Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
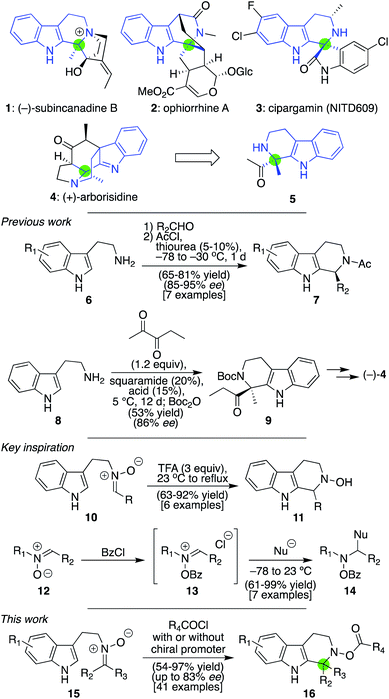
Synthesis of aza-quaternary centers via Pictet–Spengler reactions of ketonitrones†
Tessa
Lynch-Colameta
 ,
Sarah
Greta
and
Scott A.
Snyder
,
Sarah
Greta
and
Scott A.
Snyder
 *
*
Department of Chemistry, University of Chicago, 5735 S. Ellis Avenue, Chicago, IL 60637, USA. E-mail: sasnyder@uchicago.edu
First published on 16th March 2021
Abstract
Despite the array of advances that have been made in Pictet–Spengler chemistry, particularly as it relates to the synthesis of β-carboline derivatives of both natural and designed origin, the ability to use such reactions to generate aza-quaternary centers remains limited. Herein, we report a simple procedure that enables the synthesis of a variety of such products by harnessing the distinct reactivity profiles of ketonitrones as activated by commercially available acyl chlorides. Notably, the reaction process is mild, fast, and high-yielding (54–97%) for a diverse collection of substrates, including some typically challenging ones, such as indole cores with electron-deficient substituents. In addition, by deploying an acyl bromide in combination with a thiourea promoter, a catalytic, asymmetric version has been established, leading to good levels of enantioselectivity (up to 83% ee) for several ketonitrones. Finally, the resultant N–O bonds within the products can also be functionalized in several unique ways, affording valuable complementarity to existing Pictet–Spengler variants based on the use of imines.
Introduction
Given that the tetrahydro-β-carboline core is a privileged scaffold found in natural products, folk medicines, and mainline pharmaceutical agents, numerous methods have been developed for its synthesis.1 The most powerful of these, arguably, is the Pictet–Spengler reaction,2,3 especially given recent advances in asymmetric variants promoted by hydrogen bonding catalysts such as phosphoric acids and thioureas,4 as exemplified through the conversion of 6 to 7 (Scheme 1).4a Less common, though no less valuable, are β-carbolines that possess an aza-quaternary center, with 1–4 being representative cases.5 Methods to access these materials, by contrast, are far less developed.4b,6,7 Indeed, in our own recent total synthesis of (+)-4, we could not directly form the lone chiral center of 5 as a single enantiomer from achiral materials using tryptamine and a diketone reactant via a Pictet–Spengler reaction.5h Nonetheless, in a recent synthesis of the same target by Zhu and co-workers, extensive investigations ultimately identified the means to achieve an enantioselective synthesis of 9 (in 86% ee), despite requiring prolonged reaction times (12 d).5i Only one other report of an asymmetric variant involving non-spirocyclic 1,2-dicarbonyls is known, and it required stoichiometric amounts of a chiral promoter for success.6k Herein, we delineate a distinct approach for generating such aza-quaternary centers through Pictet–Spengler chemistry, both in racemic and asymmetric form, by taking advantage of the unique chemistry of nitrones. As illustrated by >40 examples, the identified process leads to the rapid and high-yielding synthesis of materials with a diverse array of electronic properties, most of which were previously unsynthesized through Pictet–Spengler reactions. Furthermore, many of these products proved accessible in enantioenriched form (up to 83% ee) when our core procedure was modified slightly with catalytic amounts of an appropriate chiral promoter. And, as demonstrated by some concluding examples, the presence of the resultant N–O bond in the products could be leveraged in several distinct ways to access additional complexity that would be difficult to obtain from materials fashioned through a traditional Pictet–Spengler approach.Attracted by this general challenge and drawing inspiration from our recent work using nitrones to forge functionalized piperidines through Mannich-type chemistry,8 we wondered if such species might also be of service here. Although a limited number of nitrone-based Pictet–Spengler reactions have been reported,9 nearly every example utilizes aldonitrones (such as 10) under conditions that tend to require strong acid and/or high temperatures to promote successful cyclization to 11;9f in the case of reported asymmetric reactions, superstoichiometric amounts of chiral promoters have been needed.9i–k To the best of our knowledge, just one example of a successful reaction with a ketonitrone, in which acetone was utilized to generate that species, has been reported in a racemic format.9d This dearth of examples may reflect that ketonitrones are generally less reactive than their aldonitrone counterparts. However, it has been shown in separate studies that if nitrones more generally, such as 12, are converted into their corresponding N-acyloxyiminium ions (13), their reactivity is greatly enhanced and can undergo nucleophilic attack to generate materials of type 14.10 We anticipated that such a mode of activation might enable Pictet–Spengler chemistry to proceed when it otherwise would not. In addition, the added acyloxy group might also at the same time confer structural advantages that would render an asymmetric version of the reaction easier to achieve than standard imine-based Pictet–Spengler reactions.
Results and discussion
Our studies began with efforts to affect a racemic version of the process, utilizing ketonitrone 19 (Table 1) as our inaugural starting material. This compound was readily prepared on gram scale over the course of three steps from tryptophol (17, see ESI† for details), and is an easily purifiable, bench-stable solid. In short order, we found that if this compound was combined with 1.05 equiv. of benzoyl chloride in the presence of 4 Å molecular sieves in CH2Cl2 at 23 °C, the desired product (20a) was obtained in 86% yield after just 30 min; X-ray crystallographic analysis confirmed its identity. Of note, the presence of molecular sieves was crucial to prevent the hydrolysis of the N-acyloxyiminium species formed in situ, which led to diminished yields.11 Pleasingly, screening of numerous acyl chlorides showed the reaction process to be just as facile irrespective of their steric bulk or electronic properties (entries 2–5), with phenylacetyl chloride providing the highest yield at 95%. We view such breadth of success with the activating partner as an attractive feature given that this group can serve as a protective device (with different means of deprotection and/or stability to subsequent reaction conditions as a result) and might provide critical functionality that could enable chiral differentiation.| Entry | R | Product | Yield [%] |
|---|---|---|---|
| a Final reactions were performed with 19 (0.25 mmol) under argon. | |||
| 1 | Ph | 20a [X-ray] | 86 |
| 2 | Me | 20b | 86 |
| 3 | t-Bu | 20c | 88 |
| 4 | OMe | 20d | 87 |
| 5 | CH2Ph | 20e | 95 |
With this initial condition screen completed, we then turned our attention to exploring the overall scope. Scheme 2 presents our efforts to evaluate distinct ketonitrones generated from hydroxylamine precursor 18 (cf.Table 1). Some nitrones were synthesized as inseparable E-/Z-mixtures, as denoted within the ESI,† but this element did not affect the outcome of these racemic reactions. As shown, a wide range of both ketonitrones and their respective products were readily synthesized, with yields for the final compounds ranging from 71–97% for 21–38 when phenylacetyl chloride was used as the activating species. These products include aryl and heteroaryl groups (21–26), with those rings containing electron-deficient groups affording elevated yields. Simple alkyl substituents could also be incorporated (27–30), yet the amount of steric hindrance is relevant to the degree of success as revealed by the decreased yield obtained for 29 (74%). Pleasingly, ether, ester, and ketone-substituted nitrones worked as well (31–34),12 as did materials leading to spirocyclic products (35–38). However, we found for the latter category that when substrates had extended ring sizes or an indanone-based system, the yields obtained for their products were significantly lower (<50%); compounds of this type are known to be challenging in the imine variant of this, and related, reactions.13 Surmising that increased hydrolysis of the N-acyloxyiminium species over prolonged reaction times might be the culprit,11 we switched the activating species to a more sterically encumbered acyl chloride (i.e. pivaloyl chloride) in hopes it would still enable sufficient intramolecular cyclization while slowing down the intermolecular decomposition/hydrolysis pathways. As proposed, the yields for 39–41 were significantly enhanced. Therefore, we recommend this activating species in particular for ketonitrone starting materials that are ineffective with smaller acyl groups. Notably, the only real limitations identified from this initial screen were that ketonitrones possessing very sterically encumbered (i.e. if R1 or R2 = t-butyl) or electron-deficient groups (i.e. if R1 or R2 = CF3) could not be prepared; as long as the ketonitrone could be made, a successful Pictet–Spengler cyclization was observed in every case.
We next examined the extent to which the electronic properties of the indole core could be modulated. As seen in Scheme 3, and as expected, those substrates possessing electron-donating groups proceeded smoothly to afford 42 and 43 in high yield (92 and 90%, respectively). To our delight, the reaction also tolerated both weak and strongly electron-withdrawing groups at either the 4-, 5-, or 6-positions, with those substrates possessing the stronger withdrawing groups only leading to an ∼10% decrease in yield overall, to afford 44–47 in 62–83% yield. In general, these latter two substrates reflect examples that have not yet been demonstrated to succeed with reasonable yield in standard, imine-based Pictet–Spengler reactions,14 highlighting how activated nitrones provide enhanced electrophilicity that can broaden scope.
Given this range of prepared racemic products possessing aza-quaternary centers, our next focus was on rendering this process asymmetric. We began by testing thioureas as enantioselective promoters, given their broad application in asymmetric catalysis, particularly as relates to anion-binding-mediated enantioselection.15 Our first effective hit came with one particular thiourea (48)16,17 using substrate 19, 4 Å molecular sieves, and benzoyl chloride as the activating group in CH2Cl2 at a concentration of 0.015 M at −78 °C (Table 2, entry 1). In this case, while the product yield was reasonable (79%), only modest enantioselectivity (51% ee) was observed. Though many acyl chlorides were subsequently screened (see ESI† Section), none provided substantial improvements over this lead. However, a solvent screen showed that if the reaction medium was changed to toluene (entry 2), enantioselectivity could be enhanced (to 64% ee) at the expense of yield (52%). A screening of additional catalysts (not shown, see ESI†) was able to increase the enantioselectivity to 81% ee, but could not provide any solution to the low yield of the reaction. Encouraged by these results and the documented effect that different anions can have on binding,15 we next changed the activating species to benzoyl bromide, and finally saw retention in yields with increases in enantioselectivity (75% and 83% ee, respectively, entry 3). We believe this finding to be significant, in that it implies our reaction may not be going through a traditional anion-binding mechanism since a switch to bromide under such a manifold would normally lead to a decrease in chiral selection.4b Hence, we propose that the catalyst itself could be interacting with the carbonyl of the N-acyloxyiminium species, with the phenyl group of the activating group likely being involved as well.18 Following extensive optimization, the reaction afforded the best results when performed using 10 mol% catalyst loading of thiourea 48 in toluene at −78 °C at a concentration of 0.01 M (entry 6).
| Entry | Solvent | BzX | M (mol L−1) | (X mol%) | Yield [%] | ee [%] |
|---|---|---|---|---|---|---|
| a Final reactions were performed with 19 (0.09 mmol) under argon. | ||||||
| 1 | CH2Cl2 | BzCl | 0.015 | 20 mol% | 79 | 51 |
| 2 | Toluene | BzCl | 0.015 | 20 mol% | 52 | 64 |
| 3 | Toluene | BzBr | 0.015 | 20 mol% | 75 | 83 |
| 4 | Toluene | BzBr | 0.015 | 10 mol% | 83 | 83 |
| 5 | Toluene | BzBr | 0.020 | 10 mol% | 83 | 78 |
| 6 | Toluene | BzBr | 0.010 | 10 mol% | 85 | 84 |
As indicated in Scheme 4, these conditions enabled effective chiral selection for ketonitrones possessing a variety of appended benzene rings, with 49–53 being obtained in good yield and enantioselectivities (80–83% ee); an X-ray crystal structure of 51 provided the means to establish their absolute configuration, with the other products being assigned based on analogy.19 Impressively, this crystallization afforded 51 with near optical purity (99% ee). These results in terms of enantioselectivities for these substrates are entirely in line with the best previous literature precedent (Scheme 1, product 9, 86% ee) for aza-quaternary center generation, where only a single substrate was probed, yet with much higher yields and significantly faster reaction times. In addition, substrates possessing a heterocyclic substituent (pyridine or thiophene) also worked well, though with decreased enantioselectivity (55% ee and 53% ee, respectively). Unlike with the racemic variant of the reaction, critical for the results of all these asymmetric cases was that their ketonitrones could be obtained as single regioisomers. By contrast, most of the alkyl–alkyl substituted nitrones that were synthesized exist as inseparable mixtures of E-/Z-isomers. For one such material where separation could be accomplished, only moderate levels of enantiocontrol (62% ee) were observed with the resultant product (56). Still, we were able to show with this substrate that E-/Z-isomers gave approximately equal, but opposite, enantioselectivities under the optimized conditions.20 The presence of an additional carbonyl group (as in 57) proved detrimental, likely due to the competing racemic background reaction, noting that all reactions appear to suffer from some degree of background reaction in eroding enantioselection. Substrates with strongly electron-withdrawing groups on the indole core (not shown) were incompatible with the asymmetric reaction conditions, affording only the undesired hydrolysis side-products.
 | ||
Scheme 4 Substrate scope for the asymmetric variant of the reaction. a Performed in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 (4 CH2Cl2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Finally, while the use of ketonitrones in this procedure does necessitate the expenditure of some supplemental synthetic operations to prepare the relevant starting materials, this investment can pay added synthetic dividends beyond substrate scope and the potential for enantiocontrol. As shown in Scheme 5, one can, of course, readily cleave the N–O bond within 20e through standard treatment with Zn in AcOH at 60 °C to generate 58 in quantitative yield.8,21 More valuable, potentially, is the ability to affect that same reaction and perform a reductive amination in the same pot, shown here by the formation of 59.8,22 Equally important, 20e can engage directly in electrophilic amination reactions.23,24 Although O-acyl hydroxylamines are commonly used in this event, the phenylacetyl group has never been tested to the best of our knowledge, with the neighboring aza-quaternary center also providing a high degree of steric bulk that might thwart success. Nevertheless, applying Johnson's conditions,23a we were able to access the desired tertiary amine 60via a copper-catalyzed electrophilic amination with a diorganozinc reagent (Ph2Zn).24 Lastly, following simple deprotection of the acyl group using LiOH·H2O to release free hydroxylamine 61,25 aldonitrone 62 was prepared through the action of HgO.8,26 This species could then be engaged by nucleophiles (such as MeMgBr27 and Et2AlCN28) or in a [3 + 2]-cycloaddition reaction29 with methyl acrylate to afford 63–65, respectively.30 All of these latter products were confirmed by X-ray crystallography as possessing the relative configurations shown.
Conclusions
In conclusion, we have developed a mild, fast, and high-yielding method to generate a variety of tetrahydro-β-carbolines bearing aza-quaternary centers by effecting Pictet–Spengler reactions of ketonitrones, rendered competent here by converting them into N-acyloxyiminium ions. This approach allows for significant substrate scope with high functional group tolerance and good to excellent yields. Moreover, we have established a catalytic, asymmetric variant of the reaction that proceeds with good enantioselectivity, along with an initial understanding of the range for its efficacy. Overall, we believe this method can be utilized in diverse synthetic applications to access compounds of value, such as those highlighted in Scheme 1, with the range of functionalizations available to the final products supporting the validity of this idea. Such efforts are the subject of current endeavors.Conflicts of interest
There are no conflicts to declare.Author contributions
S. A. S. and T. L.-C. conceived the project. S. A. S. directed the research, and S. A. S. and T. L.-C. composed the manuscript and the and the ESI Section.† T. L.-C. developed the initial reactions and explored substrate scope, while S. G. completed the synthesis of several starting materials and contributed significantly to the examples in the substrate tables.Acknowledgements
We thank Dr Alexander Filatov, Dr Andrew McNeece, and Ms Kate Jesse for X-ray analysis of our crystalline intermediates, and Dr Josh Kurutz, Dr Antoni Jurkiewicz, and Dr C. Jin Qin for assistance with NMR and mass spectrometry, respectively. We are grateful to Mr Vladislav Lisnyak for advice during initial explorations and Ms Olivia Morales for material preparation. We also wish to thank colleagues at Merck for several ureas and thioureas, as well as the Rawal group (University of Chicago) for several squaramide and thiosquaramide precursors, donated and used during our optimization efforts. Financial support for this work came from the University of Chicago and the Jeff Metcalf Summer Internship Program (Fellowship to S. G.).Notes and references
- For selected reviews on β-carbolines and their syntheses, see: (a) The Alkaloids, Chemistry and Physiology, ed. R. H. F. Manske, Academic Press, New York, vol. XX, 1981 Search PubMed; (b) B. E. Love, Org. Prep. Proced. Int., 1996, 28, 1 CrossRef CAS; (c) J. W. Grate and G. C. Frye, in Sensors Update, ed. H. Baltes, W. Gopel and J. Hesse, Wiley-VCH, Weinheim, vol. 2, 1996, p. 10 Search PubMed; (d) M. Hesse, in Alkaloids: Nature's Curse or Blessing, Wiley-VCH, Zürich, Switzerland, 2002, p. 14 Search PubMed; (e) R. H. Cao, W. L. Peng, Z. H. Wang and A. L. Xu, Curr. Med. Chem., 2007, 14, 479 CrossRef CAS PubMed; (f) J. K. Dai, W. J. Dan, U. Schneider and J. R. Wang, Eur. J. Med. Chem., 2018, 157, 622 CrossRef CAS PubMed; (g) P. Maity, D. Adhikari and A. K. Jana, Tetrahedron, 2019, 75, 965 CrossRef CAS.
- (a) A. Pictet and T. Spengler, Ber. Dtsch. Chem. Ges., 1911, 44, 2030 CrossRef CAS; (b) G. Tatsui, Yakugaku Zasshi, 1928, 48, 453 CrossRef CAS.
- For selected reviews on the Pictet–Spengler reaction, see: (a) E. D. Cox and J. M. Cook, Chem. Rev., 1995, 95, 1797 CrossRef CAS; (b) J. Stöckigt, A. P. Antonchick, F. Wu and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 8538 CrossRef PubMed; (c) R. Dalpozzo, Molecules, 2016, 21, 69 CrossRef PubMed.
- For examples based on thioureas, see: (a) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 10558 CrossRef CAS PubMed; (b) I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 13404 CrossRef CAS PubMed; (c) R. S. Klausen and E. N. Jacobsen, Org. Lett., 2009, 11, 887 CrossRef CAS PubMed; (d) N. Mittal, D. X. Sun and D. Seidel, Org. Lett., 2014, 16, 1012 CrossRef CAS PubMed; (e) R. S. Klausen, C. R. Kennedy, A. M. Hyde and E. N. Jacobsen, J. Am. Chem. Soc., 2017, 139, 12299 CrossRef CAS PubMed. For examples based on phosphoric acids, see: (f) J. Seayad, A. M. Seayad and B. List, J. Am. Chem. Soc., 2006, 128, 1086 CrossRef CAS PubMed; (g) M. J. Wanner, R. N. van der Haas, K. R. de Cuba, J. H. van Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2007, 46, 7485 CrossRef CAS PubMed; (h) N. V. Sewgobind, M. J. Wanner, S. Ingemann, R. de Gelder, J. H. van Maarseveen and H. Hiemstra, J. Org. Chem., 2008, 73, 6405 CrossRef CAS PubMed; (i) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796 CrossRef CAS PubMed; (j) C. A. Holloway, M. E. Muratore, R. I. Storer and D. J. Dixon, Org. Lett., 2010, 12, 4720 CrossRef CAS PubMed; (k) D. Huang, F. Xu, X. Lin and Y. Wang, Chem.–Eur. J., 2012, 18, 3148 CrossRef CAS PubMed; (l) S.-G. Wang, Z.-L. Xia, R.-Q. Xu, X.-J. Liu, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 7440 CrossRef CAS PubMed. For an additional case using gold under non-hydrogen bonding catalysis, see: (m) N. Glinsky-Olivier, S. Yang, P. Retailleau, V. Gandon and X. Guinchard, Org. Lett., 2019, 21, 9446 CrossRef CAS PubMed.
- For the isolation or discovery of 1–4, see: (a) J. Kobayashi, M. Sekiguchi, S. Shimamoto, H. Shigemori, H. Ishiyama and A. Ohsaki, J. Org. Chem., 2002, 67, 6449 CrossRef CAS PubMed; (b) T. Feng, K.-T. Duan, S.-J. He, B. Wu, Y.-S. Zheng, H.-L. Ai, Z.-H. Li, J. He, J.-P. Zuo and J.-K. Liu, Org. Lett., 2018, 20, 7926 CrossRef CAS PubMed; (c) B. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki, C. Fischli, A. Goh, E. K. Schmitt, P. Krastel, E. Francotte, K. Kuhen, D. Plouffe, K. Henson, T. Wagner, E. A. Winzeler, F. Petersen, R. Brun, V. Dartois, T. T. Diagana and T. H. Keller, J. Med. Chem., 2010, 53, 5155 CrossRef CAS PubMed; (d) M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. E. González-Páez, S. B. Lakshminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler and T. T. Diagana, Science, 2010, 329, 1175 CrossRef CAS PubMed; (e) S.-P. Wong, K.-W. Chone, K.-H. Lim, S.-H. Lime, Y.-Y. Low and T.-S. Kam, Org. Lett., 2016, 18, 1618 CrossRef CAS PubMed. For total syntheses of 1 and 4, see: (f) Y. Liu, S. Luo, X. Fu, F. Fang, Z. Zhuang, W. Xiong, X. Jia and H. Zhai, Org. Lett., 2006, 8, 115 CrossRef CAS PubMed; (g) K. Suzuki and H. Takayama, Org. Lett., 2006, 8, 4605 CrossRef CAS PubMed; (h) Z. Zhou, A. X. Gao and S. A. Snyder, J. Am. Chem. Soc., 2019, 141, 7715 CrossRef CAS PubMed; (i) R. Andres, Q. Wang and J. Zhu, J. Am. Chem. Soc., 2020, 142, 14726 CrossRef PubMed.
- For selected, racemic versions to access aza-quaternary centers within tetrahydro-β-carbolines, see: (a) J. B. Hester, J. Org. Chem., 1964, 29, 2864 CrossRef CAS; (b) J. P. Mayer, D. B. Davis, J. Zhang, G. Beaton, K. Bjergarde, C. M. Andersen, B. A. Goodman and C. J. Herrera, Tetrahedron Lett., 1996, 37, 5633 CrossRef CAS; (c) Y. Horiguchi, M. Nakamura, T. Saitoh and T. Sano, Chem. Pharm. Bull., 2003, 51, 1368 CrossRef CAS PubMed; (d) F.-M. Kuo, M.-C. Tseng, Y.-H. Yen and Y.-H. Chu, Tetrahedron, 2004, 60, 12075 CrossRef CAS; (e) Y. Lingam, D. M. Rao, D. R. Bhowmik, P. S. Santu, K. R. Rao and A. Islam, Tetrahedron Lett., 2007, 48, 7243 CrossRef CAS; (f) P. M. Mumford, J. J. Shiers, G. J. Tarver, J. F. Hayes and M. Shipman, Tetrahedron Lett., 2008, 49, 3489 CrossRef CAS; (g) S. V. Ryabukhin, D. M. Panov, A. S. Plaskon, A. A. Tolmachev and R. V. Smaliy, Monatsh. Chem., 2012, 143, 1507 CrossRef CAS; (h) L.-N. Wang, S.-L. Shen and J. Qu, RSC Adv., 2014, 4, 30733 RSC; (i) A. Riesco-Domínguez, N. van der Zwaluw, D. Blanco-Ania and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2017, 662 CrossRef; (j) Z. Wang, J. Niu, H. Zeng and C.-J. Li, Org. Lett., 2019, 21, 7033 CrossRef CAS PubMed. For enantioselective variants, see: (k) F. R. Bou-Hamdan and J. L. Leighton, Angew. Chem., Int. Ed., 2009, 48, 2403 CrossRef CAS PubMed; (l) J. J. Badillo, A. Silva-Garcia, B. H. Shupe, J. C. Fettinger and A. K. Franz, Tetrahedron Lett., 2011, 52, 5550 CrossRef CAS PubMed; (m) A. Duce, F. Pesciaioli, L. Gramigna, L. Bernardi, A. Mazzanti, A. Ricci, G. Bartoli and G. Bencivenni, Adv. Synth. Catal., 2011, 353, 860 CrossRef; (n) W. Dai, H. Lu, X. Li, F. Shi and S. J. Tu, Chem.–Eur. J., 2014, 20, 11382 CrossRef CAS PubMed; (o) C. Piemontesi, Q. Wang and J. Zhu, J. Am. Chem. Soc., 2016, 138, 11148 CrossRef CAS PubMed.
- While standard Pictet–Spengler reactions work well with highly reactive ketones such as 1,2-dicarbonyls, simple ketones often require long reaction times and are low-yielding due to the slow rate of imine formation and steric congestion that renders the cyclization challenging.
- V. G. Lisnyak, T. Lynch-Colameta and S. A. Snyder, Angew. Chem., Int. Ed., 2018, 57, 15162 CrossRef CAS PubMed.
- For selected racemic versions, see: (a) S.-Y. Han, M. V. Lakshmikantham and M. P. Cava, Heterocycles, 1985, 23, 1671 CrossRef CAS; (b) R. Plate, R. H. M. Van Hout, H. Behm and H. C. J. Ottenheijm, J. Org. Chem., 1987, 52, 555 CrossRef CAS; (c) J. Sandrin, S. P. Hollinshead and J. M. Cook, J. Org. Chem., 1989, 54, 5636 CrossRef CAS; (d) I. W. J. Still and J. R. Strautmanis, Can. J. Chem., 1990, 68, 1408 CrossRef CAS; (e) P. H. H. Hermkens, J. H. van Maarseveen, P. L. H. M. Cobben, H. C. J. Ottenheijm, C. G. Kruse and H. W. Scheeren, Tetrahedron, 1990, 46, 833 CrossRef CAS; (f) T. Hino, A. Hasegawa, J.-J. Liu and M. Nakagawa, Chem. Pharm. Bull., 1990, 38, 59 CrossRef CAS; (g) J. Liu, M. Nakagawa, K. Ogata and T. Hino, Chem. Pharm. Bull., 1991, 39, 1672 CrossRef CAS; (h) R. Tsuji, M. Yamanaka, A. Nishida and M. Nakagawa, Chem. Lett., 2002, 428 CrossRef CAS. For enantioselective versions, see: (i) T. Kawate, H. Yamada, T. Soe and M. Nakagawa, Tetrahedron: Asymmetry, 1996, 7, 1249 CrossRef CAS; (j) T. Kawate, H. Yamada, M. Matsumizu, A. Nishida and M. Nakagawa, Synlett, 1997, 1761 Search PubMed; (k) H. Yamada, T. Kawate, M. Matsumizu, A. Nishida, K. Yamaguchi and M. Nakagawa, J. Org. Chem., 1998, 63, 6348 CrossRef CAS PubMed. For a review, see: (l) T. Hino and M. Nakagawa, Heterocycles, 1998, 49, 499 CrossRef CAS.
- (a) H. W. Heine, R. Zibuck and W. J. A. VandenHeuvel, J. Am. Chem. Soc., 1982, 104, 3691 CrossRef CAS; (b) T. Kawakami, H. Ohtake, H. Arakawa, T. Okachi, Y. Imada and S.-I. Murahashi, Org. Lett., 1999, 1, 107 CrossRef CAS; (c) T. Kawakami, H. Ohtake, H. Arakawa, T. Okachi, Y. Imada and S.-I. Murahashi, Chem. Lett., 1999, 795 CrossRef CAS; (d) T. Kawakami, H. Ohtake, H. Awakawa, T. Okachi, Y. Imada and S.-I. Murahashi, Bull. Chem. Soc. Jpn., 2000, 73, 2423 CrossRef CAS.
- Our current sense of this pathway is the following, with the benzoylated version of the final material having been characterized:
 .
. - When we attempted to form 34, it was found that the HCl formed during the reaction could affect cyclization of the starting material directly from the ketonitrone without generation of the N-acyloxyiminium ion. Further exploration showed that compounds 30, 33, and 34 with highly favorable electronics (i.e. electron-withdrawing groups attached) could also be cyclized as their free hydroxylamines by simply reacting the selected ketonitrone under catalytic acidic conditions. In general, however, the majority of substrates were ineffective without the formation of the intermediate N-acyloxyiminium species.
- (a) J. G. Rodríguez and P. Gil-Lopetegui, J. Heterocycl. Chem., 1993, 30, 373 CrossRef; (b) Y. Horiguchi, M. Nakamura, A. Kida, H. Kodama, T. Saitoh and T. Sano, Heterocycles, 2003, 59, 691 CrossRef CAS; (c) K. Murai, T. Kobayashi, M. Miyoshi and H. Fujioka, Org. Lett., 2018, 20, 2333 CrossRef CAS PubMed; (d) H. Zeng, Z. Wang and C.-J. Li, Angew. Chem., Int. Ed., 2019, 58, 2859 CrossRef CAS PubMed.
- (a) F. Viti, S. Bellinvia and S. Demartis, WO2015/197861A1, 2015; (b) Y. Chen, L. Yihua, J. Luo, Y. Zhang, C. Zheng, W. Tong and M. Liu, CN102977091B, 2016.
- For selected, recent reviews, see: (a) Y. Takemoto, Org. Biomol. Chem., 2005, 3, 4299 RSC; (b) Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187 RSC; (c) W.-Y. Siau and J. Wang, Catal. Sci. Technol., 2011, 1, 1298 RSC; (d) O. V. Serdyuk, C. M. Heckel and S. B. Tsogoeva, Org. Biomol. Chem., 2013, 11, 7051 RSC; (e) K. Brak and E. N. Jacobsen, Angew. Chem., Int. Ed., 2013, 52, 534 CrossRef CAS PubMed; (f) X. Fang and C.-J. Wang, Chem. Commun., 2015, 51, 1185 RSC.
- For complete details of catalysts explored, please see the ESI Section.†.
- E. N. Jacobsen and S. J. Zuend, US Pat., 2013/66109A1, 2013 Search PubMed.
- For example, when AcBr was used as the activating species, the ee of the product dropped by ∼10%.
- All nitrone precursors for Scheme 4 were determined to be the E-isomers via NOESY experiments, except for the formation of product 55, which had a Z-nitrone precursor. Hence, this product was assigned as the opposite enantiomer.
- A 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E
1 mixture of E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z nitrone isomers used for synthesis of 56. When a 1
Z nitrone isomers used for synthesis of 56. When a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 mixture of E
7 mixture of E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z nitrone isomers was used, a −49% ee was obtained. Since we were unable to perform this reaction on scale, a yield for this conversion is not reported.
Z nitrone isomers was used, a −49% ee was obtained. Since we were unable to perform this reaction on scale, a yield for this conversion is not reported. - G. S. King, P. D. Magnus and H. S. Rzepa, J. Chem. Soc., Perkin Trans. 1, 1972, 437 RSC.
- R. A. da Silva, I. H. S. Estevam and L. W. Bieber, Tetrahedron Lett., 2007, 48, 7680 CrossRef CAS.
- (a) A. M. Berman and J. S. Johnson, J. Am. Chem. Soc., 2004, 126, 5680 CrossRef CAS PubMed; (b) S. Graßl, Y.-H. Chen, C. Hamze, C. P. Tüllmann and P. Knochel, Org. Lett., 2019, 21, 494 CrossRef PubMed.
- Strikingly, the use of the phenylacetyl group afforded comparable yields to the commonly utilized benzoyl group with this substrate. It is worth noting that Knochel's conditions for this event employing an organozinc chloride reagent (PhZnCl) and CoCl2 (ref. 23b) also provided the electrophilic amination product 60, but in a lower yield (57%).
- A. Banerjee and H. Yamamoto, Chem. Sci., 2019, 10, 2124 RSC.
- (a) E. Gössinger, Tetrahedron Lett., 1980, 21, 2229 CrossRef; (b) S. Cicchi, A. Goti and A. Brandi, J. Org. Chem., 1995, 60, 4743 CrossRef CAS.
- P. Merino, V. Mannucci and T. Tejero, Eur. J. Org. Chem., 2008, 3943 CrossRef CAS.
- (a) F. L. Merchan, P. Merino and T. Tejero, Tetrahedron Lett., 1995, 36, 6949 CAS; (b) P. Merino, A. Lanaspa, F. L. Merchan and T. Tejero, J. Org. Chem., 1996, 61, 9028 CrossRef CAS PubMed; (c) A. Goti, S. Cicchi, V. Mannucci, F. Cardona, F. Guarna, P. Merino and T. Tejero, Org. Lett., 2003, 5, 4235 CrossRef CAS PubMed.
- (a) S. A. Ali and M. I. M. Wazeer, J. Chem. Soc., Perkin Trans. 2, 1986, 1789 RSC; (b) S. A. Ali and M. I. M. Wazeer, J. Chem. Soc., Perkin Trans. 1, 1988, 597 RSC; (c) S. A. Ali and M. I. M. Wazeer, Tetrahedron, 1988, 44, 187 CrossRef.
- For general reviews about nucleophilic additions into nitrones, see: (a) M. Lombardo and C. Trombini, Synthesis, 2000, 6, 759 CrossRef; (b) P. Merino, S. Franco, F. L. Merchan and T. Tejero, Synlett, 2000, 442 CAS; (c) P. Merino, C. R. Chim., 2005, 8, 775 CrossRef CAS; (d) S.-I. Murahashi and Y. Imada, Chem. Rev., 2019, 119, 4684 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 2060600–2060606. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc00882j |
| This journal is © The Royal Society of Chemistry 2021 |