Open Access Article
Open Access ArticleFacile synthesis of sulfinate esters from aryl iodides via direct oxidation of thioesters†
Keisuke
Nakamura
a,
Yukiko
Kumagai
a,
Akihiro
Kobayashi
ab,
Minori
Suzuki
ab and
Suguru
Yoshida
 *a
*a
aDepartment of Biological Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan. E-mail: s-yoshida@rs.tus.ac.jp
bLaboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan
First published on 11th August 2023
Abstract
A practical method to synthesize sulfinate esters from aryl iodides is disclosed. Direct oxidation of thioesters prepared by copper-catalyzed C–S formation of aryl iodides realized the efficient synthesis of sulfinate esters. Due to the good accessibility of aryl iodides, a wide variety of sulfinate esters were prepared from easily available starting materials such as carboxylic acids and anilines.
Oxidation of thiols is a fundamental method to synthesize a wide range of organosulfurs.1 For example, disulfides, sulfinic acid esters, and sulfonic acid esters were prepared by oxidation of thiols under different oxidation conditions (Fig. 1A, top).2–4 Due to the troubling properties of thiols such as unpleasant smell and easy oxidizability under air, the limited preparation of thiols having various functionalities has significantly decreased the accessibility of organosulfurs. Thus, alternative sulfur surrogates instead of thiols have been developed so far for synthesizing highly functionalized organosulfurs.5 In this study, considering that direct transformations of thioesters as sulfur surrogates can contribute to preparing diverse organosulfurs, we paid attention to developing a new method for synthesizing sulfinic acid esters by direct oxidation of thioesters (Fig. 1A, bottom).
 | ||
| Fig. 1 (A) Oxidation methods of thiols and thioesters. (B) Transformations of sulfinate esters. (C) This work. | ||
Thioesters have served as protected thiols in the preparation of organosulfurs, where electron-withdrawing acyl groups decrease the nucleophilicity and oxidizability at the sulfur atom.6 The good stability and accessibility are clear benefits of thioesters as sulfur surrogates in organosulfur synthesis.7,8 However, the direct oxidation of thioesters is challenging due to their reduced oxidizability and possible overoxidation to organosulfur(VI) compounds.8b,9,10 Since transformations of sulfinic acid esters allowed us to synthesize highly functionalized sulfoxides and sulfides in our recent studies (Fig. 1B),11–13 we started to develop an efficient method to prepare sulfinic acid esters through the direct oxidation of thioesters. Herein, we disclose an efficient method to prepare sulfinic acid esters from aryl iodides via copper-catalyzed C–S formation followed by direct oxidation of thioesters (Fig. 1C).
We first attempted the synthesis of methyl sulfinate 3a having a tetrasubstituted benzene ring through the oxidation of thiol 2a in a four-step procedure (Fig. 2A). Indeed, diazotization of aniline 1a, C–S formation with potassium ethyl xanthate,14 hydrolysis, and oxidation of the resulting thiol 2a with N-bromosuccinimide (NBS) in methanol afforded sulfinate ester 3a only in 1% yield. This result obviously indicates that the difficulty in thiol synthesis led to the poor yield, since the preparation of multi-substituted thiols is not always easy.5 To develop an efficient method to prepare sulfinate esters, we then evaluated frontier orbital energies of thiols 2b and 2c, thioesters 4b and 4c, and sulfinate esters 3b and 3c having a methoxy or a chloro group by DFT calculations (Fig. 2B). These results support the possibility of direct oxidation of thioesters leading to sulfinate esters without overoxidation, since HOMO energies of thioesters 4b and 4c are slightly higher than those of sulfinate esters 3b and 3c, although oxidation of thioesters 3b and 3c will require higher energies than that of thiols 2b and 2c. In addition, the electron-donating methoxy group and electron-deficient chloro group significantly affect the HOMO energies. Thus, direct oxidation of thioesters providing sulfinate esters without overoxidation seems to be a challenging issue.
 | ||
| Fig. 2 (A) An attempt to synthesize 3a. (B) DFT calculations of 2b, 2c, 4b, 4c, 3b, and 3c (B3LYP/6-311+G(d,p)). See details in the ESI.† (C) Synthesis of 4b and 4c. 1,10-phen = 1,10-phenanthroline. | ||
Synthesis of methyl 4-methoxybenzenesulfinate (3b) and methyl 4-chlorobenzenesulfinate (3c) was accomplished from 4-methoxyphenyl iodide (5b) and 4-chlorophenyl iodide (5c), respectively, with thiobenzoic acid (6) catalyzed by copper under the mild conditions established by Sawada and co-workers8a (Fig. 2C). When the thiolation of 4-methoxyphenyl bromide or chloride was attempted, starting materials were recovered, which shows that diverse bromo- or chloro-substituted thioesters can be synthesized by copper-catalyzed thiolation. After screening the reaction conditions, we succeeded in the direct oxidation of S-(4-methoxyphenyl) benzothioate (4b) with NBS to provide sulfinate ester 3b efficiently (Table 1, entry 1). To our surprise, sulfinate ester 3b was not obtained when using NCS9c or NIS instead of NBS (entries 2 and 3). While treatment of thioester 4b with NBA, NBP, or NBSA also afforded sulfinate ester 3b in good yields, the oxidation of thioester 4b with DBH,2a tetrabutylammonium tribromide, and mCPBA resulted in the synthesis of sulfinate ester 3b in low yields (entries 4–9). In addition, a longer reaction time significantly decreased the yield of 3b due to overoxidation (entry 10). Not only electron-rich thioester 4b but also electron-deficient thioester 4c was successfully oxidized to yield sulfinate ester 3c efficiently. The yield was not decreased in a gram-scale synthesis of 3c, indicating the good scalability of this method. We achieved the facile synthesis of sulfinate ester 3c from thioester 4c with NBS, NBA, NBP, or NBSA (entries 11, 14, 15, and 16). In contrast, only a trace amount of sulfinate ester 3c was obtained when thioester 4c was treated with NCS or NIS (entries 12 and 13). Moreover, the overoxidation of sulfinate ester 3c by elongation of the reaction time slightly lowered the yield (entry 20). These results clearly show that NBS-facilitated oxidation allowed us to prepare sulfinate esters 3b and 3c having electron-donating and -withdrawing functional groups from odorless thioesters 4b and 4c, avoiding overoxidation and without the preparation of thiols.
| Entry | X | Oxidant | Time/h | Yield (%)a |
|---|---|---|---|---|
| a Yields based on 1H NMR analysis. b Isolated yields. c Gram-scale synthesis. See details in the ESI.† | ||||
| 1 | OMe | NBS | 1 | 82b |
| 2 | OMe | NCS | 1 | Trace |
| 3 | OMe | NIS | 1 | Trace |
| 4 | OMe | NBA | 1 | 54 |
| 5 | OMe | NBP | 1 | 67 |
| 6 | OMe | NBSA | 1 | 80 |
| 7 | OMe | DBH | 1 | 2 |
| 8 | OMe | n-Bu4NBr3 | 1 | 13 |
| 9 | OMe | mCPBA | 1 | 3 |
| 10 | OMe | NBS | 18 | 38 |
| 11 | Cl | NBS | 1 | 99b [99]b,c |
| 12 | Cl | NCS | 1 | Trace |
| 13 | Cl | NIS | 1 | Trace |
| 14 | Cl | NBA | 1 | 81 |
| 15 | Cl | NBP | 1 | 94 |
| 16 | Cl | NBSA | 1 | 90 |
| 17 | Cl | DBH | 1 | 58 |
| 18 | Cl | n-Bu4NBr3 | 1 | 20 |
| 19 | Cl | mCPBA | 1 | 0 |
| 20 | Cl | NBS | 18 | 65 |
The 2-step synthesis of sulfinate ester 3c from aryl iodide 5c was accomplished using a single silica-gel column chromatography protocol (Fig. 3A). Since easy removal of the smelly thiobenzoic acid (6) by a basic aqueous workup allowed us to synthesize sulfinate esters without unpleasant smells, the 2-step protocol for synthesizing sulfinate esters from aryl iodides has a practical advantage over the conventional synthetic method using thiols. We also succeeded in sulfinate ester synthesis through Cu-catalyzed C–S formation of aryl iodides with potassium thioacetate, which was reported by Ho and co-workers,8b followed by oxidation with NBS (Fig. 3B). It is worth noting that one-pot synthesis of sulfinate ester 3c from aryl iodide 5c was realized in the 2-step sulfinate ester synthesis using potassium thioacetate. Although both thioesters with benzoyl and acetyl groups could lead to sulfinate esters efficiently, good crystallizability of thioesters with benzoyl groups offers an advantage in purification for large-scale synthesis.
A range of sulfinate esters 9a–9c were successfully synthesized from thioester 4c by oxidation with NBS in the presence of various alcohols (Fig. 3C). For example, ethanol and 2-methoxyethanol participated in sulfinate ester synthesis when using excess alcohol in dichloromethane. Isopropyl 4-chlorobenzenesulfinate (9c) was also prepared using 2-propanol in dichloromethane, in which the yield was improved when 2-propanol was used as a solvent. Unfortunately, the synthesis of tert-butyl 4-chlorobenzenesulfinate (9d) failed due to the low nucleophilicity of bulky tert-butyl alcohol.15
We succeeded in the synthesis of diverse sulfinate esters from aryl iodides by Cu-catalyzed C–S formation and subsequent oxidation (Fig. 3D). Benzenesulfinic acid esters 3d–3l were prepared by the 2-step procedure without damaging methyl, fluoro, bromo, trifluoromethyl, and methoxycarbonyl groups. Of note, we successfully synthesized a wide variety of 2-substituted benzenesulfinate esters 3j–3m from bulky 2-substituted aryl iodides, where selective C–S formation took place at the iodo group in the presence of a bromo group. It is noteworthy that the preparation of methyl 2-bromothiophene-2-sulfinate (3n) was achieved from 2-iodothiophene by the catalytic C–S formation and following oxidation with NBS. Unfortunately, synthesis of methyl 3-pyridylsulfinate was unsuccessful due to the rapid decomposition after the oxidation step.16 Additionally, we accomplished the synthesis of S-alkyl-substituted sulfinate ester 12 from alkyl iodide 10 through a nucleophilic substitution reaction with thiourea and benzoic anhydride, followed by oxidation (Fig. 3E). These results obviously indicate that direct oxidation of thioesters will help in preparing highly functionalized sulfinate esters by virtue of the accessibility and good stability of thioesters.
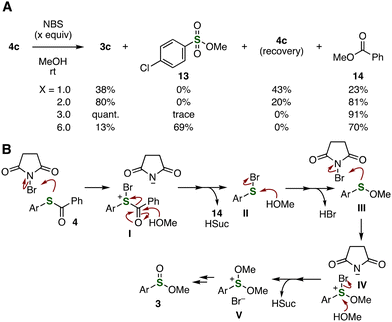
We then conducted control experiments to gain insight into the reaction mechanism of the oxidation of thioesters (Fig. 4A). When thioester 4c was treated with an equimolar amount of NBS in methanol at room temperature, sulfinate ester 3c was obtained in a moderate yield. Increasing the amount of NBS to 2.0 or 3.0 equivalents significantly improved the yields of sulfinate ester 3c, in which sulfinate ester 3c was also synthesized efficiently when using 2.0 equivalents of NBS. The oxidation of thioester 4c with 6.0 equivalents of NBS resulted in overoxidation of sulfinate ester 3c to afford sulfonate ester 13 in a high yield. In these reactions, methyl benzoate (14) was obtained as a side product. These results show that the oxidation involves reaction steps consuming two molar amounts of NBS.
On the basis of these control experiments, we proposed a reaction mechanism as shown in Fig. 4B, although oxidation mechanisms involving single-electron transfer cannot be excluded.9 First, direct oxidation of thioester 4 with NBS would take place smoothly. Benzoylation of methanol with intermediate I to furnish methyl benzoate can lead to sulfenyl bromide II, which would undergo methanolysis to generate sulfenyl ester III. Then, the second oxidation of the resulting sulfenyl ester III would proceed, followed by further methanolysis to provide sulfinate ester 3. According to the control experiments using an equimolar amount of NBS, the second oxidation will occur faster than the first oxidation of thioester 4.
Good accessibility of aryl iodides prompted us to synthesize sulfinate esters from a variety of starting materials (Fig. 5A and B). For example, sulfinate ester 3b was easily prepared from 4-methoxybenzoic acid (15) by decarboxylative iodination,17 Cu-catalyzed C–S formation, and oxidation with NBS in methanol without isolation of intermediates (Fig. 5A). We also succeeded in the synthesis of sulfinate esters 3a and 3o–3q from anilines via diazotization, denitrogenative iodination,18 C–S formation, and oxidation. Since 2-bromoanilines were easily synthesized from the corresponding anilines by electrophilic bromination, these results indicate a synthetic benefit of this newly developed method. It is worth noting that bulky methyl 2-bromo-4,6-dimethylbenzenesulfinate (3a) was prepared from 2-bromo-4,6-dimethylaniline through 2-bromo-4,6-dimethylphenyl iodide, clearly showing a great advantage over conventional methods using thiols because only 1% 3a was synthesized in only 1% from aniline 1a through thiol 2a (Fig. 2A). Compared to conventional preparation methods of sulfinate esters from other sulfur surrogates such as disulfides,19 superior accessibility of aryl iodides from broad aromatic compounds by not only aromatic electrophilic iodination but also decarboxylative and denitrogenative methods will enable us to synthesize highly functionalized sulfinate esters.
 | ||
| Fig. 5 (A) Sulfinate synthesis from 15. (B) Sulfinate synthesis from anilines. (C) Transformations of 3i. | ||
A wide range of organosulfurs such as sulfoxides having functionalities will be synthesized through the sulfinate ester synthesis developed in this study. For instance, we achieved S-arylation of sulfinate ester 3i with 4-methoxyphenylboronic acid catalyzed by palladium (Fig. 5C, top).13h Treatment of sulfinate ester 3i with triflic anhydride in the presence of allyl(trimethyl)silane provided allyl aryl sulfoxide 17, leaving the methoxycarbonyl group untouched (Fig. 5C, bottom).13f
In summary, we have developed a facile synthetic method to synthesize sulfinate esters from aryl iodides. It is worth noting that thioesters were efficiently oxidized, albeit in the presence of an electron-withdrawing carbonyl group on the sulfur atom, to afford sulfinate esters without overoxidation. A wide variety of sulfinate esters were successfully prepared from aryl iodides by the Cu-catalyzed C–S formation and the direct oxidation of the resulting thioesters. Easy removal of thiobenzoic acid by a basic aqueous workup allowed us to synthesize sulfinate esters without unpleasant smells. Due to the good accessibility of aryl iodides17,18,20 and the diverse synthetic applications of sulfinate esters, the sulfinate ester synthesis developed in this study will be applicable in broad disciplines using organosulfurs. The good accessibility of diverse aryl iodides will be a clear advantage over the conventional sulfinate ester synthesis from thiols. Further studies such as applications in the preparation of bioactive organosulfur derivatives and synthesis of bis-sulfinate esters are underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Central Glass Co., Ltd for providing Tf2O. This work was supported by the JSPS KAKENHI Grant Number JP22H02086 (S.Y.); the Uehara Memorial Foundation (S.Y.); the Tokuyama Science Foundation (S.Y.); the Ube Foundation (S.Y.); and the Inamori Research Grants (S.Y.).References
- (a) D. Schilter, Nat. Rev. Chem., 2017, 1, 0013 CrossRef; (b) M. Asnaashariisfahani, B. Azizi, M. R. P. Heravi, E. Mohammadi, S. Arshadi and E. Vessally, RSC Adv., 2022, 12, 14521 RSC.
- (a) A. Khazaei, M. A. Zolfigol and A. Rostami, Synthesis, 2004, 2959 CrossRef CAS; (b) J. L. G. Ruano, A. Parra and J. Alemán, Green Chem., 2008, 10, 706 RSC; (c) V. Rathore, A. Upadhyay and S. Kumar, Org. Lett., 2018, 20, 6274 CrossRef CAS PubMed; (d) M. Oka, R. Kozako and H. Iida, Synlett, 2021, 32, 1227 CrossRef CAS.
- (a) P. K. Shyam, Y. K. Kim, C. Lee and H.-Y. Jang, Adv. Synth. Catal., 2016, 358, 56 CrossRef CAS; (b) C. Zhou, Z. Tan, H. Jiang and M. Zhang, Green Chem., 2018, 20, 1992 RSC; (c) C. Ai, H. Shen, D. Song, Y. Li, X. Yi, Z. Wang, F. Ling and W. Zhong, Green Chem., 2019, 21, 5528 RSC; (d) H. Zhou, J. Duan, D. Xie, J. Yang, B. Ma, G. Wang, C. Wu and X.-C. Wang, Synthesis, 2020, 52, 2705 CrossRef CAS; (e) C.-H. Yang, C. Wu, J.-M. Zhang, X.-Z. Tao, J. Xu, J.-J. Dai and H.-J. Xu, Curr. Org. Synth., 2020, 17, 540 CrossRef CAS PubMed; (f) L.-A. T. Nguyen, T.-N. Le, C.-T. Duong, C.-T. Vo, F. Duus and T. X. T. Luu, J. Sulfur Chem., 2021, 42, 519 CrossRef CAS.
- C. Silva-Cuevas, C. Perez-Arrieta, L. A. Polindara-Garcia and J. A. Lujan-Montelongo, Tetrahedron Lett., 2017, 58, 2244 CrossRef CAS.
- (a) H. Liu and X. Jiang, Chem. – Asian J., 2013, 8, 2546 CrossRef CAS PubMed; (b) N. Sundaravelu, S. Sangeetha and G. Sekar, Org. Biomol. Chem., 2021, 19, 1459 RSC; (c) M. Wang and X. Jiang, ACS Sustainable Chem. Eng., 2022, 10, 671 CrossRef CAS.
- P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley, Hoboken, New Jersey, 5th Ed., 2014, pp 881–883 Search PubMed.
- X. Wang and Z.-B. Dong, Eur. J. Org. Chem., 2022, e202200452 CAS.
- (a) N. Sawada, T. Itoh and N. Yasuda, Tetrahedron Lett., 2006, 47, 6595 CrossRef CAS; (b) D. K. H. Ho, L. Chan, A. Hooper and P. E. Brennan, Tetrahedron Lett., 2011, 52, 820 CrossRef CAS.
- (a) H. Minato, H. Kodama, T. Miura and M. Kobayashi, Chem. Lett., 1977, 413 CrossRef CAS; (b) H. Minato, K. Takeda, T. Miura and M. Kobayashi, Chem. Lett., 1977, 1095 CrossRef CAS; (c) A. Nishiguchi, K. Maeda and S. Miki, Synthesis, 2006, 4131 CrossRef CAS; (d) Y. Venkatesh, K. S. Kiran, S. S. Shah, A. Chaudhuri, S. Dey and N. D. P. Singh, Org. Biomol. Chem., 2019, 17, 2640 RSC; (e) S. Park, S. Y. Kim, J. Cho, D. Jung, J. Ha, D. Seo, J. Lee, S. Lee, S. Yun, H. Lee, O. Park, B. Seo, S. Kim, M. Seol, J. Song and T. K. Park, Bioconjugate Chem., 2020, 31, 1392 CrossRef CAS PubMed.
- (a) D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701 CrossRef CAS PubMed; (b) R. Fan, C. Tan, Y. Liu, Y. Wei, X. Zhao, X. Liu, J. Tan and H. Yoshida, Chin. Chem. Lett., 2021, 32, 299 CrossRef CAS; (c) H. Yorimitsu, Chem. Rec., 2021, 21, 3356 CrossRef CAS PubMed.
- (a) K. K. Andersen, Tetrahedron Lett., 1962, 3, 93 CrossRef; (b) K. K. Andersen, W. Gaffield, N. E. Papanikolaou, J. W. Foley and R. I. Perkins, J. Am. Chem. Soc., 1964, 86, 5637 CrossRef CAS; (c) K. K. Andersen, B. Bujnicki, J. Drabowicz, M. Mikolajczyk and J. B. O'Brien, J. Org. Chem., 1984, 49, 4070 CrossRef CAS; (d) G. Solladie, J. Hutt and A. Girardin, Synthesis, 1987, 173 CrossRef CAS; (e) X. Zhang, E. C. X. Ang, Z. Yang, C. W. Kee and C.-H. Tan, Nature, 2022, 604, 298 CrossRef CAS PubMed.
- (a) B. Z. Lu, F. Jin, Y. Zhang, X. Wu, S. A. Wald and C. H. Senanayake, Org. Lett., 2005, 7, 1465 CrossRef CAS PubMed; (b) G. Maitro, S. Vogel, G. Prestat, D. Madec and G. Poli, Org. Lett., 2006, 8, 5951 CrossRef CAS PubMed; (c) G. Maitro, G. Prestat, D. Madec and G. Poli, J. Org. Chem., 2006, 71, 7449 CrossRef CAS PubMed; (d) G. Signore, S. Samaritani, C. Malanga and R. Menicagli, Synthesis, 2006, 762 CAS; (e) F. Foucoin, C. Caupène, F.-F. Lohier, J. S. de Oliveira Santos, S. Perrio and P. Metzner, Synthesis, 2007, 1315 CAS; (f) J. Wei and Z. Sun, Org. Lett., 2015, 17, 5396 CrossRef CAS PubMed; (g) D. C. Lenstra, V. Vedovato, E. F. Flegeau, J. Maydom and M. C. Willis, Org. Lett., 2016, 18, 2086 CrossRef CAS PubMed; (h) T. Jia, M. Zhang, S. P. McCollom, A. Bellomo, S. Montel, J. Mao, S. D. Dreher, C. J. Welch, E. L. Regalado, R. T. Williamson, B. C. Manor, N. C. Tomson and P. J. Walsh, J. Am. Chem. Soc., 2017, 139, 8337 CrossRef CAS PubMed; (i) L. Wang, M. Chen, P. Zhang, W. Li and J. Zhang, J. Am. Chem. Soc., 2018, 140, 3467 CrossRef CAS PubMed; (j) D. Fu, J. Dong, H. Du and J. Xu, J. Org. Chem., 2020, 85, 2752 CrossRef CAS PubMed.
- For recent transformations of sulfinate esters, see: (a) F. Yuste, A. H. Linares, V. M. Mastranzo, B. Ortiz, R. Sánchez-Obregón, A. Fraile and J. L. G. Ruano, J. Org. Chem., 2011, 76, 4635 CrossRef CAS PubMed; (b) J. A. Lujan-Montelongo, A. O. Estevez and F. F. Fleming, Eur. J. Org. Chem., 2015, 1602 CrossRef CAS PubMed; (c) A. Mohd, T. Anitha, K. R. Reddy, J. Wencel-Delord and F. Colobert, Eur. J. Org. Chem., 2019, 7836 CrossRef CAS; (d) G.-J. Li, Y.-L. Pan, Y.-L. Liu, H.-F. Xu and J.-Z. Chen, Tetrahedron Lett., 2019, 60, 151260 CrossRef CAS; (e) L. Chen, J. Zhang, Y. Wei, Z. Yang, P. Liu, J. Zhang and B. Dai, Tetrahedron, 2019, 75, 130664 CrossRef; (f) A. Kobayashi, T. Matsuzawa, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 5429 RSC; (g) A. Kobayashi, T. Matsuzawa, T. Hosoya and S. Yoshida, Chem. Lett., 2020, 49, 813 CrossRef CAS; (h) M. Suzuki, K. Kanemoto, Y. Nakamura, T. Hosoya and S. Yoshida, Org. Lett., 2021, 23, 3793 CrossRef CAS PubMed.
- E. Campaigne and S. Osborn, J. Org. Chem., 1957, 22, 561 CrossRef CAS.
- When using benzyl alcohol in the reaction of 4c, benzyl 4-chlorobenzenesulfinate was not obtained.
- We succeeded in the synthesis of S-(3-pyridyl) benzothioate from 3-pyridyl iodide in 65% yield. Although the oxidation of S-(3-pyridyl) benzothioate with NBS took place smoothly, rapid decomposition of the product obtained by silica-gel column chromatography in storage under an argon atmosphere was observed, affording structurally undetermined insoluble solids.
- G. J. P. Perry, J. M. Quibell, A. Panigrahi and I. Larrosa, J. Am. Chem. Soc., 2017, 139, 11527 CrossRef CAS PubMed.
- H. Heaney and I. T. Millar, Org. Synth., 1960, 40, 105 CrossRef CAS.
- (a) P. Brownbridge and I. C. Jowett, Synthesis, 1988, 252 CrossRef CAS; (b) M. Xia and Z.-C. Chen, Synth. Commun., 1997, 27, 1321 CrossRef CAS; (c) B. Du, Z. Li, P. Qian, J. Han and Y. Pan, Chem. - Asian J., 2016, 11, 478 CrossRef CAS PubMed.
- C.-p. Dong, K. Nakamura, T. Taniguchi, S. Mita, S. Kodama, S. Kawaguchi, A. Nomoto, A. Ogawa and T. Mizuno, ACS Omega, 2018, 3, 9814 CrossRef CAS PubMed and references cited therein.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of new compounds including NMR spectra. See DOI: https://doi.org/10.1039/d3ob01108a |
| This journal is © The Royal Society of Chemistry 2023 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NBS (5.0 equiv.) was used in the oxidation of 8.
NBS (5.0 equiv.) was used in the oxidation of 8.