DOI:
10.1039/D2QI01895K
(Research Article)
Inorg. Chem. Front., 2023,
10, 108-117
A rigid–flexible double-layer steric strategy promoting ethylene polymerization and copolymerization in alkane solvents†
Received
2nd September 2022
, Accepted 1st November 2022
First published on 1st November 2022
Abstract
In this study, a series of double-layered steric α-diimine nickel and palladium complexes containing bulky diarylmethyl moieties with remote 4-cycloalkyl and -phenyl substituents were designed and synthesized. The as-synthesized nickel complexes showed high activities (ca. 106 g mol−1 h−1) and superior thermal stability, giving access to moderately branched polyethylenes with high molecular weights and narrow molecular weight distributions at high temperatures. With the rigid–flexible double-layered steric nickel catalysts, high temperature living polymerizations at 80 °C were achieved. The polyethylene materials yielded by these nickel catalysts at 80 °C exhibited outstanding tensile mechanical and elastic recovery properties (SR up to 86%). Correspondingly, the palladium complexes displayed moderate activities in ethylene polymerization, producing moderately branched polyethylenes with high molecular weights. The polyethylene materials yielded by palladium complexes at 60 °C exhibited good tensile mechanical properties and moderate elastic recovery values. In addition, these palladium complexes could also promote ethylene-polar monomer copolymerization, albeit with modest activity, yielding polar functionalized polyethylenes with moderate levels of branching densities, molecular weights, and incorporation ratios. Overall, the rigid–flexible double-layered steric nickel and palladium complexes with remote cyclohexyl substituents were found to have significantly higher activities than their rigid–rigid double-layered steric or monolayered dibenzhydryl counterparts in alkane solvents.
1. Introduction
Due to the propensity of late-transition-metal alkyl complexes to undergo facile β-H elimination, deleterious chain transfer often occurs in late-transition-metal catalyzed olefin polymerization and copolymerization, which leads to restricted molecular weights of the resulting polymers. Consequently, over the past few years much effort has been devoted to developing catalysts which can exhibit mitigation of chain-transfer tendency and other superior properties.1–3 It has been demonstrated that the introduction of large steric substituents at the axial position of the metal center is capable of mitigating the undesired chain transfer.4–7 Meanwhile, the introduction of bulky substituents also brings about enhanced catalyst stability4–14 and provides an opportunity to modulate the branching density and topology of the resulting polymer in chain walking polymerization systems.5,6,8 For example, the bulky dibenzhydryl substituents have often been integrated into imine-based late-transition metal catalysts, which exhibit excellent ethylene polymerization performance, including suppressed chain transfer and enhanced thermal stability.15–33 Notably, the superior thermal stability of the catalysts will enable ethylene polymerization to be implemented under high temperature conditions, promising for industrial production.10
As is known, alkanes are more desirable solvents than aromatic hydrocarbons for the transition metal-catalyzed manufacture of polyethylene in industry. However, the incorporation of multiple rigid aryl groups into catalysts makes it difficult to dissolve and disperse them in an alkane medium.34 Conley and coworkers employed heterogeneous α-diimine nickel and palladium catalysts anchored on inorganic carriers to accomplish the slurry phase ethylene polymerization.38–40 With respect to the homogeneous ethylene polymerization, we hypothesized that a double-layer steric strategy combining rigidity and flexibility might address the contradiction between the catalyst's thermal stability and solubility issues (Scheme 1). Specifically, the first rigid aromatic layer was supposed to be responsible for the thermal stability of the catalyst and the primary suppression of chain transfer, while the second layer of flexible cyclohexyl substituents was expected to improve the solubility and dispersion of the catalyst in alkane solvents. Besides, this second layer might be beneficial for the suppression of chain transfer reactions as well, which was illustrated in Jian et al.'s recent work.35–37 Based on the above assumption, we designed and synthesized a series of rigid–flexible double-layered steric α-diimine nickel and palladium complexes containing cycloalkyl-substituted dibenzhydryl moieties. These newly synthesized catalysts proved to be highly efficient for ethylene (co)polymerization in alkane solvents.
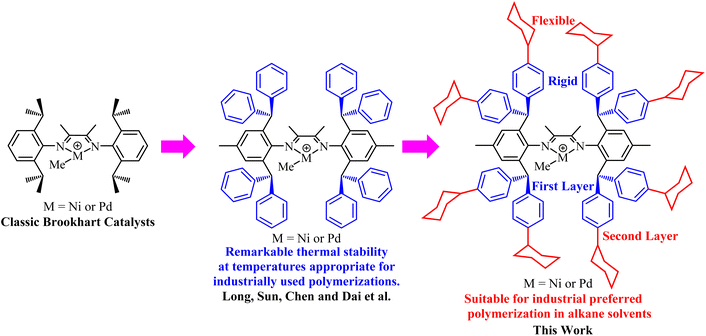 |
| | Scheme 1 Evolution of α-diimine catalysts to adapt for industrial applications in ethylene polymerization. | |
2. Results and discussion
2.1 Synthesis and characterization of α-diimine Ni(II) and Pd(II) complexes
The 4-substituted diarylmethanols O1–O3 were obtained in good yields (65–79%) by reacting ethyl formate with in situ generated phenyllithiums from bromobenzenes and butyllithium (cf. ESI-1.2†). The Friedel–Crafts alkylation of p-methylaniline with diarylmethanols O1–O3 in the presence of the Lucas reagent (ZnCl2/HCl) delivered the corresponding diarylmethylanilines A1–A3 in 54–74% yields (Scheme 2). The as-prepared unknown compounds were identified and characterized by NMR and high-resolution mass spectrometry (cf. ESI; Fig. S1–8 and Fig. S21, 22†). The double-layered steric α-diimines L1–L3 were synthesized via acid-catalyzed condensation between the diarylmethyl anilines and 2,3-butanedione in moderate yields (42–76%) (Scheme 2). L1–L3 were comprehensively examined by 1H and 13C NMR spectroscopy (cf. ESI, Fig. S9–14†) and high-resolution mass spectrometry (cf. ESI, Fig. S23 and 24†). Reactions of these pro-ligands with NiBr2(DME) (DME = dimethoxyethane) at room temperature in a glove box yielded the corresponding α-diimine Ni(II) complexes Ni1–Ni3 without further purification (46–72% yields) (Scheme 2). The Ni(II) complexes were identified by elemental analysis and MALDI-TOF MS (cf. ESI, Fig. S25–27†). Similarly, the corresponding Pd(II) complexes Pd1–Pd3 were obtained in good yields (60–67%) by treating L1–L3 with equivalent PdMeCl(COD) (COD = 1,5-cyclooctadiene), where column chromatography purification was required. These Pd(II) complexes were completely analyzed and characterized by 1H and 13C NMR spectroscopy (cf. ESI, Fig. S15–20†), elemental analysis, and MALDI-TOF-MS (cf. ESI, Fig. S28–30†) (Scheme 2). The structure of Pd1 was also verified by single-crystal X-ray diffraction (Fig. 1). Pd1 possesses an approximate square-planar geometry at the Pd(II) center and the N–Ar is perpendicular to this square-planar coordination plane which will drive the o-aryl diarylmethyl moieties parallel to the Pd(II) axial position. The methyl group and chlorine atom attached to the palladium center appeared at the same position due to the disordered processing method used. Additionally, the side and top views of the Pd1 molecular structure demonstrate that these remote cyclohexyl substituents which exist in different conformational states extend further toward the metal axial position and compel the adjacent phenyl group into misalignment. Here, classical monolayered steric dibenzhydryl α-diimine Ni(II) and Pd(II) complexes (Ni4 and Pd4) were also synthesized as described in the literature.8,10
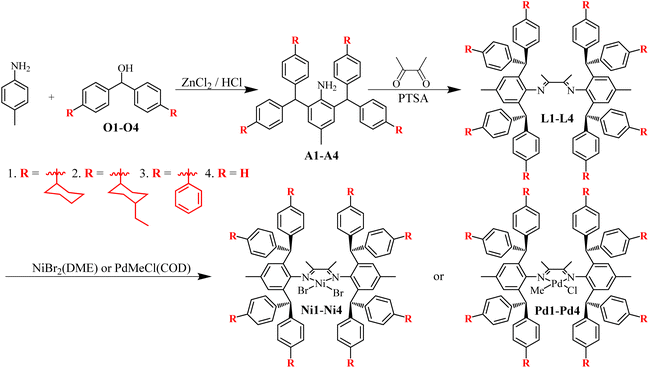 |
| | Scheme 2 Synthesis of double-layered steric α-diimine pro-ligands and the corresponding Ni(II) and Pd(II) complexes. | |
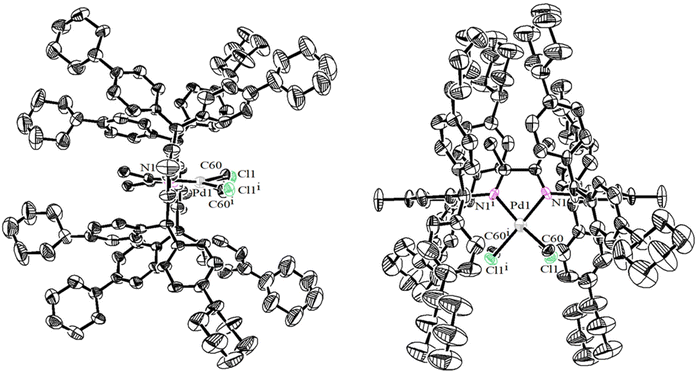 |
| | Fig. 1 Two views of the molecular structure of Pd1 determined by single-crystal X-ray diffraction (CCDC: 2194287†). Atoms are drawn at the 30% probability level and hydrogen atoms are omitted for clarity. | |
2.2 Ni(II)-catalyzed ethylene polymerization
With the addition of 500 eq. of Et2AlCl as an activator, the complexes Ni1 and Ni2 with rigid–flexible double-layered steric substituents exhibited high activities (ca. 106 g per mol Ni per h), while Ni3 with rigid–rigid double-layered steric substituents showed relatively lower activities (ca. 105 g per mol Ni per h) in ethylene polymerizations where hexanes were used as the reaction medium. All these Ni(II) complexes yielded polyethylenes with very high molecular weights (707–1002 kg mol−1) and narrow molecular weight distributions (<1.3) over a wide range of polymerization temperatures (20–80 °C). Notably, both the activity of catalysts and the molecular weight of the resulting polyethylene increased as the temperature was increased (Fig. 2a and b), indicating that these bulky Ni(II) catalysts have very good thermal stability and a strong ability to suppress chain transfer reactions. Given this, we speculated that the ethylene living polymerization at elevated temperatures might be feasible with these nickel complexes. To this end, we conducted ethylene polymerizations with a series of equal variations in the polymerization time (10, 20, 30, 40 min) at 80 °C (Fig. 3). The plots of yield versus time showed that polyethylene yields increased approximately linearly vs. time within 40 min for Ni1 and Ni2 (Fig. 3a and Table S1†). Moreover, the molecular weight of the resulting polymers also increased steadily and almost linearly with time and the molecular weight distributions were very narrow (PDI < 1.3)((Fig. 3b and Table S1†). The above results suggested that high temperature living polymerizations really occurred and also further corroborated that the deactivation of Ni1 and Ni2 and the chain transfer in ethylene polymerizations at 80 °C were effectively suppressed by the double-layered steric substituents. It is noteworthy that Ni3 with rigid–rigid double-layered steric substituents yielded higher molecular weight polyethylene in a lower yield compared to Ni1 and Ni2 with rigid–flexible double-layered steric substituents. The inferior yield could be attributed to the poor dispersion of completely rigid complex Ni3 in hexanes, which led to low initiation efficiency. Obviously, the rigid–flexible double-layer steric strategy can indeed impart superior polymerization performance to this nickel system in hexane solvents. Besides, in comparison with classical monolayered steric bulky Ni4, all the double-layered steric bulky Ni1 and Ni2 possessed superior polymerization activities and produced significantly higher molecular weight polyethylenes under identical polymerization conditions due to the poor dispersion of complex Ni4 in hexanes (entries 13 vs. 1 and 5, Table 1 and Fig. S109†).
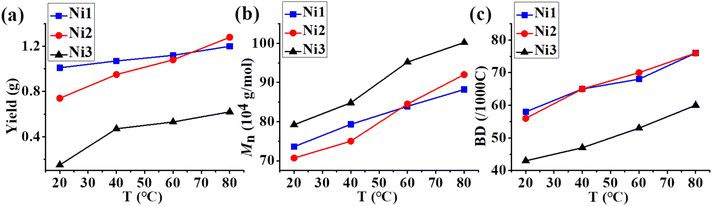 |
| | Fig. 2 Comparisons of the yield (a), molecular weight (b), and branching density (c) of polyethylenes generated with Ni1–Ni3 from 20 °C to 80 °C. | |
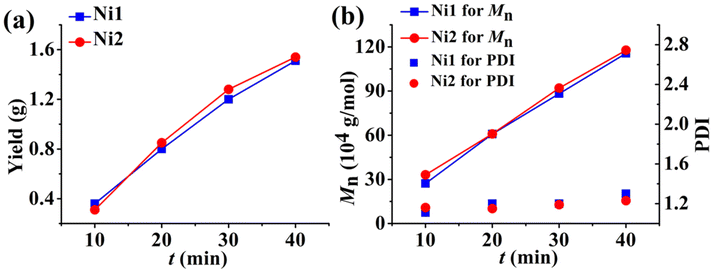 |
| | Fig. 3 Plots of the yield (a), Mn and PDI (b) versus time for Ni1 (blue) and Ni2 (red) at 80 °C. | |
Table 1 Ethylene polymerization with Ni(II) complexesa
| Ent. |
Precat. |
T/°C |
Yield/g |
Act.b |
M
n
|
M
w/Mnc |
B
|
T
m
/(°C) |
|
Conditions: Ni(II) catalyst (2 μmol), 500 eq. of Et2AlCl, 40 mL of hexanes, 6 atm, polymerization time (30 min).
Activity (Act.) = 106 g per mol Ni per h.
M
n is in the unit of 104 g mol−1, determined by SEC in trichlorobenzene at 150 °C.
B = branches per 1000 carbons, determined by 1H NMR spectroscopy, B = 1000 × 2(ICH3)/3(ICH2+CH + ICH3).
Determined by differential scanning calorimetry (DSC), broad peak.
|
| 1 |
Ni1
|
20 |
1.01 |
1.01 |
73.6 |
1.21 |
58 |
61 |
| 2 |
Ni1
|
40 |
1.07 |
1.07 |
79.3 |
1.20 |
65 |
52 |
| 3 |
Ni1
|
60 |
1.12 |
1.12 |
83.9 |
1.19 |
68 |
44 |
| 4 |
Ni1
|
80 |
1.20 |
1.20 |
88.2 |
1.20 |
76 |
36 |
| 5 |
Ni2
|
20 |
0.74 |
0.74 |
70.7 |
1.14 |
56 |
64 |
| 6 |
Ni2
|
40 |
0.95 |
0.95 |
75.0 |
1.18 |
65 |
53 |
| 7 |
Ni2
|
60 |
1.08 |
1.08 |
84.5 |
1.18 |
70 |
41 |
| 8 |
Ni2
|
80 |
1.28 |
1.28 |
92.0 |
1.19 |
76 |
34 |
| 9 |
Ni3
|
20 |
0.15 |
0.15 |
79.2 |
1.25 |
43 |
74 |
| 10 |
Ni3
|
40 |
0.47 |
0.47 |
84.8 |
1.28 |
47 |
67 |
| 11 |
Ni3
|
60 |
0.53 |
0.53 |
95.2 |
1.20 |
53 |
58 |
| 12 |
Ni3
|
80 |
0.62 |
0.62 |
100.2 |
1.23 |
60 |
45 |
| 13 |
Ni4
|
20 |
0.37 |
0.37 |
40.7 |
1.13 |
64 |
50 |
On the other hand, the polyethylenes generated by Ni1–Ni3 possessed moderate to high branching degrees (43–76/1000C) and low to medium melting points (entries 1–12, Table 1). As anticipated, the branching density ascended when the polymerization temperature was elevated (Fig. 2c). Polyethylene samples generated with rigid–flexible double-layered steric Ni1 and Ni2 exhibited a higher degree of branching accompanied by a lower melting point than those generated with rigid-rigid double-layered steric Ni3, implying that the presence of the second flexible substituent layer promoted chain walking of the nickel catalysts during ethylene polymerization. The features of the polyethylene samples suggest that this type of polyethylene may have good thermoplastic elastomer properties. Thus, the tensile strength tests of the polyethylene samples generated with Ni1–Ni3 at 80 °C (Fig. 4a–c) were performed. The examined samples displayed moderate stress-at-break values (4.6 to 10.1 MPa) and high strain-at-break values (611% to 860%). Among them, samples generated by Ni1 and Ni2 exhibited a lower Young's modulus than that generated by Ni3, because the former had a higher branching density. This further illustrates the effect of the catalyst structure on the mechanical properties of the resulting polyethylene. As is known, polyethylene samples with low stress-at-break values and high strain-at-break values may have good elastic recovery properties. Thus, these samples were subjected to hysteresis tests where each sample was expanded at 300% strain over 10 cycles. Strain recovery values (SR) can be calculated using the following formula, SR = 100(εa − εr)/εa, where εa is 300% and εr is the strain value in the 10th cycle at zero load. The polyethylene samples obtained by Ni1 and Ni2 showed excellent elastic recovery properties with SR values of 83% and 86%, respectively (Fig. 4d and e), significantly better than that (61%) obtained by Ni3. This demonstrates that the rigid–flexible double-layer steric strategy for the nickel-catalyzed polymerization of ethylene has a significant advantage in regard of the production of high-performance polyethylene thermoplastic elastomers.
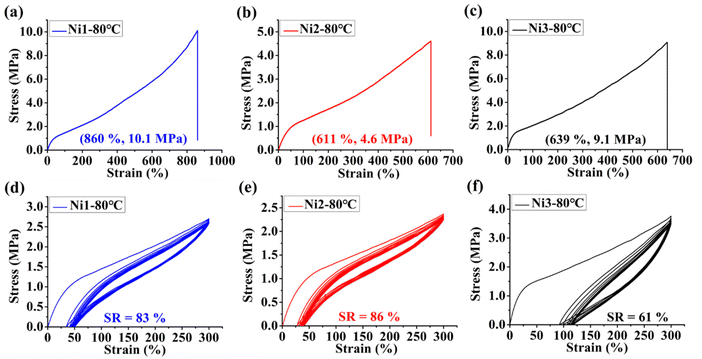 |
| | Fig. 4 Stress–strain curves for samples generated by (a) Ni1, (b) Ni2, and (c) Ni3 at 80 °C. Plots of hysteresis experiments of ten cycles at a strain of 300% for samples generated by (d) Ni1, (e) Ni2, and (f) Ni3 at 80 °C. | |
2.3 Pd(II)-catalyzed ethylene (co)polymerization
The catalytic behavior of the corresponding pallidum complexes was evaluated through in situ activation using an excess amount of sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (NaBArF). Since the second-layer flexible substituents endowed Pd1 and Pd2 with better solubility in hexanes, the activities of Pd1 and Pd2 (ca. 105 g per mol Pd per h) were significantly higher than those of Pd3 with rigid–rigid double-layered steric substituents in ethylene polymerizations using hexanes as the reaction medium (Fig. 5a and Table 2). The pre-catalyst and active Pd–Me+ of Pd1 were soluble in C6D12 and clear characteristic peaks (such as C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and Ar-CH3) could be observed in the 1H NMR spectrum (Fig. S21†). This further supports the above hypothesis. Notably, the polymerization activity of Pd1 and Pd2 showed first an ascending and then a descending trend as the reaction temperature was raised from 20 °C to 80 °C, while the activity of Pd3 kept increasing. The variation tendency of the activity of Pd1 and Pd2 results from the comprehensive influence of the temperature on the catalyst thermal stability, ethylene solubility and ethylene insertion energy barrier. Although elevating temperatures can significantly reduce the ethylene insertion barrier and thus increase the rate of chain growth, a very high temperature may deactivate the catalyst and reduce the ethylene solubility, which can greatly affect the polymerization activity. With regard to Pd3, its activity might be deeply dependent on the catalyst dispersion, and high temperatures favored the dispersion of the catalyst in hexanes. However, the overall activity still remained at a low level. Pd1–3 yielded polyethylene with high molecular weights (well above 105 g mol−1) and narrow molecular weight distributions (1.18–1.71) in ethylene polymerization. Similar to the trend of polymerization activity, the molecular weight of the polyethylene generated by Pd1–3 increased first and then decreased with the increase of temperature. This is also attributed to the change in the relative ratio of chain growth to chain transfer caused by the increase in temperature. Moreover, the molecular weights of the polyethylenes generated by Pd1 and Pd2 were significantly higher than those generated by Pd3 (Fig. 5b and Table 2), suggesting that the second layer flexible substituents facilitate the suppression of chain transfer during polymerization. Compared to classical monolayered steric bulky Pd4, the rigid–flexible double-layered steric Pd1 and Pd2 showed superior polymerization activities and produced significantly higher molecular weight polyethylenes under identical polymerization conditions (entries 13 vs. 1 and 5, Table 2). That is, the rigid–flexible double-layer steric strategy also imparts superior polymerization performance to this palladium system in hexane solvents.
N and Ar-CH3) could be observed in the 1H NMR spectrum (Fig. S21†). This further supports the above hypothesis. Notably, the polymerization activity of Pd1 and Pd2 showed first an ascending and then a descending trend as the reaction temperature was raised from 20 °C to 80 °C, while the activity of Pd3 kept increasing. The variation tendency of the activity of Pd1 and Pd2 results from the comprehensive influence of the temperature on the catalyst thermal stability, ethylene solubility and ethylene insertion energy barrier. Although elevating temperatures can significantly reduce the ethylene insertion barrier and thus increase the rate of chain growth, a very high temperature may deactivate the catalyst and reduce the ethylene solubility, which can greatly affect the polymerization activity. With regard to Pd3, its activity might be deeply dependent on the catalyst dispersion, and high temperatures favored the dispersion of the catalyst in hexanes. However, the overall activity still remained at a low level. Pd1–3 yielded polyethylene with high molecular weights (well above 105 g mol−1) and narrow molecular weight distributions (1.18–1.71) in ethylene polymerization. Similar to the trend of polymerization activity, the molecular weight of the polyethylene generated by Pd1–3 increased first and then decreased with the increase of temperature. This is also attributed to the change in the relative ratio of chain growth to chain transfer caused by the increase in temperature. Moreover, the molecular weights of the polyethylenes generated by Pd1 and Pd2 were significantly higher than those generated by Pd3 (Fig. 5b and Table 2), suggesting that the second layer flexible substituents facilitate the suppression of chain transfer during polymerization. Compared to classical monolayered steric bulky Pd4, the rigid–flexible double-layered steric Pd1 and Pd2 showed superior polymerization activities and produced significantly higher molecular weight polyethylenes under identical polymerization conditions (entries 13 vs. 1 and 5, Table 2). That is, the rigid–flexible double-layer steric strategy also imparts superior polymerization performance to this palladium system in hexane solvents.
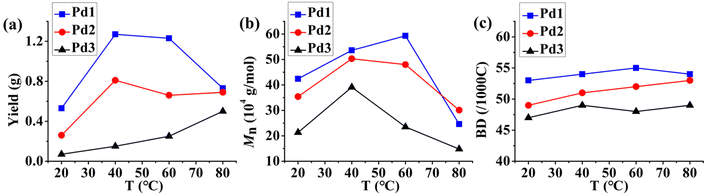 |
| | Fig. 5 Comparisons of the yield (a), molecular weight (b), and branching density (c) of polyethylene generated with Pd1–3 from 20 °C to 80 °C. | |
Table 2 Ethylene polymerization with Pd(II) complexesa
| Ent. |
Precat. |
T/°C |
Yield/g |
Act.b |
M
n
(104) |
M
w/Mnc |
B
|
T
m
/(°C) |
|
Conditions: 5 μmol pre-catalyst, 2.0 eq. of NaBArF, 40 mL of hexanes, ethylene (6 atm), 30 min.
Activity (Act.) = 105 g per mol Pd per h.
M
n is in the unit of 104 g mol−1, determined by SEC in trichlorobenzene at 150 °C.
B = branches per 1000 carbons, determined by 1H NMR spectroscopy, B = 1000 × 2(ICH3)/3(ICH2+CH + ICH3).
Determined by differential scanning calorimetry (DSC), broad peak.
|
| 1 |
Pd1
|
20 |
0.53 |
2.12 |
42.4 |
1.21 |
53 |
55 |
| 2 |
Pd1
|
40 |
1.27 |
5.08 |
53.6 |
1.35 |
54 |
56 |
| 3 |
Pd1
|
60 |
1.23 |
4.92 |
59.3 |
1.54 |
55 |
56 |
| 4 |
Pd1
|
80 |
0.73 |
2.92 |
24.6 |
1.58 |
54 |
57 |
| 5 |
Pd2
|
20 |
0.26 |
1.04 |
35.4 |
1.18 |
49 |
53 |
| 6 |
Pd2
|
40 |
0.81 |
3.24 |
50.3 |
1.30 |
51 |
54 |
| 7 |
Pd2
|
60 |
0.66 |
2.64 |
48.0 |
1.49 |
52 |
55 |
| 8 |
Pd2
|
80 |
0.69 |
2.76 |
30.1 |
1.56 |
53 |
54 |
| 9 |
Pd3
|
20 |
0.07 |
0.28 |
21.3 |
1.24 |
47 |
42, 58 |
| 10 |
Pd3
|
40 |
0.15 |
0.60 |
39.1 |
1.71 |
49 |
63 |
| 11 |
Pd3
|
60 |
0.25 |
1.00 |
23.5 |
1.58 |
48 |
61 |
| 12 |
Pd3
|
80 |
0.50 |
2.00 |
14.8 |
1.67 |
49 |
43, 59 |
| 13 |
Pd4
|
20 |
0.12 |
0.48 |
33.0 |
1.42 |
25 |
96 |
Furthermore, the polyethylenes obtained from the double-layered steric palladium-catalyzed ethylene polymerization system exhibited moderate branching densities (47–55/1000C) and medium melting points (Table 2). And the branching densities appeared to be independent of the polymerization temperature (Fig. 5c). Similar to the phenomenon observed in the nickel system, the polyethylenes generated using rigid–flexible double-layered steric Pd1 and Pd2 possessed higher branching densities accompanied by lower melting points than those generated with rigid–rigid double-layered steric Pd3. This suggests that the presence of the second flexible substituent layer also promotes chain walking of the palladium catalysts during ethylene polymerization. The polyethylene obtained with Pd1 at 60 °C (highest branching density) was chosen for the studies of mechanical properties and exhibited moderate stress-at-break values (15.5 MPa), high strain-at-break values (1059%) and an average elastic recovery value of 44% (Fig. 6).
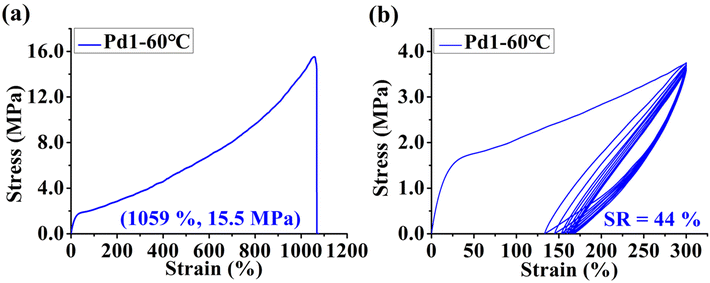 |
| | Fig. 6 Stress–strain curve for the sample generated by (a) Pd1 at 60 °C. The plot of the hysteresis experiment of ten cycles at a strain of 300% for the sample generated by (b) Pd1 at 60 °C. | |
The copolymerization of ethylene with polar monomers is the most straightforward route to access polar functionalized polyolefins.1,2 Hence, the copolymerization catalyzed by Pd1–3 was also investigated. We first implemented the copolymerization using ethylene (2 atm) and methyl acrylate (MA) (1 M and 2 M) as comonomers in hexanes. Disappointingly, the yields of palladium-catalyzed ethylene–MA copolymerization reactions were very low compared to their corresponding homopolymerization reactions (Tables 3vs.2). The modest efficiency could be attributed to the formation of a resting chelate intermediate after the insertion of methyl acrylate during the copolymerization process.2,13,14 The resulting E-MA copolymers exhibited moderate levels of molecular weights (5–15 kg mol−1), branching densities (46–66/1000C) and incorporation ratios (0.67–3.68 mol%). Generally, a higher initial MA concentration led to decreased activities and molecular weights but significantly increased incorporation ratios (entries 1–6, Table 3).
Table 3 Copolymerization of ethylene and polar comonomers with Pd(II) complexesa
| Ent. |
Precat. |
Comonomer |
[M]b (M) |
Yield (g) |
X
M
(mol %) |
M
n
(104) |
M
w/Mnd |
B
|
|
Conditions: pre-catalyst (10 μmol), NaBArF (2.0 equiv.), hexanes + polar comonomers (20 mL), ethylene (2 atm), polymerization temperature (30 °C), polymerization time (12 h).
The concentration of comonomers.
X
M = Incorporation of the comonomer; B = number of branches per 1000C, as determined by 1H NMR spectroscopy. The branches ending with functional groups were added to the total branches.
Determined by SEC in trichlorobenzene at 150 °C.
Ethylene (4 atm).
|
| 1 |
Pd1
|
MA |
1 |
0.10 |
2.01 |
1.5 |
1.45 |
55 |
| 2 |
Pd1
|
MA |
2 |
0.09 |
3.68 |
1.0 |
1.46 |
56 |
| 3 |
Pd2
|
MA |
1 |
0.07 |
0.67 |
1.2 |
1.48 |
65 |
| 4 |
Pd2
|
MA |
2 |
0.07 |
1.18 |
0.6 |
1.37 |
66 |
| 5 |
Pd3
|
MA |
1 |
0.09 |
1.00 |
0.8 |
1.72 |
46 |
| 6 |
Pd3
|
MA |
2 |
0.06 |
2.18 |
0.5 |
1.64 |
48 |
| 7e |
Pd1
|
MU |
1 |
0.46 |
3.56 |
6.6 |
1.19 |
55 |
| 8e |
Pd2
|
MU |
1 |
0.31 |
3.70 |
5.8 |
1.66 |
56 |
| 9e |
Pd3
|
MU |
1 |
0.06 |
2.89 |
4.9 |
1.22 |
52 |
| 10e |
Pd1
|
UA |
1 |
1.35 |
3.55 |
14.2 |
1.23 |
57 |
| 11e |
Pd2
|
UA |
1 |
0.36 |
4.02 |
8.5 |
1.52 |
59 |
| 12e |
Pd3
|
UA |
1 |
0.24 |
3.14 |
3.2 |
1.42 |
53 |
| 13e |
Pd4
|
MU |
1 |
0.02 |
2.88 |
4.3 |
1.46 |
28 |
Apart from MA, biomass-derived methyl 10-undecenoate (MU) and 10-undecylenic acid (UA) were used as comonomers in palladium-catalyzed copolymerization reactions with 1 M concentration and moderate ethylene pressure (4 atm) in hexanes. Among the three palladium complexes, Pd1 was found to be significantly superior to Pd2 and Pd3 in terms of yield in these copolymerization reactions (Fig. 7a). Although the exact reason is not yet clear, the cyclohexyl substituents in the second layer are supposed to be responsible for the better performance of Pd1. The reason for the poor performance of Pd2 may be that the ethyl group at the distal end of the cyclohexyl group is not conducive to the coordination and insertion of ethylene and polar monomers. Compared with E-MA copolymers, most of the resulting E-MU and E-UA copolymers exhibited higher molecular weights (32–142 kg mol−1), higher incorporation ratios (2.89–4.02 mol%) and comparable branching densities (52–59/1000C) (Fig. 7). Generally, MU-involved copolymerizations provided significantly lower yields than the corresponding copolymerizations with UA (Fig. 7a, entries 7–9 vs. entries 10–12, Table 3), probably because the presence of UA as a dimer in a non-polar solvent alleviated the toxic effect on the metal center.41,42
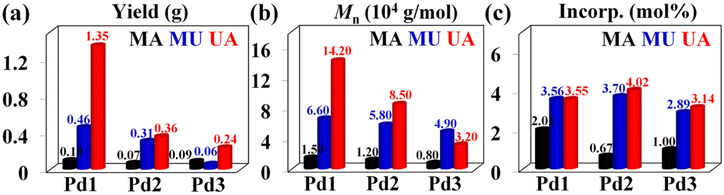 |
| | Fig. 7 Comparisons of the yield (a), molecular weight (b), and incorporation (c) of ethylene-polar monomer copolymers generated with Pd1–Pd3. | |
Notably, no matter what polar comonomer was used, Pd1 exhibited a superior catalytic ability in terms of the activity and molecular weight of the resulting copolymers, compared to Pd2 and Pd3 (Fig. 7a and b), and a higher insertion ratio was observed in Pd1-promoted E-MA copolymerization (Fig. 7c). More importantly, a comparison of the copolymerizations employing rigid–flexible double-layered steric Pd1 and Pd2 and rigid–rigid double-layered steric Pd3 reveals that the second layer flexible substituents are more favorable for polar monomer insertion than the rigid ones, which is consistent with our previous reports that flexible substituents promote the insertion of polar monomers.43,44 Further compared to classical monolayered steric bulky Pd4, the rigid–flexible double-layered steric Pd1 and Pd2 showed superior polymerization activities and generated higher molecular weight copolymers with higher insertion ratios in the E-MU copolymerization under identical polymerization conditions (entries 13 vs. 7 and 8, Table 3). This indicates that the rigid–flexible double-layer steric strategy also performs well in the copolymerization.
3. Conclusions
In summary, we synthesized a small family of double-layered steric α-diimine nickel and palladium complexes by incorporating 4-substituted diarylmethyl motifs and employed them in ethylene (co)polymerization. The nickel complexes with rigid–flexible double-layered steric substituents displayed high activities and superior thermal stability (at least 40 min at 80 °C) in ethylene polymerization. Besides, high temperature living polymerizations at 80 °C were observed with these nickel complexes. Moderately branched polyethylenes with high molecular weights (up to a million Da) and narrow molecular weight distributions (1.14–1.28) were obtained using the nickel system at high temperatures. The polyethylene materials yielded at 80 °C exhibited excellent tensile mechanical and superior elastic recovery properties (SR up to 86%). Correspondingly, the palladium complexes showed moderate activities, producing moderately branched polyethylenes with high molecular weights (up to 593 kg mol−1). The polyethylene materials yielded by the Pd(II) complexes at 60 °C exhibited good tensile mechanical properties and moderate elastic recovery values. Moreover, polar functionalized polyethylenes with moderate levels of branching density, molecular weights as well as incorporation ratios were generated in the ethylene and polar monomer copolymerizations using these palladium complexes. Most importantly, the rigid–flexible double-layer steric strategy not only endowed the α-diimine nickel and palladium complexes with outstanding thermostability, but also enhanced their solubility in alkane solvents. Compared to the rigid–rigid double-layered steric or monolayered dibenzhydryl Ni(II)/Pd(II) complexes, the rigid–flexible double-layered steric complexes with remote cyclohexyl substituents exhibited significantly superior activities in alkane solvents. The rigid–flexible double-layer steric strategy may further enable the practical application of late-transition metal catalytic systems in industry.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the Natural Science Foundation of Anhui Province (2108085Y06), the Anhui Provincial Key Laboratory Open Project Foundation (LCECSC-01), and the Natural Science Research Projects of Universities in Anhui Province (KJ2021A0066).
References
- S. D. Ittel, L. K. Johnson and M. Brookhart, Late-metal catalysts for ethylene homo-and copolymerization, Chem. Rev., 2000, 100, 1169–1204 CrossRef CAS.
- L. Guo, S. Dai, X. Sui and C. Chen, Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization, ACS Catal., 2016, 6, 428–441 CrossRef CAS.
- L. K. Johnson, C. M. Killian and M. Brookhart, New Pd(II)-and Ni(II)-based catalysts for polymerization of ethylene and α-olefins, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- D. P. Gates, S. A. Svejda, E. Oñate, C. M. Killian, L. K. Johnson, P. S. White and M. Brookhart, Synthesis of Branched Polyethylene Using (α-Diimine) nickel(II) Catalysts: Influence of Temperature, Ethylene Pressure, and Ligand Structure on Polymer Properties, Macromolecules, 2000, 33, 2320–2334 CrossRef CAS.
- S. Dai, S. Zhou, W. Zhang and C. Chen, Systematic investigations of ligand steric effects on α-diimine palladium catalyzed olefin polymerization and copolymerization, Macromolecules, 2016, 49, 8855–8862 CrossRef CAS.
- Y. Gong, S. Li, Q. Gong, S. Zhang, B. Liu and S. Dai, Systematic investigations of ligand steric effects on α-diimine nickel catalyzed olefin polymerization and copolymerization, Organometallics, 2019, 38, 2919–2926 CrossRef CAS.
- Y. Ota, S. Ito, J. Kuroda, Y. Okumura and K. Nozaki, Quantification of the steric influence of alkylphosphine-sulfonate ligands on polymerization, leading to high-molecular-weight copolymers of ethylene and polar monomers, J. Am. Chem. Soc., 2014, 136, 11898–11901 CrossRef CAS PubMed.
- S. Dai, X. Sui and C. Chen, Highly Robust Palladium(II) α–Diimine Catalysts for Slow–Chain–Walking Polymerization of Ethylene and Copolymerization with Methyl Acrylate, Angew. Chem., Int. Ed., 2015, 54, 9948–9953 CrossRef CAS.
- D. Meinhard, M. Wegner, G. Kipiani, A. Hearley, P. Reuter, S. Fischer, O. Marti and B. Rieger, New nickel(II) diimine complexes and the control of polyethylene microstructure by catalyst design, J. Am. Chem. Soc., 2007, 129, 9182–9191 CrossRef CAS PubMed.
- J. L. Rhinehart, L. A. Brown and B. K. Long, A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization, J. Am. Chem. Soc., 2013, 135, 16316–16319 CrossRef CAS PubMed.
- Y. Liao, Y. Zhang, L. Cui, H. Mu and Z. Jian, Pentiptycenyl Substituents in Insertion Polymerization with α-Diimine Nickel and Palladium Species, Organometallics, 2019, 38, 2075–2083 CrossRef CAS.
- D. Zhang, E. T. Nadres, M. Brookhart and O. Daugulis, Synthesis of highly branched polyethylene using “sandwich” (8-p-tolyl naphthyl α-diimine) nickel(II) catalysts, Organometallics, 2013, 32, 5136–5143 CrossRef CAS.
- L. Guo, W. Sun, S. Li, G. Xu and S. Dai, Bulky yet flexible substituents in insertion polymerization with α-diimine nickel and palladium systems, Polym. Chem., 2019, 10, 4866–4871 RSC.
- S. Dai, S. Li, G. Xu, Y. Liao and L. Guo, Flexible cycloalkyl substituents in insertion polymerization with α-diimine nickel and palladium species, Polym. Chem., 2020, 11, 1393–1400 RSC.
- X. Hu, S. Dai and C. Chen, Ethylene polymerization by salicylaldimine nickel(II) complexes containing a dibenzhydryl moiety, Dalton Trans., 2016, 45, 1496–1503 RSC.
- H. Liu, W. Zhao, X. Hao, C. Redshaw, W. Huang and W. Sun, 2,6-Dibenzhydryl-N-(2-phenyliminoacenaphthylenylidene)-4-methylbenzenamine Nickel Dibromides: Synthesis, Characterization, and Ethylene Polymerization, Organometallics, 2011, 30, 2418–2424 CrossRef CAS.
- J. L. Rhinehart, N. E. Mitchell and B. K. Long, Enhancing α-Diimine Catalysts for High-Temperature Ethylene Polymerization, ACS Catal., 2014, 4, 2501–2504 CrossRef CAS.
- L. Guo, K. Lian, W. Kong, S. Xu, G. Jiang and S. Dai, Synthesis of Various Branched Ultra-High-Molecular-Weight Polyethylenes Using Sterically Hindered Acenaphthene-Based α-Diimine Ni(II) Catalysts, Organometallics, 2018, 37, 2442–2449 CrossRef CAS.
- Y. Gong, S. Li, C. Tan, W. Kong, G. Xu, S. Zhang, B. Liu and S. Dai, π-π interaction effect in insertion polymerization with a-Diimine palladium systems, J. Catal., 2019, 378, 184–191 CrossRef CAS.
- S. Li, G. Xu and S. Dai, A remote nonconjugated electron effect in insertion polymerization with α-diimine nickel and palladium species, Polym. Chem., 2020, 11, 2692–2699 RSC.
- S. Dai and C. Chen, A Self-Supporting Strategy for Gas-Phase and Slurry-Phase Ethylene Polymerization using Late-Transition-Metal Catalysts, Angew. Chem., Int. Ed., 2020, 59, 14884–14890 CrossRef CAS.
- Y. Zhao, S. Li, W. Fan and S. Dai, Reversion of the Chain Walking Ability of α-Diimine Nickel and Palladium Catalysts with Bulky Diarylmethyl Substituents, J. Organomet. Chem., 2021, 932, 121649 CrossRef CAS.
- Q. Mahmood, Y. Zeng, E. Yue, G. A. Solan, T. Liang and W. Sun, Ultra-high molecular weight elastomeric polyethylene using an electronically and sterically enhanced nickel catalyst, Polym. Chem., 2017, 8, 6416–6430 RSC.
- G. Li, Y. Ge, G. Xu and S. Dai, The electronic effects on unsymmetrical Bis(imino)pyridyl iron(II) catalyzed ethylene polymerization, J. Organomet. Chem., 2020, 923, 121457 CrossRef CAS.
- S. Li and S. Dai, 8-Arylnaphthyl substituent retarding chain transfer in insertion polymerization with unsymmetrical α-diimine systems, Polym. Chem., 2020, 11, 7199–7206 RSC.
- S. Li, Y. Zhao and S. Dai, Synthesis of polyethylene thermoplastic elastomer by using robust α-diimine Ni(II) catalysts with abundant tBu substituents, J. Polym. Sci., 2021, 59, 638–645 CrossRef CAS.
- L. Guo, Y. Liu, W. Sun, Q. Du, Y. Yang, W. Kong, Z. Liu and D. Chen, Synthesis, characterization, and olefin (co)polymerization behavior of unsymmetrical α-diimine palladium complexes containing bulky substituents at 4-position of aniline moieties, J. Organomet. Chem., 2018, 877, 12–20 CrossRef CAS.
- S. Li, Z. Lu, W. Fan and S. Dai, Efficient incorporation of a polar comonomer for direct synthesis of hyperbranched polar functional ethylene oligomers, New J. Chem., 2021, 45, 4024–4031 RSC.
- L. Guo, X. Hu, W. Lu, G. Xu, Q. Liu and S. Dai, Investigations of ligand backbone effects on bulky diarylmethyl-based nickel(II) and palladium(II) catalyzed ethylene polymerization and copolymerization, J. Organomet. Chem., 2021, 952, 122046 CrossRef CAS.
- Z. Lu, G. Chang, H. Wang, K. Jing and S. Dai, A Dual Steric Enhancement Strategy in α-Diimine Nickel and Palladium Catalysts for Ethylene Polymerization and Copolymerization, Organometallics, 2022, 41, 124–132 CrossRef CAS.
- F. Zhai, J. B. Solomon and R. F. Jordan, Copolymerization of Ethylene with Acrylate Monomers by Amide-Functionalized α-Diimine Pd Catalysts, Organometallics, 2017, 36, 1873–1879 CrossRef CAS.
- X. Hu, Y. Zhang, Y. Zhang and Z. Jian, Asymmetrical Strategy Makes Significant Differences in α–Diimine Nickel and Palladium Catalyzed Ethylene (Co) Polymerizations, ChemCatChem, 2020, 12, 2497–2505 CrossRef CAS.
- J. Wang, L. Wang, H. Yu, R. S. Ullah, M. Haroon, Z. Abdin, X. Xia and R. U. Khan, Recent Progress in Ethylene Polymerization Catalyzed by Ni and Pd Catalysts, Eur. J. Inorg. Chem., 2018, 2018, 1450–1468 CrossRef CAS.
- Y. Gao, M. D. Christianson, Y. Wang, M. P. Coons, J. Chen, J. Zhang, S. Marshall, T. L. Lohr, J. Klosin and T. J. Marks, Alkane-Soluble Bis[tris(alkylphenyl)carbenium] Diborate Cocatalyst for Olefin Polymerizations, ACS Catal., 2022, 12, 7589–7597 CrossRef CAS.
- X. Hu, X. Kang and Z. Jian, Suppression of Chain Transfer at High Temperature in Catalytic Olefin Polymerization, Angew. Chem., Int. Ed., 2022, e202207363 CAS.
- X. Hu, Y. Zhang, B. Li and Z. Jian, Horizontally and Vertically Concerted Steric Strategy in α-Diimine Nickel Promoted Ethylene (Co)Polymerization, Chin. J. Chem., 2021, 39, 2829–2836 CrossRef CAS.
- J. Xia, Y. Zhang, S. Kou and Z. Jian, A concerted double-layer steric strategy enables an ultra-highly active nickel catalyst to access ultrahigh molecular weight polyethylenes, J. Catal., 2020, 390, 30–36 CrossRef CAS.
- H. Tafazolian, D. B. Culver and M. P. Conley, A Well-Defined Ni(II) α-Diimine Catalyst Supported on Sulfated Zirconia for Polymerization Catalysis, Organometallics, 2017, 36, 2385–2388 CrossRef CAS.
- J. Gao, R. W. Dorn, G. P. Laurent, F. A. Perras, A. J. Rossini and M. P. Conley, A Heterogeneous Palladium Catalyst for the Polymerization of Olefins Prepared by Halide Abstraction Using Surface R3Si+ Species, Angew. Chem., 2022, 134, e202117279 Search PubMed.
- D. B. Culver, H. Tafazolian and M. P. Conley, A Bulky Pd(II) α-Diimine Catalyst Supported on Sulfated Zirconia for the Polymerization of Ethylene and Copolymerization of Ethylene and Methyl Acrylate, Organometallics, 2018, 37, 1001–1006 CrossRef CAS.
- S. Dai, S. Li, G. Xu and C. Chen, Direct Synthesis of Polar Functionalized Polyethylene Thermoplastic Elastomer, Macromolecules, 2020, 53, 2539–2546 CrossRef CAS.
- S. Dai and C. Chen, Palladium-Catalyzed Direct Synthesis of Various Branched, Carboxylic Acid-Functionalized Polyolefins: Characterization, Derivatization, and Properties, Macromolecules, 2018, 51, 6818–6824 CrossRef CAS.
- H. Fan, Y. Liao and S. Dai, Propylene Polymerization and Copolymerization with Polar Monomers Facilitated by Flexible Cycloalkyl Substituents in α-Diimine Systems, Polymer, 2022, 254, 125076 CrossRef CAS.
- Z. Hai, Z. Lu, S. Li, Z. Cao and S. Dai, The Synergistic Effect of Rigid and Flexible Substituents in Insertion Polymerization with α-Diimine Nickel and Palladium Catalysts, Polym. Chem., 2021, 12, 4643–4653 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details for the synthetic procedures, materials, analytical methods, NMR and mass spectra of the synthesized compounds, and NMR, DSC and SEC curves of polymer and copolymer samples (PDF). CCDC 2194287 (Pd1). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi01895k |
|
| This journal is © the Partner Organisations 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  *b and
Shengyu
Dai
*b and
Shengyu
Dai
 *ab
*ab




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and Ar-CH3) could be observed in the 1H NMR spectrum (Fig. S21†). This further supports the above hypothesis. Notably, the polymerization activity of Pd1 and Pd2 showed first an ascending and then a descending trend as the reaction temperature was raised from 20 °C to 80 °C, while the activity of Pd3 kept increasing. The variation tendency of the activity of Pd1 and Pd2 results from the comprehensive influence of the temperature on the catalyst thermal stability, ethylene solubility and ethylene insertion energy barrier. Although elevating temperatures can significantly reduce the ethylene insertion barrier and thus increase the rate of chain growth, a very high temperature may deactivate the catalyst and reduce the ethylene solubility, which can greatly affect the polymerization activity. With regard to Pd3, its activity might be deeply dependent on the catalyst dispersion, and high temperatures favored the dispersion of the catalyst in hexanes. However, the overall activity still remained at a low level. Pd1–3 yielded polyethylene with high molecular weights (well above 105 g mol−1) and narrow molecular weight distributions (1.18–1.71) in ethylene polymerization. Similar to the trend of polymerization activity, the molecular weight of the polyethylene generated by Pd1–3 increased first and then decreased with the increase of temperature. This is also attributed to the change in the relative ratio of chain growth to chain transfer caused by the increase in temperature. Moreover, the molecular weights of the polyethylenes generated by Pd1 and Pd2 were significantly higher than those generated by Pd3 (Fig. 5b and Table 2), suggesting that the second layer flexible substituents facilitate the suppression of chain transfer during polymerization. Compared to classical monolayered steric bulky Pd4, the rigid–flexible double-layered steric Pd1 and Pd2 showed superior polymerization activities and produced significantly higher molecular weight polyethylenes under identical polymerization conditions (entries 13 vs. 1 and 5, Table 2). That is, the rigid–flexible double-layer steric strategy also imparts superior polymerization performance to this palladium system in hexane solvents.
N and Ar-CH3) could be observed in the 1H NMR spectrum (Fig. S21†). This further supports the above hypothesis. Notably, the polymerization activity of Pd1 and Pd2 showed first an ascending and then a descending trend as the reaction temperature was raised from 20 °C to 80 °C, while the activity of Pd3 kept increasing. The variation tendency of the activity of Pd1 and Pd2 results from the comprehensive influence of the temperature on the catalyst thermal stability, ethylene solubility and ethylene insertion energy barrier. Although elevating temperatures can significantly reduce the ethylene insertion barrier and thus increase the rate of chain growth, a very high temperature may deactivate the catalyst and reduce the ethylene solubility, which can greatly affect the polymerization activity. With regard to Pd3, its activity might be deeply dependent on the catalyst dispersion, and high temperatures favored the dispersion of the catalyst in hexanes. However, the overall activity still remained at a low level. Pd1–3 yielded polyethylene with high molecular weights (well above 105 g mol−1) and narrow molecular weight distributions (1.18–1.71) in ethylene polymerization. Similar to the trend of polymerization activity, the molecular weight of the polyethylene generated by Pd1–3 increased first and then decreased with the increase of temperature. This is also attributed to the change in the relative ratio of chain growth to chain transfer caused by the increase in temperature. Moreover, the molecular weights of the polyethylenes generated by Pd1 and Pd2 were significantly higher than those generated by Pd3 (Fig. 5b and Table 2), suggesting that the second layer flexible substituents facilitate the suppression of chain transfer during polymerization. Compared to classical monolayered steric bulky Pd4, the rigid–flexible double-layered steric Pd1 and Pd2 showed superior polymerization activities and produced significantly higher molecular weight polyethylenes under identical polymerization conditions (entries 13 vs. 1 and 5, Table 2). That is, the rigid–flexible double-layer steric strategy also imparts superior polymerization performance to this palladium system in hexane solvents.





