 3.5 mL), and stirred for 30 min. After that, 21 mL of solution of the GSH-protected AuAg NCs (pH = 8) was mixed with the TPP solution with stirring. Finally, the AIE-featured AuAg NCs@TPP could be obtained after 1 hour of reaction.Cytotoxicity assay
Mouse microglia cells (BV2) and mouse breast cancer cells (4T1) were separately seeded into 96-well U-bottom plates at a density of 1 × 104 cells per well, and then incubated with the AuAg NCs or AuAg NCs@TPP at different concentrations (0, 0.05, 0.1, 0.2, 0.4, and 0.8 mM based on metal ions). Each concentration of the NCs was tested in 8 wells. After 24 hours, the culture media were discarded, the cells were washed with PBS (pH = 7.4), and 0.1 mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) solution (0.5 mg mL−1 in DMEM) was then added into each well. After incubating for 4 hours, the medium was removed, followed by the addition of 150 μL of DMSO into each well. After shaking for additional 30 min, the absorbance of the suspension in each well was measured at 450 nm using a SpectraMax absorbance reader. The cell viability rate was estimated using the following equation: cell viability (%) = (Asample − Ablank)/(Acontrol − Ablank) × 100%, where Asample, Acontrol, and Ablank are the mean absorbance of the treated group, untreated group, and blank group, respectively.In vitro mitochondria-targeted imaging
For mitochondrial imaging of cancer cells, BV2 cells and 4T1 cells were separately seeded on 35 mm glass-bottom dishes with a density of 3 × 104 cells per well and cultured for 24 h. After that, 0.4 mM AuAg NCs or AuAg NCs@TPP were introduced into each well and incubated for 3 hours at 37 °C, followed by washing with PBS (pH = 7.4). Subsequently, 500 μL of Mito Tracker solution (20 nM) was added and incubated with the cells for 30 min. After washing with PBS, the solution was kept in PBS for imaging. Luminescence imaging of cells was performed on a FV 1000IX81 confocal laser scanning microscope, using different excitation wavelengths for each dye: the excitation at 405 nm was applied for AuAg NCs and AuAg NCs@TPP, while that at 490 nm was used for Mito Tracker.In vivo brain imaging
All animal experiments were approved by the Department of Science and Technology of Shandong Province and the Laboratory Animal Center of Qingdao Zhong Hao Biological Engineering Co., Ltd. The mice for in vivo brain imaging were obtained from theLaboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China). The AuAg NCs or AuAg NCs@TPP (70 μL, 10 mM) dissolved in ultrapure water were injected intravenously into mice. After injection, luminescence imaging was carried out on a Maestro EX in vivo fluorescence imaging system (CRi, Inc.; excitation: 440 nm, spectral imaging from 600 nm to 700 nm).Results and discussion
In this study, highly luminescent GSH-protected AuAg NCs with AIE were engineered with TPP as a luminescent probe for bioimaging, and the pristine GSH-protected AuAg NCs without surface engineering were synthesized by a simple one-pot strategy.43 As shown in Fig. 1a, the as-obtained AuAg NCs displayed a yellowish colour (item 1 in the inset) with a shoulder absorption band at ∼400 nm extending up to 500 nm in the UV-visible absorption spectrum. Interestingly, the sample of AuAg NCs shows intense orange PL with an emission peak at 610 nm under UV light irradiation (red curve, and item 2 in the inset). Both the optical absorption and the PL properties of the sample suggest the successful synthesis of ultrasmall AuAg NCs, excluding the possibility of forming large-sized non-luminescent Au nanoparticles (>3 nm) with a typical surface plasmon resonance (SPR) absorption at ∼520 nm. In addition, the TEM image reveals that all the particles of the sample are below 3 nm in core size (Fig S1, ESI†), further supporting the formation of ultrasmall AuAg NCs.Upon acquiring the AIE-featured AuAg NCs, TPP with carboxyl groups was applied for engineering the surface of NCs by reacting with the amino group of surface GSH via a simple EDC/NHS cross-linking strategy. After TPP conjugation, the as-obtained AuAgNCs@TPP inherited the same absorption band as the pristine AuAg NCs at 300–500 nm (Fig. 1a), but displayed a special absorption peak at 267 nm, which was derived from TPP (Fig. S2, ESI†), indicating the successful conjugation of TPP on the surface of AuAg NCs. This result was further supported by the FTIR result. As shown in Fig. S3 (ESI†), in the FTIR spectrum of AuAg NCs@TPP, both the amide bond peaks of the GSH-protected AuAg NCs at 1650 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1532 cm−1 (N–H bending), and 3410 cm−1 (O–H bending), and the characteristic peak of the tensile vibration of C–P in TPP at 1437 cm−1 are all observed, manifesting the co-existence of GSH and TPP molecules in AuAg NCs@TPP. Moreover, the zeta potential of the GSH-protected AuAg NCs changes from −41 ± 0.9 mV to −37.8 ± 0.1 mV after TPP conjugation (Fig. S4, ESI†), which implies that the deprotonation of conjugated TPP reduces the negative charges of the AuAg NCs.
O stretching), 1532 cm−1 (N–H bending), and 3410 cm−1 (O–H bending), and the characteristic peak of the tensile vibration of C–P in TPP at 1437 cm−1 are all observed, manifesting the co-existence of GSH and TPP molecules in AuAg NCs@TPP. Moreover, the zeta potential of the GSH-protected AuAg NCs changes from −41 ± 0.9 mV to −37.8 ± 0.1 mV after TPP conjugation (Fig. S4, ESI†), which implies that the deprotonation of conjugated TPP reduces the negative charges of the AuAg NCs.
More interestingly, the surface engineering of AuAg NCs with TPP does not alter their PL emission wavelength but enhances the PL intensity up to 1.9 times (red curves in Fig. 1a, and items 3 and 4 in the inset), which is benefited from the rigid molecular structure of TPP that constrains the non-radiation energy loss. Moreover, the conjugation of TPP on the surface of AuAg NCs also prolongs its PL lifetime from 6.13 μs to 7.19 μs, which may be because the electron-rich TPP moiety facilitates the relaxation of ligand-to-metal charge transfer (LMCT) or ligand-to-metal–metal charge transfer (LMMCT). This charge transfer can be supported by the XPS result. As shown in Fig. S5 (ESI†), while the binding energies of both Au 4f and Ag 3d components in the AuAg NCs have neglectable change after TPP conjugation, that of S 2p in the AuAg NCs@TPP sample shift up to 0.25 eV toward the lower binding energy side in comparison to that of AuAg NCs, suggesting the increased electron density of the S element in AuAu NCs@TPP. In addition, the TPP conjugation also does not alter the core size of AuAg NCs, which is evidenced from the TEM image after surface engineering (Fig. 1c). Of note, the as-developed AuAg NCs@TPP probe also shows excellent stability in PBS or solutions with different pH values or salt concentrations (Fig. S6, ESI†), ensuring their broad applicability in different bioenvironments. Taken together, the AuAg NCs@TPP probes have been demonstrated to have ultrasmall size, strong PL, long PL lifetime, proper surface chemistry, and excellent stability, which enables them to be a decent luminescent probe for in vitro and in vivo bioimaging.
The proportion of TPP on the surface of AuAg NCs plays a certain role in affecting the PL, and the feeding molar ratio of AuAg ions to TPP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 is found to be optimal for the formation of NCs with highest PL intensity (Fig. S7, ESI†). It is required to determine how many TPP molecules are present on the surface of each AuAg NC. To answer this question, we used TGA to analyse the molar ratio of AuAg ions-to-GSH and determined it to be 1
4 is found to be optimal for the formation of NCs with highest PL intensity (Fig. S7, ESI†). It is required to determine how many TPP molecules are present on the surface of each AuAg NC. To answer this question, we used TGA to analyse the molar ratio of AuAg ions-to-GSH and determined it to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.73 based on the weight ratio of GSH to AuAg species in the GSH-protected AuAg NCs (∼50.8%, see Fig. S8, ESI†). In addition, by correlating the concentration with the absorbance of TPP based on the Beer–Lambert Law (Fig. S9a, ESI†), we can determine that the molar ratio of AuAg ions-to-TPP is 1
0.73 based on the weight ratio of GSH to AuAg species in the GSH-protected AuAg NCs (∼50.8%, see Fig. S8, ESI†). In addition, by correlating the concentration with the absorbance of TPP based on the Beer–Lambert Law (Fig. S9a, ESI†), we can determine that the molar ratio of AuAg ions-to-TPP is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.77 in the AuAg NCs@TPP probe based on the absorbance difference between pristine AuAg NCs and AuAg NCs@TPP at 267 nm (Fig. S9b, ESI†). The nice match between the molar ratio of AuAg ions-to-GSH (1
0.77 in the AuAg NCs@TPP probe based on the absorbance difference between pristine AuAg NCs and AuAg NCs@TPP at 267 nm (Fig. S9b, ESI†). The nice match between the molar ratio of AuAg ions-to-GSH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7) and that of AuAg ions-to-TPP (1
0.7) and that of AuAg ions-to-TPP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.77) clearly suggests that every GSH in AuAg NCs@TPP is generally conjugated with one TPP. While the average number of metal atoms in the AuAg NCs may be around 35 according to previous findings,44 the number of TPP on the NC's surface could be determined to be about 24.
0.77) clearly suggests that every GSH in AuAg NCs@TPP is generally conjugated with one TPP. While the average number of metal atoms in the AuAg NCs may be around 35 according to previous findings,44 the number of TPP on the NC's surface could be determined to be about 24.
With the AuAg NCs@TPP-based luminescent probe in our hand, we first conducted its cytotoxicity assessment by using MTT assay in 4T1 and BV2 cell lines because the low cytotoxicity of a probe is the prerequisite for bioimaging application. As shown in Fig. 2a and Fig. S10a (ESI†), the AuAg NCs@TPP probe (0.4 mM) displayed very low cytotoxicity with viabilities of 93% for BV2 cells and 90% for 4T1 cells after 24 hours of incubation, which is comparable to that of AuAg NCs (0.4 mM) resulting in viabilities of 99% for BV2 cells and 120% for 4T1 cells. This result indicates that both pristine AuAg NCs and AuAg NCs@TPP have excellent biocompatibility, which should be attributed to the antioxidative trait of GSH as well as the strong binding of GSH to Au/Ag species that prevents the dissociation of Au/Ag species from AuAg NCs.
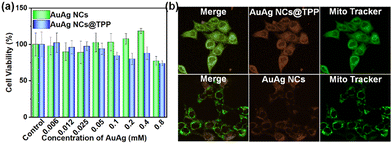 | ||
| Fig. 2 (a) In vitro cytotoxicity of AuAg NCs@TPP and pristine AuAg NCs for 4T1 cells; (b) confocal PL images of 4T1 cells co-stained with Mito-tracker and AuAg NCS@TPP or AuAg NCs (0.4 mM). | ||
Benefiting from the targeted function of TPP for subcellular mitochondria, the as-developed luminescent AuAg NCs@TPP probe performed well in targeted imaging of mitochondria. As illustrated in Fig. 2b and Fig S9b (ESI†) (upper panel), the 4T1 and BV2 cells treated with AuAg NCs@TPP and Mito-tracker dye were investigated by using a confocal PL microscope, and the result reveals that the orange PL of the AuAg NCs@TPP probe could overlap nicely with the green PL of Mito-tracker dye in the mitochondria of 4T1 and BV2 cells with Pearson's colocalization coefficients of 0.88 and 0.85, respectively. This result clearly suggests that the AuAg NCs@TPP probe holds outstanding mitochondria-targeting ability. By contrast, the colocalization experiments of pristine AuAg NCs and Mito Tracker dye in the same cell lines disclose that pristine AuAg NCs cannot accumulate into subcellular mitochondria of living cells (Fig. 2b and Fig. S10b, lower panel, ESI†), further proving the key role of surface TPP of NCs in targeting the mitochondria.
A big bonus of TPP conjugation on the NC surface is the realization of in vivo brain imaging. As shown in Fig. 3a, in the initial 6 hours from intravenous injection, the AuAg NCs@TPP probe-directed luminescence imaging of mice was unsuccessful due to the influence of self-fluorescence of mice. With a further increase in time, the NCs began to accumulate in some organs (e.g., lung and liver), making luminescence imaging possible. Particularly, further prolonging the time up to 4 days, brain imaging was achieved by using the AuAg NCs@TPP probe with a gradually increased PL intensity. This achievement of the AuAg NCs@TPP probe may be ascribed to its ultrasmall hydrodynamic size as well as amphiphilic surface chemistry, which probably facilitates its BBB penetration. However, the exploration of the detailed BBB penetration mechanism of this probe requires future comprehensive studies. For a clearer comparison, we have put the luminescence images of mice treated with AuAg NCs@TPP and pristine AuAg NCs after 4 days together in Fig. 3b. As shown, AuAg NCs@TPP achieves luminescence brain imaging of a living mouse. Unexpectedly, it seems that the pristine AuAg NCs can also do the same thing as AuAg NCs@TPP. To thoroughly clarify this abnormal phenomenon, we sacrificed the treated mice on the 4th day post-injection, and investigated the residue biodistribution of AuAg NCs and AuAg NCs@TPP in the major organs by luminescence imaging. As shown in Fig. 3c, while strong PL could be seen emitting from the liver, lungs, and brain of the mouse treated with AuAg NCs@TPP, almost no PL can be observed from the major organs in the group treated with pristine AuAg NCs, which clearly suggests the fake brain imaging by using pristine AuAg NCs as shown in Fig. 3b. The reason for the fake imaging of the brain may be that the GSH ligands of AuAg NCs enable their interaction with the bones of the mouse and subsequent deposition on the bone surface, which could be supported by a previous study that used GSH-protected Au25 NCs for targeted bone imaging.45 Finally, the mice were euthanized and dissected after injection of AuAg NCs@TPP for 10 days, and no obvious luminescence could be detected from the major organs (Fig. S11, ESI†). This result indicates that AuAg NCs@TPP and AuAg NCs may completely be excreted through the kidney within 10 days due to their ultrasmall size. Nevertheless, these preliminary results not only certify the excellent in vivo stability of the AuAg NCs@TPP probe, but also corroborate the successful surface engineering of metal NCs for both subcellular mitochondrial and brain imaging.
We also checked the in vivo biosafety of the AuAg NCs@TPP and AuAg NCs with normal mice as a reference. As shown in Fig. 4, the major organs including the heart, liver, spleen, lungs, kidneys, and brain of mice treated with the AuAg NCs@TPP probe and pristine AuAg NCs for 4 days were collected for H&E staining, and no obvious histopathological lesions were observed in any of the tissues from all the mice. Such results demonstrated that the AuAg NCs@TPP probe exhibits excellent in vivo biocompatibility and biosafety.
 | ||
| Fig. 4 H&E staining images of major organs, including the heart, liver, spleen, lungs, and kidneys, collected from the different groups of mice. | ||
Conclusions
In summary, we have developed a novel luminescent metal NC-based probe for targeted subcellular mitochondrial and brain imaging based on the ligand engineering of AIE-featured AuAg NCs with TPP. Upon conjugating TPP with the mitochondria-targeting function on the NC surface, the as-designed AuAg NCs@TPP probe exhibited several merits such as ultrasmall size, strong PL with 610 nm emission, long PL lifetime (7.193 μs), amphiphilic surface chemistry, and excellent stability, which make it suitable for targeted subcellular mitochondrial imaging and in vivo brain imaging of living mice. In addition, this probe has also been demonstrated to have low cytotoxicity and in vivo biocompatibility, which are helpful for biomedical applications. The novelty of this study is not only reflected in the design of a “killing two birds with one stone” strategy to realize both mitochondrial and brain imaging, but also embodied in the demonstration of ultrasmall metal NCs with customized functions for various biomedical applications.Author contributions
X. Yuan, X. Pan and Z. Zuo designed the experiments. X. Pan, Z. Zuo, and G. Yang conducted experiments. Discussion of results and manuscript preparation are the group efforts of X. Pan, Z. Zuo, Z. Wang, G. Yang, H. Zhu, Y. Li and X. Yuan. This project was supervised by X. Yuan.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Prof. Yiran Zheng from Soochow University and Prof. Xianfeng Zhou from QUST for valuable discussion and suggestions. This work is supported by the National Natural Science Foundation of China (22071127), the Natural Science Foundation of Shandong Province (ZR2019YQ07), and the Taishan Scholar Foundation (tsqn201812074, China).Notes and references
- Z. Li, H. Peng, J. Liu, Y. Tian, W. Yang, J. Yao, Z. Shao and X. Chen, Plant Protein-Directed Synthesis of Luminescent Gold Nanocluster Hybrids for Tumor Imaging, ACS Appl. Mater. Interfaces, 2018, 10, 83–90 CrossRef CAS.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X. D. Zhang, Atomic-Precision Gold Clusters for NIR-II Imaging, Adv. Mater., 2019, 31, e1901015 CrossRef.
- Y. Yang, Y. Yu, H. Chen, X. Meng, W. Ma, M. Yu, Z. Li, C. Li, H. Liu, X. Zhang, H. Xiao and Z. Yu, Illuminating Platinum Transportation while Maximizing Therapeutic Efficacy by Gold Nanoclusters via Simultaneous Near-Infrared-I/II Imaging and Glutathione Scavenging, ACS Nano, 2020, 14, 13536–13547 CrossRef CAS PubMed.
- X. Song, W. Zhu, X. Ge, R. Li, S. Li, X. Chen, J. Song, J. Xie, X. Chen and H. Yang, A New Class of NIR-II Gold Nanoclusters Based Protein Biolabels for In Vivo Tumor-Targeted Imaging, Angew. Chem., Int. Ed., 2021, 60, 1306–1312 CrossRef CAS.
- S. Qian, Z. Wang, Z. Zuo, X. Wang, Q. Wang and X. Yuan, Engineering Luminescent Metal Nanoclusters for Sensing Applications, Coord. Chem. Rev., 2022, 451, 214268 CrossRef CAS.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Luminescent Metal Nanoclusters: Biosensing Strategies and Bioimaging Applications, Aggregate, 2021, 2, 114–132 CrossRef.
- J. Ma, Z. Lu, C. Li, Y. Luo, Y. E. Shi, P. Alam, J. W. Y. Lam, Z. Wang and B. Z. Tang, Fluorescence Ratiometric Assay for Discriminating GSH and Cys Based on the Composites of UiO-66-NH2 and Cu Nanoclusters, Biosens. Bioelectron., 2022, 215, 114582 CrossRef CAS PubMed.
- B. Wang, M. Zhao, M. Mehdi, G. Wang, P. Gao and K.-Q. Zhang, Biomolecule-assisted Synthesis and Functionality of Metal Nanoclusters for Biological Sensing: A Review, Mater. Chem. Front., 2019, 3, 1722–1735 RSC.
- L. Qin, K. Zhang, B. Feng, P. Zhang, T. Qing and J. Fei, Proximity Sequence-dependent Spectral Conversion of Silver Nanoclusters and Construction of Ratiometric Nanoprobe, Chem. Eng. J., 2022, 441, 136001 CrossRef CAS.
- L. Qin, F. Sun, X. Ma, G. Ma, Y. Tang, L. Wang, Q. Tang, R. Jin and Z. Tang, Homoleptic Alkynyl-Protected Ag15 Nanocluster with Atomic Precision: Structural Analysis and Electrocatalytic Performance toward CO2 Reduction, Angew. Chem., Int. Ed., 2021, 60, 26136–26141 CrossRef CAS PubMed.
- S. Hossain, D. Hirayama, A. Ikeda, M. Ishimi, S. Funaki, A. Samanta, T. Kawawaki and Y. Negishi, Atomically Precise Thiolate-protected Gold Nanoclusters: Current Status of Designability of the Structure and Physicochemical Properties, Aggregate, 2022 DOI:10.1002/agt2.255.
- Q. Tang, G. Hu, V. Fung and D. E. Jiang, Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles, Acc. Chem. Res., 2018, 51, 2793–2802 CrossRef CAS.
- G. Yang, Y. Xie, Y. Wang, Y. Tang, L. L. Chng, F. Jiang, F. Du, X. Zhou, J. Y. Ying and X. Yuan, Water-soluble Cu30 Nanoclusters as A Click Chemistry Catalyst for Living Cell Labeling via Azide-alkyne Cycloaddition, Nano Res., 2023, 16, 1748–1754 CrossRef CAS.
- J. Yan, B. K. Teo and N. Zheng, Surface Chemistry of Atomically Precise Coinage-Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- C. Yao, C. Q. Xu, I. H. Park, M. Zhao, Z. Zhu, J. Li, X. Hai, H. Fang, Y. Zhang, G. Macam, J. Teng, L. Li, Q. H. Xu, F. C. Chuang, J. Lu, C. Su, J. Li and J. Lu, Giant Emission Enhancement of Solid-State Gold Nanoclusters by Surface Engineering, Angew. Chem., Int. Ed., 2020, 59, 8270–8276 CrossRef CAS.
- X. Yuan, B. Zhang, Z. Luo, Q. Yao, D. T. Leong, N. Yan and J. Xie, Balancing the Rate of Cluster Growth and Etching for Gram-Scale Synthesis of Thiolate-Protected Au25 Nanoclusters with Atomic Precision, Angew. Chem., Int. Ed., 2014, 53, 4623–4627 CrossRef CAS PubMed.
- M. Zhou, T. Higaki, G. Hu, M. Y. Sfeir, Y. Chen, D.-E. Jiang and R. Jin, Three-orders-of-magnitude Variation of Carrier Lifetimes with Crystal Phase of Gold Nanoclusters, Science, 2019, 364, 279–282 CrossRef CAS PubMed.
- N. N. M. Adnan, S. Ahmad, R. P. Kuchel and C. Boyer, Exploring the Potential of Linear Polymer Structures for the Synthesis of Fluorescent Gold Nanoclusters, Mater. Chem. Front., 2017, 1, 80–90 RSC.
- Y. Song, Y. Li, M. Zhou, X. Liu, H. Li, H. Wang, Y. Shen, M. Zhu and R. Jin, Ultrabright Au@Cu14 Nanoclusters: 71.3% Phosphorescence Quantum Yield in Non-degassed Solution at Room Temperature, Sci. Adv., 2021, 7, eabd2091 CrossRef CAS PubMed.
- J. Wang, N. Goswami, T. Shu, L. Su and X. Zhang, pH-Responsive Aggregation-induced Emission of Au Nanoclusters and Crystallization of the Au(I)–thiolate Shell, Mater. Chem. Front., 2018, 2, 923–928 RSC.
- Z. Wu, Q. Yao, O. J. H. Chai, N. Ding, W. Xu, S. Zang and J. Xie, Unraveling the Impact of Gold(I)-Thiolate Motifs on the Aggregation-Induced Emission of Gold Nanoclusters, Angew. Chem., Int. Ed., 2020, 59, 9934–9939 CrossRef CAS PubMed.
- S. S. Zhang, S. Havenridge, C. Zhang, Z. Wang, L. Feng, Z. Y. Gao, C. M. Aikens, C. H. Tung and D. Sun, Sulfide Boosting Near-Unity Photoluminescence Quantum Yield of Silver Nanocluster, J. Am. Chem. Soc., 2022, 144, 18305–18314 CrossRef CAS PubMed.
- Y. Zhou, L. Liao, S. Zhuang, Y. Zhao, Z. Gan, W. Gu, J. Li, H. Deng, N. Xia and Z. Wu, Traceless Removal of Two Kernel Atoms in a Gold Nanocluster and Its Impact on Photoluminescence, Angew. Chem., Int. Ed., 2021, 60, 8668–8672 CrossRef CAS PubMed.
- A. Yao, Y. Du, M. Han, Y. Wang, J. Hu, Q. Zhu, H. Sheng and M. Zhu, Covalence Bridge Atomically Precise Metal Nanocluster and Metal–organic Frameworks for Enhanced Photostability and Photocatalysis, Nano Res., 2023, 16, 1527–1532 CrossRef CAS.
- S. Zhou, B. Peng, Y. Duan, K. Liu, O. Ikkala and R. H. A. Ras, Bright and Photostable Fluorescent Metal Nanocluster Supraparticles from Invert Emulsions, Angew. Chem., Int. Ed., 2022, 61, e202210808 CAS.
- Y. Li, S. Qu, Y. Xue, L. Zhang and L. Shang, Cationic Antibacterial Metal Nanoclusters with Traceable Capability for Fluorescent Imaging the Nano—bio Interactions, Nano Res., 2023, 16, 999–1008 CrossRef CAS.
- X. Wang, Z. Wang, S. Fang, Y. Hou, X. Du, Y. Xie, Q. Xue, X. Zhou and X. Yuan, Injectable Ag Nanoclusters-based Hydrogel for Wound Healing via Eliminating Bacterial Infection and Promoting Tissue Regeneration, Chem. Eng. J., 2021, 420, 127589 CrossRef CAS.
- H. Tang, Q. Li, W. Yan and X. Jiang, Reversing the Chirality of Surface Ligands Can Improve the Biosafety and Pharmacokinetics of Cationic Gold Nanoclusters, Angew. Chem., Int. Ed., 2021, 60, 13829–13834 CrossRef CAS.
- X. Jiang, B. Du, S. Tang, J. T. Hsieh and J. Zheng, Photoacoustic Imaging of Nanoparticle Transport in the Kidneys at High Temporal Resolution, Angew. Chem., Int. Ed., 2019, 58, 5994–6000 CrossRef CAS PubMed.
- V. Marjomaki, T. Lahtinen, M. Martikainen, J. Koivisto, S. Malola, K. Salorinne, M. Pettersson and H. Hakkinen, Site-specific Targeting of Enterovirus Capsid by Functionalized Monodisperse Gold Nanoclusters, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1277–1281 CrossRef CAS PubMed.
- Z. Pang, W. Yan, J. Yang, Q. Li, Y. Guo, D. Zhou and X. Jiang, Multifunctional Gold Nanoclusters for Effective Targeting, Near-Infrared Fluorescence Imaging, Diagnosis, and Treatment of Cancer Lymphatic Metastasis, ACS Nano, 2022, 16, 16019–16037 CrossRef CAS PubMed.
- L. Zhang, W. Zhuang, Y. Yuan, J. Shen, W. Shi, G. Liu, W. Wu, Q. Zhang, G. Shao, Q. Mei and Q. Fan, Novel Glutathione Activated Smart Probe for Photoacoustic Imaging, Photothermal Therapy, and Safe Postsurgery Treatment, ACS Appl. Mater. Interfaces, 2022, 14, 24174–24186 CrossRef CAS.
- Y. An, Y. Ren, M. Bick, A. Dudek, E. Hong-Wang Waworuntu, J. Tang, J. Chen and B. Chang, Highly Fluorescent Copper Nanoclusters for Sensing and Bioimaging, Biosens. Bioelectron., 2020, 154, 112078 CrossRef CAS PubMed.
- Y. A. Kuo, C. Jung, Y. A. Chen, H. C. Kuo, O. S. Zhao, T. D. Nguyen, J. R. Rybarski, S. Hong, Y. I. Chen, D. C. Wylie, J. A. Hawkins, J. N. Walker, S. W. J. Shields, J. S. Brodbelt, J. T. Petty, I. J. Finkelstein and H. C. Yeh, Massively Parallel Selection of NanoCluster Beacons, Adv. Mater., 2022, 34, e2204957 CrossRef PubMed.
- Z. Lin, N. Goswami, T. Xue, O. J. H. Chai, H. Xu, Y. Liu, Y. Su and J. Xie, Engineering Metal Nanoclusters for Targeted Therapeutics: From Targeting Strategies to Therapeutic Applications, Adv. Funct. Mater., 2021, 31, 2105662 CrossRef CAS.
- K. Zheng and J. Xie, Engineering Ultrasmall Metal Nanoclusters as Promising Theranostic Agents, Trends Chem., 2020, 2, 665–679 CrossRef CAS.
- P. Sarkar, M. Saha, N. Nandi, D. K. Sahu and K. Sahu, Red-Emitting Silver Nanoclusters for Dual-Mode Detection of Cu2+ and Vitamin B12 in Living Cells, ACS Appl. Nano Mater., 2022, 5, 7670–7678 CrossRef CAS.
- W. F. Lai, W. T. Wong and A. L. Rogach, Development of Copper Nanoclusters for In Vitro and In Vivo Theranostic Applications, Adv. Mater., 2020, 32, e1906872 CrossRef PubMed.
- O. A. Wong, R. J. Hansen, T. W. Ni, C. L. Heinecke, W. S. Compel, D. L. Gustafson and C. J. Ackerson, Structure–activity Relationships for Biodistribution, Pharmacokinetics, and Excretion of Atomically Precise Nanoclusters in a Murine Model, Nanoscale, 2013, 5, 10525–10533 RSC.
- W. M. Pardridge, Drug and Gene Targeting to the Brain with Molecular Trojan Horses, Nat. Rev. Drug Discovery, 2002, 1, 131–139 CrossRef CAS PubMed.
- Q. Zhuang, H. Jia, L. Du, Y. Li, Z. Chen, S. Huang and Y. Liu, Targeted Surface-functionalized Gold Nanoclusters for Mitochondrial Imaging, Biosens. Bioelectron., 2014, 55, 76–82 CrossRef CAS PubMed.
- A. Nicol, W. Qin, R. T. K. Kwok, J. M. Burkhartsmeyer, Z. Zhu, H. Su, W. Luo, J. W. Y. Lam, J. Qian, K. S. Wong and B. Z. Tang, Functionalized AIE Nanoparticles with Efficient Deep-red Emission, Mitochondrial Specificity, Cancer Cell Selectivity and Multiphoton Susceptibility, Chem. Sci., 2017, 8, 4634–4643 RSC.
- Z. Wang, Z. Zhu, C. Zhao, Q. Yao, X. Li, H. Liu, F. Du, X. Yuan and J. Xie, Silver Doping-Induced Luminescence Enhancement and Red-Shift of Gold Nanoclusters with Aggregation-Induced Emission, Chem. – Asian J., 2019, 14, 765–769 CrossRef CAS PubMed.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, From Aggregation-induced Emission of Au(I)-thiolate Complexes to Ultrabright Au(0)@Au(I)-thiolate Core-shell Nanoclusters, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
- D. Li, Q. Liu, Q. Qi, H. Shi, E. C. Hsu, W. Chen, W. Yuan, Y. Wu, S. Lin, Y. Zeng, Z. Xiao, L. Xu, Y. Zhang, T. Stoyanova, W. Jia and Z. Cheng, Gold Nanoclusters for NIR-II Fluorescence Imaging of Bones, Small, 2020, 16, e2003851 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01125e |
| ‡ These two authors contributed equally to this article. |
| This journal is © the Partner Organisations 2023 |


