Noncovalent induced circular dichroism sensors based on a chiral metal–organic framework: chiral induction synthesis, quantitative enantioselective sensing and noncovalent sensing mechanism†
Yanyu
Zhu
,
Tianyang
Ding
,
Xu
Zhang
,
Yanan
Zhou
,
Jiahui
Yu
,
Xin
Li
,
Hanwen
Zheng
,
Zhengang
Sun
 * and
Chengqi
Jiao
*
* and
Chengqi
Jiao
*
School of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, China. E-mail: szg188@163.com; jiaochengqi1989@163.com
First published on 3rd April 2023
Abstract
As an important class of chiral optical sensors, chiral induced circular dichroism (ICD) sensors have received increasing attention. Herein, a series of monometallic and bimetallic centered metal–organic frameworks (MOFs) with the same crystal structure, namely [Cu(L)(2,2′-bipy)]·H2O (LNNU-2), [Cd(L)(2,2′-bipy)(H2O)] (LNNU-3) and [ZnxCuy(L)(2,2′-bipy)]·H2O (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 9.6
y = 9.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4–1.0
0.4–1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.0) (H2L = HOOCC6H4CH2PO(OH)(OC2H5), 2,2′-bipy = 2,2′-bipyridine), have been successfully synthesized for studying the non-covalent ICD sensing mechanism. LNNU-2 shows excellent ICD sensing performance due to the strong metal coordination interaction between LNNU-2 and chiral tryptophan (Trp). In contrast, there is no ICD effect between LNNU-3 and chiral Trp due to the absence of metal coordination interaction. The results indicate that the metal coordination interaction plays a decisive role in generating and amplifying ICD signals in this sensing system. Compared to the previously reported LNNU-1 ([Zn(L)(2,2′-bipy)]·H2O) sensor, LNNU-2 is not only capable of quantifying the enantiomeric composition of chiral Trp samples at a lower concentration but also enables highly selective and accurate determination of L-Trp concentration in water, mixed natural amino acids and simulated blood plasma components by the circular dichroism (CD) titration method. LNNU-2 also allows the accurate determination of the absolute configuration and enantiomeric excess (ee) values of two chiral aromatic amino alcohols in pure water. Additionally, the effects of different chiral amino acids as chiral inducers on the chiral induction synthesis of enantioenriched LNNU-2 were investigated. As a result, the enantioenriched (P)-LNNU-2 and (M)-LNNU-2 were successfully obtained using L-alanine (L-Ala) and D-alanine (D-Ala) as chiral inducers, respectively.
9.0) (H2L = HOOCC6H4CH2PO(OH)(OC2H5), 2,2′-bipy = 2,2′-bipyridine), have been successfully synthesized for studying the non-covalent ICD sensing mechanism. LNNU-2 shows excellent ICD sensing performance due to the strong metal coordination interaction between LNNU-2 and chiral tryptophan (Trp). In contrast, there is no ICD effect between LNNU-3 and chiral Trp due to the absence of metal coordination interaction. The results indicate that the metal coordination interaction plays a decisive role in generating and amplifying ICD signals in this sensing system. Compared to the previously reported LNNU-1 ([Zn(L)(2,2′-bipy)]·H2O) sensor, LNNU-2 is not only capable of quantifying the enantiomeric composition of chiral Trp samples at a lower concentration but also enables highly selective and accurate determination of L-Trp concentration in water, mixed natural amino acids and simulated blood plasma components by the circular dichroism (CD) titration method. LNNU-2 also allows the accurate determination of the absolute configuration and enantiomeric excess (ee) values of two chiral aromatic amino alcohols in pure water. Additionally, the effects of different chiral amino acids as chiral inducers on the chiral induction synthesis of enantioenriched LNNU-2 were investigated. As a result, the enantioenriched (P)-LNNU-2 and (M)-LNNU-2 were successfully obtained using L-alanine (L-Ala) and D-alanine (D-Ala) as chiral inducers, respectively.
Introduction
Chirality is fundamental in nature and vital in chemistry, biology, life sciences, materials sciences, and other fields.1–7 The enantiomers of chiral molecules often exhibit different pharmacological, metabolic, therapeutic or toxicological properties, but have the same physical and chemical properties in an achiral environment.8,9 Therefore, the rapid, highly selective and accurate determination of the enantiomeric composition of chiral analytes is extremely important. Over the past decades, the most commonly applied techniques have been chromatography with chiral stationary phases and nuclear magnetic resonance (NMR) analysis with chiral solvating or derivatizing agents.10–15 However, these methods are not suitable for rapid analysis, due to expensive instrumentation, time-consuming steps, and sophisticated programs. In contrast, chiral optical sensing is considered to be a promising method due to the advantages of simple operation, rapid response, high sensitivity and low cost.16–25 Chiral optical sensing can allow the enantioselective recognition of chiral molecules by monitoring changes in the fluorescence/circular dichroism (CD) signals of chiral optical sensors. Thus, the rational design and construction of chiral optical sensors is of great importance for the determination of the enantiomeric composition of chiral analytes.In recent years, chiral metal–organic frameworks (MOFs) have become an important class of chiral optical sensing materials due to their stable chiral structures, designable functional sites and specific porosity.26–39 Some optical sensors based on chiral MOFs have been reported for the enantioselective sensing of amino acids, amino alcohols, drugs, etc.26–34 Despite the considerable progress in the design and application of optical sensors based on chiral MOFs, several challenges remain: (1) relying on a single optical signal change to achieve differentiation of target enantiomers, which is susceptible to interference from external factors and has poor resistance to background interference; (2) reports on the accurate determination of enantiomeric excess (ee) values for chiral analytes are rare. It is worth noting that the induced circular dichroism (ICD) technique may provide a feasible strategy for dealing with the above problems. Strong ICD signals could be generated through covalent or noncovalent interactions between the sensor and the chiral analyte.19,40–51 The generation of red-shifted ICD signals (above 300 nm) is likely to eliminate the interference from chiral impurities when quantitative analysis of ee values is performed. Recently, a reusable, highly selective and sensitive ICD sensor (LNNU-1) based on a chiral MOF was first reported by our group.51 This ICD sensor could accurately determine the absolute configuration and enantiomeric ratio of chiral tryptophan (Trp) in water, mixed natural amino acids, and simulated blood plasma components, respectively. However, there are three unresolved problems: (1) although it has been confirmed that the ICD effect between LNNU-1 and chiral Trp is due to three simultaneous noncovalent interactions (metal coordination, hydrogen bonding and π⋯π interactions) in aqueous solution (Fig. S1†), it is necessary to further explore the effect of these noncovalent interactions on the generation and amplification of ICD signals; (2) LNNU-1 was unable to determine the concentration of chiral Trp in water or mixed chiral amino acids when using the CD titration method; (3) LNNU-1 was not capable of quantifying the enantiomeric composition of other chiral compounds by noncovalent ICD sensing. Among the above problems, the study of the noncovalent ICD sensing mechanism is very pivotal. It may provide a strategy for the targeted design and synthesis of highly sensitive ICD sensors based on chiral MOFs. Highly sensitive ICD sensors have the potential to determine the concentration of chiral Trp in water or mixed chiral amino acids, and to analyze the enantiomeric composition of other chiral compounds. Previous work has demonstrated that the π⋯π interaction is an indispensable noncovalent interaction for the ICD sensing of chiral aromatic amino acids (L-/D-Trp).51 Herein, only metal coordination and hydrogen bonding interactions in this ICD sensing system should be further investigated. Our idea is to synthesize new chiral MOFs that should be isomorphic to LNNU-1 but have other unsaturated or saturated metal centers. By observing the differences in the ICD signals between these MOFs and chiral Trp/chiral Trp derivatives, it is possible to determine the effects of metal coordination and hydrogen bonding interactions on the generation and amplification of ICD signals.
Thus, a series of monometallic and bimetalliccentered MOFs with the same crystal structure, namely [Cu(L)(2,2′-bipy)]·H2O (LNNU-2), [Cd(L)(2,2′-bipy)(H2O)] (LNNU-3) and [ZnxCuy(L)(2,2′-bipy)]·H2O (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 9.6
y = 9.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4–1.0
0.4–1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.0) (H2L = HOOCC6H4CH2PO(OH)(OC2H5), 2,2′-bipy = 2,2′-bipyridine), have been successfully synthesized. It is found that the metal coordination interaction is critical and indispensable to this ICD sensing system. The strength of ICD signals increased with the enhancement of Lewis acidity in the metal center of the above MOFs. Subsequently, LNNU-2 interacted with L-Trp and three L-Trp derivatives containing different active sites, resulting in different ICD effects. These results further confirmed that the metal coordination interaction played a decisive role in the generation of ICD signals, while the hydrogen bonding interaction only affected the amplification degree of the ICD signal. Compared with LNNU-1, LNNU-2 has a higher sensitivity for ICD sensing of chiral Trp. It allows the highly selective and accurate determination of L-Trp concentration in water, mixed natural amino acids and simulated blood plasma components by the CD titration method. Moreover, LNNU-2 achieves the accurate determination of the absolute configuration and ee values of two chiral aromatic amino alcohols in water without the involvement of any organic solvent. Inspired by the results of the noncovalent ICD sensing mechanism, we investigated the effects of different chiral amino acids as chiral inducers on the chiral induction synthesis of enantioenriched LNNU-2. Surprisingly, the enantioenriched (P)-LNNU-2 and (M)-LNNU-2 were successfully obtained using L-alanine (L-Ala) and D-alanine (D-Ala) as chiral inducers, respectively.
9.0) (H2L = HOOCC6H4CH2PO(OH)(OC2H5), 2,2′-bipy = 2,2′-bipyridine), have been successfully synthesized. It is found that the metal coordination interaction is critical and indispensable to this ICD sensing system. The strength of ICD signals increased with the enhancement of Lewis acidity in the metal center of the above MOFs. Subsequently, LNNU-2 interacted with L-Trp and three L-Trp derivatives containing different active sites, resulting in different ICD effects. These results further confirmed that the metal coordination interaction played a decisive role in the generation of ICD signals, while the hydrogen bonding interaction only affected the amplification degree of the ICD signal. Compared with LNNU-1, LNNU-2 has a higher sensitivity for ICD sensing of chiral Trp. It allows the highly selective and accurate determination of L-Trp concentration in water, mixed natural amino acids and simulated blood plasma components by the CD titration method. Moreover, LNNU-2 achieves the accurate determination of the absolute configuration and ee values of two chiral aromatic amino alcohols in water without the involvement of any organic solvent. Inspired by the results of the noncovalent ICD sensing mechanism, we investigated the effects of different chiral amino acids as chiral inducers on the chiral induction synthesis of enantioenriched LNNU-2. Surprisingly, the enantioenriched (P)-LNNU-2 and (M)-LNNU-2 were successfully obtained using L-alanine (L-Ala) and D-alanine (D-Ala) as chiral inducers, respectively.
Results and discussion
Synthesis, characterization and stability
A new chiral MOF, [Cu(L)(2,2′-bipy)]·H2O (LNNU-2), was successfully synthesized by selecting the copperII (CuII) ion with stronger Lewis acidity to replace the zincII (ZnII) ion of LNNU-1. LNNU-2 was isomorphic to LNNU-1, and the crystal structure and characterization of LNNU-2 are provided in the ESI (Tables S1 and S2 and Fig. S2†). In addition, a series of ZnII and CuII ion co-doped bimetallic MOFs isomorphic to LNNU-1, [ZnxCuy(L)(2,2′-bipy)]·H2O (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 9.6
y = 9.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4–1.0
0.4–1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.0), were also successfully obtained. All the above products were block crystals, and the color of these crystals gradually deepened with the increase in the amount of CuII ions (Fig. 1a). The powder X-ray diffraction (PXRD) patterns and Fourier transform infrared (FT–IR) spectra showed that the above bimetallic MOFs have the same crystal structure as LNNU-1 (Fig. 1b and c). Additionally, the actual molar ratios of ZnII/CuII ions in the bimetallic MOFs were detected using an inductively coupled plasma emission spectrometer (ICP). As shown in Table S3,† the content of CuII ions in the products of bimetallic MOFs increased with the increase of the amount of the raw material Cu(CH3COO)2·H2O, while the content of ZnII ions decreased with the decrease of the amount of raw material Zn(CH3COO)2·2H2O.
9.0), were also successfully obtained. All the above products were block crystals, and the color of these crystals gradually deepened with the increase in the amount of CuII ions (Fig. 1a). The powder X-ray diffraction (PXRD) patterns and Fourier transform infrared (FT–IR) spectra showed that the above bimetallic MOFs have the same crystal structure as LNNU-1 (Fig. 1b and c). Additionally, the actual molar ratios of ZnII/CuII ions in the bimetallic MOFs were detected using an inductively coupled plasma emission spectrometer (ICP). As shown in Table S3,† the content of CuII ions in the products of bimetallic MOFs increased with the increase of the amount of the raw material Cu(CH3COO)2·H2O, while the content of ZnII ions decreased with the decrease of the amount of raw material Zn(CH3COO)2·2H2O.
 | ||
| Fig. 1 (a) The images, (b) PXRD patterns and (c) FT–IR spectra of LNNU-1, LNNU-2 and the bimetallic MOFs. | ||
Subsequently, the stabilities of LNNU-2 and a series of bimetallic MOFs were investigated. LNNU-2 and bimetallic MOFs have similar thermal gravimetric (TG) curves, and thus, LNNU-2 was used as a representative (Fig. S3a and b†). Thermal gravimetric and differential thermal analysis (TG–DTA), PXRD patterns and FT–IR spectra of LNNU-2 showed that its framework structure could be stable up to 200 °C (Fig. S3a, c and d†). In addition, the PXRD patterns and FT–IR spectra of LNNU-2 after immersing in pure water and the aqueous solution of L-Trp (2.5 × 10−3 M) indicated that the framework structure of LNNU-2 remained stable (Fig. S4†). The above results indicated that LNNU-2 and a series of ZnII and CuII ion co-doped bimetallic MOFs were successfully obtained, and they could be used to study the noncovalent ICD sensing mechanism.
The noncovalent ICD sensing mechanism
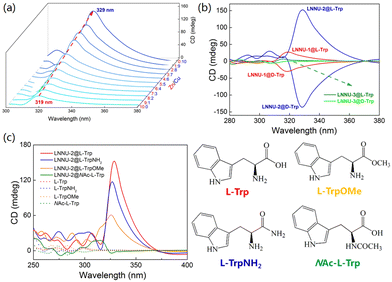
The suspensions of LNNU-1@L-Trp, LNNU-2@L-Trp and a series of bimetallic MOFs@L-Trp were analyzed by CD spectroscopy under identical conditions. As expected, the ICD signal intensities of the above suspensions increased with the increasing content of CuII ions in the MOFs, and the ICD signals were gradually red-shifted (Fig. 2a and Table S4†). In particular, the complete replacement of ZnII ions with CuII ions caused a dramatic increase in the measured intensity. The crystal structures of the above MOFs were identical, and thus, the variation in the ICD signal could be mainly attributed to the different Lewis acidities of the metal centers in the MOFs. Since the Lewis acidity of the CuII ion was stronger than that of the ZnII ion, it was more likely to interact with the nitrogen (N) atom of the amino group in Trp. This resulted in an amplification of the ICD signal. Then, if the coordination geometry of the metal center of the MOF is saturated, could it produce the ICD effect after interacting with chiral Trp? Inspired by the above idea, we tried to synthesize a new MOF with the saturated coordination mode of the metal center by replacing the ZnII ion with the cadmiumII (CdII) ion having the same d10 electron configuration to self-assemble with H2L and 2,2′-bipy. Fortunately, a new chiral MOF, [Cd(L)(2,2′-bipy)(H2O)] (LNNU-3), was successfully synthesized. Single-crystal structural analysis indicated that a lattice water molecule (O1 W) in the crystal structure of LNNU-1 became a coordination water molecule (O6) in LNNU-3 (Fig. S5 and Tables S5, S6†). Thus, the metal center of LNNU-3 adopted a saturated coordination mode, while the framework of LNNU-3 was basically the same as that of LNNU-1. The chiral characterization and stability of LNNU-3 are provided in the ESI (Fig. S6†). Then, the suspensions of LNNU-1@D/L-Trp, LNNU-2@D/L-Trp and LNNU-3@D/L-Trp were analyzed by CD spectroscopy under the same conditions (Fig. 2b). The results showed that the suspensions of LNNU-3@D/L-Trp did not produce an ICD signal due to the absence of the unsaturated coordination metal center. In contrast, the suspensions of both LNNU-1@D/L-Trp and LNNU-2@D/L-Trp produced obvious enantiomeric ICD signals. The above results indicated that the metal coordination interaction was critical and indispensable in this sensing system. The strength of the ICD signal increased with the enhancement of the Lewis acidity of the metal center of the MOFs.To further explore the effects of metal coordination and hydrogen bonding interactions on the generation and amplification of ICD signals, L-Trp and three L-Trp derivatives with different active sites were selected to interact with LNNU-2, respectively. As shown in Fig. 2c and Table S7,† the ICD signal intensity of the LNNU-2@analyte suspension followed the order LNNU-2@L-Trp > LNNU-2@L-TrpNH2 > LNNU-2@L-TrpOMe > LNNU-2@NAc-L-Trp. This result showed that the ICD signal intensity of the LNNU-2@L-Trp suspension was the strongest, indicating that multiple noncovalent interactions facilitated the output and amplification of the ICD signal. L-TrpNH2 could be regarded as a molecule formed by replacing the –COOH in L-Trp with –CONH2. Although –CONH2 could also participate in the formation of hydrogen bonding, the hydrogen bonding strength of N–H⋯O is weaker than that of O–H⋯O. Therefore, the ICD signal intensity of the LNNU-2@L-TrpNH2 suspension was slightly weaker than that of LNNU-2@L-Trp suspension. The main difference between L-TrpOMe and L-Trp was the substitution of –COOH by –COOCH3, resulting in no hydrogen bonding interaction between L-TrpOMe and LNNU-2. Therefore, the ICD signal intensity of the LNNU-2@L-TrpOMe suspension was significantly weaker than that of LNNU-2@L-Trp and LNNU-2@L-TrpNH2 suspensions. In contrast, when –NHCOCH3 completely replaced –NH2 in L-Trp, the metal coordination interaction disappeared in the sensing system, resulting in no ICD signal output in the LNNU-2@NAc-L-Trp suspension. The above results suggested that the metal coordination interaction played a decisive role in the generation of ICD signals, and the hydrogen bonding interaction only affected the amplifiication degree of the ICD signal.
Determination of L-Trp concentration
L-Trp is an essential amino acid that is very important for humans and animals, and its imbalance or deficiency may lead to several chronic diseases.52,53 Although LNNU-1 exhibited good enantioselective sensing of chiral Trp at a millimolar analytical concentration in the previous research,51 it was unable to detect the concentration of L-Trp based on the ICD response (Fig. S7†). As demonstrated in Fig. 2b and Table S4,† the ICD signal intensity of the LNNU-2@L-Trp suspension was about 5.8 times higher than that of the LNNU-1@L-Trp suspension. Therefore, LNNU-2 should have a higher sensitivity for ICD sensing of L-Trp. This result provided a possibility for achieving the accurate determination of L-Trp concentration in water using the CD titration method.Subsequently, the CD spectra of the LNNU-2 suspension (2.0 mg LNNU-2 in 2.0 mL deionized water after treating by ultrasonication for 20 minutes) were measured in situ after incremental addition of freshly prepared L-Trp solution (2.5 × 10−3 M). After each addition, the CD spectrum of the suspension was monitored. As demonstrated in Fig. 3a, the intensity of the ICD signal appearing at 325 nm gradually increased with the increase of L-Trp concentration. The linear regression equation was derived from the calibration curve, ICD (mdeg) = 0.13218[C] (μM) − 0.18606 (R2 = 0.9990), which was established based on the plot of the average value of the ICD signal intensities (325 nm) versusL-Trp concentrations in the range from 0 to 4.339 × 10−4 M (Fig. 3b). According to the limit of detection (LOD) equation54 LOD = 3σ/S (σ is the standard deviation of 10 blank measurements, and S is the slope between ICD signal intensity versusL-Trp concentration), the LOD was obtained as 2.00 × 10−5 M. A good linear relationship provided a chance to efficiently and accurately detect the concentration of L-Trp, as demonstrated by the quantitative detection results of L-Trp concentration discussed below.
Using LNNU-2 as an ICD sensor, 10 samples with different concentrations of L-Trp aqueous solutions were detected by CD spectroscopy in the range of 7.50 × 10−5 to 5.00 × 10−4 M, and the intensities of the ICD signals at 325 nm were recorded (Fig. S8 and Table S8†). By substituting the ICD signal intensity into the linear equation ICD (mdeg) = 0.13218[C] (μM) − 0.18606, the calculated L-Trp concentration was the detected concentration, which was very close to the actual concentration of the sample. As shown in Table S8,† the actual concentration of the L-Trp sample was 2.78 × 10−4 M (entry 6), while the detected concentration was 2.79 × 10−4 M. Even for the L-Trp sample with a low concentration, it showed good detection results, that is, the actual concentration in the L-Trp aqueous solution was 7.50 × 10−5 M (entry 1), and the detected concentration was 7.83 × 10−5 M. The average relative error between the detected and actual concentrations of 10 L-Trp aqueous solution samples was only 2.6%, indicating that LNNU-2 could accurately and rapidly detect the concentration of L-Trp in water using the CD titration method.
The detection of the concentration of an individual chiral amino acid in a mixture of other chiral amino acids is challenging by using chiral optical sensing techniques due to the similar properties of chiral amino acid molecules. Since LNNU-2 had high sensitivity for the ICD sensing of L-Trp in water, we tried to determine the concentration of L-Trp in a mixed aqueous solution of 19 other natural amino acids. First, we assessed the selectivity of LNNU-2 toward natural amino acids. As illustrated in Fig. S9,† the CD spectrum of the LNNU-2@L-Trp suspension showed a strong ICD signal at 329 nm, while the CD spectra of pure aqueous solutions of all natural amino acids and the suspensions of LNNU-2@other natural amino acids exhibited no ICD signals. This result indicated that LNNU-2 had a high selectivity for the ICD sensing of L-Trp in the presence of other natural amino acids and without interference from the co-existing species. Subsequently, using the same method as the above, a standard curve was established in the L-Trp concentration range from 0 to 8.333 × 10−4 M (Fig. 3c and d), and the linear equation was derived from the standard curve as ICD (mdeg) = 0.1097[C] (μM) + 4.80784 (R2 = 0.9996). The LOD of L-Trp was calculated to be 2.28 × 10−5 M. The effectiveness of the L-Trp concentration analyses was demonstrated by analyzing 10 L-Trp samples at varying concentrations in the presence of 19 other natural amino acids (Table S8 and Fig. S10†). As shown in Table S8,† the actual concentrations of the L-Trp samples were 2.58 × 10−4 M (entry 1), 3.54 × 10−4 M (entry 3), 5.54 × 10−4 M (entry 8) and 6.82 × 10−4 M (entry 10), and the detected concentrations were 2.54 × 10−4 M, 3.55 × 10−4 M, 5.53 × 10−4 M and 6.80 × 10−4 M, respectively. The average value of the relative error between the detected and actual concentrations of the 10 samples was only 0.7%. The above results indicated that LNNU-2 could be used as a highly selective ICD sensor for the accurate determination of L-Trp concentration in the presence of other natural amino acids.
Trp is the only amino acid that binds to plasma albumin and exists in a balance between albumin-bound and free forms in the peripheral circulation.53 Therefore, it is of biological significance to detect the concentration of free L-Trp in blood plasma components. Fortunately, using LNNU-2 as an ICD sensor, the determination of L-Trp concentration in simulated blood plasma components showed the same accuracy and high selectivity. A standard curve was established using the above same method, and the linear equation ICD (mdeg) = 0.11957[C] (μM) + 4.83409 (R2 = 0.9996) was obtained (Fig. 3e and f). In addition, the LOD was calculated to be 1.97 × 10−5 M. Based on the measurement and analysis of 10 L-Trp samples at varying concentrations, it was concluded that the LNNU-2 sensor could also be used to accurately determine the L-Trp concentration in simulated blood plasma components (Table S8 and Fig. S11†). For instance, the actual concentrations of three L-Trp samples were 1.85 × 10−4 M (entry 2), 6.06 × 10−4 M (entry 7) and 8.05 × 10−4 M (entry 10), and the detected concentrations were 1.85 × 10−4 M, 6.07 × 10−4 M and 8.06 × 10−4 M, respectively. The average relative error was only 0.8%. This result indicated that LNNU-2 could accurately and efficiently determine the concentration of L-Trp in simulated blood plasma components without any interference from other coexisting species.
The recyclability of sensing materials is important for assessing their practicality. Therefore, the recycling performance of LNNU-2 in water, mixed natural amino acids and simulated blood plasma components was investigated (Fig. S12a–c†). After performing the sensing experiments of LNNU-2, the suspensions of LNNU-2@L-Trp were centrifuged and washed with water several times, and then the ICD signal of the above suspensions disappeared. Subsequently, an aqueous solution of L-Trp was added to the recycled samples. The results showed that the ICD signals of LNNU-2@L-Trp suspensions can be recovered and regenerated. After five continuous cycles, the ICD signal intensities of LNNU-2@L-Trp suspensions were basically the same as those of the initial suspensions. Meanwhile, the PXRD patterns of the recycled LNNU-2 samples revealed that their crystal structure was intact after five cycles (Fig. S12d†). The experimental results indicated that LNNU-2 has excellent recyclability.
Quantitative enantioselective sensing of chiral Trp
Using the CD titration method, LNNU-2 achieved the accurate determination of L-Trp concentration at a submillimolar concentration. Compared to LNNU-1, LNNU-2 was expected to serve as an ICD sensor for determining the absolute configuration and ee value of the chiral Trp sample at a lower concentration. As demonstrated in Fig. S13a,† a calibration curve was constructed using samples containing Trp (2.5 × 10−4 M) at varying ee values (+100, +80, +60, +40, +20, 0, −20, −40, −60, −80 and −100%). Notably, a good linear relationship between the average value of the ICD signal intensities (325 nm) and the ee values of Trp samples was observed at submillimolar concentrations. The linear regression equation was calculated from the calibration curve, ICD (mdeg) = 0.32289 ee (%) + 1.8991 (R2 = 0.9992). Thus, the ICD signal intensity at 325 nm could be used for the determination of the ee values of chiral Trp samples. In addition, the absolute configuration of the sample could be correctly assigned based on the direction of the ICD signal. The validity of the ICD sensing assay was demonstrated by analyzing 10 Trp samples at varying enantiomeric compositions in water (Table 1 and Fig. S13b†). For instance, sensing a mixture containing Trp with an ee value of 96% (entry 1) gave an exact ee value of 95.9%, and it was also able to determine the enantiomeric ratio of the sample at the same time (Table 1). By comparing the reference spectra (Fig. 2b), the absolute configuration of the major enantiomer was determined to be the L configuration. Notably, the absolute configurations of the major enantiomers of the 10 Trp samples were correctly assigned, and the average absolute error between the detected and the actual ee value was only 0.9%.| Sample composition | Sensing results | ||||||
|---|---|---|---|---|---|---|---|
| Entry | Abs. config. | %ee | Ratio L/D | Abs. config.a | %eeb | Ratio L/D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
%ee absolute error |
| a By comparison of the sign of the ICD with the reference spectra (Fig. 2b). b Based on ICD signal intensities at 325 nm. | |||||||
| 1 | L | 96.0 | 98.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
L | 95.9 | 97.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1 2.1 |
0.1 |
| 2 | D | −96.0 | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98.0 98.0 |
D | −95.5 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97.8 97.8 |
0.5 |
| 3 | L | 90.0 | 95.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 5.0 |
L | 88.5 | 94.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.7 5.7 |
1.5 |
| 4 | D | −55.0 | 22.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77.5 77.5 |
D | −55.1 | 22.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77.6 77.6 |
0.1 |
| 5 | L | 80.0 | 90.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.0 10.0 |
L | 81.1 | 90.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.4 9.4 |
1.1 |
| 6 | D | −40.0 | 30.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.0 70.0 |
D | −40.9 | 29.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.5 70.5 |
0.9 |
| 7 | L | 70.0 | 85.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.0 15.0 |
L | 68.8 | 84.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.6 15.6 |
1.2 |
| 8 | D | −30.0 | 35.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65.0 65.0 |
D | −29.0 | 35.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64.5 64.5 |
1.0 |
| 9 | L | 50.0 | 75.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.0 25.0 |
L | 50.6 | 75.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.7 24.7 |
0.6 |
| 10 | D | −20.0 | 40.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60.0 60.0 |
D | −18.5 | 40.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59.3 59.3 |
1.5 |
Quantitative enantioselective sensing of chiral aromatic amino alcohols
The ICD sensing mechanism indicated that if the functional sites of LNNU-2 highly matched with the active sites of the chiral analyte, multiple noncovalent interactions might be generated between LNNU-2 and the chiral analyte. Then, the noncovalent interactions might result in the emergence of a strong ICD response, which could achieve the determination of the absolute configuration and ee value of the chiral analyte. Inspired by this work, two chiral aromatic amino alcohols, namely, 2-phenylglycinol (1) and (−)-cis-1-amino-2-indanol (2), were selected for the noncovalent ICD sensing study (Fig. 4a and b).LNNU-2 (2.0 mg) was dispersed in an aqueous solution (3.0 mL, 3.0 × 10−3 M) of each of the two chiral aromatic amino alcohol enantiomers and sonicated for 20 minutes before CD analysis. As expected, the ICD signals were detected between LNNU-2 and two chiral aromatic amino alcohols, respectively. The CD spectrum of LNNU-2@(S)-1 exhibited a strong positive ICD signal centered at 565 nm and a weak negative ICD signal at 686 nm, while the spectrum of LNNU-2@(R)-1 exhibited opposite ICD signals (Fig. 4c). As demonstrated in Fig. 4d, the CD spectrum of LNNU-2@(1R,2S)-2 showed a strong positive ICD signal at 603 nm and two weak negative ICD signals at 513 nm and 710 nm, respectively. As expected, the opposite ICD signals were observed in the spectrum of LNNU-2@(1S,2R)-2. Since multiple ICD signals were generated during the ICD sensing process, the strongest ICD signal was selected for the determination of the absolute configuration and ee value of the chiral aromatic amino alcohol sample. Thus, a calibration curve was established based on the plot of the average value of the ICD signal intensities (565 nm) versus ee values of samples of 1, and then the linear regression equation, ICD (mdeg) = 0.21725 ee (%) + 0.23068 (R2 = 0.9978) (Fig. 4e and f), was derived. Similarly, the linear regression equation ICD (mdeg) = 0.06673 ee (%) − 0.1155 (R2 = 0.9993) was derived from the calibration curve based on the plot of the average value of the ICD signal intensities (603 nm) versus ee values of samples of 2 (Fig. 4g and h).
Based on the good linear relationship, the absolute configuration and ee analyses of the practical sample were further performed simultaneously using the LNNU-2 sensor. Six samples of 1 and 2 with different enantiomeric compositions were prepared in pure water, respectively. The absolute configuration was determined using the sign of the Cotton effect, and the ICD signal intensities at 565 nm for 1 and 603 nm for 2 were used to quantify ee values. As shown in Table 2 and Fig. S14,† the absolute configurations of the major enantiomers of the above samples could be correctly assigned, and the ee values were also accurately established. The average absolute error between the detected and the actual ee values of the six samples of both 1 and 2 was 1.2%. The results indicated that LNNU-2 could be used as an ICD sensor for the rapid and accurate quantification of the enantiomeric compositions of two chiral aromatic amino alcohols in water without the involvement of any organic solvent.
| Sample 1 composition | Sensing results | ||||||
|---|---|---|---|---|---|---|---|
| Entry | Abs. config. | %ee | Ratio R/S | Abs. config.a | %eeb | Ratio R/Sb | %ee absolute error |
| 1 | R | −88.0 | 94.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 6.0 |
R | −88.9 | 94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
0.9 |
| 2 | S | 85.0 | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92.5 92.5 |
S | 83.6 | 8.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91.8 91.8 |
1.4 |
| 3 | R | −48.0 | 74.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.0 26.0 |
R | −47.4 | 73.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.3 26.3 |
0.6 |
| 4 | S | 60.0 | 20.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80.0 80.0 |
S | 61.7 | 19.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80.9 80.9 |
1.7 |
| 5 | R | −32.0 | 66.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34.0 34.0 |
R | −32.9 | 66.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.5 33.5 |
0.9 |
| 6 | S | 48.0 | 26.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74.0 74.0 |
S | 46.2 | 26.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73.1 73.1 |
1.8 |
| Sample 2 composition | Sensing results | ||||||
|---|---|---|---|---|---|---|---|
| Entry | Abs. config. | %ee | Ratio 1R,2S/1S,2R | Abs. config.a | %eeb | Ratio 1R,2S/1S,2Rb | %ee absolute error |
| a By comparison of the sign of the ICD with the reference spectra (Fig. 4c and d). b Based on ICD signal intensities at 565 nm for 1 and 603 nm for 2. | |||||||
| 1 | 1R,2S | 90.0 | 95.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 5.0 |
1R,2S | 88.2 | 94.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9 5.9 |
1.8 |
| 2 | 1S,2R | −92.0 | 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96.0 96.0 |
1S,2R | −91.5 | 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95.8 95.8 |
0.5 |
| 3 | 1R,2S | 49.0 | 74.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.5 25.5 |
1R,2S | 47.4 | 73.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26.3 26.3 |
1.6 |
| 4 | 1S,2R | −66.0 | 17.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83.0 83.0 |
1S,2R | −64.7 | 17.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82.4 82.4 |
1.3 |
| 5 | 1R,2S | 28.0 | 64.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.0 36.0 |
1R,2S | 27.4 | 63.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.3 36.3 |
0.6 |
| 6 | 1S,2R | −40.0 | 30.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70.0 70.0 |
1S,2R | −38.4 | 30.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69.2 69.2 |
1.6 |
Chiral induction synthesis of enantioenriched LNNU-2
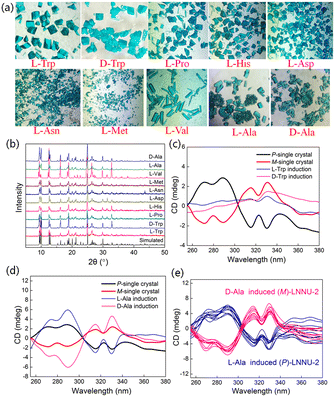
Single-crystal structural analysis revealed that LNNU-2 crystallized in a chiral space group of P212121 with a Flack absolute configuration parameter of 0.029(4), indicating the enantiomeric purity of the single crystal (Table S1†).55 Although the phosphonic acid ligand (H2L) had a chiral phosphorus (P) center, the raw material used to synthesize H2L was achiral, resulting in H2L becoming a racemate. Therefore, the chirality of bulk samples of LNNU-2 should be random. To determine the chiral purity of LNNU-2, 20 single-crystal samples were randomly picked from the same batch and their solid-state CD spectra were recorded. Each single-crystal sample was found to be CD active, and 13 of them were in the M-configuration and 7 in the P-configuration (Fig. S15†). Apparently, two enantiomers (M and P) were present in the bulk LNNU-2 samples with a slight excess of the M-configuration.Although many chiral MOFs with novel structures and excellent properties have been successfully obtained, the synthesis of homochiral or enantioenriched MOFs remains a challenge.
Chiral induction is an effective method for the synthesis of homochiral or enantioenriched MOFs, and the appropriate interactions between chiral inducers and achiral building blocks are very important for chiral induction synthesis.56 Inspired by the results of the noncovalent ICD sensing mechanism, chiral Trp could be a candidate for chiral induction due to the existence of multiple noncovalent interactions between LNNU-2 and chiral Trp. Therefore, we attempted to split two enantiomers of LNNU-2 to obtain enantioenriched (P)-LNNU-2 and (M)-LNNU-2 using L-Trp and D-Trp as chiral inducers, respectively. The synthesis of enantioenriched LNNU-2 was carried out by replacing deionized water with an aqueous solution of L-Trp (or D-Trp) at a concentration of 2.5 × 10−3 M, while the other original synthesis conditions of LNNU-2 remained unchanged. As a result, blue block crystals were obtained, and the PXRD patterns of products showed that they had the same framework structure as LNNU-2 (Fig. 5a and b). However, solid-state CD spectra of the products showed that they had no CD activity either induced with L-Trp or D-Trp (Fig. 5c). This result indicated that the strong noncovalent interactions between LNNU-2 and chiral Trp were not favorable for the synthesis of enantioenriched LNNU-2. Therefore, using the same synthesis method, other different types of chiral amino acids were selected as chiral inducers for the chiral induction synthesis of LNNU-2 (Table S9†).
As shown in Fig. 5a, the LNNU-2 products induced by chiral amino acids were all blue block crystals, and the PXRD patterns of products confirmed that their framework structures were the same as that of LNNU-2 (Fig. 5b). Solid-state CD spectra showed that LNNU-2 bulk samples induced by L-proline (L-Pro), L-histidine (L-His) and L-aspartate (L-Asn) had Cotton effects similar to that of the single-crystal sample of (M)-LNNU-2 (Fig. S16a†). The only difference was that all CD spectra of these products had an additional positive CD signal near 260 nm. This suggested that the above products might not be enantioenriched but enantiomeric excess (M > P). In contrast, when L-aspartic acid (L-Asp), L-methionine (L-Met) and L-valine (L-Val) were selected as chiral inducers, the solid-state CD spectra of bulk samples of the products showed no CD activity, indicating that they were all racemates (Fig. S16b†). Surprisingly, the solid-state CD spectra of the products inducted by L-Ala exhibited a Cotton effect opposite to that of the single-crystal sample of (M)-LNNU-2, suggesting the successful synthesis of (P)-LNNU-2 samples (Fig. 5d). Meanwhile, single-crystal structural analysis of the products revealed the presence of P-helical chains in the crystal structure, which had mirror symmetry with the M-configuration (Fig. S17†). A Flack parameter of 0.025(4) further verified that the P-configuration was determined correctly (Table S10†).55 To determine the chiral purity of bulk samples of the products induced by L-Ala, the solid-state CD spectra of 10 bulk samples randomly picked from the same batch were measured (Fig. 5e). The results showed the same Cotton effects as that of the single-crystal sample of (P)-LNNU-2. The above results indicated that the enantioenriched (P)-LNNU-2 samples were successfully synthesized using L-Ala as a chiral inducer. Similarly, the enantioenriched (M)-LNNU-2 samples were also successfully obtained upon chiral induction of D-Ala. The solid-state CD spectra of 10 bulk samples randomly picked from the same batch had the same Cotton effect as that of the single-crystal sample with the M-configuration (Fig. 5e). Furthermore, two parallel syntheses of LNNU-2 were carried out using L-Ala and D-Ala as the chiral inducers, respectively. The solid-state CD spectra of 5 bulk samples randomly picked from each synthesis were the same as before (Fig. S18†). The results of crystal structure analyses and solid-state CD spectra demonstrated that two enantiomers of LNNU-2, (P)-[Cu(L)(2,2′-bipy)]·H2O ((P)-LNNU-2) and (M)-[Cu(L)(2,2′-bipy)]·H2O ((M)-LNNU-2), were successfully obtained by chiral induction, and L-Ala and D-Ala were suitable chiral inducers for the synthesis of enantioenriched LNNU-2 samples.
The above results suggested that using different chiral amino acids as chiral inducers resulted in products with the same framework structure but different chiral features. Apparently, different amino acid inducers have different chiral induction effects on the synthesis of LNNU-2. It was found that the strong noncovalent interactions between LNNU-2 and chiral Trp reduced the degree of enantiomeric excess of LNNU-2, and the products changed from a slight enantiomeric excess to racemic. This suggested that too strong interactions between chiral inducers and building blocks were not conducive to the formation of enantioenriched products. Among the different types of chiral amino acids used as chiral inducers, only chiral Ala induced the enantioenriched LNNU-2. Compared with other chiral amino acids, chiral Ala is the simplest chiral amino acid in terms of the molecular structure and low positional resistance (Table S9†). Compared with chiral Trp, the molecular structure of chiral Ala lacked an aromatic group, so there was no π⋯π interaction between chiral Ala and LNNU-2. This weakened the degree of noncovalent interactions between the chiral inducer and the building blocks of LNNU-2. Instead, it is more favorable to generate the enantioenriched LNNU-2 in chiral induction synthesis. It could be seen that the rational control of the degree of noncovalent interactions between chiral inducers and framework building blocks might have a significant effect on the generation of enantioenriched products. Although we could not explain exactly why the enantioenriched LNNU-2 was successfully obtained upon chiral induction of L-Ala and D-Ala, the above-mentioned studies could provide ideas for screening suitable chiral inducers for the synthesis or splitting of chiral MOFs.
Conclusions
In summary, two new chiral MOFs, namely [Cu(L)(2,2′-bipy)]·H2O (LNNU-2) and [Cd(L)(2,2′-bipy)(H2O)] (LNNU-3), and a series of bimetallic MOFs, [ZnxCuy(L)(2,2′-bipy)]·H2O (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 9.6
y = 9.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4–1.0
0.4–1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.0), were successfully designed and synthesized for the study of the noncovalent ICD sensing mechanism. By observing the differences in ICD signals between the above MOFs and L-Trp or between LNNU-2 and L-Trp and its derivatives, the effects of noncovalent interactions on the generation and amplification of ICD signals were investigated. The results indicated that multiple noncovalent interactions facilitated the output and amplification of the ICD signal. In this ICD sensing system, the metal coordination interaction played a decisive role in the generation of ICD signals, while the hydrogen bonding interaction only affected the amplification degree of the ICD signal. The strength of the ICD signal increased with the enhancement of the Lewis acidity of the metal center of the MOFs. Thus, LNNU-2 showed improved ICD sensing performance compared to the previously reported LNNU-1 sensor. LNNU-2 allowed the quantification of the enantiomeric composition of the chiral Trp sample at a lower concentration. More importantly, the amplified ICD signal of the LNNU-2@L-Trp suspension enabled highly selective and accurate determination of L-Trp concentration in water, mixed natural amino acids and simulated blood plasma components, respectively. Based on the noncovalent ICD sensing mechanism, LNNU-2 also achieved rapid and accurate determination of the absolute configuration and ee values of the chiral aromatic amino alcohols in pure water. In addition, different chiral amino acids were selected as chiral inducers for the chiral induction synthesis of enantioenriched LNNU-2. Remarkably, LNNU-2 was successfully split into enantioenriched (P)-LNNU-2 and (M)-LNNU-2 upon chiral induction of L-Ala and D-Ala. This work provides new ideas for the rational design of MOF-based noncovalent ICD sensors and the screening of suitable chiral inducers for chiral induction synthesis.
9.0), were successfully designed and synthesized for the study of the noncovalent ICD sensing mechanism. By observing the differences in ICD signals between the above MOFs and L-Trp or between LNNU-2 and L-Trp and its derivatives, the effects of noncovalent interactions on the generation and amplification of ICD signals were investigated. The results indicated that multiple noncovalent interactions facilitated the output and amplification of the ICD signal. In this ICD sensing system, the metal coordination interaction played a decisive role in the generation of ICD signals, while the hydrogen bonding interaction only affected the amplification degree of the ICD signal. The strength of the ICD signal increased with the enhancement of the Lewis acidity of the metal center of the MOFs. Thus, LNNU-2 showed improved ICD sensing performance compared to the previously reported LNNU-1 sensor. LNNU-2 allowed the quantification of the enantiomeric composition of the chiral Trp sample at a lower concentration. More importantly, the amplified ICD signal of the LNNU-2@L-Trp suspension enabled highly selective and accurate determination of L-Trp concentration in water, mixed natural amino acids and simulated blood plasma components, respectively. Based on the noncovalent ICD sensing mechanism, LNNU-2 also achieved rapid and accurate determination of the absolute configuration and ee values of the chiral aromatic amino alcohols in pure water. In addition, different chiral amino acids were selected as chiral inducers for the chiral induction synthesis of enantioenriched LNNU-2. Remarkably, LNNU-2 was successfully split into enantioenriched (P)-LNNU-2 and (M)-LNNU-2 upon chiral induction of L-Ala and D-Ala. This work provides new ideas for the rational design of MOF-based noncovalent ICD sensors and the screening of suitable chiral inducers for chiral induction synthesis.
Author contributions
Z. S. and C. J. conceived the concept and directed the project. Y. Z. designed the study, conducted most of the experiments, wrote the original draft and revised the manuscript. T. D. performed the experiments. X. Z., Y. Z., J. Y. and X. L. collected and analyzed the data. H. Z., C. J. and Z. S. modified the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21371085 and 21903011), the Central Government-guided Local Scientific and Technological Development Fund, the Dalian Youth Science and Technology Star Fund (2021RQ112), and the State Key Laboratory of Fine Chemicals, Dalian University of Technology (KF2216).References
- G. Pályi, Biological Chirality, Elsevier, London, 2019 Search PubMed.
- V. Davankov, Chirality as an Inherent General Property of Matter, Chirality, 2006, 18, 459–461 CrossRef CAS PubMed.
- D. P. Glavin, A. S. Burton, J. E. Elsila, J. C. Aponte and J. P. Dworkin, The Search for Chiral Asymmetry as a Potential Biosignature in our Solar System, Chem. Rev., 2020, 120, 4660–4689 CrossRef CAS PubMed.
- P. Peluso and B. Chankvetadze, Recognition in the Domain of Molecular Chirality: From Noncovalent Interactions to Separation of Enantiomers, Chem. Rev., 2022, 122, 13235–13400 CrossRef CAS PubMed.
- F. Pop, N. Zigon and N. Avarvari, Main-Group-Based Electro- and Photoactive Chiral Materials, Chem. Rev., 2019, 119, 8435–8478 CrossRef CAS PubMed.
- J. Liu, S. Mukherjee, F. Wang, R. A. Fischer and J. Zhang, Homochiral Metal-Organic Frameworks for Enantioseparation, Chem. Soc. Rev., 2021, 50, 5706–5745 RSC.
- A. Gheorghe, M. A. Tepaske and S. Tanase, Homochiral Metal-Organic Frameworks as Heterogeneous Catalysts, Inorg. Chem. Front., 2018, 5, 1512–1523 RSC.
- I. D. Rukhlenko, N. V. Tepliakov, A. S. Baimuratov, S. A. Andronaki, Y. K. Guńko, A. V. Baranov and A. V. Fedorov, Completely Chiral Optical Force for Enantioseparation, Sci. Rep., 2016, 6, 36884 CrossRef CAS PubMed.
- A. Gogoi, N. Mazumder, S. Konwer, H. Ranawat, N.-T. Chen and G.-Y. Zhuo, Enantiomeric Recognition and Separation by Chiral Nanoparticles, Molecules, 2019, 24, 1007 CrossRef CAS PubMed.
- D. C. Patel, M. F. Wahab, D. W. Armstrong and Z. S. Breitbach, Advances in High-Throughput and High-Efficiency Chiral Liquid Chromatographic Separations, J. Chromatogr. A, 2016, 1467, 2–18 CrossRef CAS PubMed.
- I. Ilisz, A. Péter and W. Lindner, State-of-the-Art Enantioseparations of Natural and Unnatural Amino Acids by High-Performance Liquid Chromatography, Trends Anal. Chem., 2016, 81, 11–22 CrossRef CAS.
- V. Schurig, Practice and Theory of Enantioselective Complexation Gas Chromatography, J. Chromatogr. A, 2002, 965, 315–356 CrossRef CAS PubMed.
- L. Yang, T. Wenzel, R. T. Williamson, M. Christensen, W. Schafer and C. J. Welch, Expedited Selection of NMR Chiral Solvating Agents for Determination of Enantiopurity, ACS Cent. Sci., 2016, 2, 332–340 CrossRef CAS PubMed.
- Y. Zhao and T. M. Swager, Simultaneous Chirality Sensing of Multiple Amines by 19F NMR, J. Am. Chem. Soc., 2015, 137, 3221–3224 CrossRef CAS PubMed.
- G. Storch, M. Haas and O. Trapp, Attracting Enantiomers: Chiral Analytes that are Simultaneously Shift Reagents Allow Rapid Screening of Enantiomeric Ratios by NMR Spectroscopy, Chem. – Eur. J., 2017, 23, 5414–5418 CrossRef CAS PubMed.
- Z. Chen, Q. Wang, X. Wu, Z. Li and Y.-B. Jiang, Optical Chirality Sensing Using Macrocycles, Synthetic and Supramolecular Oligomers/Polymers, and Nanoparticle Based Sensors, Chem. Soc. Rev., 2015, 44, 4249–4263 RSC.
- S. Thoonen and C. Hua, Chiral Detection with Coordination Polymers, Chem. – Asian J., 2021, 16, 890–901 CrossRef CAS PubMed.
- L. Pu, Enantioselective Fluorescent Recognition of Free Amino Acids: Challenges and Opportunities, Angew. Chem., Int. Ed., 2020, 59, 21814–21828 CrossRef CAS PubMed.
- W. Ma, L. Xu, L. Wang, C. Xu and H. Kuang, Chirality-Based Biosensors, Adv. Funct. Mater., 2019, 29, 1805512 CrossRef.
- X. Zhang, J. Yin and J. Yoon, Recent Advances in Development of Chiral Fluorescent and Colorimetric Sensors, Chem. Rev., 2014, 114, 4918–4959 CrossRef CAS PubMed.
- Z. A. De los Santos, C. C. Lynch and C. Wolf, Optical Chirality Sensing with an Auxiliary-free Earth-abundant Cobalt Probe, Angew. Chem., Int. Ed., 2019, 58, 1198–1202 CrossRef CAS PubMed.
- D. Wu, Y. Yu, J. Zhang, L. Guo and Y. Kong, Chiral Poly(ionic liquid) with Nonconjugated Backbone as a Fluorescent Enantioselective Sensor for Phenylalaninol and Tryptophan, ACS Appl. Mater. Interfaces, 2018, 10, 23362–23368 CrossRef CAS PubMed.
- Z.-Z. Weng, H. Xu, W. Zhang, G.-L. Zhuang, L.-S. Long, X.-J. Kong and L.-S. Zheng, Enantioselective Recognition and Separation of C2 Symmetric Substances via Chiral Metal-Organic Frameworks, ACS Appl. Mater. Interfaces, 2021, 13, 37412–37421 CrossRef CAS PubMed.
- B. Li, J. Zhang, L. Li and G. Chen, Rapid and Sensitive Method for Chiroptical Sensing of α-Amino Acids via Click-Like Labeling with o-Phthalaldehyde and p-Toluenethiol, Chem. Sci., 2021, 12, 2504–2508 RSC.
- L. Pu, Chemoselective and Enantioselective Fluorescent Identification of Specific Amino Acid Enantiomers, Chem. Commun., 2022, 58, 8038–8048 RSC.
- Z. Sharifzadeh, K. Berijani and A. Morsali, Chiral Metal-Organic Frameworks Based on Asymmetric Synthetic Strategies and Applications, Coord. Chem. Rev., 2021, 445, 214083 CrossRef CAS.
- M. M. Wanderley, C. Wang, C.-D. Wu and W. Lin, A Chiral Porous Metal-Organic Framework for Highly Sensitive and Enantioselective Fluorescence Sensing of Amino Alcohols, J. Am. Chem. Soc., 2012, 134, 9050–9053 CrossRef CAS PubMed.
- T.-Y. Liu, X.-L. Qu and B. Yan, A Sensitive Metal-Organic Framework Nanosensor with Cation-Introduced Chirality for Enantioselective Recognition and Determination of Quinine and Quinidine in Human Urine, J. Mater. Chem. C, 2020, 8, 14579–14586 RSC.
- P. Chandrasekhar, A. Mukhopadhyay, G. Savitha and J. N. Moorthy, Remarkably Selective and Enantiodifferentiating Sensing of Histidine by a Fluorescent Homochiral Zn–MOF Based on Pyrene-Tetralactic Acid, Chem. Sci., 2016, 7, 3085–3091 RSC.
- Q. Zhang, M. Lei, F. Kong and Y. Yang, A Water-Stable Homochiral Luminescent MOF Constructed from an Achiral Acylamide-Containing Dicarboxylate Ligand for Enantioselective Sensing of Penicillamine, Chem. Commun., 2018, 54, 10901–10904 RSC.
- Z. Han, K. Wang, Y. Guo, W. Chen, J. Zhang, X. Zhang, G. Siligardi, S. Yang, Z. Zhou, P. Sun, W. Shi and P. Cheng, Cation-Induced Chirality in a Bifunctional Metal-Organic Framework for Quantitative Enantioselective Recognition, Nat. Commun., 2019, 10, 5117 CrossRef PubMed.
- S. Li, Y. Zhou and B. Yan, Zirconium Metal Organic Framework-Based Hybrid Sensors with Chiral and Luminescent Centers Fabricated by Postsynthetic Modification for the Detection and Recognition of Tryptophan Enantiomers, Inorg. Chem., 2022, 61, 9615–9622 CrossRef CAS PubMed.
- Y. W. Zhao, Y. Wang and X. M. Zhang, Homochiral MOF as Circular Dichroism Sensor for Enantioselective Recognition on Nature and Chirality of Unmodified Amino Acids, ACS Appl. Mater. Interfaces, 2017, 9, 20991–20999 CrossRef CAS PubMed.
- R. X. Yao, H. H. Fu, B. Yu and X. M. Zhang, Chiral Metal-Organic Frameworks Constructed from Four-fold Helical Chain SBUs for Enantioselective Recognition of α-Hydroxy/Amino Acids, Inorg. Chem. Front., 2018, 5, 153–159 RSC.
- C.-X. Tan, K.-W. Yang, J.-Q. Dong, Y.-H. Liu, Y. Liu, J.-W. Jiang and Y. Cui, Boosting Enantioselectivity of Chiral Organocatalysts with Ultrathin Two-Dimensional Metal-Organic Framework Nanosheets, J. Am. Chem. Soc., 2019, 141, 17685–17695 CrossRef CAS PubMed.
- Y.-W. Zhao and X.-M. Zhang, The Construction of Helicate Metal–Organic Nanotubes and Enantioselective Recognition, J. Mater. Chem. C, 2020, 8, 4453–4460 RSC.
- Y.-H. Han, Y.-C. Liu, X.-S. Xing, C.-B. Tian, P. Lin and S.-W. Du, Chiral Template Induced Homochiral MOFs Built from Achiral Components: SHG Enhancement and Enantioselective Sensing of Chiral Alkamines by Ion Exchange, Chem. Commun., 2015, 51, 14481–14484 RSC.
- Y.-W. Zhao, L.-E. Guo, F.-Q. Zhang, J. Yao and X.-M. Zhang, Turn-On Fluorescence Enantioselective Sensing of Hydroxyl Carboxylic Enantiomers by Metal–Organic Framework Nanosheets with a Homochiral Tetracarboxylate of Cyclohexane Diamide, ACS Appl. Mater. Interfaces, 2021, 13, 20821–20829 CrossRef CAS PubMed.
- X. Zhao, E. T. Nguyen, A. N. Hong, P. Feng and X. Bu, Chiral Isocamphoric Acid: Founding a Large Family of Homochiral Porous Materials, Angew. Chem., Int. Ed., 2018, 57, 7101–7105 CrossRef CAS PubMed.
- S. Allenmark, Induced Circular Dichroism by Chiral Molecular Interaction, Chirality, 2003, 15, 409–422 CrossRef CAS PubMed.
- M. Quan, X.-Y. Pang and W. Jiang, Circular Dichroism Based Chirality Sensing with Supramolecular Host–Guest Chemistry, Angew. Chem., Int. Ed., 2022, 61, e202201258 CAS.
- H. Fenniri, B. L. Deng and A. E. Ribbe, Helical Rosette Nanotubes with Tunable Chiroptical Properties, J. Am. Chem. Soc., 2002, 124, 11064–11072 CrossRef CAS PubMed.
- K. W. Bentley and C. Wolf, Stereodynamic Chemosensor with Selective Circular Dichroism and Fluorescence Readout for in Situ Determination of Absolute Configuration, Enantiomeric Excess, and Concentration of Chiral Compounds, J. Am. Chem. Soc., 2013, 135, 12200–12203 CrossRef CAS PubMed.
- F. Biedermann and W. M. Nau, Noncovalent Chirality Sensing Ensembles for the Detection and Reaction Monitoring of Amino Acids, Peptides, Proteins, and Aromatic Drugs, Angew. Chem., Int. Ed., 2014, 53, 5694–5699 CrossRef CAS PubMed.
- M. Hasan, V. N. Khose, A. D. Pandey, V. Borovkov and A. V. Karnik, Tailor-Made Supramolecular Chirogenic System Based on Cs-Symmetric Rigid Organophosphoric Acid Host and Amino Alcohols: Mechanistic Studies, Bulkiness Effect, and Chirality Sensing, Org. Lett., 2016, 18, 440–443 CrossRef CAS PubMed.
- S. L. Pilicer, P. R. Bakhshi, K. W. Bentley and C. Wolf, Biomimetic Chirality Sensing with Pyridoxal-5′-phosphate, J. Am. Chem. Soc., 2017, 139, 1758–1761 CrossRef CAS PubMed.
- W. Zuo, Z. Huang, Y. Zhao, W. Xu, Z. Liu, X.-J. Yang, C. Jia and B. Wu, Chirality Sensing of Choline Derivatives by a Triple Anion Helicate Cage through Induced Circular Dichroism, Chem. Commun., 2018, 54, 7378–7381 RSC.
- K. Liu, G. Du, L. Ye and L. Jiang, Chiroptical Nanoprobe for Highly Selective Recognition of Histidine Enantiomers in Aqueous Media, Sens. Actuators, B, 2019, 284, 55–62 CrossRef CAS.
- E. Nelson, J. S. S. K. Formen and C. Wolf, Rapid Organocatalytic Chirality Analysis of Amines, Amino Acids, Alcohols, Amino Alcohols and Diols with Achiral Iso(thio)cyanate Probes, Chem. Sci., 2021, 12, 8784–8790 RSC.
- Z. A. De los Santos, S. MacAvaney, K. Russell and C. Wolf, Tandem Use of Optical Sensing and Machine Learning for the Determination of Absolute Configuration, Enantiomeric and Diastereomeric Ratios, and Concentration of Chiral Samples, Angew. Chem., Int. Ed., 2020, 59, 2440–2448 CrossRef CAS PubMed.
- Y.-Y. Zhu, Y.-N. Zhou, X. Zhang, Z.-G. Sun and C.-Q. Jiao, Homochiral MOF as Chiroptical Sensor for Determination of Absolute Configuration and Enantiomeric Ratio of Chiral Tryptophan, Adv. Opt. Mater., 2021, 9, 2001889 CrossRef CAS.
- Y. Zhou and J. Yoon, Recent Progress in Fluorescent and Colorimetric Chemosensors for Detection of Amino Acids, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- J. P. Ruddick, A. K. Evans, D. J. Nutt, S. L. Lightman, G. A. W. Rook and C. A. Lowry, Tryptophan Metabolism in the Central Nervous System: Medical Implications, Expert Rev. Mol. Med., 2006, 8, 1–27 CrossRef PubMed.
- Analytical Methods Committee, Recommendations for the Definition, Estimation and Use of the Detection Limit, Analyst, 1987, 112, 199–204 RSC.
- H. D. Flack and G. Bernardinelli, The Use of X-ray Crystallography to Determine Absolute Configuration, Chirality, 2008, 20, 681–690 CrossRef CAS PubMed.
- R. E. Morris and X. Bu, Induction of Chiral Porous Solids Containing only Achiral Building Blocks, Nat. Chem., 2010, 2, 353–361 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic details, PXRD, FT-IR and CD spectra, thermal analysis, the results of ICP measurements and L-Trp concentration determination. CCDC 1964191, 1964192 and 1964196. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00251a |
| This journal is © the Partner Organisations 2023 |