Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular polysulfide polymers cross-linked by metal–ligand interactions†
Yuichiro
Kobayashi
 *abc,
Daiki
Kitano
a,
Ryuto
Nishimura
a,
Yuki
Yamagishi
a,
Akiyoshi
Horiguchi
a and
Hiroyasu
Yamaguchi
*abc,
Daiki
Kitano
a,
Ryuto
Nishimura
a,
Yuki
Yamagishi
a,
Akiyoshi
Horiguchi
a and
Hiroyasu
Yamaguchi
 *abc
*abc
aDepartment of Macromolecular Science, Graduate School of Science, Osaka University, 1-1 Machikaneyama, Toyonaka, Osaka 560-0043, Japan. E-mail: kobayashiy11@chem.sci.osaka-u.ac.jp; hiroyasu@chem.sci.osaka-u.ac.jp
bInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ISC-OTRI), Osaka University, Suita, Osaka 567-0871, Japan
cProject Research Center for Fundamental Sciences, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan
First published on 12th May 2023
Abstract
We report the first synthesis of a supramolecular cross-linked polysulfide polymer containing bipyridine (poly(LS-bpy)) using metal–ligand interactions. Complexing the bipyridine part in the poly(LS-bpy) and a metal ion realizes a supramolecular cross-linked polysulfide polymer. The molecular weight of the supramolecular cross-linked polysulfide polymer is 29 times higher than that of a polysulfide polymer obtained from conventional polymerization. In addition, the addition of chelating agents and a metal ion can control the disassembly and assembly of the supramolecular cross-linked polysulfide polymer, respectively. The supramolecular cross-linked polysulfide polymer remarkably improves the thermal stability, which should be useful for applications as a polysulfide polymer material.
Sulfur, which is obtained as a by-product of crude oil refining, is used in the production of chemical substances such as sulfuric acid, fertilisers, and rubber.1 However, several million tons of unused sulfur are discarded annually, and its effective utilization is needed desperately.2 One solution is to develop polymeric materials using discarded sulfur.3,4 They also exhibit functions not found in carbon polymers such as a high capacitance.5–11 Although heating elemental sulfur (S8) generates polysulfide polymers, it is difficult to prepare polymers with a high molecular weight because depolymerization occurs at room temperature.4,10,11 In addition, they have a low solubility in general organic solvents. To overcome these problems, a copolymerization technique using S8 and an organic compound was developed.12–16 Using radical copolymerization and a condensation technique involving a vinyl monomer and a bifunctional monomer, respectively, polysulfide polymers with excellent self-healing properties or battery recyclability have been produced.4,17,18 However, the molecular weight of most polysulfide polymers was less than 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Higher molecular weight polysulfide polymers show higher solvent resistance and thermal properties.19 Recently, preparation of high-molecular weight polysulfide polymers by inverse vulcanization20 or supramolecular assembly21–24 has been reported, and the polysulfide polymers exhibit excellent mechanical and electrochemical properties.20,25 The development of new techniques for producing high-molecular weight polysulfide polymer is desired.
000. Higher molecular weight polysulfide polymers show higher solvent resistance and thermal properties.19 Recently, preparation of high-molecular weight polysulfide polymers by inverse vulcanization20 or supramolecular assembly21–24 has been reported, and the polysulfide polymers exhibit excellent mechanical and electrochemical properties.20,25 The development of new techniques for producing high-molecular weight polysulfide polymer is desired.
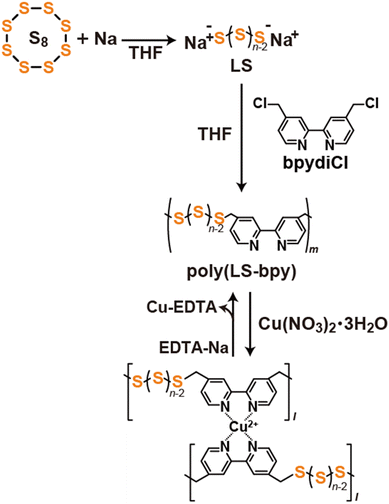
In this study, we report the synthesis of a supramolecular cross-linked polysulfide polymer by introducing non-covalent bondable sites. Bipirydine (bpy) is introduced into the polysulfide polymer (poly(LS-bpy)), and a coordination bond forms between bpy and Cu(II), producing a supramolecular cross-linked polysulfide polymer (poly(LS-bpyCu)) (Fig. 1). Note that the molecular weight of poly(LS-bpyCu) is Mn = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Mn/Mw = 2.20), demonstrating that our methodology is feasible to prepare a polysulfide polymer with a high molecular weight. Because the formation and dissociation of coordination bond is reversible, adding Cu(II) and a chelating agent such as ethylenediaminetetraacetic acid disodium salt (EDTA-Na) can control the assembly and disassembly of poly(LS-bpyCu), respectively. Moreover, poly(LS-bpyCu) shows an improved thermal stability, indicating that the resulting poly(LS-bpyCu) holds promise as a new polysulfide material.
000 (Mn/Mw = 2.20), demonstrating that our methodology is feasible to prepare a polysulfide polymer with a high molecular weight. Because the formation and dissociation of coordination bond is reversible, adding Cu(II) and a chelating agent such as ethylenediaminetetraacetic acid disodium salt (EDTA-Na) can control the assembly and disassembly of poly(LS-bpyCu), respectively. Moreover, poly(LS-bpyCu) shows an improved thermal stability, indicating that the resulting poly(LS-bpyCu) holds promise as a new polysulfide material.
S8 and sodium (Na) were mixed in THF, and the mixture was stirred at 75 °C to obtain linear sulfide (LS). 4,4′-Bis(chloromethyl)-2,2′-bipyridyl (bpydiCl) was added to the LS solution, and the mixture was stirred at r.t. for 24 h. The obtained suspension was filtered. The filtrate was purified by separation GPC to obtain poly(LS-bpy) (Fig. 1 and Scheme S1†). The preparation of poly(LS-bpy) was confirmed by 1H and 13C NMR, GPC, MALDI-TOF MS measurements, and elemental analysis (Fig. S1–S4†). The sulfur content in poly(LS-bpy) was 44%, and the average sulfur chain length (n) in poly(LS-bpy) calculated from the sulfur content was n = 4.0.
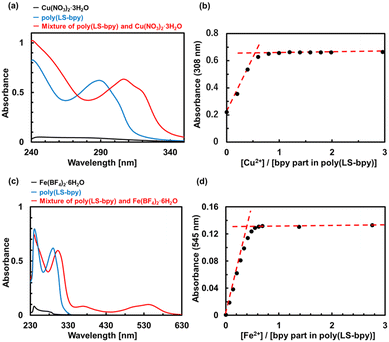
To prepare a supramolecular cross-linked polysulfide polymer with a metal–ligand interaction (poly(LS-bpyCu)) (Fig. 1 and Scheme S2†), we investigated whether the bpy part in poly(LS-bpy) and Cu(II) formed a complex. In the UV-Vis measurements, the addition of Cu(NO3)2·3H2O into the poly(LS-bpy) solution produced a new peak at 308 nm (Fig. 2a and S6†). This peak was also observed in a mixture of bpy and Cu(NO3)2·3H2O (Fig. S7†), showing that the metal–ligand complex of bpy and Cu(II) formed.23,26 Titration plot of abs. at 308 nm of Cu(NO3)2·3H2O and poly(LS-bpy) suggested that the bpy part in poly(LS-bpy) forms a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Cu(II) (Fig. 2b). In addition, the UV-Vis spectrum of a mixed solution of a sulfur compound in which benzyl groups were introduced at both ends of LS (LS-Bn, Scheme S3 and Fig. S8–S10†) and Cu(NO3)2·3H2O did not generate a new peak compared with LS-Bn and Cu(NO3)2·3H2O alone (Fig. S11†), confirming that the sulfur part does not interact with Cu(II). Because bpy can form a complex with not only Cu(II) but also Fe(II), we mixed poly(LS-bpy) and Fe(BF4)2·6H2O (Scheme S4 and Fig. S12†). In the UV-Vis spectrum, new peaks at 370 nm and 545 nm were observed, indicating poly(LS-bpyFe) is prepared (Fig. 2b and S14†). In the case of poly(LS-bpyFe), titration experiments of Fe(BF4)2·6H2O and poly(LS-bpy) suggested that the bpy part in poly(LS-bpy) forms a 3
1 complex with Cu(II) (Fig. 2b). In addition, the UV-Vis spectrum of a mixed solution of a sulfur compound in which benzyl groups were introduced at both ends of LS (LS-Bn, Scheme S3 and Fig. S8–S10†) and Cu(NO3)2·3H2O did not generate a new peak compared with LS-Bn and Cu(NO3)2·3H2O alone (Fig. S11†), confirming that the sulfur part does not interact with Cu(II). Because bpy can form a complex with not only Cu(II) but also Fe(II), we mixed poly(LS-bpy) and Fe(BF4)2·6H2O (Scheme S4 and Fig. S12†). In the UV-Vis spectrum, new peaks at 370 nm and 545 nm were observed, indicating poly(LS-bpyFe) is prepared (Fig. 2b and S14†). In the case of poly(LS-bpyFe), titration experiments of Fe(BF4)2·6H2O and poly(LS-bpy) suggested that the bpy part in poly(LS-bpy) forms a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Fe(II) (Fig. 2d, S15, and S16†). These results show that a supramolecular cross-linked polysulfide polymer is formed based on the metal–ligand interactions between bpy and the metal-ion.
1 complex with Fe(II) (Fig. 2d, S15, and S16†). These results show that a supramolecular cross-linked polysulfide polymer is formed based on the metal–ligand interactions between bpy and the metal-ion.
Gel permeation chromatography (GPC) measurements were performed to determine the molecular weight of the supramolecular cross-linked polysulfide polymer. In the case of poly(LS-bpyFe), GPC measurements could not be performed because precipitation occurred one day after mixing poly(LS-bpy) and Fe(BF4)2·6H2O (Fig. S12†) and the precipitate did not dissolve. In contrast, poly(LS-bpyCu) was soluble in DMSO. This is probably due to the difference in the cross-link density. Since Fe(II) and Cu(II) form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
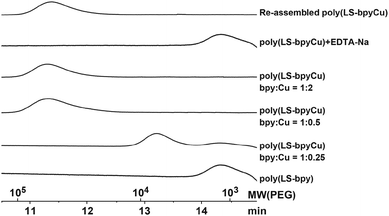
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with bpy, respectively, the cross-linking density of poly(LS-bpyFe) is higher than that of poly(LS-bpyCu). Therefore, the solubilities of poly(LS-bpyFe) and poly(LS-bpyCu) differ. A high molecular weight peak in the GPC measurements occurs upon the addition of Cu(NO3)2·3H2O to poly(LS-bpy) (Fig. 3). The molecular weight initially increases with the addition of Cu(II). It reaches a maximum at a 1
2 complexes with bpy, respectively, the cross-linking density of poly(LS-bpyFe) is higher than that of poly(LS-bpyCu). Therefore, the solubilities of poly(LS-bpyFe) and poly(LS-bpyCu) differ. A high molecular weight peak in the GPC measurements occurs upon the addition of Cu(NO3)2·3H2O to poly(LS-bpy) (Fig. 3). The molecular weight initially increases with the addition of Cu(II). It reaches a maximum at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixing ratio of Cu(II) and the bpy part in poly(LS-bpy). The molecular weight did not change even if additional Cu(II) was added. Note that the molecular weight of poly(LS-bpyCu) Mn = 72
2 mixing ratio of Cu(II) and the bpy part in poly(LS-bpy). The molecular weight did not change even if additional Cu(II) was added. Note that the molecular weight of poly(LS-bpyCu) Mn = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Mn/Mw = 2.20) is 29 times higher than that of poly(LS-bpy) Mn = 2500 (Mn/Mw = 1.25) (Fig. 3). This is one of the largest molecular weights of polysulfide polymers reported.4,9,10,27–31 These results demonstrate that our methodology is feasible to prepare soluble polysulfide polymers with a high molecular weight.
000 (Mn/Mw = 2.20) is 29 times higher than that of poly(LS-bpy) Mn = 2500 (Mn/Mw = 1.25) (Fig. 3). This is one of the largest molecular weights of polysulfide polymers reported.4,9,10,27–31 These results demonstrate that our methodology is feasible to prepare soluble polysulfide polymers with a high molecular weight.
Since poly(LS-bpyCu) is formed via metal–ligand interactions between bpy and Cu(II), poly(LS-bpyCu) should disassemble upon adding a competitive ligand. We mixed poly(LS-bpyCu) and an excess amount of EDTA-Na in DMSO at r.t. for one day and performed GPC measurements. The peak derived from poly(LS-bpyCu) disappears, and a peak similar to that of poly(LS-bpy) is observed (Fig. 3). This result indicates that poly(LS-bpyCu) disassembles into poly(LS-bpy) due to de-metalation of Cu(II) from the metal–ligand complex between bpy and Cu(II) by the addition of EDTA-Na (Fig. 1). Mixing the disassembled poly(LS-bpy) with Cu(II) generates a high molecular weight peak (Fig. 3a), indicating that poly(LS-bpy) and Cu(II) re-assemble to form poly(LS-bpyCu) (Fig. 1). This disassembly and re-assembly of poly(LS-bpyCu) were also confirmed by UV-Vis measurements (Fig. S17†). Thus, poly(LS-bpyCu) freely assembles and disassembles.
Understanding the thermal properties of the obtained supramolecular cross-linked polysulfide polymer is important for potential applications as a polysulfide material. We studied the thermal stability of poly(LS-bpyCu) by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) measurements. In the DSC trace, the glass transition temperature observed at 50° for poly(LS-bpy) was not observed for poly(LS-bpyCu) due to cross-linking between polymers with metal–ligand coordination bonds (Fig. S18†). The TGA decomposition trace of the polymer part of poly(LS-bpyCu) is 249 °C, which is 71 °C higher than that of the poly(LS-bpy) (Fig. S19–S21†). This is consistent with earlier reports, which showed that the cross-linked polysulfide polymer tends to have a higher thermal stability than the linear polysulfide polymer.32,33 The linear type supramolecular polysulfide polymer did not show improved thermal properties.23 Due to the improved solubility and thermal stability, our supramolecular cross-linked polysulfide polymer may help realize functional plastics based on polysulfide polymers.
In summary, this study reports the first preparation of a supramolecular cross-linked polysulfide polymer. The concept of supramolecular cross-linking is a useful synthetic strategy for high molecular weight polymers. Supramolecular cross-linking should significantly improve, the thermal stability of the supramolecular cross-linked polysulfide polymer.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by JSPS KAKENHI Grant Number 22H02145, 22K19066, and 20K21225. This work was supported by Noguchi institute, Fuso Innovative Technology Fund, Masuya Memorial Basic Research Foundation, Iketani Science and Technology Foundation, Tonen General Sekiyu Research/Development Encouragement & Scholarship Foundation, Fuso Innovative Technology Fund, Arai Science and Technology Foundation, and Asahi Glass Foundation. We wish to thank Mr Iijima and Ms Hirai is the member of the Analytical Instrument Facility, Graduate School of Science, Osaka University, for the discussion about elemental analysis data. We also wish to thank Prof. Y. Takashima and Prof. A. Harada of Osaka University for access to the MALDI-TOF MS and GPC. We also wish to thank Dr Y. Tani of Osaka University for access to the separation GPC. We also wish to thank Ms Miyake of Osaka University for access to the pyrolysis-GC-MS.References
- T. Rauchfuss, Nat. Chem., 2011, 3, 648–648 CrossRef CAS PubMed.
- J. A. Ober, US Geological Survey Open-File Report 02-298, 2002, pp. 1–56 Search PubMed.
- J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 CrossRef CAS PubMed.
- Y. Y. Zhang, R. S. Glass, K. Char and J. Pyun, Polym. Chem., 2019, 10, 4078–4105 RSC.
- J. S. Jorczak and E. M. Fettes, Ind. Eng. Chem., 1951, 43, 324–328 CrossRef CAS.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- A. Kausar, S. Zulfiqar and M. I. Sarwar, Polym. Rev., 2014, 54, 185–267 CrossRef CAS.
- A. Manthiram, Y. Z. Fu, S. H. Chung, C. X. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- J. J. Griebel, R. S. Glass, K. Char and J. Pyun, Prog. Polym. Sci., 2016, 58, 90–125 CrossRef CAS.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC.
- R. Steudel and T. Chivers, Chem. Soc. Rev., 2019, 48, 3279–3319 RSC.
- J. C. Patrick, Trans. Faraday Soc., 1936, 32, 0347–0357 RSC.
- P. D. Bartlett and H. Kwart, J. Am. Chem. Soc., 1952, 74, 3969–3973 CrossRef CAS.
- B. R. Currell, A. J. Williams, A. J. Mooney and B. J. Nash, Adv. Chem. Ser., 1975, 1–17 CAS.
- S. Penczek, R. Slazak and A. Duda, Nature, 1978, 273, 738–739 CrossRef CAS.
- M. Schmidt and E. Weissflog, Angew. Chem., Int. Ed. Engl., 1978, 17, 51–52 CrossRef.
- Y. M. Xin, H. Peng, J. Xu and J. Y. Zhang, Adv. Funct. Mater., 2019, 29, 1808989 CrossRef.
- S. J. Tonkin, C. T. Gibson, J. Campbell, D. Lewis, A. Karton, T. Hasell and J. M. Chalker, Chem. Sci., 2020, 11, 5537–5546 RSC.
- Y. L. Zhang, L. S. Shao, D. Y. Dong and Y. H. Wang, RSC Adv., 2016, 6, 17163–17171 RSC.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y. E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- Y. Kobayashi, A. Harada and H. Yamaguchi, Chem. Commun., 2020, 56, 13619–13622 RSC.
- Y. Kobayashi, Polym. J., 2021, 53, 505–513 CrossRef CAS.
- Y. Yamagishi, Y. Kobayashi, A. Horiguchi, D. Kitano and H. Yamaguchi, ChemistrySelect, 2022, 7, e202103991 CrossRef CAS.
- Y. Kobayashi, Y. Yamagishi, R. Nishimura, C. L. Xiao, D. Kitano, A. Horiguchi, S. Hashimoto and H. Yamaguchi, J. Sulfur Chem., 2023 DOI:10.1080/17415993.2023.2183773.
- P. Y. Yan, W. Zhao, S. J. Tonkin, J. M. Chalker, T. L. Schiller and T. Hasell, Chem. Mater., 2022, 34, 1167–1178 CrossRef CAS.
- H. J. Park, J. H. Kwon, T. S. Cho, J. M. Kim, I. H. Hwang, C. Kim, S. Kim, J. Kim and S. K. Kim, J. Inorg. Biochem., 2013, 127, 46–52 CrossRef CAS PubMed.
- P. Y. Yan, W. Zhao, B. W. Zhang, L. Jiang, S. Petcher, J. A. Smith, D. J. Parker, A. I. Cooper, J. X. Lei and T. Hasell, Angew. Chem., Int. Ed., 2020, 59, 13371–13378 CrossRef CAS PubMed.
- J. M. Scheiger, C. Direksilp, P. Falkenstein, A. Welle, M. Koenig, S. Heissler, J. Matysik, P. A. Levkin and P. Theato, Angew. Chem., Int. Ed., 2020, 59, 18639–18645 CrossRef CAS PubMed.
- M. Karayilan, T. S. Kleine, K. J. Carothers, J. J. Griebel, K. M. Frederick, D. A. Loy, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, J. Polym. Sci., 2020, 58, 35–41 CrossRef CAS.
- K. S. Kang, A. Phan, C. Olikagu, T. Lee, D. A. Loy, M. Kwon, H. J. Paik, S. J. Hong, J. Bang, W. O. Parker, M. Sciarra, A. R. Angelis and J. Pyun, Angew. Chem., Int. Ed., 2021, 60, 22900–22907 CrossRef CAS PubMed.
- L. A. Ko, Y. S. Huang and Y. A. Lin, ACS Appl. Polym. Mater., 2021, 3, 3363–3372 CrossRef CAS.
- A. S. M. Ghumman, M. M. Nasef, M. R. Shamsuddin and A. Abbasi, Polym. Polym. Compos., 2021, 29, 1333–1352 CAS.
- M. Arslan, B. Kiskan, E. C. Cengiz, R. Demir-Cakan and Y. Yagci, Eur. Polym. J., 2016, 80, 70–77 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H, 13C, MALDI-TOF MS, elemental analysis, GPC, UV-Vis, and thermal properties. See DOI: https://doi.org/10.1039/d3py00151b |
| This journal is © The Royal Society of Chemistry 2023 |