Open Access Article
Open Access ArticleRuthenium(II) polypyridyl complexes with π-expansive ligands: synthesis and cubosome encapsulation for photodynamic therapy of non-melanoma skin cancer†
Gina Elena
Giacomazzo‡
a,
Michele
Schlich‡
 b,
Luca
Casula‡
b,
Luca
Casula‡
 b,
Luciano
Galantini
b,
Luciano
Galantini
 cd,
Alessandra
Del Giudice
cd,
Alessandra
Del Giudice
 cd,
Giangaetano
Pietraperzia
ae,
Chiara
Sinico
b,
Francesca
Cencetti
cd,
Giangaetano
Pietraperzia
ae,
Chiara
Sinico
b,
Francesca
Cencetti
 f,
Sara
Pecchioli
f,
Sara
Pecchioli
 f,
Barbara
Valtancoli
a,
Luca
Conti
f,
Barbara
Valtancoli
a,
Luca
Conti
 *a,
Sergio
Murgia
*a,
Sergio
Murgia
 *bd and
Claudia
Giorgi
*bd and
Claudia
Giorgi
 *a
*a
aDepartment of Chemistry “Ugo Schiff”, University of Florence, Via della Lastruccia 3, 50019 Sesto Fiorentino (FI), Italy. E-mail: luca.conti@unifi.it; claudia.giorgi@unifi.it
bDepartment of Life and Environmental Sciences, University of Cagliari, 09124 Cagliari (CA), Italy. E-mail: murgias@unica.it
cDepartment of Chemistry, University of Rome La Sapienza, P.le A. Moro 5, 00185 Rome, Italy
dCSGI, Consorzio Interuniversitario per lo Sviluppo dei Sistemi a Grande Interfase, 50019, Sesto Fiorentino (FI), Italy
eEuropean Laboratory for Non-Linear Spectroscopy (LENS), Via Nello Carrara 1, 50019, Sesto Fiorentino (FI), Italy
fDepartment of Experimental and Clinical Biomedical Sciences “Mario Serio”, 50134, Florence, Italy
First published on 6th April 2023
Abstract
In photodynamic therapy (PDT), Ru(II) polypyridyl complexes (RPCs) featuring the popular π-expansive benzo[i]dipyrido[3,2-a:2′,3′-c]phenazine (dppn) ligand have attracted much attention, mainly due to the good singlet oxygen sensitizing properties imparted by this peculiar ligand. However, notwithstanding the intriguing perspectives, much remains to be explored about the use of RPC-based photosensitizing agents (PSs) with more than a dppn ligand in their scaffolds. Herein, two bis-heteroleptic RPCs of the general formula [Ru(dppn)2L]n+ (L = 4,4′-dimethyl-2,2′-bipyridine, n = 2, Ru1 or 2,2′-bipyridine-4,4′-dicarboxylate, n = 0, Ru2) were prepared in good yields by adopting an alternative synthetic approach to previously reported methods. The optimal singlet oxygen sensitizing properties and capabilities to interact with DNA displayed by Ru1 and Ru2 were paralleled by a potent light-triggered toxicity (λmax = 462 nm) exerted on squamous epithelial carcinoma cells. To improve the biopharmaceutical properties of these compounds, Ru1 and Ru2 were encapsulated into cubosomes, soft nanoparticles with a lyotropic liquid crystalline core. In vitro studies probed the effectiveness of these formulations against light-irradiated cancer cells and confirmed intracellular ROS generation as the mechanism likely to be responsible for the observed PDT efficacy. This work highlights the potential of [Ru(dppn)2L]-based PSs in PDT, beyond providing a general and straightforward synthetic route for the preparation of this class of compounds. To the best of our knowledge, this is also the first example of the encapsulation of a RPC into cubosome nanostructures, paving the way for the development of nano-formulations with augmented biopharmaceutical properties for PDT application.
Introduction
Photodynamic therapy (PDT) continues to attract increasing attention thanks to the encouraging results that its application has led to the treatment of a variety of cancers, spanning from skin tumors to lung, bladder and prostate cancers,1,2 as well as bacterial infections.3,4 The main advantage of this therapeutic approach, which consists of the light activation of a prodrug, called a photosensitizer (PS), to produce harmful reactive oxygen species (ROS), is represented by the complete spatio-temporal control over drug activation, which provides a precious chance to limit the severe side effects normally occurring with standard chemotherapeutics.Ruthenium(II) polypyridyl complexes (RPCs) have been largely employed in the research of suitable PSs in PDT, with the Mc Farland compound TLD1433 being the first Ru(II)-based PS to enter human clinical trials for bladder cancer.5–8 The interest towards this versatile class of compounds can be attributed to its rich chemical–physical repertoire, which includes a variety of excited-state electronic configurations accessible with light, good singlet oxygen sensitizing properties, and the capacity to interact with key biological targets (such as DNA or proteins).9–11 Of relevance is that a fine choice of ligands in their octahedral geometries permits convenient modulation of the photophysical, photochemical, and photobiological properties of the resulting RPCs, in an effort to improve cellular uptake,12,13 shift the absorption profiles towards red,14 confer targeting ability,15,16 and boost 1O2 sensitization. With regard to the latter aim, as prolonged excited state lifetimes are important for efficient energy transfer to molecular oxygen to form 1O2, changing the nature of the lowest-lying excited state from metal-to-ligand charge-transfer (3MLCT) to long-lived intraligand 3IL states represents a suitable way to endow the resulting RPCs with augmented cytotoxicity.6,17 Following this strategy, over the past few years much interest has been devoted to the use of the π-expansive benzo[i]dipyrido[3,2-a:2′,3′-c]phenazine (dppn) ligand in the rational design of RPC-based PSs. Indeed, this peculiar ligand has been largely exploited not only to improve the photobiological activity of the resulting compounds, via the population of long-lived dppn-centered 3ππ* excited states, but also to shift their 1MLCT absorption towards longer wavelengths18 and, given its known DNA-intercalating properties, to strengthen their interaction with the nucleic acid.19–21 A recent example of this was reported by Zhao and coworkers, who showed the benefits derived from the substitution of a bpy (bipyridine) unit by a dppn ligand in their tris-heteroleptic RPC-based PSs.22
Notwithstanding the advantages derived from the use of dppn, it is surprising that PSs containing two dppn ligands simultaneously coordinated to a Ru(II) center have been only sparingly explored,23,24 while numerous examples of dppn-containing RPCs for PDT are reported in the literature25–31 (some of them have also been applied for compounds at the boundary between PDT and photoactivated chemotherapy PACT).32,33 Such net discrepancy between RPCs containing one and two dppn units would be related to synthetic issues concerning the preparation of the latter compounds by the general procedures for bis-heteroleptic Ru(II) complexes,34,35 involving the intermediate [(dppn)2RuCl2] which is scarcely soluble in most organic solvents.
In addition, the potential anticancer activity of these systems might be limited by their common hydrophobic nature, leading to poor bioavailability and compromised therapeutic outcomes. To overcome these limits, nanocarriers have been widely investigated as a formative approach to increase the water solubility of insoluble drug candidates, prevent drug degradation, and enhance their delivery.36–38 In the midst of the innovative exploited nano-systems, great interest has arisen around cubosomes, also known as bicontinuous cubic liquid crystalline nanoparticles with a three-dimensional arrangement of the lipid bilayer forming a honeycomb-like inner structure. Compared to single-bilayer liposomes, cubosomes are characterized by an inner portion completely filled with the lipid matrix, providing a greater hydrophobic volume and thus a higher loading efficiency.39 Despite the fact that some investigations illustrated a possible cubosome cytotoxicity,40,41 appropriate formulation strategies and administration at lower concentrations can be used to achieve the desired therapeutic effects. In fact, recent studies have proven their useful biomedical applications for diagnostic purposes, anticancer activity, and PDT.42–46
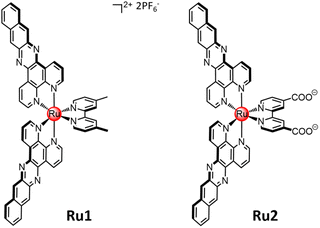
Prompted by this scenario, herein we explored the potential as PSs of two Ru(II) compounds featuring two dppn ligands simultaneously coordinated to the Ru(II) centers; [Ru(dppn)2(dmbpy)]2+ (Ru1) and [Ru(dppn)2(dcbpy2−)] (Ru2) (dmbpy = 4,4′-dimethyl-2,2′-bipyridine, dcbpy2− = 2,2′-bipyridine-4,4′-dicarboxylate) (Chart 1). Besides the Ru(dppn)2-core, different dmbpy and dcbpy2− ancillary ligands were chosen to provide a potential synthetic platform for obtaining differently functionalized (dppn)2-RPCs, to investigate their possible influence on the chemical–physical and biological properties of the resulting compounds.
Ru1 and Ru2 were prepared by adopting a straightforward synthetic route where the dppn ligands were allowed to react with Ru(II)-intermediates in the last step of the reaction, thus avoiding the use of [Ru(dppn)2Cl2] and leading to the production of RPCs in good yields. These systems exhibited promising features as PSs, by virtue of optimal singlet oxygen sensitizing properties and capacity to interact with DNA, and for this reason their phototoxicity and biocompatibility were tested on non-melanoma skin cancer cells in vitro, a model tumor selected for the feasibility of its treatment by photodynamic therapy.47 To further ascertain their potential as PSs, Ru1 and Ru2 were also encapsulated in monoolein-based cubosomes stabilized with Pluronic F108. Following a preliminary investigation of the obtained formulations, Ru2-cubo was then selected for further development including a thorough physicochemical characterization and the assessment of its phototoxic activity against epidermoid carcinoma cells.
The results herein discussed may provide fundamental knowledge for the design of novel and highly performant PSs based on (dppn)2-containing RPCs. Moreover, to the best of our knowledge, this study reports the first example of the encapsulation of a RPC into cubosome nanostructures, thus paving the way for the development of pharmaceutically viable nano-formulations for PDT applications.
Results and discussion
Synthesis and characterization of ruthenium compounds
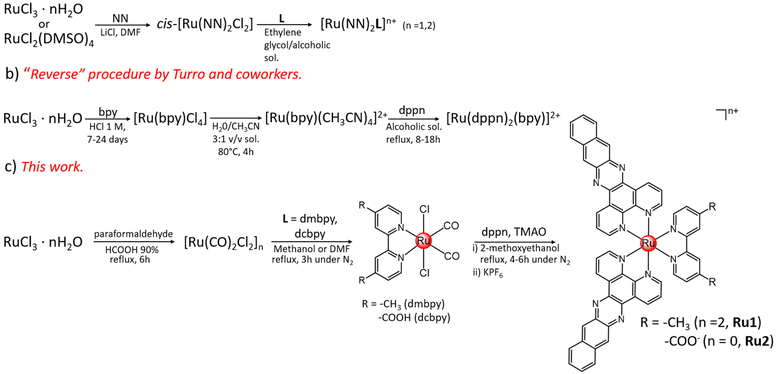
Ru(II) complexes Ru1 and Ru2 were obtained via stepwise ligand addition following the synthetic route shown in Scheme 1c. In this synthetic approach, the polymeric precursor [Ru(CO)2Cl2]n was first prepared by the reaction of the commercial RuCl3·nH2O with paraformaldehyde in formic acid. Then, this compound was allowed to react with the bidentate ligands in refluxing methanol (dmbpy) or DMF (dcbpy), affording the trans-Cl[Ru(dmbpy)(CO)2Cl2] and trans-Cl[Ru(dcbpy)(CO)2Cl2] intermediates, with yields of 75 and 55%, respectively. In the latter case, DMF was chosen as the solvent because of the limited solubility of dcbpy in methanol.48 Finally, two equivalents of dppn ligands were added to solutions of the trans-Cl[RuL(CO)2Cl2] (L = 4,4′-dimethyl-2,2′-bipyridine or dmbpy, 2,2′-bipyridine-4,4′-dicarboxylic acid or dcbpy) intermediates in 2-methoxyethanol, and in the presence of trimethylamine N-oxide (TMAO), to favour the detachment of the strongly coordinated CO ligands49 and allow their replacement by the bidentate dppn ligands. The addition of aqueous KPF6 led to the precipitation of the hexafluorophosphate salt of Ru1 whereas Ru2 precipitated as a neutral product from the reaction mixture. Ru1 and Ru2 were obtained, after purification by flash chromatography, in 78% and 50% yields, respectively. The identity of the obtained compounds was confirmed by 1H, 13C, COSY and HSQC NMR and high-resolution mass spectrometry (HR-MS) analysis (see the ESI, Fig. S1–S10†). 1H NMR signal assignment is reported in the ESI;† recording of 13C and HSQC spectra of Ru2 was prevented by its poor solubility in (CD3)2SO.As shown in Scheme 1, it can be highlighted that, compared to the most employed synthetic approach used for the preparation of bis-heteroleptic complexes with the general formula [Ru(NN)2L]2+ (N,N = polypyridyl bidentate ligand)50,51 (Scheme 1a), commonly obtained by the reaction of [Ru(NN)2Cl2] with a third chelate ligand (L), in the method of this work the two dppn ligands are allowed to react with the Ru(II)-scaffolds only in the last step of the reaction. This would permit to overcome solubility issues arising from the use of the [Ru(dppn)2Cl2] intermediate. A similar “reverse” concept was also previously applied by Turro and coworkers in the synthesis of a rare example of a (dppn)2-containing RPC reported in the literature, namely [Ru(bpy)(dppn)2][PF6]2,24 which was indeed obtained by the reaction of dppn with [Ru(bpy)(CH3CN)4]2+ in the last reaction step (Scheme 1b). However, long reaction times (in the order of 7–24 days) were required by this route to prepare the intermediate [Ru(bpy)(CH3CN)4]2+ using RuCl3·nH2O as the starting material.52,53
In light of these considerations, the synthetic strategy employed in this work may provide an alternative and straightforward way for the preparation of RPCs featuring the general formula [Ru(dppn)2L]n+ (L = variously functionalized bidentate polypyridyl chelates), in good yields and reaction times.
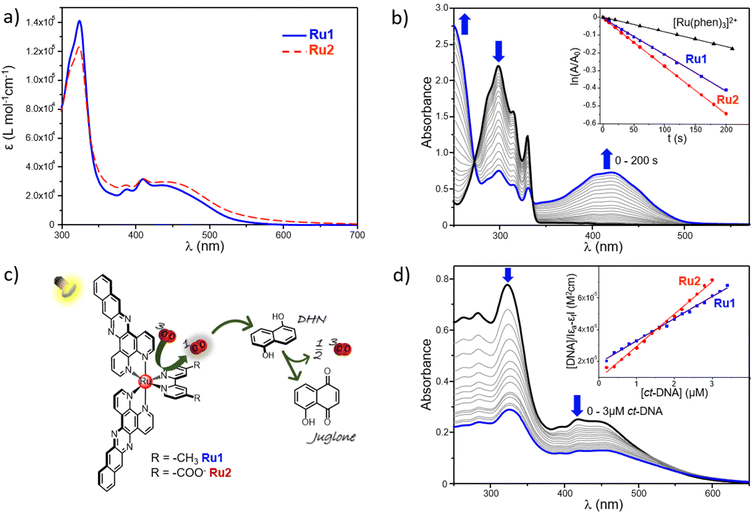
The electronic absorption spectra of Ru1 and Ru2 in acetonitrile are shown in Fig. 1a, whereas their molar extinction coefficients (ε) at different absorption maxima (λmax) are listed in Table 1. As shown, besides the intense intraligand π → π* transitions at 280–330 nm, both complexes display a double humped absorption at ∼387 and ∼410 nm, which is typical of the dppn centered π → π* transitions, plus a broad 1MLCT absorption centered at ∼445 nm, in good agreement with those of dppn-containing RPCs reported in the literature.24,54 It can also be noted that the absorptions relative to the dppn-centered transitions of Ru1 and Ru2 are more intense than the corresponding ones reported for the parental compound [Ru(bpy)2dppn]2+,24 as expected due to the presence of two dppn units in their Ru(II) scaffolds. On the other hand, Ru1 and Ru2 resulted to be weakly luminescent, with the highest emission being displayed by Ru2 in acetonitrile and ethanol (Fig. S11, ESI†).
| Compound | λ abs nm−1 (ε × 103 M−1 cm−1)a | K obs | ϕ Δ (1O2)b | K b (×106 M−1) | pKa |
|---|---|---|---|---|---|
| a Determined in acetonitrile. b Determined for air-saturated acetonitrile solutions of Ru(II) complexes. | |||||
| Ru1 | 324 (146.1), 387 (25.3), 409 (32.2), 440 (26.6) | 1.85 × 10−3 | 0.54 ± 0.06 | 7.49 × 105 | –– |
| Ru2 | 323 (123.2), 387 (27.8), 410 (32.4), 445 (30.0) | 2.71 × 10−3 | 0.50 ± 0.07 | 2.34 × 106 | pKa1, pKa2°3.6 ± 0.3, pKa2* 4.6 ± 0.4 |
Finally, given the presence of the ionizable dcbpy ligand in Ru2, the acid–base behavior of this complex in water was examined by means of spectrophotometric titrations, as described in the ESI.† Similar to what was previously reported for a parental dcbpy-containing Ru(II) complex,55 of the two possible protonation equilibria only pKa2 values of 3.6 ± 0.3 and 4.6 ± 0.4, respectively, for the ground (pKa2°) and the excited state (pKa2*) (Table 1) were determined by these measurements. This, along with the presence of two inflection points in the fluorescence titrations, suggested that the first pKa value was too low to be accurately determined. The higher value found for pKa2* relative to pKa2° would be in line with the higher basicity of the complex in the excited state. It should also be noted that these data confirm that the carboxylic functions of Ru2 are likely to be fully deprotonated at neutral pH, conferring an overall total neutral charge to the complex. Therefore, along with the different nature of their ancillary ligands, it can be envisaged that the different charges of metal complexes in physiological media (+2 for Ru1, 0 for Ru2) might have an influence on their biological behavior and interaction with cubosome nanostructures (vide infra).
Singlet oxygen sensitizing properties of Ru(II)-complexes and DNA interaction
A crucial requisite for a candidate PS for PDT applications relies on its ability to trigger the formation of harmful reactive species under light-irradiation, such as the highly oxidant singlet oxygen 1O2, the classical warhead of PDT produced as the result of type-II-based pathways.56The singlet oxygen sensitizing properties of Ru(II) complexes Ru1 and Ru2 were first assessed spectrophotometrically, by employing 1,5-dihydroxynaphtalene (DHN) as an indirect reporter for singlet oxygen. Indeed, in the presence of 1O2, DHN is promptly and quantitatively oxidized to give 5-hydroxy-1,4-naphthalenedione (Juglone), thus allowing to easily follow the photoexcitation process by monitoring the decrease of the DHN absorption band, centered at 297 nm, and the simultaneous increase of the broad Juglone band at around 427 nm (see Fig. 1c for a schematic illustration of the 1O2 analysis by the DHN method for Ru1 and Ru2 complexes).
Fig. 1b shows the absorption spectra of an acetonitrile solution containing Ru1 and DHN subjected to increasing irradiation times (LED emitting at 434 nm, 160 mW), light-exposure determined the progressive decrease of the DHN absorption band along with the simultaneous increase of that of Juglone, thus clearly demonstrating the photosensitizing properties of Ru1. It should also be noted that the appearance of two clear isosbestic points in the UV-Vis titration, at ∼280 and 330 nm, ruled out the formation of long-lived intermediates or byproducts. An analogous behavior was displayed by Ru2, as reported in Fig. S13 of the ESI.† Compared to [Ru(phen)3]2+, taken as a reference RPC for 1O2 sensitization, both Ru1 and Ru2 exhibited remarkably higher photosensitizing features. This can be easily appreciated from the corresponding semilogaritmic plots of ln(A/Ao) over the irradiation time frame (A0 and A are the absorbance values at 297 nm at time “zero” and at a generic time “t”) reported in the inset of Fig. 1b, in which can be evidenced, for example, that a similar amount of 1O2 was produced within 65–75 s by Ru1 and Ru2, and in more than 200 s by [Ru(phen)3]2+. In detail, Ru1 and Ru2 displayed a comparable potency, as denoted by the slight differences emerging between their relative rate constants for the DHN photooxidation processes (kobs), of 1.85 × 10−3 and 2.71 × 10−3, respectively (Table 1). In addition to the indirect DHN method, the 1O2 sensitizing properties of Ru1 and Ru2 were further probed through direct measurement of the phosphorescence signal of 1O2 at 1270 nm, induced by irradiation of air-saturated acetonitrile solutions of ruthenium complexes. This allowed us to determine the relative quantum yields of 1O2 generation (ϕΔ), which are listed, along with the one of [Ru(phen)3]2+ for comparison (ϕΔ = 0.38 ± 0.06), in Table 1. As shown, ϕΔ values of 0.54 ± 0.06 and 0.50 ± 0.07 were respectively obtained for Ru1 and Ru2, thus confirming that the simultaneous presence of two dppn units into the Ru(II) scaffolds confers to these complexes a potent and comparable ability to sensitize the formation of singlet oxygen, in well agreement with the results of the UV-Vis analysis.
Since it is known that 1O2 rapidly reacts with the surrounding biological substrates (estimated half-life <40 ns, range of action in the order of 20 nm),57 leading to an extremely localized oxidative damage, the ability of a PS to effectively interact with a desired biological target may be important for its potential application in PDT, as it would ensure drug localization in close proximity to the target to be treated, strengthening the oxidative damage induced by ROS sensitization. This, along with the known DNA intercalating properties imparted by the π-expansive dppn ligands, prompted us to consider the affinity of the studied RPCs with the nucleic acid. The DNA-binding abilities of Ru1 and Ru2 were evaluated on calf thymus (ct-DNA) through UV-Vis analysis, by monitoring the changes in the absorption profiles of the aqueous solution of RPCs buffered at pH 7.4 induced by increasing concentrations of ct-DNA. As shown in Fig. 1d for a 10 μM solution of Ru1, the addition of ct-DNA resulted in a strong hypochromism in both the MLCT and π → π* absorption bands of the metal complex, with a reduction of approximately 50 and 65% of their relative intensities in the presence of only 3 μM DNA. No blue or red shift was observed upon the addition of DNA and a very similar trend was also observed in the case of Ru2 (see Fig. S14, ESI†). The intrinsic binding constants (Kb) of Ru1 and Ru2 were calculated from titration data (see the inset of Fig. 1d for a comparison between the two RPCs) as described in the ESI† and the resulting values are reported in Table 1. As shown, Kb values of 7.49 × 105 M−1 and 2.34 × 106 M−1 were respectively obtained for Ru1 and Ru2, thus confirming the ability of these systems to strongly interact with DNA under abiotic conditions. It can be noted that these values are in line with the ones reported for other dppn-containing ruthenium complexes (Kb in the order of 106 M−1)54,58,59 and though not conclusive, together with the large extent of hypochromism observed, they hint at the intercalation as the most likely binding mode for these complexes. Moreover, the possible beneficial role played by the presence of a second dppn ligand in strengthening the interaction of complexes with the biopolymer is particularly evidenced by comparing Ru1 with its mono-dppn containing analogue, [Ru(dmbpy)(dppn)]2+, for which a lower Kb, of almost 5.8-fold has been reported.28
Cytotoxicity and photoactivity of Ru(II)-complexes
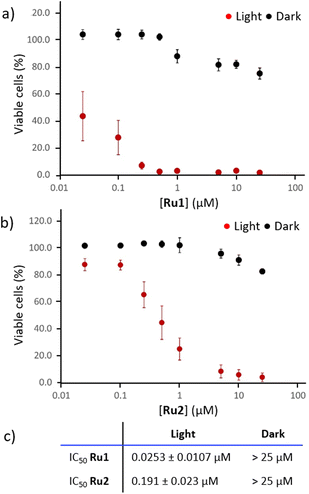
To be qualified as a potential agent for photodynamic therapy, newly developed photosensitizers should be biologically inert in the dark, but highly cytotoxic when exposed to light of a given wavelength.60 This simple mechanism allows for selective action against the light-exposed area (i.e. the tumor), abolishing the systemic toxicity typically associated with traditional chemotherapeutic drugs.46 Here, the anticancer activity of Ru1 and Ru2 was evaluated on A431 cells, an in vitro model of human epidermoid carcinoma.As shown in Fig. 2, both compounds were found to be well tolerated by cells when no light was provided to the culture dishes. A slight difference between their in dark toxicities was observed, thus indicating that seemingly small modifications on the groups gathered on the bpy moieties of complexes (methyl or carboxylic functions) may influence their toxicity. In details, cell viability was reduced to 75.1 ± 4.1% and 82.4 ± 2.0% upon exposure to the highest dose of Ru1 and Ru2 (25 μM) in the dark, respectively. Conversely, 30-minutes of irradiation with an LED array (λmax = 462 nm, 18 mW cm−2) induced potent activation of the complexes, triggering complete cell death (viability < 10%) at concentrations of 0.25 μM (Ru1, Fig. 2a) and 5 μM (Ru2, Fig. 2b): Fig. 2c summarizes the IC50 values calculated from in vitro experiments. As shown, both Ru1 and Ru2 displayed high photo-toxic indexes (PI, defined as IC50 in the dark/IC50 upon irradiation), with values exceeding 988 and 130, respectively. From a translational point of view, higher PIs are predictive of a larger therapeutic window, with limited off-target cytotoxicity and enhanced on-target potency. Of note, notwithstanding the lack of data for the phototoxicity of dppn-containing Ru(II) complexes in A431 cells, it can be highlighted that the in vitro therapeutic outcomes of complexes of this study are ones of the highest among those reported in the literature for the PDT effect of dppn-containing RPCs.23,25,61–63
Cubosome loading and characterization
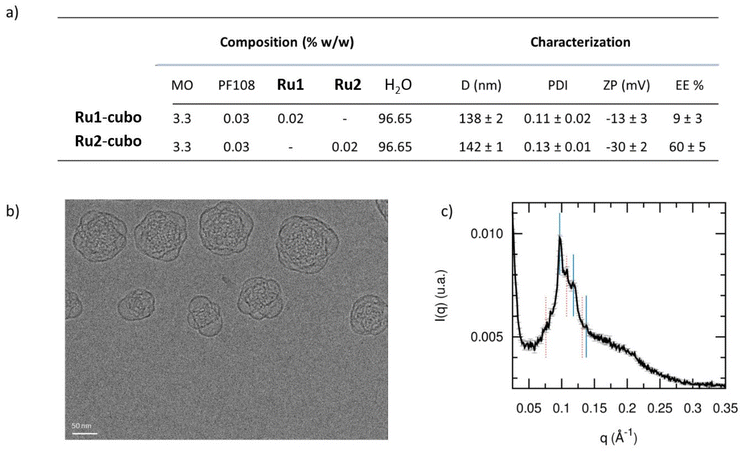
The in vitro results highlighted the promising activity of the obtained systems in PDT. Nevertheless, their poor aqueous solubility would not be compatible with direct administration to a patient. In fact, self-aggregation phenomena might occur due to the high hydrophobicity of these systems, leading to low bioavailability, possible off-target activation and reduction of their photosensitivity and photophysical properties.64 The encapsulation of PSs into nanocarriers is a well-known technique used to overcome these issues and to facilitate their biomedical application.65 In this study, we prepared Ru1 and Ru2 cubosome-loaded formulations using monoolein (MO) as the molecular building block and PF108 as the stabilizing agent. In line with previous results,66 the obtained samples were fluid aqueous dispersions with a milky macroscopic appearance. Cubosomes, here proposed as PS carriers, were prepared as described in paragraph 4.1 of the ESI† and characterized in terms of encapsulation efficiency and colloidal properties, namely size, size distribution and zeta potential.Unencapsulated PSs were removed by exhaustive dialysis, then cubosomes were dissolved in methanol and the drug content was spectrophotometrically quantified. The results revealed high encapsulation values of Ru2 (60%), whereas the amount of encapsulated Ru1 was 9%. Besides their different structures, the two complexes also display different overall charges (at neutral pH Ru1 features a double positive charge whereas Ru2 is likely to be present in its neutral form) and this can be reasonably assumed to affect the encapsulation efficiency into cubosomes. Indeed, the production procedure and the excipients employed were identical for both formulations, the encapsulated PS being the sole difference.
As for the colloidal properties, DLS analysis revealed the presence of nanoparticles with an average diameter of approximately 138 and 142 nm, for Ru1-cubo and Ru2-cubo respectively (Fig. 3a). Both formulations showed a narrow size distribution with PDI values below 0.2. Concerning the nanoparticle zeta potential, we recorded a value of −9 mV for Ru1-cubo and −30 mV for Ru2-cubo, thus indicating a superior stabilization of the latter.
We monitored the average diameter, PDI and zeta potential over a period of 30 days, for a medium-term stability study of the colloidal systems (Fig. S16, ESI†). The size distribution study revealed optimal stability of Ru2-cubo, since the mean diameter did not vary appreciably during the 30 days on storage at 25 °C, with an average diameter of approximately 140 nm during the whole study. The PDI and zeta potential were almost constant, confirming the retention of the fairly narrow size distribution on storage. Conversely, the average diameter of Ru1-cubo increased from 138 nm (day 0) to 235 nm (day 30), the zeta potential moved to lower values, while PDI values were almost steady.
Given the obtained preliminary results of Ru1-cubo, namely, low encapsulation efficiency and an increase of the average diameter over 30 days of storage, we selected Ru2-cubo for further characterization and in vitro bioactivity tests. Firstly, we evaluated the nanoparticles morphology of Ru2-cubo by means of cryo-transmission electron microscopy (Cryo-TEM). As shown in Fig. 3b, cubosomes appear as spherical nanoparticles with an internal structure characterized by a dark matrix and bright spots, which represent the lipid phase and the water channels respectively. We then evaluated the inner nanostructure of Ru2-cubo through small angle X-ray scattering. Particularly, the recorded SAXS pattern shown in Fig. 3c strongly suggests the simultaneous presence of two bicontinuous cubic phases, the Pn3 m and the Im3 m, respectively characterized by lattice parameters of 92 ± 1 Å and 117 ± 1 Å and water channel radii of 38 ± 1 Å and 37 ± 1 Å. In fact, the coexistence of the two phases is often observed when MO cubosomes are stabilized with Pluronics.66,67
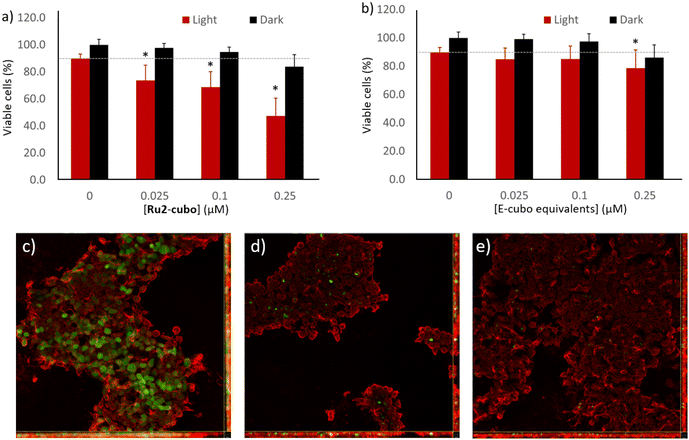
Cytotoxicity, photoactivity and ROS production of cubosomes-encapsulated Ru2
In addition to promoting solubility and stability, nanoencapsulation of photosensitizers in soft colloids has shown to improve the management of cancer in previous studies, as it allows targeted delivery and favors bio-membranes crossing.68–71 When designing novel PS-loaded nanoparticles, it is critical to assess that the biological activity of the cargo is retained upon nano-encapsulation, and that no unspecific toxicity comes from the nanoparticle itself (i.e. empty vector). For such reasons, we tested the cytotoxicity (in the dark) and phototoxicity (upon LED illumination) of Ru2-cubo on the previously described epidermoid carcinoma model, comparing the results with the effect triggered by empty cubosomes (E-cubo, not loaded with PS). Ru2-cubo sensitized cancer cells to light even at a very low concentration of 0.025 μM ([Ru2]), with more than 50% reduction of cell viability at a dose of 0.25 μM (Fig. 4a). Calculated IC50 for Ru2-cubo was 0.268 ± 0.079 μM. The slightly higher IC50 of Ru2-cubo compared to free Ru2 is expected for a nano-encapsulated molecule and can be partially explained by the lower intracellular localization of ruthenium, evidenced by inductively coupled plasma atomic emission spectrometry (ICP-AES), when entrapped in the soft lipid matrix (Fig. S17, ESI†). As expected, treatment with Ru2-cubo was efficacious only when coupled with LED irradiation, as cells incubated in the dark did not show signs of sufferance. The risk of unspecific toxicity of other components of the nanoformulation (i.e. monoolein and PF108) was ruled out by exposing cells to E-cubo under the same conditions (Fig. 4b).To obtain preliminary information about the mechanism of the observed phototoxicity, we first investigated the production of intracellular ROS upon PDT using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay.72 When applied to the cell culture, the cell permeable probe DCFH-DA crosses the cell membrane and it is deacetylated by cytosolic esterases into a non-fluorescent metabolite (DCFH). In the presence of intracellular ROS, the metabolite can be oxidized to produce highly fluorescent 2′,7′-dichlorofluorescein (DCF). The amount of ROS produced by the cells in response to PDT can be estimated by measuring the green fluorescence intensity of DCF localized within the cell body.
A431 cells were treated with Ru2-cubo, supplied with the DCFH-DA probe and exposed to light to trigger the activation of the photosensitizer. The dose of Ru2-cubo (100 nM of Ru2) was selected to allow for the observation of ROS generation, limiting the extent of cell toxicity. Immediately after irradiation, cells were fixed, and their membranes were stained with WGA for microscopy observation. Representative images acquired by confocal laser scanning microscopy allow to observe a diffuse green fluorescence in almost 87.4% of the cells exposed Ru2-cubo + light (Fig. 4c). Conversely, the treatment with Ru2-cubo in the dark did not induce significant production of ROS, as the amount of green signal detectable (Fig. 4d) was comparable to the one observed in a well of untreated cells (control, Fig. 4e). More specifically, the percentage of ROS-producing cells calculated through image analysis was 1.3% and 0.3% for Ru2-cubo in the dark and for untreated cells, respectively.
We then inspected the intracellular distribution of Ru2-cubo into A431 cells by employing laser-scanning confocal microscopy (Fig. S18, ESI†). Despite the ability of the PS to bind DNA, our results indicated a modest localization of Ru2-cubo within the nuclei, at least after 1 hour of incubation of A431 cells. This was also observed for the non-encapsulated metal complex, thus suggesting that ROS oxidation of other types of macromolecules, such as proteins or membrane lipids, rather than DNA, is likely to be the cause of the observed phototoxicity under our experimental conditions. These oxidized species, in addition to losing their function, can initiate the pro-apoptotic and pro-necrotic cascade, resulting in cell damage and death.73
Interestingly, it can also be noted that, in contrast to the free metal complex, which, after 1 hour of incubation, evidenced a random distribution in the cellular cytosol, after the same incubation time Ru2-cubo was rather found to be finely localized in discrete areas.
Overall, these results confirm that the cytotoxic effect observed upon PDT with Ru2-cubo would be related to the capacity of this system to effectively trigger the production of intracellular ROS, as expected due to the good singlet oxygen sensitizing properties of Ru2.
Conclusions
In this study, we explored the potential as PSs for PDT applications of two novel Ru(II) complexes, Ru1 and Ru2, characterized by two π-expansive dppn units simultaneously coordinated to their Ru(II) centers. The synthetic route followed for the preparation of these complexes may represent a valid alternative to commonly employed methods and can be potentially harnessed for the preparation of bis-heteroleptic RPCs of the general formula [Ru(dppn)2L]2+ (L = variously functionalized bpy ligands), whose chemical–physical and photobiological properties can be finely modulated by tuning the nature of their bidentate chelates. The simultaneous presence of two dppn ligands conferred to Ru1 and Ru2 optimal singlet oxygen sensitizing features and DNA-interaction capabilities, which were paralleled by a potent light-triggered toxicity exerted on squamous epithelial carcinoma cells, with PI values exceeding 988 (Ru1) and 130 (Ru2).Given their scarce solubility in physiological media, which would preclude their direct administration for therapeutic use, Ru1 and Ru2 were encapsulated into cubosomes, chosen as soft nanoparticles to obtain Ru(II)-formulations with improved biopharmaceutical properties. Among the resulting hybrid systems, Ru2-cubo displayed superior encapsulation efficiency and stability as compared to Ru1-cubo, thus hinting at a subtle role played by the nature of the ancillary ligands and/or the overall charge of RPCs. For this reason, we focused our attention on the former system, which was further characterized and subjected to bioactivity investigations. Our results probed the effectiveness of Ru2-cubo, as denoted by the photoactivity observed even at a very low drug concentration, whereas mechanistic studies confirmed that intracellular ROS generation was likely responsible for the Ru2-cubo-mediated PDT efficacy.
An important aspect that deserves consideration is that soft matter nanoparticles are prone to phase transition/degradation when dispersed in fluids of biological interest.74 However, several studies evidenced that monoolein-based cubosomes are rather stable when incubated in fetal bovine serum solution,75,76 while when dispersed in human plasma77 after 15 min they start to evolve towards a different kind of nanoparticle known as hexosomes, characterized by a hexagonal inner nanostructure.78,79 Indeed, at least one investigation proved that after 10/15 min from i.v. administration in mice, cubosomes are non-altered and able to reach all the biological compartments without the release of the imaging agent they carried.
In conclusion, the results herein discussed highlight the great potential of RPCs featuring two π-expansive dppn ligands as photosensitizing agents in the blooming field of research of PDT. Going beyond providing a simple and general synthetic route for the preparation of this class of compounds, to the best of our knowledge this work also reports the first RPC to be encapsulated into cubosome nanostructures, providing fundamental knowledge about the design of pharmaceutically viable Ru(II)-cubosome formulations for PDT applications.
Author contributions
G. E. G., M. S. and L. Casula: investigation and data curation, L. G., A. D. G., C. S., G. P., F. C., S. P. and B. V.: investigation, S. M. and C. G.: supervision and project administration, L. Conti.: writing the original draft and project administration. G. E. G., M. S., L. Casula, L. Conti, C. S., B. V., C. G. and S. M. are inventors on a pending patent application pertaining to the ruthenium polypyridyl complexes described in this work. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors would like to thank Dr Annalisa Guerri, from the Department of Chemistry Ugo Schiff of the University of Florence for the Cryo-TEM measurements. UniCA-Progetti biennali di Ateno Finanziati dalla Fondazione di Sardegna 2018 (CUP F741I19000950007) is gratefully acknowledged for (partial) financial support.References
- G. Gunaydin, M. E. Gedik and S. Ayan, Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status, Front. Chem., 2021, 9, 686303 CrossRef CAS PubMed.
- J. H. Correia, J. A. Rodrigues, S. Pimenta, T. Dong and Z. Yang, Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions, Pharmaceutics, 2021, 13, 1332 CrossRef CAS PubMed.
- L. Karner, S. Drechsler, M. Metzger, A. Hacobian, B. Schädl, P. Slezak, J. Grillari and P. Dungel, Antimicrobial Photodynamic Therapy Fighting Polymicrobial Infections-a Journey from: In Vitro to in Vivo, Photochem. Photobiol. Sci., 2020, 19, 1332–1343 CrossRef CAS PubMed.
- G. Boccalini, L. Conti, C. Montis, D. Bani, A. Bencini, D. Berti, C. Giorgi, A. Mengoni and B. Valtancoli, Methylene Blue-Containing Liposomes as New Photodynamic Anti-Bacterial Agents, J. Mater. Chem. B, 2017, 5, 2788–2797 RSC.
- L. Conti, E. Macedi, C. Giorgi, B. Valtancoli and V. Fusi, Combination of Light and Ru(II) Polypyridyl Complexes: Recent Advances in the Development of New Anticancer Drugs, Coord. Chem. Rev., 2022, 469, 214656 CrossRef CAS.
- S. Monro, K. L. Colón, H. Yin, J. Roque, P. Konda, S. Gujar, R. P. Thummel, L. Lilge, C. G. Cameron and S. A. McFarland, Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433, Chem. Rev., 2019, 119(2), 797–828 CrossRef CAS PubMed.
- Y. Wu, S. Li, Y. Chen, W. He and Z. Guo, Recent Advances in Noble Metal Complex Based Photodynamic Therapy, Chem. Sci., 2022, 13, 5085–5106 RSC.
- C. Mari, V. Pierroz, S. Ferrari and G. Gasser, Combination of Ru(II) Complexes and Light: New Frontiers in Cancer Therapy, Chem. Sci., 2015, 6, 2660–2686 RSC.
- A. Notaro and G. Gasser, Monomeric and Dimeric Coordinatively Saturated and Substitutionally Inert Ru(II) Polypyridyl Complexes as Anticancer Drug Candidates., Chem. Soc. Rev., 2017, 46, 7317–7337 RSC.
- L. Conti, G. E. Giacomazzo, B. Valtancoli, M. Perfetti, A. Privitera, C. Giorgi, P. S. Sfragano, I. Palchetti, S. Pecchioli, P. Bruni and F. Cencetti, Highly Charged Ru(II) Polypyridyl Complexes as Photosensitizer Agents in Photodynamic Therapy of Epithelial Ovarian Cancer Cells, Int. J. Mol. Sci., 2022, 23(21), 13302 CrossRef CAS PubMed.
- L. Conti, A. Mengoni, G. E. Giacomazzo, L. Mari, M. Perfetti, C. Fagorzi, L. Sorace, B. Valtancoli and C. Giorgi, Exploring the Potential of Highly Charged Ru(II)- and Heteronuclear Ru(II)/Cu(II)-Polypyridyl Complexes as Antimicrobial Agents, J. Inorg. Biochem., 2021, 220, 111467 CrossRef CAS PubMed.
- Z. Y. Yan, J. Chen, J. Shao, Z. Q. Jiao, T. S. Tang, M. Tang, Z. G. Sheng, L. Mao, R. Huang, C. H. Huang, Z. H. Zhang, H. M. Su and B. Z. Zhu, The Cell-Impermeable Ru(II) Polypyridyl Complex as a Potent Intracellular Photosensitizer under Visible Light Irradiation via Ion-Pairing with Suitable Lipophilic Counter-Anions, Free Radicals Biol. Med., 2021, 171, 69–79 CrossRef CAS PubMed.
- S. Estalayo-Adrián, S. Blasco, S. A. Bright, G. J. McManus, G. Orellana, D. C. Williams, J. M. Kelly and T. Gunnlaugsson, Water-Soluble Amphiphilic Ruthenium(II) Polypyridyl Complexes as Potential Light-Activated Therapeutic Agents, Chem. Commun., 2020, 56, 9332–9335 RSC.
- J. Karges, F. Heinemann, F. Maschietto, M. Patra, O. Blacque, I. Ciofini, B. Spingler and G. Gasser, A Ru(II) Polypyridyl Complex Bearing Aldehyde Functions as a Versatile Synthetic Precursor for Long-Wavelength Absorbing Photodynamic Therapy Photosensitizers, Bioorg. Med. Chem., 2019, 27(12), 2666–2675 CrossRef CAS PubMed.
- L. K. McKenzie, M. Flamme, P. S. Felder, J. Karges, F. Bonhomme, A. Gandioso, C. Malosse, G. Gasser and M. Hollenstein, A Ruthenium-Oligonucleotide Bioconjugated Photosensitizing Aptamer for Cancer Cell Specific Photodynamic Therapy, RSC Chem. Biol., 2022, 3, 85–95 RSC.
- M. Lin, S. Zou, X. Liao, Y. Chen, D. Luo, L. Ji and H. Chao, Ruthenium(II) Complexes as Bioorthogonal Two-Photon Photosensitizers for Tumour-Specific Photodynamic Therapy against Triple-Negative Breast Cancer Cells, Chem. Commun., 2021, 57, 4408–4411 RSC.
- A. Chettri, J. A. Roque, K. R. A. Schneider, H. D. Cole, C. G. Cameron, S. A. McFarland and B. Dietzek, It Takes Three to Tango: The Length of the Oligothiophene Chain Determines the Nature of the Long-Lived Excited State and the Resulting Photocytotoxicity of a Ruthenium(II) Photodrug, ChemPhotoChem, 2021, 5(5), 421–425 CrossRef CAS PubMed.
- E. C. Glazer, Panchromatic Osmium Complexes for Photodynamic Therapy: Solutions to Existing Problems and New Questions, Photochem. Photobiol., 2017, 93(5), 1326–1328 CrossRef CAS PubMed.
- A. Zamora, E. Wachter, M. Vera, D. K. Heidary, V. Rodríguez, E. Ortega, V. Fernández-Espín, C. Janiak, E. C. Glazer, G. Barone and J. Ruiz, Organoplatinum(II) Complexes Self-Assemble and Recognize AT-Rich Duplex DNA Sequences, Inorg. Chem., 2021, 60(4), 2178–2187 CrossRef CAS PubMed.
- L. E. Joyce, J. D. Aguirre, A. M. Angeles-Boza, A. Chouai, P. K. L. Fu, K. R. Dunbar and C. Turro, Photophysical Properties, DNA Photocleavage, and Photocytotoxicity of a Series of Dppn Dirhodium(II,II) Complexes, Inorg. Chem., 2010, 49(12), 5371–5376 CrossRef CAS PubMed.
- R. N. Akhimie, J. K. White and C. Turro, Dual Photoreactivity of a New Rh2(II,II) Complex for Biological Applications, Inorg. Chim. Acta, 2017, 454, 149–154 CrossRef CAS PubMed.
- S. Li, J. Zhao, X. Wang, G. Xu, S. Gou and Q. Zhao, Design of a Tris-Heteroleptic Ru(II) Complex with Red-Light Excitation and Remarkably Improved Photobiological Activity, Inorg. Chem., 2020, 59(15), 11193–11204 CrossRef CAS PubMed.
- L. Wang, H. Yin, M. A. Jabed, M. Hetu, C. Wang, S. Monro, X. Zhu, S. Kilina, S. A. McFarland and W. Sun, π-Expansive Heteroleptic Ruthenium(II) Complexes as Reverse Saturable Absorbers and Photosensitizers for Photodynamic Therapy, Inorg. Chem., 2017, 56(6), 3245–3259 CrossRef CAS PubMed.
- B. Peña, N. A. Leed, K. R. Dunbar and C. Turro, Excited State Dynamics of Two New Ru(II) Cyclometallated Dyes: Relation to Cells for Solar Energy Conversion and Comparison to Conventional Systems, J. Phys. Chem. C, 2012, 116(42), 17095–17101 CrossRef.
- B. A. Albani, B. Peña, N. A. Leed, N. A. B. G. De Paula, C. Pavani, M. S. Baptista, K. R. Dunbar and C. Turro, Marked Improvement in Photoinduced Cell Death by a New Tris-Heteroleptic Complex with Dual Action: Singlet Oxygen Sensitization and Ligand Dissociation, J. Am. Chem. Soc., 2014, 136(49), 17095–17101 CrossRef CAS PubMed.
- C. Reichardt, S. Monro, F. H. Sobotta, K. L. Colón, T. Sainuddin, M. Stephenson, E. Sampson, J. Roque, H. Yin, J. C. Brendel, C. G. Cameron, S. A. McFarland and B. Dietzek, Predictive Strength of Photophysical Measurements for in Vitro Photobiological Activity in a Series of Ru(II) Polypyridyl Complexes Derived from π-Extended Ligands, Inorg. Chem., 2019, 58(5), 3156–3166 CrossRef CAS PubMed.
- F. Haddache, A. Le Goff, B. Reuillard, K. Gorgy, C. Gondran, N. Spinelli, E. Defrancq and S. Cosnier, Label-Free Photoelectrochemical Detection of Double-Stranded HIV DNA by Means of a Metallointercalator-Functionalized Electrogenerated Polymer, Chem. – Eur. J., 2014, 20(47), 15555–15560 CrossRef CAS PubMed.
- S. Vidhisha, K. L. Reddy, Y. P. Kumar, M. Srijana and S. Satyanarayana, Synthesis, Characterization, Antibacterial Activity and Investigation of DNA Binding for Ru(II) Molecular “Light Switch” Complexes, Int. J. Pharm. Sci. Rev. Res., 2014, 25(1), 197–205 Search PubMed.
- H. Yin, M. Stephenson, J. Gibson, E. Sampson, G. Shi, T. Sainuddin, S. Monro and S. A. McFarland, In Vitro Multiwavelength PDT with 3IL States: Teaching Old Molecules New Tricks, Inorg. Chem., 2014, 53(9), 4548–4559 CrossRef CAS PubMed.
- Q. X. Zhou, W. H. Lei, J. R. Chen, C. Li, Y. J. Hou, X. S. Wang and B. W. Zhang, A New Heteroleptic Ruthenium(II) Polypyridyl Complex with Long-Wavelength Absorption and High Singlet-Oxygen Quantum Yield., Chem. – Eur. J., 2010, 16(10), 3157–3165 CrossRef CAS PubMed.
- C. W. Jiang, H. Chao, R. H. Li, H. Li and L. N. Ji, Syntheses, Characterization and Third-Order Nonlinear Optical Properties of Ruthenium(II) Complexes Containing 2-Phenylimidazo-[4,5-f][1,10]Phenanthroline and Extended Diimine Ligands, Polyhedron, 2001, 20(17), 2187–2193 CrossRef CAS.
- L. N. Lameijer, T. G. Brevé, V. H. S. van Rixel, S. H. C. Askes, M. A. Siegler and S. Bonnet, Effects of the Bidentate Ligand on the Photophysical Properties, Cellular Uptake, and (Photo)Cytotoxicity of Glycoconjugates Based on the [Ru(Tpy)(NN)(L)]2 + Scaffold, Chem. – Eur. J., 2018, 24(11), 2709–2717 CrossRef CAS PubMed.
- N. Toupin, S. J. Steinke, S. Nadella, A. Li, T. N. Rohrabaugh, E. R. Samuels, C. Turro, I. F. Sevrioukova and J. J. Kodanko, Photosensitive Ru(II) Complexes as Inhibitors of the Major Human Drug Metabolizing Enzyme CYP3A4, J. Am. Chem. Soc., 2021, 143(24), 9191–9205 CrossRef CAS PubMed.
- A. C. Munteanu, A. Notaro, M. Jakubaszek, J. Cowell, M. Tharaud, B. Goud, V. Uivarosi and G. Gasser, Synthesis, Characterization, Cytotoxic Activity, and Metabolic Studies of Ruthenium(II) Polypyridyl Complexes Containing Flavonoid Ligands, Inorg. Chem., 2020, 59(7), 4424–4434 CrossRef CAS PubMed.
- G. E. Shillito, S. E. Bodman, J. I. Mapley, C. M. Fitchett and K. C. Gordon, Accessing a Long-Lived 3LC State in a Ruthenium(II) Phenanthroline Complex with Appended Aromatic Groups, Inorg. Chem., 2020, 59(23), 16967–16975 CrossRef CAS PubMed.
- K. Sztandera, M. Gorzkiewicz and B. Klajnert-Maculewicz, Nanocarriers in Photodynamic Therapy—in Vitro and in Vivo Studies, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12(3), 1509 Search PubMed.
- A. Q. Annu, B. Nabi, S. Kotta, J. K. Narang, S. Baboota and J. Ali, Role of Nanocarriers in Photodynamic Therapy, Photodiagn. Photodyn. Ther., 2020, 30, 101782 CrossRef PubMed.
- F. Lai, M. Schlich, R. Pireddu, F. Corrias, A. Fadda and C. Sinico, Production of Nanosuspensions as a Tool to Improve Drug Bioavailability: Focus on Topical Delivery, Curr. Pharm. Des., 2015, 21(42), 6089–6103 CrossRef CAS PubMed.
- S. Murgia, S. Biffi and R. Mezzenga, Recent Advances of Non-Lamellar Lyotropic Liquid Crystalline Nanoparticles in Nanomedicine, Curr. Opin. Colloid Interface Sci., 2020, 48, 28–39 CrossRef CAS.
- J. Barauskas, C. Cervin, M. Jankunec, M. Špandyreva, K. Ribokaite, F. Tiberg and M. Johnsson, Interactions of Lipid-Based Liquid Crystalline Nanoparticles with Model and Cell Membranes, Int. J. Pharm., 2010, 391(1–2), 248–291 Search PubMed.
- J. C. Bode, J. Kuntsche, S. S. Funari and H. Bunjes, Interaction of Dispersed Cubic Phases with Blood Components, Int. J. Pharm., 2013, 448(1), 87–95 CrossRef CAS PubMed.
- S. Murgia, S. Bonacchi, A. M. Falchi, S. Lampis, V. Lippolis, V. Meli, M. Monduzzi, L. Prodi, J. Schmidt, Y. Talmon and C. Caltagirone, Drug-Loaded Fluorescent Cubosomes: Versatile Nanoparticles for Potential Theranostic Applications, Langmuir, 2013, 29(22), 6673–6679 CrossRef CAS PubMed.
- F. D. Victorelli, L. Salvati Manni, S. Biffi, B. Bortot, H. H. Buzzá, V. Lutz-Bueno, S. Handschin, G. Calixto, S. Murgia, M. Chorilli and R. Mezzenga, Potential of Curcumin-Loaded Cubosomes for Topical Treatment of Cervical Cancer, J. Colloid Interface Sci., 2022, 620, 419–430 CrossRef CAS PubMed.
- S. Jenni, G. Picci, M. Fornasier, M. Mamusa, J. Schmidt, Y. Talmon, A. Sour, V. Heitz, S. Murgia and S. C. Caltagirone, Multifunctional Cubic Liquid Crystalline Nanoparticles for Chemo- A Nd Photodynamic Synergistic Cancer Therapy, Photochem. Photobiol. Sci., 2020, 19, 674–680 CrossRef CAS PubMed.
- U. Bazylińska, D. Wawrzyńczyk, J. Kulbacka, G. Picci, L. S. Manni, S. Handschin, M. Fornasier, C. Caltagirone, R. Mezzenga and S. Murgia, Hybrid Theranostic Cubosomes for Efficient NIR-Induced Photodynamic Therapy, ACS Nano, 2022, 16(4), 5427–5438 CrossRef PubMed.
- U. Bazylińska, J. Kulbacka, J. Schmidt, Y. Talmon and S. Murgia, Polymer-Free Cubosomes for Simultaneous Bioimaging and Photodynamic Action of Photosensitizers in Melanoma Skin Cancer Cells, J. Colloid Interface Sci., 2018, 522, 163–173 CrossRef PubMed.
- M. C. F. Simões, J. J. S. Sousa and A. A. C. C. Pais, Skin Cancer and New Treatment Perspectives: A Review, Cancer Lett., 2015, 357(1), 8–42 CrossRef PubMed.
- M. Kubeil, R. R. Vernooij, C. Kubeil, B. R. Wood, B. Graham, H. Stephan and L. Spiccia, Studies of Carbon Monoxide Release from Ruthenium(II) Bipyridine Carbonyl Complexes upon UV-Light Exposure, Inorg. Chem., 2017, 56(10), 5941–5952 CrossRef CAS PubMed.
- N. Nickita, M. J. Belousoff, A. I. Bhatt, A. M. Bond, G. B. Deacon, G. Gasser and L. Spiccia, Synthesis, Structure, Spectroscopic Properties, and Electrochemical Oxidation of Ruthenium(II) Complexes Incorporating Monocarboxylate Bipyridine Ligands, Inorg. Chem., 2007, 46(21), 8638–8651 CrossRef CAS PubMed.
- A. Notaro, M. Jakubaszek, S. Koch, R. Rubbiani, O. Dömötör, E. A. Enyedy, M. Dotou, F. Bedioui, M. Tharaud, B. Goud, S. Ferrari, E. Alessio and G. Gasser, A Maltol-Containing Ruthenium Polypyridyl Complex as a Potential Anticancer Agent, Chem. – Eur. J., 2020, 26(20), 4997–5009 CrossRef CAS PubMed.
- E. Wachter, D. K. Heidary, B. S. Howerton, S. Parkin and E. C. Glazer, Light-Activated Ruthenium Complexes Photobind DNA and Are Cytotoxic in the Photodynamic Therapy Window, Chem. Commun., 2012, 48, 9649–9651 RSC.
- R. A. Krause, Synthesis of Mixed Complexes of Ruthenium(II) with 2,2′-Dipyridyl, Inorg. Chim. Acta, 1977, 22, 209–213 CrossRef CAS.
- A. Petroni and L. D. Slep, Etchenique, R. Ruthenium(II) 2,2′-Bipyridyl Tetrakis Acetonitrile Undergoes Selective Axial Photocleavage., Inorg. Chem., 2008, 47(3), 951–956 CrossRef CAS PubMed.
- H. K. Saeed, P. J. Jarman, S. Archer, S. Sreedharan, I. Q. Saeed, L. K. Mckenzie, J. A. Weinstein, N. J. Buurma, C. G. W. Smythe and J. A. Thomas, Homo– and Heteroleptic Phototoxic Dinuclear Metallo–Intercalators Based on Ru II (Dppn) Intercalating Moieties: Synthesis, Optical, and Biological Studies, Angew. Chem., Int. Ed., 2017, 56(41), 12628–12633 CrossRef CAS PubMed.
- T. Shimidzu, T. Iyoda and K. Izaki, Photoelectrochemical Properties of Bis(2,2′-Bipyridine)(4,4′-Dicarboxy-2,2′-Bipyridine) Ruthenium(II) Chloride, J. Phys. Chem., 1985, 89(4), 642–645 CrossRef CAS.
- C. Imberti, P. Zhang, H. Huang and P. J. Sadler, New Designs for Phototherapeutic Transition Metal Complexes, Angew. Chem., Int. Ed., 2020, 59(1), 61–73 CrossRef CAS PubMed.
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Mechanisms in Photodynamic Therapy: Part One - Photosensitizers, Photochemistry and Cellular Localization, Photodiagn. Photodyn. Ther., 2004, 1(4), 279–293 CrossRef CAS PubMed.
- Y. Sun, L. E. Joyce, N. M. Dickson and C. Turro, Efficient DNA Photocleavage by [Ru(Bpy)2(Dppn)]2+ with Visible Light, Chem. Commun., 2010, 46, 2426–2428 RSC.
- S. P. Foxon, C. Metcalfe, H. Adams, M. Webb and J. A. Thomas, Electrochemical and Photophysical Properties of DNA Metallo-Intercalators Containing the Ruthenium(II) Tris(1-Pyrazolyl)Methane Unit., Inorg. Chem., 2007, 46(2), 409–416 CrossRef CAS PubMed.
- D. Van Straten, V. Mashayekhi, H. S. de Bruijn, S. Oliveira and D. J. Robinson, Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions, Cancers, 2017, 9(2), 1–54 Search PubMed.
- L. M. Lifshits, J. A. Roque, P. Konda, S. Monro, H. D. Cole, D. V. Dohlen, S. Kim, G. Deep, R. P. Thummel, C. G. Cameron, S. Gujar and S. A. McFarland, Near-infrared absorbing Ru(II) complexes act as immunoprotective photodynamic therapy (PDT) agents against aggressive melanoma, Chem. Sci., 2020, 11, 11740 RSC.
- L. N. Lameijer, S. L. Hopkins, T. G. Brevé, S. H. C. Askes and S. Bonnet, D-Versus L-Glucose Conjugation: Mitochondrial Targeting of a Light-Activated Dual-Mode-of Action Ruthenium-Based Anticancer Prodrug, Chem. – Eur. J., 2016, 22, 18484–18491 CrossRef CAS PubMed.
- S. Li, J. Zhao, X. Wang, G. Xu, S. Gou and Q. Zhao, Design of a Tris-Heteroleptic Ru(II) Complex with Red-Light Excitation and Remarkably Improved Photobiological Activity, Inorg. Chem., 2020, 59, 11193–11204 CrossRef CAS PubMed.
- S. Moghassemi, A. Dadashzadeh, R. B. Azevedo, O. Feron and C. A. Amorim, Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system, J. Controlled Release, 2021, 339(10), 75–90 CrossRef CAS PubMed.
- C. F. de Freitas, D. S. Pellosi and A. L. Tessaro, 8-Lipid-based nanoparticles in photodynamic therapy, in Nanomaterials for Photodynamic Therapy, 2023, 203–226 Search PubMed.
- M. Fornasier, S. Biffi, B. Bortot, P. Macor, A. Manhart, F. R. Wurm and S. Murgia, Cubosomes Stabilized by a Polyphosphoester-Analog of Pluronic F127 with Reduced Cytotoxicity, J. Colloid Interface Sci., 2020, 580, 286–297 CrossRef CAS PubMed.
- A. M. Falchi, A. Rosa, A. Atzeri, A. Incani, S. Lampis, V. Meli, C. Caltagirone and S. Murgia, Effects of Monoolein-Based Cubosome Formulations on Lipid Droplets and Mitochondria of HeLa Cells, Toxicol. Res., 2015, 4, 1025–1036 CrossRef CAS.
- J. Guo, M. Schlich, J. F. Cryan and C. M. O'Driscoll, Targeted Drug Delivery via Folate Receptors for the Treatment of Brain Cancer: Can the Promise Deliver?, J. Pharm. Sci., 2017, 106(12), 3413–3420 CrossRef CAS PubMed.
- Z. Zhen, W. Tang, C. Guo, H. Chen, X. Lin, G. Liu, B. Fei, X. Chen, B. Xu and J. Xie, Ferritin Nanocages to Encapsulate and Deliver Photosensitizers for Efficient Photodynamic Therapy against Cancer, ACS Nano, 2013, 7(8), 6988–6996 CrossRef CAS PubMed.
- S. Demartis, G. Rassu, S. Murgia, L. Casula, P. Giunchedi and E. Gavini, Improving Dermal Delivery of Rose Bengal by Deformable Lipid Nanovesicles for Topical Treatment of Melanoma, Mol. Pharm., 2021, 18(11), 4046–4057 CrossRef CAS PubMed.
- L. Conti, S. Ciambellotti, G. E. Giacomazzo, V. Ghini, L. Cosottini, E. Puliti, M. Severi, E. Fratini, F. Cencetti, P. Bruni, B. Valtancoli, C. Giorgi and P. Turano, Ferritin Nanocomposites for the Selective Delivery of Photosensitizing Ruthenium-Polypyridyl Compounds to Cancer Cells, Inorg. Chem. Front., 2022, 9, 1070–1081 RSC.
- S. Wang, A. Riedinger, H. Li, C. Fu, H. Liu, L. Li, T. Liu, L. Tan, M. J. Barthel, G. Pugliese, F. De Donato, M. Scotto D'Abbusco, X. Meng, L. Manna, H. Meng and T. Pellegrino, Plasmonic Copper Sulfide Nanocrystals Exhibiting Near-Infrared Photothermal and Photodynamic Therapeutic Effects, ACS Nano, 2015, 9(2), 1788–1800 CrossRef CAS PubMed.
- M. Redza-Dutordoir and D. A. Averill-Bates, Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species, Biochim. Biophys. Acta, 2016, 1863(12), 2977–2992 CrossRef CAS PubMed.
- A. Yaghmur and H. Mu, Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles, Acta Pharm. Sin. B, 2021, 11(4), 871–885 CrossRef CAS PubMed.
- S. Deshpande, E. Venugopal, S. Ramagiri, J. R. Bellare, G. Kumaraswamy and N. Singh, ACS Appl. Mater. Interfaces, 2014, 6(19), 17126–17133 CrossRef CAS PubMed.
- A. Gupta, T. Stait-Gardner, L. de Campo, L. J. Waddington, N. Kirby, W. S. Price and M. J. Moghaddam, J. Mater. Chem. B, 2014, 2, 1225–1233 RSC.
- J. C. Bode, J. Kuntsche, S. S. Funari and H. Bunjes, Int. J. Pharm., 2013, 448(1), 87–95 CrossRef CAS PubMed.
- C. Caltagirone, M. Arca, A. M. Falchi, V. Lippolis, V. Meli, M. Monduzzi, T. Nylander, A. Rosa, J. Schmidt, Y. Talmond and S. Murgia, RSC Adv., 2015, 5, 23443–23449 RSC.
- V. Meli, C. Caltagirone, C. Sinico, F. Lai, A. M. Falchi, M. Monduzzi, M. Obiols-Rabasa, G. Picci, A. Rosa, J. Schmidt, Y. Talmone and S. Murgia, New J. Chem., 2017, 41, 1558–1565 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Methods, supplementary figures and schemes, and the spectra of compounds. See DOI: https://doi.org/10.1039/d2qi02678c |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |