Use of an N-heterocyclic carbene (NHC)-based interacting Lewis pair for the synthesis of a cyclic poly(alkyl acrylate) via chain-growth polymerization and subsequent ring-closing without extreme dilution†
Abstract
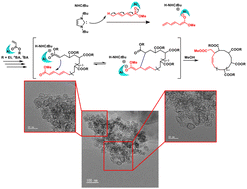
The pairing of 1,3-di-tert-butylimidazol-2-ylidene (NHCtBu) and methylaluminum bis(2,6-di-tert-butyl-4-methylphenoxide) (MAD) acted as an interacting Lewis pair (ILP) for anionic polymerization of several acrylates. The ILP showed higher polymerization activity for ethyl acrylate (EA) than for n-butyl acrylate (n-BA) or t-butyl acrylate (t-BA). The slow initiation to n-BA and t-BA was beneficial for chain polymerization initiated by deprotonated methyl sorbate (MS) and subsequent ring-closure, because direct initiation by NHCtBu was necessarily suppressed. In fact, when we used a 1 : 1 mixture of NHCtBu with MS and t-BA as the initiator and the monomer, respectively, anionic polymerization occurred in toluene and after monomer depletion the ring closure of the polymer molecules occurred without the need for highly dilute conditions because of the presence of the α-terminal MS unit neighbor during polymerization. Both matrix assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry and TEM images indicated a cyclic topology of the product and the aggregates. TEM images also indicated that the aggregation of cyclic poly(t-BA) in toluene solution was suppressed compared with that of cyclic poly(n-BA).


 Please wait while we load your content...
Please wait while we load your content...