Open Access Article
Open Access ArticleLarge anion binding in water
Khaleel I.
Assaf
 *ab and
Werner M.
Nau
*ab and
Werner M.
Nau
 *a
*a
aConstructor University, School of Science, Campus Ring 1, 28759 Bremen, Germany. E-mail: wnau@constructor.university
bDepartment of Chemistry, Faculty of Science, Al-Balqa Applied University, 19117 Al-Salt, Jordan. E-mail: khaleel.assaf@bau.edu.jo
First published on 7th August 2023
Abstract
Large water-soluble anions with chaotropic character display surprisingly strong supramolecular interactions in water, for example, with macrocyclic receptors, polymers, biomembranes, and other hydrophobic cavities and interfaces. The high affinity is traced back to a hitherto underestimated driving force, the chaotropic effect, which is orthogonal to the common hydrophobic effect. This review focuses on the binding of large anions with water-soluble macrocyclic hosts, including cyclodextrins, cucurbiturils, bambusurils, biotinurils, and other organic receptors. The high affinity of large anions to molecular receptors has been implemented in several lines of new applications, which are highlighted herein.
Introduction
The binding of “mineral water” anions such as F−, Cl−, CO32−, SO42−, PO43−, and NO3− in water has been in the focus of anion binding by supramolecular receptors, among others, due to their biological and environmental importance. These common anions are strongly solvated in aqueous solution, which presents a challenge for the supramolecular design of the associated receptors.1–4 In contrast, the binding of large anions such as I−, SCN−, ClO4−, and PF6−, which have a lower charge density and are weakly hydrated, has received comparably less attention, among others, due to the fact that they play, with the notable exception of I−, less critical natural roles.5–9 On the other hand, the diametrically opposed effects that the two groups of anions can exert on biological systems have been recognized since long by the Hofmeister series, which conventionally classifies the character of ions as being salting-out (kosmotropic) or salting-in (chaotropic) in nature.10 Recently, the supramolecular chemistry of long-known synthetic cluster anions of the borate-11,12 and polyoxometalate-type13 has moved into the focus, driven by the observation that their affinity with hydrophobic binding sites does exceed,14 against expectation, those of hydrophobic guest molecules.15–17 These large anions were recognized to expand the traditional Hofmeister series as so-called superchaotropic anions,14,18–34 and their peculiar supramolecular chemistry has revived the interest in large anion binding in water.14,25,26,35–49 The present review provides a short historical background and a summary of the most recent developments in this emerging field. Amphiphilic anions, which are composed of long hydrophobic chains and kosmotropic head groups, such as sodium dodecyl sulphate, are also large anions in the metric sense, but display entirely different properties and are not the subject of this review. Also excluded from further discussion are hydrophobic anions, such as Ph4B−, which in contrast to superchaotropic anions display positive hydration free energies.18Historical developments in large anion binding and initial empirical observations (up to 2015)
Cyclodextrins17,50,51 (CDs, Fig. 1) are the oldest and most intensively studied class of macrocyclic hosts, and they are also the first ones that were found to display sizable affinity towards large anions, despite their known propensity to preferentially complex hydrophobic guests inside their cavity.5–8,52–54 The first qualitative observation for the binding of a large anion, namely I−, by α-CD was made by Schlenk and Sand 60 years back.5 Soon after that, the first binding constant (Ka) between ClO4− and α-CD was determined by Cramer et al., who found that this large anion competed with the binding of 4-nitrophenolate.55 This binding could be readily followed by optical titration through the color change of the organic guest, incidentally also setting up the putative first example for an indicator displacement assay in host–guest chemistry. The binding was sizable for an inorganic anion (Ka = 29 M−1), but insufficiently high to draw attention at that time. Cramer also established the binding of NO3− with α-CD, while no binding was observed for SO42− and PO43−.55 Several subsequent studies showed that α-CD also binds many other anions, such as PF6−, I3−, and SCN−, with a sizable binding affinity,56–59 while for anions such as F−, Cl−, and Br−, the affinity was very low, if at all detectable.60,61 In addition, the next larger homologous host, β-CD, also displayed a sizable but lower affinity to large anions.6,8 The study by Wojcik and Rohrbach from 1975 reported a range of 10–29 M−1 for the binding of SCN−, I−, and ClO4− to α-CD. Matsui et al. used NMR spectroscopy to study the complexation-induced chemical shifts of the CD protons (including α-, β-, and γ-CD) for different anions,52 which again revealed a preferential binding of certain anions (N3−, I−, SCN−, and ClO4−), with the most stable inclusion complexes being observed with α-CD. In contrast, anions such as F−, HCO3−, H2PO4−, HPO42−, and SO42− showed no or negligible changes in the chemical shifts. Similarly, Taraszewska and Wójcik studied systematically the complexation of inorganic anions with β-CD.8 Although the reported association constants of the anions were lower (<9 M−1) than for α-CD (up to 29 M−1), the weakly hydrated anions showed stronger binding than strongly hydrated ones (such as PO43−), Table 1.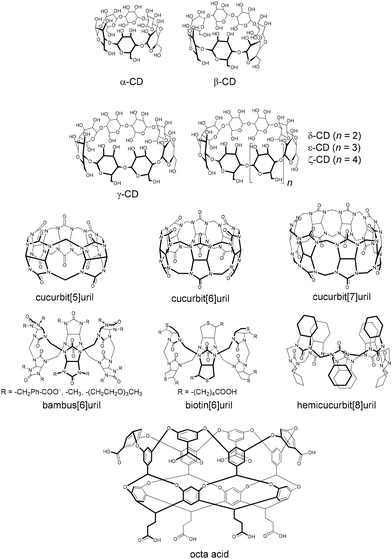 | ||
| Fig. 1 Chemical structures of macrocyclic receptors with sizable affinity to large anions in aqueous solution. | ||
| Host | Anion | Radiusa/pm | K a × 103/M−1 | Techniqueb | Yearc | Ref. |
|---|---|---|---|---|---|---|
a Taken from ref. 106 and 107.
b The applied techniques for the determination of association constants are abbreviated as follows: cond. stands for conductometric titration; NMR stands for 1H NMR titration; calor. stands for calorimetry titration; optical stands for competitive optical titration (UV-Vis or fluorescence spectroscopy); electr. stands for electrochemical (potentiometric) titration; and ITC stands for isothermal titration calorimetry.
c Year refers to the first report.
d Given as an averaged value from different reports.
e Approximate values based on the calculated molecular volume using HyperChem(TM) Professional 8.0 software,108 with the assumption of a spherical shape from which the radius was obtained.
f From ref. 18.
g Calculated from the diameters obtained from XRD structures.
h Very weak binding; Ka was not determined.
i Hexakis(6-deoxy-6-amino-)-α-CD.
j Mono[6-(1-pyridinio)-6-deoxy]-α-CD.
k Values correspond to the cluster core; calculated from the diameters obtained from the geometry-optimized structures.
l Mono[6-(1-pyridinio)-6-deoxy]-β-CD.
m Heptakis(6-deoxy-6-amino-)-β-CD.
n 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complexation was reported.
o Mono[6-(1-pyridinio)-6-deoxy]-γ-CD.
p Octakis(6-deoxy-6-amino)-γ-CD.
q Functionalized bambusuril with R = –CH2Ph-COO−, see Fig. 1.
r Functionalized bambusuril with R = –(CH2CH2O)3CH3, see Fig. 1. 2 host–guest complexation was reported.
o Mono[6-(1-pyridinio)-6-deoxy]-γ-CD.
p Octakis(6-deoxy-6-amino)-γ-CD.
q Functionalized bambusuril with R = –CH2Ph-COO−, see Fig. 1.
r Functionalized bambusuril with R = –(CH2CH2O)3CH3, see Fig. 1.
|
||||||
| α-CDj | Br− | 196 | 0.01 | NMR | 1993 | 98 |
| α-CDj | NO3− | 200 | 0.02 | NMR | 1993 | 98 |
| α-CD | SCN− | 213 | 0.03d | NMR, cond., calor. | 1975 | 7, 52, 53, 56 and 58 |
| α-CDj | SCN− | 213 | 0.36 | NMR | 1993 | 98 |
| α-CD | I− | 220 | 0.02d | NMR, cond., calor. | 1967 | 7, 52, 53, 55, 56, 58 and 60 |
| α-CDj | I− | 220 | 0.20 | NMR | 1993 | 98 |
| α-CD | ClO4− | 240 | 0.04d | NMR, cond., calor. | 1967 | 7, 52, 53, 55–58, 61 and 82 |
| α-CDj | ClO4− | 240 | 0.33 | NMR | 1993 | 98 |
| α-CD | CF3SO3− | 255e | 0.04 | Calor. and optical | 1996 | 57 |
| α-CD | I3− | 285 | 270d | Electr. | 1947 | 64 and 99–101 |
| α-CD | PF6− | 295 | 0.04 | Calor. and optical | 1996 | 57 |
| α-CDi | Fe(CN)64− | 343 | 300 | Electr. | 1996 | 102 |
| α-CDi | Fe(CN)63− | 380 | 2 | Electr. | 1996 | 102 |
| α-CD | B10H102− | 393f | 0.04 | NMR | 2019 | 37 |
| α-CD | B12H122− | 400f | 0.1 | NMR | 2015 | 14 |
| α-CD | Re6S8(CN)64− | 580g | 0.73 | ITC | 2019 | 81 |
| α-CD | Re6Se8(CN)64− | 590g | 0.03 | ITC | 2019 | 81 |
| α-CD | Re6Te8(CN)64− | 600g | —h | ITC | 2019 | 81 |
| β-CD | SCN− | 213 | 0.01d | Cond., NMR, calor., | 1975 | 6–8, 52, 56 and 58 |
| β-CD | I− | 220 | 0.01d | Cond., NMR, optical | 1977 | 7, 52, 56 and 103 |
| β-CDl | I− | 220 | 0.04 | NMR | 1993 | 98 |
| β-CD | ClO4− | 240 | 0.01d | NMR, calor., optical | 1977 | 6–8, 52, 56, 57 and 103 |
| β-CD | CF3SO3− | 255 | 0.06 | Calor. | 1996 | 57 |
| β-CD | I3− | 285 | 3.03 | Electr. | 1947 | 99, 100 and 64 |
| β-CD | PF6− | 295 | 0.09 | Calor. | 1996 | 57 |
| β-CDm | Fe(CN)64− | 343 | 800 | Electr. | 1996 | 102 |
| β-CDm | Fe(CN)63− | 380 | 4 | Electr. | 1996 | 102 |
| β-CD | B10H102− | 393f | 0.11 | NMR | 2019 | 37 |
| β-CD | B12H122− | 400f | 0.2 | NMR | 2015 | 14 |
| β-CD | Ph4B− | 425e | 0.01 | Electrophoresis | 2001 | 104 |
| β-CD | B21H18− | 425k | 130 | ITC | 2020 | 85 |
| β-CD | Re6S8(CN)64− | 580g | 0.88 | ITC | 2019 | 81 |
| β-CD | COSAN-1 | 583k | 26 | ITC | 2019 | 35 |
| β-CD | COSAN-2 | 583k | 710 | ITC | 2019 | 35 |
| β-CD | COSAN-3 | 583k | 21 | ITC | 2019 | 35 |
| β-CD | COSAN-4 | 583k | 19 | ITC | 2019 | 35 |
| β-CD | COSAN-5 | 583k | 24 | ITC | 2019 | 35 |
| β-CD | COSAN-6 | 583k | 38 | ITC | 2019 | 35 |
| β-CD | COSAN-7 | 583k | 180 | ITC | 2019 | 35 |
| β-CD | COSAN-8 | 583k | 55 | ITC | 2019 | 35 |
| β-CD | COSAN-9 | 583k | 59 | ITC | 2019 | 35 |
| β-CD | Re6Se8(CN)64− | 590g | 0.75 | ITC | 2019 | 81 |
| β-CD | Re6Te8(CN)64− | 600g | 0.08 | ITC | 2019 | 81 |
| β-CD | PMO12O403− | 692f | 2 | ITC | 2015 | 46 |
| γ-CD | SCN− | 213 | 4.1 | NMR | 1997 | 52 |
| γ-CD | I− | 220 | 4.9 | NMR | 1997 | 52 |
| γ-CDo | I− | 220 | 0.02 | NMR | 1993 | 98 |
| γ-CD | I3− | 285 | 0.5 | Electr. | 1985 | 64 |
| γ-CDp | Fe(CN)64− | 343 | 1000 | Electr. | 1996 | 102 |
| γ-CDp | Fe(CN)63− | 380 | 5 | Electr. | 1996 | 102 |
| γ-CD | B10H102− | 393f | 0.06 | NMR | 2019 | 37 |
| γ-CD | B12H12S2− | 400f | 9.2 | ITC | 2015 | 14 |
| γ-CD | Ph4B− | 425e | 108 | Electrophoresis | 2001 | 104 |
| γ-CD | B21H18− | 425k | 1800 | ITC | 2020 | 85 |
| γ-CD | H3NB10Cl9− | 500k | 5.9 | ITC | 2019 | 36 |
| γ-CD | Me3NB10Cl9− | 500k | 77 | ITC | 2019 | 36 |
| γ-CD | B12Cl122− | 525 | 17 | ITC | 2015 | 14 |
| γ-CD | H3NB10Br9− | 530k | 2.4 | ITC | 2019 | 36 |
| γ-CD | Mr3NB10Br9− | 530k | 430 | ITC | 2019 | 36 |
| γ-CD | Me3NB10I9− | 558k | 78 | ITC | 2019 | 36 |
| γ-CD | H3NB10I9− | 558k | 3.8 | ITC | 2019 | 36 |
| γ-CD | B12Br122− | 560f | 960 | ITC | 2015 | 14 |
| γ-CD | Re6S8(CN)64− | 580g | 0.9 | ITC | 2018 | 83 |
| γ-CD | COSAN-1 | 583k | 191 | ITC | 2019 | 35 |
| γ-CD | COSAN-2 | 583k | 3000 | ITC | 2019 | 35 |
| γ-CD | COSAN-3 | 583k | 300 | ITC | 2019 | 35 |
| γ-CD | COSAN-4 | 583k | 3600, 90n | ITC | 2019 | 35 |
| γ-CD | COSAN-5 | 583k | 1300 | ITC | 2019 | 35 |
| γ-CD | COSAN-6 | 583k | 6, 8000n | ITC | 2019 | 35 |
| γ-CD | COSAN-7 | 583k | 72 | ITC | 2019 | 35 |
| γ-CD | COSAN-8 | 583k | 12 | ITC | 2019 | 35 |
| γ-CD | COSAN-9 | 583k | 720 | ITC | 2019 | 35 |
| γ-CD | H2W12O406− | 590g | 0.02 | NMR | 2021 | 93 |
| γ-CD | BW12O405− | 590g | 1.03 | ITC | 2021 | 93 |
| γ-CD | SiW12O404− | 590g | 17.2, 0.56n | ITC | 2021 | 93 |
| γ-CD | PW12O403− | 590g | 2.92 | ITC | 2021 | 93 |
| γ-CD | SiW11MoO404− | 590g | 17.7, 0.371n | ITC | 2021 | 94 |
| γ-CD | PW11VO404− | 590g | 8.5, 0.22n | ITC | 2021 | 94 |
| γ-CD | PW11VO405− | 590g | 0.164 | ITC | 2021 | 94 |
| γ-CD | SiW11MoO405− | 590g | 0.478 | ITC | 2021 | 94 |
| γ-CD | SiW11VO405− | 590g | 0.618 | ITC | 2021 | 94 |
| γ-CD | SiW11VO406− | 590g | —h | ITC | 2021 | 94 |
| γ-CD | SiW11MoO406− | 590g | —h | ITC | 2021 | 94 |
| γ-CD | Re6Se8(CN)64− | 590g | 1.5, 13n | ITC | 2018 | 83 |
| γ-CD | B12I122− | 590f | 67 | ITC | 2015 | 14 |
| γ-CD | Re6Te8(CN)64− | 600g | 38, 13n | ITC | 2018 | 83 |
| γ-CD | PMO12O403− | 692f | 40, 1n | ITC | 2015 | 46 |
| γ-CD | P2W18O626− | 701f | 3.2, 0.37n | ITC | 2017 | 25 |
| δ-CD | B12Cl122− | 525f | 2500 | ITC | 2016 | 44 |
| δ-CD | B12Br122− | 560f | 2600 | ITC | 2016 | 44 |
| δ-CD | B12I122− | 590f | 67 | ITC | 2016 | 44 |
| ε-CD | B12Cl122− | 525f | 29 | ITC | 2016 | 44 |
| ε-CD | B12Br122− | 560f | 140 | ITC | 2016 | 44 |
| ε-CD | B12I122− | 590f | 2100 | ITC | 2016 | 44 |
| ζ-CD | B12Cl122− | 525f | 2 | ITC | 2016 | 44 |
| ζ-CD | B12Br122− | 560f | 6 | ITC | 2016 | 44 |
| ζ-CD | B12I122− | 590f | 8 | ITC | 2016 | 44 |
| Bambusurilq | F− | 133 | 0.1 | NMR | 2015 | 45 |
| Bambusurilq | F− | 133 | 0.01 | ITC | 2015 | 45 |
| Bambusurilq | Cl− | 181 | 0.91 | NMR | 2015 | 45 |
| Bambusurilq | Cl− | 181 | 0.4 | ITC | 2015 | 45 |
| Bambusurilq | CN− | 191 | 1.1 | NMR | 2015 | 45 |
| Bambusurilq | Br− | 196 | 140 | NMR | 2015 | 45 |
| Bambusurilq | Br− | 196 | 33 | ITC | 2015 | 45 |
| Bambusurilq | NO3− | 200 | 480 | NMR | 2015 | 45 |
| Bambusurilq | I− | 220 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
NMR | 2015 | 45 |
| Bambusurilq | I− | 220 | 2000 | ITC | 2015 | 45 |
| Bambusurilq | BF4− | 230 | 4300 | NMR | 2015 | 45 |
| Bambusurilq | ClO4− | 240 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
NMR | 2015 | 45 |
| Bambusurilq | IO4− | 250 | 6.5 | NMR | 2015 | 45 |
| Bambusurilq | ReO4− | 260 | 30 | NMR | 2015 | 45 |
| Bambusurilq | PF6− | 295 | 2200 | NMR | 2015 | 45 |
| Bambusurilr | Cl− | 181 | 1.2 | ITC | 2022 | 77 |
| Bambusurilr | CN− | 191 | 21 | NMR | 2022 | 77 |
| Bambusurilr | N3− | 195 | 21 | ITC | 2022 | 77 |
| Bambusurilr | Br− | 196 | 120 | ITC | 2022 | 77 |
| Bambusurilr | NO3− | 200 | 330 | ITC | 2022 | 77 |
| Bambusurilr | SCN− | 213 | 1200 | ITC | 2022 | 77 |
| Bambusurilr | I− | 220 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
ITC | 2022 | 77 |
| Bambusurilr | SeCN− | 229e | 2800 | ITC | 2022 | 77 |
| Bambusurilr | BF4− | 230 | 1300 | ITC | 2022 | 77 |
| Bambusurilr | ClO4− | 240 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
ITC | 2022 | 77 |
| Bambusurilr | IO4− | 250 | 56 | ITC | 2022 | 77 |
| Bambusurilr | ReO4− | 260 | 470 | ITC | 2022 | 77 |
| Bambusurilr | PF6− | 295 | 1600 | ITC | 2022 | 77 |
| Bambusurilr | [Au(CN)2]− | 320 | 1300 | ITC | 2022 | 77 |
| Bambusurilr | [Ag(CN)2]− | 330 | 13 | ITC | 2022 | 77 |
| Biotin[6]uril | Cl− | 181 | 0.06 | NMR | 2015 | 47 |
| Biotin[6]uril | Cl− | 181 | 0.03 | ITC | 2015 | 47 |
| Biotin[6]uril | N3− | 195 | 0.79 | NMR | 2015 | 47 |
| Biotin[6]uril | N3− | 195 | 0.4 | ITC | 2015 | 47 |
| Biotin[6]uril | Br− | 196 | 1 | NMR | 2015 | 47 |
| Biotin[6]uril | Br− | 196 | 0.5 | ITC | 2015 | 47 |
| Biotin[6]uril | NO3− | 200 | 0.08 | NMR | 2015 | 47 |
| Biotin[6]uril | NO3− | 200 | 0.05 | ITC | 2015 | 47 |
| Biotin[6]uril | CNO− | 203 | 0.01 | NMR | 2015 | 47 |
| Biotin[6]uril | CNO− | 203 | 0.06 | ITC | 2015 | 47 |
| Biotin[6]uril | SCN− | 213 | 32 | NMR | 2015 | 47 |
| Biotin[6]uril | SCN− | 213 | 13 | ITC | 2015 | 47 |
| Biotin[6]uril | I− | 220 | 4d | NMR | 2015 | 47 |
| Biotin[6]uril | I− | 220 | 2.5 | ITC | 2015 | 47 |
| Biotin[6]uril | SeCN− | 229e | 20 | NMR | 2015 | 47 |
| Biotin[6]uril | SeCN− | 229e | 10 | ITC | 2015 | 47 |
| Biotin[6]uril | ClO4− | 240 | 0.5 | NMR | 2015 | 47 |
| Biotin[6]uril | ClO4− | 240 | 0.25 | ITC | 2015 | 47 |
| Octa acid | NO3− | 200 | <0.01 | NMR | 2011 | 66 |
| Octa acid | SCN− | 213 | 0.03 | NMR | 2011 | 66 |
| Octa acid | SCN− | 213 | 0.04 | ITC | 2016 | 105 |
| Octa acid | ClO3− | 220e | <0.01 | NMR | 2011 | 66 |
| Octa acid | I− | 220 | 0.01 | NMR | 2011 | 66 |
| Octa acid | I− | 220 | 0.02 | ITC | 2016 | 105 |
| Octa acid | BH3CN− | 225e | 0.15 | ITC | 2016 | 105 |
| Octa acid | ClO4− | 240 | 0.10 | NMR | 2011 | 66 |
| Octa acid | ClO4− | 240 | 0.1 | ITC | 2016 | 105 |
| Octa acid | N(CN)2− | 240e | 0.04 | ITC | 2016 | 105 |
| Octa acid | IO4− | 250 | 0.24 | ITC | 2016 | 105 |
| Octa acid | CF3SO3− | 255e | 0.31 | ITC | 2016 | 105 |
| Octa acid | CH3SO2S− | 260e | 0.66 | ITC | 2016 | 105 |
| Octa acid | ReO4− | 260 | 0.37 | ITC | 2016 | 105 |
| Octa acid | Cl2CHCO2− | 270e | 0.05 | ITC | 2016 | 105 |
| Octa acid | Cl3CCO2− | 283e | 6.34 | ITC | 2016 | 105 |
| Octa acid | PF6− | 295 | 0.79 | ITC | 2016 | 105 |
The original study by Cramer et al. did not allude to the factors responsible for the large anion binding by CDs. Subsequently, through the investigation of a series of different anions, an empirical relationship between their binding affinities and their chaotropic (water-structure breaking) character emerged.8,52,58 Wojcik and Rohrbach related the trend in binding constants of anions to their B− viscosity coefficient,58 a coefficient of the linear term of the concentration-dependence of proton relaxation rates, which has later become an accepted parameter for the water-structural entropic properties (chaotropicity) of ions.62,63 A linear correlation between the binding constants58 of the anions and their B− coefficient was established. Matsui et al. explicitly interpreted the differential binding of anions to CDs in terms of their chaotropic or “anti-chaotropic” character,52 a qualification which has later been replaced by the term “kosmotropic”, but a detailed interpretation in terms of the underlying intermolecular interactions remained elusive. Similarly, Taraszewska and Wójcik studied systematically the complexation of inorganic anions with β-CD8 and related the magnitude of their affinities to their position in the Hofmeister series as well.
The higher binding affinity of the anions to CDs is also related to the size/shape complementarity, which is reflected in the higher affinity of the large anions (I−, SCN−, and ClO4−),52 because only those are able to fill the hydrophobic cavity to a significant extent. The binding constant of I3− decreased from α- to β- to γ-CD.64 Due to the large cavity size of γ-CD, only few examples of the binding of classical anions by this host have been reported.52,64 A summary of anion binding to CDs is shown in Table 1, which clearly indicates that the binding affinity of anions to CDs increases with the chaotropicity/size of the anions. However, even though independent correlations between the binding affinities of inorganic anions with CDs and their chaotropicity became apparent, the unusual affinity pattern received little attention, presumably because the absolute binding affinities were too small to have major practical implications.
While studying Hofmeister effects on the binding of an organic guest (adamantanecarboxylate) with an aromatic host (octa acid, Fig. 1),65,66 it eventually emerged that large anions, e.g. ClO4−, showed also sizable binding to this macrocycle (95 M−1).66,67 The chaotropic anion competed with the binding of adamantanecarboxylate in the same manner as Cramer had previously observed for the 4-nitrophenolate-α-CD couple.55 The binding affinity of adamantane carboxylic acid to octa acid was found to slightly increase in the presence of kosmotropic anions such as F−, an observation which was linked to the enhanced hydrophobic effect caused by the presence of such anions. In contrast, chaotropic anions such as SCN− and ClO4− lowered the binding affinity of adamantane carboxylic acid, which was attributed to the ability of chaotropic anions to weaken the hydrophobic effect and, thereby, the competition with the hydrophobic guest for the hydrophobic cavity of octa acid.66 Although the driving force for ClO4− binding was not discussed, it transpired that the phenomenon of large anion binding to hydrophobic pockets was more general and not limited to CDs.68 One tentative explanation involved the presence of high-energy water inside these hosts, which could account for an “intrinsic” driving force that would drive the encapsulation process regardless of the precise chemical nature or the charge status of a suitably sized spherical guest.69,70 In 2010, upon successful synthesis of a new macrocyclic host derived from a glycoluril derivative through an acid condensation reaction with formaldehyde, Sindelar and co-workers noticed by X-ray diffraction of the obtained hexamer receptor (bambusuril, Fig. 1) that the cavity of this molecular container accommodated a Cl− ion.71 The macrocycle was later intensively investigated for its binding to halides with high affinity and selectivity (see below).38,39,72–77
Recent developments in large anion binding and mechanistic interpretations (after 2015)
The tentative idea that the release of high-energy water from the macrocycles presents the major driving force for the complexation of large anions needed to be abandoned in 2015 when an exceptionally strong binding of several large anions to different macrocycles (Fig. 1) was established by several research groups.14,45–47 The affinities reached micromolar values and rivaled, e.g. for CDs,14 the affinities of hydrophobic guests, which until then had been considered as the tightest binding motifs.15,78,79Besides classical large anions with chaotropic character such as I−, PF6−, and ClO4−, which showed much stronger binding to bambusuril and biotinuril than to CDs,45,47 two classes of very large, highly charged, synthetic anions, namely boron clusters and polyoxometalates (Fig. 2), were found to complex very strongly to γ-CD14,25,26,35–37,40,46,80 as well as to the larger homologues (ε-CD, δ-CD, and ζ-CD,44 see Fig. 1). In the course of two independent investigations, it turned out, by employing classical Hofmeister salting-in14 and cloud point measurements,21,23 that the large cluster anions behave as superchaotropic anions which expand the Hofmeister series to the chaotropic side and which outperform classical chaotropic anions such as ClO4− in regard to their salting-in properties.21
 | ||
| Fig. 2 Molecular structures and size comparison of large (chaotropic) anions with sizable binding affinity to macrocyclic receptors in water. | ||
While it became apparent that the binding strength to the hydrophobic cavity increased with the chaotropicity of the anion (up to the turning point where the anion size largely exceeded the cavity size),14,44,81 the underlying driving forces remained more difficult to pinpoint. A first hint came from the thermochemical signature of the binding process, which showed that the binding of the superchaotropic anions is enthalpically driven.14 In retrospect, one of the first studies that pointed to an enthalpic driving force for the complexation of large inorganic anions by macrocycles was published in 1973, in which the binding of ClO4− to α-CD had shown a favorable enthalpic contribution of −9.7 kcal mol−1 and an unfavorable entropy.82 Similar signatures were reported on several occasions.52,56,57
The effect became very pronounced when large anions were investigated.14,25,44,83 For example, the thermochemical trend was also observed for the complexation of dodecaborate anions with CDs, and it was for the first time linked to their chaotropic character; the generic driving force was accordingly referred to as the chaotropic effect, to differentiate from the entropically driven (classical) hydrophobic effect.14,44 Following the theory of Marcus for the aqueous solvation of ions, the large favorable enthalpy and unfavorable entropy were related to water structure recovery upon the relocation of the anion into the cavity as well as to the dispersion interaction between the highly polarizable guests and the cavity walls. Roughly speaking, the larger and more polarizable an anion, the lower its charge density, and the better delocalized its charge, the higher its chaotropicity (Fig. 2), until a turning point, when an ion adopts more hydrophobic ionic character (such as BPh4−) on a continuous scale of aqueous solvation.18
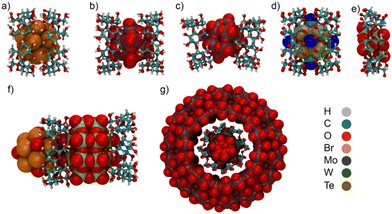
Cyclodextrins as receptors for boron cluster anions
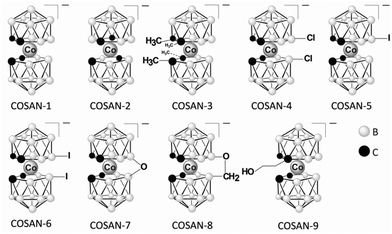
Dodecaborate anions of the type B12X122− (X: H, Cl, Br, and I, Fig. 2) form stable complexes with CDs, in particular with γ-CD, in aqueous solution,14 as reflected in their high binding affinities (e.g., ca. 106 M−1 for B12Br122− with γ-CD, see Table 1 for the binding affinity of other clusters).14 The single-crystal X-ray structure of the B12Br122−/γ-CD complex is shown in Fig. 3a. Although the chaotropic effect has in common with the hydrophobic effect that the involved guests are weakly hydrated, they are conceptually distinct in that chaotropic anions interfere differently with the solvation-shell water structure than the hydrophobic species do. The contrasting solvation behavior is borne out by the diametrically opposed thermodynamic fingerprints of boron cluster anions versus hydrophobic residues.18 Since chaotropic agents are thought to “break” the water structure in the solvation shell (as assessed through large positive water-structural contributions to the hydration entropy), one may argue that their relocation into the hydrophobic cavity is not characterized by the positive entropic dehydration component characteristic of the hydrophobic effect, but rather by a strongly negative enthalpic one. High binding affinities of boron cluster anions have also been obtained for large-ring CDs (δ-, ε-, and ζ-CD).84 For example, the binding affinity of B12Br122− with δ-CD (2.60 × 106versus 0.96 × 106 M−1) even exceeded that with γ-CD, while B12I122− was found to strongly associate with the large ζ-CD cavity, with Ka = 2.1 × 106 M−1, Table 1.44 A weaker binding affinity was obtained for the smaller B10H102− cluster with CDs,37 while the binding affinity of the larger B21H18− cluster to CDs exceeded that of B12Br122−.85 Other boron cluster anions of the type [H3NB10X9]− and [Me3NB10X9]− also showed sizable binding affinity to γ-CD. The complexation of the mono-anionic ammonium-substituted decaborate derivatives with γ-CD was, however, in general weaker than that of the di-anionic dodecaborate clusters (Table 1).36 The variation of binding constants pointed to an interesting structure–activity relationship.Very recently, metallacarboranes, another class of sandwich inorganic boron clusters, have been found to form stable complexes with CDs in aqueous solution.35 Cobalt bis(1,2-dicarbollide) and its derivatives (COSANs, Fig. 4), as mono-anionic clusters, associate strongly with the cavity of β- and γ-CD, Table 1.35 The complexation of COSANs with CDs was investigated by NMR and UV−visible spectroscopy, as well as mass spectrometry, all of which confirmed the strong binding. Isothermal titration calorimetry (ITC) revealed high association constants up to 106 M−1.35 The interaction between COSAN and the glucose moiety has also been studied in the context of biopolymeric interactions,86 and they were found to bind to cationic peptides, which enables their use as membrane carriers.87 Recently, the ability of COSANs to solubilize hydrophobic substances in water has been reported.88 At first sight, this mimics the “salting-in” feature known for chaotropic and superchaotropic ions,14 but the mechanism turned out to be more involved in this case.
Cyclodextrins as receptors for polyoxometalates and related inorganic cluster anions
In 2015, Stoddart and co-workers investigated the complexation of polyoxometalates with CDs.46 The binding of PMO12O403− with β- and γ-CD was established in solution and in the solid state. 1H NMR experiments confirmed the formation of inclusion complexes, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometry with β- and γ-CD, respectively. The association constants were measured by ITC. The obtained binding affinity with β-CD was found to be 2 × 103 M−1, while the two-step sequential binding fitting for the complexation with γ-CD resulted in Ka1 = 4 × 104 and Ka2 = 1 × 103 M−1.46 The crystal structures of the PMO12O403− complexes with γ-CD and β-CD are shown in Fig. 3b and 3c. The complexation of PMO12O403− with (chiral) CDs was recognized to induce chirality of the encapsulated cluster in aqueous solution.89
1 host–guest stoichiometry with β- and γ-CD, respectively. The association constants were measured by ITC. The obtained binding affinity with β-CD was found to be 2 × 103 M−1, while the two-step sequential binding fitting for the complexation with γ-CD resulted in Ka1 = 4 × 104 and Ka2 = 1 × 103 M−1.46 The crystal structures of the PMO12O403− complexes with γ-CD and β-CD are shown in Fig. 3b and 3c. The complexation of PMO12O403− with (chiral) CDs was recognized to induce chirality of the encapsulated cluster in aqueous solution.89
Three-component organic–inorganic systems were constructed based on the supramolecular interaction between γ-CD and the Dawson-type polyoxometalate, P2W18O626−, and a cationic cluster [Ta6Br12(H2O)6]2+.25 X-ray analysis (Fig. 3f) revealed that the cationic cluster is trapped between two CD units, while the anionic cluster facilitates the assembly by linking the CD units from the narrow rim.25 A two-site binding model was used to analyse the ITC data, Table 1. The P2W18O626− anion afforded association constants of 3.2 × 103 and 3.7 × 102 M−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometries, respectively. Higher affinity values were obtained for the inclusion complexes of the cationic cluster, 1.5 × 105 M−1 for the 1
1 host–guest stoichiometries, respectively. Higher affinity values were obtained for the inclusion complexes of the cationic cluster, 1.5 × 105 M−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1.3 × 105 M−1 for the 2
1 and 1.3 × 105 M−1 for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometry. In a similar manner, hierarchical architectures were prepared by using γ-CD as a host in combination with the metallic cationic cluster [{Re6Se8}(H2O)6]2+ as well as the anionic ones [{Re6Se8}(CN)6]4− and P2W18O626−.90 The solid-state structures of the ternary assemblies indicated that two γ-CD units interact with P2W18O626− through the primary rim (partial inclusion), while the [{Re6Se8}(H2O)6]2+ cluster is positioned at the secondary rim. For the anionic [{Re6Se8}(CN)6]4− cluster, a 2
1 host–guest stoichiometry. In a similar manner, hierarchical architectures were prepared by using γ-CD as a host in combination with the metallic cationic cluster [{Re6Se8}(H2O)6]2+ as well as the anionic ones [{Re6Se8}(CN)6]4− and P2W18O626−.90 The solid-state structures of the ternary assemblies indicated that two γ-CD units interact with P2W18O626− through the primary rim (partial inclusion), while the [{Re6Se8}(H2O)6]2+ cluster is positioned at the secondary rim. For the anionic [{Re6Se8}(CN)6]4− cluster, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest inclusion complex is formed, in which the cluster remains entrapped within two γ-CD units facing each other from the wider rims, while the polyoxometalate acts as a linker that connects the CD units through the association with the outer surface.90 Another three-component hybrid assembly of the [Mo154O462H14(H2O)70]14− molecular wheel (Mo154) encapsulating a sandwich-type complex between P2W18O626− and two γ-CDs was reported (Fig. 3g), which revealed the association of these large anions with CDs in aqueous solution by NMR and solid-state structural analysis.26
1 host–guest inclusion complex is formed, in which the cluster remains entrapped within two γ-CD units facing each other from the wider rims, while the polyoxometalate acts as a linker that connects the CD units through the association with the outer surface.90 Another three-component hybrid assembly of the [Mo154O462H14(H2O)70]14− molecular wheel (Mo154) encapsulating a sandwich-type complex between P2W18O626− and two γ-CDs was reported (Fig. 3g), which revealed the association of these large anions with CDs in aqueous solution by NMR and solid-state structural analysis.26
Water-soluble salts of anionic chalcogenide octahedral rhenium clusters (Re6X8(CN)64−, X = S, Se, Te) form stable inclusion complexes with CDs in aqueous solution.81,83,91 A partial inclusion of the large anions was obtained for Re6X8(CN)64− with α- and β-CD, while deeper inclusion complexes were observed with γ-CD.81,83 In the case of α-CD, only the Re6S8(CN)64− anion was found to be complexed between two CD units near the secondary rim. The Re6X8(CN)64− anions are partially embedded between the β-CD rings through their primary rims. Similar to the single-crystal structure of B12Br122−/γ-CD, the Re6Se8(CN)64− and Re6Te8(CN)64− clusters were entrapped between two γ-CD rings (Fig. 3d), while the smallest anion, Re6S8(CN)64−, formed a partial inclusion complex with γ-CD, in which the anion interacts with the secondary rims of γ-CD. In 1H NMR aqueous-solution spectra, the complexation-induced chemical shifts confirmed the formation of inclusion complexes, as indicated by the large downfield shifts of the inner protons of γ-CD. In addition, 77Se and 125Te NMR experiments supported the complexation pattern in solution. The binding affinities of the anions to γ-CD were determined using ITC experiments, which showed that the complexation is enthalpically driven, in agreement with the chaotropic effect.83 With the smallest CD homologue, α-CD, only the smallest cluster, Re6S8(CN)64−, showed a sizable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding affinity of 730 M−1. The larger clusters showed much weaker binding affinity to α-CD. The complexation of the three clusters to β-CD showed size complementarity, that is, the binding constants decreased with increasing size of the cluster, Table 1.81 The affinity of Re6S8(CN)64− to β-CD (Ka = 900 M−1) was slightly higher than that to α-CD. Interestingly, the oxidized form of the rhenium cluster showed higher affinity to β-CD (Ka1:1 = 2.3 × 105 M−1) compared to the parent one.91 This difference in binding affinity can be attributed to the higher chaotropicity of the oxidized form (Re6S8(CN)63−), induced by its lower charge density.91 Most recently, Leclerc et al. reported the formation of a 1
1 binding affinity of 730 M−1. The larger clusters showed much weaker binding affinity to α-CD. The complexation of the three clusters to β-CD showed size complementarity, that is, the binding constants decreased with increasing size of the cluster, Table 1.81 The affinity of Re6S8(CN)64− to β-CD (Ka = 900 M−1) was slightly higher than that to α-CD. Interestingly, the oxidized form of the rhenium cluster showed higher affinity to β-CD (Ka1:1 = 2.3 × 105 M−1) compared to the parent one.91 This difference in binding affinity can be attributed to the higher chaotropicity of the oxidized form (Re6S8(CN)63−), induced by its lower charge density.91 Most recently, Leclerc et al. reported the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exclusion complex between γ-CD and a Preyssler-type anion, [NaP5W30O110]14−.92 The large and highly charged anion interacts with the primary face of the γ-CD as revealed by single-crystal X-ray diffraction and 1H NMR experiments.
1 exclusion complex between γ-CD and a Preyssler-type anion, [NaP5W30O110]14−.92 The large and highly charged anion interacts with the primary face of the γ-CD as revealed by single-crystal X-ray diffraction and 1H NMR experiments.
Structural analysis in the solid-state indicated the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes between Lindqvist M6O192− ions and γ-CD (Fig. 3f), in which the anion is encapsulated inside the cavity. 1H NMR analysis also confirmed the complexation.80 Very recently, Yao et al. studied the complexation of Keggin-type polyoxotungstate anions with γ-CD in aqueous solution.93 Despite the fact that the investigated anions have similar structure and size, their binding affinity to γ-CD was found to decrease with increasing charge density on the anions, following the order H2W12O406− < BW12O405− < SiW12O404− < PW12O403−.93 Similarly, the host–guest complexation of [XW11MO40]n− Keggin-type anions (X = P or Si and M = MoV/VI or VIV/V) by γ-CD, in which the anionic net charge can vary from 3− to 6−, indicated that the binding affinity is highest for the anion with lower charge density.94 Such trends can be directly related to the variation of their chaotropicity, which decreases with increasing charge.18 γ-CD was also found to form host–guest complexes with metal chloride clusters ([M6Cl14]2−, M = Mo and W).95
1 inclusion complexes between Lindqvist M6O192− ions and γ-CD (Fig. 3f), in which the anion is encapsulated inside the cavity. 1H NMR analysis also confirmed the complexation.80 Very recently, Yao et al. studied the complexation of Keggin-type polyoxotungstate anions with γ-CD in aqueous solution.93 Despite the fact that the investigated anions have similar structure and size, their binding affinity to γ-CD was found to decrease with increasing charge density on the anions, following the order H2W12O406− < BW12O405− < SiW12O404− < PW12O403−.93 Similarly, the host–guest complexation of [XW11MO40]n− Keggin-type anions (X = P or Si and M = MoV/VI or VIV/V) by γ-CD, in which the anionic net charge can vary from 3− to 6−, indicated that the binding affinity is highest for the anion with lower charge density.94 Such trends can be directly related to the variation of their chaotropicity, which decreases with increasing charge.18 γ-CD was also found to form host–guest complexes with metal chloride clusters ([M6Cl14]2−, M = Mo and W).95
The cationic cluster, [Nb6Cl12(H2O)6]2+, binds to γ-CD with an association constant of 2.2 × 103 M−1 in water.96 Despite its cationic nature, the complexation thermodynamics showed a large favorable enthalpic energy gain counterbalanced by a strong entropic penalty, a trend that is similar to the binding of superchaotropic anions, and which is consistent with the chaotropic effect. Cationic palladium(II)-oxo clusters, [PdII6O12M8{(CH3)2AsO2}16(H2O)8]4+ (M = CeIV and ThIV), have been recently synthesized and showed strong binding affinity (Ka > 105 M−1) to negatively charged macrocyclic hosts, namely 4-sulfonatocalix[n]arenes (n = 4, 6, and 8).97 The binding events were again enthalpically driven. This observation may allow the classification of such large cations (e.g. [{Re6Se8}(H2O)6]2+, [Nb6Cl12(H2O)6]2+, and [Ta6Br12(H2O)6]2+) as being superchaotropic in nature, thereby constituting the first examples of the area of “large cation binding in water” that is not further scrutinized here.
Cucurbiturils, bambusurils, and biotinurils
Cucurbit[n]urils, Fig. 1, are water-soluble macrocyclic host molecules that are known for their high binding affinity towards cationic and neutral guest molecules, Fig. 1.109–111 Their cavity is classified as hydrophobic and it is accessible through two identical carbonyl rims. Examples of the encapsulation of anions inside CBs are rare,112–119 which can be attributed to the high barrier required to cross the negatively charged openings. Although there is no evidence for the inclusion of anions inside CBs in solution, single-crystal X-ray structures revealed several cases.118 For example, chloride and nitrate ions were found inside the cavity of CB5 and its permethylated derivatives, in which the complexation is assisted by the docking of the counter cations at the rims.113–115In contrast to CBn, bambusurils (Fig. 1) have been recognized as anion receptors in both organic and aqueous solutions.38,45,71–73,120 The X-ray structure of bambus[6]uril revealed anions inside its cavity, such as Cl−, which indeed acts as a template in the synthesis of bambusurils.72 Cl− can be displaced by other halides, such as I−, in a mixture of methanol and chloroform, as confirmed by 1H NMR experiments.71 The recognition propensity of a water-soluble bambusuril was tested with various anions (F−, Cl−, Br−, I−, CN−, IO4−, ReO4−, NO3−, PF6−, BF4−, and ClO4−−) in water, see Table 1. The measured association constants were surprisingly high (Ka > 102 M−1) even for highly solvated ions, such as F−, and reached values of 107 M−1 for I− and ClO4−.45 ITC data in water verified a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode in solution and showed that the complexation was associated with large favourable enthalpy values compensated by entropic penalties.45 Systematic analysis of the binding of halides to a bambusuril derivative, soluble in various solvents, was also performed.120 The obtained results indicated that halide binding in water and chloroform is invariably driven by favorable enthalpic effects, modulated by an entropic penalty, while in alcohols and nonpolar solvents both a favorable enthalpy and entropy contribute to anion binding, most pronounced for large anions.120
1 binding mode in solution and showed that the complexation was associated with large favourable enthalpy values compensated by entropic penalties.45 Systematic analysis of the binding of halides to a bambusuril derivative, soluble in various solvents, was also performed.120 The obtained results indicated that halide binding in water and chloroform is invariably driven by favorable enthalpic effects, modulated by an entropic penalty, while in alcohols and nonpolar solvents both a favorable enthalpy and entropy contribute to anion binding, most pronounced for large anions.120
The acid-mediated reaction between biotin and formaldehyde in hydrochloric acid led to the selective formation of a 6 + 6 macrocycle (biotin[6]uril, Fig. 1).121 A templating effect of the halide anions was assigned as the driving force for hexamer formation in H2SO4. In analogy to bambusuril, biotin[6]uril binds a variety of inorganic anions in water.47,121 The affinity followed the order Cl− < NO3− < ClO4− < N3− < Br− < I− < SeCN− < SCN−, Table 1. With the exception of ClO4−, which may be too large, this ordering follows again the chaotropicity of the anions. The thermodynamics of complexation was found to be enthalpically favorable and entropically unfavourable.47 The single-crystal X-ray structure of I− entrapped inside the cavity of biotin[6]uril is shown in Fig. 5.
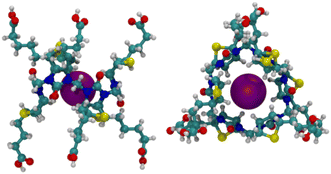 | ||
| Fig. 5 Single-crystal X-ray structure of biotin[6]uril encapsulating an iodide ion.121 | ||
Hemicucurbit[n]urils are another congener of the cucurbituril family.122–124 Their formation (n = 6 and 8) during the synthesis was found to be controlled by the presence of anions (templating effect), such that the formation of cyclohexanohemicucurbit[6]uril (cycHC[6]) is predominant with halides, while carboxylates and PF6− selectively facilitate the formation of cycHC[8].125 The chiral (all-R)-cyclohexanohemicucurbit[8]uril was found to selectively bind large anions in methanol.123 The highest association constant was measured for SbF6− (Ka = 2.5 × 105 M−1). In the gas phase, the order SbF6− ≈ PF6− > ReO4− > ClO4− > SCN− > BF4− > HSO4− > CF3SO3− for anion complexation was obtained, Fig. 6.
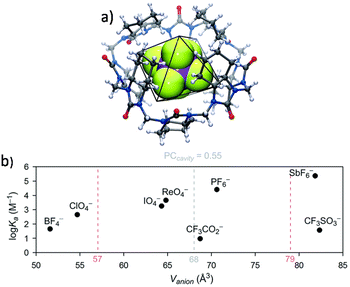 | ||
Fig. 6 (a) Crystal structure of the cyclohexanohemicucurbit[8]uril·SbF6− complex. (b) Binding affinities (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) of large anions to cyclohexanohemicucurbit[8]uril as a function of the anion volume (Vanion). Note the clear trend of increasing affinity with the anion size when the two perfluorinated organic ions at the bottom are excluded. Reproduced from ref. 123 with permission from the Royal Society of Chemistry. Ka) of large anions to cyclohexanohemicucurbit[8]uril as a function of the anion volume (Vanion). Note the clear trend of increasing affinity with the anion size when the two perfluorinated organic ions at the bottom are excluded. Reproduced from ref. 123 with permission from the Royal Society of Chemistry. | ||
It is worth mentioning that the association of chaotropic anions with macrocyclic hosts is not limited to the interaction with macrocyclic cavities and the formation of inclusion-type complexes, but it applies similarly to the interaction with the outer walls, to form exclusion-type of complexes.118,126–140 For example, superchaotropic anions, such as polyoxometalates and dodecaborate clusters, associate with the hydrophobic exterior of CBn macrocycles (Fig. 7), which results in the formation of hybrid aggregates.134–136
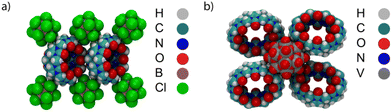 | ||
| Fig. 7 Single-crystal X-ray structure of the exclusion complexes of superchaotropic anions with CB7. (a) CB7-B12Cl122− and (b) CB8-V18O4212−. | ||
Octa acid and other macrocyclic hosts
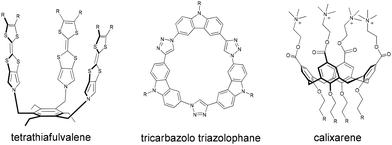
Gibb and co-workers found that chaotropic anions have sizable affinity to the hydrophobic concavity of the octa acid host (Fig. 1).65–67,105 An extended series of anions (as sodium salts) was studied (F−, SO42−, AcO−, Cl−, Br−, NO3−, ClO3−, I−, SCN−, and ClO4−).67,68,105,1411H NMR displacement experiments provided evidence for the actual inclusion of large anions into octa acid, while ITC experiments revealed association constants (1–100 M−1, Table 1) in the order NO3− < ClO3− < I− < SCN− ≪ ClO4−. The binding of other anions to octa acid was further investigated by 1H NMR and ITC.105 The largest affinities were measured for Cl3CCO2− (Ka = 6340 M−1) and PF6− (Ka = 790 M−1). The thermodynamic signature of the binding of these anions is in line with the chaotropic effect established for CDs.Examples of the binding of large anions to other molecular receptors and cages have also been documented.142–148 The stability of dodecaborate anions with CDs and tetrathiafulvalene (Fig. 8) was established in the gas phase.149 Nitschke and co-workers reported on the encapsulation of dianionic clusters, Mo6O192− and B12F122−, inside a metal–organic cage in acetonitrile with relatively high binding constants of 2.9 × 103 and 2.2 × 103 M−1, respectively, which were one order of magnitude higher than those measured for monoanionic guests, such as BPh4−, CB11H12−, and B(C6F5)4−, which pointed to a dominant electrostatic effect.150 The Mo6O192− cluster was found to form an inclusion complex with a coordination cage in organic solvents as supported by NMR and ESI-MS results.151 The association of conventional chaotropic anions with other molecular receptors was also observed, in non-aqueous solution. The weakly coordinating anion PF6− showed very high affinity to the tricarbazolo triazolophane macrocycle (Fig. 8) in organic solvents.152 The C5-symmetric penta-t-butylpentacyanopentabenzo[25]annulene macrocycle, known as cyanostar, showed also high association constants for large anions (e.g., PF6− and ClO4−).153 Evidently, since the aqueous desolvation effect does not play a role here, dispersion interactions of the large anions must also be important.
Potential applications of large anion binding
The earliest application of a supramolecular host–guest complex of a large anion is the famous color reaction of I− (I3−) with starch that has been discovered more than 200 years ago.154 Although not macrocyclic but helical in structure, starch is the polymeric equivalent of the oligomeric CDs, such that the driving force for large anion binding is the same: the chaotropic effect.With large superchaotropic anions a new high-affinity binding motif or anchoring unit for CDs has been identified, which opens several lines of applications.41,43,49,96,155,156 For example, the dodecaborate anion B12H11SH2−, as an auxiliary anchor residue, has been tethered to an organic moiety to synthesize water-soluble anchor dyes with high affinity to CDs.43 Upon complexation with CDs, dodecaborate-substituted dyes showed marked changes in their UV-visible and fluorescence properties, which enabled their application for indicator displacement assays.43 Another carboxyfluorescein dye of this type, with B12Br11OR2− as the anchoring unit, has also been reported.157 Other hybrid organic–inorganic derivatives of dodecaborate clusters were synthesized by tethering nitroanilines to the B12H122− anion.158 These new aniline-substituted dodecaborates showed higher basicity than the parent nitroanilines and were able to form stable inclusion complexes with β- and γ-CD.158
Larsen and Beeren have recently exploited this affinity in biotechnology, namely, in a templated directed synthesis of CDs (Fig. 9).49,159 For example, each CD, namely, α-, β-, and γ-CD, can be selectively obtained in high yields in the presence of sodium dodecyl sulfate, 1-adamantane carboxylic acid, and NaBPh4, respectively, as templating guest molecules of the amphiphilic or hydrophobic ionic type. Interestingly, large ring CDs (δ-CD and ε-CD) could also be synthesized by treating smaller CD homologues, such as α-CD with cyclodextrin glycosyl transferase (CGTase) in the presence of dodecaborate anions (e.g. Cs2B12I12), where they serve as a template (Fig. 9), owing to their high affinity to large-ring CDs.44 In this manner, halogenated dodecaborate cluster anions serve as templates for the practical synthesis of these precious large-ring CD homologues.
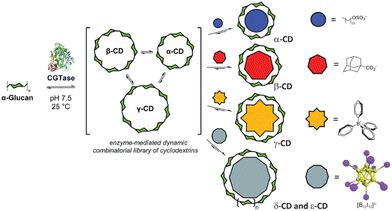 | ||
| Fig. 9 Template-selective synthesis of CDs in the presence of differently sized anionic guest molecules. Reproduced from ref. 49 with permission from the Royal Society of Chemistry. | ||
The largest dodecaborate anion, B12I122−, is also a promising X-ray contrast agent for medical diagnostics (owing to the heavy-atom content) but it is known to induce hemolysis at high mM concentrations.160,161 Hollow and Johnstone have recently reported that the complexation of the iodinated cluster with a permethylated γ-CD derivative prevents hemolysis, due to complexation.160 The authors also reported the XRD structure of the B12I122−·γ-CD complex which bears close similarity to that of B12Br122−·γ-CD (Fig. 3a).
CDs have also been used as templates for the synthesis of polyoxometalates. The Lindqvist M6O192− ions (M = Mo or W) were found to be formed in the presence of γ-CD, despite their low aqueous solubility.80 Recently, Falaise et al. have shown that the Mo154 polyoxometalate can readily be made in the presence of γ-CD as a templating agent.27 Host–guest complexation between the host and molybdenum-halide anionic clusters in aqueous solution was successfully applied to generate luminescent and redox-active materials.162 In detail, [Mo6Xi8Cl6]2− ions with Xi = Cl, Br or I are known to undergo hydrolysis in aqueous solution, and consequently form precipitates. This, however, could be suppressed, in particular for the brominated and iodinated clusters, upon complexation with γ-CD due to the formation of stable host–guest inclusion complexes, as indicated by 1H NMR experiments.162 Furthermore, the host–guest complexes were assembled in the presence of Al3+-based polycations, which did not affect the structure of the γ-CD/[Mo6Xi8Cl6]2− complex, and showed no effect on the optical and electrochemical properties of the [Mo6Xi8Cl6]2− clusters.162 Similarly, the hydrolytic stability of [W6X8Cl6]2− (X = Br and I) was enhanced upon encapsulation inside two γ-CD rings.163
Another application has recently been reported using bambusuril functionalized with an aza-crown ether, which can be utilized for the extraction of simple inorganic salts from aqueous solution to organic solvents (chloroform).164 Dodecaborate anions were found to facilitate the preparation of stable and dispersed gold nanoparticles (AuNPs), which allowed for the further fabrication of the nanoparticle surface through supramolecular host–guest complexation.41 For example, gold nanoparticles stabilized by B12H11SH2− anions were functionalized by an amphiphilic calixarene macrocycle (Fig. 8).41 The cationic calixarene derivative forms a stable complex with B12H11SH2− clusters, which allowed for the assembly of mono- and bilayers of calixarene on the surface of AuNPs in a controlled manner (Fig. 10a). Zhang and co-workers have also reported on the ability of the CB7-B12H122− assembly to reduce noble metals, for example Pt4+, to the zerovalent form, and subsequently the formation of nanoparticles which can be used for applications in catalysis.165 Similarly, Qi et al. prepared metal nanomaterials based on the assembly of B12H122−-capped nanoparticles with CD-functionalized Fe3O4 (Fig. 10b). The new nano-composites showed remarkable catalytic activity and recyclability in the selective reduction of nitroaromatic compounds.166
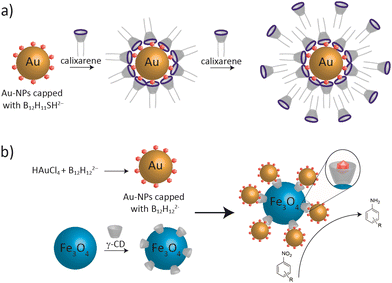 | ||
| Fig. 10 (a) Assembly of a calixarene derivative on B12H11SH2− coated AuNPs.41 (b) The preparation of B12H12-capped gold nanoparticles and CD-functionalized Fe3O4 and their assembly as a catalyst for the reduction of nitroaromatics.166 | ||
The propensity of large anions to associate with the exterior of macrocyclic hosts (see above) leads to the formation of higher-order aggregates (precipitation) and, thus, enables applications in the direction of separation and isolation. CBn/dodecaborate assemblies have been used as adsorbent materials for the preferential removal of organic dyes from aqueous solution.167 Qi et al. used the CBn/B12H122− assembly to prepare metal-cluster supramolecular composites as catalysts for Suzuki reactions.168 Heptamolybdate (Mo7O246−) showed a preferential association with the outer surface of CBn.135 In detail, the Mo7O246− anion forms an exclusion complex with CB7 in aqueous solution, which precipitates out. The finding has led to a new strategy for the separation of 99mTc from 99Mo solutions.
The propensity of superchaotropic anions to form precipitates with CBn macrocycles by outer-wall binding has been initially described for dodecaborate clusters as a manifestation of the chaotropic effect.134 Since then, this macrocycle-induced precipitation has been exploited for the separation of different precious metal complexes.169–171 For example, gold can be separated through the high binding of [AuBr4]− and [AuCl4]− to CB6 as well as α-CD, while PtCl62− forms also an exclusion complex with CB6. The resulting complexes immediately precipitate, allowing their separation. The superchaotropic anions thereby act as a “supramolecular glue”,134 made possible by their large size and external binding. Interestingly, the binding shows high selectivity, because PtCl62− can be separated from PdCl42− and RhCl63−.170 Very likely, this is related to the fact that PtCl62− is the anion with the highest chaotropicity in this large-anion set, because PdCl42− is less chaotropic on account of its smaller size, while RhCl63− is less chaotropic on account of its higher charge. These structure–activity relationships in large anion binding in water are emerging only now, and will help design refined applications in the future.
Recently, Wang et al. have explored the supramolecular assembly of CB8 with a dodecaborate derivative for image-guided photodynamic therapy applications (Fig. 11).172 The B12H122− cluster was monofunctionalized with an RGD cell-binding sequence through polyethylene glycol units for targeted therapy purposes. The modified cluster can assemble at the exterior of CB8, which is prefilled with methylene blue, in aqueous solution, to form nanoparticles. Methylene blue served as a photosensitizer dye. The nanoparticles were found to accumulate in the tumor region when injected in mice through the tail vein. The release of methylene blue from the cavity could then be triggered through competitive binding using a peptide with an aromatic N-terminus.
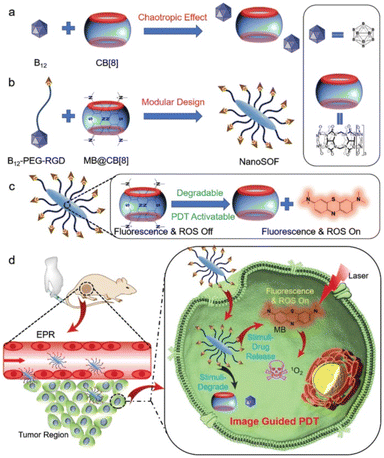 | ||
| Fig. 11 Schematic illustration for the implementation of the chaotropic effect for the design of nano-carriers for image-guided photodynamic therapy application. (a) the association of the chaotropic B12H122− anion to CB8, (b) molecular assembly of the functionalized B12H122− cluster and CB8, (c) activation of supramolecular photosensitizer, and (d) the working principle of the assembly as smart drug carrier for image-guided photodynamic therapy in vivo. Reproduced with permission from ref. 172 Copyright 2020 John Wiley and Sons, Inc. | ||
The self-assembly of β-CD with SiW12O404− provides a versatile tool to prepare metal–organic frameworks (MOFs).173 The structure of the (KCl4)Na7[(β-CD)3(SiW12O40)]·9H2O composite indicated that the anionic polyoxometalate is captured by three β-CD units. The new hybrid-MOF possesses high catalytic activity for the chemical oxidation of alcohols, alkenes, and thiophene to their corresponding products in the presence of H2O2.173 MOFs have also been synthesized based on the interaction between the polyoxometalate PW12O403− and α-CD as well as a polyoxopalladate (P10Pd15.5O5019−) and γ-CD.174 Although the large size of the anionic clusters does not allow the formation of inclusion-type complexes, the propensity of the large ions to associate with the outer walls of CDs drives the assembly into MOFs.174 Other examples have recently been reported.175 The strong interaction between the native CDs and Anderson–Evans type polyoxometalates, namely [AlMo6O18(OH)6]3−, was used to design infinite CD-channels linked through a polyoxometalate.175
While the development of chemosensors for kosmotropic anions presents an important challenge in supramolecular chemistry,2,176–182 sensing applications for large chaotropic and superchaotropic anions are of comparably lesser interest, because most of them are purely synthetic in nature (BF4−, PF6−, boron cluster anions, polyoxometalates), and therefore of limited environmental or biological concern. An exception is the perchlorate anion, ClO4−, which is formed as a by-product from oxidative processes of chloride ions or chlorinated hydrocarbons, and for which several specific receptors have been reported.183
Conclusions
The research field of anion recognition has experienced considerable developments in the past two decades. Despite a large number of reports on the binding of natural (small) inorganic anions by synthetic receptors, their relatively weak binding properties frequently limit the application potential to areas where the ions themselves serve either as analytes (ion sensing) or as cargos (ion transport) rather than as supramolecular building blocks on their own. The emergence of large cluster anions as tight binders for several macrocycles in 2015 has allowed their utilization as new assembly motifs in aqueous solution that have afforded, during the past 10 years, a wide spectrum of complex supramolecular architectures and several applications, ranging from the design of functional materials for catalysis to separation agents. Large anions, in particular those of the boron cluster and polyoxometalate type, display superchaotropic properties, which allow them to interact with extraordinary affinity with hydrophobic surfaces, and in particular with the hydrophobic cavity of macrocyclic hosts, which can be traced back to a combination of desolvation and dispersion effects. Since the two types of superchaotropic anions are emerging key ingredients for solid-state batteries184,185 and photocatalytic water-splitting,186 large anion binding could also become relevant for future energy-related applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the DFG through projects NA-868/14 (chaotropic effect of boron cluster anions) and NA-868/17 (chaotropic effect of polyoxometalates).References
- J. Y. C. Lim and P. D. Beer, Chem, 2018, 1, 731–783 Search PubMed.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS PubMed.
- Y. Liu, A. Sengupta, K. Raghavachari and A. H. Flood, Chem, 2017, 3, 411–427 CAS.
- X. Wu, P. Wang, W. Lewis, Y.-B. Jiang and P. A. Gale, Nat. Commun., 2022, 13, 4623 CrossRef CAS PubMed.
- H. Schlenk and D. M. Sand, J. Am. Chem. Soc., 1961, 83, 2312–2320 CrossRef CAS.
- A. Buvari and L. Barcza, Inorg. Chim. Acta, 1979, 33, L179–L180 CrossRef CAS.
- H. Hoiland, L. H. Hald and O. J. Kvammen, J. Solution Chem., 1981, 10, 775–784 CrossRef.
- J. Taraszewska and J. Wójcik, Supramol. Chem., 1993, 2, 337–343 CrossRef CAS.
- Y. Wu, C. Zhang, S. Fang, D. Zhu, Y. Chen, C. Ge, H. Tang and H. Li, Angew. Chem., Int. Ed., 2022, 61, e202209078 CAS.
- F. Hofmeister, Arch. Exp. Pathol. Pharmakol., 1888, 24, 247–260 CrossRef.
- J. Plesek, Chem. Rev., 1992, 92, 269–278 CrossRef CAS.
- J. Cebula, K. Fink, J. Boratyński and T. M. Goszczyński, Coord. Chem. Rev., 2023, 477, 214940 CrossRef CAS.
- E. Coronado and C. J. Gómez-García, Chem. Rev., 1998, 98, 273–296 CrossRef CAS PubMed.
- K. I. Assaf, M. S. Ural, F. Pan, T. Georgiev, S. Simova, K. Rissanen, D. Gabel and W. M. Nau, Angew. Chem., Int. Ed., 2015, 54, 6852–6856 CrossRef CAS PubMed.
- J. Voskuhl, M. Waller, S. Bandaru, B. A. Tkachenko, C. Fregonese, B. Wibbeling, P. R. Schreiner and B. J. Ravoo, Org. Biomol. Chem., 2012, 10, 4524–4530 RSC.
- F. Schibilla, J. Voskuhl, N. A. Fokina, J. E. P. Dahl, P. R. Schreiner and B. J. Ravoo, Chem. – Eur. J., 2017, 23, 16059–16065 CrossRef CAS PubMed.
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1918 CrossRef CAS PubMed.
- K. I. Assaf and W. M. Nau, Angew. Chem., Int. Ed., 2018, 57, 13968–13981 CrossRef CAS PubMed.
- P. Schmid, T. Buchecker, A. Khoshsima, D. Touraud, O. Diat, W. Kunz, A. Pfitzner and P. Bauduin, J. Colloid Interface Sci., 2021, 587, 347–357 CrossRef CAS PubMed.
- M. Hohenschutz, I. Grillo, C. Dewhurst, P. Schmid, L. Girard, A. Jonchère, O. Diat and P. Bauduin, J. Colloid Interface Sci., 2021, 603, 141–147 CrossRef CAS PubMed.
- T. Buchecker, P. Schmid, S. Renaudineau, O. Diat, A. Proust, A. Pfitzner and P. Bauduin, Chem. Commun., 2018, 54, 1833–1836 RSC.
- A. Malinenko, A. Jonchère, L. Girard, S. Parrès-Maynadié, O. Diat and P. Bauduin, Langmuir, 2017, 34, 2026–2038 CrossRef PubMed.
- B. Naskar, O. Diat, V. Nardello-Rataj and P. Bauduin, J. Phys. Chem. C, 2015, 119, 20985–20992 CrossRef CAS.
- V. Ďorďovič, Z. Tošner, M. Uchman, A. Zhigunov, M. Reza, J. Ruokolainen, G. Pramanik, P. Cígler, K. Kalíková, M. Gradzielski and P. Matějíček, Langmuir, 2016, 32, 6713–6722 CrossRef PubMed.
- M. A. Moussawi, N. Leclerc-Laronze, S. Floquet, P. A. Abramov, M. N. Sokolov, S. Cordier, A. Ponchel, E. Monflier, H. Bricout, D. Landy, M. Haouas, J. Marrot and E. Cadot, J. Am. Chem. Soc., 2017, 139, 12793–12803 CrossRef CAS PubMed.
- M. A. Moussawi, M. Haouas, S. Floquet, W. E. Shepard, P. A. Abramov, M. N. Sokolov, V. P. Fedin, S. Cordier, A. Ponchel, E. Monflier, J. Marrot and E. Cadot, J. Am. Chem. Soc., 2017, 139, 14376–14379 CrossRef CAS PubMed.
- C. Falaise, S. Khlifi, P. Bauduin, P. Schmid, W. Shepard, A. A. Ivanov, M. N. Sokolov, M. A. Shestopalov, P. A. Abramov, S. Cordier, J. Marrot, M. Haouas and E. Cadot, Angew. Chem., Int. Ed., 2021, 60, 14146–14153 CrossRef CAS PubMed.
- E. W. Kent, E. M. Lewoczko and B. Zhao, Polym. Chem., 2021, 12, 265–276 RSC.
- B. Kang, H. Tang, Z. Zhao and S. Song, ACS Omega, 2020, 5, 6229–6239 CrossRef CAS PubMed.
- P. Matějíček, Curr. Opin. Colloid Interface Sci., 2020, 45, 97–107 CrossRef.
- D. Tu, H. Yan, J. Poater and M. Solà, Angew. Chem., Int. Ed., 2020, 59, 9018–9025 CrossRef CAS PubMed.
- M. Hohenschutz, I. Grillo, O. Diat and P. Bauduin, Angew. Chem., Int. Ed., 2020, 59, 8084–8088 CrossRef CAS PubMed.
- P. Schmid, X. Graß, P. Bahadur, I. Grillo, O. Diat, A. Pfitzner and P. Bauduin, Colloids Interfaces, 2022, 6, 16 CrossRef CAS.
- W. Wei, Adv. Sci., 2023, 2302057 CrossRef PubMed.
- K. I. Assaf, B. Begaj, A. Frank, M. Nilam, A. S. Mougharbel, U. Kortz, J. Nekvinda, B. Grüner, D. Gabel and W. M. Nau, J. Org. Chem., 2019, 84, 11790–11798 CrossRef CAS PubMed.
- S. El Anwar, K. I. Assaf, B. Begaj, M. A. Samsonov, Z. Růžičková, J. Holub, D. Bavol, W. M. Nau, D. Gabel and B. Grűner, Chem. Commun., 2019, 55, 13669–13672 RSC.
- M. Diab, S. Floquet, M. Haouas, P. A. Abramov, X. López, D. Landy, A. Damond, C. Falaise, V. Guérineau, D. Touboul, D. Naoufal and E. Cadot, Eur. J. Inorg. Chem., 2019, 2019, 3373–3382 CrossRef CAS.
- T. Lizal and V. Sindelar, Isr. J. Chem., 2018, 58, 326–333 CrossRef CAS.
- V. Havel, M. Babiak and V. Sindelar, Chem. – Eur. J., 2017, 23, 8963–8968 CrossRef CAS PubMed.
- S. M. Eyrilmez, E. Bernhardt, J. Z. Dávalos, M. Lepšík, P. Hobza, K. I. Assaf, W. M. Nau, J. Holub, J. M. Oliva-Enrich, J. Fanfrlík and D. Hnyk, Phys. Chem. Chem. Phys., 2017, 19, 11748–11752 RSC.
- K. I. Assaf, A. Hennig, S. Peng, D.-S. Guo, D. Gabel and W. M. Nau, Chem. Commun., 2017, 53, 4616–4619 RSC.
- J. L. Pursell and C. J. Pursell, J. Phys. Chem. A, 2016, 120, 2144–2149 CrossRef CAS PubMed.
- K. I. Assaf, O. Suckova, N. Al Danaf, V. von Glasenapp, D. Gabel and W. M. Nau, Org. Lett., 2016, 18, 932–935 CrossRef CAS PubMed.
- K. I. Assaf, D. Gabel, W. Zimmermann and W. M. Nau, Org. Biomol. Chem., 2016, 14, 7702–7706 RSC.
- M. A. Yawer, V. Havel and V. Sindelar, Angew. Chem., Int. Ed., 2015, 54, 276–279 CrossRef CAS PubMed.
- Y. Wu, R. Shi, Y.-L. Wu, J. M. Holcroft, Z. Liu, M. Frasconi, M. R. Wasielewski, H. Li and J. F. Stoddart, J. Am. Chem. Soc., 2015, 137, 4111–4118 CrossRef CAS PubMed.
- M. Lisbjerg, B. E. Nielsen, B. O. Milhoj, S. P. A. Sauer and M. Pittelkow, Org. Biomol. Chem., 2015, 13, 369–373 RSC.
- T. Buchecker, P. Schmid, I. Grillo, S. Prévost, M. Drechsler, O. Diat, A. Pfitzner and P. Bauduin, J. Am. Chem. Soc., 2019, 141, 6890–6899 CrossRef CAS PubMed.
- D. Larsen and S. R. Beeren, Chem. Sci., 2019, 10, 9981–9987 RSC.
- H. Dodziuk, Cyclodextrins and Their Complexes, Wiley-VCH, Weinheim, 2006 Search PubMed.
- G. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254–2266 RSC.
- Y. Matsui, M. Ono and S. Tokunaga, Bull. Chem. Soc. Jpn., 1997, 70, 535–541 CrossRef CAS.
- J. N. Spencer, Q. He, X. M. Ke, Z. Q. Wu and E. Fetter, J. Solution Chem., 1998, 27, 1009–1019 CrossRef CAS.
- Y. Domi, K. Ikeura, K. Okamura, K. Shimazu and M. D. Porter, Langmuir, 2011, 27, 10580–10586 CrossRef CAS PubMed.
- F. Cramer, W. Saenger and H. C. Spatz, J. Am. Chem. Soc., 1967, 89, 14–20 CrossRef CAS.
- R. I. Gelb, L. M. Schwartz, M. Radeos and D. A. Laufer, J. Phys. Chem., 1983, 87, 3349–3354 CrossRef CAS.
- L. A. Godinez, B. G. SchulzeFiehn, S. Patel, C. M. Criss, J. D. Evanseck and A. E. Kaifer, Supramol. Chem., 1996, 8, 17–22 CrossRef CAS.
- J. F. Wojcik and R. P. Rohrbach, J. Phys. Chem., 1975, 79, 2251–2253 CrossRef CAS.
- H. Høiland, L. H. Hald and O. J. Kvammen, J. Solution Chem., 1981, 10, 775–784 CrossRef.
- A. Hersey, B. H. Robinson and H. C. Kelly, J. Chem. Soc., Faraday Trans. 1, 1986, 82, 1271–1287 RSC.
- I. Sanemasa, M. Fujiki and T. Deguchi, Bull. Chem. Soc. Jpn., 1988, 61, 2663–2665 CrossRef CAS.
- Y. Marcus, J. Solution Chem., 1994, 23, 831–848 CrossRef CAS.
- H. D. B. Jenkins and Y. Marcus, Chem. Rev., 1995, 95, 2695–2724 CrossRef CAS.
- J. P. Diard, E. Saintaman and D. Serve, J. Electroanal. Chem., 1985, 189, 113–120 CrossRef CAS.
- B. C. Gibb, Isr. J. Chem., 2011, 51, 798–806 CrossRef CAS.
- C. L. D. Gibb and B. C. Gibb, J. Am. Chem. Soc., 2011, 133, 7344–7347 CrossRef CAS PubMed.
- R. S. Carnegie, C. L. D. Gibb and B. C. Gibb, Angew. Chem., Int. Ed., 2014, 53, 11498–11500 CrossRef CAS PubMed.
- M. R. Sullivan, W. Yao, D. Tang, H. S. Ashbaugh and B. C. Gibb, J. Phys. Chem. B, 2018, 122, 1702–1713 CrossRef CAS PubMed.
- F. Biedermann, W. M. Nau and H.-J. Schneider, Angew. Chem., Int. Ed., 2014, 53, 11158–11171 CrossRef CAS PubMed.
- F. Biedermann, V. D. Uzunova, O. A. Scherman, W. M. Nau and A. De Simone, J. Am. Chem. Soc., 2012, 134, 15318–15323 CrossRef CAS PubMed.
- J. Svec, M. Necas and V. Sindelar, Angew. Chem., Int. Ed., 2010, 49, 2378–2381 CrossRef CAS PubMed.
- V. Havel, J. Svec, M. Wimmerova, M. Dusek, M. Pojarova and V. Sindelar, Org. Lett., 2011, 13, 4000–4003 CrossRef CAS PubMed.
- Á. Révész, D. Schröder, J. Svec, M. Wimmerová and V. Sindelar, J. Phys. Chem. A, 2011, 115, 11378–11386 CrossRef PubMed.
- V. Havel and V. Sindelar, ChemPlusChem, 2015, 80, 1601–1606 CrossRef CAS PubMed.
- V. Havel, M. A. Yawer and V. Sindelar, Chem. Commun., 2015, 51, 4666–4669 RSC.
- E. Solel, M. Singh, O. Reany and E. Keinan, Phys. Chem. Chem. Phys., 2016, 18, 13180–13185 RSC.
- C. Rando, J. Vázquez, J. Sokolov, Z. Kokan, M. Nečas and V. Šindelář, Angew. Chem., Int. Ed., 2022, 61, e202210184 CrossRef CAS PubMed.
- M. R. Eftink, M. L. Andy, K. Bystrom, H. D. Perlmutter and D. S. Kristol, J. Am. Chem. Soc., 1989, 111, 6765–6772 CrossRef CAS.
- S. Moghaddam, C. Yang, M. Rekharsky, Y. H. Ko, K. Kim, Y. Inoue and M. K. Gilson, J. Am. Chem. Soc., 2011, 133, 3570–3581 CrossRef CAS PubMed.
- C. Falaise, M. A. Moussawi, S. Floquet, P. A. Abramov, M. N. Sokolov, M. Haouas and E. Cadot, J. Am. Chem. Soc., 2018, 140, 11198–11201 CrossRef CAS PubMed.
- A. A. Ivanov, C. Falaise, K. Laouer, F. Hache, P. Changenet, Y. V. Mironov, D. Landy, Y. Molard, S. Cordier, M. A. Shestopalov, M. Haouas and E. Cadot, Inorg. Chem., 2019, 58, 13184–13194 CrossRef CAS PubMed.
- E. A. Lewis and L. D. Hansen, J. Chem. Soc., Perkin Trans. 2, 1973, 2081–2085 RSC.
- A. A. Ivanov, C. Falaise, P. A. Abramov, M. A. Shestopalov, K. Kirakci, K. Lang, M. A. Moussawi, M. N. Sokolov, N. G. Naumov, S. Floquet, D. Landy, M. Haouas, K. A. Brylev, Y. V. Mironov, Y. Molard, S. Cordier and E. Cadot, Chem. – Eur. J., 2018, 24, 13467–13478 CrossRef CAS PubMed.
- C. Sonnendecker, S. Thürmann, C. Przybylski, F. D. Zitzmann, N. Heinke, Y. Krauke, K. Monks, A. A. Robitzki, D. Belder and W. Zimmermann, Angew. Chem., Int. Ed., 2019, 58, 6411–6414 CrossRef CAS PubMed.
- K. I. Assaf, J. Holub, E. Bernhardt, J. M. Oliva-Enrich, M. I. Fernández Pérez, M. Canle, J. A. Santaballa, J. Fanfrlík, D. Hnyk and W. M. Nau, ChemPhysChem, 2020, 21, 971–976 CrossRef CAS PubMed.
- T. Merhi, A. Jonchère, L. Girard, O. Diat, M. Nuez, C. Viñas and P. Bauduin, Chem. – Eur. J., 2020, 26, 13935–13947 CrossRef CAS PubMed.
- Y. Chen, A. Barba-Bon, B. Grüner, M. Winterhalter, M. A. Aksoyoglu, S. Pangeni, M. Ashjari, K. Brix, G. Salluce, Y. Folgar-Cameán, J. Montenegro and W. M. Nau, J. Am. Chem. Soc., 2023, 145, 13089–13098 CrossRef CAS PubMed.
- I. Chazapi, O. Diat and P. Bauduin, J. Colloid Interface Sci., 2023, 638, 561–568 CrossRef CAS PubMed.
- B. Zhang, W. Guan, F. Yin, J. Wang, B. Li and L. Wu, Dalton Trans., 2018, 47, 1388–1392 RSC.
- A. A. Ivanov, C. Falaise, A. A. Shmakova, N. Leclerc, S. Cordier, Y. Molard, Y. V. Mironov, M. A. Shestopalov, P. A. Abramov, M. N. Sokolov, M. Haouas and E. Cadot, Inorg. Chem., 2020, 59, 11396–11406 CrossRef CAS PubMed.
- A. A. Ivanov, C. Falaise, D. Landy, M. Haouas, Y. V. Mironov, M. A. Shestopalov and E. Cadot, Chem. Commun., 2019, 55, 9951–9954 RSC.
- N. Leclerc, M. Haouas, C. Falaise, S. Al Bacha, L. Assaud and E. Cadot, Molecules, 2021, 26, 5126 CrossRef CAS PubMed.
- S. Yao, C. Falaise, A. A. Ivanov, N. Leclerc, M. Hohenschutz, M. Haouas, D. Landy, M. A. Shestopalov, P. Bauduin and E. Cadot, Inorg. Chem. Front., 2021, 8, 12–25 RSC.
- S. Yao, C. Falaise, S. Khlifi, N. Leclerc, M. Haouas, D. Landy and E. Cadot, Inorg. Chem., 2021, 60, 7433–7441 CrossRef CAS PubMed.
- A. A. Ivanov, P. A. Abramov, M. Haouas, Y. Molard, S. Cordier, C. Falaise, E. Cadot and M. A. Shestopalov, Inorganics, 2023, 11, 11 CrossRef.
- A. A. Ivanov, T. N. Pozmogova, A. O. Solovieva, T. S. Frolova, O. I. Sinitsyna, O. V. Lundovskaya, A. R. Tsygankova, M. Haouas, D. Landy, E. Benassi, L. V. Shestopalova, C. Falaise, E. Cadot, M. A. Shestopalov, P. A. Abramov and M. N. Sokolov, Chem. – Eur. J., 2020, 26, 7479–7485 CrossRef CAS PubMed.
- S. Bhattacharya, A. Barba-Bon, T. A. Zewdie, A. B. Müller, T. Nisar, A. Chmielnicka, I. A. Rutkowska, C. J. Schürmann, V. Wagner, N. Kuhnert, P. J. Kulesza, W. M. Nau and U. Kortz, Angew. Chem., Int. Ed., 2022, 61, e202203114 CrossRef CAS PubMed.
- P. Mu, T. Okada, N. Iwami and Y. Matsui, Bull. Chem. Soc. Jpn., 1993, 66, 1924–1928 CrossRef CAS.
- J. R. Rohrbach, Unpublished Ph.D. dissertation, cited by K. Takeo and T. Kuge, Die Stärke, 10 (1972) 331., Columbia University, New York, 1955.
- H. Dube, Ph.D dissertation, Iowa State University, Ames, Lowa, 1947.
- J. A. Thoma and D. French, J. Phys. Chem., 1961, 65, 1825–1828 CrossRef CAS.
- L. A. Godinez, J. Lin, M. Munoz, A. W. Coleman and A. E. Kaifer, J. Chem. Soc., Faraday Trans., 1996, 92, 645–650 RSC.
- R. P. Rohrbach, L. J. Rodriguez, E. M. Eyring and J. F. Wojcik, J. Phys. Chem., 1977, 81, 944–948 CrossRef CAS.
- T. Nhujak and D. M. Goodall, Electrophoresis, 2001, 22, 117–122 CrossRef CAS PubMed.
- P. Sokkalingam, J. Shraberg, S. W. Rick and B. C. Gibb, J. Am. Chem. Soc., 2016, 138, 48–51 CrossRef CAS PubMed.
- Y. Marcus, Ion Properties, Marcel Dekker, New York, 1997, vol. 1 Search PubMed.
- Y. Marcus, J. Solution Chem., 1994, 23, 831–848 CrossRef CAS.
- HyperChem(TM) Professional 8.0, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA Search PubMed.
- K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394–418 RSC.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed.
- D. Shetty, J. K. Khedkar, K. M. Park and K. Kim, Chem. Soc. Rev., 2015, 44, 8747–8761 RSC.
- P. Thuéry, Inorg. Chem., 2009, 48, 825–827 CrossRef PubMed.
- J.-X. Liu, L.-S. Long, R.-B. Huang and L.-S. Zheng, Cryst. Growth Des., 2006, 6, 2611–2614 CrossRef CAS.
- Y.-Q. Zhang, Q.-J. Zhu, S.-F. Xue and Z. Tao, Molecules, 2007, 12, 1325–1333 CrossRef CAS PubMed.
- J.-X. Liu, L.-S. Long, R.-B. Huang and L.-S. Zheng, Inorg. Chem., 2007, 46, 10168–10173 CrossRef CAS PubMed.
- D. G. Samsonenko, O. A. Gerasko, A. V. Virovets and V. P. Fedin, Russ. Chem. Bull., 2005, 54, 1557 CrossRef CAS.
- J. Liu, Y. Gu, R. Lin, W. Yao, X. Liu and J. Zhu, Supramol. Chem., 2010, 22, 130–134 CrossRef CAS.
- M. Zhang, R.-L. Lin, W.-Q. Sun and J.-X. Liu, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 173–187 CrossRef CAS.
- A. Flinn, G. C. Hough, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1992, 31, 1475–1477 CrossRef.
- T. Fiala, K. Sleziakova, K. Marsalek, K. Salvadori and V. Sindelar, J. Org. Chem., 2018, 83, 1903–1912 CrossRef CAS PubMed.
- M. Lisbjerg, B. M. Jessen, B. Rasmussen, B. E. Nielsen, A. Ø. Madsen and M. Pittelkow, Chem. Sci., 2014, 5, 2647–2650 RSC.
- R. Aav, E. Shmatova, I. Reile, M. Borissova, F. Topić and K. Rissanen, Org. Lett., 2013, 15, 3786–3789 CrossRef CAS PubMed.
- S. Kaabel, J. Adamson, F. Topic, A. Kiesila, E. Kalenius, M. Oeren, M. Reimund, E. Prigorchenko, A. Lookene, H. J. Reich, K. Rissanen and R. Aav, Chem. Sci., 2017, 8, 2184–2190 RSC.
- Y. Miyahara, K. Goto, M. Oka and T. Inazu, Angew. Chem., Int. Ed., 2004, 43, 5019–5022 CrossRef CAS PubMed.
- E. Prigorchenko, M. Öeren, S. Kaabel, M. Fomitšenko, I. Reile, I. Järving, T. Tamm, F. Topić, K. Rissanen and R. Aav, Chem. Commun., 2015, 51, 10921–10924 RSC.
- S.-C. Qiu, Q. Li, K. Chen, Y.-Q. Zhang, Q.-J. Zhu and Z. Tao, Inorg. Chem. Commun., 2016, 72, 50–53 CrossRef CAS.
- X.-W. Cui, S.-Y. Chen, C.-Z. Wang, W.-X. Zhao, T. Sun, X.-L. Ni, Y.-Q. Zhang and Z. Tao, Chin. Chem. Lett., 2016, 27, 173–177 CrossRef CAS.
- Y. Zhao, L.-L. Liang, K. Chen, N.-N. Ji, X.-J. Cheng, X. Xiao, Y.-Q. Zhang, S.-F. Xue, Q.-J. Zhu, N. Dong and Z. Tao, Dalton Trans., 2014, 43, 929–932 RSC.
- B.-X. Han, C.-Z. Wang, Y. Zhao, K. Chen, X. Xiao, Q.-J. Zhu, S.-F. Xue, Y.-Q. Zhang and Z. Tao, Eur. J. Inorg. Chem., 2014, 2014, 831–835 CrossRef CAS.
- B.-X. Han, C.-Z. Wang, K. Chen, X. Xiao, Z. Tao, S.-F. Xue, Y.-Q. Zhang and Q.-J. Zhu, CrystEngComm, 2014, 16, 1615–1619 RSC.
- X.-J. Cheng, N.-N. Ji, Y. Zhao, L.-L. Liang, X. Xiao, Y.-Q. Zhang, S.-F. Xue, Q.-J. Zhu and Z. Tao, CrystEngComm, 2014, 16, 144–147 RSC.
- Y. Zhao, L.-L. Liang, K. Chen, T. Zhang, X. Xiao, Y.-Q. Zhang, Z. Tao, S.-F. Xue and Q.-J. Zhu, CrystEngComm, 2013, 15, 7987–7998 RSC.
- X.-L. Ni, X. Xiao, H. Cong, L.-L. Liang, K. Cheng, X.-J. Cheng, N.-N. Ji, Q.-J. Zhu, S.-F. Xue and Z. Tao, Chem. Soc. Rev., 2013, 42, 9480–9508 RSC.
- W. Wang, X. Wang, J. Cao, J. Liu, B. Qi, X. Zhou, S. Zhang, D. Gabel, W. M. Nau, K. I. Assaf and H. Zhang, Chem. Commun., 2018, 54, 2098–2101 RSC.
- T. Goel, N. Barooah, M. B. Mallia, A. C. Bhasikuttan and J. Mohanty, Chem. Commun., 2016, 52, 7306–7309 RSC.
- X. Fang, P. Kögerler, L. Isaacs, S. Uchida and N. Mizuno, J. Am. Chem. Soc., 2009, 131, 432–433 CrossRef CAS PubMed.
- Y. Huang, R.-H. Gao, M. Liu, L.-X. Chen, X.-L. Ni, X. Xiao, H. Cong, Q.-J. Zhu, K. Chen and Z. Tao, Angew. Chem., Int. Ed., 2020, 60, 15166–15191 CrossRef PubMed.
- X. Zhao, Z. Yang, A. V. Kuklin, G. V. Baryshnikov, H. Ågren, W. Wang, X. Zhou and H. Zhang, J. Mater. Chem. A, 2020, 8, 13086–13094 RSC.
- X. Zhao, M. Zheng, Z. Zhang, Y. Wang, Y. Zhou, X. Zhou and H. Zhang, J. Mater. Chem. A, 2021, 9, 16427–16435 RSC.
- J.-H. Hu, R. Cen, M. Liu, P.-H. Shan, T. J. Prior, C. Redshaw, Y. Huang, Z. Tao and X. Xiao, Inorg. Chem. Commun., 2022, 142, 109663 CrossRef CAS.
- J. H. Jordan, C. L. D. Gibb, A. Wishard, T. Pham and B. C. Gibb, J. Am. Chem. Soc., 2018, 140, 4092–4099 CrossRef CAS PubMed.
- X. Kuang, X. Wu, R. Yu, J. P. Donahue, J. Huang and C.-Z. Lu, Nat. Chem., 2010, 2, 461–465 CrossRef CAS PubMed.
- Y. Liu, C. Hu, A. Comotti and M. D. Ward, Science, 2011, 333, 436 CrossRef CAS PubMed.
- Y. R. Hristova, M. M. J. Smulders, J. K. Clegg, B. Breiner and J. R. Nitschke, Chem. Sci., 2011, 2, 638–641 RSC.
- F. Sommer, Y. Marcus and S. Kubik, ACS Omega, 2017, 2, 3669–3680 CrossRef CAS PubMed.
- F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Nat. Rev. Chem., 2019, 3, 204–222 CrossRef.
- A. Borissov, I. Marques, J. Y. C. Lim, V. Félix, M. D. Smith and P. D. Beer, J. Am. Chem. Soc., 2019, 141, 4119–4129 CrossRef CAS PubMed.
- N. Cheng, Y. Chen, X. Wu and Y. Liu, Chem. Commun., 2018, 54, 6284–6287 RSC.
- J. Warneke, C. Jenne, J. Bernarding, V. A. Azov and M. Plaumann, Chem. Commun., 2016, 52, 6300–6303 RSC.
- W. J. Ramsay, F. T. Szczypiński, H. Weissman, T. K. Ronson, M. M. J. Smulders, B. Rybtchinski and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 5636–5640 CrossRef CAS PubMed.
- M. Han, J. Hey, W. Kawamura, D. Stalke, M. Shionoya and G. H. Clever, Inorg. Chem., 2012, 51, 9574–9576 CrossRef CAS PubMed.
- S. Lee, B. E. Hirsch, Y. Liu, J. R. Dobscha, D. W. Burke, S. L. Tait and A. H. Flood, Chem. – Eur. J., 2016, 22, 560–569 CrossRef CAS PubMed.
- S. Lee, C.-H. Chen and A. H. Flood, Nat. Chem., 2013, 5, 704–710 CrossRef CAS PubMed.
- J. J. Colin and H. F. Gaultier de Claubry, Ann. Chim., 1814, 90, 87–100 Search PubMed.
- F. Yang, M. Moors, D. A. Hoang, S. Schmitz, M. Rohdenburg, H. Knorke, A. Charvat, X.-B. Wang, K. Y. Monakhov and J. Warneke, ACS Appl. Nano Mater., 2022, 5, 14216–14220 CrossRef CAS.
- B. Qi, C. Wu, X. Li, D. Wang, L. Sun, B. Chen, W. Liu, H. Zhang and X. Zhou, ChemCatChem, 2018, 10, 2285–2290 CrossRef CAS.
- J. Zhang, D. Gabel, K. I. Assaf and W. M. Nau, Org. Lett., 2022, 24, 9184–9188 CrossRef CAS PubMed.
- T. Marei, M. K. Al-Joumhawy, M. A. Alnajjar, W. M. Nau, K. I. Assaf and D. Gabel, Chem. Commun., 2022, 58, 2363–2366 RSC.
- A. Erichsen, D. Larsen and S. R. Beeren, Front. Chem., 2021, 9, 582 Search PubMed.
- S. E. Hollow and T. C. Johnstone, Chem. Commun., 2022, 58, 2375–2378 RSC.
- A. Barba-Bon, G. Salluce, I. Lostalé-Seijo, K. I. Assaf, A. Hennig, J. Montenegro and W. M. Nau, Nature, 2022, 603, 637–642 CrossRef CAS PubMed.
- C. Falaise, A. A. Ivanov, Y. Molard, M. Amela-Cortes, M. A. Shestopalov, M. Haouas, E. Cadot and S. Cordier, Mater. Horiz., 2020, 7, 2399–2406 RSC.
- A. A. Ivanov, M. Haouas, D. V. Evtushok, T. N. Pozmogova, T. S. Golubeva, Y. Molard, S. Cordier, C. Falaise, E. Cadot and M. A. Shestopalov, Inorg. Chem., 2022, 61, 14462–14469 CrossRef CAS PubMed.
- K. Maršálek and V. Šindelář, Org. Lett., 2020, 22, 1633–1637 CrossRef PubMed.
- X. Zhao, M. Zheng, Z. Zhang, Y. Wang, Y. Zhou, X. Zhou and H. Zhang, J. Mater. Chem. A, 2021, 9, 16427–16435 RSC.
- B. Qi, C. Wu, X. Li, D. Wang, L. Sun, B. Chen, W. Liu, H. Zhang and X. Zhou, ChemCatChem, 2018, 10, 2285–2290 CrossRef CAS.
- W. Wang, X. Wang, C. Xiang, X. Zhou, D. Gabel, W. M. Nau, K. I. Assaf and H. Zhang, ChemNanoMat, 2019, 5, 124–129 CrossRef CAS.
- B. Qi, X. Li, L. Sun, B. Chen, H. Chen, C. Wu, H. Zhang and X. Zhou, Nanoscale, 2018, 10, 19846–19853 RSC.
- R.-L. Lin, Y.-P. Dong, M. Tang, Z. Liu, Z. Tao and J.-X. Liu, Inorg. Chem., 2020, 59, 3850–3855 CrossRef CAS PubMed.
- H. Wu, Y. Wang, L. O. Jones, W. Liu, L. Zhang, B. Song, X.-Y. Chen, C. L. Stern, G. C. Schatz and J. F. Stoddart, Angew. Chem., Int. Ed., 2021, 60, 17587–17594 CrossRef CAS PubMed.
- A. Ponchel and E. Monflier, Nat. Commun., 2023, 14, 1283 CrossRef CAS PubMed.
- W. Wang, B. Qi, X. Yu, W.-Z. Li, Z. Yang, H. Zhang, S. Liu, Y. Liu and X.-Q. Wang, Adv. Funct. Mater., 2020, 30, 2004452 CrossRef CAS.
- L. Ni, H. Li, H. Xu, C. Shen, R. Liu, J. Xie, F. Zhang, C. Chen, H. Zhao, T. Zuo and G. Diao, ACS Appl. Mater. Interfaces, 2019, 11, 38708–38718 CrossRef CAS PubMed.
- P. Yang, W. Zhao, A. Shkurenko, Y. Belmabkhout, M. Eddaoudi, X. Dong, H. N. Alshareef and N. M. Khashab, J. Am. Chem. Soc., 2019, 141, 1847–1851 CrossRef CAS PubMed.
- S. Khlifi, J. Marrot, M. Haouas, W. E. Shepard, C. Falaise and E. Cadot, J. Am. Chem. Soc., 2022, 144, 4469–4477 CrossRef CAS PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS PubMed.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648–3663 RSC.
- N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716–11754 CrossRef CAS PubMed.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- J. Pancholi and P. D. Beer, Coord. Chem. Rev., 2020, 416, 213281 CrossRef CAS.
- J. Zhao, D. Yang, X.-J. Yang and B. Wu, Coord. Chem. Rev., 2019, 378, 415–444 CrossRef CAS.
- L. K. Macreadie, A. M. Gilchrist, D. A. McNaughton, W. G. Ryder, M. Fares and P. A. Gale, Chem, 2022, 8, 46–118 CAS.
- T. Guchhait, S. Roy, M. Das and S. P. Jena, J. Mol. Struct., 2023, 136195 CrossRef CAS.
- W. S. Tang, K. Yoshida, A. V. Soloninin, R. V. Skoryunov, O. A. Babanova, A. V. Skripov, M. Dimitrievska, V. Stavila, S.-i. Orimo and T. J. Udovic, ACS Energy Lett., 2016, 1, 659–664 CrossRef CAS.
- A. Gigante, L. Duchêne, R. Moury, M. Pupier, A. Remhof and H. Hagemann, ChemSusChem, 2019, 12, 4832–4837 CrossRef CAS PubMed.
- F. M. Toma, A. Sartorel, M. Iurlo, M. Carraro, P. Parisse, C. Maccato, S. Rapino, B. R. Gonzalez, H. Amenitsch, T. Da Ros, L. Casalis, A. Goldoni, M. Marcaccio, G. Scorrano, G. Scoles, F. Paolucci, M. Prato and M. Bonchio, Nat. Chem., 2010, 2, 826–831 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |