Tailoring photoluminescence and multifunctionalities of lanthanide coordination complexes employing ligand-controlled aggregation states†
Jun
Wang‡
 *a,
Qianbo
Zhang‡
a,
Zhiming
Chen
a,
Xin
Lan
a,
Wenjing
Shi
a and
Zhiqiang
Li
*a,
Qianbo
Zhang‡
a,
Zhiming
Chen
a,
Xin
Lan
a,
Wenjing
Shi
a and
Zhiqiang
Li
 *b
*b
aSchool of Chemistry and Materials Science, Guizhou Normal University, Guiyang 550025, China. E-mail: wjc@gznu.edu.cn
bChemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China. E-mail: zhiqiangli@hebut.edu.cn
First published on 25th August 2023
Abstract
Developing lanthanide(III) ion (Ln3+) coordination complexes to mimic sunlight and achieve dynamic anti-counterfeiting is cutting-edge research, but it remains a significant challenge for luminescence color tuning and advanced information handling. Herein, a precisely designed ligand (TL) displays controllable monomers and excimers in different environments, resulting in the colorimetric properties of Ln3+ complexes (TL-Eu/Tb). When the sensitizer resides in the dimeric state, only TL-Eu gives strong luminescence, including outstanding WLE. Instead, in the monomeric state, TL-Eu/Tb emits red/green luminescence, and Eu3+/Tb3+ co-doped films demonstrate tunable luminescence properties and satisfactory WLE (CIE coordinates of (0.33, 0.33)) can be obtained from a customized film with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (Eu3+/Tb3+) molar ratio. Interestingly, the resulting WLE materials could be utilized in the preparation of white light-emitting-diodes (WLEDs) with superior color quality and have shown potential for advanced anti-counterfeiting applications due to their multi-stimuli responsiveness. This work provides a promising strategy for fabricating smart materials that utilize the controllable aggregation of sensitized ligands.
9 (Eu3+/Tb3+) molar ratio. Interestingly, the resulting WLE materials could be utilized in the preparation of white light-emitting-diodes (WLEDs) with superior color quality and have shown potential for advanced anti-counterfeiting applications due to their multi-stimuli responsiveness. This work provides a promising strategy for fabricating smart materials that utilize the controllable aggregation of sensitized ligands.
Introduction
Effective light control is crucial for meeting the growing energy demand.1 Natural sunlight can curb the circadian rhythm of organisms and further influence human metabolism.2 White light emission (WLE) can mimic natural sunlight since it contains multicolor components over the entire visible spectral region. High-quality WLE, with an International Commission on Illumination (CIE) coordinate of (0.33, 0.33), a modest correlated color temperature (CCT) in the range 2500–6500 K, and a high color rendering index (>80),3 is highly desirable to cater to advanced applications in surgery, photography, and museum exhibition.4–6 To date, the common commercial WLED suffers from a low CRI; therefore, the fabrication of WLE materials with a high CRI (>90) remains a great challenge.7,8Lanthanide(III) (Ln3+) coordination complexes have triggered ever-increasing interest in their practical applications and fundamental importance.5–7,9–12 The combination of ligands and Ln3+-centered emissions makes them ideal for white lighting systems.13 Considerable WLE materials have been reported so far. Although the single lanthanide (Ln3+)-doped approach is efficient and highly desirable, it is challenging and has been rarely studied, limiting its widespread application due to unsatisfactory light color purity.5,14–17 Another popular strategy is the co-doping of multiple Ln3+, where ligand-centered blue emission combines with complementary co-doped Eu3+ and Tb3+ emission.18,19 However, based on these strategies, WLE is observed only in solution/the solid state/the doped state, which may be ascribed to the aggregation states of ligands (e.g., aggregation-induced quenching/emission).20 Combined with these traditional strategies, employing sensitized ligands (fluorophores) with tunable aggregated states may open a new approach for the desired optical performance,2,21–23 thus exhibiting advantages such as flexible luminescence tunability, easy processability for the device, and multi-functionalities. Interestingly, 1,8-naphthalimide (NI) features well-tailored photophysical properties by molecular engineering and in the physical state (monomers and excimers).24 Moreover, NI possesses a short-lived (<1 ns) blue emission of singlet state (S1) and suitable T1 energy (∼18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1),25 matching energy transfer to the Eu3+ 5D0 state (17
500 cm−1),25 matching energy transfer to the Eu3+ 5D0 state (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) according to Latva's and Reinhoudt's rules.26,27 Hence, NI/Eu3+ complexes are ideal platforms to form WLE. Unfortunately, only the closer WLE is observed in these systems.28,29
500 cm−1) according to Latva's and Reinhoudt's rules.26,27 Hence, NI/Eu3+ complexes are ideal platforms to form WLE. Unfortunately, only the closer WLE is observed in these systems.28,29
In addition, dynamic luminescence is propitious to enhance the security of anti-counterfeiting techniques due to the excitation wavelength/chemical species-dependent luminescence color changes.30,31 Unquestionably, considering the sharp emission bands, the long luminescence lifetime of Ln3+, and the diversity of sensitizers, Ln3+-based complexes would be the most promising dynamic anti-counterfeiting materials. For example, Yan et al. reported an excited light and chemical stimulus-responsive Eu3+-functionalized hydrogel film for dynamic anti-counterfeiting.32 We recently also developed UV and visible light-controlled anti-counterfeiting materials for reversible multiple information encryption and decryption based on the supramolecular assembly of an anionic Eu3+ coordination polymer and a cationic diarylethene derivative.33 These investigations exemplify that anti-counterfeiting materials with high-level security should be elaborately designed. However, multimodal dynamic anti-counterfeiting with high-level security is still challenging.
Herein, we developed Ln3+ coordination complexes for photoluminescence color tuning by controlling the monomer and excimer-centered emission of sensitized ligands. To fabricate Ln3+ complexes, we first prepared a multifunctional ligand (TL) incorporating NI and terpyridine motifs (Fig. 1), along with a reference ligand (RL). The non-conjugated skeleton of TL prevented a decrease of triplet state (T1) energy due to co-facial stacking,34,35 guaranteeing efficient energy transfer from TL to Eu3+. In addition, as a fine Ln3+ ligand, terpyridine endowed the assemblies with multi-stimuli responsiveness.18,36,37 Consequently, in solution and in the doped state, TL-Eu showed red emission. While in the solid state, thanks to the blue-green emission of dimeric TL and Eu3+ red emission, the resultant WLE was achieved. The obtained WLED displayed ideal CIE coordinates of (0.32, 0.34), a moderate CCT of 6192 K, and a superior CRI of 94. In contrast, TL-Tb only showed strong Tb3+ characteristic emission in the doped state. Furthermore, Eu3+/Tb3+ co-doped polymethyl methacrylate (PMMA) (TL-Eu/Tb@PMMA) films displayed tailorable luminescence, and the WLE with CIE coordinates (0.33, 0.33) was obtained from a customized film with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (Eu3+/Tb3+). The TL-Eu/Tb@PMMA-based WLED also gave a satisfactory CRI value (80). More interestingly, based on the multi-stimuli responsiveness, solid-state TL-Eu/Tb was developed for dynamic anti-counterfeiting.
9 (Eu3+/Tb3+). The TL-Eu/Tb@PMMA-based WLED also gave a satisfactory CRI value (80). More interestingly, based on the multi-stimuli responsiveness, solid-state TL-Eu/Tb was developed for dynamic anti-counterfeiting.
 | ||
| Fig. 1 Ligand-controlled (a) luminescence properties and (b) anti-counterfeiting applications of lanthanide complexes. | ||
Results and discussion
Aggregation behaviors of TL
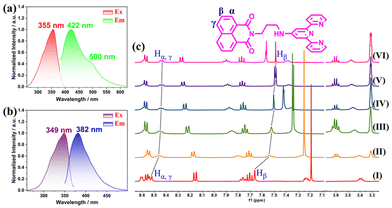
RL/TL was synthesized and well characterized (Fig. S1–S5†). The photophysical properties of TL were initially investigated. As shown in Fig. S6,†TL in solution displayed a π–π* transition of terpyridine36 at 282 nm and NI moieties38 at 335 nm. Their absorbance dramatically increased versus concentration from 1.0 to 100 μM, which could be attributed to the π–π stacking interaction.39 Furthermore, TL (10 μM) showed monomer emission (382 nm) when excited at 349 nm in solution, Fig. 2a. While solid-state TL manifested bathochromic emission, reaching 422 nm with shoulder emission at 500 nm upon 355 nm excitation (Fig. 2b), which was characteristic excimer emission of NI.24,38 To intensively study the aggregation behavior, 1H NMR titration experiments were performed, Fig. 2c. With the increase of CD3OD fractions from 0 to 50%, the signals of Hα–γ on NI exhibited obvious π–π stacking interaction triggered upfield shifts.21,40 These observations reasonably confirm that TL monomers/excimers dominantly exist in solution/in the solid state, respectively. Consistent with our expectation, the monomer displayed blue emission and the excimer showed blue-green emission in the solid state. As a proof of conclusion, the monomer-centered emission of the doped PMMA film (TL@PMMA) could also be detected,41 as presented in Fig. S7.†Fabrication and photophysical properties of TL-Eu/Tb in solution
First, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (TL: Eu3+/Tb3+) complexation between TL and Eu3+/Tb3+ was confirmed by Job's plot (Fig. S8†), which was further supported by ESI-MS titration experiments. In particular, the peak at m/z 486.19 could be assigned to [TL + H]+ (Fig. S4†), and after addition of Eu3+, the molecular ion peak [2TL + Eu3+ + 3NO3− + 3H+]3+ (Fig. S9†) was perceived at m/z 437.19. Besides, TL–Tb formation was testified by the peaks at 415.21 of [2TL + Tb3+ + NO3− + H+ + 3H2O]3+ (Fig. S10†). Furthermore, due to the coordinated interaction between Ln3+ and terpyridine, the C
1 (TL: Eu3+/Tb3+) complexation between TL and Eu3+/Tb3+ was confirmed by Job's plot (Fig. S8†), which was further supported by ESI-MS titration experiments. In particular, the peak at m/z 486.19 could be assigned to [TL + H]+ (Fig. S4†), and after addition of Eu3+, the molecular ion peak [2TL + Eu3+ + 3NO3− + 3H+]3+ (Fig. S9†) was perceived at m/z 437.19. Besides, TL–Tb formation was testified by the peaks at 415.21 of [2TL + Tb3+ + NO3− + H+ + 3H2O]3+ (Fig. S10†). Furthermore, due to the coordinated interaction between Ln3+ and terpyridine, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N IR peak at 1653 cm−1 shifted to ∼1620 cm−1 (Fig. S11†).32 Moreover, the corresponding association constants (Ka) for TL-Eu/Tb were found to be 1.36 × 1013 and 1.14 × 1014 (Fig. S12†). The existing results jointly signify that each Ln3+ has been coordinated with two terpyridine motifs to form TL-Eu/Tb complexes in the solution.
N IR peak at 1653 cm−1 shifted to ∼1620 cm−1 (Fig. S11†).32 Moreover, the corresponding association constants (Ka) for TL-Eu/Tb were found to be 1.36 × 1013 and 1.14 × 1014 (Fig. S12†). The existing results jointly signify that each Ln3+ has been coordinated with two terpyridine motifs to form TL-Eu/Tb complexes in the solution.
The photoluminescence of TL-Eu/Tb (10 μM) was further investigated in different solvents. In CHCl3–CH3CN (v/v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solution, upon 354 nm irradiation, TL-Tb showed blue emission at 384 nm and negligible Tb3+ characteristic luminescence, and for TL-Eu, except for the blue emission, strong Eu3+ characteristic emission was also inspected (Fig. S13†); the blue and red emissions hybridized together, resulting in pink hybrid fluorescence in CHCl3 and CH2Cl2 solutions, indicative of the dispersed TL state in these complexes. In contrast, both emission peaks faded away with the increase of solvent polarity, and a blue color in CH3OH and no emission in DMF and DMSO were observed, respectively, which could be responsible for the formation of detrimental aggregation of TL,41 giving rise to (1) the decrease of T1 energy and (2) the aggregation-induced fluorescence quenching in strong polar solvents (ACQ effect), supported by their photographs (Fig. S14†).34
3) solution, upon 354 nm irradiation, TL-Tb showed blue emission at 384 nm and negligible Tb3+ characteristic luminescence, and for TL-Eu, except for the blue emission, strong Eu3+ characteristic emission was also inspected (Fig. S13†); the blue and red emissions hybridized together, resulting in pink hybrid fluorescence in CHCl3 and CH2Cl2 solutions, indicative of the dispersed TL state in these complexes. In contrast, both emission peaks faded away with the increase of solvent polarity, and a blue color in CH3OH and no emission in DMF and DMSO were observed, respectively, which could be responsible for the formation of detrimental aggregation of TL,41 giving rise to (1) the decrease of T1 energy and (2) the aggregation-induced fluorescence quenching in strong polar solvents (ACQ effect), supported by their photographs (Fig. S14†).34
Photoluminescence properties of solid-state TL-Eu/Tb
In the solid state of TL-Eu/Tb, the experimental powder X-ray diffraction (PXRD) patterns (Fig. S15†) showed broad amorphous peaks in the 2θ range from 11° to 24°, while no crystalline peak (2θ < 10°) was observed, suggesting the formation of lanthanide coordination polymers.11 Correspondingly, the aggregated nanoplates were perceived from SEM, Fig. 3, which was in excellent agreement with the analysis of PXRD. The assemblies were made up of C, N, O and Eu/Tb (Fig. 3b and d), which were further confirmed from energy dispersive X-ray mapping images (Fig. S16†). Notably, TL-Eu/Tb exhibited excellent stability, and the decomposition temperatures were over 280 °C (Fig. S17†). | ||
| Fig. 3 (a and c) SEM images and (b and d) EDX mapping images of C, N, O and Eu/Tb elements of solid-state TL-Eu/Tb, respectively. | ||
Considering the dual emission of dimeric TL and Eu3+-centered red emission in the solid state, we speculated that TL-Eu is preferable to tune full-spectrum emission. As expected, the luminescence comprised the excimer and Eu3+-centered emission under excitation at 384 nm, covering the entire visible spectral region, Fig. 4a. In this context, the 400–575 nm broadband was attributed to the dimeric TL π–π* transition,28 while the well-separated ones centered at 580, 593, 618, 650 and 686 nm stemmed from Eu3+ 5D0 → 7FJ (J = 1, 2, 3 and 4) transitions, respectively. Therein, the intensity ratio of the electronic-dipole and magnetic-dipole [(5D0 → 7F2)/(5D0 → 7F1)] was 1.79, showing a relatively low-symmetry coordination microenvironment.32 These observations testify that Eu3+ can be sensitized by dimeric TL, and the WLE would be observed solely based on TL-Eu.
Indeed, similar luminescence profiles were obtained when the excitation wavelength varied from 350 to 410 nm, Fig. S18.† The intensity of excimers and Eu3+ emissions was first increased and then decreased, reaching their maximum at 384 nm, respectively. These changeable spectroscopic properties, combining the color coordinates (x,y) (Fig. S19†), CCT and CRI values (Table S1†), illustrate that TL-Eu exhibits excitation wavelength-dependent emission, which is responsible for color tunability. The emission colors almost stayed in the white light zone.
Then, outstanding WLE was acquired with CIE coordinates of (∼0.34, ∼0.33) and CCT of (∼5500 K) under irradiation at 354–360 nm (Fig. 4b–d), which were almost consistent with the standard parameters. The color purity was superior to those of the analogs (Table S2†). The contributing color components for WLE were dimeric TL π–π* (blue-green, ∼16%) and 5D0 → 7FJ (J = 1–4) transitions (red, ∼84%) of Eu3+,5 Table S3.† Due to the incomplete energy transfer from TL to Eu3+, the TL lifetime decreased from 14.48 ns to 10.39 ns (Fig. S20† and Fig. 4e). The Eu3+-centered absolute fluorescence quantum yield and lifetime were measured at 6.40% and 0.56 ms (Fig. 4f), respectively. Interestingly, the TL-Eu-coated blue LED chip (365 nm) emitted bright WLE (Fig. 5) with CIE coordinates of (0.32, 0.34) and a CCT of 6192 K, and the CRI value (94) was the highest among the reported single Ln3+-coated WLED devices (Table S4†).
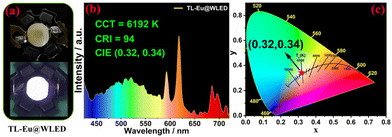 | ||
| Fig. 5 (a) Images of a 365 nm LED with coated TL-Eu when a LED is off and on, and (b and c) the corresponding emission spectra and CIE coordinates of a WLED, respectively. | ||
As for TL-Tb, only the excimer emission at 476 nm was perceived without Tb3+-centered emission under 366 nm light, which was very similar to that of TL-Gd (Fig. S21 and S22†). Unquestionably, both the WLE and TL-centered emission of TL-Tb were responsible for the formation of excimers via π–π stacking of NI, which was further demonstrated with the help of the luminescence performance of RL-Eu/Tb. The RL structure was similar to that of TL, except for the absence of NI moieties. The coordinated interaction between Ln3+ and RL was testified by IR spectra and is depicted in Fig. S23.†RL-Eu showed red emission in the solid state, while no blue-green emission was detected at different excitation wavelengths (Fig. S24†). In contrast, RL-Tb exhibited emission peaks at 490, 544, 590, and 625 nm, which were ascribed to the Tb3+ 5D4–7FJ (J = 6, 5, 4 and 3) transitions at different excitation wavelengths (Fig. S25†). Thus, the ligand states could finely control the luminescent colors of TL-Eu/Tb.
Photoluminescence properties of PMMA films
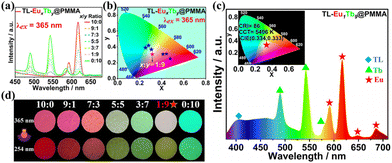
As we know, the doping of Ln3+ complexes into PMMA matrices not only restrains the formation of aggregates, but also enhances the luminescence properties due to the auxiliary sensitization of Ln3+,15,42 thereby resulting in Tb3+ characteristic emission, thanks to the elevation of TL T1 energy.34 Thus, as a control, we investigated the photophysical properties of TL@PMMA and TL-Eu/Tb@PMMA films. Consequently, the films revealed good transparency with tunable colors at different excitation wavelengths, as presented in Fig. 6. It is worth noting that, among the different TL states, the solid state possessed the highest emission wavelength (422 nm), followed by the doped state in PMMA (398 nm), and in solution (382 nm), similar to those of the emissions in the corresponding state of TL-Eu, Fig. S26 and S27.† Due to the dominant monomer in the TL doped state, both Eu3+/Tb3+-centered red/green emissions could be obtained by sensitized luminescence (Fig. 6k and l). Compared to the solid-state TL and TL-Eu, the TL-centered fluorescence lifetimes (τ = 4.95/2.53 ns) in TL-Eu/Tb@PMMA films were further decreased, while the Eu3+/Tb3+-centered lifetime (τ = 1.11/0.66 ms) increased (Table S5 and Fig. S28 and S29†), respectively. Hence, the incomplete energy transfer from TL to Eu3+/Tb3+ would enable co-doped films to modulate the expected WLE.Based on the trichromatic (RGB) principle, the luminescent colors could be precisely tailored from Eu3+/Tb3+ co-doped films (TL-EuxTby@PMMA) by altering the doping molar ratio (x/y). Delightfully, with the decrease in x/y (from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, total amount: 1 mmol), the Eu3+ red emission intensity decreased continuously, while that of Tb3+ green emission increased gradually, causing a color change from red to green (Fig. 7). As expected, TL-Eu1Tb9@PMMA emitted pure WLE with standardized CIE coordinates of (0.33, 0.33) upon 361 nm excitation. The quantum yields (3.98%/1.52% for Eu3+/Tb3+) and the lifetimes (1.02 ms/0.89 ms for Eu3+/Tb3+) are listed in Fig. S30 and Table S5.† Besides, by simply coating the resulting film on the surface of a commercial blue LED chip, the WLED was fabricated and it emitted white light at a voltage of 3.0 V. The corresponding CIE, CCT and CRI values were found to be (0.32, 0.37), 5937 K and 80, respectively. Compared with the WLED, the TL-Eu-coated WLED displays superior color quality (see Table S5 and Fig. S31†).
10, total amount: 1 mmol), the Eu3+ red emission intensity decreased continuously, while that of Tb3+ green emission increased gradually, causing a color change from red to green (Fig. 7). As expected, TL-Eu1Tb9@PMMA emitted pure WLE with standardized CIE coordinates of (0.33, 0.33) upon 361 nm excitation. The quantum yields (3.98%/1.52% for Eu3+/Tb3+) and the lifetimes (1.02 ms/0.89 ms for Eu3+/Tb3+) are listed in Fig. S30 and Table S5.† Besides, by simply coating the resulting film on the surface of a commercial blue LED chip, the WLED was fabricated and it emitted white light at a voltage of 3.0 V. The corresponding CIE, CCT and CRI values were found to be (0.32, 0.37), 5937 K and 80, respectively. Compared with the WLED, the TL-Eu-coated WLED displays superior color quality (see Table S5 and Fig. S31†).
Energy transfer mechanism
To gain deep insight into the energy transfer mechanism (the so-called S1 → T1 → Ln3+ energy transfer sensitization route14,26), the low-temperature phosphorescence and UV-Vis absorbance spectra of the solid-state Gd complex (TL-Gd) were recorded and are illustrated in Fig. 8. TL-Gd presented the maximum absorption wavelength at 325 nm, implying that S1 energy was estimated to be 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 cm−1. At 77 K, TL-Gd showed significant phosphorescence emission (494 nm) when excited at 380 nm, and T1 energy was calculated as 20
490 cm−1. At 77 K, TL-Gd showed significant phosphorescence emission (494 nm) when excited at 380 nm, and T1 energy was calculated as 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 243 cm−1. The energy gap ΔE(ST) (8247 cm−1) between S1 and T1 was more than 5000 cm−1, which indicated that efficient intersystem crossing (ISC) was possible according to Reinhoudt's rule.27 Furthermore, the energy gap between T1 and the lowest Eu3+ excited state (5D0 17
243 cm−1. The energy gap ΔE(ST) (8247 cm−1) between S1 and T1 was more than 5000 cm−1, which indicated that efficient intersystem crossing (ISC) was possible according to Reinhoudt's rule.27 Furthermore, the energy gap between T1 and the lowest Eu3+ excited state (5D0 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) was 2743 cm−1, ranging from 2500 to 4000 cm−1 and meeting Latva's empirical rule,26 which could sensitize Eu3+ due to the antenna effect.43 Namely, efficient energy transfer from S1 to T1 and then to the 5D0 state of Eu3+ gives rise to f–f emission. Instead, the Tb3+ excited state (5D4 20
500 cm−1) was 2743 cm−1, ranging from 2500 to 4000 cm−1 and meeting Latva's empirical rule,26 which could sensitize Eu3+ due to the antenna effect.43 Namely, efficient energy transfer from S1 to T1 and then to the 5D0 state of Eu3+ gives rise to f–f emission. Instead, the Tb3+ excited state (5D4 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) energy was slightly higher than that of the T1 energy of TL, which is responsible for the ligand-centered blue-green emission of solid-state TL-Tb, Fig. S21.†
500 cm−1) energy was slightly higher than that of the T1 energy of TL, which is responsible for the ligand-centered blue-green emission of solid-state TL-Tb, Fig. S21.†
Applications in anti-counterfeiting
Unquestionably, the external multi-stimuli could elevate the security level of smart anti-counterfeiting materials.31,32 It should be noted that the solid-state TL-Eu/Tb featured excitation wavelength-dependent luminescence (Fig. S18 and S21†). Besides, considering the dynamic Ln–N interactions,18,37 the coordination interactions between TL-Eu and metal ions were studied based on spectral analysis in CHCl3–CH3CN binary solution. Excitingly, under 354 nm irradiation, Eu3+ luminescence quenching triggered by ten heavy metal ions (Zn2+, Fe2+, Cr3+, Co2+, Fe3+, Hg2+, Cu2+, Pb2+, Ni2+ and Cd2+) could be observed, resulting in different color changes (Fig. 9). The detailed sensing properties are discussed in the ESI (from Fig. S32 to S51, and Table S6†). Based on the above multi-stimuli responses, the developed TL-Eu/Tb system could be further explored for potential anti-counterfeiting applications.Benefiting from the strong luminescence, red and green luminescent inks of TL-Eu/Tb were prepared and a series of anti-counterfeiting patterns were fabricated with higher security for information encryption and decryption. First, the binary codes “0” and “1” were written down with green and red luminescent inks, as presented in Fig. 10a, respectively. The encryption information of “GZNU” characters was not identified under daylight and was retrieved under 254 nm illumination in a dark field. Also, taking the “Guizhou” characters that were imprinted with red inks as another example (Fig. 10b), they were stored in “CGouliozrhtouul” by scrambled letters with UV 254 nm light on. The right sequence letters of “Guizhou” with red luminescence could only be distinguished under 365 nm irradiation. Furthermore, a great artistic painting was employed as an information storage system to improve anti-counterfeiting security, Fig. 10c. The colorful painting was invisible in daylight and fully presented under 254 nm illumination. Upon irradiation with 365 nm, only those painted with red ink were left, while others disappeared.
Interestingly, the patterned information could be changed by a chemical species stimulus. Attributed to the stimuli-response to Zn2+, when both the decrypted information of “Guizhou” and artistic painting were immersed in Zn2+ solution (20 μM), their luminescence disappeared under 365 nm UV light, meaning that the expected information could be erased (Fig. S52†). In contrast, when they were exposed to HCl gas, due to the dissociation of Ln3+ complexes by protonation of terpyridine motifs,36 the scrambled letters “CGouliozrhtouul” and the whole artistic painting emerged again, but the light emitted by them transferred to dimeric ligand-centered green luminescence with UV 254/365 nm light on. Excitedly, the original information could be recovered after rewriting with DPA ink when a UV lamp (254 nm) was turned on (Fig. 10b and c), which would be explained by the competition coordination between DPA and Ln3+.44 These results indicate that TL-Eu/Tb can be employed to fabricate information encryption–decryption–erasing–recovering materials.
Experimental
Materials
Unless otherwise noted, all commercial chemicals were used without further purification. 1,8-Naphthalic anhydride (NI), 1,3-diaminopropane, 4′-chloro-2,2′:6′,2′′-terpyridine, 2,6-pyridine dicarboxylic acid (DPA) and poly(methyl methacrylate) (PMMA) were obtained from Aladdin (Shanghai, China). Ln(NO3)3·6H2O (Ln3+ = Eu3+, Tb3+, Gd3+, 99.99%) and deuterium reagents (CDCl3 and CD3OD) were purchased from Innochem (Beijing, China). Other analytical metal ions (mercury dichloride, sodium chloride, potassium chloride, magnesium chloride, calcium chloride, copper(II) chloride dihydrate, cadmium chloride, iron(III) chloride hexahydrate, cobaltous chloride, nickel chloride, chromium chloride hexahydrate, zinc sulfate heptahydrate, iron(II) sulfate heptahydrate and lead nitrate were used as cation (Hg2+, Na+, K+, Mg2+, Ca2+, Cu2+, Cd2+, Fe3+, Co2+, Ni2+, Cr3+, Zn2+, Fe2+ and Pb2+) sources in other experiments, respectively.Characterization
The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker instrument at 25 °C (1H: 400 MHz, 13C: 100 MHz), and CDCl3 and CD3OD were used as the NMR solvents, respectively. The contents of Ln3+ were determined by using an inductively coupled plasma optical emission spectrometer (ICP-OES, Agilent 5110). Elemental analysis was executed using an Elementar analysis system (UNICUBE). High-resolution mass spectrometry (HRMS) was performed using an ion-Spec QFT-ESI MS in the ESI mode. Fourier transform infrared spectra were recorded on a Thermo Scientific Nicolet 6700 FT-IR spectrometer (Sugar Land, TX, USA) with the KBr pellet technique. Scanning electron microscopy (SEM) images were obtained using a Hitachi Regulus 8100. Powder X-ray diffraction (PXRD) was investigated through Rigaku Smartlab. Thermogravimetric analysis (TGA) was carried out under an N2 atmosphere from room temperature to 900 °C using a Netzsch STA449F3 analyzer at a heating rate of 10 °C min−1. Shimadzu UV-2550 and FL970 fluorescence spectrometers (Techcomp, China) were used to obtain UV-vis absorption and fluorescence spectra, respectively. Low temperature (77 K) phosphorescence spectra, quantum yields and fluorescence lifetimes were recorded by using an Edinburgh Instruments FLS1000 near-infrared spectrometer. The luminescent images were obtained by using a Huawei Mate 30 in a UV box (WFH-203B, China) at room temperature. The corresponding CIE, CCT and CRI values of the WLED were obtained using MPS2000 (China).Synthesis of TL and lanthanide complexes
The synthesis procedures of RL/TL, TL-Eu/Tb/Gd, and RL-Eu/Tb complexes can be found in the ESI.†Fabrication of luminescent PMMA films
PMMA (1.0 g) and TL/TL-Eu/TL-Tb (5 mg) were dissolved in 20 mL of acetone and 2 mL of DMF and stirred for 1 h. Then, the resulting mixture was dropped into a Teflon mold to obtain the corresponding films (TL/TL-Eu/TL-Tb@PMMA). For co-doped Eu3+/Tb3+ films, the Eu3+/Tb3+ heteronuclear complexes were first synthesized with different molar ratios (x/y) from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (total amount: 1 mmol), and then, 5 mg of heteronuclear complexes were doped into 1.0 g of PMMA to prepare co-doped films (TL-EuxTby@PMMA).42
10 (total amount: 1 mmol), and then, 5 mg of heteronuclear complexes were doped into 1.0 g of PMMA to prepare co-doped films (TL-EuxTby@PMMA).42
Preparation of WLED lamps
According to Wang's report as a reference,45 the WLED lamps were prepared with commercial violet light chips (365 nm, 3.2 V) as the excitation source and the obtained WLE materials as coatings. First, the obtained WLE materials (TL-Eu or TL-Eu1Tb9@PMMA) were dispersed in an epoxy resin AB adhesive (equal mass of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a weight ratio of 1
1) with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, the above mixtures were coated on the surface of commercial chips and the WLED lamps were obtained after drying at 50 °C for 12 h.
1. Then, the above mixtures were coated on the surface of commercial chips and the WLED lamps were obtained after drying at 50 °C for 12 h.
Fabrication of luminescent inks
5 mg of TL-Eu/Tb or 2,6-pyridine dicarboxylic acid (DPA) was dissolved in 1 mL of ethanol and 1 mL of ethanediol and sonicated for a few minutes. Then, the mixture solution was injected into a neutral refill.Conclusions
In summary, this work has successfully demonstrated that the luminescence performance of Ln3+ complexes (TL-Eu/Tb) can be precisely regulated by controlling the monomeric and dimeric states of sensitized ligands. Specifically, our findings indicate that dispersed ligands in solution and doped states can sensitize the luminescence of Eu3+/Tb3+ ions. However, the dimeric ligand only sensitizes Eu3+ luminescence and provides single-component WLE. A TL-Eu-coated WLED lamp displays superior color quality (CIE coordinates of (0.32, 0.34), a CCT of 6192 K, and a CRI of 94, respectively). Remarkably, when TL-Eu/Tb complexes were co-doped into the PMMA matrix, the customized TL-Eu1Tb9@PMMA film exhibited pure WLE with CIE coordinates of (0.33, 0.33). Also, a satisfactory WLED device (CIE coordinates of (0.32, 0.37), a CCT of 5937 K, and a CRI of 80, respectively) was prepared. In addition, TL-Eu/Tb complexes were found to be effective in anti-counterfeiting applications due to their multi-stimuli responsiveness. Overall, this study provides a promising strategy for designing color-tunable lanthanide luminescence and WLE materials with promising applications in WLED lighting.Author contributions
J. Wang and Q. Zhang contributed equally to this work. J. Wang: supervision, writing – review and editing, and funding acquisition; Q. Zhang: experiment, data curation, visualization and investigation; Z. Chen: formal analysis; X. Lan and W. Shi: validation; and Z. Li: supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 22161012), the Science and Technology Program of Guizhou Province (Grant No. Qiankehejichu-ZK[2021]General 066), and the Academic New Seedling Foundation Project of Guizhou Normal University (Grant No. Qianshixinmiao-[2022]07).References
- J. Cho, J. H. Park, J. K. Kim and E. F. Schubert, White Light-Emitting Diodes: History, Progress, and Future, Laser Photonics Rev., 2017, 11, 1600147 CrossRef.
- J. Zhang, X. Zhao, H. Shen, J. W. Y. Lam, H. Zhang and B. Z. Tang, White-Light Emission from Organic Aggregates: A Review, Adv. Photonics, 2022, 4, 014001 CAS.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Luminescent Functional Metal-Organic Frameworks, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- M. Pan, W.-M. Liao, S.-Y. Yin, S.-S. Sun and C.-Y. Su, Single-Phase White-Light-Emitting and Photoluminescent Color-Tuning Coordination Assemblies, Chem. Rev., 2018, 118, 8889–8935 CrossRef CAS PubMed.
- S. V. Eliseeva, E. V. Salerno, B. A. Lopez Bermudez, S. Petoud and V. L. Pecoraro, Dy3+ White Light Emission Can Be Finely Controlled by Tuning the First Coordination Sphere of Ga3+ /Dy3+ Metallacrown Complexes, J. Am. Chem. Soc., 2020, 142, 16173–16176 CrossRef CAS PubMed.
- Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian and B. Chen, A Luminescent Mixed-Lanthanide Metal Organic Framework Thermometer, J. Am. Chem. Soc., 2012, 134, 3979–3982 CrossRef CAS PubMed.
- H. Yu, J. Liu, S. Bao, G. Gao, H. Zhu, P. Zhu and G. Wang, Luminescent Lanthanide Single Atom Composite Materials: Tunable Full-Color Single Phosphor and Applications in White LEDs, Chem. Eng. J., 2022, 430, 132782 CrossRef CAS.
- Y. Li, Q. Chen, L.-H. Xie, K. Wang, M. Zhao and J.-R. Li, Single-Phase White-Light Phosphors Based on a Bicarbazole-Based Metal−Organic Framework with Encapsulated Dyes, ACS Mater. Lett., 2022, 4, 2345–2351 CrossRef CAS.
- X. Liu, B. Li, W. Wang, Z. Li and Q. Xiong, Hydrogels with Both Mechanical Strength and Luminescence Anisotropy, Inorg. Chem. Front., 2022, 9, 4194–4200 RSC.
- Y. Liu, W. Dan and B. Yan, A Light-Operated Dual-Mode Method for Neuroblastoma Diagnosis Based on a Tb-MOF: from Biometabolite Detection to Logic Devices, Inorg. Chem. Front., 2023, 10, 1660–1670 RSC.
- T. Feng, Y. Ye, X. Liu, H. Cui, Z. Li, Y. Zhang, B. Liang, H. Li and B. Chen, A Robust Mixed–Lanthanide PolyMOF Membrane for Ratiometric Temperature Sensing, Angew. Chem., Int. Ed., 2020, 59, 21752–21757 CrossRef CAS PubMed.
- Q. Ma, M. Zhang, C. Yao, Y. Du and D. Yang, Supramolecular Hydrogel with Luminescence Tunability and Responsiveness Based on Co-doped Lanthanide and Deoxyguanosine Complex, Chem. Eng. J., 2020, 394, 124894 CrossRef CAS.
- Q. Tang, S. Liu, Y. Liu, D. He, J. Miao, X. Wang, Y. Ji and Z. Zheng, Color Tuning and White Light Emission via in Situ Doping of Luminescent Lanthanide Metal−Organic Frameworks, Inorg. Chem., 2014, 53, 289–293 CrossRef CAS PubMed.
- S. SeethaLekshmi, A. R. Ramya, M. L. P. Reddy and S. Varughese, Lanthanide Complex-Derived White-Light Emitting Solids: A Survey on Design Strategies, J. Photochem. Photobiol., C, 2017, 33, 109–131 CrossRef CAS.
- J. Wang, X. Li, H. Chu, J. He and Z. Chen, Research Progress in the White Light-Emitting Lanthanide-Based Complex/Coordination Polymer Materials, Chin. J. Org. Chem., 2019, 39, 3399–3413 CrossRef CAS.
- S. Mund and S. Vaidyanathan, New Isomeric Ancillary Ligands and their Eu(III) Complexes: a Single Component White Light Emissive Phosphor and their Applications in Red/White Smart LEDs, Electronic Noses, and Temperature Sensing, J. Mater. Chem. C, 2022, 10, 7201–7215 RSC.
- W.-M. Liao, C.-J. Li, X. Wu, J.-H. Zhang, Z. Wang, H.-P. Wang, Y.-N. Fan, M. Pan and C.-Y. Su, Homometallic Ln(III)-Complexes from an ILCT Ligand with Sensitized Vis-NIR Emission, Excitation-Dependent PL Color Tuning and White-Light Emission, J. Mater. Chem. C, 2018, 6, 3254–3259 RSC.
- P. Chen, Q. Li, S. Grindy and N. Holten-Andersen, White-Light-Emitting Lanthanide Metallogels with Tunable Luminescence and Reversible Stimuli-Responsive Properties, J. Am. Chem. Soc., 2015, 137, 11590–11593 CrossRef CAS PubMed.
- A. E. Psalti, D. Andriotou, S. A. Diamantis, A. Chatz-Giachia, A. Pournara, M. J. Manos, A. Hatzidimitriou and T. Lazarides, Mixed-Metal and Mixed-Ligand Lanthanide Metal-Organic Frameworks Based on 2,6-Naphthalenedicarboxylate: Thermally Activated Sensitization and White-Light Emission, Inorg. Chem., 2022, 61, 11959–11972 CrossRef CAS PubMed.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: New Vistas at the Aggregate Level, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS PubMed.
- Q. Li, Y. Liu, P. Liu, L. Shangguan, H. Zhu and B. Shi, Solvent-Controlled Assembly of Pillar[5]Arene-Based Supramolecular Networks Via π–π Interactions for White Light Modulation, Org. Chem. Front., 2020, 7, 399–404 RSC.
- W. Zhou, Y. Chen, Q. Yu, P. Li, X. Chen and Y. Liu, Photo-Responsive Cyclodextrin/Anthracene/Eu3+ Supramolecular Assembly for a Tunable Photochromic Multicolor Cell Label and Fluorescent Ink, Chem. Sci., 2019, 10, 3346–3352 RSC.
- J. Leng, H. Li, P. Chen, W. Sun, T. Gao and P. Yan, Aggregation-Induced White-Light Emission from the Triple-Stranded Dinuclear Sm(III) Complex, Dalton Trans., 2014, 43, 12228–12235 RSC.
- Y. Luo, K. Zhang, Z. Ding, P. Chen, X. Peng, Y. Zhao, K. Chen, C. Li, X. Zheng, Y. Huang, X. Pu, Y. Liu, S.-J. Su, X. Hou and Z. Lu, Ultra-Fast Triplet-Triplet-Annihilation-Mediated High-Lying Reverse Intersystem Crossing Triggered by Participation of nπ*-Featured Excited States, Nat. Commun., 2022, 13, 6892 CrossRef CAS.
- A. H. Shelton, I. V. Sazanovich, J. A. Weinstein and M. D. Ward, Controllable Three-Component Luminescence from a 1,8-Naphthalimide/Eu(III) Complex: White Light Emission from a Single Molecule, Chem. Commun., 2012, 48, 2749–2751 RSC.
- M. Latva, H. Takalo, V.-M. Mukkala, C. Matachescu, J. C. Rodriguez-Ubis and J. Kankare, Correlation Between the Lowest Triplet State Energy Level of the Ligand and Lanthanide(III) Luminescence Quantum Yield, J. Lumin., 1997, 75, 149–169 CrossRef CAS.
- F. J. Steemers, W. Verboom, D. N. Reinhoudt, E. B. van der Tol and J. W. Verhoeven, New Sensitizer-Modified Calix[4]arenes Enabling Near-UV Excitation of Complexed Luminescent Lanthanide Ions, J. Am. Chem. Soc., 1995, 117, 9408–9414 CrossRef CAS.
- A. T. O'Neil, A. Chalard, J. Malmström and J. A. Kitchen, White Light and Colour-Tunable Emission from a Single Component Europium-1,8-Naphthalimide Thin Film, Dalton Trans., 2023, 52, 2255–2261 RSC.
- J. Zhang, H. Li, P. Chen, W. Sun, T. Gao and P. Yan, A New Strategy for Achieving White-Light Emission of Lanthanide Complexes: Effective Control of Energy Transfer from Blue-Emissive Fluorophore to Eu(III) Centres, J. Mater. Chem. C, 2015, 3, 1799–1806 RSC.
- C. Chen, X. Pang, Y. Li and X. Yu, Dual Lewis Acid- and Base-Responsive Terpyridine-Based Hydrogel: Programmable and Spatiotemporal Regulation of Fluorescence for Chemical-Based Information Security, Inorg. Chem., 2023, 62, 2105–2115 CrossRef CAS PubMed.
- X. Yuan, E. Cui, K. Liu, F. Artizzu, X. Liao, J. Zhao, J. Tang, J. Liu and Y. Liu, Triple-Mode upconversion emission for dynamic multicolor luminescent anti-Counterfeiting, J. Colloid Interface Sci., 2023, 641, 961–971 CrossRef CAS PubMed.
- X. Xu, J. Wang and B. Yan, Facile Fabrication of Luminescent Eu(III) Functionalized HOF Hydrogel Film with Multifunctionailities: Quinolones Fluorescent Sensor and Anticounterfeiting Platform, Adv. Funct. Mater., 2021, 31, 2103321 CrossRef CAS.
- Z. Li, X. Liu, G. Wang, B. Li, H. Chen, H. Li and Y. Zhao, Photoresponsive Supramolecular Coordination Polyelectrolyte as Smart Anticounterfeiting Inks, Nat. Commun., 2021, 12, 1363 CrossRef CAS PubMed.
- Q.-S. Zhang, S.-C. Wang, X.-H. Xiong, P.-Y. Fu, X.-D. Zhang, Y.-N. Fan and M. Pan, High-Temperature and Dynamic RGB (Red–Green–Blue) Long–Persistent Luminescence in an Anti–Kasha Organic Compound, Angew. Chem., Int. Ed., 2022, 134, e202205556 CrossRef.
- Y. Zhou, H.-Y. Zhang, Z.-Y. Zhang and Y. Liu, Tunable Luminescent Lanthanide Supramolecular Assembly Based on Photoreaction of Anthracene, J. Am. Chem. Soc., 2017, 139, 7168–7171 CrossRef CAS PubMed.
- D. Zhao, J. Yang, X. Tian, J. Wei, Q. Li and Y. Wang, Self-Healing Metallo-Supramolecular Polymers Showing Luminescence OFF/ON Switching Based on Lanthanide Ions and Terpyridine Moieties, Chem. Eng. J., 2022, 434, 134806 CrossRef CAS.
- Y. Ma, F. Yu, S. Zhang, P. She, S. Liu, W. Huang and Q. Zhao, In Situ Control of Multicolor Luminescence over the Entire Visible Spectral Region in a Single Chromophore by Sol–Gel Transformation and Dynamic Metal–Ligand Coordination, CCS Chem., 2020, 2, 2437–2444 Search PubMed.
- Y. Sheng, J. Ma, S. Liu, Y. Wang, C. Zhu and Y. Cheng, Strong and Reversible Circularly Polarized Luminescence Emission of a Chiral 1,8-Naphthalimide Fluorophore Induced by Excimer Emission and Orderly Aggregation, Chem. – Eur. J., 2016, 22, 9519–9522 CrossRef CAS PubMed.
- Y. Zhang, Y. Su, H. Wu, Z. Wang, C. Wang, Y. Zheng, X. Zheng, L. Gao, Q. Zhou, Y. Yang, X. Chen, C. Yang and Y. Zhao, Large-Area, Flexible, Transparent, and Long-lived Polymer-Based Phosphorescence Films, J. Am. Chem. Soc., 2021, 143, 13675–13685 CrossRef CAS PubMed.
- Q. Lin, X.-W. Guan, Y.-M. Zhang, J. Wang, Y.-Q. Fan, H. Yao and T.-B. Wei, Spongy Materials Based on Supramolecular Polymer Networks for Detection and Separation of Broad-Spectrum Pollutants, ACS Sustainable Chem. Eng., 2019, 7, 14775–14784 CrossRef CAS.
- X. Shan, W. Chi, H. Jiang, Z. Luo, C. Qian, H. Wu and Y. Zhao, Monomer and Excimer Emission in a Conformational and Stacking–Adaptable Molecular System, Angew. Chem., 2023, 135, e202215652 CrossRef.
- W. Chen, R. Fan, H. Zhang, Y. Dong, P. Wang and Y. Yang, Tunable White-Light Emission PMMA-Supported Film Materials Containing Lanthanide Coordination Polymers: Preparation, Characterization, and Properties, Dalton Trans., 2017, 46, 4265–4277 RSC.
- M. Yuan, Q. Tang, Y. Lu, Z. Zhong, X.-H. Li, S.-M. Liu, X.-W. Sun and S.-X. Liu, Using the Luminescence and Ion Sensing Experiment of a Lanthanide Metal−Organic Framework to Deepen and Extend Undergraduates’ Understanding of the Antenna Effect, J. Chem. Educ., 2019, 96, 1256–1261 CrossRef CAS.
- D. W. R. Balkenende, S. Coulibaly, S. Balog, Y. C. Simon, G. L. Fiore and C. Weder, Mechanochemistry with Metallosupramolecular Polymers, J. Am. Chem. Soc., 2014, 136, 10493–10498 CrossRef CAS PubMed.
- J. Wang, Y. Li, X. Li, J. Pan, D. Wang, S. Wei, C. Wang and J. Li, Energy Transfer Mechanism of Carboxymethyl Chitosan-Eu3+/Tb3+ Complex Materials and Application in Multicolor LED, Carbohydr. Polym., 2023, 315, 120981 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi01071f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2023 |