A two-dimensional luminescent HOF containing an interpenetrating network structure with dual function: highly sensitive detection of methotrexate and multi-level information encryption†
Chunyu
Yang
,
Xin
Xu
 and
Bing
Yan
and
Bing
Yan
 *
*
School of Chemical Science and Engineering, Tongji University, Siping Road 1239, Shanghai 200092, China. E-mail: byan@tongji.edu.cn
First published on 12th April 2023
Abstract
In recent years, hydrogen-bonded organic frameworks (HOFs) have been used in fluorescence sensing and anti-counterfeiting applications because of their mild preparation conditions, excellent optical properties and biocompatibility, but dual-functional HOFs based on the same response mechanism have rarely been reported. Methotrexate (MTX) is a broad-spectrum anti-tumor drug. However, as the therapeutic window for MTX is narrow in the actual treatment process, it is particularly critical to monitor therapeutic drug concentrations in the human body to ensure accurate dosing. This paper reports a luminescent HOF with imidazole groups containing a two-dimensional interpenetrating structural network. It has favorable water stability and therefore holds promise as a fluorescent probe for the detection of methotrexate levels in humans. HOF–TIA prepared by a simple solvent volatilization method enables rapid and highly sensitive detection of MTX. The detection limit of MTX was 0.022 μM in aqueous solution and 0.061 μM in serum. The detection mechanisms of HOF–TIA toward MTX and adenosine monophosphate (AMP) were investigated and the color change phenomenon of HOF–TIA on encountering MTX and AMP was applied to the multi-level encryption of information. This study has not only produced a new HOF containing imidazole groups but also provided a typical example of a dual-function optical platform synchronously applied for fluorescence sensing and anti-counterfeiting.
Introduction
Hydrogen-bonded organic frameworks (HOFs) are a type of porous crystalline solid composed of organic or metal–organic building blocks that are linked together by weak hydrogen bonds, electrostatic interactions, π–π interactions, and van der Waals force interactions.1 HOFs have been widely used in chemical sensing,2,3 anti-counterfeiting,4 bioimaging,5 gas storage and separation,6–8 catalysis,9 proton conduction,10–12etc. Hydrogen bonds are weak and reversible in comparison to covalent and coordination bonds. Thus, HOF materials have certain distinct advantages, such as softer preparation conditions,13 better solution processing performance,14 and greater biocompatibility.15 In the majority of HOF cases, the carboxyl and amino groups on organic building blocks promote the formation of hydrogen bonds, however, reports of HOFs relying on heterocyclic groups to establish hydrogen bonds are rare. In addition, for luminescent HOFs, strong hydrogen bonding interactions, and dense-stacking are advantageous in lowering non-radiative transitions. Luminescent HOFs (LHOFs) typically possess chromophores with distorted structures, which can demonstrate aggregation-induced emission characteristics and avoid the aggregation-caused quenching (ACQ) effect.16 Besides that, some LHOFs can exploit additional hydrogen bond motif's high space resistance to lessen the building blocks’ ACQ effect successfully.17 Hence, the preparation of LHOFs with a new structure remains a major challenge.Methotrexate (MTX) has shown superior efficacy in osteosarcoma and breast cancer.18 In the field of systemic lupus erythematosus, dermatomyositis, and other rheumatic diseases, MTX also has a pivotal position.19 As a folate reductase inhibitor, MTX mainly hinders dihydrofolate reductase, impeding the conversion of dihydrofolate to tetrahydrofolate, which ultimately accounts for the inhibition of the transfer of the one-carbon group in DNA biosynthesis.20 Nevertheless, ingesting MTX sometimes results in toxic side effects such as myelosuppression and pulmonary, renal, and hepatic disorders.21 To ensure minimal toxic effects, careful monitoring of MTA in serum is particularly essential in clinics and pharmaceutics. To date, approaches like luminescence sensing,22–24 electrochemistry,25 liquid chromatography-tandem mass spectrometry26 and enzyme multiplied immunoassay technique27 have been developed, in which the luminescence sensing technique exhibit several advantages: (i) quick and easy to prepare; (ii) low cost and convenient to operate; and (iii) abundant visualization response types, such as color shift, including enhancement and quenching. For instance, Yan's team prepared a Tb3+ functionalized HOF film by a simple electrodeposition method to detect SO2 content in the ratio mode;28 Chen and coworkers developed HOF-20, which can selectively detect aniline content in an aqueous solution by fluorescence enhancement.29 Based on the above advantages of luminescence sensing, it is necessary and feasible to use LHOFs as fluorescent probes for the detection of MTX in various environments.
Governments and businesses worldwide invest massive money, effort, and experience to protect their intellectual property rights from infringement. In the last few years, counterfeit consumer goods have had a considerable negative economic impact. The widespread distribution of counterfeit goods has a negative impact on the economy, health, defense, and many other areas.30 Smart responsive luminescent materials (SRLMs) mostly have adjustable optical properties in response to different species, such as chemical reagents, medicines, pH, temperature, light, etc. From the aspect of information security and anti-counterfeiting technologies, SRLMs exhibit significant applied potential owing to their multiple luminescence responses, which can be utilized to realize multilevel information encryption.31 Additionally, some luminescent materials have visible-range absorption, and the encryption patterns are simpler to decipher in sunlight, impacting the encryption security level. In order to attain higher security for anti-counterfeiting and information storage, it is urgent to explore new-style SRLMs with simple preparation and low cost. The luminescence properties of HOFs make them particularly desirable for chemical methods for anti-counterfeiting, and the key to encrypting and decrypting information is to leverage the change in fluorescence intensity and the displacement of emission peaks by various chemicals. Although HOFs have been reported in fluorescence sensing and chemical anti-counterfeiting, few examples have been published to achieve dual functionality based on the same response mechanism.
Inspired by the above, we prepared an unreported HOF–TIA and tried to develop a dual-functional platform based on new HOF materials, including anticancer drug sensing and a multi-level anti-counterfeiting platform. HOF–TIA with imidazole groups was prepared by the ‘one step’ method via simple solvent evaporation. It was discovered during the investigation of the fluorescence sensing capabilities of HOF–TIA. MTX aqueous solution could greatly quench the fluorescence of HOF–TIA, and the intensity of its emission had good linearity in the concentration range from 5 × 10−4 M to 5 × 10−7 M, with a detection limit of 0.022 μM. Adenosine monophosphate (AMP) in aqueous solution makes the emission peak of HOF–TIA to redshift, and its detection limit was 0.298 μM. The emission intensity of HOF–TIA in AMP solution displayed an excellent linear relationship with the concentration, in the concentration range from 1 × 10−3 M to 3 × 10−6 M. In addition, we explored the fluorescence response mechanism by competitive absorption, photoinduced electron transfer (PET) and exciplex formation. The detection limit in serum was 0.061 μM, demonstrating the feasibility of MTX concentration detection in humans. To further establish the optical application of HOF–TIA, we synthesized an anti-counterfeiting ink using HOF–TIA as the raw material and prepared a digital anti-counterfeiting array and a three-dimensional multi-level anti-counterfeiting platform based on a keypad codebook using the different response mechanisms of HOF–TIA to AMP and MTX. The anti-counterfeiting platform in this instance can be applied to the packaging of MTX drugs as a security label. This work not only constructs a novel easy-to-prepare imidazole-based HOF but also provides a representative and successful case for a HOF to be simultaneously applied for fluorescence sensing and chemical anti-counterfeiting.
Experimental section
Synthesis of HOF–TIA
50 mg of TIA powder was dissolved in methanol (10 mL) by stirring for about 10 min at room temperature. The mixture changed to a faint yellow clarified solution. The solution was then filtered and collected using filter paper and left to evaporate at room temperature while methanol evaporated and the crystals grew slowly. After filtration and drying at room temperature, the HOF–TIA crystals were collected. Yield: 75%.Fluorescence sensing experiments
2 mg of HOF–TIA powder was weighed and placed in a test tube, 10−3 M MTX was added, then the emission spectra were recorded after one minute. Fluorescence detection experiments were carried out using similar methods, and solutions of the same concentration of the interfering substances (urea, glucose, sodium bicarbonate, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, histidine, serine, leucine, lysine, proline, cysteine, and phenylalanine) were prepared to investigate the selectivity of HOF–TIA toward MTX. To determine the DL of MTX, MTX solutions at concentrations of 5 × 10−4, 1 × 10−4, 5 × 10−5, 1 × 10−5, 5 × 10−6, 1 × 10−6, and 5 × 10−7 M were added to test tubes containing 2 mg HOF–TIA, allowed to stand for 1 min, and the corresponding emission spectra were obtained under identical control measurement circumstances. Following the same method, the AMP solution was prepared and its emission spectra were recorded.Serum sample determination
Goat serum and acetonitrile were combined and shaken violently for two minutes before being centrifuged. The supernatant was filtered, collected, and then diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 with deionized water.
20 with deionized water.
Fabrication of the luminescent ink and the anticounterfeiting procedure of encryption and decryption
The luminous ink was manufactured from ethylene glycol (EG), deionized water, and polyvinyl alcohol (PVA). First, 1 g of polyvinyl alcohol was added to 8 mL of water, and the mixture was then heated and agitated at 80 °C until it became transparent. In the second phase, EG (PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG = 5
EG = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)32 was added to the aforementioned solution and vigorously stirred at room temperature for 1 h. Finally, 16 mg of HOF–TIA powder was added to the solution, sonicated for 20 min, and mixed at room temperature until well diffused.
1)32 was added to the aforementioned solution and vigorously stirred at room temperature for 1 h. Finally, 16 mg of HOF–TIA powder was added to the solution, sonicated for 20 min, and mixed at room temperature until well diffused.
Results and discussion
Design and characterization of HOF materials
As already explained, the molecular units assemble HOFs through hydrogen bonding interactions. Most organic molecular ligands include amide, carboxylic acid, sulfonic acid, pyridine, urea and pyrazole groups. The imidazole groups found in the monomer tris-(4-imidazolyl phenyl) amine (TIA), which was chosen for this study, can stimulate the formation of HOFs. By simply evaporating the transparent aqueous solution of TIA, HOF–TIA was produced. The primary building block of HOF–TIA is the TIA molecule, which comprises three imidazole groups and three benzene rings in distinct planes. There were intermolecular hydrogen bonds O1–H1⋯N1 and O3–H3⋯O1 between the crystalline HOF–TIA system, which had angles of 172.25° and 173.64°, respectively (Fig. 1a). In addition, the three imidazoles of each monomer that form a hydrogen bond are not coplanar; their angles, respectively, are 5.154°, 69.865°, and 64.635° (Fig. 1b). Each TIA molecule was linked to other two TIA molecules via hydrogen bonds with H2O (Fig. 1c), which are interspersed with one another to create a 2-dimensional (2D) layer along the a axis and arranged in the ABAB mode (Fig. 1d). There are perfectly aligned micropores along this route, with pore sizes of approximately 4.31 × 3.55 Å2 (Fig. 1e). Additionally, it is visible that adjacent TIA molecular layers have opposing molecular stacking patterns and that 2D layers are placed in parallel to produce a three-dimensional (3D) structure along the a axis (Fig. S1†). The pores generated by three TIA molecules with the same arrangement are crossed by a molecule with a different arrangement, which is another indication of the structural characteristics of HOF–TIA (Fig. S2†).The chemical properties of HOF–TIA have also been investigated and recorded. It is clear from the powder X-ray diffraction (PXRD) comparison diagram that the theoretical and experimental materials’ diffraction peak positions are consistent. This indicates that HOF–TIA has been effectively manufactured (Fig. S3†). HOF–TIA has advantageous water stability, and its frame can be immersed in water for 48 h without being destroyed (Fig. S4†). In various pH aqueous solutions (ranging from 3 to 11), HOF–TIA can also maintain structural integrity (Fig. S5†). In the infra-red (IR) spectrum of HOF–TIA, there is a wide absorption band at 3120 cm−1, which is caused by the C–H stretching vibration of the benzene ring on TIA, and a strong absorption peak at 1515 cm−1, which is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N skeleton stretching vibration of the imidazole group in TIA. Besides that, near 827 cm−1 is the absorption peak of the C–H out-of-plane bending vibration in the para-disubstituted benzene ring of the TIA molecule, and certain absorption peaks in the region of 1309–1220 cm−1 may be caused by the N–H stretching vibration peak of tert-N (Fig. S6†). Besides, the thermal stability of HOF–TIA was determined by the thermogravimetric (TG) curve. Fig. S7† suggests that the HOF–TIA molecular fragment starts to decompose at about 350 °C. The pore size distribution graph (Fig. S8†) shows that the pore size of HOF–TIA is mainly concentrated within 1–2 nm. It confirms that HOF–TIA is a porous framework material with predominantly microporous pores.
N skeleton stretching vibration of the imidazole group in TIA. Besides that, near 827 cm−1 is the absorption peak of the C–H out-of-plane bending vibration in the para-disubstituted benzene ring of the TIA molecule, and certain absorption peaks in the region of 1309–1220 cm−1 may be caused by the N–H stretching vibration peak of tert-N (Fig. S6†). Besides, the thermal stability of HOF–TIA was determined by the thermogravimetric (TG) curve. Fig. S7† suggests that the HOF–TIA molecular fragment starts to decompose at about 350 °C. The pore size distribution graph (Fig. S8†) shows that the pore size of HOF–TIA is mainly concentrated within 1–2 nm. It confirms that HOF–TIA is a porous framework material with predominantly microporous pores.
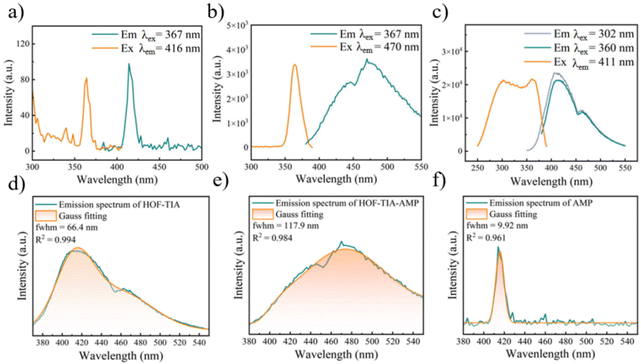
The photoluminescence (PL) properties of HOF–TIA solid powder at room temperature were investigated. At λex = 372 nm, the radiative transition of the HOF–TIA powder's S1 → S0 energy level is responsible for the large emission band at 424 nm, as seen in Fig. S10.† Bright blue light is produced by HOF–TIA under UV light, which is consistent with the coordinates shown in the CIE diagram at that wavelength (Fig. S11†). In addition, at λem = 424 nm, HOF–TIA possesses a broad excitation band (S0 → S1) near 372 nm (Fig. S12†). The peak at 424 nm is selected for the fluorescence lifetime diagram (Fig. S13†). It is found that the short fluorescence lifetime τ1 and long fluorescence lifetime τ2 are 1.18 μs and 13.01 μs, respectively, and the average lifetime is 7.193 μs. To explore the PL properties of its aqueous solution, the emission intensity was detected at different temperatures, and the fluorescence emission spectra were obtained and compared (Fig. S14†). In the excitation spectrum of the aqueous suspension of HOF–TIA, two peaks appeared at 360 nm and 303 nm. both the optimal excitation wavelength and the optimal emission wavelength were shifted, and the peak in the emission spectrum was shifted to 411 nm (Fig. S15†). It is clear that the aqueous solution's luminous characteristics achieve high temperature and acid–base stability. According to the aforementioned facts, HOF–TIA is a reliable blue fluorescent material.
Fluorescence detection of MTX
Because MTX lacks specificity for tumor cells, it is also lethal to some normal tissues with high proliferation rates, so it has great toxicity and side effects.33 In order to prevent the side effects caused by excessive intake of MTX, HOF–TIA, as a blue fluorescent sensor, can be developed to detect the content of MTX in the serum of patients and control its intake in time. The following 14 major serum constituents were chosen to demonstrate that HOF–TIA had strong selectivity to MTX: urea, glucose, sodium bicarbonate, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, histidine, serine, leucine, lysine, proline, cysteine, and phenylalanine. The HOF–TIA powder was added to the solution prepared by MTX and other interfering substances at a concentration of 10−3 M, and the emission spectra of the suspension were recorded. It can be found that only MTX has a significant quenching effect on the emission peak of HOF–TIA at 411 nm, and the presence of other substances has no significant effect on the quenching effect of MTX. The MTX solution and other interfering solutions were combined with HOF–TIA powder at a concentration of 10−3 M, and the suspensions’ emission spectra were recorded. It has been discovered that only MTX significantly reduces the peak emission of HOF–TIA at 411 nm (Fig. 2a). The HOF–TIA cannot be massively diminished by the remaining solution. More intuitively, after adding HOF–TIA to MTX under ultraviolet light irradiation, the solution quickly changes from bright blue to colorless. In order to achieve highly selective sensing of MTX in actual samples, interference experiments were carried out in the presence of several other serum components (Fig. 2b). MTX and other solutions were combined at identical molar concentrations to acquire their emission spectra, which could be used to investigate its anti-interference. Comparatively to MTX applied alone, the other components had no effect on the luminescence intensity of HOF–TIA, proving that the quenching effect of MTX on HOF–TIA fluorescence possesses strong anti-interference properties. The sensitivity and DL are two of the most important parameters for a fluorescence sensor. The concentrations of the solutions ranged from 5 × 10−7 M to 5 × 10−4 M, and each received 2 mg of MTX. Fig. 2c shows that as the MTX concentration rises, the emission peak intensity of the suspension decreases. Under the 360 nm UV light, the color shift of the suspension from blue to colorless is noticeable. It is worth noticing that in this concentration range, the emission peak intensity and concentration showed a superior linear connection (I/I0 = −0.3223![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMTX − 1.0629), and its R2 is 0.995. The DL of MTX determined by HOF–TIA was 0.022 μM by using formula
CMTX − 1.0629), and its R2 is 0.995. The DL of MTX determined by HOF–TIA was 0.022 μM by using formula| 3.3/DL = Ii/Ci, | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMTX − 1.4425), and the R2 was 0.990 (Fig. 2d). The DL was determined to be 0.061 μM using formula (1). Moreover, we have compared the DL obtained by this method with other works, shown in Table S3.† Therefore, the DL of the detection can reach the criteria of detecting whether MTX elimination is delayed. The response time diagram showed that the quenching could be achieved in 5.6 s after the addition of MTX (Fig. S16†). Based on the above information, HOF–TIA can achieve rapid and high-sensitivity detection of MTX in a serum environment.
CMTX − 1.4425), and the R2 was 0.990 (Fig. 2d). The DL was determined to be 0.061 μM using formula (1). Moreover, we have compared the DL obtained by this method with other works, shown in Table S3.† Therefore, the DL of the detection can reach the criteria of detecting whether MTX elimination is delayed. The response time diagram showed that the quenching could be achieved in 5.6 s after the addition of MTX (Fig. S16†). Based on the above information, HOF–TIA can achieve rapid and high-sensitivity detection of MTX in a serum environment.
Fluorescence detection of AMP
As mentioned above, during the de novo synthesis of purine nucleotides, the presence of MTX inhibits dihydrofolate reductase, which blocks the transfer of single carbon units and thus inhibits the formation of purine nucleotides. In this regard, we found that AMP aqueous solution can quench the fluorescence of HOF–TIA and convert HOF–TIA from blue to cyan, while guanosine monophosphate (GMP), which is also converted from inosine monophosphate, has no effect on HOF–TIA. The same concentration (5 × 10−4 M) of GMP solution and AMP solution were selected at 1 mL each, and HOF–TIA (2 mg) was added. It was found that fluorescence quenching was obvious in the AMP solution, while there was no noticeable change in the GMP solution, and the comparative emission spectra were consistent with the phenomenon (Fig. S17†). AMP was dissolved in 1 mL aqueous solution to prepare a series of solutions with concentration gradient changes (3 × 10−3 M−3 × 10−6 M), and 2 mg HOF–TIA was added to record these emission spectra. When the concentration increases, it is clear from Fig. 3a that the light from the ultraviolet lamp gradually changes from blue to cyan.To illustrate the phenomenon, the emission spectrum of HOF–TIA suspension with 5 × 10−4 M AMP was compared with that of the HOF–TIA aqueous suspension without AMP under 360 nm excitation. While the HOF–TIA emission peak at 411 nm was dramatically reduced in intensity and red-shifted to 444 nm, the emission peak at 466 nm was only slightly red-shifted to 476 nm and slowly decreased in intensity (Fig. S18†). According to the related CIE diagram, the color transitioned from dark blue to cyan (Fig. S19†). In addition, the emission spectra showed that the emission peaks of the mixed solution decreased under the excitation of 303 nm and 360 nm, its concentration and emission intensity exhibited a positive linear relationship (I (λex = 360 nm) = −15044![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CAMP − 5904; I (λex = 303 nm) = −23548
CAMP − 5904; I (λex = 303 nm) = −23548![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CAMP − 7546), their respective R2 are 0.993 and 0.990 (Fig. 3a and b). According to formula (1), the DL of AMP under 303 nm excitation is 0.298 μM and the DL of AMP under 360 nm excitation is 0.316 μM. Thus HOF–TIA can effectively distinguish AMP and GMP, and achieve high-sensitivity detection of the AMP content in an aqueous solution.
CAMP − 7546), their respective R2 are 0.993 and 0.990 (Fig. 3a and b). According to formula (1), the DL of AMP under 303 nm excitation is 0.298 μM and the DL of AMP under 360 nm excitation is 0.316 μM. Thus HOF–TIA can effectively distinguish AMP and GMP, and achieve high-sensitivity detection of the AMP content in an aqueous solution.
Mechanism of HOF–TIA response to MTX
By demonstrating that the skeleton was still intact after the addition of MTX, the PXRD patterns ruled out the chance of structural collapse (Fig. S20†). Dynamic quenching and static quenching are frequent quenching processes. In addition to measuring the lifetimes of HOF–TIA and HOF–TIA mixtures with MTX, fluorescence lifetime fitting curves were recorded and compared. The short fluorescence lifetime τ1 = 0.95 μs for HOF–TIA and the long fluorescence lifetime τ2 = 10.44 μs were obtained and the average fluorescence lifetime was calculated to be τ = 5.86 μs (Fig. 4a). The mixture containing MTX had long and short lifetimes of τ1 = 0.87 μs and τ2 = 9.55 μs respectively and its average lifetime was τ = 4.92 μs (Fig. 4b). The shortened fluorescence lifetime indicates that it was a dynamic quenching process. The molecular fragment of HOF–TIA and the structural formula of MTX are shown in Fig. S21.† For the mixed state systems (HOF–TIA + MTX), the highest occupied molecular orbital (HOMO) localized to the HOF–TIA molecular fragment and lowest unoccupied molecular orbital (LUMO) was found to be populated on MTX for their respective systems. This molecular orbital assignment suggests that excited electron transfer from the donor HOF–TIA to the acceptor MTX is possible (Fig. S22†). The fact that the donor has a higher LUMO energy level than the acceptor, enabling the transfer of excited electrons from the donor to the electron-deficient acceptor, is generally acknowledged as the primary driving force for PET as a common quenching mechanism for dynamic quenching. The HOMO and LUMO energy levels of MTX and the HOF–TIA monomer were calculated using density functional theory (DFT), and it was discovered that MTX's LUMO energy level was considerably lower than that of HOF–TIA's, illustrating an electron transfer that caused fluorescence quenching (Fig. 4d). Comparing the UV absorption pattern of MTX and the excitation pattern of HOF–TIA, it was discovered that there was a significant overlap between 250 and 360 nm (Fig. 4c), indicating that MTX could partially absorb the energy of HOF–TIA excitation and that a mechanism of competitive absorption also existed in this process. These findings revealed that the coexistence of PET and competitive absorption is the most probable reason for the quenching of HOF–TIA fluorescence by MTX.Mechanism of HOF–TIA response to AMP
The quenching of the fluorescence of HOF–TIA by AMP was described as being followed by obvious emission light changes in the section on AMP detection. In order to explore this quenching mechanism, an in-depth discussion was carried out. Firstly, by comparing the fluorescence lifetime of HOF–TIA and HOF–TIA with AMP solution, it was discovered that the average lifetime decreased from τ = 5.86 μs to τ = 4.65 μs, illustrating evident dynamic quenching (Fig. S23†). Consequently, we noted that there was little overlap when we compared the UV absorption spectrum of AMP with the excitation spectrum of HOF–TIA, effectively ruling out the possibility of competitive absorption (Fig. S24†). Through the analysis of experimental data and literature review, we hypothesized that when the HOF–TIA suspension was added to AMP solution, HOF–TIA combined with AMP to create a new exciplex HOF–TIA–AMP, and its fluorescence changed from dark blue to cyan. In order to verify this conjecture, the emission spectra and excitation spectra of HOF–TIA, HOF–TIA–AMP, and AMP were recorded respectively. Among them, HOF–TIA–AMP and HOF–TIA have a wide emission band at 410 nm–470 nm, while the emission band of AMP is narrower (Fig. 5a–c). As previously stated, HOF–TIA–AMP exhibits a red shift in both emission peaks under 360 nm excitation compared to HOF–TIA (Fig. S18†). According to Gaussian fitting, the full width at half maximum (fwhm) of the AMP–HOF–TIA was 9.92 nm (R2 = 0.961). The fwhm of the emission spectrum of HOF–TIA was 66.4 nm (R2 = 0.994), and the fwhm of the emission spectrum of HOF–TIA–AMP was 117.9 nm (R2 = 0.984) (Fig. 5d–f). Based on the aforementioned results, it can be deduced that HOF–TIA and AMP bind to each other to form the new exciplex HOF–TIA–AMP.Application in information encryption and decryption
Manufacturing goods must designate the authenticity of items with security labels and serial numbers in order to fight fraud and protect consumer rights. The merits of SRLMs such as strong visibility, variety of colors, and low-cost offer a fresh perspective on information security.36 Here, a chemical anti-counterfeiting technology was suggested to further develop the practical optical application of HOFs. The luminescent anti-counterfeiting ink was prepared by using polyvinyl alcohol, ethylene glycol, deionized water, and HOF–TIA (Fig. 6b). A number of high-security information encryption and decryption anti-counterfeiting platforms were built using HOF–TIA and the color-changing fluorescence response of various chemicals. As previously indicated, AMP and blue-emitting HOF–TIA can be combined to create a new exciplex HOF–TIA–AMP, whereas MTX quenches the blue light of HOF–TIA and turns it colorless. It is worth mentioning the HOF–TIA–AMP exhibits cyan fluorescence at 360 nm excitation, and it exhibits the same blue fluorescence as HOF–TIA at 310 nm excitation (Fig. 7a). Inspired by this phenomenon, a digital encryption and decryption anti-counterfeiting array was constructed, and its encryption process is shown in Fig. 6a–c. First, a template with four ‘8’ grooves was prepared, and the AMP solution was added dropwise to the green portion and the MTX solution to the yellow portion, and to the remaining portion was added deionized water, at this time, it could be seen that there was no fluorescence under UV light of any wavelength. Next, the brush dipped in HOF–TIA was applied evenly to all areas of the template. As depicted in the schematic picture, when exposed to 360 nm ultraviolet light, the areas where MTX solution was added dropwise displayed blue fluorescence while the area where MTX solution was added could not display any color because it quenched the fluorescence of HOF–TIA. The digital array obtained by mirror flipping was ‘0839’. Only the cyan pattern was identified to produce the digital array of ‘2150’ under 360 nm UV light. The digital code was ‘0215’ after flipping (Fig. 6c). Adding the option of error information was conducive to confusing the information received by people without keys and ensuring the security of encrypted information in transmission. Since 360 nm and 310 nm have different fluorescence patterns, these two wavelengths can be used as two keys to transmit information. However, only 360 nm UV irradiation is the correct key to decrypt the correct code ‘0215’, and if the receiver lacks the key or the key is incorrect, the wrong code ‘0839’ will be received under UV light.Due to their portability and quick response times, smartphones are frequently employed in information encryption and decryption.37 Inspired by this, a three-dimensional (3D) multilevel anti-counterfeiting fluorescent label based on a keyboard codebook was developed so as to further elaborate the HOF–TIA material's optical anti-counterfeiting capabilities. This label was scanned by the mobile phone's decryption system, which converted the 3D visualization information into an appropriate code. The codebook's correct password and the order of decryption are highlighted in Fig. 7b (first, second, and third). The final four columns of the three-row English alphabet keyboard were where the password book is drawn from. Fig. 7c illustrates the process of scanning the first-level fluorescence 3D information step by step from a smartphone and further integrating it into the correct code ‘T J U’. The patterns corresponding to ‘T’, ‘J’, and ‘U’ were stored in advance in the decryption system of a smartphone as a standard to be scanned by the phone. First, the HOF–TIA ink was filled in the blue area as directed, and at this time, the first level of information is obtained under 360 nm UV light. Then, the AMP solution was added dropwise to the codebook according to the decryption sequence (V B M) described in the first reception code, and the second level of fluorescent 3D information is obtained, which is deciphered and recognized as ‘T’ by the smartphone decryption system. Similarly, according to the second received code (J L M) and the third received code (H P; changing the wavelength of ultraviolet light to 310 nm), the third level and fourth level of information were obtained respectively, and the codes ‘J’ and ‘U’ were obtained after scanning. The decryption system of the cell phone integrates according to the identification sequence to obtain the correct code of ‘T J U’ transmitted by the 3D multi-level anti-counterfeiting system (Fig. 7d). The merits of the above 3D anti-counterfeiting system are as follows: constructing a keyboard codebook increases the difficulty of decoding; the pattern must be added in the order of encoding, otherwise the pattern is unreadable, and the 3D visualized fluorescent pattern can only be decrypted within the decryption system set in advance by the smartphone. Therefore, it has a higher level of information security. The aforementioned findings demonstrate that the HOF–TIA ink can be employed as a novel fluorescent anti-counterfeiting material for the encryption and decryption of complex optical information.
Conclusions
To sum up, an unreported luminescent HOF containing imidazole groups with a 2D interpenetrating structural network has been fabricated in this study. HOF–TIA was synthesized simply and rapidly by solvent volatilization. It exhibited favorable optical stability in aqueous solution and its emission intensity was almost independent of temperature (ranging from 25 °C to 40 °C) and pH (ranging from 3 to 11). In addition, the MTX solution caused quenching of HOF–TIA fluorescence, so HOF–TIA could be used as a fluorescent probe for the detection of methotrexate levels in humans. The detection limit of MTX in aqueous solution was 0.022 μM and in serum it was 0.061 μM. It was found that AMP could cause HOF–TIA fluorescence to quench and change color, allowing the detection of AMP concentration in aqueous solution with a detection limit of 0.298 μM. Therefore, we studied the quenching mechanism of MTX and AMP on HOF–TIA and applied the color change phenomenon of HOF–TIA upon encountering MTX and AMP to the multi-level encryption of information and produced the HOF–TIA ink to achieve a high-security anti-counterfeiting platform. This study not only prepares a new HOF containing imidazole groups but also provides a facile strategy for a dual-function optical platform for both fluorescence sensing and anti-counterfeiting, successfully expanding the scope of optical applications of the HOF.Author contributions
B. Y. designed the experiment and revised the manuscript. X. X. grew the single crystal and recorded the crystal structure. C. Y. prepared the materials, measured the photoluminescence properties, recorded the pictures and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (21971194) and the Developing Science Funds of Tongji University. The authors also thank Dr Wenyan Dan for measurement data of the HOF's crystal structure.References
- R. B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Multifunctional Porous Hydrogen-Bonded Organic Framework Materials, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC.
- X. Xu and B. Yan, Bioinspired HOF-Based Luminescent Skin Sensor with Triple Mechanochromism Responses for Recognition and Collection of Human Biophysical Signals, Mater. Horiz., 2023 10.1039/D3MH00096F.
- M. R. di Nunzio, I. Hisaki and A. Douhal, HOFs under Light: Relevance to Photon-Based Science and Applications, J. Photochem. Photobiol., C, 2021, 47, 100418 CrossRef CAS.
- X. Xu, J. Wang and B. Yan, Facile Fabrication of Luminescent Eu(III) Functionalized HOF Hydrogel Film with Multifunctionailities: Quinolones Fluorescent Sensor and Anticounterfeiting Platform, Adv. Funct. Mater., 2021, 31, 2103321 CrossRef CAS.
- Y. Liang, R. An, P. Du, P. Lei and H. Zhang, NIR-activated Upconversion Nanoparticles/Hydrogen-Bonded Organic Framework Nanocomposites for NIR-II Imaging-guided Cancer Therapy, Nano Today, 2023, 48, 101751 CrossRef CAS.
- B. Wang, R. B. Lin, Z. Zhang, S. Xiang and B. Chen, Hydrogen-Bonded Organic Frameworks as a Tunable Platform for Functional Materials, J. Am. Chem. Soc., 2020, 142, 14399–14416 CrossRef CAS.
- D. W. Kang, M. Kang, H. Kim, J. H. Choe, D. W. Kim, J. R. Park, W. R. Lee, D. Moon and C. S. Hong, A Hydrogen-Bonded Organic Framework (HOF) with Type IV NH3 Adsorption Behavior, Angew. Chem., Int. Ed., 2019, 58, 16152 CrossRef CAS PubMed.
- Q. Yin, Y. L. Li, L. Li, J. Lü, T. F. Liu and R. Cao, Novel Hierarchical Meso-Microporous Hydrogen-Bonded Organic Framework for Selective Separation of Acetylene and Ethylene versus Methane, ACS Appl. Mater. Interfaces, 2019, 11, 17823–17827 CrossRef CAS PubMed.
- S. Cai, Z. An and W. Huang, Recent Advances in Luminescent Hydrogen-Bonded Organic Frameworks: Structures, Photophysical Properties, Adv. Funct. Mater., 2022, 32, 2207145 CrossRef CAS.
- S. C. Pal, D. Mukherjee, R. Sahoo, S. Mondal and M. C. Das, Proton-Conducting Hydrogen-Bonded Organic Frameworks, ACS Energy Lett., 2021, 6, 4431–4453 CrossRef CAS.
- Y. Sun, J. Wei, Z. Fu, M. Zhang, S. Zhao, G. Xu, C. Li, J. Zhang and T. Zhou, Bio-Inspired Synthetic Hydrogen-Bonded Organic Frameworks for Efficient Proton Conduction, Adv. Mater., 2023, 35, 2208625 CrossRef CAS.
- J. Yang, J. Wang, B. Hou, X. Huang, T. Wang, Y. Bao and H. Hao, Porous Hydrogen-Bonded Organic Frameworks (HOFs): From Design to Potential Applications, Chem. Eng. J., 2020, 399, 125873 CrossRef CAS.
- Z. J. Lin, S. A. R. Mahammed, T. F. Liu and R. Cao, Multifunctional Porous Hydrogen-Bonded Organic Frameworks: Current Status and Future Perspectives, ACS Cent. Sci., 2022, 8, 1589–1608 CrossRef CAS.
- S. Feng, Y. Shang, Z. Wang, Z. Kang, R. Wang, J. Jiang, L. Fan, W. Fan, Z. Liu, G. Kong, Y. Feng, S. Hu, H. Guo and D. Sun, Fabrication of a Hydrogen-Bonded Organic Framework Membrane through Solution Processing for Pressure-Regulated Gas Separation, Angew. Chem., Int. Ed., 2020, 59, 3840–3845 CrossRef CAS PubMed.
- Y. H. Luo, X. T. He, D. L. Hong, C. Chen, F. H. Chen, J. Jiao, L. H. Zhai, L. H. Guo and B. W. Sun, A Dynamic 3D Hydrogen–Bonded Organic Frameworks with Highly Water Affinity, Adv. Funct. Mater., 2018, 18, 1804822 CrossRef.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS.
- I. Hisaki, Y. Suzuki, E. Gomez, Q. Ji, N. Tohnai, T. Nakamura and A. Douhal, Acid Responsive Hydrogen-Bonded Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 2111–2121 CrossRef CAS PubMed.
- W. Katchamart, J. Trudeau, V. Phumethum and C. Bombardier, Efficacy and Toxicity of Methotrexate (MTX) Monotherapy Versus MTX Combination Therapy with Non-biological Disease-modifying Antirheumatic Drugs in Rheumatoid Arthritis: a Systematic Review and Meta-analysis, Ann. Rheum. Dis., 2009, 68, 1105 CrossRef CAS PubMed.
- D. S. Shewach and R. D. Kuchta, Introduction to Cancer Chemotherapeutics, Chem. Rev., 2009, 109, 2859–2861 CrossRef CAS PubMed.
- S. C. Howard, J. McCormick, C. H. Pui, R. K. Buddington and R. D. Harvey, Preventing and Managing Toxicities of High-Dose Methotrexate, Oncologist, 2016, 21, 1471–1482 CrossRef CAS.
- M. Chen, J. Tang, W. Luo, Z. Zhang, Y. Zhu, R. Wang, H. Yang and X. Chen, Core-Shell-Satellite Microspheres-Modified Glass Capillary for Microsampling and Ultrasensitive SERS Spectroscopic Detection of Methotrexate in Serum, Sens. Actuators, B, 2018, 275, 267–276 CrossRef CAS.
- X. Jiang, D. Q. Feng, G. Liu, D. Fan and W. Wang, A Fluorescent Switch Sensor for Detection of Anticancer Drug and ctDNA Based on the Glutathione Stabilized Gold Nanoclusters, Sens. Actuators, B, 2016, 232, 276–282 CrossRef CAS.
- J. He, J. Wang, M. Zhang and G. Shi, Selection of a Structure-Switching Aptamer for the Specific Methotrexate Detection, ACS Sens., 2021, 6, 2436–2441 CrossRef CAS PubMed.
- L. Yang, J. Ge, D. Ma, J. Tang, H. Wang and Z. Li, MoS2 Quantum Dots as Fluorescent Probe for Methotrexate Detection, Spectrochim. Acta, Part A, 2022, 279, 121443 CrossRef CAS PubMed.
- F. Wang, Y. Wang, K. Lu, X. Wei and B. Ye, Sensitive Determination of Methotrexate at Nano-Au Self-Assembled Monolayer Modified Electrode, J. Electroanal. Chem., 2012, 674, 83–89 CrossRef CAS.
- E. Sonemoto, N. Kono, R. Ikeda, M. Wada, Y. Ueki and K. Nakashima, Practical Determination of Methotrexate in Serum of Rheumatic Patients by LC-MS/MS, Biomed. Chromatogr., 2012, 26, 1297–1300 CrossRef CAS PubMed.
- M. P. Borgman, M. F. Hiemer, A. R. Molinelli, J. C. Ritchie and S. A. Jortani, Improved Sensitivity for Methotrexate Analysis using Enzyme Multiplied Immunoassay Technique on the Siemens Viva-E instrument, Ther. Drug Monit., 2012, 34, 193–197 CrossRef CAS PubMed.
- X. Xu, W. Ma and B. Yan, An Electrodeposited Nano-Porous and Neural Network-like Ln@HOF Film for SO2 Gas Quantitative Detection via Fluorescent Sensing and Machine Learning, J. Mater. Chem. A, 2021, 9, 26391–26400 RSC.
- B. Wang, R. He, L. H. Xie, Z. J. Lin, X. Zhang, J. Wang, H. Huang, Z. Zhang, K. S. Schanze, J. Zhang, S. Xiang and B. Chen, Microporous Hydrogen-Bonded Organic Framework for Highly Efficient Turn-Up Fluorescent Sensing of Aniline, J. Am. Chem. Soc., 2020, 142, 12478–12485 CrossRef CAS PubMed.
- A. M. Kaczmarek, Y. Y. Liu, C. Wang, B. Laforce, L. Vincze, P. Van Der Voort, K. Van Hecke and R. Van Deun, Lanthanide “Chameleon” Multistage Anti-Counterfeit Materials, Adv. Funct. Mater., 2017, 27(20), 1700258 CrossRef.
- M. Tanioka, S. Kamino, A. Muranaka, Y. Ooyama, H. Ota, Y. Shirasaki, J. Horigome, M. Ueda, M. Uchiyama, D. Sawada and S. Enomoto, Reversible Near-Infrared/Blue Mechanofluorochromism of Aminobenzopyranoxanthene, J. Am. Chem. Soc., 2015, 137, 6436–6439 CrossRef CAS.
- T. M. D. Cao, T. T. G. Le, S. Turrell, M. Ferrari, Q. V. Lam and T. T. V. Tran, Luminescent Ink Based on Upconversion of NaYF4:Er, Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment, Molecules, 2021, 26, 1041 CrossRef CAS PubMed.
- S. Kumagai, S. Takahashi, M. Takahashi, T. Saito, K. Yoshida, M. Katayama, S. Mukohara, N. Amano, A. Onishi, M. Shinohara and S. Hatachi, Frio129 Development of a Prediction Model for Maximum Methotrexate (MTX) Dose without Hepatotoxicity using an Index of Erythrocyte MTX-Polyglutamate (MTXPG) Levels Speculated by Clinical and Genetic Markers, Ann. Rheum. Dis., 2020, 79, 646–647 CrossRef.
- I. D. Goldman and L. H. Matherly, The Cellular Pharmacology of Methotrexate, Pharmacol. Ther., 1985, 28, 77–102 CrossRef CAS PubMed.
- Y. Yang, X. Wang, J. Tian and Z. Wang, Renal Function and Plasma Methotrexate Concentrations Predict Toxicities in Adults Receiving High-Dose Methotrexate, Med. Sci. Monit., 2018, 24, 7719–7726 CrossRef CAS PubMed.
- S. Li, Q. Zhu, J. Xiahou and J. G. Li, Polyhedron Engineering by Chemical Unit Co-substitution in LaAlO3:0.02Pb2+ to Generate Multimode and Condition-Sensitive Luminescence for Dynamic Anticounterfeiting, Chem. Eng. J., 2022, 450, 138440 CrossRef CAS.
- M. You, M. Lin, S. Wang, X. Wang, G. Zhang, Y. Hong, Y. Dong, G. Jin and F. Xu, Three-Dimensional Quick Response Code Based on Inkjet Printing of Upconversion Fluorescent Nanoparticles for Drug Anti-Counterfeiting, Nanoscale, 2016, 8, 10096–10104 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Refinement details and additional figures. CCDC 2221028. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00444a |
| This journal is © the Partner Organisations 2023 |