Base-promoted Conia-ene cyclization of propargyl amides†
Abstract
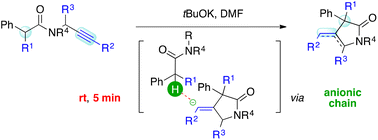
We report a tBuOK-promoted synthesis of 1,3-dihydro-2H-pyrrol-2-one and 4-methylenepyrrolidin-2-one systems via Conia-ene like intramolecular cyclization. The method features extremely short reaction times (5 min) and mild reaction conditions (rt), enabling the trapping of a propargyl unit by an amide enolate. An intriguing anionic chain mechanism is at work, which can trigger the isomerization of an exo-alkene giving access to the otherwise elusive endo-product.


 Please wait while we load your content...
Please wait while we load your content...