Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel stereoisomeric lignin-derived polycarbonates: towards the creation of bisphenol polycarbonate mimics†
Xianyuan
Wu‡
 a,
Dan
Xu‡
b,
Mario
De bruyn‡
b,
Gregor
Trimmel
a,
Dan
Xu‡
b,
Mario
De bruyn‡
b,
Gregor
Trimmel
 c and
Katalin
Barta
c and
Katalin
Barta
 *ab
*ab
aStratingh Institute for Chemistry, University of Groningen, Groningen, The Netherlands. E-mail: katalin.barta@uni-graz.at
bDepartment of Chemistry, Organic and Bioorganic Chemistry, University of Graz, Heinrichstrasse 28/II, 8010 Graz, Austria
cInstitute for Chemistry and Technology of Materials (ICTM), NAWI Graz, Graz University of Technology, Stremayrgasse 9, 8010 Graz, Austria
First published on 17th January 2023
Abstract
In this work, we have described a family of bio-based polycarbonates (PC-MBC) based on the unique lignin-derived aliphatic diol 4,4′-methylenebiscyclohexanol (MBC) that was sustainably sourced from lignin oxidation mixture. The detailed structure analysis of these polycarbonates has been confirmed by a series of 2D NMR (HSQC and COSY) characterizations. Depending on the stereoisomerism of MBC, the PC-MBC displayed a wide achievable Tg range of 117–174 °C and high Td5% of >310 °C by variation of the ratio of the stereoisomers of MBC, offering great substitution perspectives towards a bisphenol-containing polycarbonates. Nonetheless, the most here presented PC-MBC polycarbonates were film-forming and transparent.
Polycarbonates (PC) are an important class of polymers, which over recent years have found broad applications towards a wide range of topical fields such as electronics, construction materials, 3D printing, automotive, aircraft, and (bio)medical applications.1–4 Central to this are their excellent thermal and mechanical properties, impact resistance, and optical features.4 Originally, polycarbonates were obtained by reacting diols with phosgene, a notoriously toxic gas. Also, this process uses vast amounts of methylene chloride solvent and tends to lead to the formation of stoichiometric amounts of chlorine salts. Aside this technology, a range of alternatives were developed notably (a) melt transesterification and polycondensation of a diol with a suitable carbonate;5,6 (b) the copolymerization of carbon dioxide (CO2) with an epoxide;7,8 (c) polymerization of suitable polyol diallyl carbonates9 and (d) ring-opening polymerization (ROP) of cyclic carbonate monomers.10–12
Bisphenol A (BPA) polycarbonate (PC) is a very important polymer material as it displays excellent thermal properties characterized by a Tg of 147 °C.13,14 Also, it is well known for its excellent miscibility, transparency, processability, and durability – all characteristics which largely relate to its stable amorphous structure.15 However, it has also been revealed that BPA-PC is sensitive to (UV-)light and hydrothermal aging, which makes for a small yet steady release of BPA monomer in the environment.16 This has been a concern as BPA has been identified as an endocrine disrupting chemical.17 Additionally, it has been shown that the presence of higher BPA levels carries, among others, a higher risk of heart disease, diabetes, and elevated liver enzymes.18,19 Recent years has seen the development of a range of BPA alternatives such as BPF [formaldehyde (F)-based], BPAF (hexafluoroacetone-based) (BPAF) and BPS (sulfur trioxide – based).20,21 To date the latter compound, commonly known as bisphenol S, is the most applied bisphenol A analogue in so called BPA-free products, while it displays a higher heat and photo resistance.20 However, scientific studies are increasingly showing that all these bisphenol analogues are little benign themselves, and sometimes even equally harmful than the original BPA.20,22 This all makes that a high demand exists for the development of truly benign bisphenol A alternatives and this preferentially from renewable resources such as lignocellulosic biomass.23,24 To date the most common bio-based BPA-alternative is based on the use of bisguaiacol (BG) instead of bisphenol (BP).13,25–27 Most revealing, depending on the exact BG regioisomer, a lower to non-existent estrogen activity is being observed. Additionally, BG-based polycarbonates display similar thermal and mechanical properties than BP-based ones.28 However, alternatives for BP-based polycarbonates with high Tg such as encountered with PC-BPA (Tg,147 °C) remain to date somewhat elusive.13 Other bio-based polycarbonates with a wide range of thermal and mechanical properties have also been developed, the central molecular unit typically being epoxides, bisphenols or diols.21 Some illustrative examples are poly(1,4-cyclohexadiene oxide carbonate) (Tg ∼ 115 °C),29 poly(limonene-8,9-oxide carbonate) (Tg: ∼140 °C),30 poly(limonene carbonate) (Tg: ∼130 °C),31 and poly(isosorbide carbonate) (Tg ∼ 170 °C).32
The effect of stereoisomerism on the properties of polymers is presently still a lesser investigated topic.33 To date the main effect of changing the cis–trans isomerism of cyclohexane rings in polymer backbones regards changes in crystallinity.34 This is though a most important feature, as differences in the degree of crystallinity strongly influence the thermal and mechanical properties.34 Exemplary is the case of 1,4-cyclohexane-dimethanol (14CHDM) enriched PET, where addition of trans or cis 14CHDM is shown to markedly enhance the thermal properties and even induce crystallinity or amorphism.35
In this work, we report a range of novel bio-based, polycyclic, non-aromatic polycarbonates to which the Tg varies between 117 and 174 °C by variation of the ratio of the stereoisomers of the central structural monomer: lignin-derived 4,4′-methylenebiscyclohexanol (MBC). Importantly, this range covers the Tg values of a wide range of bisphenol-based polycarbonates. Most of the MBC-based polycarbonates are film-forming and transparent. To the MBC monomer, our group previously reported an elegant synthesis starting from lignin-derived compounds.36,37 (see also Fig. 1A).
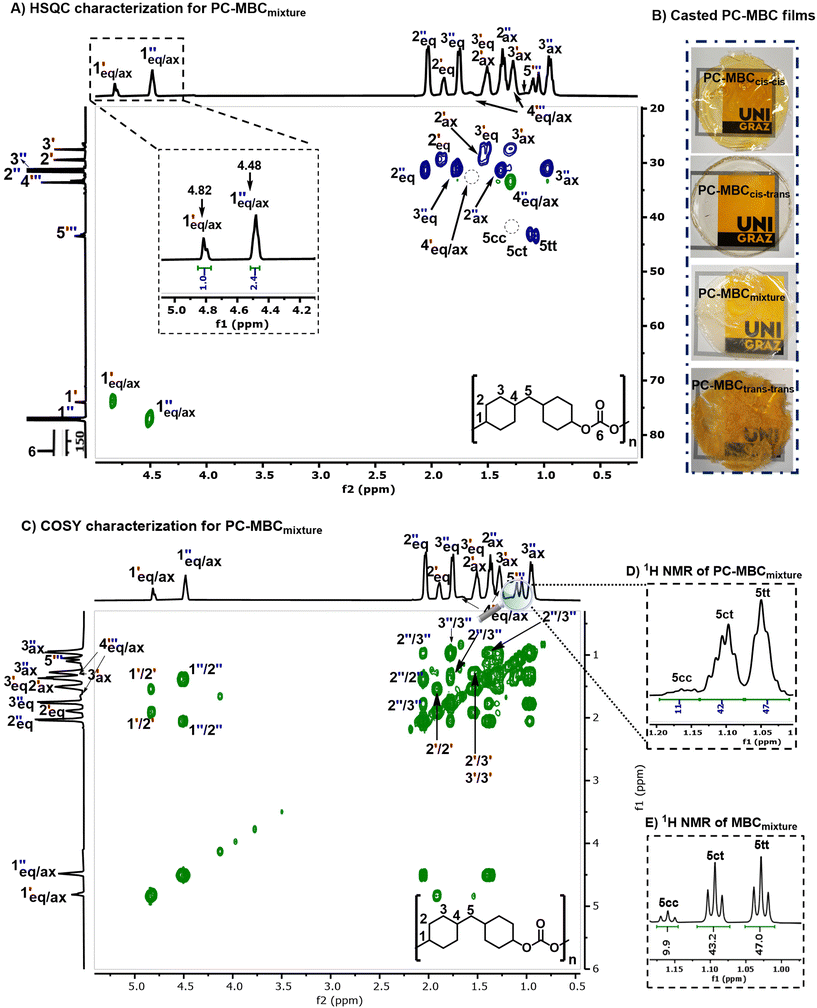
All here synthesized polycarbonates (PC-MBC) were prepared in accordance with a literature reported one-pot, two-step melt polymerization process (Fig. 1B).6 Their structural analysis was performed by FT-IR (Fig. 3A) and NMR spectroscopy (1H NMR, 13C NMR, 2D HSQC and 2D COSY) (see ESI Note 3†). More specifically, FT-IR characterization revealed the clear absence of the MBC-OH stretching vibration at 3250 cm−1 as well as the presence of a carbonate carbonyl stretching vibration band at 1730 cm−1, thus confirming the successful copolymerization of MBC with diphenyl carbonate (DPC) to yield PC-MBC. Additionally, 1H NMR spectroscopy showed the downfield shift of the 1H C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) –OH signals of MBC at 3.54 ppm (cis–cis & cis–trans) and 3.94 ppm (cis–trans & trans–trans) to respectively 4.5 and 4.8 ppm (Fig. 2A), which is evidence of the formation of a C
–OH signals of MBC at 3.54 ppm (cis–cis & cis–trans) and 3.94 ppm (cis–trans & trans–trans) to respectively 4.5 and 4.8 ppm (Fig. 2A), which is evidence of the formation of a C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) –C
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.37 Furthermore, with the numerical integration of the 1H C
O group.37 Furthermore, with the numerical integration of the 1H C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) –C
–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks of PC-MBC being no different from the one of the MBC 1H C
O peaks of PC-MBC being no different from the one of the MBC 1H C![[H with combining low line]](https://www.rsc.org/images/entities/b_char_0048_0332.gif) –OH peaks (ratio: 1
–OH peaks (ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4), non-preferential insertion of the MBC stereoisomers into the polymer chain can be inferred (Fig. S3†). Convincingly, the integration value of MBC's bridging methylene protons in PC-MBC shows nearly the same ratio of (11
2.4), non-preferential insertion of the MBC stereoisomers into the polymer chain can be inferred (Fig. S3†). Convincingly, the integration value of MBC's bridging methylene protons in PC-MBC shows nearly the same ratio of (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) (Fig. 3D) as with the original MBC stereoisomer mixture (Fig. 3E).
47) (Fig. 3D) as with the original MBC stereoisomer mixture (Fig. 3E).
 | ||
| Fig. 3 Structural eludication of PC-MBC. (A) FTIR spectroscopy of MBC and PC-MBCmixture. (B) XRD patterns of PC-MBCcis–cis, PC-MBCcis–trans, PC-MBCmixture and PC-MBCtrans–trans. | ||
The polymeric properties of the PC-MBC polycarbonates were gauged by GPC analysis and DSC/TGA. GPC revealed Mw values between ∼23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 for PC-MBCmixture (stereoisomer ratio 11
000 g mol−1 for PC-MBCmixture (stereoisomer ratio 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) and ∼28
47) and ∼28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 for PC-MBCcis–trans, and PC-MBCcis–cis. PC-MBCtrans–trans exhibits a somewhat lower value of 19
000 g mol−1 for PC-MBCcis–trans, and PC-MBCcis–cis. PC-MBCtrans–trans exhibits a somewhat lower value of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 due to the presence of oligomers (Fig. S44†). DSC analysis revealed a profound effect of the MBC stereoisomerism on the thermal properties. And indeed, per Table 1 the Tg of PC-MBCcis–cis is with 117 °C significantly lower than the ones obtained for PC-MBCcis–trans (141 °C) and PC-MBCtrans–trans (174 °C) and this despite PC-MBCtrans–trans displaying the lowest molecular weight. The higher Tg value for PC-MBCtrans–trans is in line with the existing literature which tends to link higher Tg values to higher trans contents.38–43 The Tg of PC-MBCmixture (stereoisomer ratio 11
200 g mol−1 due to the presence of oligomers (Fig. S44†). DSC analysis revealed a profound effect of the MBC stereoisomerism on the thermal properties. And indeed, per Table 1 the Tg of PC-MBCcis–cis is with 117 °C significantly lower than the ones obtained for PC-MBCcis–trans (141 °C) and PC-MBCtrans–trans (174 °C) and this despite PC-MBCtrans–trans displaying the lowest molecular weight. The higher Tg value for PC-MBCtrans–trans is in line with the existing literature which tends to link higher Tg values to higher trans contents.38–43 The Tg of PC-MBCmixture (stereoisomer ratio 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) takes an intermediate value of 125 °C, which upon variation of the MBC stereoisomer ratios can be likely broadly varied in the 117–174 °C window. It is noteworthy that the Tg value of PC-MBCcis–trans (141 °C) is very close to the one of PC-BPA (147 °C for a Mw of 126 kDa and 134 °C for a Mw of 16 kDa).13 Additionally, the Tg of PC-MBCcis–cis (117 °C) is near equal to the one of PC-BPF (114 °C) and the Tg of PC-MBCmixture (125 °C) mimics the one of PC-BGA (126 °C).13 Very importantly, tailored targeting of the Tg value of PC-MBC to the ones of other bisphenol/bisguaiacol polycarbonates, by variation of the MBC stereoisomer ratios, is a realistic option.
47) takes an intermediate value of 125 °C, which upon variation of the MBC stereoisomer ratios can be likely broadly varied in the 117–174 °C window. It is noteworthy that the Tg value of PC-MBCcis–trans (141 °C) is very close to the one of PC-BPA (147 °C for a Mw of 126 kDa and 134 °C for a Mw of 16 kDa).13 Additionally, the Tg of PC-MBCcis–cis (117 °C) is near equal to the one of PC-BPF (114 °C) and the Tg of PC-MBCmixture (125 °C) mimics the one of PC-BGA (126 °C).13 Very importantly, tailored targeting of the Tg value of PC-MBC to the ones of other bisphenol/bisguaiacol polycarbonates, by variation of the MBC stereoisomer ratios, is a realistic option.
| Entry | Products | Yieldb [%] | M w [g mol−1] | M n [g mol−1] | Đ | T m [°C] | T d [°C] | T g [°C] |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 2.5 mmol MBC, 2.5 mmol DPC, 1 mol% titanium(IV) butoxide (TBT) catalyst, 190 °C N2/1 h, 230 °C/1 h under vacuum 1 mba. b Yield (%) = weight of collect product/weight of theoretical product×100%. c Molecular weight distribution was determined by GPC. d T d = temperature of decomposition – as determined by TGA characterization. e T g = glass transition temperature and Tm = melting temperature were determined by DSC characterization. | ||||||||
| 1 | PC-MBCcis–cis | 80.5 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.81 | N/A | 319 | 117 |
| 2 | PC-MBCmixture stereoisomer ratio (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) 47) |
87.4 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.8 | N/A | 326 | 125 |
| 3 | PC-MBCcis–trans | 84.0 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.54 | N/A | 322 | 141 |
| 4 | PC-MBCtrans–trans | 79.0 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
7900 | 2.44 | 212 | 331 | 174 |
It is noteworthy that XRD analysis revealed that the main feature of all PC-MBC polymers is a halo at a 2θ-value of 18°, a characteristic feature for amorphous materials (Fig. 3). However, PC-MBCtrans–trans has a distinct crystalline fraction represented by a series of reflections making it thus semicrystalline. This is also confirmed by DSC as it is the sole polymer with a small melting point at 212 °C (Table 1). The PC-MBCmixture [(stereoisomer ratio 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47)] also shows a small reflection indicating the presence of a very small crystalline fraction. Given though the low degree of crystallinity of PC-MBCtrans–trans, it is realistic to assume that fast cooling from the melt could yields a predominantly amorphous material. All this is in accordance with other types of polycarbonates being amorphous, the exception being some stereospecific polycarbonates.44 Explanatory to our observations could be the rigidity of the MBC molecules and the tendency of especially MBCtrans–trans to engage in intermolecular interactions. For all here evaluated PC-MBC polymers, the decomposition temperature (Td) is well above 300 °C, the highest recorded value being 331 °C for PC-MBCtrans–trans (Table 1).
47)] also shows a small reflection indicating the presence of a very small crystalline fraction. Given though the low degree of crystallinity of PC-MBCtrans–trans, it is realistic to assume that fast cooling from the melt could yields a predominantly amorphous material. All this is in accordance with other types of polycarbonates being amorphous, the exception being some stereospecific polycarbonates.44 Explanatory to our observations could be the rigidity of the MBC molecules and the tendency of especially MBCtrans–trans to engage in intermolecular interactions. For all here evaluated PC-MBC polymers, the decomposition temperature (Td) is well above 300 °C, the highest recorded value being 331 °C for PC-MBCtrans–trans (Table 1).
In conclusion, we have demonstrated strong influences of stereoisomerism on the crystallinity and thermal properties of a range of MBC-based polycarbonates. More specifically, depending on the MBC stereoisomerism (ratio), the Tg value of the polymer can be tuned within a remarkably wide temperature range [117 to 174 °C] and high Td5% of >310 °C. Importantly, most here presented MBC-based polycarbonates are film-forming and transparent, this holding real potential to the possible substitution of a wide range of polycarbonates such as PC-BPA, PC-BGA and PC-BPF. Also, due to the presence of only aliphatic cyclic structures in the polymeric backbone, the here presented PC-MBC polycarbonates are expected to be less susceptible to degradation by UV-light, which would constitutes an important advantage over PC-BPA and other bisphenol/bisguaiacol-containing polycarbonates. Future work should focus on scaling of the MBC synthesis strategies as well as optimizing of the purification protocols to allow easy access to all MBC isomers in pure form, and the corresponding polycarbonates. With these materials in hand, the polycarbonates should be subjected to further testing of UV resistance, as well as in-depth evaluation toward industrially relevant applications.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
K. B. is grateful for financial support from the European Research Council: ERC Starting Grant 2015 (CatASus) 638076 and ERC Proof of Concept Grant 2019 (PURE) 875649. This work is part of the research programme Talent Scheme (Vidi) with project number 723.015.005, which is partly financed by The Netherlands Organization for Scientific Research (NWO). X. W. is grateful for financial support from the China Scholarship Council (grant number 201808330391). The authors thank Thomas Rath and Karin Bartl for experimental support.References
-
D. G. LeGrand and J. T. Bendler, Handbook of Polycarbonate Science and Technology, Marcel Dekker, Inc., 2000 Search PubMed
.
- J. Feng, R. X. Zhuo and X. Z. Zhang, Prog. Polym. Sci., 2012, 37, 211–236 CrossRef CAS
.
- J. T. Cantrell, S. Rohde, D. Damiani, R. Gurnani, L. DiSandro, J. Anton, A. Young, A. Jerez, D. Steinbach, C. Kroese and P. G. Ifju, Rapid Prototyp. J., 2017, 23, 811–824 CrossRef
.
- D. J. Brunelle, ACS Symp. Ser., 2005, 898, 1–5 CAS
.
- R. Vanderhenst and S. A. Miller, Green Mater., 2013, 1, 64–78 CrossRef
.
- Y. H. Wu, C. C. Wang and C. Y. Chen, J. Polym. Res., 2020, 27, 246 CrossRef CAS
.
- Y. Q. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed
.
- O. Hauenstein, S. Agarwal and A. Greiner, Nat. Commun., 2016, 7, 11862 CrossRef CAS PubMed
.
-
J. C. Carl and R. L. Haynes, EP0144782A2, 1985
.
- W. Yu, E. Maynard, V. Chiaradia, M. C. Arno and A. P. Dove, Chem. Rev., 2021, 121, 10865–10907 CrossRef CAS
.
- F. Suriano, O. Coulembier, J. L. Hedrick and P. Dubois, Polym. Chem., 2011, 2, 528–533 RSC
.
- K. Tezuka, K. Komatsu and O. Haba, Polym. J., 2013, 45, 1183–1187 CrossRef
.
- S. F. Koelewijn, D. Ruijten, L. Trullemans, T. Renders, P. Van Puyvelde, H. Witters and B. F. Sels, Green Chem., 2019, 21, 6622–6633 RSC
.
- T. Hoeks, J. Goossens, H. Vermeulen and A. A. G. Shaikh, Polym. Eng. Sci., 2022, 62, 1377–1385 CrossRef
.
- E. J. Hoekstra and C. Simoneau, Crit. Rev. Food Sci. Nutr., 2013, 53, 386–402 CrossRef PubMed
.
- https://backend.orbit.dtu.dk/ws/portalfiles/portal/110762088/BPA_MST_project_No_1710_2015.pdf .
- B. S. Rubin, J. Steroid Biochem., 2011, 127, 27–34 CrossRef
.
- Y. Ma, H. H. Liu, J. X. Wu, L. Yuan, Y. Q. Wang, X. D. Du, R. Wang, P. W. Marwa, P. Petlulu, X. H. Chen and H. Z. Zhang, Environ. Res., 2019, 176, 108575 CrossRef
.
- A. Tarafdar, R. Sirohi, P. A. Balakumaran, R. Reshmy, A. Madhavan, R. Sindhu, P. Binod, Y. Kumar, D. Kumar and S. J. Sim, J. Hazard. Mater., 2022, 423, 127097 CrossRef CAS PubMed
.
- M. Thoene, E. Dzika, S. Gonkowski and J. Wojtkiewicz, Nutrients, 2020, 12, 532 CrossRef CAS
.
- F. Liguori, C. Moreno-Marrodan and P. Barbaro, Chem. Soc. Rev., 2020, 49, 6329–6363 RSC
.
- J. R. Rochester and A. L. Bolden, Environ. Health Perspect., 2015, 123, 643–650 CrossRef CAS
.
- S. Cui, J. Borgemenke, Y. Qin, Z. Liu and Y. Li, Adv. Bioenergy, 2019, 4, 183–208 CAS
.
- H. T. H. Nguyen, P. X. Qi, M. Rostagno, A. Feteha and S. A. Miller, J. Mater. Chem. A, 2018, 6, 9298–9331 RSC
.
- F. Koelewijn, S. Van den Bosch, T. Renders, W. Schutyser, B. Lagrain, M. Smet, J. Thomas, W. Dehaen, P. Van Puyvelde, H. Witters and B. F. Sels, Green Chem., 2017, 19, 2561–2570 RSC
.
-
K. H. Reno, I. Joseph Francis Stanzione, R. P. Wool, J. M. Sadler, J. J. LaScala and E. D. Hernandez, US10723684B2, 2015
.
- B. G. Harvey, A. J. Guenthner, H. A. Meylemans, S. R. L. Haines, K. R. Lamison, T. J. Groshens, L. R. Cambrea, M. C. Davis and W. W. Lai, Green Chem., 2015, 17, 1249–1258 RSC
.
- Y. Peng, K. H. Nicastro, T. H. Epps and C. Q. Wu, J. Agric. Food Chem., 2018, 66, 11775–11783 CrossRef CAS PubMed
.
- M. Winkler, C. Romain, M. A. R. Meier and C. K. Williams, Green Chem., 2015, 17, 300–306 RSC
.
- C. L. Li, T. Veldhuis, B. Reuvers, R. J. Sablong and C. E. Koning, Polym. Int., 2020, 69, 24–30 CrossRef CAS
.
- O. Hauenstein, M. Reiter, S. Agarwal, B. Rieger and A. Greiner, Green Chem., 2016, 18, 760–770 RSC
.
- O. Gomez Miranda-Jimenez Aberasturi, A. Centeno-Pedrazo, S. P. Fernandez, R. R. Alonso, S. Medel, J. M. Cuevas, L. G. Monsegue, S. De Wildeman, E. Benedetti, D. Klein, H. Henneken and J. R. Ochoa-Gomez, Green Chem. Lett. Rev., 2021, 14, 533–543 Search PubMed
.
- J. H. Liu, Y. Zhang, H. Phan, A. Sharenko, P. Moonsin, B. Walker, V. Promarak and T. Q. Nguyen, Adv. Mater., 2013, 25, 3645–3650 CrossRef CAS PubMed
.
- J. C. Worch, H. Prydderch, S. Jimaja, P. Bexis, M. L. Becker and A. P. Dove, Nat. Rev. Chem., 2019, 3, 514–535 CrossRef CAS
.
- J. G. Wang, X. Q. Liu, Z. Jia, L. Y. Sun, Y. J. Zhang and J. Zhu, Polymer, 2018, 137, 173–185 CrossRef
.
- X. Y. Wu, M. V. Galkin and K. Barta, Chem. Catal., 2021, 1, 1360–1362 CrossRef
.
- X. Y. Wu, M. De bruyn, G. Trimmel, K. Zangger and K. Barta, ACS Sustainable Chem. Eng., 2022 DOI:10.1021/acssuschemeng.2c05998
.
- B. Vanhaecht, B. Rimez, R. Willem, M. Biesemans and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 1962–1971 CrossRef
.
- A. Celli, P. Marchese, L. Sisti, D. Dumand, S. Sullalti and G. Totaro, Polym. Int., 2013, 62, 1210–1217 CrossRef
.
- A. Celli, P. Marchese, S. Sullalti, C. Berti and G. Barbiroli, Macromol. Chem. Phys., 2011, 212, 1524–1534 CrossRef
.
- C. Berti, A. Celli, P. Marchese, E. Marianucci, G. Barbiroli and F. Di Credico, Macromol. Chem. Phys., 2008, 209, 1333–1344 CrossRef
.
- C. Berti, A. Celli, P. Marchese, E. Marianucci, S. Sullalti and G. Barbiroli, Macromol. Chem. Phys., 2010, 211, 1559–1571 CrossRef
.
- B. Vanhaecht, M. N. Teerenstra, D. R. Suwier, R. Willem, M. Biesemans and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 833–840 CrossRef
.
- S. J. Liu and X. H. Wang, Curr. Opin. Green Sustainable Chem., 2017, 3, 61–66 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py01523d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |