A dense 3d–4f metal–organic framework with “gas pockets” for highly efficient CH4/N2 separation†
Li-Min
Zhu
a,
Wen-Liang
Li
a,
Tian-Ran
Li
a,
Lin-Ping
Shi
a,
Li-Ting
Li
a,
Zhao-Quan
Yao
*a,
Hong-Liang
Huang
 *b,
Jiong-Peng
Zhao
*b,
Jiong-Peng
Zhao
 *a and
Fu-Chen
Liu
*a and
Fu-Chen
Liu
 *a
*a
aSchool of Chemistry and Chemical Engineering, TKL of Organic Solar Cells and Photochemical Conversion, Tianjin University of Technology, Tianjin 300384, China. E-mail: yaozq@email.tjut.edu.cn; zhaojp@tjut.edu.cn; fcliu@tjut.edu.cn
bSchool of Chemistry and Chemical Engineering, Tiangong University, Tianjin 300387, China. E-mail: huanghongliang@tiangong.edu.cn
First published on 21st March 2023
Abstract
In this work, we report a multi-component MOF [CuCe L(Cl4-bdc)0.5(H2O)2·(H2O)6]n (L = 1H-pyrazole-3,4,5-tricarboxylic acid, Cl4-bdc = 2,3,5,6-tetrachloroterephthalate) with a pillar-layered structure. In the structure, the polydentate ligand (L) linked CuII and CeIII atoms constitute 3d–4f layers that serve as the pedestal base and the chlorinated Cl4-bdc ligands acting as bolsters are installed between the layers through CeIII. The moderate pore size and the unique chloride decorated surface of channels endow this MOF with excellent separation ability for methane (CH4) and nitrogen (N2). According to the adsorption tests, this MOF exhibits a high adsorption capacity for CH4 (28.41 cm3/cm3) at 298 K and 1 bar, while the N2 adsorption capacity is only 3.43 cm3/cm3. The DFT calculations demonstrate that the adjacent Cl4-bdc in the network can act as “gas pockets” or nano traps to immobilize the CH4 molecules effectively through multiple interactions between Cl atoms and CH4. The high performance of this MOF in CH4/N2 separation has been verified by the outstanding IAST selectivity of 13.32 and breakthrough experiments. This work provides a new perspective for capturing CH4 from coal-mine gas to recover fuel and reduce greenhouse gas emissions.
Due to the rapid consumption of fossil fuels, energy and environmental problems have to be solved urgently in this century. Conventional natural gas (mainly composed of methane) is considered to be an ideal alternative to petroleum fuels because of its abundance and cleanliness.1 Unconventional natural gas (such as coal bed methane (CBM), landfill gas, and shale gas) also produces a large amount of methane, which is an excellent complement to conventional natural gas.2 Currently, due to the lack of suitable purification methods, a large amount of methane is introduced into air, which not only brings about a serious greenhouse effect (21 times that from CO2) but also causes a waste of energy.3 Therefore, utilization of this low-quality natural gas is extremely important. Generally, in unconventional natural gas, the concentration of nitrogen (N2) is much higher than that of methane (e.g. the methane concentration in CBM is less than 30%), which limits its direct utilization.2,3 So, the separation of CH4/N2 mixtures to obtain high purity methane has important industrial value. Currently, the prevalent industrial approach to separate methane from CH4/N2 mixtures is cryogenic distillation (boiling point: 112 K for CH4 and 77 K for N2) which is not only an energy-intensive process but also a burden to the environment.4 Thus, a cost- and energy-efficient CH4/N2 separation process is urgently desired.
Compared with traditional cryogenic distillation methods, adsorbent-based gas adsorption using porous materials is an economical and energy-efficient method.5 Many traditional porous materials, such as zeolites and porous carbons, have been extensively studied for CH4 purification.6–12 Although these kinds of porous material adsorbents have been widely reported and have exhibited prominent adsorption separation performance toward CH4/N2 mixtures, their selectivity and yields are still low. The main reason is the similar physical properties of CH4 and N2, including polarizability and kinetic diameters (3.8 Å for CH4 and 3.64 Å for N2).13–23 As a new class of crystalline porous materials, metal–organic frameworks (MOFs) featuring diverse structures, programmable topology, and tailorable and modifiable porosity offer an ideal platform for gas adsorption and separation.3,24–26 Besides, the crystalline nature of MOFs also provides an opportunity to investigate the gas adsorption mechanisms. Up to now, various methods have been used to improve the separation performance of CH4/N2 in MOF systems including the design and synthesis of new structures, decorating with functional groups, compositing with other materials etc. By using these methods, although the interaction between CH4 and frameworks has been improved, the adsorption and separation performances are still unsatisfactory.18,20,27,28 Inspired by the vital effect of R–H⋯X (X = heteroatoms in MOFs) interaction in hydrocarbon (alkene and alkane, alkene and alkyne) separation,29–34 decorating heteroatoms in the inner wall of channels or frameworks might endow the frameworks with a stronger binding effect for CH4. Besides, considering the non-polar nature of CH4 molecules, anchoring heteroatoms to specific sites (e.g. cages or nanopores) using the inherent confinement effect to enhance the host framework–guest CH4 molecule interaction might be a promising protocol (Scheme 1).
In this work, a novel pillar-layered MOF [CuCeL(Cl4-bdc)0.5(H2O)2 (H2O)6]n (1) (L: 3,4,5-pyrazoletricarboxylate; Cl4-bdc: 2,3,5,6-tetrachloroterephthalate) has been constructed successfully. In this structure, L connected CeIII and CuII atoms form 3d–4f layers, while the Cl4-bdc ligands which act as pillars are installed between layers through CeIII to form the 3D-pillar-layered framework with obvious 1D channels (9.0 Å × 9.5 Å) along the a-axis. On the sides of the channels, a large number of small hallways constructed by the adjacent Cl4-bdc ligands were formed (5.0 Å × 7.0 Å × 9.5 Å). According to the result of DFT calculation, due to the synergistic effect between the confinement effect and abundant C–H⋯Cl interactions in these small hallways, the methane molecules can be trapped in these confined spaces effectively. As a result, 1 presents a high adsorption capacity for CH4 (28.41 cm3/cm3 at 298 K) and a high IAST selectivity of 13.32 for CH4/N2 separation.
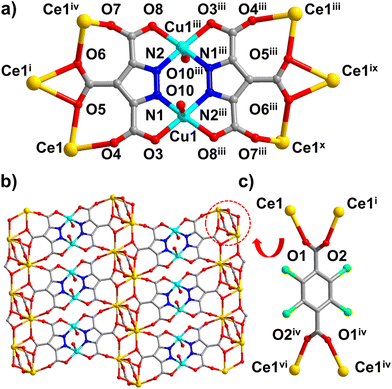
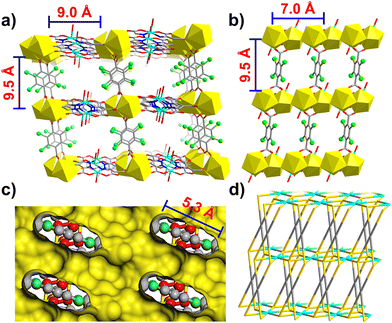
Single-crystal X-ray diffraction study on a suitable single crystal of [CuCeL(Cl4-bdc)0.5(H2O)2·(H2O)6]n at 298 K revealed that 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S1†) with high densities of 2.145 g cm−3 and 1.700 g cm−3 for the complex and the activated framework, respectively. The asymmetric unit cell has one CeIII, one CuII, one L ligand, half Cl4-bdc ligand, two coordinated water molecules and six free water molecules (Fig. S1†). As shown in Fig. 1a, each Cu ion presents a pyramidal coordination geometry: two Cu1 are chelated by two L ligands to form a Cu2N4O4 coordination plane, in which N1, O3, and N2iii, O8iii are assigned to two L ligands, respectively. The axial positions of each Cu ion are occupied by one water molecule (O10). In this manner, two Cu ions (Cu1 and Cu1iii) are bridged by two L ligands forming a Cu2L2 plane (Fig. 1a, Table S2†). The residual carboxyl oxygen atoms in L are coordinated to six Ce ions in the chelating mode to form the 3d–4f Cu–Ce layer (Fig. 1b). In this layer structure, each CeIII ion is coordinated with five oxygen atoms which can be assigned to three L ligands (O4, O5, O6, O7) and a water molecule (O9), respectively. However in the perpendicular direction of the layer, each CeIII ion is coordinated with four oxygen atoms which come from two Cl4-bdc ligands (O1 and O2) (Fig. 1c and S2†). Consequently, the Cl4-bdc ligands serve as pillars to sustain the neighbouring 3d–4f layers into a 3D framework with 1D open channels along the a and c axes (Fig. 2a–c).
(Table S1†) with high densities of 2.145 g cm−3 and 1.700 g cm−3 for the complex and the activated framework, respectively. The asymmetric unit cell has one CeIII, one CuII, one L ligand, half Cl4-bdc ligand, two coordinated water molecules and six free water molecules (Fig. S1†). As shown in Fig. 1a, each Cu ion presents a pyramidal coordination geometry: two Cu1 are chelated by two L ligands to form a Cu2N4O4 coordination plane, in which N1, O3, and N2iii, O8iii are assigned to two L ligands, respectively. The axial positions of each Cu ion are occupied by one water molecule (O10). In this manner, two Cu ions (Cu1 and Cu1iii) are bridged by two L ligands forming a Cu2L2 plane (Fig. 1a, Table S2†). The residual carboxyl oxygen atoms in L are coordinated to six Ce ions in the chelating mode to form the 3d–4f Cu–Ce layer (Fig. 1b). In this layer structure, each CeIII ion is coordinated with five oxygen atoms which can be assigned to three L ligands (O4, O5, O6, O7) and a water molecule (O9), respectively. However in the perpendicular direction of the layer, each CeIII ion is coordinated with four oxygen atoms which come from two Cl4-bdc ligands (O1 and O2) (Fig. 1c and S2†). Consequently, the Cl4-bdc ligands serve as pillars to sustain the neighbouring 3d–4f layers into a 3D framework with 1D open channels along the a and c axes (Fig. 2a–c).
As shown in Fig. 2a, the framework of 1 has moderate 1D channels with a rectangular cross-section in dimensions of 9.0 × 9.5 Å along the a-axis. The chlorinated Cl4-bdc ligands which serve as pillars are located on either side of the channels and provide a large number of chloride atoms on the inner wall of channels. Besides, between the two neighbouring CuII–CeIII layers, the adjacent Cl4-bdc ligands also form small cavities or confined spaces in dimensions of 9.5 × 7.0 × 5.8 Å which are vertically distributed on both sides of the one-dimensional channel (Fig. 2c). Due to the narrow size of these cavities and abundant chlorine binding sites, various gas molecules with a small size could access the cavity and be trapped within these “gas pockets”. In addition, PLATON analysis shows that the effective free volume of 1 can reach up to 43.3% of the crystal volume after squeezing out free water molecules.35 To further investigate the structure, the topological simplification of the structure was carried out. As shown in Fig. 2d, the Cu2L2 units which connected to six CeIII ions can be viewed as six connected nodes, while the Cl4-bdc ligands which coordinated with four CeIII ions can be viewed as four-connected nodes. The CeIII ions could be viewed as five-connected nodes. Finally, the whole framework can be simplified as a 4,5,6-connected topological network using the Schläfli notation {42;84}20{48;62}2{46;66;83}.36–38
Due to the moderate pore size, large porosity, and exposed halogen atoms in specific cavities, this MOF may have a special interaction with some gas molecules and is beneficial in the field of adsorption and separation. Before the adsorption investigation, the purity of 1 and the rigidity of the framework were verified by PXRD and IR under different conditions (Fig. 3a and Fig. S3†). The PXRD data reveal that all diffraction peaks in the experimental patterns match well with the simulation, suggesting the high purity of the synthesized sample. Furthermore, the PXRD patterns of the samples which were immersed in H2O, acetonitrile, and DMF solutions for 24 hours are also consistent with those obtained by simulation. Even for the sample activated at 146 °C for 8 h, both the PXRD pattern and FT-IR spectra matched well with those of the pristine sample which indicates that 1 could keep the framework intact under these conditions (Fig. 3a and S3†). The thermogravimetric analysis (TGA) of this MOF reveals an obvious weight loss of 20% when the temperature was increased to 152 °C, corresponding to the loss of coordinated and free water molecules in channels (calculated to be 20.7%) and the framework did not collapse until 320 °C, which suggested the good thermal stability of the MOF. The TGA curve of the activated sample showed that almost all water molecules had been removed under the activation process. And the slight mass loss at a low temperature indicated there were still some residues in the channel, which may be attributed to the adsorption of water or VOCs in the air of the activated sample during the sample transfer process.39 Besides, the framework remained intact until 320 °C, which further proves the excellent stability and rigidity of the framework.
 | ||
| Fig. 3 (a) Powder X-ray diffraction patterns of 1 under different conditions. (b) TGA curves of 1 before and after activation. | ||
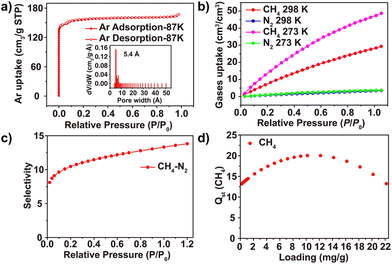
Due to the smaller molecular radius and the better packing ability on the inner surface of Ar than those of diatomic N2 molecules, the 87 K Ar adsorption measurement was carried out for investigating the permanent porosity of 1. As shown in Fig. 4a, 1 exhibits a reversible type-I isotherm with the characteristic of microporosity, and the Brunauer–Emmett–Teller (BET) surface area was estimated to be 536.63 m2 g−1. The experimental total pore volume was determined to be 0.214 cm3 g−1 (calculated by the single point method at P/P0 = 0.98). Using nonlocal density functional theory, the pore size distribution was calculated to be 5.4 Å, which is consistent with the value from SCXRD data. Owing to the microporous nature and the unique chlorinated “gas pocket” structure, the adsorption capacity for CH4 gas of 1 was tested at 273 and 298 K, respectively. As shown in Fig. 4b and Fig. S4,† the CH4 absorption at 273 K and 298 K can reach up to 47.36 cm3/cm3 and 28.41 cm3/cm3, respectively. However under the same conditions, the adsorption capacities for N2 are only 3.67 cm3/cm3 (273 K) and 3.43 cm3/cm3 (298 K). The isosteric enthalpies of adsorption (Qst) of this MOF for CH4 were calculated from the virial equation based on the isotherms collected at 273 and 298 K. As shown in Fig. 4d, the initial Qst value for CH4 of 1 is 13.2 kJ mol−1. Besides, with the increase of adsorption capacity, the Qst values for CH4 exhibit a turnover behaviour.
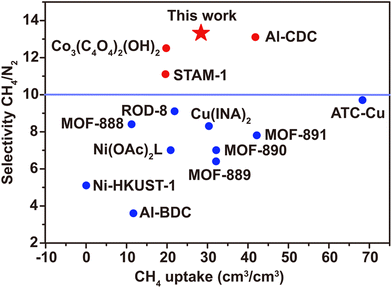
For an industrial process the volume adsorption capacity is more important than the mass adsorption capacity, and the volume adsorption capacity for CH4 is a key metric for evaluating the separation effect. As shown in Fig. 5, the adsorption capacity of 1 (28.41 cm3/cm3) exceeds that of Co3(C4O4)2(OH)2 (19.81 cm3/cm3), ROD-8 (21.89 cm3/cm3), and Ni (OAc)2L (20.92 cm3/cm3) and is comparable to that of Cu(NIA)2, MOF-890 and MOF-889 (Table S3†) but is smaller than that of MOF-891, Al-CDC and ACT-Cu.14,40,41 In order to verify the separation ability of 1 for CH4/N2 separation, the ideal adsorption solution theory (IAST) was used to investigate its CH4/N2 selectivity. After calculation and fitting, the IAST selectivity of 1 is shown in Fig. 4c, which indicates a gradually increasing trend in the whole pressure range, and the maximum selectivity at normal pressure is 13.32, which is rare in published MOFs and higher than that of Co3(C4O4)2(OH)2 (12.5), STAM-1 (11.1) and Al-CDC (13.1) in the first echelon (detailed information can be found in the ESI (Table S3†)).
In addition, DFT calculations were used to investigate the interactions between the MOF and CH4 molecules. As shown in Fig. 6, multiple interactions occurred between CH4 and adjacent Cl4-bdc with a binding energy of CH4 of −13.95 kJ mol−1, and the distances between the hydrogen atoms of CH4 and the Cl atoms in Cl4-bdc ranged from 3.154 to 4.359 Å, which is in the range of the van der Waals’ (vdW) interaction. Besides, the adjacent Cl4-bdc ligands can form confined spaces which not only exhibit multipoint vdW interactions between CH4 and the host framework but also enhance the interaction by the confinement effect. DFT calculations show that the surface C–Cl plays an important role in the enhancement of the adsorption selectivity of the MOF toward CH4/N2 mixtures.
 | ||
| Fig. 6 (a and b) Preferential adsorption sites and binding energies of CH4 in the MOF obtained from the DFT-optimized structural model; BE stands for binding energy in (b). | ||
Static adsorption and selectivity calculation indicated that 1 has great advantages in the separation of CH4/N2. To further confirm the separation performance of 1 for mixed gases in practical applications, two consecutive cycles of breakthrough experiments of 1 for a CH4/N2 (50/50, v/v) mixture were carried out. As shown in Fig. 7, the adsorption time of CH4 was longer than that of N2 during the process. N2 first broke through the packed column, whereas CH4 was preferentially adsorbed by 1 and broke through the column after a longer time delay which means that a clear separation between CH4 and N2 was achieved. Besides, during these two consecutive cycles of breakthrough experiments, the separation ability of 1 remains almost unchanged which demonstrates the good circulation stability of 1 in actual application. The above results indicate that 1 possesses good thermal/chemical stability, high separation selectivity for CH4/N2 and good recyclability which make this MOF have potential application in CH4 purification.
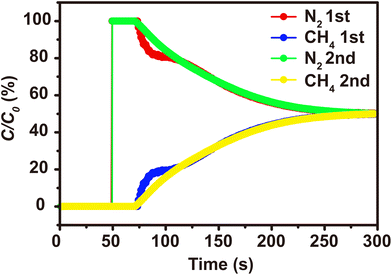 | ||
Fig. 7 Experimental breakthrough curves of N2/CH4 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1 v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 298 K during two consecutive cycles (red and blue for the 1st cycle; green and yellow for the 2nd cycle). 1) at 298 K during two consecutive cycles (red and blue for the 1st cycle; green and yellow for the 2nd cycle). | ||
In conclusion, a pillar-layered multi-component metal–organic framework [CuCeL(Cl4-bdc)0.5(H2O)2·(H2O)6]n was constructed. Through the coordination bonds between Cl4-bdc ligands and adjacent 3d–4f layers, 1D open channels along the a-axis and small confined cavities along the sides of channels were constructed precisely. Due to the synergistic effect between the abundant Cl atoms in Cl4-bdc which can form multiple C–H⋯Cl interactions and the confinement effect which enhances the interaction between guest molecules and host framework, this MOF exhibits a strong binding effect for CH4. More strikingly, this synergistic effect can enhance the CH4 affinity and endow 1 with great separation ability for CH4/N2. As a result, 1 presents a high CH4 adsorption capacity of 28.41 cm3/cm3 at 298 K and high IAST selectivity of 13.32 for CH4/N2 separation. DFT calculations show that the confined cavity which is decorated by chlorine atoms is crucial to binding CH4 molecules and can be used as a molecular trap for CH4. The breakthrough experiments revealed that the MOF-based CH4 nano-trap is a new benchmark for CH4/N2 separation and this MOF can be a potential material for capturing CH4 from coal-mine methane to recover fuel and reduce greenhouse gas emissions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21571139, 22035003, 22001132, 22271217).References
- Y. He, W. Zhou, G. Qian and B. Chen, Methane storage in metal-organic frameworks, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC.
- D. Saha, H. A. Grappe, A. Chakraborty and G. Orkoulas, Postextraction separation, on-board storage, and catalytic conversion of methane in natural gas: a review, Chem. Rev., 2016, 116, 11436–11499 CrossRef CAS PubMed.
- M. Chang, Y. Zhao, D. Liu, J. Yang, J. Li and C. Zhong, Methane-trapping metal–organic frameworks with an aliphatic ligand for efficient CH4/N2 separation, Sustainable Energy Fuels, 2020, 4, 138–142 RSC.
- S. M. Wang, M. Shivanna and Q. Y. Yang, Nickel-based metal-organic frameworks for coal-bed methane purification with record CH4/N2 selectivity, Angew. Chem., Int. Ed., 2022, 61, e202201017 CAS.
- H. Wang, Y. Liu and J. Li, Designer metal-organic frameworks for size-exclusion-based hydrocarbon separations: progress and challenges, Adv. Mater., 2020, 32, 2002603 CrossRef CAS PubMed.
- J. Liu, H. Shang, J. Yang, J. Wang, J. Li and S. Deng, Novel zeolite/carbon monolith adsorbents for efficient CH4/N2 separation, Chem. Eng. J., 2021, 426, 130163 CrossRef CAS.
- R. Tang, Q. Dai, W. Liang, Y. Wu, X. Zhou, H. Pan and Z. Li, Synthesis of novel particle rice-based carbon materials and its excellent CH4/N2 adsorption selectivity for methane enrichment from Low-rank natural gas, Chem. Eng. J., 2020, 384, 123388 CrossRef CAS.
- Z. Yang, D. Wang, Z. Meng and Y. Li, Adsorption separation of CH4/N2 on modified coal-based carbon molecular sieve, Sep. Purif. Technol., 2019, 218, 130–137 CrossRef CAS.
- Y. Wu, D. Yuan, D. He, J. Xing, S. Zeng, S. Xu, Y. Xu and Z. Liu, Decorated traditional zeolites with subunits of metal-organic frameworks for CH4 /N2 separation, Angew. Chem., Int. Ed., 2019, 58, 10241–10244 CrossRef CAS PubMed.
- S. Xu, W. C. Li, C. T. Wang, L. Tang, G. P. Hao and A. H. Lu, Self-pillared ultramicroporous carbon nanoplates for selective separation of CH4 /N2, Angew. Chem., Int. Ed., 2021, 60, 6339–6343 CrossRef CAS PubMed.
- Q. Shi, J. Wang, H. Shang, H. Bai, Y. Zhao, J. Yang, J. Dong and J. Li, Effective CH4 enrichment from N2 by SIM-1 via a strong adsorption potential SOD cage, Sep. Purif. Technol., 2020, 230, 115850 CrossRef CAS.
- M. Chang, T. Yan, Y. Wei, J.-X. Wang, D. Liu and J.-F. Chen, Enhancing CH4 capture from coalbed methane through tuning van der Waals affinity within isoreticular Al-based metal–organic frameworks, ACS Appl. Mater. Interfaces, 2022, 14, 25374–25384 CrossRef CAS PubMed.
- Z. Niu, X. Cui, T. Pham, P. C. Lan, H. Xing, K. A. Forrest, L. Wojtas, B. Space and S. Ma, A metal-organic framework based methane nano-trap for the capture of coal-mine methane, Angew. Chem., Int. Ed., 2019, 58, 10138–10141 CrossRef CAS PubMed.
- C. E. Kivi, B. S. Gelfand, H. Dureckova, H. T. K. Ho, C. Ma, G. K. H. Shimizu, T. K. Woo and D. Song, 3D porous metal-organic framework for selective adsorption of methane over dinitrogen under ambient pressure, Chem. Commun., 2018, 54, 14104–14107 RSC.
- D. Saha, G. Orkoulas, S. Yohannan, H. C. Ho, E. Cakmak, J. Chen and S. Ozcan, Nanoporous boron nitride as exceptionally thermally stable adsorbent: role in efficient separation of light hydrocarbons, ACS Appl. Mater. Interfaces, 2017, 9, 14506–14517 CrossRef CAS PubMed.
- K. X. Yao, Y. Chen, Y. Lu, Y. Zhao and Y. Ding, Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading, Carbon, 2017, 122, 258–265 CrossRef CAS.
- X. Ren, T. Sun, J. Hu and S. Wang, Highly enhanced selectivity for the separation of CH4 over N2 on two ultra-microporous frameworks with multiple coordination modes, Microporous Mesoporous Mater., 2014, 186, 137–145 CrossRef CAS.
- P. T. K. Nguyen, H. T. D. Nguyen, H. Q. Pham, J. Kim, K. E. Cordova and H. Furukawa, Synthesis and selective CO2 capture properties of a series of hexatopic linker-based metal–organic frameworks, Inorg. Chem., 2015, 54, 10065–10072 CrossRef CAS PubMed.
- X. W. Liu, Y. Guo, A. Tao, M. Fischer, T. J. Sun, P. Z. Moghadam, D. Fairen-Jimenez and S. D. Wang, “Explosive” synthesis of metal-formate frameworks for methane capture: an experimental and computational study, Chem. Commun., 2017, 53, 11437–11440 RSC.
- J. Hu, T. Sun, X. Liu, Y. Guo and S. Wang, Separation of CH4/N2 mixtures in metal–organic frameworks with 1D micro-channels, RSC Adv., 2016, 6, 64039–64046 RSC.
- Y. Guo, J. Hu, X. Liu, T. Sun, S. Zhao and S. Wang, Scalable solvent-free preparation of [Ni3(HCOO)6] frameworks for highly efficient separation of CH4 from N2, Chem. Eng. J., 2017, 327, 564–572 CrossRef CAS.
- A. Jayaraman, A. J. Hernandez-Maldonado, R. T. Yang, D. Chinn, C. L. Munson and D. H. Mohr, Clinoptilolites for nitrogen/methane separation, Chem. Eng. Sci., 2004, 59, 2407–2417 CrossRef CAS.
- D. Saha, Z. Bao, F. Jia and S. Deng, Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A, Environ. Sci. Technol., 2010, 44, 1820–1826 CrossRef CAS PubMed.
- H. Noh, C.-W. Kung, T. Islamoglu, A. W. Peters, Y. Liao, P. Li, S. J. Garibay, X. Zhang, M. R. DeStefano, J. T. Hupp and O. K. Farha, Room temperature synthesis of an 8-connected Zr-based metal–organic framework for top-down nanoparticle encapsulation, Chem. Mater., 2018, 30, 2193–2197 CrossRef CAS.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Microporous metal-organic framework materials for gas separation, Chem, 2020, 6, 337–363 CAS.
- S. Wang, H. Reinsch, N. Heymans, M. Wahiduzzaman, C. Martineau-Corcos, G. De Weireld, G. Maurin and C. Serre, Toward a rational design of titanium metal-organic frameworks, Matter, 2020, 2, 440–450 CrossRef CAS.
- M. Chang, J. Ren, Q. Yang and D. Liu, A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation, Chem. Eng. J., 2021, 408, 127294 CrossRef CAS.
- X. Jia, N. Yuan, L. Wang, J. Yang and J. Li, (CH3)2NH–assisted synthesis of high–purity Ni–HKUST–1 for the adsorption of CO2, CH4, and N2, Eur. J. Inorg. Chem., 2018, 8, 1047–1052 CrossRef.
- R. B. Lin, L. Li, H. L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Molecular sieving of ethylene from ethane using a rigid metal-organic framework, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, A metal-organic framework-based splitter for separating propylene from propane, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- H. Wang, X. Dong, V. Colombo, Q. Wang, Y. Liu, W. Liu, X. L. Wang, X. Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Tailor-made microporous metal-organic frameworks for the full separation of propane from propylene through selective size exclusion, Adv. Mater., 2018, 30, 1805088 CrossRef PubMed.
- B. Liang, X. Zhang, Y. Xie, R. B. Lin, R. Krishna, H. Cui, Z. Li, Y. Shi, H. Wu, W. Zhou and B. Chen, An ultramicroporous metal-organic framework for high sieving separation of propylene from propane, J. Am. Chem. Soc., 2020, 142, 17795–17801 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, P. Zhang, J. Hu, R. Lin, Q. Deng, Z. Zeng, H. Xing, S. Deng and B. Chen, Optimizing pore space for flexible-robust metal-organic framework to boost trace acetylene removal, J. Am. Chem. Soc., 2020, 142, 9744–9751 CrossRef CAS PubMed.
- T. Ke, Q. Wang, J. Shen, J. Zhou, Z. Bao, Q. Yang and Q. Ren, Molecular sieving of C2-C3 alkene from alkyne with tuned threshold pressure in robust layered metal-organic frameworks, Angew. Chem., Int. Ed., 2020, 59, 12725–12730 CrossRef CAS PubMed.
- Y. T. Zhang, X. L. Wang, E. L. Zhou, X. S. Wu, B. Q. Song, K. Z. Shao and Z. M. Su, Polyoxovanadate-based organic-inorganic hybrids: from {V5O9Cl} clusters to nanosized octahedral cages, Dalton Trans., 2016, 45, 3698–3701 RSC.
- M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets, Acc. Chem. Res., 2009, 41, 1782–1789 CrossRef PubMed.
- V. Robins, S. J. Ramsden and S. T. Hyde, 2D hyperbolic groups induce three-periodic euclidean reticulations, Eur. Phys. J. B, 2004, 39, 365–375 CrossRef CAS.
- A. P. Shevchenko, I. A. Blatov, E. V. Kitaeva and V. A. Blatov, Local coordination versus overall topology in crystal structures: deriving knowledge from crystallographic databases, Cryst. Growth Des., 2016, 17, 774–785 CrossRef.
- L.-H. Xie, X.-M. Liu, T. He and J.-R. Li, Metal-Organic Frameworks for the Capture of Trace Aromatic Volatile Organic Compounds, Chem, 2018, 4, 1911–1927 CAS.
- L. Li, L. Yang, J. Wang, Z. Zhang, Q. Yang, Y. Yang, Q. Ren and Z. Bao, Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework, AIChE J., 2018, 64, 3681–3689 CrossRef CAS.
- R.-J. Li, M. Li, X.-P. Zhou, S. W. Ng, M. O'Keeffe and D. Li, ROD-8, a rod MOF with a pyrene-cored tetracarboxylate linker: framework disorder, derived nets and selective gas adsorption, CrystEngComm, 2014, 16, 6291–6295 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional crystallographic data. CCDC 2223795. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00235g |
| This journal is © the Partner Organisations 2023 |