Utility of redox-active ligands for reversible multi-electron transfer in uranyl(vi) complexes†
Abstract
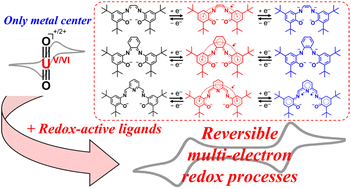
In most cases, the redox activity of a UVIO22+ complex is regarded as metal-centered phenomena, because uranium has small energy gaps amongst the 5f/6d/7s subshells, thereby exhibiting a wide range of oxidation states, commonly from +III to +VI or in some cases even +I or +II. While a wide variety of redox-active ligands are known for use as transition metal complexes including multi-electron reduction that could facilitate inert bond or small molecule activation, only a few such examples are known for UVIO22+. In this study, three UVIO22+ complexes bearing α-diimine-, o-quinonediimine- and 2,6-diiminopyridine-based ligands were synthesized, which exhibited two redox couples in the range of −0.79 V to −2.02 V vs. Fc+/0 to give singly- and doubly-reduced complexes by stepwise reduction. Unique electronic transitions of UVIO22+ complexes with a variety of low-lying excited states helped us to combine spectroelectrochemistry and time-dependent density functional theory (TD-DFT) calculations which complemented each other to assign the redox-active site in these UVIO22+ complexes, i.e., whether or not a ligand of interest becomes redox-active. During all the redox processes observed here, the ligands employed are found to be exclusively redox-active, i.e., non-innocent, whereas the centered UVIO22+ is just “spectating” and remains unchanged, i.e., innocent. Whereas the double reduction of the UVIO22+ complexes usually involves breaking of strong U![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O bonds, in the present examples this is not required and therefore a basis for the synthesis of new types of uranium molecular catalysts and magnetic materials may be found.
O bonds, in the present examples this is not required and therefore a basis for the synthesis of new types of uranium molecular catalysts and magnetic materials may be found.


 Please wait while we load your content...
Please wait while we load your content...