Open Access Article
Open Access ArticlePrimary nitro compounds: progress in the synthesis of isoxazoles by condensation with aldehydes or activated ketones
Francesco
De Sarlo
a and
Fabrizio
Machetti
 *b
*b
aDipartimento di Chimica “Ugo Schiff” dell’ Università degli Studi di Firenze, Via della Lastruccia 13, 50019 Sesto Fiorentino, Firenze, Italy
bIstituto di Chimica dei Composti Organometallici del Consiglio Nazionale delle Ricerche c/o Dipartimento di Chimica “Ugo Schiff” dell’ Università degli Studi di Firenze, Via della Lastruccia 13, 50019 Sesto Fiorentino, Firenze, Italy. E-mail: fabrizio.machetti@unifi.it; fabrizio.machetti@cnr.it
First published on 21st August 2023
Abstract
Among the known strategies directed towards the synthesis of isoxazole derivatives, the reactions of aldehydes with primary nitro compounds deserve a comprehensive treatment, including the historical development as well as the more recent applications. The reactions of aldehydes with primary nitro compounds in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio have been shown to lead to isoxazoline-N-oxides or isoxazole derivatives, via β-dinitro derivatives. Several modifications of the process allowed the formation of products bearing substituents at various positions of the heterocyclic ring with control of regioselectivity. Ketones are reported to react with primary nitro compounds, only if activated (β-diketones, α-nitroketones, or strained ketones), to give isoxazole derivatives. Symmetric 2,4-dinitroglutarates formed from aromatic aldehydes and nitroacetate undergo ring closure to form isoxazole derivatives or, according to reaction conditions, 5-hydroxy-6-oxo-4-aryl-6H-1,2-oxazine-3-carboxylates (“oxazinones”), by loss of alcohol instead of water. Isoxazole-4-carbaldehydes are obtained by the reaction of 3-oxetanone with primary nitro compounds.
2 molar ratio have been shown to lead to isoxazoline-N-oxides or isoxazole derivatives, via β-dinitro derivatives. Several modifications of the process allowed the formation of products bearing substituents at various positions of the heterocyclic ring with control of regioselectivity. Ketones are reported to react with primary nitro compounds, only if activated (β-diketones, α-nitroketones, or strained ketones), to give isoxazole derivatives. Symmetric 2,4-dinitroglutarates formed from aromatic aldehydes and nitroacetate undergo ring closure to form isoxazole derivatives or, according to reaction conditions, 5-hydroxy-6-oxo-4-aryl-6H-1,2-oxazine-3-carboxylates (“oxazinones”), by loss of alcohol instead of water. Isoxazole-4-carbaldehydes are obtained by the reaction of 3-oxetanone with primary nitro compounds.
1. Introduction
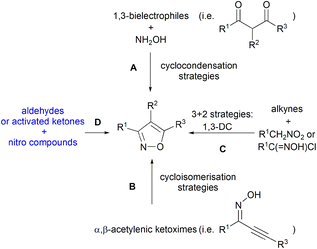
The isoxazole ring represents one of the privileged structures in organic and medicinal chemistry, and there have been an increasing number of studies on isoxazole-containing compounds.1 Various starting materials are used for their preparation, either as a single substrate (B) or in combination (A, C, and D) (Scheme 1). One of the most popular approaches for their synthesis involves the cyclocondensation of hydroxylamine with 1,3-bielectrophiles, mainly β-diketones (Scheme 1, A). In a similar manner, three-carbon 1,3-difunctionalised units with sp or sp2 carbons such as propargyl ketones, α,β-unsaturated ketones, enaminones, β-chloro/alkylthioenones, α,β-unsaturated nitriles, or α-oxoketenedithioacetals (Scheme 1, A) have been widely employed in this context.2 The cycloisomerisation of α,β-acetylenic ketoximes is related to these methods and in recent years has emerged as a powerful tool for the synthesis of isoxazoles (Scheme 1, B).3A quite different approach for the synthesis of these heterocycles involves the use of alkynes and nitro compounds or hydroximoyl chlorides (Scheme 1, C). These substrates give the isoxazole ring through 1,3-DC (1,3-dipolar cycloaddition), often generating the dipole in situ. The synthetic methodologies for the generation of the dipole have evolved over the years by using different ways according to the Mukaiyama reaction,4 Huisgen protocols,5 and Machetti–De Sarlo reaction.6
Most of these reactions have been extensively discussed in many reviews and book chapters7 and their recent development has been described.8
Aldehydes have been known for a long time to react with primary nitro compounds in excess to produce isoxazole derivatives, and the process has been the object of investigations that allowed the identification of several intermediates and an accepted mechanism (Scheme 1, D). Moreover, several activated ketones are known to react with primary nitro compounds; usually, a cycloaddition step is assumed to rationalise the formation of isoxazole derivatives (section 4). Although some of these reactions have been mentioned among the known isoxazole preparations, a review of the reactions of nitro compounds with carbonyl derivatives (aldehydes or ketones) is lacking (Scheme 1, D). Moreover, recent developments of this procedure introduced modifications that allowed the selective preparation of polysubstituted isoxazole derivatives.
This review is aimed at comprehensively summarising the development of synthetic preparations of functionalised isoxazoles which utilise aldehydes or ketones with nitro compounds as starting substrates. We expect that this review will be useful for medicinal and synthetic organic chemists and will also stimulate further interesting reaction design and developments in this area.
2. Aldehydes with nitro compounds
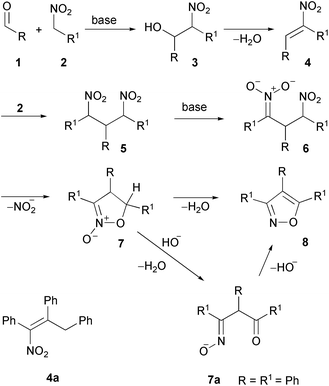
Primary nitro compounds contain the C–N–O sequence which has been embedded into an isoxazole ring since a long time ago; thus 3,4,5-trimethylisoxazole was obtained from nitroethane on treatment with alkalis. Although no mechanism was offered for this complex reaction, the “doctrine of tautomerism” was invoked as possibly contributing to a future rationalisation.9 1-Nitropropane behaves similarly, but not nitromethane, which undergoes deeper transformations.Aldehydes condense with primary nitro compounds under basic conditions, the ease of the reaction depending on the substituent geminal to the nitro group. In excess of nitro compounds, the resulting unsaturated nitro derivative can react with a second molecule, thus leading to β-dinitro compounds which are considered as key intermediates to isoxazole derivatives.
2.1. Arylnitromethanes and primary nitroalkanes
In general, the reactions of aldehydes with primary nitro derivatives in the presence of bases lead to a variety of products such as 2-hydroxy nitro compounds 3,10 condensed nitroalkenes 4,10 β-dinitro compounds 5, isoxazoline-N-oxides 7,11 and isoxazoles 8. The complete reaction sequence is reported in Scheme 2 but intermediates have been isolated, depending on substituents and reaction conditions. Condensation of benzaldehyde (1, R = Ph) with phenylnitromethane (2, R1 = Ph) in the presence of bases (aliphatic amines) was found to lead to several products, besides benzylidenephenylnitromethane (4, R = R1 = Ph). Among them, 3,4,5-triphenylisoxazole (8, R = R1 = Ph) was later identified, as a result of nitrous acid and water elimination, on heating in a strongly basic medium (Scheme 2).12,13This reaction sequence has been the object of many studies over the past century and several side products and possible intermediates containing nitro groups have been identified, depending on experimental conditions, such as 1,3-dinitro-1,2,3-triphenylpropane (5, R = R1 = Ph), nitrobenzylstilbene (4a),12 and 3,4,5-triphenylisoxazoline-2-oxide (7, R = R1 = Ph)14,15 (Scheme 2). According to a later study,16 the route via nitrobenzylstilbene 4a has been disproved as 4a and its β,γ-isomer prepared by unambiguous routes were not shown to be converted into 3,4,5-triphenyl-2-isoxazoline-2-oxide under any conditions. Intramolecular removal of NO2− was shown to occur in the nitronate anion (6, R = R1 = Ph) by a “displacement mechanism”, thus leading to isoxazoline-2-oxide (7, R = R1 = Ph). Base abstraction of 5-H converts 7 into 8via ring-opening to the β-oximino ketone anion 7a.16,17
Replacement of phenyl with other aryl groups either in the starting materials 1 and/or 2 or in intermediate 4 leads to variously substituted isoxazole derivatives 8. Thus, treatment of anisaldehyde 1 (R = 4-MeO-C6H4) with nitrostilbene 4 (R = R1 = Ph) is reported to afford mixtures of isoxazole derivatives with one to three anisyl residues, and this result is ascribed to the reversibility of condensations under the experimental conditions employed (base and heat).18 Similarly, 3,5-di(o-tolyl)-4-phenylisoxazole was obtained from 4 (R = C6H5 and R1 = o-tolyl) and 3,5-diphenyl-4-(o-tolyl)isoxazole from 4 (R = o-tolyl and R1 = C6H5).19 Other isoxazoline N-oxide 7 and isoxazole 8 derivatives with various substituents at the C-4 and/or C-5 position (p-bromophenyl, anisyl, piperonyl, and o-chlorophenyl) are reported but scarcely documented.15 However, product 4 (R = 2-NO2-C6H4 and R1 = Ph) from o-nitrobenzaldehyde 1 (R = 2-NO2-C6H4) and phenylnitromethane 2 (R1 = Ph) is reported to give with further phenylnitromethane the dinitro compound 1,2-dinitro-1,2-diphenyl-3-(2-NO2-C6H4) propane (structural isomer of 5).20 This leads eventually to 3,4-diphenyl-5-(2-NO2-C6H4)-isoxazole. This anomalous regioisomerism has been rationalised later.21 Other examples of analogous regioisomerism have been reported, but scarcely documented.15,22 Selectivity control has been achieved by replacing the second mole of 2 by pyridinium ylides23 (see section 3.1).
Many reactions either from nitroalkanes, other aldehydes or intermediates, fitting in Scheme 2, have been reported. The results depend on substituents and experimental conditions. Several aromatic aldehydes, furfuraldehyde and pivalaldehyde (1, R = Ar, 2-furfuryl, t-Bu) undergo the reaction shown in Scheme 2 (NaOH heating) with nitroethane or 1-nitropropane (2, R1 = Me, Et) to form isoxazole derivatives 8, without evidenced intermediates. The isoxazoles 8 were obtained in good yields except for pivalaldehyde (6%). The reaction failed using aldehydes with α-protons or α,β-unsaturations.21 Zeolite-catalysed condensation of aromatic aldehydes 1 with alkylnitromethanes 2 afforded 2-alkyl-2-nitrostyrene derivatives (4, R = Ar and R1 = alkyl), with substituted isoxazole 8 being observed as a minor product when R = 4-ClC6H4 and R1 = Me or Et.24 The product obtained from nitroethane with 6-bromopiperonal (by heating in ethanol in the presence of n-butylamine and Na2CO3) was investigated by 13C NMR spectroscopy and found to be 3,5-dimethyl-4(6-bromopiperonyl)isoxazole (8, R = 6-bromopiperonyl, R1 = Me, 3% yield); no intermediates were evidenced.25
β-Dinitroalkanes 5, prepared by another route, undergo the same reactions of Scheme 2 to form 8 (R = H, R1 = Me, Et). Two regioisomeric isoxazoles are produced when the dinitro intermediate is not symmetric, as reported for 2,4-dinitroheptane.26 Disodium 3,5-alkanedinitronates (salts of 6, R = H and R1 = alkyl, Scheme 2) are stable in basic solution, yielding 3,5-dialkylisoxazoles (8, R = H and R1 = alkyl) only upon acidification.14 No intermediates were intercepted in this case. 3,5-Dialkylisoxazole 8 (R = H, R1 = Et) is formed from disodium heptane-3,5-bisnitronate (stabilised by H-bonding) upon acidification.
Nitroethane and phenylnitromethane (2, R1 = Me and Ph) are reported to react with several aromatic (R = 4-XC6H4, X = 4-OMe, 4-Cl, and 4-NMe2) and heteroaromatic aldehydes (R = 3-furyl and 3-indolyl) 1 under treatment with basic alumina and microwave irradiation for a few minutes at 140 °C, affording 3,5-dimethyl-4-arylisoxazoles 8 (R1 = Me, R = Ar and heteroaryl) or 3,5-diphenyl-4-arylisoxazoles 8 (R1 = Ph, R = Ar and heteroaryl) in excellent yields.27
Heterocyclic aldehydes (1, R = heteroaryl) react with nitroethane (2, R1 = Me) in the presence of n-butylamine to form the intermediate nitroalkene 4 (R = heteroaryl and R1 = Me) which then affords isoxazoles 8 (R = heteroaryl and R1 = Me) with more nitroethane in alkaline hydroalcoholic solution (during 21 days at r.t.) (Scheme 3). The long reaction time and ambient temperature avoided the formation of by-products and the resinification of the reaction mixture. Nitrobutane reacts similarly (2, R = n-Pr).28
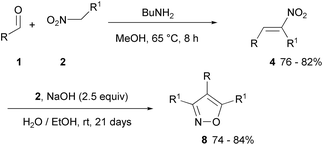 | ||
| Scheme 3 Isoxazole derivatives from nitroethane or nitrobutane (R1 = Me and n-Pr) and heterocyclic aldehydes (R = Het). | ||
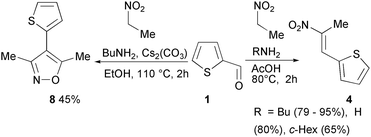
The reaction between nitroethane and thiophene-2-carbaldehyde can be selected as an illustrative case (Scheme 4). The reaction yields isoxazole 8 (45%, R1 = Me and R = 2-thienyl) by heating with an inorganic base (Cs2CO3),29 while it yields the Henry product 4 (65–95%, R1 = Me and R = 2-thienyl) by heating with ammonium acetates (acetic acid and n-butylamine or other amines like cyclohexylamine or NH3).30
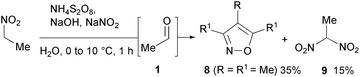
The oxidation of nitroalkanes in an alkaline environment leads to the formation of aldehydes, which can react with excess nitroalkanes to give isoxazoles.31 Thus, the oxidation of a nitroethane anion with ammonium persulfate in the presence of a double excess of NaNO2 produces acetaldehyde (1, R = Me), which then gives 3,4,5-trimethylisoxazole (8, R = R1 = Me) along with 1,1-dinitroethane 9 (Scheme 5).32
2.2. Nitroacetic esters
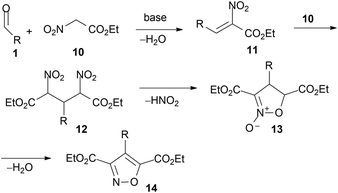
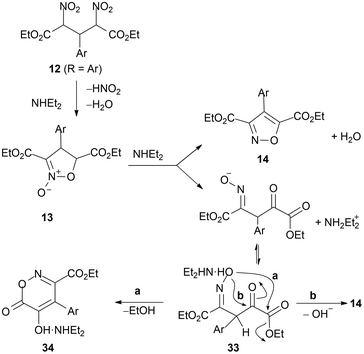
Aliphatic aldehydes react with ethyl nitroacetate 10 in the presence of amines.33 For aromatic derivatives, Schiff bases are preferred as starting materials.34–36Either dinitroglutarates 12, isoxazole N-oxide derivatives 13, isoxazole 3,5-dicarboxylic esters 14, or their derivatives are possibly obtained (depending on experimental conditions). All these fit in the accepted reaction path, analogous to the sequence reported above (Scheme 2), as illustrated in Scheme 6.
Substituted nitro acrylic esters 1137 have been reported38,39 while a general preparative procedure has been described starting from aldehydes and ethyl nitroacetate catalysed by TiCl4 and amine.40,41
Nitrocinnamates (11, R = aryl) undergo Michael addition to 12 with excess nitroacetate, but can be intercepted by cycloaddition with good dipolarophiles (e.g. organic azides), as reported.42
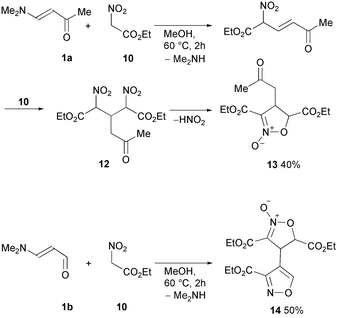
The same intermediates (12, R = alkyl, aryl or heteroaryl) are reported to be obtained from Schiff bases with ethyl nitroacetate and converted into isoxazole derivatives 14, in excess n-butylamine. No intermediate N-oxides were observed and the products were obtained as butylamides, in some cases, hydrolysed to 3,5-dicarboxylic acids.43,44 Similar reactions with nitroacetates are reported for enaminoketone 1a (enamine of aldehyde 1, R = CH2COCH3) leading to N-oxide 13 in 40% yield via intermediate 1245 (Scheme 7). As for enamino aldehyde 1b (enamine of aldehyde 1, R = CH2CHO), isoxazole 14 is directly afforded in 50% yield (Scheme 7).
Several further reports deal with the conversion of 12 into 1446,47 and reactions of nitroacetates with aldehydes according to Scheme 6. Thus, from indole aldehyde (1, R = 3-indolyl), the corresponding N-oxide 13 (as dibenzyl ester instead of ethyl ester, 51%) and isoxazole 14 (as dibenzyl ester instead of ethyl ester, 61%) were obtained,48 while from aldose derivatives49 the corresponding N-oxides 13 were obtained. From benzaldehyde and aliphatic aldehydes, 2,4-dinitroglutarates 12 (R = alkyl, C6H5), N-oxides 13 (R = alkyl, C6H5) and isoxazoles 14 (R = alkyl, C6H5) were obtained.17,50 However, during reactions from 12 to 13 or to 14, esters may be converted into acids,48,50 or amides, when amines are used as bases.17,23,50
Aromatic aldehydes are reported to condense with ethyl nitroacetate (10) in water with catalytic DABCO (1,4-diazabicyclo[2.2.2]octane) on ultrasonication (40 °C, 24 h) to give 3,5-dicarbethoxy-4-arylisoxazoline N-oxides 13 (R = Ar) (20 examples, 72–92% yields). At a higher temperature of 80 °C, 3,5-dicarbethoxy-4-arylisoxazoles 14 (R = Ar) are obtained (4 examples, 86–90% yields) as a result of dehydration of the corresponding N-oxides 13. The same reaction (40 °C, 24 h) on aliphatic aldehydes gives 3,5-dicarbethoxy-4-alkylisoxazoline N-oxides 13 (R = alkyl), besides the deoxidised products (3,5-dicarbethoxy-4-alkylisoxazolines) for the less hindered alkyl substituents such as ethyl, propyl, and butyl (64–76% yields). The formation of isoxazolines (in some cases even predominantly over their N-oxides) remains unexplained.51
Procedures are reported starting from intermediate α-nitrocinnamates 11 (R = Ar, from 15 with loss of methanol) with nitroacetate 10,23 thus providing access to a variety of derivatives, including enantioselective preparation of N-oxides 13.52 Incidentally, conversion of ethyl p-methyl-α-nitrocinnamate 11 (R = p-tolyl) into 4-p-tolyl-3,5-dicarbethoxyisoxazole 14 (R = p-tolyl) has been observed in the course of a study on reactions of α-nitrocinnamates with nucleophiles.53 Isoxazole 14 was obtained on a small scale with an almost quantitative yield (Scheme 8).
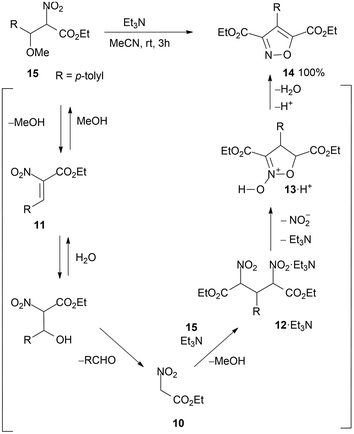 | ||
| Scheme 8 Intermediate α-nitrocinnamate (R = p-tolyl) reacts with ethyl nitroacetate in situ, affording 3,5-dicarboethoxy-4-p-tolylisoxazole. | ||
Phenylglyoxal intermediate, obtained by in situ oxidation of acetophenone (Z = Ph, Scheme 9), is reported to react with nitroacetate 10 to give 4-benzoyl-3,5-dicarbethoxyisoxazole (14, R = COPh) in 85% yield. The process is successfully applied to several substituted acetophenones and heterocyclic analogues to give 14 (R = COZ, 16 examples, 54–85% yields).54 The same reaction starting from styrene or analogues gave products 14 (R = COZ, seven examples, 62–78% yields).
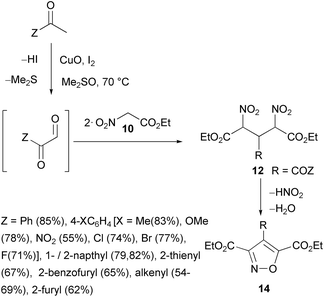 | ||
| Scheme 9 In situ preparation of glyoxals and a reaction with ethyl nitroacetate, affording 3,5-dicarboethoxy-4-acylisoxazoles. | ||
2.3. Nitro ketones
Nitroacetone (16) reacts with aryl(heteroaryl)aldehydes at the position away from the nitro group,55,56 thus giving nitroenone 17 (Scheme 10). Nitroenones 18 have been prepared from the Schiff bases of aromatic aldehydes with n-butylamine; the addition product with nitroacetone (16), in acetic anhydride, affords 18 (Scheme 10, R = aryl, 5 examples, including 2-furyl).56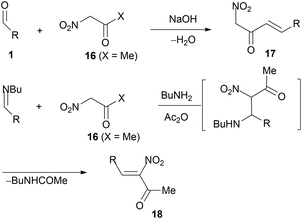 | ||
| Scheme 10 Formation of 4-nitroenones from aldehydes and preparation of 2-nitroenones from Schiff bases. | ||
Other nitroenones 18 are reported under different experimental conditions: Al2O3 in CH2Cl2 at room temperature,57 thionyl chloride in ethanol at rt or heating58,59 and β-alanine and acetic acid in benzene under reflux;60–65 phosphoryl chloride in ethanol at room temperature;66 acetic anhydride at 120–130 °C,67 (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine and 4-nitro-benzoic acid in dichloromethane;68 and 4-methyl-morpholine and titanium tetrachloride at 0 °C.69
They can be isolated as they do not undergo Michael addition with nitro ketones under the reported conditions.
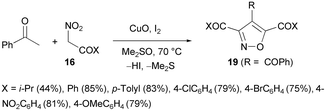
However, phenylglyoxal generated in situ from acetophenone (Scheme 11) reacts with two moles of α-nitro ketones 16 affording 4-benzoyl-3,5-diacylisoxazoles (19, R = PhCO, seven examples) in good yields for benzoylnitromethanes (X = Ar) and moderate yields in the case of aliphatic nitro ketone (X = i-Pr).54
2.4. Nitromethane
The use of nitromethane, in combination with aldehydes, in the synthesis of isoxazoles has only recently been reported.70 Nitromethane is a highly polar liquid with a weakly nucleophilic nature and is commonly used as a solvent.71 It is the simplest nitro compound and also the least acidic.72 Despite this, it can be deprotonated and used in the Henry reaction. These reactions are the first step towards the formation of the isoxazole ring but contrary to what one might expect, the condensation product of aldehydes with nitromethane does not turn out to be isoxazole 8 (R1 = H, Scheme 2).In fact, reactions of nitromethane (ten-fold excess) with aromatic aldehydes (21 examples, the reaction failed with 4-carboxybenzaldehyde) and heteroaromatic aldehydes (4 examples), under Cu(OAc)2 catalysis in the presence of ammonium iodide, are reported to give 3-nitro-4-arylisoxazoles 20 (Scheme 12).
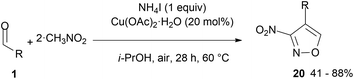 | ||
| Scheme 12 3-Nitroisoxazole derivatives from aromatic aldehydes and excess nitromethane under Cu(OAc)2 (0.2 equiv.) and NH4I (1 equiv.) mediation. | ||
The process has been investigated in detail using 4-chlorobenzaldehyde (R = 4-Cl-C6H4, R1 = H, Scheme 2). The proposed reaction mechanism follows part of the sequence reported above for primary nitro compounds, with the formation of 1-nitrostyrenes (4, R = Ar, R1 = H, Scheme 2), which with a second molecule of nitromethane and NH4I (1 equiv) afford 5 (R = Ar, R1 = H, Scheme 2) that follows a different evolution under Cu(OAc)2 catalysis.70,73 The anion of 5, rather than losing NO2−, undergoes intramolecular cyclisation and rearrangement to isoxazole derivatives, thus explaining the change of the sequence C–N–O of CH3NO2 into the C–O–N unit of the isoxazole ring. In fact, deuterated nitromethane leads to the formation of isoxazole 20 deuterated in C-5. Compared to the general reaction in Scheme 2, the mechanism should involve oxidation at some stage while the details remain unclear.
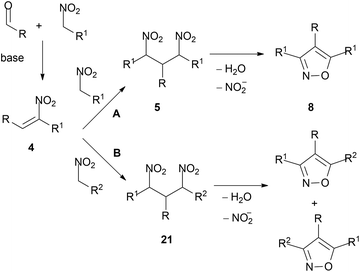
3. Regioisomerism
3.1. Selectivity via pyridinium salts
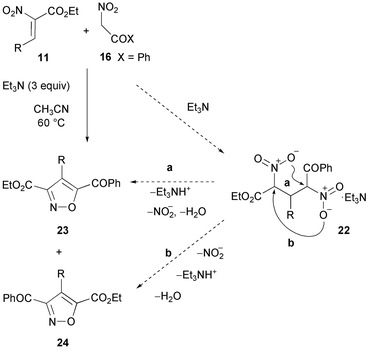
The reactions illustrated in Schemes 1 and 5 carried out in excess of nitro compounds give respectively symmetrical β-dinitro intermediates 5 and 12; therefore the substituents at positions C-3 and C-5 of the products are identical (Scheme 13, A).From the reactions of intermediate nitroalkene derivatives 4 with nitromethane derivatives, unsymmetrical β-dinitro intermediates 21 are obtained when R2 ≠ R1. These undergo in principle two alternative ring closure paths, leading to regioisomeric products with the substituents at positions 3 and 5 exchanged (Scheme 13, B). Several examples are known. Thus, the reactions of α-nitrocinnamates 11 (R = C6H5, p-BrC6H4, 2-thienyl) with α-nitroacetophenone 16 in excess of Et3N give mixtures in which the 5-benzoyl regioisomers 23 are predominant (69–78%) vs. the 3-benzoyl regioisomers (24, <6%). This result is rationalised via the preferred evolution of intermediate 22 because the carbon atom adjacent to the keto group is more electrophilic than the one adjacent to the ester (Scheme 14).23 Analogous regioisomeric mixtures have been reported later.52
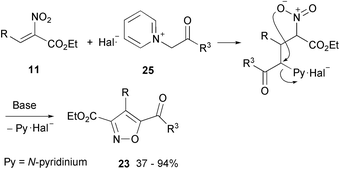
Improved selectivity has been achieved by replacing the second activated primary nitro compound by pyridinium ylides74 obtained from α-bromoesters or α-bromoketones. Thus, a better leaving group selectively drives the ring closure: the reaction between α-nitrocinnamate and analogues (11, R = C6H5, p-Br-C6H5, 2-thienyl) with pyridinium ylides (25, R3 = COMe, COPh, COOEt, and Z = Br, Cl) leads to a single isoxazole derivative 23 (as illustrated in Scheme 15).23
Moreover, excellent results are reported by a three-component procedure, using aromatic aldehydes, ethyl nitroacetate (10) and pyridinium salts 25 (12 examples, 50–92% yields).
The use of pyridinium salts in this context has been extended to α-nitrostilbenes (with N-oxide intermediates):75–77 different substitutions at positions 3 and 5 are selectively ensured.
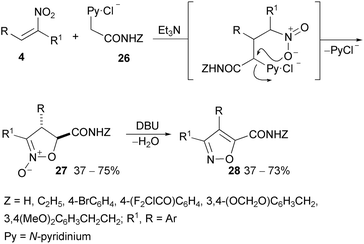
Thus, from aromatic or heteroaromatic aldehydes (10 examples) with phenylnitromethane or 4-methoxyphenylnitromethane intermediates, substituted nitrostilbenes 4 are obtained. These, with ethoxycarbonyl methylpyridinium bromide, selectively afford 3,4-diarylisoxazoline N-oxide-5-carboxylates.77 Similarly, α-nitrostilbenes with pyridinium acetamides (26) afford 3,4-diarylisoxazole-5-carboxyamides (28, 9 examples) via intermediate N-oxides (27) (Scheme 16).76
Note that dehydration to isoxazoles requires in this case a very strong base (DBU) whereas N-oxides from nitrocinnamates are easily converted into isoxazoles, owing to the presence of two EW groups.75
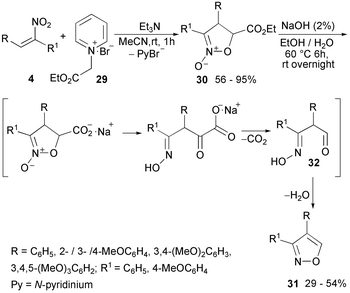
A subsequent implementation of the procedure, using the ethoxycarbonyl methylpyridinium salt 29, yielded a series of methoxy-substituted diarylisoxazoline N-oxides 30, which were converted into the corresponding 5-unsubstituted diarylisoxazoles 31via a one-pot hydrolysis/(N–O) cleavage/decarboxylation/cyclisation sequence (Scheme 17). A detailed mechanism of decarboxylation in a basic environment was not elucidated. However, a 1H NMR reaction mixture control at a half-time reaction revealed the presence of oxyimino aldehydes 32.77,78 Isoxazoles 31 were tested for antimitotic antitubulin activity in a sea urchin embryo model. A structure–activity relationship study revealed that isoxazoles 31 with an unsubstituted benzene ring at the C-3 of the isoxazole ring provide the appropriate configuration for the molecule to exhibit an antiproliferative effect by a microtubule destabilising mode of action.78
3.2. Selectivity via oxazinones
Another strategy to achieve selectivity in isoxazole synthesis from aldehydes and nitroacetates has been developed by means of intermediates 5-hydroxy-6-oxo-4-aryl-6H-1,2-oxazine-3-carboxylates (oxazinones) (34, Scheme 18).As reported above (Scheme 8), aldehydes with ethyl nitroacetate in the presence of a secondary amine give products 12, 13, and 14, and their derivatives (Dornow and his co-workers). A recent re-examination of the process79 has shown that dinitroglutarate, under appropriate reaction conditions, undergoes a different ring-closure to oxazinones 34 (Scheme 18) and a general synthesis of these compounds is reported from nitroacetic esters and aromatic aldehydes (1, R = Ar) in acetonitrile and Et2NH (Baranov and his co-workers).79,80
The two CO groups differentiated in oxazinones prompted the way for selectively preparing isoxazole-5-carboxyamide derivatives by a reaction of oxazinones with amines (methylamine, pyrrolidine, cycloexylamine, benzylamine, and morfoline). Thus, the regioselective preparation of 4-arylisoxazole-3,5-dicarboxylic acid derivatives 35 is described, including ester (Me, Et, and i-Pr) at C-3 with amide at C-5 (over 60 examples) and unsymmetrical diamides (2 examples) (Scheme 19).80
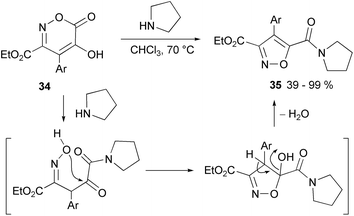 | ||
| Scheme 19 Example of 3-carbethoxy-5-carboxamide isoxazole derivatives from oxazinones and pyrrolidine. | ||
The Dornow reaction is updated34 with further insight into its mechanism. It may be noted that one of the 3,4-diaryl-5-ethoxycarbonylisoxazoline N-oxides reported above, 30 (R1 = CO2Et, R = [2,5-(MeO)2-3,4-OCH2OC6H],76 has been reported to be converted into the corresponding oxazinone (33%) on treatment with a very strong base (DBU) in MeCN.76
4. Activated ketones with nitro compounds
Unlike aldehydes, only activated ketones are reported to react with primary nitro compounds to afford isoxazole derivatives.4.1 Enolisable ketones
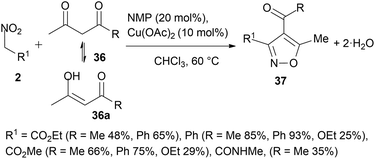
The treatment of activated nitro compounds 2 (2-nitroacetates, N-methyl-2-nitroacetamide and phenylnitromethane) with enolisable ketones 36 and 36a (1,3-diketones and 1,3-ketoesters) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
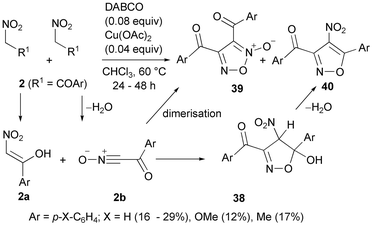
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with catalytic tertiary amines such as N-methylpiperidine (NMP) in the presence of a Cu(II) salt allowed the synthesis of highly functionalised isoxazoles 37 by a cycloaddition-condensation process (Scheme 20).81,82 5-Methyl trisubstituted isoxazoles were selectively obtained with high yields (85–93%) when only ketone groups were present in the substrates (R1 = Ph, R = Me, Ph, Scheme 20). In this context, enolisable ketones could be considered synthetic equivalents of alkynes (Scheme 1, D). This procedure avoids the use of nitrile oxide intermediates (Scheme 1, C) requiring dehydrating agents that would interfere with the dipolarophile. An intriguing case is the reaction of benzoylnitromethanes under the above conditions (Scheme 21).81,83 Here, the nitro compound as an enolised dipolarophile 2a reacts with nitrile oxide 2b derived from another mole of 2 to give selectively, after the loss of water from intermediates 38, the corresponding 4-nitro-isoxazole cycloadducts 40. A concomitant process was observed with the formation of furoxanes 39 derived from the dimerisation of nitrile oxides 2b. Similarly, long-chain alkanoylnitromethanes 41 (3 examples) in the presence of potassium fluoride as a catalytic base gave 4-nitro-isoxazoles 42 in low yields, likely as a result of cycloaddition of the nitro compound (or possibly the corresponding nitrile oxide) to the enolic form of another mole of the nitro compound and dehydration (Scheme 22). A preferred solvent is anhydrous tert-butanol since the use of secondary alcohol or the presence of water further decreases the reaction yield with the formation of acids.84 Diacyl furoxanes 39, unlike the isomeric isoxazole 40, were found to be peculiar prodrugs for the inhibition of GPX4 (glutathione peroxidase 4). The molecular mechanism of action of these small molecules, which are nothing more than masked nitrile oxides 2b, consists of covalent binding that leads to the desired inhibition effect.83
1 ratio with catalytic tertiary amines such as N-methylpiperidine (NMP) in the presence of a Cu(II) salt allowed the synthesis of highly functionalised isoxazoles 37 by a cycloaddition-condensation process (Scheme 20).81,82 5-Methyl trisubstituted isoxazoles were selectively obtained with high yields (85–93%) when only ketone groups were present in the substrates (R1 = Ph, R = Me, Ph, Scheme 20). In this context, enolisable ketones could be considered synthetic equivalents of alkynes (Scheme 1, D). This procedure avoids the use of nitrile oxide intermediates (Scheme 1, C) requiring dehydrating agents that would interfere with the dipolarophile. An intriguing case is the reaction of benzoylnitromethanes under the above conditions (Scheme 21).81,83 Here, the nitro compound as an enolised dipolarophile 2a reacts with nitrile oxide 2b derived from another mole of 2 to give selectively, after the loss of water from intermediates 38, the corresponding 4-nitro-isoxazole cycloadducts 40. A concomitant process was observed with the formation of furoxanes 39 derived from the dimerisation of nitrile oxides 2b. Similarly, long-chain alkanoylnitromethanes 41 (3 examples) in the presence of potassium fluoride as a catalytic base gave 4-nitro-isoxazoles 42 in low yields, likely as a result of cycloaddition of the nitro compound (or possibly the corresponding nitrile oxide) to the enolic form of another mole of the nitro compound and dehydration (Scheme 22). A preferred solvent is anhydrous tert-butanol since the use of secondary alcohol or the presence of water further decreases the reaction yield with the formation of acids.84 Diacyl furoxanes 39, unlike the isomeric isoxazole 40, were found to be peculiar prodrugs for the inhibition of GPX4 (glutathione peroxidase 4). The molecular mechanism of action of these small molecules, which are nothing more than masked nitrile oxides 2b, consists of covalent binding that leads to the desired inhibition effect.83
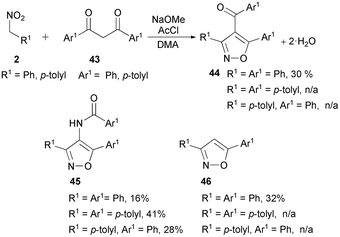
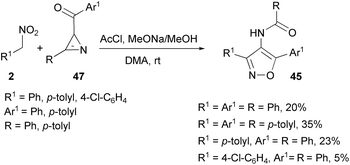
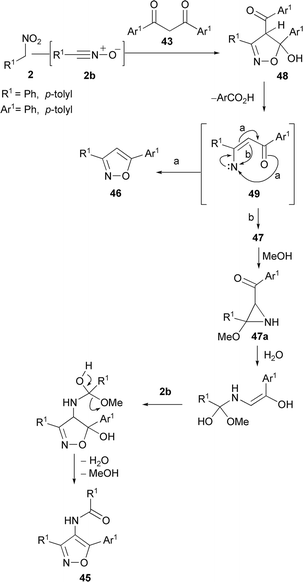
In a different way, the reaction of aryl nitromethanes 2 with dibenzoylmethanes 43 in the presence of stoichiometric acetyl chloride and sodium methoxide in dimethylacetamide (DMA) leads to the formation of a mixture of cycloadducts. Thus, direct cycloadduct 4-acyl isoxazoles 44 were obtained, albeit in low yields, along with 4-benzamidoisoxazoles 45 and 3,5 disubstituted isoxazoles 46 (Scheme 23).85
The above process is related to the reaction of keto-azirines 47 with aryl nitromethanes. Under the same reaction conditions as above, keto azirines 47 gave 4-benzamidoisoxazoles 45 along with isoxazole 46 (Scheme 24). The plausible mechanism for their formation (Scheme 23) is reported in Scheme 25.
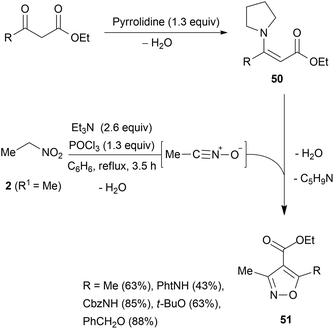
Nitrile oxide generated from 2 reacts with dibenzoylmethanes in 1,3-DC to give isoxazoline intermediates 48. A fission of the N–O bond under basic conditions followed by elimination of benzoic acid affords nitrene intermediates 49 which isomerise in MeOH to aziridines 47a. Isomerisation of nitrene 49 is not selective and the formation of isoxazole 46 is observed along with isoxazole 45. The latter is obtained by water C–C bond breaking of aziridines 47a to an olefin intermediate which undergoes 1,3-DC with dipole 2b followed by H2O and MeOH elimination. Another approach for circumventing the use of a dehydrating agent in the presence of an enolisable ketone was reported. The pyrrolidine enamine of ethyl acetoacetate (50, R = Me) was reacted (benzene reflux) with acetonitrile oxide, generated in situ from nitroethane, to give selectively the corresponding 4-carboxyisoxazoles 51 in modest yields. The procedure has been extended to protected γ-amino esters (N-phthaloyl and N-carbobenzyloxy) or protected γ-hydroxy-β-keto esters (O-tert-butyl and O-benzyl) (Scheme 26).86
4-Carboethoxy isoxazole 51 (R = Me) was designed as a precursor for the synthesis of α-cyclopiazonic acid87 and as a precursor of isoxazole-chenodeoxycholic acid hybrids. The latter were synthesised and evaluated for their lipid-lowering effects.88
4.2 Strained ketones
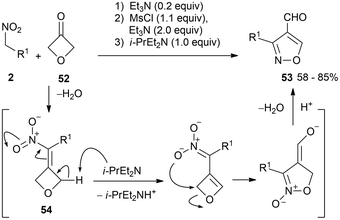
Condensation of 3-oxetanone 52 with various primary nitro derivatives (either aliphatic or aromatic) is reported, through an intriguing cascade sequence in a one-pot procedure. A variety of 3-substituted isoxazole-4-carbaldehydes 53 are obtained in high overall yields (16 examples). They originate by rearrangement of nitromethyleneoxetane intermediates 54 after deprotonation with a suitable base (Scheme 27).895. Conclusions
There are various reported methods for preparing isoxazoles, but few are general and versatile. Many methods suffer from low functional group tolerance and poor selectivity and yields. From each case, it is therefore necessary to choose the most suitable method for preparing the desired isoxazole derivatives. Studies on the title reactions carried out in the last century have highlighted the steps involved and their preparative potential.One of the advantages of using the combination of nitro compounds with activated aldehydes or ketones is the possibility of preparing 3,4,5-trisubstituted isoxazoles (i.e. processes reported in Schemes 8 and 17) and 3,4-disubstituted isoxazoles selectively (i.e. processes reported in Schemes 12, 17, and 27). The latter are difficult to obtain with other current methods and consequently rarely used as building blocks in the drug discovery process.
In recent years, modified procedures starting from intermediates have added synthetic value allowing the preparation of variously substituted products. Thus, the method has been employed for the preparation of a variety of selectively substituted isoxazoline N-oxides and isoxazole derivatives, affording compounds and libraries useful in medicinal chemistry. The reaction between activated ketones and nitro compounds leads to isoxazoles only under certain conditions, the most effective of which involves using a copper catalyst to control the process.
Many improvements in these methods have been described in this review. Their observation suggests that the future development of new and more efficient methods must focus on the identification of intermediates. These allow the control of the reaction selectivity and efficiency when isolated or replaced by suitable synthetic equivalents (i.e. intermediates in Scheme 17). The use of new catalysts, hopefully, simple in structure and cheap, to activate the process and direct it selectively towards the products, is another area of development.
Author contributions
All authors contributed equally to this work.Conflicts of interest
There are no conflicts to declare.References
- (a) J. Zhu, J. Mo, H.-z. Lin, Y. Chen and H.-p. Sun, Bioorg. Med. Chem., 2018, 26, 3065–3075 CrossRef CAS PubMed; (b) T. Haino and T. Hirao, Chem. Lett., 2020, 49, 574–584 CrossRef CAS; (c) C. Lamberth, J. Heterocycl. Chem., 2018, 55, 2035–2045 CrossRef CAS.
- B. J. Wakefield, Isoxazoles, in Science of Synthesis, ed. E. Schaumann, Georg Thieme Verlag, Stuttgart, 2002, vol. 11, ch. 9, pp. 229–288 Search PubMed.
- (a) See section 2 in D. X. Duc and V. C. Dung, Curr. Org. Chem., 2021, 25, 2938–2989 CrossRef CAS and section 3 in F. Hua and M. Szostak, Adv. Synth. Catal., 2015, 357, 2583–2614 Search PubMed; (b) J. Li, Z. Lin, W. Wu and H. Jiang, Org. Chem. Front., 2020, 7, 2325–2348 RSC.
- T. Mukaiyama and T. Hoshino, J. Am. Chem. Soc., 1960, 82, 5339–5342 CrossRef CAS.
- M. Christl and R. Huisgen, Chem. Ber., 1973, 106, 3345–3367 CrossRef.
- (a) F. De Sarlo and F. Machetti, Condensation of Primary Nitro Compounds to Isoxazole Derivatives: Stoichiometric to Catalytic, in Methods and Applications of Cycloaddition Reactions in Organic Syntheses, Wiley, New York, 2014, pp. 205–222 Search PubMed; (b) L. Cecchi, F. De Sarlo and F. Machetti, Chem. – Eur. J., 2008, 14, 7903–7912 CrossRef CAS PubMed; (c) L. Guideri, F. De Sarlo and F. Machetti, Chem. – Eur. J., 2013, 19, 665–677 CrossRef CAS PubMed.
- (a) D. Giomi, F. M. Cordero and F. Machetti, Isoxazoles, in Comprehensive Heterocyclic Chemistry III, 2008, vol. 4, pp. 365–485 Search PubMed; (b) D. Giomi, F. M. Cordero and F. Machetti, Isoxazoles, in Comprehensive Heterocyclic Chemistry IV, 2021, vol. 4, pp. 308–434 Search PubMed.
- (a) F. M. Cordero, L. Lascialfari and F. Machetti, Five-membered ring systems with O and N atoms, in Progress in Heterocyclic Chemistry, 2023, vol. 34, pp. 355–386 Search PubMed; (b) F. M. Cordero, L. Lascialfari and F. Machetti, Five-membered ring systems with O and N atoms, in Progress in Heterocyclic Chemistry, 2021, vol. 33, pp. 311–340 Search PubMed; (c) F. M. Cordero, L. Lascialfari and F. Machetti, Five-membered ring systems with O and N atoms, in Progress in Heterocyclic Chemistry, 2021, vol. 32, pp. 365–395 Search PubMed; (d) F. M. Cordero, L. Lascialfari and F. Machetti, Five-membered ring systems with O and N atoms, in Progress in Heterocyclic Chemistry, 2020, vol. 31, pp. 399–429 Search PubMed.
- W. R. Dunstan and T. S. Dymond, J. Chem. Soc., Trans., 1891, 410–433 RSC ; see p. 430.
- N. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001, pp. 30–44 Search PubMed.
- (a) A. Chatterjee, S. C. Jha and N. N. Joshi, Tetrahedron Lett., 2002, 43, 5287–5289 CrossRef CAS; (b) K. Harada, E. Kaji, K. Sasaki and S. Zen, Heterocycles, 1996, 42, 289–304 CrossRef CAS.
- E. Knoevenagel and L. Walter, Ber. Dtsch. Chem. Ges., 1904, 37, 4502–4510 CrossRef CAS.
- F. Heim, Ber. Dtsch. Chem. Ges., 1911, 44, 2016–2022 CrossRef CAS.
- E. P. Kohler and G. R. Barrett, J. Am. Chem. Soc., 1924, 46, 2105–2113 CrossRef CAS.
- D. E. Worrall, J. Am. Chem. Soc., 1935, 46, 2299–2301 CrossRef.
- A. T. Nielsen and T. G. Archibald, J. Org. Chem., 1969, 34, 984–991 CrossRef CAS.
- E. Kaji and S. Zen, Chem. Pharm. Bull., 1980, 28, 479–486 CrossRef CAS.
- J. Meisenheimer and K. Weibezahn, Ber. Dtsch. Chem. Ges., 1921, 54, 3195–3206 CrossRef.
- J. Meisenheimer, O. Beißwenger, H. O. Kauffmann, U. Kummer and J. Link, Justus Liebigs Ann. Chem., 1929, 468, 202–258 CrossRef CAS.
- P. Ruggli and B. Hegedüs, Helv. Chim. Acta, 1939, 22, 405–410 CrossRef CAS.
- W. M. Best, E. L. Ghisalberti and M. Powell, J. Chem. Res., Synop., 1998, 388–389 RSC.
- N. Campbell, W. Anderson and J. Gilmore, J. Chem. Soc., 1940, 446–451 RSC.
- K.-P. Chen, Y.-J. Chen and C.-P. Chuang, Eur. J. Org. Chem., 2010, 5292–5300 CrossRef CAS.
- R. Ballini, F. Bigi, E. Gogni, R. Maggi and G. Sartori, J. Catal., 2000, 191, 348–353 CrossRef CAS.
- G. M. Buchan and A. Turner, J. Chem. Soc., Perkin Trans. 1, 1975, 2115–2117 RSC.
- H. Feuer and S. Markofsky, J. Org. Chem., 1964, 29, 935–938 CrossRef CAS.
- M. Kidwai and P. Sapra, Org. Prep. Proced. Int., 2001, 33, 381–386 CrossRef CAS . It must be noted that the R residues in the figure on page 382 for nitro compound 1 of this reference were erroneously reported as Me and Ph but are Et and Benzyl, respectively. Also in the text, the authors referred to nitromethane, but it is nitroethane. See for the corrected interpretation ChemInform, 2001, 32(44), 139.
- L. A. Shumilova, M. K. Korsakov, M. V. Dorogov and E. E. Shalygina, Izv. Akad. Nauk, Ser. Khim., 2014, 63, 118–122 CAS . [Russ. Chem. Bull., Int. Ed., 2014, 63, 118–122].
- G. Noronha, J. Cao, C. P. Chow, C. C. Mak, M. S. S. Palanki, E. Dneprovskaia, A. McPherson, V. P. Pathak, J. Renick and B. Zeng, WO, 49028, 2009, (see pages 86, 87, and 92) Search PubMed.
- (a) A. J. Byrne, S. A. Bright, D. Fayne, J. P. McKeown, T. McCabe, B. Twamley, C. Williams and M. J. Meegan, Med. Chem., 2018, 14, 181–199 CAS; (b) M. A. El-Atawy, F. Ferretti and F. Ragaini, Eur. J. Org. Chem., 2017, 1902–1910 CrossRef CAS; (c) G. Vallejos, A. Fierro, M. C. Rezende, S. Sepulveda-Boza and M. Reyes-Parada, Bioorg. Med. Chem., 2005, 13, 4450–4457 CrossRef CAS PubMed; (d) K. Goodman, K. Marks Jr. and L. Lee, J. Med. Chem., 1992, 35, 280–285 CrossRef PubMed.
- A. H. Pagano and H. Shechter, J. Org. Chem., 1970, 35, 295–303 CrossRef CAS.
- N. A. Petrova, M. B. Shcherbinin, A. G. Bazanov and I. V. Tselinskii, Russ. J. Org. Chem., 2007, 43, 646–651 CrossRef CAS.
- A. Dornow and A. Frese, Ann. Chem., 1953, 581, 211 CAS.
- A. Dornow and G. Wiehler, Ann. Chem., 1952, 578, 113 CAS.
- A. Dornow and A. Frese, Ann. Chem., 1952, 578, 122 CAS.
- A. Dornow and H. Menzel, Ann. Chem., 1954, 588, 40–44 CAS.
- Stereochemistry of nitroesters is disregarded.
- R. F. C. Brown and G. V. Meehan, Aust. J. Chem., 1968, 21, 1681–1699 Search PubMed.
- For example, see: R. I. Baichurin, L. V. Baichurina, N. I. Aboskalova and V. M. Berestovitskaya, Zh. Obshch. Khim., 2013, 83, 1547–1554 Search PubMed . [Russ. J. Gen. Chem., 2013, 83, 1764–1770].
- W. Lehnert, Tetrahedron, 1972, 28, 663–666 CrossRef CAS.
- For other examples using TiCl4, see: (a) R. S. Fornicola, E. Oblinger and J. Montgomery, J. Org. Chem., 1998, 63, 3528–3529 CrossRef CAS; (b) L. He, G. S. C. Srikanth and S. L. Castle, J. Org. Chem., 2005, 70, 8140–8147 CrossRef CAS PubMed; (c) R. S. Fornicola, E. Oblinger and J. Montgomery, Tetrahedron Lett., 1999, 40, 8337–8341 CrossRef CAS.
- J. Thomas, J. John, N. Parekh and W. Dehaen, Angew. Chem., Int. Ed., 2014, 53, 10155–10159 CrossRef CAS PubMed.
- S. Umezawa and S. Zen, Bull. Chem. Soc. Jpn., 1961, 33, 890–891 Search PubMed.
- S. Umezawa and S. Zen, Bull. Chem. Soc. Jpn., 1963, 36, 1150–1154 CrossRef CAS.
- Z. A. Krasnaya, T. S. Stytsenko, E. P. Prokof'ev, I. P. Yakovlev and V. F. Kucherov, Izv. Akad. Nauk SSSR, Ser. Khim., 1974, 845–853 CAS . [Bull. Acad. Sc. USSR Div. Chem. Sc., 1974, 23, 809–816].
- S. Umezawa and S. Zen, Bull. Chem. Soc. Jpn., 1960, 33, 1016–1017 CrossRef CAS.
- S. Zen and S. Umezawa, Bull. Chem. Soc. Jpn., 1963, 36, 1146–1149 CrossRef CAS.
- L. K. Vinograd and N. N. Suvorov, Khim. Geterotsikl. Soedin., 1970, 1505–1507 CAS . [Chem. Heterocycl. Compd. USSR, 1970, 1403–1405].
- E. Kaji, H. Ichikawa and S. Zen, Bull. Chem. Soc. Jpn., 1979, 2928–2932 CrossRef CAS.
- S. Zen and M. Koyama, Bull. Chem. Soc. Jpn., 1971, 2882 CrossRef CAS.
- A. Rouf, E. Şahin and C. Tanyeli, Tetrahedron, 2017, 73, 331–337 CrossRef CAS.
- S. C. Sahoo and S. C. Pan, Eur. J. Org. Chem., 2019, 1385–1389 CrossRef CAS.
- H. Asahara, A. Sofue, Y. Kuroda and N. Nishiwaki, J. Org. Chem., 2018, 83, 13691–13699 CrossRef CAS PubMed.
- Y. Yang, M. Gao, C. Deng, D.-X. Zhang, L.-M. Wu, W.-M. Shu and A.-X. Wu, Tetrahedron, 2012, 68, 6257–6262 CrossRef CAS.
- C. Harries, Justus Liebigs Ann. Chem., 1901, 319, 230–256 CrossRef CAS.
- A. Dornow and W. Sassenberg, Justus Liebigs Ann. Chem., 1957, 602, 14–23 CrossRef CAS.
- S. Fioravanti, L. Pellacani and M. C. Vergari, Org. Biomol. Chem., 2012, 10, 524–528 RSC.
- R. I. Baichurin, L. M. Alizada, N. I. Aboskalova and S. V. Makarenko, Zh. Obshch. Khim., 2018, 88, 39–44 Search PubMed . [Russ. J. Gen. Chem., 2018, 88, 36–40].
- N. I. Aboskalova, A. S. Polyanskaya, V. M. Berestovitskaya and N. A. Fatkulova, Russ. J. Org. Chem., 1996, 32, 129–130 Search PubMed.
- M. I. Budagyants, M. K. Shakhova and G. I. Samokhvalov, Zh. Org. Khim., 1969, 5, 1857–1860 CAS . [J. Org. Chem. USSR, 1969, 5, 1803–1805].
- V. S. Velezheva, Y. V. Erofeev and N. N. Suvorov, Zh. Org. Khim., 1980, 16, 2157–2163 CAS . [J. Org. Chem. USSR, 1980, 16, 1839–1844].
- V. M. Berestovitskaya, N. I. Aboskalova, E. A. Ishmaeva, S. V. Bakhareva, G. A. Berkova, Y. A. Vereshchagina, A. V. Fel'gendler and G. R. Fattakhova, Zh. Obshch. Khim., 2001, 71, 2049–2056 Search PubMed . [Russ. J. Gen. Chem., 2001, 71, 1942–1949].
- B. J. Stokes, S. Liu and T. G. Driver, J. Am. Chem. Soc., 2011, 133, 4702–4705 CrossRef CAS PubMed.
- V. M. Berestovitskaya, R. I. Baichurin, N. I. Aboskalova, L. V. Baichurina, E. V. Trukhin, A. V. Fel'gendler and M. A. Gensirovskaya, Zh. Obshch. Khim., 2016, 86, 936–943 Search PubMed . [Russ. J. Gen. Chem., 2016, 86, 1266–1273].
- R. I. Baichurin, A. A. Reshetnikov, V. D. Sergeev, N. I. Aboskalova and S. V. Makarenko, Zh. Obshch. Khim., 2019, 89, 666–670 CrossRef . [Russ. J. Gen. Chem., 2019, 89, 865–869].
- R. I. Baichurin, A. A. Fedorushchenko, N. I. Aboskalova, L. V. Baichurina, A. V. Felgendler and S. V. Makarenko, Zh. Obshch. Khim., 2019, 89, 671–683 CrossRef . [Russ. J. Gen. Chem., 2019, 89, 870–880].
- A. V. Fel'gendler, N. I. Aboskalova and V. M. Berestovitskaya, Zh. Obshch. Khim., 2000, 70, 1158–1164 Search PubMed . [Russ. J. Gen. Chem., 2000, 70, 1087–1093].
- J. O. Guevara-Pulido, J. M. Andrés and R. l. Pedrosa, Eur. J. Org. Chem., 2014, 8072–8076 CrossRef CAS.
- J. M. Melot, F. Texier-Boullet and A. Foucaud, Tetrahedron, 1988, 8, 2215–2224 CrossRef.
- M. Fu, H. Li, M. Su, Z. Cao, Y. Liu, Q. Liu and C. Guo, Adv. Synth. Catal., 2019, 361, 3420–3429 CrossRef CAS.
- B. Sheldon, Markofsky “Nitro Compounds, Aliphatic”, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Wienheim, 2002 Search PubMed.
- F. G. Bordwell and A. V. Satish, J. Am. Chem. Soc., 1994, 116, 8885–8889 CrossRef CAS.
- G. Cancheng, F. Meiqiang and G. Xin, CN, 108658883, 2018 Search PubMed.
- O. Tsuge, S. Kanemasa and S. Takenaka, Bull. Chem. Soc. Jpn., 1985, 58, 3137–3157 CrossRef CAS.
- N. B. Chernysheva, A. S. Maksimenko, F. A. Andreyanov, V. P. Kislyi, Y. A. Strelenko, V. N. Khrustalev, M. N. Semenova and V. V. Semenov, Tetrahedron, 2017, 73, 6728–6735 CrossRef CAS.
- A. S. Maksimenko, V. P. Kislyi, N. B. Chernysheva, Y. A. Strelenko, Y. V. Zubavichus, V. N. Khrustalev, M. N. Semenova and V. V. Semenov, Eur. J. Org. Chem., 2019, 4260–4270 CrossRef CAS.
- N. B. Chernysheva, A. S. Maksimenko, F. A. Andreyanov, V. P. Kislyi, Y. A. Strelenko, V. N. Khrustalev, M. N. Semenova and V. V. Semenov, Eur. J. Med. Chem., 2018, 146, 511–518 CrossRef CAS PubMed.
- E. A. Silyanova, V. I. Ushkarov, A. V. Samet, A. S. Maksimenko, I. A. Koblov, V. P. Kislyi, M. N. Semenova and V. V. Semenov, Mendeleev Commun., 2022, 32, 120–122 CrossRef CAS.
- M. S. Baranov and I. V. Yampolsky, Tetrahedron Lett., 2013, 54, 628–629 CrossRef CAS.
- A. Yu Smirnov, E. R. Zaitseva, O. A. Belozerova, R. S. Alekseyev, N. S. Baleeva, M. B. Zagudaylova, A. A. Mikhaylov and M. S. Baranov, J. Org. Chem., 2019, 84, 15417–15428 CrossRef.
- E. Trogu, L. Cecchi, F. De Sarlo, L. Guideri, F. Ponticelli and F. Machetti, Eur. J. Org. Chem., 2009, 5971–5978 CrossRef CAS.
- A. Baglieri, L. Meschisi, F. De Sarlo and F. Machetti, Eur. J. Org. Chem., 2016, 4643–4655 CrossRef CAS.
- J. K. Eaton, R. A. Ruberto, A. Kramm, V. S. Viswanathan and S. L. Schreiber, J. Am. Chem. Soc., 2019, 141, 20407–20415 CrossRef CAS.
- R. F. Love and F. G. Duranleau, US, 4061651, 1977 Search PubMed.
- S. Zen, K. Harada, H. Nakamura and Y. Iitaka, Bull. Chem. Soc. Jpn., 1988, 61, 2881–2884 CrossRef CAS.
- R. C. F. Jones, G. Bhalay, P. A. Carter, K. A. M. Duller and S. I. E. Vulto, J. Chem. Soc., Perkin Trans. 1, 1994, 2513–2514 RSC.
- V. A. Moorthie, E. M. McGarrigle, R. Stenson and V. K. Aggarwal, ARKIVOC, 2007,(v), 139–151 Search PubMed.
- R. Qiua, G. Luoa, X. Lia, F. Zheng, H. Li, J. Zhang, Q. Youa and H. Xiang, Bioorg. Med. Chem. Lett., 2018, 28, 2879–2884 CrossRef PubMed.
- J. A. Burkhard, B. H. Tchitchanov and E. M. Carreira, Angew. Chem., Int. Ed., 2011, 50, 5379–5382 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |