Open Access Article
Open Access ArticleMelilite oxychalcogenide Sr2FeGe2OS6: a phase-matching IR nonlinear optical material realized by isomorphous substitution†
He-Di
Yang‡
abc,
Sheng-Hua
Zhou‡
abd,
Mao-Yin
Ran
abd,
Xin-Tao
Wu
 abd,
Hua
Lin
abd,
Hua
Lin
 *abd and
Qi-Long
Zhu
*abd and
Qi-Long
Zhu
 *abd
*abd
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: linhua@fjirsm.ac.cn; qlzhu@fjirsm.ac.cn
bFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350002, China
cCollege of Chemistry, Fuzhou University, Fujian 350002, China
dUniversity of the Chinese Academy of Sciences, Beijing 100049, China
First published on 20th February 2023
Abstract
Transition-metal-based chalcogenides have recently emerged as greatly promising infrared nonlinear optical (IR-NLO) candidates due to their unique structural chemistries and rich optical properties. However, Fe-based IR-NLO chalcogenides with phase-matching (PM) features have not yet been reported. In this work, a new non-centrosymmetric (NCS) melilite oxychalcogenide, Sr2FeGe2OS6, has been prepared by an isomorphous substitution method, and the relationships between the microscopic crystal structure and macroscopic NLO activity were systematically investigated. Sr2FeGe2OS6 adopts the tetragonal space group of P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m (no. 113) and features a unique two-dimensional structure with Cairo pentagonal tiling layers formed by the alternating connection of [Ge2OS6] dimers and [FeS4] tetrahedra via corner-sharing and with the charge-balanced Sr2+ cations between these layers. Excitingly, Sr2FeGe2OS6 is the first Fe-based example capable of achieving PM in the IR-NLO chalcogenide system and displays an outstanding IR-NLO comprehensive performance, including a wide energy gap (Eg = 2.24 eV), sufficient second-harmonic-generation (SHG) efficiency (deff = 5.89 pm V−1 at 2050 nm) and strong laser-induced damage threshold (LIDT = 14.42 MW cm−2). Deeper structural and theoretical analysis suggests that the ordered arrangement of NLO-active motifs, [Ge2OS6] dimers and [FeS4] tetrahedra, makes significant contributions to the strong deff and large birefringence (Δn). This work not only demonstrates a PM Fe-based NCS material for the first time but also puts forward a new design avenue for high-performance IR-NLO materials.
21m (no. 113) and features a unique two-dimensional structure with Cairo pentagonal tiling layers formed by the alternating connection of [Ge2OS6] dimers and [FeS4] tetrahedra via corner-sharing and with the charge-balanced Sr2+ cations between these layers. Excitingly, Sr2FeGe2OS6 is the first Fe-based example capable of achieving PM in the IR-NLO chalcogenide system and displays an outstanding IR-NLO comprehensive performance, including a wide energy gap (Eg = 2.24 eV), sufficient second-harmonic-generation (SHG) efficiency (deff = 5.89 pm V−1 at 2050 nm) and strong laser-induced damage threshold (LIDT = 14.42 MW cm−2). Deeper structural and theoretical analysis suggests that the ordered arrangement of NLO-active motifs, [Ge2OS6] dimers and [FeS4] tetrahedra, makes significant contributions to the strong deff and large birefringence (Δn). This work not only demonstrates a PM Fe-based NCS material for the first time but also puts forward a new design avenue for high-performance IR-NLO materials.
Introduction
Nonlinear optical (NLO) technology plays an increasingly important role in materials analysis, high-resolution imaging, laser communication and other scientific research studies.1–5 In addition, pulse lasers, optical modulators and optical memory based on NLO technology are generally applied in today's industrial society and life.6,7 The existing commercial inorganic infrared (IR) NLO crystals AgGaS2, AgGaSe2, and ZnGeP2 cannot meet the increasing market demand because of their inherent defects of low laser-induced damage threshold (LIDT), non-phase-matching (NPM) behaviour and two-photon absorption, respectively.8–10 Therefore, it is crucial to design and synthesize new compounds that can combine non-centrosymmetric (NCS) structures and excellent comprehensive characteristics.Transition-metal (TM) elements show a variety of physical and chemical properties due to their unique electronic structures, which hold a place of importance in the NLO field.11–14 To date, more than 500 TM-based IR-NLO materials have been reported, which show extraordinary advantages as potential powerful candidates. First, benefiting from the second-order Jahn–Teller effect, they can reduce the energy barrier and then form a variety of coordination modes. Second, three common [TMQ2], [TMQ3] and [TMQ4] basic building units (BBUs) in this system not only exist independently, but can also combine with others to form one-dimensional (1D) chains, two-dimensional (2D) layers and three-dimensional (3D) frameworks. Third, they have low melting points and good physical and chemical stabilities, indicating the easy availability of large-size crystals. At present, promising TM-based IR-NLO materials are mainly based on the d10 elements, while other d-orbit TM-centered products are relatively few, especially Fe-based materials.15–17 More than one thousand Fe-based NCS compounds of various types have been reported in the past century, for example, chalcogenides (e.g., FeMo2S4,18 Cu1.068Fe1.068S2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19), oxides (e.g., FeGaO3,20 BiFeO3
19), oxides (e.g., FeGaO3,20 BiFeO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21), phosphides (e.g., LaFexCo2−xP2,22 An2Fe12P7 (An = U, Th)23), halides (e.g., Na2Fe2F7,24 LiMnFeF6
21), phosphides (e.g., LaFexCo2−xP2,22 An2Fe12P7 (An = U, Th)23), halides (e.g., Na2Fe2F7,24 LiMnFeF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25), and oxyhalides (e.g., CaFeO2Cl,26 KFe(C2O4)F
25), and oxyhalides (e.g., CaFeO2Cl,26 KFe(C2O4)F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27). However, two- or multi-photon absorption is a frequent occurrence because of their narrow band gaps (Eg < 2.0 eV),28,29 so most of them have been excluded from the NLO field.
27). However, two- or multi-photon absorption is a frequent occurrence because of their narrow band gaps (Eg < 2.0 eV),28,29 so most of them have been excluded from the NLO field.
In 2010, Li's group synthesized the first NLO-active ferroborate crystal, K2Fe2B2O7 (space group P321 (no. 150)),30 which possesses [FeO4] and [BO3] BBUs in its 2D layered structure and displays weak second harmonic generation (SHG) efficiency (deff = 0.4 × KDP). In 2019, Mei's group reported a germanium-based sulfide, Ba6Cu2FeGe4S16 (space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d (no. 220)).31 Its 3D structure is composed of [GeS4] and [Ge(Cu/Fe)3S13] units and a [BaS8] dodecahedron and has an Eg of 1.72 eV and a deff of 1.5 × AgGaSe2. Then, Wang's group discovered a quaternary K2FeGe3S8,32 which adopts the space group of P21 (no. 4), constructed from 1D [FeGeS4] chains connected by [Ge2S4] units. K2FeGe3S8 exhibits a large Eg (2.1 eV) and a small deff (0.25 × AgGaS2). Recently, Cu2FeSiS4 with antiferromagnetic and weak SHG susceptibility has been reported by Tan's group,33 which crystallizes in Pmn21 (no. 31) and displays a wurtzite structure composed of hexagonally close packed S and inserted metal ions. Unfortunately, all of these show normal dispersion n(2w) > n(w), indicating NPM behaviour.34 Generally, a NPM nature dramatically decreases their ultimate NLO output efficiency and hinders their practical applications. For IR-NLO materials, phase-matching (PM) is a critical factor to realize applications in IR lasers and is beneficial for increasing energy conversion efficiency.35–39
3d (no. 220)).31 Its 3D structure is composed of [GeS4] and [Ge(Cu/Fe)3S13] units and a [BaS8] dodecahedron and has an Eg of 1.72 eV and a deff of 1.5 × AgGaSe2. Then, Wang's group discovered a quaternary K2FeGe3S8,32 which adopts the space group of P21 (no. 4), constructed from 1D [FeGeS4] chains connected by [Ge2S4] units. K2FeGe3S8 exhibits a large Eg (2.1 eV) and a small deff (0.25 × AgGaS2). Recently, Cu2FeSiS4 with antiferromagnetic and weak SHG susceptibility has been reported by Tan's group,33 which crystallizes in Pmn21 (no. 31) and displays a wurtzite structure composed of hexagonally close packed S and inserted metal ions. Unfortunately, all of these show normal dispersion n(2w) > n(w), indicating NPM behaviour.34 Generally, a NPM nature dramatically decreases their ultimate NLO output efficiency and hinders their practical applications. For IR-NLO materials, phase-matching (PM) is a critical factor to realize applications in IR lasers and is beneficial for increasing energy conversion efficiency.35–39
As hotspots of recent research, oxychalcogenides have demonstrated that the structural evolution from a single anion unit to a heteroanionic unit can enhance anisotropy and achieve a good balance between wide Eg and sufficiently large deff.40–43 Among them, melilite, AE2MM′2OS6 (where M and M′ represent a variety of metal elements in divalent to tetravalent states), has attracted considerable attention owing to its structural flexibility at each crystallographic site.44–48 These NCS oxychalcogenides adopt the tetragonal crystal system with the space group of P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m (no. 113), which displays alternating 2D [MM′2OS6]4− layers with the charge-balanced AE2+ cations occupying the space between these layers. In this work, a new NCS melilite oxychalcogenide, Sr2FeGe2OS6, has been prepared by an isomorphous substitution method. Excitingly, it is the first Fe-based example capable of achieving PM in the IR-NLO chalcogenide system, and displays an outstandingly comprehensive IR-NLO performance, including wide Eg (2.24 eV), sufficient deff (5.89 pm V−1) and strong LIDT (14.42 MW cm−2). Moreover, the relationships between the microscopic crystal structure and macroscopic NLO activity were systematically investigated.
21m (no. 113), which displays alternating 2D [MM′2OS6]4− layers with the charge-balanced AE2+ cations occupying the space between these layers. In this work, a new NCS melilite oxychalcogenide, Sr2FeGe2OS6, has been prepared by an isomorphous substitution method. Excitingly, it is the first Fe-based example capable of achieving PM in the IR-NLO chalcogenide system, and displays an outstandingly comprehensive IR-NLO performance, including wide Eg (2.24 eV), sufficient deff (5.89 pm V−1) and strong LIDT (14.42 MW cm−2). Moreover, the relationships between the microscopic crystal structure and macroscopic NLO activity were systematically investigated.
Experimental section
Synthesis and characterization
The raw materials were purchased from Aladdin (SrO, AR; FeCl2, 2.5N; Ge, 5N; S, 3.5N; Ba, 3N; KI, 4N) and stored in an Ar-filled glovebox. Single crystals of Sr2FeGe2OS6 were grown by the flux method. A mixture of 2 mmol SrO (93 mg), 1 mmol FeCl2 (57 mg), 2 mmol Ge (65 mg), 6 mmol S (86 mg), and 1 mmol Ba (38 mg) was weighed and an additional 300 mg of KI was added as the reactive flux. The mixture was placed in a 7 mm inner-diameter silica crucible and then in a vacuum-sealed silica tube with 11 mm inner-diameter. The samples were gradually heated to 673 K, held for 15 h, and then heated to 1073 K at a rate of 10 K h−1, held for 3 days, and then slowly cooled to 623 K at a rate of 3 K h−1. High-quality black-red block crystals (approximately 90% yield based on Fe) for single-crystal X-ray diffraction measurements were obtained after washing with 95% ethanol and being manually selected for characterization. Single-crystal X-ray diffraction (XRD) data for Sr2FeGe2OS6 were collected at 100 K using a Rigaku Oxford Hybrid Pixel Array diffractometer by Ga Kα radiation (λ = 1.3405 Å). Semiquantitative microprobe analyses were performed via a field emission scanning electron microscope (JSM6700F, operating at 10 kV) equipped with an energy dispersive X-ray spectroscope (EDS, Oxford INCA). Thermogravimetry (TG) analysis was performed under a flowing N2 atmosphere at 313–1273 K using a NETZSCH STA 449C simultaneous analyzer. X-ray photoelectron spectroscopy (XPS) spectra were recorded using ESCALAB 250Xi equipment with C 1s at 284.8 eV as the internal standard. Diffuse-reflectance spectra were recorded using a PerkinElmer LAMBDA 950 spectrophotometer at 200–2500 nm. The SHG and LIDT measurements using AgGaS2 as the reference were implemented by the Kurtz–Perry method (2050 nm)49 and the single pulse measurement method (1064 nm),50 respectively. Theoretical investigations were also performed based on the density functional theory (DFT) method (refer to the ESI† for the detailed experimental section).Results and discussion
Single-crystal XRD data for Sr2FeGe2OS6 are well indexed to the tetragonal system (space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m (no. 113)) and categorized into melilite structure type. There are one Sr, one Ge, one Fe, one O and two S crystallographically independent atoms in the unit cell (Table S1†). The crystallographic parameters and diagrammatic sketch of the structure can be found in Table 1 and Fig. 1, respectively. As shown in Fig. 1, Sr2FeGe2OS6 features a 2D {[FeGe2OS6]}4− pentagonal tiling layer stacked in the same mode along the c axis with inserted Sr2+ to realize charge balance. In the structure of Sr2FeGe2OS6, adjacent [GeOS3] tetrahedra are linked with each other via corner-sharing O atoms and polymerize into [Ge2OS6] dimers. Two [FeS4] units bridge with one [Ge2OS6] dimer and one [GeOS3] tetrahedron by corner-sharing S atoms to form a pentagonal ring, which further interlinks to form a 2D {[FeGe2OS6]}4− layer with 6.1470 (2) Å layer spacing. The bond length in the [FeS4] tetrahedron is dFe–S = 2.3252 Å, which is similar to dFe–S values of 2.332 (3)–2.374 (4) Å in K2FeGe3S8
21m (no. 113)) and categorized into melilite structure type. There are one Sr, one Ge, one Fe, one O and two S crystallographically independent atoms in the unit cell (Table S1†). The crystallographic parameters and diagrammatic sketch of the structure can be found in Table 1 and Fig. 1, respectively. As shown in Fig. 1, Sr2FeGe2OS6 features a 2D {[FeGe2OS6]}4− pentagonal tiling layer stacked in the same mode along the c axis with inserted Sr2+ to realize charge balance. In the structure of Sr2FeGe2OS6, adjacent [GeOS3] tetrahedra are linked with each other via corner-sharing O atoms and polymerize into [Ge2OS6] dimers. Two [FeS4] units bridge with one [Ge2OS6] dimer and one [GeOS3] tetrahedron by corner-sharing S atoms to form a pentagonal ring, which further interlinks to form a 2D {[FeGe2OS6]}4− layer with 6.1470 (2) Å layer spacing. The bond length in the [FeS4] tetrahedron is dFe–S = 2.3252 Å, which is similar to dFe–S values of 2.332 (3)–2.374 (4) Å in K2FeGe3S8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 and 2.320 (1)–2.332 (1) Å in K10Fe4Sn4S17.51 The detailed information about other important bond lengths and angles are described in Table S2 and Fig. S1.† Interestingly, we found that the M–S bond length may be negatively correlated with the electronegativity of M by comparing the local structures of the reported melilite oxychalcogenides Sr2MM′2OS6 (M = Mn, Co, Zn, Cd; M′ = Ge, Sn) (Table S3†).44–48
32 and 2.320 (1)–2.332 (1) Å in K10Fe4Sn4S17.51 The detailed information about other important bond lengths and angles are described in Table S2 and Fig. S1.† Interestingly, we found that the M–S bond length may be negatively correlated with the electronegativity of M by comparing the local structures of the reported melilite oxychalcogenides Sr2MM′2OS6 (M = Mn, Co, Zn, Cd; M′ = Ge, Sn) (Table S3†).44–48
 | ||
| Fig. 1 Crystal structure of Sr2FeGe2OS6: (a) schematic illustration of the structure along the b axis and (b) view along the ab-plane. | ||
| Empirical formula | Sr2FeGe2OS6 |
|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|, wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | |
| Formula weight | 584.63 |
| Temperature (K) | 100(2) |
| Crystal system | Tetragonal |
| Space group |
P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m (no. 113) 21m (no. 113) |
| a (Å) | 9.4182(2) |
| b (Å) | 9.4182(2) |
| c (Å) | 6.1470(2) |
| V (Å3) | 545.25(3) |
| Z | 2 |
| D c (g cm−3) | 3.561 |
| μ (mm−1) | 26.599 |
| GOOF on F2 | 1.066 |
| R 1, wR2 (I > 2σ(I))a | 0.0217, 0.0519 |
| R 1, wR2 (all data) | 0.0219, 0.0521 |
| CCDC number | 2227377 |
| Largest diff. peak and hole (e Å−3) | 1.092, −0.497 |
The black-red block crystals of Sr2FeGe2OS6 were obtained with a high yield through the flux method at 1123 K. The experimental powder XRD pattern is consistent with the simulated one (Fig. 2a), indicating that high phase purity of the title compound can be obtained according to the aforementioned synthesis process. XPS measurements were accomplished to elucidate the oxidation state of Fe ions. Fig. 2b shows the Fe 2p core levels of XPS narrow-scan spectra and Fig. S2† reveals the XPS data of other elements in Sr2FeGe2OS6. As shown in Fig. 2b, there are two peaks in the Fe 2p core level spectra, which can be assigned to Fe 2p1/2 and Fe 2p3/2. These two signals are similar to those of Fe2+ in FeO52 and Fe2SiO4,53 indicating that the oxidation states of the Fe ions are +2. These results show that the precise formula of the ionic valence state is (Sr2+)2(Fe2+)(Ge4+)2(O2−)(S2−)6. Moreover, the uniform distribution and atomic ratio of Sr/Fe/Ge/O/S = 1.9/1/1.9/1/6.2 approximate with the target value and were determined as shown in the results of SEM and EDS mapping analyses (Fig. S3†). The distribution of O beyond the crystal was probably affected by the O-containing conductive substrate and its light atomic weight compared with other elements. The TG analysis curves indicated that Sr2FeGe2OS6 has good thermal stability up to 1071 K under an N2 atmosphere (Fig. 2c) but starts to decompose with apparent weight loss at higher temperatures. This is in accordance with the results of powder XRD analysis and impurities of SrGeO3 and Fe0.96S were detected (as shown in Fig. S4†). As depicted in the room-temperature UV-vis absorption spectra (Fig. 2d), the deduced Eg of Sr2FeGe2OS6 is 2.24 eV using the equation for an indirect bandgap semiconductor,54 which corresponds to its black-red crystal color (the inset picture in Fig. 2d). We compared the Eg value with those of typical Fe-containing materials31,32,51–63 according to a detailed literature survey, as shown in Fig. 2e, and found that Sr2FeGe2OS6 displays the largest Eg among the 16 reported compounds based on experimental measurements. This phenomenon also illustrates that oxychalcogenides as IR-NLO candidates have advantage in the Eg compared with sulfides. Besides, the wide Eg is comparable to those of commercial IR-NLO materials AgGaS2 (2.56 eV),64 AgGaSe2 (1.83 eV)65 and ZnGeP2 (2.0 eV).66 However, this value is narrower than those of the 7 existing melilite materials AE2MM′2OS6 (Table S3†),44–48e.g., 2.77–3.73 eV, which can be attributed to the strong optical absorption in the UV-vis region caused by the d–d charge transitions of d6 Fe2+.32,51
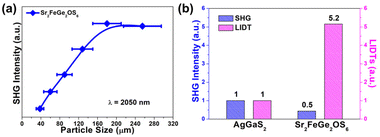
In general, the NCS structure is the paramount precondition for IR-NLO materials. The SHG signals of the target material were assessed via the Kurtz–Perry method49 with 2050 nm irradiation (10 mJ). As depicted in Fig. 3(a), the SHG intensities present an upward trend with increasing particle size and then approach saturation, which reveals that Sr2FeGe2OS6 possesses good PM nature. As far as we know, it is the first example of Fe-based compounds that has typical type-I PM behaviour. Significantly, this good PM behaviour is one of the preliminary requirements in being able to realize IR-NLO application. Moreover, the relative SHG efficiency of the title compound was compared with that of a sample of the typical commercial IR-NLO material AgGaS2. As shown in Fig. 3(b), Sr2FeGe2OS6 exhibits a moderate SHG response, which is almost 0.5 times as large as that of the reference in the particle size range of 150–210 μm. Its SHG efficiency ranks among the middle of the reported melilite materials AE2MM′2OS6 (deff = 0.3–2.1 × AgGaS2, see Table S3† for details)44–48 and is comparable with other d-block metal-containing oxychalcogenides, such as RE3NbS3O4 (RE = Sm, Gd; deff = 0.3–0.4 × AgGaS2),67 Sr4Pb1.5Sb5O5Se8 (deff = 0.25 × AgGaS2),68 AEGeOS2 (AE = Sr, Ba; deff = 0.4–0.5 × AgGaS2),69 and Ba2SnSi2SO7 (deff = 0.6 × AgGaS2).70 The powder LIDT of Sr2FeGe2OS6 has also been tested as another important index to evaluate its NLO performance via the particle size range of 150–210 μm under a 1064 nm laser and the experimental result is about 14.42 MW cm−2. It is clear that the LIDT of the title compound is significantly larger by 5 times that of AgGaS2, indicating its positive thermal conduction capability. Owing to the LIDT being generally consistent with the value of Eg, the title compound has a relatively smaller LIDT than most other melilite materials AE2MM′2OS6 (Table S3†) (e.g., LIDT = 6–21 × AgGaS2),44–48 but excellent heat tolerance among the reported Fe-based materials can be speculated.
Systematic theoretical calculations based on DFT as an effective means to discover the structure–activity relationship have been analysed in detail. The results displayed in Fig. 4a suggest an indirect semiconducting state with a calculated Eg of 1.94 eV for Sr2FeGe2OS6 along the Q–Z direction between the highest valence band (VB) and the lowest conduction band (CB). This relatively smaller value compared with the experimental value (Eg = 2.24 eV) is mainly ascribed to the common problem of DFT calculation.71–73 Besides, the first Brillouin zone with high symmetry points of the title compound is provided in Fig. S5.† The Perdew–Burke–Ernzerhof (PBE) approach74 was used to calculate the partial densities of states (PDOS) of Sr2FeGe2OS6 (Fig. 4b). From −10 eV to the Fermi level (EF = 0 eV), S 3p, Fe 3d, O 2p, and Ge 4s orbitals make a major contribution and the Fe 3d, S 3p, Ge 4p, and Ge 4s orbitals contribute mostly in the energy field ranging from 0 eV to +10 eV. Therefore, it can be deduced that the charge transitions responsible for the optical Eg absorption could be determined by [GeOS3] and [FeS4] BBUs, i.e., the 2D {[FeGe2OS6]4−}n layered structure.
Space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m of Sr2FeGe2OS6 belongs to the asymmetrical point group of 2m, and thus, only has one non-vanishing independent SHG coefficient d14 under the constraint of Kleinman symmetry.75 The calculated frequency-dependent SHG coefficient d14 at 2050 nm (ca. 0.61 eV) is 11.79 pm V−1 (the upper panel of Fig. 4c), which is slightly larger than the experimental result (Fig. 3b). The absorption of output frequency-doubled light at 1025 nm was possibly the main reason. As plotted in the lower panel of Fig. 4c, the calculated birefringence Δn (Δn = nz − nx) value was 0.127 at 2050 nm, which is obviously greater than that of AgGaS2 (Δn = 0.04) under the same conditions.76–78 Besides, such a large Δn exceeds that of the melilite structure type Sr2MGe2OS6 (M = Co, Mn, Zn; Δn = 0.064–0.124),44–46,48 indicating that Sr2FeGe2OS6 can theoretically realize PM in the IR region. Moreover, the minimum PM cut-off wavelengths on the basis of the refractive-index dispersion curves were calculated using the formula nx(2w) = nz(w).79–82 As shown in Fig. 4d, the shortest cut-off edge of SHG light was evaluated to be 600 nm under the restriction of type-I PM conditions.
21m of Sr2FeGe2OS6 belongs to the asymmetrical point group of 2m, and thus, only has one non-vanishing independent SHG coefficient d14 under the constraint of Kleinman symmetry.75 The calculated frequency-dependent SHG coefficient d14 at 2050 nm (ca. 0.61 eV) is 11.79 pm V−1 (the upper panel of Fig. 4c), which is slightly larger than the experimental result (Fig. 3b). The absorption of output frequency-doubled light at 1025 nm was possibly the main reason. As plotted in the lower panel of Fig. 4c, the calculated birefringence Δn (Δn = nz − nx) value was 0.127 at 2050 nm, which is obviously greater than that of AgGaS2 (Δn = 0.04) under the same conditions.76–78 Besides, such a large Δn exceeds that of the melilite structure type Sr2MGe2OS6 (M = Co, Mn, Zn; Δn = 0.064–0.124),44–46,48 indicating that Sr2FeGe2OS6 can theoretically realize PM in the IR region. Moreover, the minimum PM cut-off wavelengths on the basis of the refractive-index dispersion curves were calculated using the formula nx(2w) = nz(w).79–82 As shown in Fig. 4d, the shortest cut-off edge of SHG light was evaluated to be 600 nm under the restriction of type-I PM conditions.
In order to determine which microscopic NLO active units provide the most forceful contribution to the macroscopic NLO performance from the view of the micro-structure, we reveal a cutoff-energy-dependent SHG coefficient (Fig. 5a) accompanied by partial-charge-density maps (Fig. 5b) in terms of the so-called length-gauge formalism.83 From the calculation results presented in Fig. 5, the remarkable increase of d14 in the VB-1, CB-1, and CB-3 intervals can be directly observed, indicating that these energy ranges contribute significantly to the SHG intensity. In accordance with the associated PDOS (Fig. 4b), the VB-1 region is dominated by S 3p, Fe 3d, and O 2p states, the CB-1 region mainly consists of Fe 3d, S 3p, and Ge 4s states, while S 3p and Ge 4p states produce the major contribution to the CB-3 region. Consequently, the strong SHG response for Sr2FeGe2OS6 can be assigned to the [GeOS3] and [FeS4] BBUs, which are the source of increased SHG efficiency.
 | ||
| Fig. 5 (a) Variation of static coefficient d14 along with cut-off energy (eV). (b) Projection of the partial charge density maps in the VB-1, CB-1 and CB-3 intervals. | ||
Conclusions
In summary, a new NCS melilite oxychalcogenide, Sr2FeGe2OS6, was successfully designed and prepared through an isomorphous substitution strategy. Sr2FeGe2OS6 features a 2D {[FeGe2OS6]4−}n layered structure formed by corner-sharing [Ge2OS6] dimers and [FeS4] tetrahedra with Sr2+ embedded in these layers. Experimental results from a powder sample of Sr2FeGe2OS6 indicate that it possesses a wide Eg (2.24 eV), sufficient deff (ca. 0.5 × AgGaS2) and strong LIDT (ca. 5.2 × AgGaS2). Detailed theoretical investigations illustrate that the unique arrangement of NLO-active motifs, [Ge2OS6] dimers and [FeS4] tetrahedra, mainly contributes to the strong deff and large Δn of Sr2FeGe2OS6. Furthermore, Sr2FeGe2OS6 is the first PM Fe-based IR-NLO example to be realized by isomorphous substitution. These findings not only broaden the horizon of the NCS transition-metal-based chalcogenide system, but also provide a feasible strategy to discover high-performance IR-NLO candidates.Author contributions
H. D. Yang prepared the samples, designed and carried out the experiments, and wrote the manuscript. S. H. Zhou carried out the theoretical calculations. M. Y. Ran helped solve the structure of the title compound. X. T. Wu put forward suggestions about the structure–property relationship. H. Lin and Q. L. Zhu analyzed the results and edited the manuscript. All the authors have approved the final version of the manuscript. H. D. Yang and S. H. Zhou contributed equally to this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22175175, 22193043), the Fujian Science and Technology Innovation Laboratory for Optoelectronic Information of China (2021ZR118), and the Natural Science Foundation of Fujian Province (2022L3092) and the Youth Innovation Promotion Association CAS (2022303). We thank Professor Bing-Xuan Li at FJIRSM for helping with the NLO measurements and Professor Yong-Fan Zhang at Fuzhou University for helping with DFT calculations.References
- V. Petrov, Frequency down-conversion of solid-state laser sources to the mid-infrared spectral range using non-oxide nonlinear crystals, Prog. Quantum Electron., 2015, 42, 1–106 CrossRef.
- Structure-Property Relationships in Nonlinear Optical Crystals II The IR Region, ed. X.-T. Wu and L. Chen, Structure and Bonding, Series ed. D. M. Mingos, Springer, New York, 2012, p. 145 Search PubMed.
- V. A. Serebryakov, E. V. Boiko, N. N. Petrishchev and A. V. Yan, Medical applications of mid-TR lasers: problems and prospects., J. Opt. Technol., 2010, 77, 6–17 CrossRef CAS.
- F. J. Duarte, Tunable Laser Applications, CRC Press, Boca Raton, FL, 2nd edn, 2008, ch. 2, 9, and 12 Search PubMed.
- D. N. Nikogosyan, Nonlinear Optical Crystals: A Complete Survey, Springer-Science, New York, 2005 Search PubMed.
- Y. Li, J. Luo and S. Zhao, Local Polarity-Induced Assembly of Second-Order Nonlinear Optical Materials, Acc. Chem. Res., 2022, 55, 3460–3469 CrossRef CAS PubMed.
- U. Keller, Recent developments in compact ultrafast lasers, Nature, 2003, 424, 831–838 CrossRef CAS PubMed.
- A. Harasaki and K. Kato, New Data on the Nonlinear Optical Constant, Phase-Matching, and Optical Damage of AgGaS2, Jpn. J. Appl. Phys, 1997, 36, 700–703 CrossRef CAS.
- G. D. Boyd, E. Buehler and F. G. Storz, Linear and nonlinear optical properties of ZnGeP2 and CdSe, Appl. Phys. Lett., 1971, 18, 301–304 CrossRef CAS.
- G. C. Catella, L. R. Shiozawa, J. R. Hietanen, R. C. Eckardt, R. K. Route, R. S. Feigelson, D. G. Cooper and C. L. Marquardt, Mid-IR absorption in AgGaSe2 optical parametric oscillator crystals, Appl. Opt., 1993, 32, 3948–3951 CrossRef CAS PubMed.
- M.-Y. Ran, A.-Y. Wang, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, Recent progress in the design of IR nonlinear optical materials by partial chemical substitution: structural evolution and performance, Coord. Chem. Rev., 2023, 484, 215059 CrossRef.
- F. Liang, L. Kang, Z. Lin, Y. Wu and C. Chen, Analysis and prediction of mid-IR nonlinear optical metal sulfides with diamond-like structures, Coord. Chem. Rev., 2017, 333, 57–70 CrossRef CAS.
- S.-P. Guo, Y. Chi and G.-C. Guo, Recent achievements on middle and far-infrared second-order nonlinear optical materials, Coord. Chem. Rev., 2017, 335, 44–57 CrossRef CAS.
- P. S. Halasyamani, Asymmetric Cation Coordination in Oxide Materials: Influence of Lone-Pair Cations on the Intra-octahedral Distortion in d0 Transition Metals, Chem. Mater., 2004, 16, 3586–3592 CrossRef CAS.
- H. Chen, W.-B. Wei, H. Lin and X.-T. Wu, Transition-metal-based chalcogenides: A rich source of infrared nonlinear optical materials, Coord. Chem. Rev., 2021, 448, 214154 CrossRef CAS.
- G. Li, Z. Yang, J. Li and S. Pan, A review of the AI2BIICIVDVI4 family as infrared nonlinear optical materials: the effect of each site on the structure and optical properties, Chem. Commun., 2020, 56, 11565–11576 RSC.
- H. Chen, M.-Y. Ran, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, A comprehensive review on metal chalcogenides with three-dimensional frameworks for infrared nonlinear optical applications, Coord. Chem. Rev., 2022, 470, 214706 CrossRef CAS.
- J. Guillevic, L. Jy and D. Grandjean, Etude Structurale de Combinaisons Sulfurees et Seleniees du Molybdene. IV. Structures Cristallines de CoMo2S4 et de FeMo2S4, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 111–117 CrossRef CAS.
- K. Sato and T. Teranishi, Optical Absorption Spectrum of a Thin CuFeS2 Film, J. Phys. Soc. Jpn., 1976, 40, 297–298 CrossRef CAS.
- J. M. D. Coey, E. Devlin and R. J. Gambino, Noncrystalline ferromagnetic irongallium oxide, J. Appl. Phys., 1982, 53, 7810–7812 CrossRef CAS.
- Y.-H. Chu, L. W. Martin, M. B. Holcomb and R. Ramesh, Controlling magnetism with multiferroics, Mater. Today, 2007, 10, 16–23 CrossRef CAS.
- K. Kovnir, V. O. Garlea, C. M. Thompson, H. D. Zhou, W. M. Reiff, A. Ozarowski and M. Shatruk, Spin-Glass Behavior in LaFexCo2−xP2 Solid Solutions: Interplay Between Magnetic Properties and Crystal and Electronic Structures, Inorg. Chem., 2011, 50, 10274–10283 CrossRef CAS PubMed.
- W. Jeitschko, U. Meisen and J. Albering, Actinoid transition metal phosphides An2T12P7 (An = Th, U; T = Fe, Co, Ni) and arsenides An2T12As7 (An = Th, U; T = Co, Ni) with Zr2Fe12P7 type structure, Dalton Trans., 2010, 39, 6067–6073 RSC.
- O. Yakubovich, V. Urusov, W. Massa, G. Frenzen and D. Babel, Structure of Na2Fe2F7 and Structural Relations in the Family of Weberites Na2MIIMIIIF7, Z. Anorg. Allg. Chem., 1993, 619, 1909–1919 CrossRef CAS.
- G. Courbion, C. Jacoboni and R. Depape, The Dimorphism of LiMnFeF6: A New Kind of Cationic Order in the Structural Type Na2SiF6, J. Solid State Chem., 1982, 45, 127–134 CrossRef CAS.
- E. Parthe and S. Z. Hu, CaFeO2Cl and Ca2FeO3Cl with higher space group ymmetry, a reevaluation, J. Solid State Chem., 2003, 174, 165–166 CrossRef CAS.
- K. Tustain, L. Farrar, W. Yao, P. Lightfoot, I. da Silva, M. T. F. Telling and L. Clark, Materialization of a Geometrically Frustrated Magnet in a Hybrid Coordination Framework: A Study of the Iron(II) Oxalate Fluoride Framework, KFe(C2O4)F, Inorg. Chem., 2019, 58, 11971–11977 CrossRef CAS PubMed.
- H. D. Yang, M. Y. Ran, S. H. Zhou, X. T. Wu, H. Lin and Q. L. Zhu, Rational design via dual-site aliovalent substitution leads to an outstanding IR nonlinear optical material with well-balanced comprehensive properties, Chem. Sci., 2022, 13, 10725–10733 RSC.
- S. Pascal, S. David, C. Andraud and O. Maury, Near-infrared dyes for two-photon absorption in the short-wavelength infrared: strategies towards optical power limiting, Chem. Soc. Rev., 2021, 50, 6613–6658 RSC.
- Y. Wang and R. K. Li, K2Fe2B2O7: A transparent nonlinear optical crystal with frustrated magnetism, J. Solid State Chem., 2010, 183, 1221–1225 CrossRef CAS.
- W. Cao, D. Mei, Y. Yang, Y. Wu, L. Zhang, Y. Wu, X. He, Z. Lin and F. Huang, From CuFeS2 to Ba6Cu2FeGe4S16: rational band gap engineering achieves large second-harmonic-generation together with high laser damage threshold, Chem. Commun., 2019, 55, 14510–14513 RSC.
- B. Ji, K. Pandey, C. P. Harmer, F. Wang, K. Wu, J. Hu and J. Wang, Centrosymmetric or Noncentrosymmetric? Transition Metals Talking in K2TGe3S8 (T = Co, Fe), Inorg. Chem., 2021, 60, 10603–10613 CrossRef CAS PubMed.
- Z. T. Messegee, J. S. Cho, A. J. Craig, V. O. Garlea, Y. Xin, C. J. Kang, T. E. Proffen, H. Bhandari, J. C. Kelly, N. J. Ghimire, J. A. Aitken, J. I. Jang and X. Tan, Multifunctional Cu2TSiS4 (T = Mn and Fe): Polar Semiconducting Antiferromagnets with Nonlinear Optical Properties, Inorg. Chem., 2023, 62, 530–542 CrossRef CAS PubMed.
- M. M. Chen, S. H. Zhou, W. B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, Phase Matchability Transformation in the Infrared Nonlinear Optical Materials with Diamond–Like Frameworks, Adv. Opt. Mater., 2022, 10, 2102123 CrossRef CAS.
- H. Lin, L. Chen, J. S. Yu, H. Chen and L. M. Wu, Infrared SHG Materials CsM3Se6 (M = Ga/Sn, In/Sn): Phase Matcha-bility Controlled by Dipole Moment of the Asymmetric Building Unit, Chem. Mater., 2017, 29, 499–503 CrossRef CAS.
- Y. X. Zhang, B. X. Li, H. Lin, Z. J. Ma, X. T. Wu and Q. L. Zhu, Impressive Second Harmonic Generation Response in a Novel Phase-Matchable NLO-active MOF Derived from Achiral Precursors, J. Mater. Chem. C, 2019, 7, 6217–6221 RSC.
- H. Chen, Y. Y. Li, B. X. Li, P. F. Liu, H. Lin, Q. L. Zhu and X. T. Wu, Salt-Inclusion Chalcogenide [Ba4Cl2][ZnGa4S10]: Rational Design of an IR Nonlinear Optical Material with Superior Comprehensive Performance Derived from AgGaS2, Chem. Mater., 2020, 32, 8012–8019 CrossRef CAS.
- H. Lin, W.-B. Wei, H. Chen, X.-T. Wu and Q.-L. Zhu, Rational design of infrared nonlinear optical chalcogenides by chemical substitution, Coord. Chem. Rev., 2020, 406, 213150 CrossRef CAS.
- M.-M. Chen, S.-H. Zhou, W. Wei, M.-Y. Ran, B. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, RbBiP2S6: A Promising IR Nonlinear Optical Material with a Giant Second-Harmonic Generation Response Designed by Aliovalent Substitution, ACS Mater. Lett., 2022, 4, 1264–1269 CrossRef CAS.
- M. Y. Ran, Z. J. Ma, H. Chen, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, Partial Isovalent Anion Substitution to Access Remarkable Second-Harmonic Generation Response: A Generic and Effective Strategy for Design of Infrared Nonlinear Optical Materials, Chem. Mater., 2020, 32, 5890–5896 CrossRef CAS.
- Y. F. Shi, W. B. Wei, X. T. Wu, H. Lin and Q. L. Zhu, Recent progress in oxychalcogenides as IR nonlinear optical materials, Dalton Trans., 2021, 50, 4112–4118 RSC.
- Y. Zhang, H. Wu, Z. Hu and H. Yu, Oxychalcogenides: A Promising Materials Class for Nonlinear Optical Crystals with Mixed-anion Groups, Chem. – Eur. J., 2022, e202203597 Search PubMed.
- M.-Y. Ran, S.-H. Zhou, W. Wei, B. Li, X.-T. Wu, H. Lin and Q.-L. Zhu, Rational Design of a Rare-Earth Oxychalcogenide Nd3[Ga3O3S3][Ge2O7] with Superior Infrared Nonlinear Optical Performance, Small, 2023, 19, 2300248 CrossRef PubMed.
- M.-Y. Ran, S.-H. Zhou, B. Li, W. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, Enhanced Second-Harmonic-Generation Efficiency and Birefringence in Melilite Oxychalcogenides Sr2MGe2OS6 (M = Mn, Zn, and Cd), Chem. Mater., 2022, 34, 3853–3861 CrossRef CAS.
- R. Wang, F. Liang, X. Liu, Y. Xiao, Q. Liu, X. Zhang, L. M. Wu, L. Chen and F. Huang, Heteroanionic Melilite Oxysulfide: A Promising Infrared Nonlinear Optical Candidate with a Strong Second-Harmonic Generation Response, Sufficient Birefringence, and Wide Bandgap, ACS Appl. Mater. Interfaces, 2022, 14, 23645–23652 CrossRef CAS.
- X. Tian, X. Zhang, Y. Xiao, X. Wu, B. Zhang, D. Yang and K. Wu, From oxides to oxysulfides: the mixed-anion GeS3O unit induces huge improvement in the nonlinear optical effect and optical anisotropy for potential nonlinear optical materials, RSC Adv., 2022, 12, 16296–16300 RSC.
- Y. Cheng, H. Wu, H. Yu, Z. Hu, J. Wang and Y. Wu, Rational Design of a Promising Oxychalcogenide Infrared Nonlinear Optical Crystal, Chem. Sci., 2022, 13, 5305–5310 RSC.
- N. Zhang, Q. T. Xu, Z. H. Shi, M. Yang and S. P. Guo, Characterizations and Nonlinear-Optical Properties of Pentanary Transition-Metal Oxysulfide Sr2CoGe2OS6, Inorg. Chem., 2022, 61, 17002–17006 CrossRef CAS PubMed.
- S. K. Kurtz and T. T. Perry, A Powder Technique for the Evaluation of Nonlinear Optical Materials, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- M.-J. Zhang, X.-M. Jiang, L.-J. Zhou and G.-C. Guo, Two phases of Ga2S3: promising infrared second-order nonlinear optical materials with very high laser induced damage thresholds, J. Mater. Chem. C, 2013, 1, 4754–4760 RSC.
- O. Palchik, R. G. Iyer, C. G. Canlas, D. P. Weliky and M. G. Kanatzidis, K10M4M’4S17 (M = Mn, Fe, Co, Zn; M′ = Sn, Ge) and Cs10Cd4Sn4S17: Compounds with a Discrete Supertetrahedral Cluster, Z. Anorg. Allg. Chem., 2004, 630, 2237–2247 CrossRef CAS.
- P. S. Bagus, C. J. Nelin, C. R. Brundle, B. V. Crist, N. Lahiri and K. M. Rosso, Combined multiplet theory and experiment for the Fe 2p and 3p XPS of FeO and Fe2O3, J. Chem. Phys., 2021, 154, 094709 CrossRef CAS PubMed.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- M. A. Butler, Photoelectrolysis and physical properties of the semiconducting electrode WO2, J. Appl. Phys., 1977, 48, 1914–1920 CrossRef CAS.
- M. Beraich, H. Shaili, E. Benhsina, Z. Hafidi, S. Mansouri, M. Taibi, F. Bentiss, A. Guenbour, A. Bellaouchou, A. Mzerd, A. Zarrouk and M. Fahoume, Preparation and characterization of Cu2FeGeS4 thin-film synthesized via spray ultrasonic method–DFT study, Mater. Lett., 2020, 275, 128070 CrossRef CAS.
- L. Zhang, D. Mei, Y. Wu, C. Shen, W. Hu, L. Zhang, J. Li, Y. Wu and X. He, Syntheses, structures, optical properties, and electronic structures of Ba6Cu2GSn4S16 (G = Fe, Ni) and Sr6D2FeSn4S16 (D = Cu, Ag), J. Solid State Chem., 2019, 272, 69–77 CrossRef CAS.
- X. Zheng, B. Sciacca, E. C. Garnett and L. Zhang, AgFeS2-Nanowire-Modified BiVO4 Photoanodes for Photoelectrochemical Water Splitting, ChemPlusChem, 2016, 81, 1075–1082 CrossRef CAS PubMed.
- Y.-Y. Li, P.-F. Liu, L. Hu, L. Chen, H. Lin, L.-J. Zhou and L.-M. Wu, Strong IR NLO Material Ba4MGa4Se10Cl2: Highly Improved Laser Damage Threshold via Dual Ion Substitution Synergy, Adv. Opt. Mater., 2015, 3, 957–966 CrossRef CAS.
- J. He, Z. Wang, X. Zhang, Y. Cheng, Y. Gong, X. Lai, C. Zheng, J. Lin and F. Huang, Synthesis, structure, magnetic and photoelectric properties of Ln3M0.5M′Se7 (Ln = La, Ce, Sm; M = Fe, Mn; M′ = Si, Ge) and La3MnGaSe7, RSC Adv., 2015, 5, 52629–52635 RSC.
- Y. Li, T. Zhang, Y. Qin, T. Day, G. Jeffrey Snyder, X. Shi and L. Chen, Thermoelectric transport properties of diamond-like Cu1−xFe1+xS2 tetrahedral compounds, J. Appl. Phys., 2014, 116, 203705 CrossRef.
- W. Yin, W. Wang, L. Kang, Z. Lin, K. Feng, Y. Shi, W. Hao, J. Yao and Y. Wu, Ln3FeGaQ7: A new series of transition-metal rare-earth chalcogenides, J. Solid State Chem., 2013, 202, 269–275 CrossRef CAS.
- K. Feng, W. Wang, R. He, L. Kang, W. Yin, Z. Lin, J. Yao, Y. Shi and Y. Wu, K2FeGe3Se8: a new antiferromagnetic iron selenide, Inorg. Chem., 2013, 52, 2022–2028 CrossRef CAS PubMed.
- J. A. Brant, C. dela Cruz, J. Yao, A. P. Douvalis, T. Bakas, M. Sorescu and J. A. Aitken, Field-Induced Spin-Flop in Antiferromagnetic Semiconductors with Commensurate and Incommensurate Magnetic Structures: Li2FeGeS4 (LIGS) and Li2FeSnS4 (LITS), Inorg. Chem., 2014, 53, 12265–12274 CrossRef CAS PubMed.
- H. Lin, L. Chen, L. J. Zhou and L. M. Wu, Functionalization based on the substitutional flexibility: strong middle IR nonlinear optical selenides AXII4XIII5Se12, J. Am. Chem. Soc., 2013, 135, 12914–12921 CrossRef CAS PubMed.
- M. C. Ohmer and R. Pandey, Emergence of Chalcopyrites as Nonlinear Optical Materials, MRS Bull., 1998, 23, 16–22 CrossRef CAS.
- L. Bai, Z. Lin, Z. Wang, C. Chen and M. H. Lee, Mechanism of linear and nonlinear optical effects of chalcopyrite AgGaX2 (X=S, Se, and Te) crystals, J. Chem. Phys., 2004, 120, 8772–8778 CrossRef CAS PubMed.
- X. Lian, Z. T. Lu, W. D. Yao, S. H. Yang, W. Liu, R. L. Tang and S. P. Guo, Structural Transformation and Second-Harmonic-Generation Activity in Rare-Earth and d0 Transition-Metal Oxysulfides RE3NbS3O4 (RE = Ce, Sm, Gd, Dy), Inorg. Chem., 2021, 60, 10885–10889 CrossRef CAS PubMed.
- Y. Wang, M. Luo, P. Zhao, X. Che, Y. Cao and F. Huang, Sr4Pb1.5Sb5O5Se8: a new mid-infrared nonlinear optical material with a moderate SHG response, CrystEngComm, 2020, 22, 3526–3530 RSC.
- X. Zhang, Y. Xiao, R. Wang, P. Fu, C. Zheng and F. Huang, Synthesis, crystal structures and optical properties of noncentrosymmetric oxysulfides AeGeS2O (Ae = Sr, Ba), Dalton Trans., 2019, 48, 14662–14668 RSC.
- Y.-F. Shi, Z. Ma, B.-X. Li, X. Wu, H. Lin and Q.-L. Zhu, Phase matching achieved by isomorphous substitution in IR nonlinear optical material Ba2SnSSi2O7 with an undiscovered [SnO4S] functional motif, Mater. Chem. Front., 2022, 6, 3054–3061 RSC.
- K. Burke, Perspective on density functional theory, J. Chem. Phys., 2012, 136, 150901 CrossRef.
- K. Govaerts, R. Saniz, B. Partoens and D. Lamoen, van der Waals bonding and the quasiparticle band structure of SnO from first principles, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 235210 CrossRef.
- N. E. Christensen, A. Svane and E. L. Peltzer y Blancá, Electronic and structural properties of SnO under pressure, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 014109 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- D. A. Kleinman, Nonlinear Dielectric Polarization in Optical Media, Phys. Rev., 1962, 126, 1977–1979 CrossRef CAS.
- M. Y. Li, B. X. Li, H. Lin, Z. J. Ma, L. M. Wu, X. T. Wu and Q. L. Zhu, Sn2Ga2S5: A Polar Semiconductor with Exceptional Infrared Nonlinear Optical Properties Originating from the Combined Effect of Mixed Asymmetric Building Motifs, Chem. Mater., 2019, 31, 6268–6275 CrossRef CAS.
- C. Liu, S.-H. Zhou, Y. Xiao, C. Zhang, H. Lin and Y. Liu, Aliovalent-cation-substitution-induced structure transformation: a new path toward high-performance IR nonlinear optical materials, J. Mater. Chem. C, 2021, 9, 15407–15414 RSC.
- M.-Y. Ran, Z. Ma, X.-T. Wu, H. Lin and Q.-L. Zhu, Ba2Ge2Te5: a ternary NLO-active telluride with unusual one-dimensional helical chains and giant second-harmonic-generation tensors, Inorg. Chem. Front., 2021, 8, 4838–4845 RSC.
- H. Lin, Y. Y. Li, M. Y. Li, Z. J. Ma, L. M. Wu, X. T. Wu and Q. L. Zhu, Centric-to-acentric structure transformation induced by a stereochemically active lone pair: a new insight for design of IR nonlinear optical materials, J. Mater. Chem. C, 2019, 7, 4638–4643 RSC.
- M. Y. Li, Z. J. Ma, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, HgCuPS4: An Exceptional Infrared Nonlinear Optical Material with Defect Diamond-like Structure, Chem. Mater., 2020, 32, 4331–4339 CrossRef CAS.
- M.-M. Chen, Z. Ma, B.-X. Li, W.-B. Wei, X.-T. Wu, H. Lin and Q.-L. Zhu, M2As2Q5 (M = Ba, Pb; Q = S, Se): A source of infrared nonlinear optical materials with excellent overall performance activated by multiple discrete arsenate anions, J. Mater. Chem. C, 2021, 9, 1156–1163 RSC.
- Y. Xiao, M. M. Chen, Y. Y. Shen, P. F. Liu, H. Lin and Y. Liu, A3Mn2Sb3S8 (A = K, Rb): A new type of multifunctional infrared nonlinear optical materials based on unique three-dimensional open frameworks, Inorg. Chem. Front., 2021, 8, 2835–2843 RSC.
- C. Aversa and J. E. Sipe, Nonlinear optical susceptibilities of semiconductors: Results with a length-gauge analysis, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 14636–14645 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental and theory results, together with additional tables and figures. CCDC 2227377. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi02733j |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2023 |