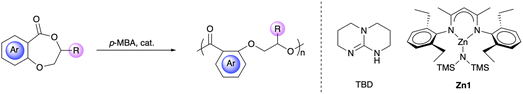
Leveraging the monomer structure for high-performance chemically recyclable semiaromatic polyesters†
Hua-Zhong
Fan
,
Xing
Yang
,
Yan-Chen
Wu
,
Qing
Cao
,
Zhongzheng
Cai
 * and
Jian-Bo
Zhu
* and
Jian-Bo
Zhu
 *
*
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, Chengdu 610064, People's Republic of China. E-mail: zzcai@scu.edu.cn; jbzhu@scu.edu.cn
First published on 6th January 2023
Abstract
The development of inexpensive and high-performance chemically recyclable polymers serves as a promising strategy for solving the issues regarding plastic pollution. In the current work, we prepared a series of aromatic monomers (DHB-R and DHN-R, R = Me, Et) derived from aromatic hydroxy acids and epoxides. Ring-opening polymerization of these monomers afforded the semiaromatic polyesters P(DHB-R) and P(DHN-R) (R = Me, Et) with high molecular weights and narrow dispersity. Remarkably, these polymers showed high thermal stability with 335 °C < Td < 350 °C. Changing the benzene ring to a naphthalene ring on the polymer backbone led to a significant improvement in the glass transition temperature (Tg), from 49 to 100 °C. P(DHB-Me) behaved as a brittle and strong material, whereas P(DHB-Et) displayed excellent ductility with an elongation at break of 762.63 ± 94.40%. More importantly, P(DHB-R) and P(DHN-R) could be effectively and selectively depolymerized into the corresponding monomers, establishing a circular plastics economy.
Introduction
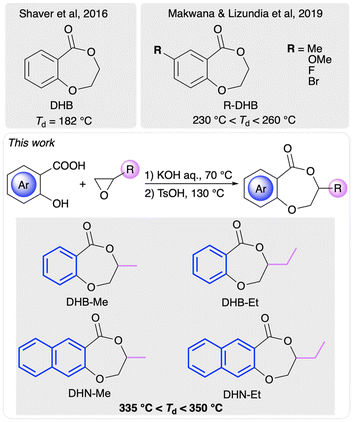
Since their commercial production in the mid-twentieth century, plastics have become indispensable products in our daily lives. However, the accumulation of consumed plastic products has led to critical environmental problems due to their poor degradation and recyclability.1–3 Extensive research focused on chemical recycling of polymers to monomers (CRM) has been devoted to enabling a circular plastics economy and sustainable development.4–35 In a pioneering work, poly (γ-butyrolactone) from the ring-opening polymerization (ROP) of “non-polymerizable” butyrolactone was discovered to quantitatively depolymerize into its monomer γ-butyrolactone upon being heated at 220–300 °C for one hour,4,36 manifesting the potential of its chemical recycling in the circular plastics economy and promoting the development of this field. However, most of the currently studied polymer systems have involved aliphatic polyesters37–48 and chemically recyclable aromatic polyesters have been less extensively investigated. As one of the most popular thermoplastic polyesters in our daily lives, polyethylene terephthalate (PET) has been widely utilized in fibers, resins, and filming owing to its outstanding properties associated with the aromatic moieties on the polymer backbone.49,50 However, its degradation and recycling requires harsh reaction conditions and inevitably suffers from side reactions.51–53 Moreover, it is difficult to isolate and purify the mixed products of decomposed PET.54–59 Therefore, designing new chemically recyclable aromatic polyesters is essential.In 2016, Shaver and co-workers reported the ROP of 2,3-dihydro-5H-1,4-benzodioxepin (DHB) to synthesize the analogues of PET (PDHB), which could be successfully recycled to the monomer DHB in dilute solution with the catalyst salen-Al (Fig. 1).60,61 However, the low thermal stability of PDHB (Td ∼ 180 °C) limited its further application. In a following work, Makwana, Lizundia and co-workers investigated the substituent effect on the meta-position of benzene ring, and revealed a resulting P(R-DHB) showing an improved thermal stability, specifically with a Td in the range of 230 to 260 °C.62 In 2021, the Li research group designed three constitutional isomers of benzo-thia-caprolactones with great chemical recyclability to monomers under bulk thermal conditions.63 The resulting semiaromatic polyesters displayed Td values ranging from 180 to 280 °C. In the meantime, our laboratory developed a facile one-pot synthetic method for preparing a library of benzo-thia-caprolactones with various substituents from thiosalicylic acid and epoxides as the reactants.64 The thermal and mechanical properties of the resulting aromatic polyesters could be tailored by subjecting them to functionalization and stereocomplexation to achieve high thermal stability (285 °C < Td < 310 °C) and polyolefin-like tensile performance.
 | ||
| Fig. 1 Representative examples of semiaromatic monomers based on 2,3-dihydro-5H-1,4-benzodioxepin (DHB). | ||
To further develop the PDHB system to meet practical applications as chemically recyclable polymers, we aimed to enhance its thermal and mechanical properties by leveraging the monomer structure. Motivated by our previous monomer design strategy based on epoxides,64–66 we were able to take advantage of the inexpensive and bio-derived salicylic acid and epoxides to synthesize DHB-Me and DHB-Et using a convenient two-step method. Furthermore, 3-substituted 2,3-dihydro-5H-naphthodioxepin-5-ones (DHN-R, R = Me, Et) were also prepared from 3-hydroxy-2-naphthoic acid and epoxides (Fig. 1). To our delight, these functionalized DHB/DHN-based monomers underwent efficient ROP and produced semiaromatic polyesters with excellent thermal stability (Td > 335 °C) and tunable thermal and mechanical properties, while maintaining chemical recyclability.
Results and discussion
All four monomers were readily prepared on the gram scale by using a two-step synthetic route (Scheme S1†). DHB-Me and DHB-Et were synthesized from natural salicylic acid and corresponding epoxides in 46–56% overall yields. Combining the two reagents in the presence of potassium hydroxide gave an intermediate product, which underwent cyclization when treated with p-toluenesulfonic acid. Replacing salicylic acid with 3-hydroxy-2-naphthoic acid afforded DHN-Me and DHN-Et in 44–65% yields. Owing to the commercial availability of enantiopure (R)-propylene oxide, we were able to obtain (R)-DHB-Me and (R)-DHN-Me using a similar procedure. This synthetic method can endow polymers with diverse functional groups and precise stereocenters.As 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) has been reported to be an effective organocatalyst for ROP of cyclic esters,67–76 TBD-catalyzed ROP of monomers was first investigated in bulk conditions. The polymerization of DHB-Me with a [DHB-Me]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 100
[I] ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reached 71% conversion within 1 h at 70 °C (Table 1, entry 1). As a comparison, DHB-Et with a bulkier ethyl substituent exhibited lower reactivity, approaching 69% conversion in 3 h under similar conditions (Table 1, entry 9). At an increased [monomer]
1 reached 71% conversion within 1 h at 70 °C (Table 1, entry 1). As a comparison, DHB-Et with a bulkier ethyl substituent exhibited lower reactivity, approaching 69% conversion in 3 h under similar conditions (Table 1, entry 9). At an increased [monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 500
[I] ratio of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the polymerizations occurred with 76% and 60% conversions of, respectively, DHB-Me and DHB-Et in 12 h (Table 1, entries 2 and 10). The number-average molecular weights (Mns) of the resulting polymers were much lower than the calculated values (Mn, theos), indicating that this TBD catalytic polymerization system was not well controlled.
1, the polymerizations occurred with 76% and 60% conversions of, respectively, DHB-Me and DHB-Et in 12 h (Table 1, entries 2 and 10). The number-average molecular weights (Mns) of the resulting polymers were much lower than the calculated values (Mn, theos), indicating that this TBD catalytic polymerization system was not well controlled.
| Entry | Monomer | Cat. | [M]/[I]/[Cat.] | Time/h | Conv.b/% | M n, theo /kDa | M n, SEC /kDa | Đ | T g /°C | T d /°C |
|---|---|---|---|---|---|---|---|---|---|---|
| a The polymerizations of DHB-Me and DHB-Et were conducted at bulk conditions, 70 °C; and the polymerizations of DHN-Me and DHN-Et were carried out in toluene solution (2 M) at 100 °C. b Calculated from the 1H NMR spectrum in CDCl3. c M n, theo = MW (monomer) × [M]0/[I]0 × Conv. + MW (terminal group). d Number-average molecular weight (Mn) and dispersity index (Đ = Mw/Mn) determined from the results of size exclusion chromatography (SEC) performed at 40 °C in THF. e T g measured from the results of differential scanning calorimetry (DSC), specifically from the second heating-scan curves with a cooling and second heating rate of 10 °C min−1. f T d measured from the results of thermal gravimetric analysis (TGA) carried out at a heating rate of 10 °C min−1. | ||||||||||
| 1 | DHB-Me | TBD | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 71 | 12.6 | 9.1 | 1.22 | — | — |
| 2 | DHB-Me | TBD | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 76 | 67.7 | 35.0 | 1.25 | 64 | 340 |
| 3 | DHB-Me | Zn1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 64 | 57.0 | 45.0 | 1.34 | 65 | 342 |
| 4 | DHB-Me | Zn1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 75 | 134 | 97.6 | 1.19 | 65 | 343 |
| 5 | (R)-DHB-Me | TBD | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 77 | 13.7 | 10.7 | 1.20 | 60 | 336 |
| 6 | (R)-DHB-Me | Zn1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 60 | 53.4 | 36.6 | 1.25 | 63 | 345 |
| 7 | (S)-DHB-Me | TBD | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 77 | 13.7 | 10.6 | 1.17 | 58 | 335 |
| 8 | (S)-DHB-Me | TBD | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 63 | 56.1 | 36.6 | 1.23 | — | — |
| 9 | DHB-Et | TBD | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 69 | 13.2 | 12.4 | 1.20 | — | — |
| 10 | DHB-Et | TBD | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 60 | 57.6 | 31.1 | 1.24 | 48 | 342 |
| 11 | DHB-Et | Zn1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 64 | 61.5 | 73.9 | 1.23 | 49 | 349 |
| 12 | DHB-Et | Zn1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 68 | 130 | 123 | 1.17 | 50 | 339 |
| 13 | DHN-Me | Zn1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 74 | 21.3 | 20.0 | 1.25 | — | — |
| 14 | DHN-Me | Zn1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 66 | 75.3 | 66.0 | 1.13 | 118 | 336 |
| 15 | DHN-Me | Zn1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 | 64 | 146 | 88.7 | 1.18 | 114 | 345 |
| 16 | (R)-DHN-Me | Zn1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 74 | 16.9 | 14.9 | 1.38 | 109 | - |
| 17 | (R)-DHN-Me | Zn1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 91 | 104 | 42.5 | 1.57 | 121 | 349 |
| 18 | (S)-DHN-Me | Zn1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 71 | 16.2 | 13.1 | 1.39 | 110 | — |
| 19 | DHN-Et | Zn1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 55 | 13.3 | 15.9 | 1.23 | — | — |
| 20 | DHN-Et | Zn1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 54 | 65.4 | 44.6 | 1.13 | 100 | 344 |
| 21 | DHN-Et | Zn1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 52 | 126 | 66.7 | 1.10 | — | — |
To improve the polymerization efficiency of this system, the organometallic complex Zn177 was investigated as the catalyst. Gratifyingly, Zn1 displayed improved activity for the ROPs of DHB-Me and DHB-Et with turnover frequencies (TOFs) ranging from 125 to 320 h−1 (Table 1, entries 3, 4, 11 and 12). Impressively, P(DHB-Et) with a high Mn of 123 kDa and relatively narrow dispersity of 1.17 was obtained when using a [DHB-Et]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn1]
[Zn1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 1000
[I] ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 12). The ROPs of DHN-Me and DHN-Et were carried out with Zn1 as a catalyst and p-tolylmethanol as an initiator in toluene at 100 °C, due to their high melting temperatures and poor solubility levels. Monomer conversions of 64% and 52% were achieved for DHN-Me and DHN-Et at a [monomer]
1 (Table 1, entry 12). The ROPs of DHN-Me and DHN-Et were carried out with Zn1 as a catalyst and p-tolylmethanol as an initiator in toluene at 100 °C, due to their high melting temperatures and poor solubility levels. Monomer conversions of 64% and 52% were achieved for DHN-Me and DHN-Et at a [monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn1]
[Zn1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 1000
[I] ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, demonstrating TOFs of 91.4 h−1 and 86.7 h−1, respectively. The produced P(DHN-Me) and P(DHN-Et) showed a range of Mns from 15.9 to 88.7 kDa and a relatively narrow distribution of molecular weights (Đ < 1.25). Additionally, enantiopure monomers (R)-DHB-Me and (R)-DHN-Me underwent polymerization in a similar fashion as did racemic monomers and afforded isotactic P[(R)-DHB-Me] and P[(R)-DHN-Me] with Mns of 36.6 and 42.5 kg mol−1, respectively (Table 1, entries 6 and 17). Their isotactic arrangements were confirmed using 13C NMR spectroscopy, with the observation of only one set of resonances (Fig. S19 and S27†).
1, demonstrating TOFs of 91.4 h−1 and 86.7 h−1, respectively. The produced P(DHN-Me) and P(DHN-Et) showed a range of Mns from 15.9 to 88.7 kDa and a relatively narrow distribution of molecular weights (Đ < 1.25). Additionally, enantiopure monomers (R)-DHB-Me and (R)-DHN-Me underwent polymerization in a similar fashion as did racemic monomers and afforded isotactic P[(R)-DHB-Me] and P[(R)-DHN-Me] with Mns of 36.6 and 42.5 kg mol−1, respectively (Table 1, entries 6 and 17). Their isotactic arrangements were confirmed using 13C NMR spectroscopy, with the observation of only one set of resonances (Fig. S19 and S27†).
The linear structure and high fidelity of p-methylbenzyl terminal groups of the produced polymer were evaluated using matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Fig. S34†). The MALDI-TOF mass spectrum of a P(DHB-Me) sample prepared from a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of [DHB-Me]
1 ratio of [DHB-Me]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn1]
[Zn1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] displayed a series of molecular ion peaks showing a neighboring spacing of 178.38, which was consistent with the molar mass of the DHB-Me repeat unit. The intercept of a line fitted to the data was found to be 145.17, matching the total mass of chain ends plus the mass of Na+ [Mend = 122.17 (CH3C6H4CH2OH) g mol−1 + 23 (Na+) g mol−1], confirming the linear structure of CH3C6H4CH2O–[DHB-Me]n–H.
[I] displayed a series of molecular ion peaks showing a neighboring spacing of 178.38, which was consistent with the molar mass of the DHB-Me repeat unit. The intercept of a line fitted to the data was found to be 145.17, matching the total mass of chain ends plus the mass of Na+ [Mend = 122.17 (CH3C6H4CH2OH) g mol−1 + 23 (Na+) g mol−1], confirming the linear structure of CH3C6H4CH2O–[DHB-Me]n–H.
Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) were utilized to assess the thermal properties of the produced polymers. All these polymers exhibited excellent thermal stability—with the onset decomposition temperature (Td), defined as the temperature at 5% weight loss, ranging from 336 °C to 349 °C (Fig. 2a). These values were about 160 °C higher than that of P(DHB),61 highlighting the significant improvement in thermal stability resulting from including the substitutions in P(DHB).
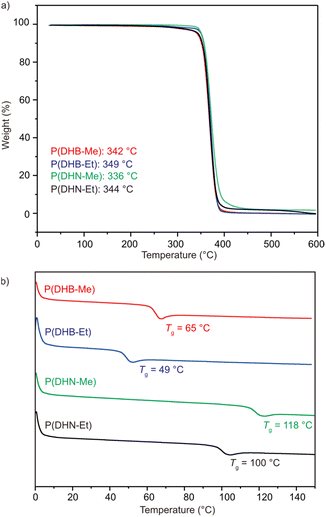 | ||
| Fig. 2 Thermal properties of the produced semiaromatic polyesters. (a) TGA curves of P(DHB-Me), P(DHB-Et), P(DHN-Me), and P(DHN-Et). (b) DSC curves of P(DHB-Me), P(DHB-Et), P(DHN-Me), and P(DHN-Et). | ||
P(DHB-Me) and P(DHB-Et) showed glass transition temperature (Tg) values of 65 °C and 49 °C, respectively. In contrast, P(DHN-Me) and P(DHN-Et) showed higher Tg values of 118 °C and 100 °C (Fig. 2b), which could be attributed to the more rigid naphthalene structures in these two polymers. The absence of a melting transition revealed the amorphous feature of these polymers. Initially, we hypothesized that the lack of crystallinity was due to their atactic structures. The thermal transitions of isotactic P[(R)-DHB-Me] and P[(R)-DHB-Me] were subsequently investigated. Surprisingly, melting transitions were observed neither for P[(R)-DHB-Me] nor for P[(R)-DHN-Me] (Fig. S43 and S46†). Compared with the previously reported sulfur-containing systems,64 we inferred that the heteroatoms in the system might be responsible for the crystallinity of the material. We investigated the stereocomplexation of the polymers by mixing isotactic (R)-polymer and (S)-polymer in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (approximately 100 mg in total). Unfortunately, no stereocomplexation was observed for either of these systems. DSC and PXRD analyses were carried out to characterize the formation of stereocomplexes (Fig. S56†). However, melting transitions were neither observed for the isotactic polymers nor for their blends. Additionally, the absence of sharp peaks for (R)-polymer, (S)-polymer, and their mixture in their PXRD patterns further confirmed the amorphous character of the polymers.
1 molar ratio (approximately 100 mg in total). Unfortunately, no stereocomplexation was observed for either of these systems. DSC and PXRD analyses were carried out to characterize the formation of stereocomplexes (Fig. S56†). However, melting transitions were neither observed for the isotactic polymers nor for their blends. Additionally, the absence of sharp peaks for (R)-polymer, (S)-polymer, and their mixture in their PXRD patterns further confirmed the amorphous character of the polymers.
The enhanced thermal properties of these semiaromatic polyesters encouraged us to investigate their mechanical properties. Uniaxial tensile tests were performed on hot-pressed dogbone-shape films at an extension rate of 10 mm min−1 (Fig. 3). P(DHB-Me) behaved as a stiff material with high tensile strength (σB = 33.69 ± 5.39 MPa), Young's modulus (E = 2.17 ± 0.36 GPa), and elongation at break (εB = 10.91 ± 2.15%). In comparison to P(DHB-Me), the isotactic P[(R)-DHB-Me] and P[(S)-DHB-Me] displayed similar tensile performances (Table S4 and Fig. S58†). In contrast, P(DHB-Et) displayed a σB value of 3.16 ± 0.57 MPa and an εB of 762.63 ± 94.40%. The distinct tensile performances of P(DHB-Me) and P(DHB-Et) manifested the profound impact of pendant groups on the mechanical properties of polymers. We hypothesized that the ethyl substituents in P(DHB-Et) were more flexible than the methyl side chain in P(DHB-Me), and that this difference in flexibility contributed to a significant difference between the packings of the polymer chains. Moreover, the Tg value of P(DHB-Et) was close to room temperature, which also led to the improved ductility of the P(DHB-Et) film measured at room temperature. P(DHN-Me) and P(DHN-Et) appeared to be brittle with an εB < 5% and σB > 35 MPa. We believe that the strength and brittleness of these polymers were due to the rigidity of their naphthalene moieties. Interestingly, attempts to improve the ductility of P(DHB-Me) via copolymerization with DHB-Et was unsuccessful. A copolymer P(DHB-Me-Co-DHB-Et) with only 7 mol% DHB-Me was prepared by carrying out a one-pot copolymerization of DHB-Me and DHB-Et in a [DHB-Me]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DHB-Et]
[DHB-Et]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn1]
[Zn1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] feed ratio of 50
[I] feed ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000
1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (with the composition determined from 1H NMR analysis, Fig. S32†). This P(DHB-Me-co-DHB-Et) copolymer (Mn = 57.5 kDa, Đ = 1.13) performed as a hard and tough material like P(DHB-Me) with σB = 38.16 ± 3.56 MPa and εB = 5.86 ± 0.65%.
1 (with the composition determined from 1H NMR analysis, Fig. S32†). This P(DHB-Me-co-DHB-Et) copolymer (Mn = 57.5 kDa, Đ = 1.13) performed as a hard and tough material like P(DHB-Me) with σB = 38.16 ± 3.56 MPa and εB = 5.86 ± 0.65%.
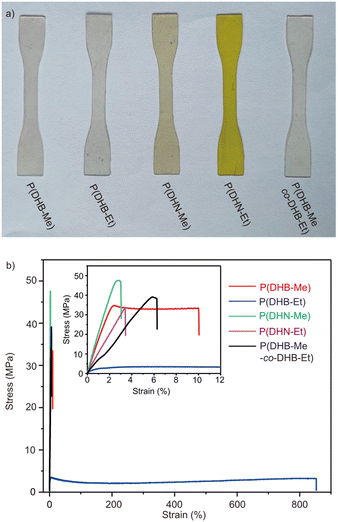 | ||
| Fig. 3 (a) Images of the polymer films used for the tensile test. (b) Stress–strain curves of the produced polymers. | ||
Prior to performing the chemical recycling study, we investigated the thermodynamics of the ROP of DHB-Me at [DHB-Me]0 = 2 M (Fig. S63†). The thermodynamic parameters 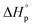 and
and 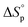 were calculated to be −14.8 kJ mol−1 and −43.3 J mol−1 K−1, respectively. The ceiling temperature Tc at an initial monomer concentration of 1 mol L−1 was determined to be 69 °C according to the equation
were calculated to be −14.8 kJ mol−1 and −43.3 J mol−1 K−1, respectively. The ceiling temperature Tc at an initial monomer concentration of 1 mol L−1 was determined to be 69 °C according to the equation 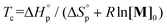 , indicative of excellent recyclability. Chemical recycling to monomers of these synthesized polymers were next tested in dilute solution. With 5 mol% Zn1 loading, the depolymerizations of P(DHB-Me) and P(DHB-Et) underwent nearly quantitative conversions to DHB-Me and DHB-Et in 30 minutes at 120 °C (Fig. 4 and Fig. S64†), while P(DHN-Me) and P(DHN-Et) showed moderately lower yields of 80% and 83% at 140 °C (Fig. S65 and S66†). When 10 mol% TBD was used as a catalyst, DHN-Me and DHN-Et were selectively recycled with high conversions of 93% and 98%, respectively (Table S10†). These results demonstrated a high efficiency for the chemical recycling of these synthesized polymers.
, indicative of excellent recyclability. Chemical recycling to monomers of these synthesized polymers were next tested in dilute solution. With 5 mol% Zn1 loading, the depolymerizations of P(DHB-Me) and P(DHB-Et) underwent nearly quantitative conversions to DHB-Me and DHB-Et in 30 minutes at 120 °C (Fig. 4 and Fig. S64†), while P(DHN-Me) and P(DHN-Et) showed moderately lower yields of 80% and 83% at 140 °C (Fig. S65 and S66†). When 10 mol% TBD was used as a catalyst, DHN-Me and DHN-Et were selectively recycled with high conversions of 93% and 98%, respectively (Table S10†). These results demonstrated a high efficiency for the chemical recycling of these synthesized polymers.
Conclusions
In summary, we have reported a facile synthetic route to prepare functionalized DHB-based monomers from aromatic hydroxy acids and epoxides. These monomers underwent efficient polymerization and afforded semiaromatic polyesters with high Mn values and narrow dispersity. Importantly, all these polymer products demonstrated high thermal stability with Td > 335 °C. The introduction of naphthalene groups into the polymer main chain enhanced the Tg values of P(DHN-R) significantly (Tg > 100 °C). Interestingly, P(DHB-Et) performed as a flexible material with an εB of 762.63 ± 94.40%, while P(DHB-Me) appeared to be strong and brittle with σB = 33.69 ± 5.39 MPa, E = 2.17 ± 0.36 Gpa, and εB = 10.91 ± 2.15%—indicative of the potential applications of these polymers, from elastomers to thermoplastics. We also found that the produced polymers could be efficiently recycled, i.e., converted back to their corresponding monomers with high yields. This functionalization method involving introducing epoxide building blocks into the PDHB system was also found to strongly modulate the thermal and mechanical properties of the materials.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51903177 and 22071163), the “1000 Youth Talents Program”, and the Fundamental Research Funds for the Central Universities (YJ201924 and YJ202209).References
- S. B. Borrelle, J. Ringma, K. L. Law, C. C. Monnahan, L. Lebreton, A. McGivern, E. Murphy, J. Jambeck, G. H. Leonard, M. A. Hilleary, M. Eriksen, H. P. Possingham, H. De Frond, L. R. Gerber, B. Polidoro, A. Tahir, M. Bernard, N. Mallos, M. Barnes and C. M. Rochman, Science, 2020, 369, 1515–1518 CrossRef CAS PubMed.
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- R. Geyer, in Plastic Waste and Recycling, ed. T. M. Letcher, Academic Press, 2020, pp. 13–32, DOI:10.1016/B978-0-12-817880-5.00002-5.
- M. Hong and E. Y. X. Chen, Nat. Chem., 2016, 8, 42–49 CrossRef CAS PubMed.
- J.-B. Zhu, E. M. Watson, J. Tang and E. Y. X. Chen, Science, 2018, 360, 398–403 CrossRef CAS PubMed.
- B. A. Abel, R. L. Snyder and G. W. Coates, Science, 2021, 373, 783–789 CrossRef CAS PubMed.
- Y.-M. Tu, X.-M. Wang, X. Yang, H.-Z. Fan, F.-L. Gong, Z. Cai and J.-B. Zhu, J. Am. Chem. Soc., 2021, 143, 20591–20597 CrossRef CAS PubMed.
- Y. Liu, H. Zhou, J.-Z. Guo, W.-M. Ren and X.-B. Lu, Angew. Chem., Int. Ed., 2017, 56, 4862–4866 CrossRef CAS PubMed.
- W. C. Ellis, Y. Jung, M. Mulzer, R. Di Girolamo, E. B. Lobkovsky and G. W. Coates, Chem. Sci., 2014, 5, 4004–4011 RSC.
- D. J. Saxon, E. A. Gormong, V. M. Shah and T. M. Reineke, ACS Macro Lett., 2021, 10, 98–103 CrossRef CAS PubMed.
- J. P. Lutz, O. Davydovich, M. D. Hannigan, J. S. Moore, P. M. Zimmerman and A. J. McNeil, J. Am. Chem. Soc., 2019, 141, 14544–14548 CrossRef CAS PubMed.
- Y. Liu, Y. Jia, Q. Wu and J. S. Moore, J. Am. Chem. Soc., 2019, 141, 17075–17080 CrossRef CAS PubMed.
- P. R. Christensen, A. M. Scheuermann, K. E. Loeffler and B. A. Helms, Nat. Chem., 2019, 11, 442–448 CrossRef CAS PubMed.
- C. Shi, M. L. McGraw, Z.-C. Li, L. Cavallo, L. Falivene and E. Y. X. Chen, Sci. Adv., 2020, 6, eabc0495 CrossRef CAS PubMed.
- D. Sathe, J. Zhou, H. Chen, H.-W. Su, W. Xie, T.-G. Hsu, B. R. Schrage, T. Smith, C. J. Ziegler and J. Wang, Nat. Chem., 2021, 13, 743–750 CrossRef CAS PubMed.
- K. A. Stellmach, M. K. Paul, M. Xu, Y.-L. Su, L. Fu, A. R. Toland, H. Tran, L. Chen, R. Ramprasad and W. R. Gutekunst, ACS Macro Lett., 2022, 11, 895–901 CrossRef CAS PubMed.
- R. M. Cywar, N. A. Rorrer, H. B. Mayes, A. K. Maurya, C. J. Tassone, G. T. Beckham and E. Y. X. Chen, J. Am. Chem. Soc., 2022, 144, 5366–5376 CrossRef CAS PubMed.
- J. Yuan, W. Xiong, X. Zhou, Y. Zhang, D. Shi, Z. Li and H. Lu, J. Am. Chem. Soc., 2019, 141, 4928–4935 CrossRef CAS PubMed.
- W. Xiong, W. Chang, D. Shi, L. Yang, Z. Tian, H. Wang, Z. Zhang, X. Zhou, E.-Q. Chen and H. Lu, Chem, 2020, 6, 1831–1843 CAS.
- H. S. Wang, N. P. Truong, Z. Pei, M. L. Coote and A. Anastasaki, J. Am. Chem. Soc., 2022, 144, 4678–4684 CrossRef CAS PubMed.
- Y.-T. Guo, C. Shi, T.-Y. Du, X.-Y. Cheng, F.-S. Du and Z.-C. Li, Macromolecules, 2022, 55, 4000–4010 CrossRef CAS.
- M. Mohadjer Beromi, C. R. Kennedy, J. M. Younker, A. E. Carpenter, S. J. Mattler, J. A. Throckmorton and P. J. Chirik, Nat. Chem., 2021, 13, 156–162 CrossRef CAS PubMed.
- J. D. Feist and Y. Xia, J. Am. Chem. Soc., 2020, 142, 1186–1189 CrossRef CAS PubMed.
- J.-Z. Zhao, T.-J. Yue, B.-H. Ren, Y. Liu, W.-M. Ren and X.-B. Lu, Macromolecules, 2022, 55, 8651–8658 CrossRef CAS.
- Y. Wang, M. Li, J. Chen, Y. Tao and X. Wang, Angew. Chem., Int. Ed., 2021, 60, 22547–22553 CrossRef CAS PubMed.
- G.-Q. Tian, Z.-H. Yang, W. Zhang, S.-C. Chen, L. Chen, G. Wu and Y.-Z. Wang, Green Chem., 2022, 24, 4490–4497 RSC.
- Q. Zhang, Y. Deng, C.-Y. Shi, B. L. Feringa, H. Tian and D.-H. Qu, Matter, 2021, 4, 1352–1364 CrossRef CAS.
- G.-W. Yang, Y. Wang, H. Qi, Y.-Y. Zhang, X.-F. Zhu, C. Lu, L. Yang and G.-P. Wu, Angew. Chem., Int. Ed., 2022, 61, e202210243 CAS.
- T. M. McGuire, A. C. Deacy, A. Buchard and C. K. Williams, J. Am. Chem. Soc., 2022, 144, 18444–18449 CrossRef CAS PubMed.
- X. Liao, F.-C. Cui, J.-H. He, W.-M. Ren, X.-B. Lu and Y.-T. Zhang, Chem. Sci., 2022, 13, 6283–6290 RSC.
- C. Li, R. J. Sablong, R. A. T. M. van Benthem and C. E. Koning, ACS Macro Lett., 2017, 6, 684–688 CrossRef CAS PubMed.
- Z Cai, Y. Liu, Y.-H. Tao and J.-B. Zhu, Acta Chim. Sin., 2022, 80, 1165–1182 CrossRef.
- W. Zhang, J. Dai, Y.-C. Wu, J.-X. Chen, S.-Y. Shan, Z. Cai and J.-B. Zhu, ACS Macro Lett., 2022, 11, 173–178 CrossRef CAS PubMed.
- G. W. Coates and Y. D. Y. L. Getzler, Nat. Rev. Mater., 2020, 5, 501–516 CrossRef CAS.
- Y. Yu, B. Gao, Y. Liu and X.-B. Lu, Angew. Chem., Int. Ed., 2022, 61, e202204492 CAS.
- M. Hong and E. Y. X. Chen, Angew. Chem., Int. Ed., 2016, 55, 4188–4193 CrossRef CAS PubMed.
- L. Cederholm, P. Olsén, M. Hakkarainen and K. Odelius, Polym. Chem., 2020, 11, 4883–4894 RSC.
- G. W. Fahnhorst and T. R. Hoye, ACS Macro Lett., 2018, 7, 143–147 CrossRef CAS PubMed.
- J. Li, F. Liu, Y. Liu, Y. Shen and Z. Li, Angew. Chem., Int. Ed., 2022, 61, e202207105 CAS.
- C. Li, L. Wang, Q. Yan, F. Liu, Y. Shen and Z. Li, Angew. Chem., Int. Ed., 2022, 61, e202201407 CAS.
- X. Tang and E. Y. X. Chen, Chem, 2019, 5, 284–312 CAS.
- X.-L. Li, R. W. Clarke, J.-Y. Jiang, T.-Q. Xu and E. Y. X. Chen, Nat. Chem., 2022 DOI:10.1038/s41557-022-01077-x.
- J. Su, G. Xu, B. Dong, R. Yang, H. Sun and Q. Wang, Polym. Chem., 2022, 13, 5897–5904 RSC.
- R. M. Rapagnani, R. J. Dunscomb, A. A. Fresh and I. A. Tonks, Nat. Chem., 2022, 14, 877–883 CrossRef CAS PubMed.
- L. Cederholm, J. Wohlert, P. Olsén, M. Hakkarainen and K. Odelius, Angew. Chem., Int. Ed., 2022, 61, e202204531 CAS.
- J. Bruckmoser, S. Remke and B. Rieger, ACS Macro Lett., 2022, 11, 1162–1166 CrossRef CAS PubMed.
- D. K. Schneiderman, M. E. Vanderlaan, A. M. Mannion, T. R. Panthani, D. C. Batiste, J. Z. Wang, F. S. Bates, C. W. Macosko and M. A. Hillmyer, ACS Macro Lett., 2016, 5, 515–518 CrossRef CAS PubMed.
- J. Dai, W. Xiong, M.-R. Du, G. Wu, Z. Cai and J.-B. Zhu, Sci. China: Chem., 2023, 66, 251–258 CrossRef CAS.
- M. Niaounakis, in Recycling of Flexible Plastic Packaging, ed. M. Niaounakis, William Andrew Publishing, 2020, pp. 1–20, DOI:10.1016/B978-0-12-816335-1.00001-3.
- J. C. C. Yeo, J. K. Muiruri, W. Thitsartarn, Z. Li and C. He, Mater. Sci. Eng., C, 2018, 92, 1092–1116 CrossRef CAS PubMed.
- S. W. Lee, M. Ree, C. E. Park, Y. K. Jung, C. S. Park, Y. S. Jin and D. C. Bae, Polymer, 1999, 40, 7137–7146 CrossRef CAS.
- D. A. Schiraldi, in Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, 2004, pp. 243–265, DOI:10.1002/0470090685.ch6.
- F. Zhang, F. Wang, X. Wei, Y. Yang, S. Xu, D. Deng and Y.-Z. Wang, J. Energy Chem., 2022, 69, 369–388 CrossRef CAS.
- H. Wang, Z. Li, Y. Liu, X. Zhang and S. Zhang, Green Chem., 2009, 11, 1568–1575 RSC.
- Q. Wang, X. Yao, S. Tang, X. Lu, X. Zhang and S. Zhang, Green Chem., 2012, 14, 2559–2566 RSC.
- K. Fukushima, J. M. Lecuyer, D. S. Wei, H. W. Horn, G. O. Jones, H. A. Al-Megren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. McNeil, J. E. Rice and J. L. Hedrick, Polym. Chem., 2013, 4, 1610–1616 RSC.
- C. Jehanno, I. Flores, A. P. Dove, A. J. Müller, F. Ruipérez and H. Sardon, Green Chem., 2018, 20, 1205–1212 RSC.
- C. S. Nunes, M. J. Vieira da Silva, D. Cristina da Silva, A. d. R. Freitas, F. A. Rosa, A. F. Rubira and E. C. Muniz, RSC Adv., 2014, 4, 20308–20316 RSC.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, Angew. Chem., Int. Ed., 2021, 60, 6710–6717 CrossRef CAS PubMed.
- J. P. MacDonald and M. P. Shaver, Polym. Chem., 2016, 7, 553–559 RSC.
- E. Lizundia, V. A. Makwana, A. Larrañaga, J. L. Vilas and M. P. Shaver, Polym. Chem., 2017, 8, 3530–3538 RSC.
- V. A. Makwana, A. Larrañaga, J. L. Vilas and E. Lizundia, Polym. Degrad. Stab., 2019, 169, 108984 CrossRef.
- L.-G. Li, Q.-Y. Wang, Q.-Y. Zheng, F.-S. Du and Z.-C. Li, Macromolecules, 2021, 54, 6745–6752 CrossRef CAS.
- H.-Z. Fan, X. Yang, J.-H. Chen, Y.-M. Tu, Z. Cai and J.-B. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202117639 CAS.
- X. Yang, W. Zhang, H.-Y. Huang, J. Dai, M.-Y. Wang, H.-Z. Fan, Z. Cai, Q. Zhang and J.-B. Zhu, Macromolecules, 2022, 55, 2777–2786 CrossRef CAS.
- X. Yang, H.-Z. Fan, Z. Cai, Q. Zhang and J.-B. Zhu, Chin. J. Chem., 2022, 40, 2973–2980 CrossRef CAS.
- R. C. Pratt, B. G. G. Lohmeijer, D. A. Long, R. M. Waymouth and J. L. Hedrick, J. Am. Chem. Soc., 2006, 128, 4556–4557 CrossRef CAS PubMed.
- L. Simón and J. M. Goodman, J. Org. Chem., 2007, 72, 9656–9662 CrossRef PubMed.
- B. G. G. Lohmeijer, R. C. Pratt, F. Leibfarth, J. W. Logan, D. A. Long, A. P. Dove, F. Nederberg, J. Choi, C. Wade, R. M. Waymouth and J. L. Hedrick, Macromolecules, 2006, 39, 8574–8583 CrossRef CAS.
- X. Sun, J. P. Gao and Z. Y. Wang, J. Am. Chem. Soc., 2008, 130, 8130–8131 CrossRef CAS PubMed.
- R. Todd, S. Tempelaar, G. Lo Re, S. Spinella, S. A. McCallum, R. A. Gross, J.-M. Raquez and P. Dubois, ACS Macro Lett., 2015, 4, 408–411 CrossRef CAS PubMed.
- S. Moins, C. Henoumont, J. De Winter, A. Khalil, S. Laurent, S. Cammas-Marion and O. Coulembier, Polym. Chem., 2018, 9, 1840–1847 RSC.
- R. M. Shakaroun, P. Jéhan, A. Alaaeddine, J.-F. Carpentier and S. M. Guillaume, Polym. Chem., 2020, 11, 2640–2652 RSC.
- A. Pascual, H. Sardon, A. Veloso, F. Ruipérez and D. Mecerreyes, ACS Macro Lett., 2014, 3, 849–853 CrossRef CAS PubMed.
- Y.-T. Guo, W. Xiong, C. Shi, F.-S. Du and Z.-C. Li, Polym. Chem., 2022, 13, 4490–4501 RSC.
- D. Ragno, G. Di Carmine, M. Vannini, O. Bortolini, D. Perrone, S. Buoso, M. Bertoldo and A. Massi, Polym. Chem., 2022, 13, 1350–1358 RSC.
- M. Cheng, A. B. Attygalle, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 11583–11584 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py01491b |
| This journal is © The Royal Society of Chemistry 2023 |