Photocatalytic water oxidation over LaWO0.6N2.4 mesoporous single crystals under visible and near-infrared light illumination†
Lin
Yang
,
Hui
Duan
* and
Xiaoxiang
Xu
 *
*
Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, Shanghai, 200092, China. E-mail: dhui@tongji.edu.cn; xxxu@tongji.edu.cn; Tel: +86-21-65982670 Tel: +86-21-65986919
First published on 26th June 2023
Abstract
Narrow-bandgap perovskite oxynitrides emerge as a promising class of inorganic photocatalysts to store solar energy in chemical fuels. However, conventional synthetic routes generally introduce a high defect concentration in these compounds, particularly at grain boundaries (GBs), which also intercept charge transportation, thus severely undermining the photocatalytic performance. Herein, we demonstrate that GB-free porous single crystals (PSCs) of narrow bandgap semiconductor LaWO0.6N2.4 can be prepared by the topotactic conversion of BiLaWO6. Due to a high structural homogeneity and porosity, LaWO0.6N2.4 PSCs deliver a good photocatalytic activity for oxidizing water into O2 even under near-infrared light illuminations. Under optimal conditions, an apparent quantum efficiency (AQE) value as high as 0.13% at 800 ± 20 nm were achieved, being the first near-infrared-light active oxynitride for photocatalytic water oxidation thus far. Steady overall water splitting into stoichiometric H2 and O2 has also been realized in a Z-scheme system employing LaWO0.6N2.4 PSCs as the O2-evolution moiety under visible light insolation. These results not only justify that PSCs serve as an ideal platform to trigger the photocatalytic performance of oxynitrides with high defects content but also attract great attention upon W-based perovskite oxynitrides for solar energy conversions.
Introduction
Water cleavage over particulate photocatalysts offers a promising means to store intermittent solar energy into hydrogen energy, which is the ideal fuel for the future.1–5 To this end, narrow bandgap semiconductors that can harvest a significant portion of the solar spectrum, i.e., visible to near infrared, are strongly desired.6–12 However, narrow bandgap semiconductors can generally produce low energetic photocarriers; thereby, it is very challenging to drive the uphill-type water splitting reactions.13,14 For instance, a semiconductor with light absorption threshold of 800 nm can only produce photocarriers with an energy gap of 1.55 eV, being very close to the minimal demand of 1.23 eV for overall water splitting reactions. Thus, it becomes imperative to minimize energy loss during photocarrier transportation and transfer steps over narrow bandgap semiconductors for photocatalytic water splitting reactions.Among various narrow bandgap semiconductors reported, d0-type transition metal perovskite oxynitrides, i.e., AM(O,N)3 (A = Ca, Sr, Ba, and La; M = Ti, Nb, Ta, and W), have gained considerable attention as photocatalysts for water splitting owing to their intensive visible light absorption, chemical robustness, and photocatalytic activity for water redox reactions.15–18 In particular, LaWO0.6N2.4 shows strong light absorption from visible to near infrared region as an ideal photocatalyst to convert solar energy.19,20 This is partly attributed to its high N/O ratio that substantially uplifts the valence band top, thereby reducing the threshold for light harvest. Notwithstanding such appealing properties, LaWO0.6N2.4 is subject to poor photocatalytic activities.21 This can be understood by the high electronegativity of W6+ that is susceptible to induce a high concentration of defects (W5+, W4+etc.) during high-temperature ammonolysis. These defects have been widely recognized as charge trapping and recombination centers that greatly undermine the photocatalytic activity.18,22,23 Things become even worse as powdery LaWO0.6N2.4 often contains copious grain boundaries (GBs). These GBs not only are enriched with defects but also serve as interceptions to the photocarrier transportation across neighboring particles.24–29 Accordingly, it implicates a useful strategy to upgrade the photocatalytic performance of LaWO0.6N2.4 by eliminating the GBs as well as defects.
In general, LaWO0.6N2.4 is prepared by high-temperature ammonolysis using shapeless precursor powders of La2W2O9, La4W3O15 and amorphous metal oxides.19–21 The irregular precursor particles with random exposure of crystal facets are characterized with uncontrolled grain growth during high-temperature ammonolysis, resulting in abundant GBs in the product. Seeking alternative precursors with specific exposure of crystal facets would be a useful means to regulate grain growth as well as to avoid GBs. We have previously used Sillén–Aurivillius type oxyhalides as the precursors to prepare porous single crystals (PSCs) of perovskite oxynitrides that are free of GBs.30–33 In this work, we adopt a layered compound BiLaWO6 as the precursor to synthesize LaWO0.6N2.4 PSCs for the first time. BiLaWO6 contains perovskite blocks and can be topotactically transformed into LaWO0.6N2.4 during high-temperature ammonolysis. The as-prepared LaWO0.6N2.4 PSCs do not contain GBs and are active to drive water oxidation reactions under visible and even near infrared light illumination. Its potential as a water splitting photocatalyst is also exemplified by a Z-scheme overall water splitting system that employs LaWO0.6N2.4 PSCs as the O2-evolution moiety.
Experimental
Materials synthesis
Materials characterization
The freshly prepared sample powders were subject to a series of analysis to understand their physical and chemical properties. X-ray powder diffraction (XRD) analysis was conducted on a Bruker D8 Focus diffractometer (Bruker, Germany) to check the phase purity and crystal structure using Cu Kα1 (λ = 1.5406 Å) and Cu Kα2 (λ = 1.5444 Å) as the incident radiation. The general step size and duration time for data collection were set as 0.01 and 0.1 s at each step, respectively. The XRD data collected were used for Rietveld refinement based on General Structure Analysis System (GSAS) software package to investigate the crystal structure.35 A pseudo-Voigt function was adopted for profile fitting and the first type Chebyshev polynomial was applied for background fitting. The oxygen and nitrogen content in sample powders were determined through an Elemental Analyzer (Unicube, Elementar, Germany). The cations in the samples were quantified by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 720ES). Sample powders were further inspected under a field emission scanning electron microscope (Hitachi S4800, Japan) equipped with an energy-dispersive X-ray spectroscopy (EDS) analysis system and a transmission electron microscope (TEM, JEOL JEM-2100, Japan). The Raman spectra of the as-obtained sample powders were collected on a UV Raman spectrometer (Renishaw inVia). The specific surface area of the sample powders was calculated according to the data collected on a NOVA 2200e adsorption instrument (Quantachrome, USA) using the Brunauer–Emmett–Teller (BET) model. Ultraviolet-visible-near infrared diffuse reflectance spectra (UV-Vis-NIR DRS) were collected on a UV-Vis-NIR spectrophotometer (Agilent Cary 5000) equipped with an integrating sphere. The non-absorbing reference material was BaSO4. An X-ray photoelectron spectrometer (XPS, AXIS Ultra DLD, Japan) with a monochromatic Al Kα X-ray source was used to examine the surface state of the sample powders. The adventitious carbon C 1s peak at 284.7 eV was adopted for signal adjustment.36 XPS PEAKFIT software was used for XPS data fitting. The fitting was performed by referring to Gaussian–Lorentzian (Lorentzian weighting of 20%) type peak shape and a Shirley-type background. Mott–Schottky (MS) experiments were conducted on a Zahner electrochemical workstation based on a three-electrode configuration setup. The working electrodes were fabricated through the electrophoretic deposition method using fluorine-doped tin oxide (FTO) glasses as the conductive substrate.37 The prepared photoelectrode, Pt foil (10 × 10 mm), and Ag/AgCl electrode (saturated KCl) were used as the working, counter, and reference electrodes, respectively. Aqueous KOH solution (0.1 M, pH = 13) was applied as the electrolyte. Flat band potentials were obtained by extracting the capacitance data from the electrochemical impedance spectra (EIS) at 500, 1000, and 2000 Hz within a potential window of −0.3 to 0.8 V vs. RHE.Photocatalytic water splitting
Photocatalytic water-splitting experiments were conducted in a top-irradiation-type reactor connected to a gas-closed circulation and evacuation system (Labsolar-6A, Perfect Light). In brief, proper amounts of CoOx were thermally loaded onto sample powders as a cocatalyst by referring to a previous report.15 Subsequently, 0.1 g sample powders loaded with CoOx cocatalyst and 0.2 g La2O3 (pH buffer, Aladdin, 99.9%) were dispersed into 100 mL silver nitrate aqueous solution (0.05 M) under constant magnetic stirring. The so-formed suspensions were transferred into the reactor. The reactor temperature was maintained at 281 K using a water jacket. After evacuation for 45 min to remove the dissolved air, the reactor was illuminated from the top by a 300 W xenon lamp (PLX-SXE300, Perfect Light) coupled with either a UV (λ ≥ 420 nm) or visible light (λ ≥ 800 nm) cutoff filter. For the determination of apparent quantum efficiency (AQE), bandpass filters at 420 nm, 450 nm, 500 nm, 550 nm, 600 nm, 700 nm, and 800 nm (Perfect Light) were respectively used to obtain monochromatic light. The photon flux was quantitatively determined by a quantum meter (Apogee MP-300, USA). The gas component within the reactor was analyzed by an on-line gas chromatograph (GC2014C, SHIMADZU, Japan) installed with a 5 Å molecular sieve column, TCD detector, and Ar as the carrier gas. The AQE for oxygen production was calculated based on the following equation (eqn (1)). | (1) |
For Z-scheme water splitting, 50 mg Ru-loaded SrTiO3:Rh and 50 mg CoOx-loaded LaWO0.6N2.4 were ultrasonically dispersed into 100 mL FeCl3 aqueous solution (2 mM). Several drops of HCl aqueous solution (0.1 M) were added to adjust the pH value to about 2.5. Pre-illumination lasted for about 1 h was conducted before overall water splitting experiment, which induces the formation of Fe2+/Fe3+ redox couple by the partial conversion of Fe3+ to Fe2+. The Z-scheme water splitting experiments were performed similar to previous experiments using a 300 W xenon lamp (PLX-SXE300, Perfect Light) coupled with a UV cutoff filter (λ ≥ 420 nm).
Results and discussion
Crystal structures and morphologies
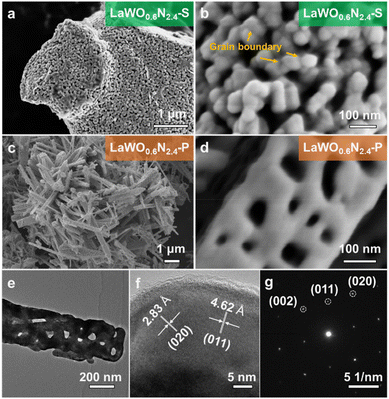
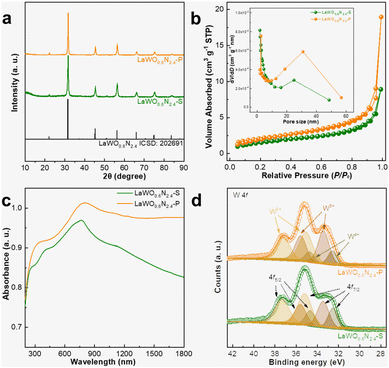
The precursor BiLaWO6 is an isostructural compound to Bi2WO6, an archetypal Aurivillius compound with the general formula [Bi2O2][An−1BnO3n+1] (n = 1). The structure of BiLaWO6 comprises alternative stacking of [Bi(La)2O2]2+ fluorite layer and [WO4]2− perovskite layer.38,39 WO6 polyhedrons in the BiLaWO6 are connected in a corner-type manner, which is topotactically similar to those of LaWO0.6N2.4 (Fig. 1b). X-ray powder diffraction (XRD) analysis and corresponding Rietveld refinement confirm the successful preparation of LaWO0.6N2.4 using BiLaWO6 as the precursor (Fig. 1a, b, Fig. S1 and Table S1†). The possible transformation mechanism is proposed as follows: BiLaWO6 undergoes gradual Bi loss during the high-temperature ammonolysis since Bi3+ cations are susceptible to reduction into elemental Bi, which subsequently evaporates at high temperatures. The residual perovskite blocks then stack facilely without substantial atom migrations/rearrangements to form LaWO0.6N2.4 (Fig. 1c). As no long-range migration of La and W atoms are involved during transformation, the product LaWO0.6N2.4 particles can retain the morphologies of the precursor. This is evidently supported by the field-emission scanning electron microscopic (FE-SEM) characterization (Fig. 1d, e, and Fig. S2†). As can be seen from the FE-SEM images, the ribbon-like morphologies of BiLaWO6 are well-maintained in the product LaWO0.6N2.4, thus verifying our proposed mechanisms. The high porosity of LaWO0.6N2.4 particles can be attributed to the evaporation of Bi as well as O/N replacements during high-temperature ammonolysis. The product LaWO0.6N2.4 is then denoted as LaWO0.6N2.4-P to be discriminated from the one produced from La2W2O9 (denoted as LaWO0.6N2.4-S).Although LaWO0.6N2.4 can be produced from different precursors (Fig. 3a, Fig. S3 and Table S2†), their microstructures are distinct and are strongly correlated with the precursors used. The LaWO0.6N2.4-S powders contain irregularly-shaped grains with a size of ∼50 nm and are randomly compacted to form bulky particles as large as several microns (Fig. 2a). Although LaWO0.6N2.4-S powders are porous, GBs between neighbouring grains can be easily identified (Fig. 2b). This is in sharp contrast to LaWO0.6N2.4-P, which contains no GBs (Fig. 2c–e). This is also confirmed by TEM analysis. As can be seen from Fig. 2e, a single LaWO0.6N2.4-P particle contains three-dimensionally interconnected pores and is lacking of GBs in its skeletons. The single LaWO0.6N2.4-P particle is of high crystallinity whose crystal structure is coherent throughout the entire particle, as revealed by the high-resolution TEM image (HRTEM) (Fig. 2f) and selected area electron diffraction (SAED) pattern (Fig. 2g). The high porosity of both LaWO0.6N2.4-P and LaWO0.6N2.4-S is further confirmed by BET analysis, which shows hysteresis between the desorption and adsorption profiles (Fig. 3b). The pore size distribution analysis (Fig. 3b inset and Table S3†) indicates that most pores fall into the region of mesopores (<50 nm). These results jointly suggest that LaWO0.6N2.4 PSCs can be successfully prepared from BiLaWO6via the topotactic transformation mechanisms.
Surface state and UV-Vis-NIR spectra
Given the distinct morphologies between LaWO0.6N2.4-S and LaWO0.6N2.4-P, we continue our exploration on their optical and surface properties. The UV-Vis-NIR DRS spectra of LaWO0.6N2.4-S and LaWO0.6N2.4-P powders are illustrated in Fig. 3c. Both compounds exhibit intense absorption in the measured range from 250 nm to 1800 nm, which is consistent with the black color of the sample powders. In particular, LaWO0.6N2.4-P appears to have stronger light absorption than LaWO0.6N2.4-S although their structure and composition are essentially identical. The slightly higher light absorption of LaWO0.6N2.4-P might be rationalized by its higher porosity than LaWO0.6N2.4-S, which favors more light reflection in the inner part of the particle. The bandgap is then deduced by a modified Tauc plot analysis method, which suggests a light absorption threshold of ∼1.2 eV of both compounds (Fig. S4†), consistent with the value reported.19,21 The intense absorption above 1050 nm is therefore assignable to various types of defect absorption, which is frequently noticed for oxynitrides. The existence of high concentration of defects is also indicated by XPS analysis shown in Fig. 3d, Fig. S5, and Table S4.† Specifically, the W 4f state contains three distinct spin–orbit pairs assignable to the W6+, W5+, and W4+ species.19 Nevertheless, there is slight variation in the content of these species between LaWO0.6N2.4-S and LaWO0.6N2.4-P. For instance, LaWO0.6N2.4-P has a high W5+ content (41.5%) while LaWO0.6N2.4-S is enriched with W4+ (20.7%). These differences might be related to the different precursors used for synthesis. BiLaWO6 might serve partially as a sacrificial agent due to the reduction of Bi3+, which helps to reduce the risks of W6+ reduction.Photocatalytic performance and band edge positions
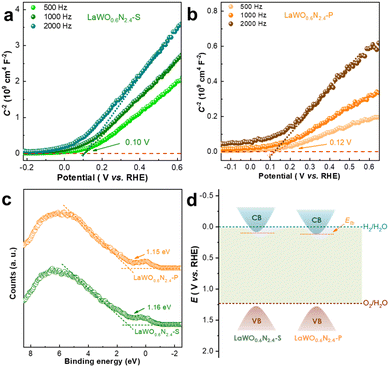
The photocatalytic performance of LaWO0.6N2.4-P and LaWO0.6N2.4-S was then evaluated by comparing their O2-evolution activity using visible (λ ≥ 420 nm) and infrared light (λ ≥ 800 nm) illumination. 1 wt% CoOx was thermally deposited as a cocatalyst and AgNO3 was used as the electron scavenger. Control experiments without any key component of either photocatalyst, light sources, or water did not produce any detectable oxygen gases, thereby excluding any O2-evolving spontaneous reactions. Instantaneous oxygen signals were detected when sample powders were illuminated in the presence of AgNO3 aqueous solution, confirming that real photocatalytic processes (Fig. 4 and Fig. S6†). A concomitant N2-evolution was also observed for both samples, which was attributed to photooxidative self-decomposition similar to other oxynitrides.19,40,41 Impressively, LaWO0.6N2.4-P photocatalyzed more than 3-fold O2-evolution when compared with LaWO0.6N2.4-S (Fig. 4a). As both compounds are of high structural and compositional similarity, such improved activity and stability is probably related to the peculiar microstructures of PSCs. More importantly, LaWO0.6N2.4-P is capable of photocatalytic water oxidation into O2 under infrared light illumination (λ ≥ 800 nm), underscoring the capability to substantially extend the useable bandwidth of solar spectrum (Fig. 4b).The photocatalytic activity of LaWO0.6N2.4-P was further optimized by varying the amounts of CoOx cocatalyst deposited (Fig. S6†). 1 wt% CoOx loading delivered the highest activity and was adopted for the determination of apparent quantum efficiency (AQE). The action spectra of LaWO0.6N2.4-P, i.e., AQE vs. wavelength, are illustrated in Fig. 4c, and the data for calculating AQE were tabulated in Table S5.† The LaWO0.6N2.4-P approaches an AQE as high as 0.13% at 800 ± 20 nm, which is the first near-infrared-light active oxynitride for photocatalytic water oxidation thus far. The large deviation of the AQE profile with UV-Vis-NIR spectra can be explained by the defect's high absorption that superimposed the intrinsic light absorption of LaWO0.6N2.4. The repeated photocatalytic test was performed in 0.05 M sodium persulfate aqueous solution, which suggests a durable production of O2 from LaWO0.6N2.4-P (Fig. S7†). The attempts to photocatalyze water reduction into H2 failed using both LaWO0.6N2.4-P and LaWO0.6N2.4-S. This can be explained by the inappropriate conduction band edge alignment of LaWO0.6N2.4, which is more positive than the water reduction potential, as revealed by the band edge analysis (Fig. 5). Mott–Schottky analysis (Fig. 5a and b) combined with XPS valence band scan data (Fig. 5c) suggests that the conduction and valence band edge of LaWO0.6N2.4 set approximately at 0.10 V and 1.3 V vs. RHE. A schematic illustration of band edge position is shown in Fig. 5d.
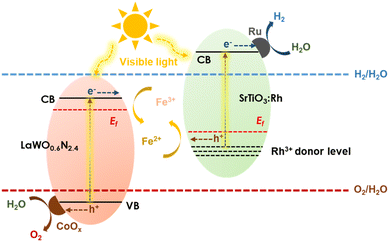
From the band edge alignment, one can quickly realize that LaWO0.6N2.4 cannot be a single-component photocatalyst for overall water splitting but can be an O2-evolution photocatalyst for Z-scheme overall water splitting. As an exemplification, LaWO0.6N2.4-P was combined with Rh-doped SrTiO3 (SrTiO3:Rh), a typical H2-evolution photocatalyst, to fabricate a Z-scheme system (Fig. 6). The so-formed system was capable of overall water splitting into stoichiometric H2/O2 evolution under visible light illumination (λ ≥ 420 nm) in the presence of the Fe2+/Fe3+ redox couple (Fig. 4d). Although XRD and FE-SEM analysis (Fig. S8 and S9†) suggest nearly no structural changes of LaWO0.6N2.4-P before and after photocatalytic experiment, N2 evolution as well as XPS analysis (Fig. S10†) indicate that the following self-decomposition reactions occur.
| 2N3− + 6h+ → N↑2 | (R1) |
| W4+ + h+ → W5+ | (R2) |
| W5+ + h+ → W6+ | (R3) |
The self-decomposition reactions might be related to the poor oxidation power of photogenerated holes in LaWO0.6N2.4 whose valence band edge is close to the water oxidation potential. A more active cocatalyst would be useful to promote water oxidation reactions rather than oxidative self-decomposition reactions and will be our future work.
Conclusion
In summary, a narrow bandgap semiconductor, LaWO0.6N2.4, has been successfully synthesized in the form of porous single crystals (LaWO0.6N2.4 PSCs) from the topotactic conversion of BiLaWO6. Photocatalytic tests suggest that it demonstrates superior activities for catalyzing water oxidation reactions under visible and infrared light illuminations. An AQE value as high as 0.13% at 800 ± 20 nm was recorded under optimal conditions, being the first near-infrared-light active oxynitride for photocatalytic water oxidation thus far. Moreover, its potential application was exemplified by constructing a Z-scheme system employing LaWO0.6N2.4 PSCs as the O2-evolution moiety to achieve steady overall water splitting. This work not only provide us a porous single-crystaline LaWO0.6N2.4 with triggered photocatalytic performance for water oxidation reactions but also throws light on W-based perovskite oxynitrides with narrow bandgaps for solar energy conversions.Author contributions
Mr Lin Yang performed the experiments. Dr Hui Duan analyzed the data. Prof. Xiaoxiang Xu administered the project and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China (Grant No. 51972233 and 52172225) and the Fundamental Research Funds for the Central Universities.References
- M. Thangamuthu, Q. S. Ruan, P. O. Ohemeng, B. Luo, D. W. Jing, R. Godin and J. W. Tang, Polymer photoelectrodes for solar fuel production: progress and challenges, Chem. Rev., 2022, 122, 11778–11829 CrossRef CAS PubMed.
- X. P. Tao, Y. Zhao, S. Y. Wang, C. Li and R. G. Li, Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts, Chem. Soc. Rev., 2022, 51, 3561–3608 RSC.
- T. Takata, J. Z. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Photocatalytic water splitting with a quantum efficiency of almost unity, Nature, 2020, 581, 411–414 CrossRef CAS PubMed.
- Y. B. Chen, X. Y. Feng, Y. Liu, X. J. Guan, C. Burda and L. J. Guo, Metal oxide-based tandem cells for self-biased photoelectrochemical water splitting, ACS Energy Lett., 2020, 5, 844–866 CrossRef CAS.
- S. Ye, C. M. Ding, M. Y. Liu, A. Q. Wang, Q. G. Huang and C. Li, Water oxidation catalysts for artificial photosynthesis, Adv. Mater., 2019, 31, 1902069 CrossRef CAS PubMed.
- Z. Wang, Y. Inoue, T. Hisatomi, R. Ishikawa, Q. Wang, T. Takata, S. S. Chen, N. Shibata, Y. Ikuhara and K. Domen, Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles, Nat. Catal., 2018, 1, 756–763 CrossRef CAS.
- Q. Wang, M. Nakabayashi, T. Hisatomi, S. Sun, S. Akiyama, Z. Wang, Z. H. Pan, X. Xiao, T. Watanabe, T. Yamada, N. Shibata, T. Takata and K. Domen, Oxysulfide photocatalyst for visible-light-driven overall water splitting, Nat. Mater., 2019, 18, 827–832 CrossRef CAS PubMed.
- S. Lin, H. W. Huang, T. Y. Ma and Y. H. Zhang, Photocatalytic oxygen evolution from water splitting, Adv. Sci., 2021, 8, 2002458 CrossRef CAS PubMed.
- R. R. Pan, M. Hu, J. Liu, D. F. Li, X. D. Wan, H. Z. Wang, Y. M. Li, X. M. Zhang, X. L. Wang, J. Jiang and J. T. Zhang, Two-dimensional all-in-one sulfide monolayers driving photocatalytic overall water splitting, Nano Lett., 2021, 21, 6228–6236 CrossRef CAS PubMed.
- H. H. Li, J. D. Xiao, J. J. M. Vequizo, T. Hisatomi, M. Nakabayashi, Z. H. Pan, N. Shibata, A. Yamakata, T. Takata and K. Domen, One-step excitation overall water splitting over a modified Mg-doped BaTaO2N photocatalyst, ACS Catal., 2022, 12, 10179–10185 CrossRef CAS.
- K. H. Chen, J. D. Xiao, J. J. M. Vequizo, T. Hisatomi, Y. W. Ma, M. Nakabayashi, T. Takata, A. Yamakata, N. Shibata and K. Domen, Overall water splitting by a SrTaO2N-based photocatalyst decorated with an Ir-promoted Ru-based cocatalyst, J. Am. Chem. Soc., 2023, 145, 3839–3843 CrossRef CAS PubMed.
- K. Maeda, D. L. Lu and K. Domen, Oxidation of Water under Visible-light irradiation over modified BaTaO2N photocatalysts promoted by tungsten species, Angew. Chem., Int. Ed., 2013, 52, 6488–6491 CrossRef CAS PubMed.
- T. Hisatomi, C. Katayama, Y. Moriya, T. Minegishi, M. Katayama, H. Nishiyama, T. Yamada and K. Domen, Photocatalytic oxygen evolution using BaNbO2N modified with cobalt oxide under photoexcitation up to 740 nm, Energy Environ. Sci., 2013, 6, 3595–3599 RSC.
- J. Seo, D. Ishizuka, T. Hisatomi, T. Takata and K. Domen, Effect of Mg2+ substitution on the photocatalytic water splitting activity of LaMgxNb1−xO1+3xN2−3x, J. Mater. Chem. A, 2021, 9, 8655–8662 RSC.
- F. X. Zhang, A. Yamakata, K. Maeda, Y. Moriya, T. Takata, J. Kubota, K. Teshima, S. Oishi and K. Domen, Cobalt-modified porous single-crystalline LaTiO2N for highly efficient water oxidation under visible light, J. Am. Chem. Soc., 2012, 134, 8348–8351 CrossRef CAS PubMed.
- S. H. Wei, G. Zhang and X. X. Xu, Activating BaTaO2N by Ca modifications and cobalt oxide for visible light photocatalytic water oxidation reactions, Appl. Catal., B, 2018, 237, 373–381 CrossRef CAS.
- Y. W. Wang, Y. Y. Kang, H. Z. Zhu, G. Liu, J. T. S. Irvine and X. X. Xu, Perovskite oxynitride solid solutions of LaTaON2-CaTaO2N with greatly enhanced photogenerated charge separation for solar-driven overall water splitting, Adv. Sci., 2021, 8, 2003343 CrossRef CAS PubMed.
- Y. W. Wang, S. H. Wei and X. X. Xu, SrTaO2N-CaTaO2N solid solutions as efficient visible light active photocatalysts for water oxidation and reduction, Appl. Catal., B, 2020, 263, 118315 CrossRef CAS.
- K. Kawashima, M. Hojamberdiev, H. Wagata, E. Zahedi, K. Yubuta, K. Domen and K. Teshima, Two-step synthesis and visible-light-driven photocatalytic water oxidation activity of AW(O,N)3 (A = Sr, La, Pr, Nd and Eu) perovskites, J. Catal., 2016, 344, 29–37 CrossRef CAS.
- W. J. Li, D. Li, X. Gao, A. Gurlo, S. Zander, P. Jones, A. Navrotsky, Z. J. Shen, R. Riedel and E. Ionescu, A study on the thermal conversion of scheelite-type ABO4 into perovskite-type AB(O,N)3, Dalton Trans., 2015, 44, 8238–8246 RSC.
- K. Kawashima, Y. Liu, J. H. Kim, B. R. Wygant, I. Cheng, H. Celio, O. Mabayoje, J. Lin and C. B. Mullins, Infrared light-driven LaW(O,N)3 OER photoelectrocatalysts from chloride flux-grown La4W3O15 templating precursors, ACS Appl. Energy Mater., 2019, 2, 913–922 CrossRef CAS.
- J. D. Xiao, J. J. M. Vequizo, T. Hisatomi, J. Rabeah, M. Nakabayashi, Z. Wang, Q. Xiao, H. H. Li, Z. H. Pan, M. Krause, N. Yin, G. Smith, N. Shibata, A. Bruckner, A. Yamakata, T. Takata and K. Domen, Simultaneously tuning the defects and surface properties of Ta3N5 nanoparticles by Mg-Zr codoping for significantly accelerated photocatalytic H2 evolution, J. Am. Chem. Soc., 2021, 143, 10059–10064 CrossRef CAS PubMed.
- Y. Q. Xiao, C. Feng, J. Fu, F. Z. Wang, C. L. Li, V. F. Kunzelmann, C. M. Jiang, M. Nakabayashi, N. Shibata, I. D. Sharp, K. Domen and Y. B. Li, Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation, Nat. Catal., 2020, 3, 932–940 CrossRef CAS.
- J. Fu and S. E. Skrabalak, Enhanced Photoactivity from Single-crystalline SrTaO2N nanoplates synthesized by topotactic nitridation, Angew. Chem., Int. Ed., 2017, 56, 14169–14173 CrossRef CAS PubMed.
- S. S. Chen, T. Takata and K. Domen, Particulate photocatalysts for overall water splitting, Nat. Rev. Mater., 2017, 2, 17050 CrossRef CAS.
- M. Xiao, B. Luo, M. Q. Lyu, S. C. Wang and L. Z. Wang, Single-crystalline nanomesh tantalum nitride photocatalyst with improved hydrogen-evolving performance, Adv. Energy Mater., 2018, 8, 1701605 CrossRef.
- C. A. Geiger, M. Grodzicki and G. Amthauer, The crystal chemistry and Fe-II-site properties of aluminosilicate garnet solid solutions as revealed by Mossbauer spectroscopy and electronic structure calculations, Phys. Chem. Miner., 2003, 30, 280–292 CrossRef CAS.
- C. Funke, T. Behm, R. Helbig, E. Schmid and S. Wurzner, Novel combination of orientation measurements and transmission microscopy for experimental determination of grain boundary miller indices in silicon and other semiconductors, J. Microsc., 2012, 246, 70–76 CrossRef CAS PubMed.
- L. Jin, F. Y. Cheng, H. Li and K. Xie, Porous tantalum nitride single crystal at two-centimeter scale with enhanced photoelectrochemical performance, Angew. Chem., Int. Ed., 2020, 59, 8891–8895 CrossRef CAS PubMed.
- L. Yang, Q. Y. Fu, L. N. Wang, J. X. Yu and X. X. Xu, Liberating photocarriers in mesoporous single-crystalline SrTaO2N for efficient solar water splitting, Appl. Catal., B, 2022, 304, 120934 CrossRef CAS.
- S. F. Chang, J. X. Yu, R. Wang, Q. Y. Fu and X. X. Xu, LaTaON2 mesoporous single crystals for efficient photocatalytic water oxidation and Z-scheme overall water splitting, ACS Nano, 2021, 15, 18153–18162 CrossRef CAS PubMed.
- L. Yang, J. X. Yu, Q. Y. Fu, L. L. Kong and X. X. Xu, Mesoporous single-crystalline SrNbO2N: expediting charge transportation to advance solar water splitting, Nano Energy, 2022, 95, 107059 CrossRef CAS.
- J. X. Yu and X. X. Xu, LaNbON2 mesoporous single crystals with expedited photocarrier separation for efficient visible-light-driven water redox reactions, J. Catal., 2022, 413, 858–869 CrossRef CAS.
- C. W. Dong, S. Y. Lu, S. Y. Yao, R. Ge, Z. D. Wang, Z. Wang, P. F. An, Y. Liu, B. Yang and H. Zhang, Colloidal synthesis of ultrathin monoclinic BiVO4 nanosheets for Z-scheme overall water splitting under visible light, ACS Catal., 2018, 8, 8649–8658 CrossRef CAS.
- B. H. Toby, EXPGUI, a graphical user interface for GSAS, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- R. Hesse, M. Weiss, R. Szargan, P. Streubel and R. Denecke, Improved peak-fit procedure for XPS measurements of inhomogeneous samples-Development of the advanced Tougaard background method, J. Electron Spectrosc. Relat. Phenom., 2015, 205, 29–51 CrossRef CAS.
- R. Abe, M. Higashi and K. Domen, Facile Fabrication of an efficient oxynitride TaON photoanode for overall water splitting into H2 and O2 under visible light irradiation, J. Am. Chem. Soc., 2010, 132, 11828–11829 CrossRef CAS PubMed.
- A. Watanabe, Polymorphism in Bi2WO6, J. Solid State Chem., 1982, 41, 160–165 CrossRef CAS.
- A. Watanabe, Synthesis and lattice-parameters of rare-earth bismuth tungstates, BiLnWO6 and their solid-solutions, Mater. Res. Bull., 1980, 15, 1473–1477 CrossRef CAS.
- A. Kasahara, K. Nukumizu, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, LaTiO2N as a visible-light (≤600 nm)-driven photocatalyst (2), J. Phys. Chem. B, 2003, 107, 791–797 CrossRef CAS.
- S. H. Wei and X. X. Xu, Boosting photocatalytic water oxidation reactions over strontium tantalum oxynitride by structural laminations, Appl. Catal., B, 2018, 228, 10–18 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns, SEM-EDS analyses; Raman spectra; Tauc plot analysis; XPS spectra; photocatalytic data; refined unit cell parameters and bandgap values; elemental compositions; BET analysis. See DOI: https://doi.org/10.1039/d3qi00924f |
| This journal is © the Partner Organisations 2023 |