Open Access Article
Open Access ArticleDirect reductive amination of functionalized aldehydes with aniline derivatives of purines and 7-deazapurines†
José-María
Orduña
 ,
Natalia
del Río
,
Natalia
del Río
 and
María-Jesús
Pérez-Pérez
and
María-Jesús
Pérez-Pérez
 *
*
Instituto de Química Médica (IQM, CSIC), Juan de la Cierva 3, 28006 Madrid, Spain. E-mail: mjperez@iqm.csic.es
First published on 15th June 2023
Abstract
Reductive amination plays a key role in the medicinal chemistry toolbox since it allows the mono alkylation of an amine or aniline. In this work, reductive amination of functionalized aldehydes with aniline derivatives of adenine and closely related 7-deazapurines has been successfully performed using H-cube technology so that imine formation and its reduction are performed “in situ”. The set-up procedure surmounts some of the drawbacks of “in batch” protocols by avoiding the handling of reductant reagents, long reaction times and tedious work-ups. The here described procedure allows a high conversion into the reductive amination products together with an easy work-up by just evaporation. More interestingly, this set-up does not require the presence of acids so that acid-sensitive protecting groups can be present both at the aldehyde and at the heterocycle.
1. Introduction
Nucleoside derivatives of purines and 7H-pyrrolo[2,3-d]pyrimidines (also named as 7-deazapurines) are considered privileged structures in medicinal chemistry.1–3 Related to them, N-9-arylpurines and, by analogy, 7-phenyl-7H-pyrrolo[2,3-d]pyrimidines4,5 represent a particularly interesting group of compounds for which different biological studies have been described including antiviral6 or anticancer activities.4,7–9 Kinases10–12 and methyl transferases13,14 are among the most studied targets for these compounds, still their biological applications are in continuous expansion.15,16 Moreover, the interest on this type of compounds favors the development of efficient methods for their synthesis, as recently reported for the oxidative coupling of purines with N-heterocycles.17Reductive amination is a well-established procedure to perform the mono-alkylation of amines and is widely used in the synthesis of biologically active compounds.18 The reductive amination of aldehydes involves two steps: the formation of the imine intermediate and the subsequent reduction, steps that can be performed sequentially or “in situ”. For the reduction step, the most used procedures involve either borohydride complexes or hydrogenation in the presence of a catalyst. In most cases, the presence of an acid in the reduction step improves the conversion towards the N-alkyl derivative.
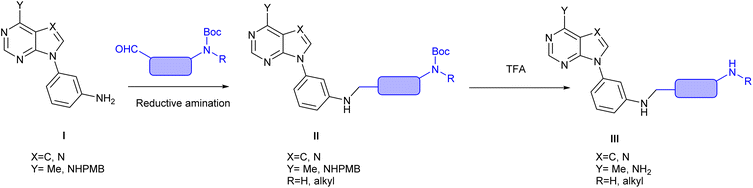
Our group has reported on the antiviral activity of 3-substituted aryl derivatives of purines and analogous heterocycles.19–23 In the course of our research, we became interested in the synthesis of aniline derivatives of general formula III. Such compounds could be obtained by the reductive amination of the corresponding aldehydes with the aniline derivatives of the purine and purine-like compounds (I, as shown in Fig. 1), although to the best of our knowledge, only few examples have been recently reported.24 We selected aldehydes with Boc-protected amines since this protecting group should be stable under the reductive amination conditions.
In the synthetic strategy represented in Fig. 1, and in order to access to adenine or 7-deazaadenine derivatives (III, Y = NH2, X = N or C, respectively), the amino group at the heterocyclic base must be masked prior to the reductive amination step. We selected the p-methoxybenzylamino group (I, Y = NHPMB) as the precursor of the amino group at the base since the PMB moiety could be removed by treatment with TFA, under conditions were the Boc-group would also be released. However, the presence of acid-sensitive protecting groups both at the heterocyclic base and at the aldehyde precluded the use of acidic conditions in the reductive amination reaction, thus we anticipated the requirement of quite harsh conditions for the reaction to take place in reasonable yields. In this sense, the H-cube technology allows generation “in situ” of a high pressure of hydrogen and application of high temperature (up to 150 °C) so that harsh conditions can be applied. Additionally, since the catalyst is included in a cartridge, no handling of solid catalysts or filtration is required, so that the work up is simple by evaporation of the solvent.
Here we describe the synthesis of compounds of general formula II (Fig. 1) by performing the amination reaction of I with a variety of aldehydes using the H-cube technology. This procedure gives access to new N-alkylated aniline derivatives of purines and 7-deazapurines.
2. Results and discussion
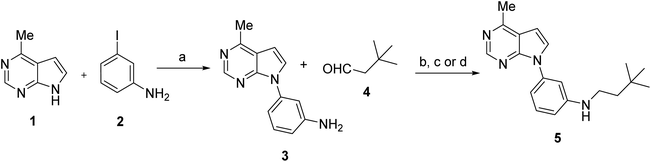
To address the reductive amination leading to N-alkylated derivatives of arylpurines, we selected two simple substrates to set up conditions: the aniline 3, that can be easily obtained through a Buchwald–Hartwig coupling14 of 4-methyl-7H-pyrrolo[2,3-d]pyrimidine (1) and 3-iodoaniline (2), and 3,3-dimethyl butanal (4), as the aldehyde, based on its stability and high boiling point (Scheme 1).We first assayed the reductive amination by performing the reaction between the aniline 3 and the aldehyde 4 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in dichloromethane using sodium sulphate (5 equiv.) as drying agent, and sodium triacetoxyborohydride (STAB-H, 5 equiv.) as reducing agent at rt overnight. Under these conditions, the conversion towards 5 determined by HPLC analysis was just 25%. This moderate conversion is in agreement with recently reported results on similar anilines of purine derivatives.24
1 ratio in dichloromethane using sodium sulphate (5 equiv.) as drying agent, and sodium triacetoxyborohydride (STAB-H, 5 equiv.) as reducing agent at rt overnight. Under these conditions, the conversion towards 5 determined by HPLC analysis was just 25%. This moderate conversion is in agreement with recently reported results on similar anilines of purine derivatives.24
This poor conversion made us consider to perform the reduction by hydrogenation using an H-cube equipment, which allows generation of H2 “in situ” and the application of high pressure and/or temperature. Thus, the initial assay carried out in the H-cube Pro™ involved a solution of aniline (3) and aldehyde (4) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in MeOH at 0.05 M, pumped with a flow rate of 0.5 mL min−1, applying a moderate temperature (65 °C) and pressure (20 Bar), employing a 30 mm CatCart™ loaded with 10% Pd/C. Under these conditions of direct reductive amination, the conversion towards the product 5 was improved up to 54%. Encouraged by these favorable results, we increased the pressure to 40 Bar, leading to an almost quantitative conversion towards product 5 (92% as determined by HPLC). So, the use of the H-cube reactor had not only facilitated the work up of the reaction, but had led to almost a 4-fold improvement in conversion towards the reductive amination product compared to reaction performed with STAB-H.
1 ratio in MeOH at 0.05 M, pumped with a flow rate of 0.5 mL min−1, applying a moderate temperature (65 °C) and pressure (20 Bar), employing a 30 mm CatCart™ loaded with 10% Pd/C. Under these conditions of direct reductive amination, the conversion towards the product 5 was improved up to 54%. Encouraged by these favorable results, we increased the pressure to 40 Bar, leading to an almost quantitative conversion towards product 5 (92% as determined by HPLC). So, the use of the H-cube reactor had not only facilitated the work up of the reaction, but had led to almost a 4-fold improvement in conversion towards the reductive amination product compared to reaction performed with STAB-H.
According to our working plan, we were interested in applying this procedure to highly functionalized aldehydes incorporating amino and/or ester groups (Scheme 2). As representative aldehydes, we selected N-Boc protected aldehydes where the amino group was either in a chain (6), or in a cycle (7); aldehydes derived from conveniently protected amino acids, such as 8, since amino acids are interesting functionalizing groups in medicinal chemistry;25 and finally an unsaturated aldehyde with an ethyl ester group (9), so that, under the hydrogenation conditions, the reduction of the double bond would also occur. The results obtained are shown in Scheme 2.
Starting with the reaction between the aniline 3 and the aldehyde 6, and applying the same conditions described above for the synthesis of 5 (CatCart™ Pd/C (10%, 30 mm), 65 °C, 40 Bar, flow: 0.5 mL min−1, 0.05 M in MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio), the conversion towards the reductive amination product 10 was quite poor (30%). The “flow-like” characteristics of H-cube made us considered a recirculation set-up,26,27 so that the reaction mixture could be pumped several times through the catalyst (a schematic representation is available as Fig. S1†). In this way, the conversion of the aniline towards the N-alkylaniline was followed by HPLC and the recirculation was stopped once the conversion did not progress. By following this recirculation approach, after 60 minutes, the conversion towards 10 had significantly improved (67%), allowing the isolation of 10 in 58% yield. By applying this recirculation protocol to the reaction between 3 and the aldehyde 7, an almost quantitative conversion was observed after 1 hour, and the product 11 was isolated in 88% yield. When these conditions were assayed for the reaction between 3 and the aldehyde 8, a very low conversion towards the N-substituted aniline 12 was observed after 60 minutes. Thus, the temperature was increased up to 100 °C and the reaction time extended up to 90 min leading a real improvement in the conversion towards 12 (76%), that was isolated in 73% yield. Finally, the reaction between 3 and the unsaturated aldehyde 9, under recirculation and at 65 °C, afforded the expected ester 13 with good conversion and yield after one hour. Thus, by applying a recirculation set-up and increasing the temperature when the conversion was poor, the substituted aniline derivatives 10–13 were obtained in yields higher than 57%.
1 molar ratio), the conversion towards the reductive amination product 10 was quite poor (30%). The “flow-like” characteristics of H-cube made us considered a recirculation set-up,26,27 so that the reaction mixture could be pumped several times through the catalyst (a schematic representation is available as Fig. S1†). In this way, the conversion of the aniline towards the N-alkylaniline was followed by HPLC and the recirculation was stopped once the conversion did not progress. By following this recirculation approach, after 60 minutes, the conversion towards 10 had significantly improved (67%), allowing the isolation of 10 in 58% yield. By applying this recirculation protocol to the reaction between 3 and the aldehyde 7, an almost quantitative conversion was observed after 1 hour, and the product 11 was isolated in 88% yield. When these conditions were assayed for the reaction between 3 and the aldehyde 8, a very low conversion towards the N-substituted aniline 12 was observed after 60 minutes. Thus, the temperature was increased up to 100 °C and the reaction time extended up to 90 min leading a real improvement in the conversion towards 12 (76%), that was isolated in 73% yield. Finally, the reaction between 3 and the unsaturated aldehyde 9, under recirculation and at 65 °C, afforded the expected ester 13 with good conversion and yield after one hour. Thus, by applying a recirculation set-up and increasing the temperature when the conversion was poor, the substituted aniline derivatives 10–13 were obtained in yields higher than 57%.
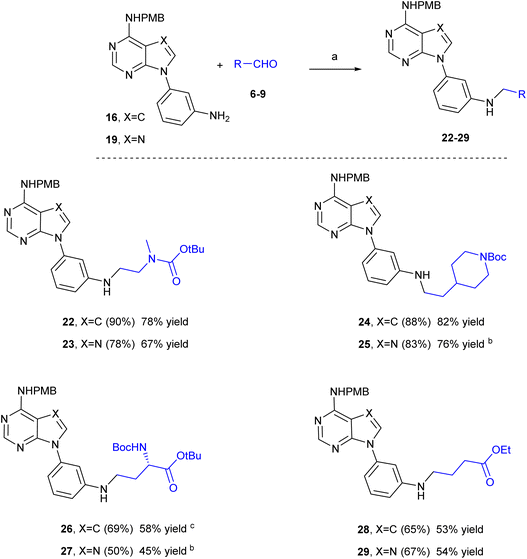
Our next step was to apply this reductive amination reaction to adenine-like derivatives. As mentioned in the introduction, a p-methoxylbenzylamino group was chosen as the precursor of the NH2 at the heterocyclic base since it could be removed by treatment with TFA and at the same time it should remain unaltered during the reductive amination reaction. Thus, treatment of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine 14 with p-methoxybenzyl amine in a sealed tube in isopropanol at 100 °C overnight led to the 4-substituted derivative 15 (Scheme 3). Reaction of 15 with 3-iodoaniline, following a synthetic procedure analogous to that described for the synthesis of 3, afforded the aniline derivative 16. On the other hand, and following a synthetic procedure described by us for the synthesis of 9-arylpurines,28 4,6-dichloropyrimidin-5-amine (17) reacted with 3-nitroaniline under MWI at 150 °C for 10 minutes to provide the pyrimidine derivative 18 in 67% yield. Reaction of 18 and trimethylorthoformate at 120 °C under MWI for 1 hour afforded the arylpurine 19. Then, the chlorine at position 6 in 19 was replaced by p-methoxylbenzylamino group by reaction with the corresponding benzylamine under MWI at 100 °C for 1 hour. Finally, reduction of the nitro group of 20 by treatment with SnCl2 in a EtOH/EtOAc mixture under reflux for 2 h afforded the aniline derivative 21.
Unfortunately, the solubility of the anilines 16 and 21 in MeOH was poor, even at the diluted standard conditions of 0.05 M. Since it is crucial that the reaction mixture is completely solubilized to avoid any clogging in the H-cube reactor, the solubility of 16 and 21 was tested in different mixtures of MeOH/DMF, being completely soluble with a MeOH/DMF ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then, aldehydes 6 to 9 were submitted to reductive amination reaction with the aniline derivatives 16 and 21 as shown in Scheme 4. To our surprise, the reaction between the aldehyde 6 and the 7-deazapurine aniline 16 after one-hour of recirculation under the previously used parameters (Pd/C (10%, 30 mm), 65 °C, 40 Bar, 0.5 mL min−1, 0.05 M in MeOH/DMF (4
1). Then, aldehydes 6 to 9 were submitted to reductive amination reaction with the aniline derivatives 16 and 21 as shown in Scheme 4. To our surprise, the reaction between the aldehyde 6 and the 7-deazapurine aniline 16 after one-hour of recirculation under the previously used parameters (Pd/C (10%, 30 mm), 65 °C, 40 Bar, 0.5 mL min−1, 0.05 M in MeOH/DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1
1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) showed only a 30% conversion towards 22. Interestingly, the HPLC chromatogram did not show any decomposition products, thus we decided to increase the temperature up to 100 °C and the pressure up to 60 Bar. Under these new conditions, the conversion of the aniline 16 towards the N-alkylaniline 22 improved significantly (90% conversion), so that the reductive amination product 22 was isolated in 78% yield.
1 molar ratio) showed only a 30% conversion towards 22. Interestingly, the HPLC chromatogram did not show any decomposition products, thus we decided to increase the temperature up to 100 °C and the pressure up to 60 Bar. Under these new conditions, the conversion of the aniline 16 towards the N-alkylaniline 22 improved significantly (90% conversion), so that the reductive amination product 22 was isolated in 78% yield.
These new parameters of pressure and temperature were used for the reaction of 16 with aldehydes 7 to 9, as well as for the reaction of the purine 21 with the aldehydes 6 to 9 (Scheme 4). The corresponding reductive amination products 23–29 were isolated in yields ranging from 50% to 88% in most cases after 60 min of recirculation. The only exceptions were compounds 25 and 27, that required 90 minutes of reaction, and compound 26, for which recirculation was maintained up to 120 min. The results obtained deserve some comments: (1) the aniline 16 afforded better conversions and isolated yields of the corresponding reductive amination products (22, 24, 26 and 28) than those obtained with the aniline 21 (products 23, 25, 27 and 29); (2) reactions involving the amino acid derivative 8 required longer reaction times and afforded lower conversions, but still the isolated yields of the resulting compounds 26 and 27 were 58 and 45%, respectively, (3) the esters 28 and 29 were obtained with good yields while no unsaturated derivatives were detected by HPLC.
In general, the conversions towards the reductive amination products obtained with the 7-deazaadenine and adenine derivatives (16 and 21, respectively) were lower than those obtained with the 6-methyl-7-deazapurine derivative 3. A possible explanation is that the presence of additional N atoms in 16 and 21 could affect the efficacy of the catalyst. Besides this, the solvent has been changed from neat MeOH in the case of the aniline 3 to a mixture of MeOH/DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the case of 16 and 21. To check if the solvent composition could affect the reaction, the aniline 3 was reacted with the aldehyde 4 in a single run, at 40 bar and 65 °C using MeOH/DMF (4
1) in the case of 16 and 21. To check if the solvent composition could affect the reaction, the aniline 3 was reacted with the aldehyde 4 in a single run, at 40 bar and 65 °C using MeOH/DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent. Under these conditions, the percentage of conversion into the product 5 was 70%, lower than the 92% achieved when, under the same conditions (see d in Scheme 1), neat MeOH was used as the solvent. Thus, the nature of the solvent affects the outcome of the reaction.
1) as solvent. Under these conditions, the percentage of conversion into the product 5 was 70%, lower than the 92% achieved when, under the same conditions (see d in Scheme 1), neat MeOH was used as the solvent. Thus, the nature of the solvent affects the outcome of the reaction.
It has been reported that nitroarenes can be used instead of anilines as starting materials for reductive aminations to provide N-substituted anilines.29–31 Thus, we decided to apply this shortcut to access to the substituted aniline 25 starting from the nitroarene 20 and the aldehyde 7. Unfortunately, the nitroderivative 20 was completely insoluble either in MeOH or MeOH/DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), so it had to be dissolved in neat DMF. Thus, an equimolecular 0.05 M solution of 20 and 7 in DMF was recirculated through the H-cube at 0.5 mL min−1, 100 °C and 60 bar, and the reaction was followed by HPLC/MS. Although the reduction of the nitro group in 20 towards the aniline 21 took place in the first 30 minutes, the conversion towards the reductive amination product 25 was quite poor reaching a 25% conversion after 4 hours of recirculation. Thus, in our case, using the nitroarene as starting material does not provide an advantage compared to the use of the aniline.
1), so it had to be dissolved in neat DMF. Thus, an equimolecular 0.05 M solution of 20 and 7 in DMF was recirculated through the H-cube at 0.5 mL min−1, 100 °C and 60 bar, and the reaction was followed by HPLC/MS. Although the reduction of the nitro group in 20 towards the aniline 21 took place in the first 30 minutes, the conversion towards the reductive amination product 25 was quite poor reaching a 25% conversion after 4 hours of recirculation. Thus, in our case, using the nitroarene as starting material does not provide an advantage compared to the use of the aniline.
As an additional step we considered of interest to evaluate if the compounds obtained could be subjected to a second reductive amination reaction to obtain unsymmetrically disubstituted anilines. To this end, we performed the reductive amination reaction between the N-alkylaniline 5 and the aldehyde 7 in MeOH at 100 °C and 60 bar (Scheme 5). After 90 minutes, a 18% conversion towards 30 was obtained. However, increasing the temperature up to 150 °C and keeping the pressure at 60 bar, improved the conversion towards 30 at 48% after 3 h. Although the conversion is moderate, this result illustrates that this reaction sequence can be applied to obtain unsymmetrically disubstituted anilines incorporating Boc-protecting groups.
 | ||
| Scheme 5 Synthesis of 30 through a second reductive amination reaction. (a) H-cube, 10% Pd/C (CatCart™, 30 mm), 150 °C, 60 Bar, 0.5 mL min−1, MeOH 0.05 M, recirculation (3 h), 46% (isolated yield). | ||
Finally, and in order to confirm that the here described synthetic strategy would lead to new adenine derivatives, compound 23 was chosen as a model substrate to carry out the concomitant deprotection of both the PMB and the Boc group. Following described conditions for removal of the PMB group,32 treatment of 23 in neat TFA at 70 °C overnight afforded the fully deprotected adenine derivative 31 in 89% yield (Scheme 6).
3. Conclusions
Reductive amination represents the most applied approach for the monoalkylation of primary amines. However, when applied to aniline derivatives of purine and purine-like heterocyclic bases even with simple aldehydes such as butanal and employing metal hydrides for the reduction step, very low yields of the substituted anilines were obtained. As an alternative we use the H-cube flow reactor technology for direct amination that combines high pressure of H2 generated “in situ” with high temperatures while it avoids the handling of metal hydrides. Moreover, a recirculation set-up allows to pump back the reaction mixture into the system till conversion towards the reductive amination product does not further evolve. In general, the HPLC chromatograms of the reactions were clean so that reaction mixtures were easily purified. As shown with the examples here reported, the set-up procedure allows the presence of acid sensitive groups both at the heterocyclic base and at the aldehyde so that highly functionalized N-monosubstituted anilines have been obtained without the need of acid catalysts. Moreover, when an unsaturated aldehyde was used, reduction of the double bond also took place as expected leading to the corresponding saturated derivate. Interestingly, the mono-alkylated derivatives can be subjected to a second reductive amination reaction in order to obtain unsymmetrically disubstituted derivatives. Still, this second reaction required more drastic conditions. Finally, the here obtained aniline derivatives can be further explored for medicinal chemistry applications including those therapeutic areas where purine and purine-like compounds are considered privileged scaffolds (i.e. kinases or methyltransferases).4. Experimental section
Melting points were measured on a M170 apparatus (Mettler Toledo, Columbus, Ohio, USA) apparatus and are uncorrected. The elemental analysis was performed with a CHN-O-RAPID instrument (Heraus, Hanau, Germany). The elemental compositions of the compounds agreed within ±0.4% of the calculated values.1H and 13C NMR spectra were recorded on a Varian INNOVA (now Agilent, Santa Clara, CA, USA) a Varian INNOVA-400 operating at 399 MHz (1H) and 99 MHz (13C), respectively, and a VARIAN SYSTEM-500 operating at 499 MHz (1H) and 125 MHz (13C), respectively. Monodimensional 1H and 13C spectra were obtained using standard conditions. Chemical shifts were recorded in units of parts per million and referenced to residual solvent peaks (CHCl3: 7.26 ppm for 1H NMR, 77.16 ppm for 13C NMR; DMSO-d6: 2.50 ppm for 1H NMR, 39.51 ppm for 13C NMR).
Reductive amination reactions were performed with a standard H-Cube Pro™ flow reactor (ThalesNano Technology, Inc. Budapest, Hungary) equipped with a 30 mm cartridge loaded with 10% Pd/C.
Microwave reactions were performed using the Biotage Initiator 2.0 single-mode cavity instrument from Biotage (Uppsala). Experiments were carried out in sealed microwave process vials using the standard absorbance level (400 W maximum power). The temperature was measured with an IR sensor on the outside of the reaction vessel.
Compounds were also analysed by HPLC/MS with a e2695 LC (Waters, Milford, Massachusetts, USA), coupled to a Waters 2996 photodiode array detector and a Waters Micromass ZQ. The column used is a Waters SunFire C18 2.1 × 50 mm, 3.5 μm, and the mobile phases were A: acetonitrile and B: H2O, together with a constant 5% of C (H2O with 2% formic acid) to assure 0.1% of formic acid along the run. When required, high-resolution mass spectrometry (HRMS) analysis was performed using a Q-TOF instrument (QTOF Bruker Impact II).
The conversion of starting material to reaction products was followed by HPLC analysis performed in Agilent 1120 compact LC, column ACE 5 C18-300 (15 cm × 4.6 mm), UV detection was performed at λ = 254 nm, and the flow rate was 1 mL min−1, using as mobile phase A CH3CN and as mobile phase B H2O (containing 0.05% TFA).
Analytical TLC was performed on silica gel 60 F254 (Merck, Dramstand, Germany)-precoated plates (0.2 mm). Spots were detected under UV light (254 nm) and/or charring with ninhydrin or phosphomolybdic acid.
Separations on silica gel were performed by preparative centrifugal circular thin-layer chromatography (CCTLC) on a Chromatotron® (Kiesegel 60 PF254 gipshaltig (Merck)), with a layer thickness of 1 and 2 mm and a flow rate of 4 or 8 mL min−1, respectively.
General procedure for the reaction of anilines with aldehydes in MeOH (general procedure A)
A solution containing the aniline (0.4 mmol) and the aldehyde (0.4 mmol) in 8 mL of MeOH was recirculated through a 30 mm CatCart™ in the H-cube at 40 Bar, 100% of Hydrogen production, at 65 °C and a flow rate of 0.5 mL min−1. The product conversion was followed by HPLC-UV analysis at 254 nm. Reaction times longer than 30 min means that recirculation has been used. Once the reaction ends, the pressure was released, and the system was further eluted with 40 mL of MeOH. Volatiles were removed and the residue was purified as specified for each compound.General procedure for the reaction of anilines with aldehydes in MeOH/DMF (general procedure B)
A solution containing the aniline (0.4 mmol) and the aldehyde (0.4 mmol) in 8 mL of MeOH/DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture was recirculated through a 30 mm CatCart™ in the H-cube at 60 Bar, 100% of Hydrogen production, at 100 °C and a flow rate of 0.5 mL min−1. The product conversion was followed by HPLC-UV analysis at 254 nm. Once the reaction ends, the pressure was released, and the system was further eluted with 40 mL of pure MeOH. Volatiles were removed and the residue was purified as specified for each compound.
1) mixture was recirculated through a 30 mm CatCart™ in the H-cube at 60 Bar, 100% of Hydrogen production, at 100 °C and a flow rate of 0.5 mL min−1. The product conversion was followed by HPLC-UV analysis at 254 nm. Once the reaction ends, the pressure was released, and the system was further eluted with 40 mL of pure MeOH. Volatiles were removed and the residue was purified as specified for each compound.
3-(4-Methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)aniline (3)
A solution containing 4-methyl-7H-pyrrolo[2,3-d]pyrimidine (500 mg, 3.75 mmol), 3-iodoaniline (980 mg, 4.51 mmol), K3PO4 (1.75 g, 8.27 mmol), copper iodide (50 mg, 0.26 mmol) and trans-N,N′-dimethylcyclohexane-1,2-diamine (80 mg, 0.56 mmol) in 1,4-dioxane (12 mL) was degassed with argon for 30 minutes and then, heated at 100 °C for 16 h. The resulting mixture was cooled to rt, filtered through a Celite pad, and washed with EtOAc (50 mL). Then, water (20 mL) was added, and the aqueous phase was further extracted with EtOAc (3 × 15 mL). The combined organic phases were washed with brine solution (25 mL), dried over anhydrous sodium sulfate, filtered, and evaporated. The product was purified by chromatography on silica gel column (EtOAc) to yield 3 (769 mg, 91% yield) as a pale orange solid. Mp: 141–143 °C. MS (ES, positive mode): m/z 225 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 2.73 (s, 3H), 3.84 (br s, 2H), 6.55–6.78 (m, 2H), 6.97 (ddd, J = 8.0, 2.1, 0.9 Hz, 1H), 7.05 (t, J = 2.1 Hz, 1H), 7.23 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 3.7 Hz, 1H), 8.77 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.6, 100.5, 110.6, 113.8, 118.8, 127.8, 130.3, 138.5, 147.6, 150.1, 151.9, 159.8. Anal. calc. for C13H12N4: C, 69.62; H, 5.39; N, 24.98. Found: C, 69.37; H, 5.55; N, 24.81.N-(3,3-Dimethylbutyl)-3-(4-methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)aniline (5)
Following the general procedure A, 3 (90 mg, 0.40 mmol) reacted with 3,3-dimethylbutanal (40 mg, 0.40 mmol) in MeOH (8 mL). After 30 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (5, 104 mg, 85% yield) as a gray, white solid. Mp: 125–127 °C. MS (ES, positive mode): m/z 309 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 0.97 (s, 9H), 1.29–2.12 (m, 2H), 2.77 (s, 3H), 3.01–3.36 (m, 2H), 6.59 (ddd, J = 8.3, 2.3, 0.9 Hz, 1H), 6.67 (d, J = 3.7 Hz, 1H), 6.92 (ddd, J = 7.9, 2.1, 0.9 Hz, 1H), 6.95 (t, J = 2.1 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.5, 29.6, 30.0, 40.2, 43.3, 100.3, 108.2, 111.3, 112.3, 118.7, 128.0, 130.1, 138.5, 149.6, 150.1, 151.8, 159.6. Anal. calc. for C19H24N4: C, 73.99; H, 7.84; N, 18.17. Found: C, 73.70; H, 7.91; N, 18.00.
3). The compound was obtained (5, 104 mg, 85% yield) as a gray, white solid. Mp: 125–127 °C. MS (ES, positive mode): m/z 309 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 0.97 (s, 9H), 1.29–2.12 (m, 2H), 2.77 (s, 3H), 3.01–3.36 (m, 2H), 6.59 (ddd, J = 8.3, 2.3, 0.9 Hz, 1H), 6.67 (d, J = 3.7 Hz, 1H), 6.92 (ddd, J = 7.9, 2.1, 0.9 Hz, 1H), 6.95 (t, J = 2.1 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.5, 29.6, 30.0, 40.2, 43.3, 100.3, 108.2, 111.3, 112.3, 118.7, 128.0, 130.1, 138.5, 149.6, 150.1, 151.8, 159.6. Anal. calc. for C19H24N4: C, 73.99; H, 7.84; N, 18.17. Found: C, 73.70; H, 7.91; N, 18.00.
tert-Butyl methyl(2-((3-(4-methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino) ethyl) carbamate (10)
Following the general procedure A, 3 (86 mg, 0.38 mmol) reacted with N-Boc-(methylamino)acetaldehyde (69 mg, 0.38 mmol) in MeOH (8 mL). After 60 min, volatiles were removed and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (10, 84 mg, 58% yield) as a pale-yellow oil. MS (ES, positive mode): m/z 382 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.45 (s, 9H), 2.77 (s, 3H), 2.90 (s, 3H), 3.33 (t, J = 6.0 Hz, 2H), 3.52 (br s, 2H), 6.59 (dd, J = 1.7, 8.4 Hz, 1H), 6.68 (d, J = 3.7 Hz, 1H), 6.88–7.05 (m, 2H), 7.30 (t, J = 8.1 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.80 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.4, 28.4, 34.9, 41.9, 42.6, 47.9, 79.9, 100.4, 107.7, 108.0, 111.2, 112.3, 112.6, 118.7, 128.1, 128.4, 130.2, 132.0, 138.5, 150.1, 151.6, 159.5. Anal. calc. for (C21H27N5O2·0.5H2O): C, 64.59; H, 7.23; N, 17.94. Found: C, 64.69; H, 7.13; N, 18.36.
3). The compound was obtained (10, 84 mg, 58% yield) as a pale-yellow oil. MS (ES, positive mode): m/z 382 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.45 (s, 9H), 2.77 (s, 3H), 2.90 (s, 3H), 3.33 (t, J = 6.0 Hz, 2H), 3.52 (br s, 2H), 6.59 (dd, J = 1.7, 8.4 Hz, 1H), 6.68 (d, J = 3.7 Hz, 1H), 6.88–7.05 (m, 2H), 7.30 (t, J = 8.1 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.80 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.4, 28.4, 34.9, 41.9, 42.6, 47.9, 79.9, 100.4, 107.7, 108.0, 111.2, 112.3, 112.6, 118.7, 128.1, 128.4, 130.2, 132.0, 138.5, 150.1, 151.6, 159.5. Anal. calc. for (C21H27N5O2·0.5H2O): C, 64.59; H, 7.23; N, 17.94. Found: C, 64.69; H, 7.13; N, 18.36.
tert-Butyl 4-(2-((3-(4-methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino)ethyl)piperidine-1-carboxylate (11)
Following the general procedure A, 3 (90 mg, 0.40 mmol) reacted with tert-butyl 4-(2-oxoethyl)piperidine-1-carboxylate (90 mg, 0.4 mmol) in MeOH (8 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (11, 153 mg, 88% yield) as a white solid. Mp: 132–135 °C. MS (ES, positive mode): m/z 436 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.02–1.30 (m, 2H), 1.45 (s, 9H), 1.53–1.66 (m, 3H), 1.70 (d, J = 13.0 Hz, 2H), 2.69 (t, J = 12.9 Hz, 2H), 2.78 (s, 3H), 3.20 (t, J = 6.9 Hz, 2H), 3.82 (br s, 1H), 4.09 (s, 2H), 6.59 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 6.68 (d, J = 3.7 Hz, 1H), 6.93 (ddd, J = 7.8, 2.1, 0.9 Hz, 1H), 6.97 (t, J = 2.2 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.5, 28.5, 32.1, 33.8, 36.1, 41.2, 43.9, 79.3, 100.4, 108.3, 111.3, 112.5, 118.7, 128.0, 130.2, 138.5, 149.3, 150.1, 151.8, 154.9, 159.7. Anal. calc. for (C25H33N5O2·0.5H2O): C, 67.54; H, 7.71; N, 15.75. Found: C, 67.94; H, 7.55; N, 15.67.
3). The compound was obtained (11, 153 mg, 88% yield) as a white solid. Mp: 132–135 °C. MS (ES, positive mode): m/z 436 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.02–1.30 (m, 2H), 1.45 (s, 9H), 1.53–1.66 (m, 3H), 1.70 (d, J = 13.0 Hz, 2H), 2.69 (t, J = 12.9 Hz, 2H), 2.78 (s, 3H), 3.20 (t, J = 6.9 Hz, 2H), 3.82 (br s, 1H), 4.09 (s, 2H), 6.59 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 6.68 (d, J = 3.7 Hz, 1H), 6.93 (ddd, J = 7.8, 2.1, 0.9 Hz, 1H), 6.97 (t, J = 2.2 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.5, 28.5, 32.1, 33.8, 36.1, 41.2, 43.9, 79.3, 100.4, 108.3, 111.3, 112.5, 118.7, 128.0, 130.2, 138.5, 149.3, 150.1, 151.8, 154.9, 159.7. Anal. calc. for (C25H33N5O2·0.5H2O): C, 67.54; H, 7.71; N, 15.75. Found: C, 67.94; H, 7.55; N, 15.67.
tert-Butyl (S)-2-((tert-butoxycarbonyl)amino)-4-((3-(4-methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)-amino)butanoate (12)
Following the general procedure A, at 100 °C, 3 (89 mg, 0.40 mmol) reacted with 8 (see ESI† for its synthesis) (109 mg, 0.40 mmol) in MeOH (8 mL). After 90 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (12, 140 mg, 73% yield) as a white solid. Mp: 61–63 °C. MS (ES, positive mode): m/z 482 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H), 1.45 (s, 9H), 1.83 (m, 1H), 2.17 (m, 1H), 2.78 (s, 3H), 3.30 (m, 2H), 4.08–4.58 (m, 2H), 5.25 (d, J = 8.1 Hz, 1H), 6.61 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 6.68 (d, J = 3.7 Hz, 1H), 6.90–7.08 (m, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.4, 28.0, 28.3, 32.7, 40.0, 52.0, 80.0, 82.4, 100.4, 108.4, 111.6, 112.6, 118.7, 128.1, 130.2, 138.5, 149.0, 150.1, 151.7, 155.7, 159.5, 171.7. Anal. calc. for (C26H35N5O4·0.5H2O): C, 63.65; H, 7.40; N, 14.28. Found: C, 64.01; H, 7.37; N, 14.23.
3). The compound was obtained (12, 140 mg, 73% yield) as a white solid. Mp: 61–63 °C. MS (ES, positive mode): m/z 482 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H), 1.45 (s, 9H), 1.83 (m, 1H), 2.17 (m, 1H), 2.78 (s, 3H), 3.30 (m, 2H), 4.08–4.58 (m, 2H), 5.25 (d, J = 8.1 Hz, 1H), 6.61 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 6.68 (d, J = 3.7 Hz, 1H), 6.90–7.08 (m, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.4, 28.0, 28.3, 32.7, 40.0, 52.0, 80.0, 82.4, 100.4, 108.4, 111.6, 112.6, 118.7, 128.1, 130.2, 138.5, 149.0, 150.1, 151.7, 155.7, 159.5, 171.7. Anal. calc. for (C26H35N5O4·0.5H2O): C, 63.65; H, 7.40; N, 14.28. Found: C, 64.01; H, 7.37; N, 14.23.
Ethyl 4-((3-(4-methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino) butanoate (13)
Following the general procedure A, 3 (90 mg, 0.40 mmol) reacted with ethyl-4-oxobut-2-enoate (51 mg, 0.40 mmol) in MeOH (8 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (13, 88 mg, 65% yield) as a pale-yellow oil. MS (ES, positive mode): m/z 339 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.25 (t, J = 7.1 Hz, 3H), 1.98 (m, 2H), 2.44 (t, J = 7.1 Hz, 2H), 2.77 (s, 3H), 3.23 (t, J = 6.9 Hz, 2H), 4.02 (br s, 1H), 4.14 (q, J = 7.1 Hz, 2H), 6.90–7.08 (m, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.2, 21.5, 24.5, 31.9, 43.3, 60.6, 100.4, 108.3, 111.4, 112.6, 118.7, 128.1, 130.2, 138.5, 149.2, 150.1, 151.7, 159.6, 173.4. Anal. calc. for (C19H22N4O2·0.33H2O): C, 66.26; H, 6.63; N, 16.27. Found: C, 66.67; H, 6.66 N, 16.56.
3). The compound was obtained (13, 88 mg, 65% yield) as a pale-yellow oil. MS (ES, positive mode): m/z 339 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.25 (t, J = 7.1 Hz, 3H), 1.98 (m, 2H), 2.44 (t, J = 7.1 Hz, 2H), 2.77 (s, 3H), 3.23 (t, J = 6.9 Hz, 2H), 4.02 (br s, 1H), 4.14 (q, J = 7.1 Hz, 2H), 6.90–7.08 (m, 2H), 7.29 (t, J = 8.0 Hz, 1H), 7.46 (d, J = 3.7 Hz, 1H), 8.81 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.2, 21.5, 24.5, 31.9, 43.3, 60.6, 100.4, 108.3, 111.4, 112.6, 118.7, 128.1, 130.2, 138.5, 149.2, 150.1, 151.7, 159.6, 173.4. Anal. calc. for (C19H22N4O2·0.33H2O): C, 66.26; H, 6.63; N, 16.27. Found: C, 66.67; H, 6.66 N, 16.56.
N-(4-Methoxybenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (15)
To a solution of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (1 g, 6.51 mmol) in isopropyl alcohol (32 mL), (4-methoxyphenyl)methanamine (846 μL, 6.51 mmol) and DIPEA (1.10 mL, 6.51 mmol) were added. The mixture was stirred at 100 °C for 16 h, cooled and filtered. The isolated solid was washed with isobutanol (2 × 10 mL) and hexane (2 × 20 mL) and the compound thus obtained (15, 900 mg, 54% yield) as a white solid, was used without further purification. MS (ES, positive mode): m/z 255 (M + H)+. 1H NMR data are in agreement with those previously described, but synthesized through a different chemical procedure.337-(3-Aminophenyl)-N-(4-methoxybenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (16)
To a solution of 15 (500 mg, 1.97 mmol), copper iodide (5.2 mg, 0.03 mmol) and K3PO4 (834 mg, 3.93 mmol) in 1,4-dioxane (10 mL), 3-iodoaniline (237 μL, 1.97 mmol) and trans-N,N′-dimethylcyclohexane-1,2-amine (47 μL, 0.31 mmol) was added. The mixture was degassed with argon for 30 min, and then heated at 100 °C for 16 h. The resulting mixture was cooled to rt, diluted with EtOAc (20 mL) and filtered through a Celite pad. The filtrate obtained was washed with H2O (3 × 10 mL) and brine solution (20 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness. The product was purified by chromatography on silica gel column (hexane/EtOAc/MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), and obtained (16, 544 mg, 80% yield) as a white solid. Mp: 201–203 °C. MS (ES, positive mode): m/z 346 (M + H)+. 1H NMR (400 MHz, DMSO-d6) δ: 3.72 (s, 3H), 4.67 (d, J = 6.0 Hz, 2H), 5.32 (s, 2H), 6.53 (dd, J = 8.1, 2.3 Hz, 1H), 6.77–6.84 (m, 2H), 6.85–6.91 (m, 2H), 7.01 (t, J = 2.1 Hz, 1H), 7.12 (t, J = 8.0 Hz, 1H), 7.26–7.32 (m, 2H), 7.41 (d, J = 3.6 Hz, 1H), 8.06 (t, J = 6.0 Hz, 1H), 8.18 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 43.1, 55.6, 100.5, 104.2, 109.5, 111.2, 112.4, 114.2, 124.3, 129.1, 129.9, 132.6, 139.2, 149.5, 150.0, 152.6, 156.8, 158.7. Anal. calc. for (C20H19N5O. 0.25 H2O): C, 68.65; H, 5.62; N, 20.02. Found: C, 68.79; H, 5.59 N, 19.70.
0.1), and obtained (16, 544 mg, 80% yield) as a white solid. Mp: 201–203 °C. MS (ES, positive mode): m/z 346 (M + H)+. 1H NMR (400 MHz, DMSO-d6) δ: 3.72 (s, 3H), 4.67 (d, J = 6.0 Hz, 2H), 5.32 (s, 2H), 6.53 (dd, J = 8.1, 2.3 Hz, 1H), 6.77–6.84 (m, 2H), 6.85–6.91 (m, 2H), 7.01 (t, J = 2.1 Hz, 1H), 7.12 (t, J = 8.0 Hz, 1H), 7.26–7.32 (m, 2H), 7.41 (d, J = 3.6 Hz, 1H), 8.06 (t, J = 6.0 Hz, 1H), 8.18 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 43.1, 55.6, 100.5, 104.2, 109.5, 111.2, 112.4, 114.2, 124.3, 129.1, 129.9, 132.6, 139.2, 149.5, 150.0, 152.6, 156.8, 158.7. Anal. calc. for (C20H19N5O. 0.25 H2O): C, 68.65; H, 5.62; N, 20.02. Found: C, 68.79; H, 5.59 N, 19.70.
6-Chloro-N4-(3-nitrophenyl)pyrimidine-4,5-diamine (18)
A solution of 4,6-dichloropyrimidin-5-amine (500 mg, 3.05 mmol), 3-nitroaniline (420 mg, 3.05 mmol) and concentrated HCl (150 μL) in isobutanol (12 mL) was heated under MWI at 150 °C for 10 minutes. The resulting solid was filtered and washed with isobutanol (2 × 10 mL), and later with hexane (2 × 20 mL), and the compound obtained (18, 542 mg, 68% yield) as an amorphous yellow solid, was used without further purification. MS (ES, positive mode): m/z 266 (M + H)+ with a chloro isotopic pattern. 1H NMR (400 MHz, DMSO-d6) δ: 6.30 (br s, 2H), 7.62 (t, J = 8.2 Hz, 1H), 7.87 (ddd, J = 8.2, 2.3, 0.9 Hz, 1H), 7.98 (s, 1H), 8.20 (ddd, J = 8.2, 2.2, 1.0 Hz, 1H), 8.79 (t, J = 2.2 Hz, 1H), 9.29 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 114.4, 117.1, 126.1, 126.4, 130.3, 139.2, 141.7, 144.6, 148.4, 148.5.6-Chloro-9-(3-nitrophenyl)-9H-purine (19)
A solution of 18 (2.0 g, 7.55 mmol) in trimethylorthoformate (15 mL) and concentrated HCl (600 μL) was heated under MWI at 120 °C for 1 hour. The resulting solid was filtered and washed with hexane (20 mL) and the compound obtained (19, 1.6 g, 80% yield) as a yellow-orange solid used, was used without further purification. MS (ES, positive mode): m/z 276 (M + H)+ with a chloro isotopic pattern. 1H NMR (400 MHz, DMSO-d6) δ: 7.96 (t, J = 8.2 Hz, 1H), 8.38 (ddd, J = 8.3, 2.3, 0.9 Hz, 1H), 8.42 (ddd, J = 8.1, 2.1, 0.9 Hz, 1H), 8.90 (t, J = 2.2 Hz, 1H), 8.92 (s, 1H), 9.28 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 118.7, 123.4, 130.1, 131.6, 132.1, 135.5, 146.6, 148.7, 150.2, 152.0, 152.9.9-(3-Nitrophenyl)-N-(4-methoxybenzyl)-9H-purin-6-amine (20)
A solution of 19 (825 mg, 3.06 mmol), p-methoxybenzylamine (411 mg, 391 μL, 3.06 mmol) and DIPEA (387 mg, 521 μL, 3.06 mmol), in isopropanol (15 mL) was heated under MWI at 120 °C for 30 minutes. The resulting solid was filtered and washed with isopropanol (2 × 15 mL) and hexane (2 × 25 mL) and the compound obtained (20, 1.02 g, 90% yield) as a yellow solid, Mp: 176–178 °C. MS (ES, positive mode): m/z 377 (M + H)+. 1H NMR (400 MHz, DMSO-d6) δ: 3.70 (s, 3H3), 4.66 (s, 2H), 6.86 (m, 2H), 7.30 (m, 2H), 7.88 (t, J = 8.2 Hz, 1H), 8.28 (ddd, J = 8.3, 2.3, 0.9 Hz, 1H), 8.34 (s, 1H), 8.43 (dt, J = 8.2, 1.5 Hz, 1H), 8.53 (br s, 1H), 8.81 (s, 1H), 8.94 (t, J = 2.2 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 42.9, 55.5, 114.1, 117.6, 122.2, 128.9, 129.0, 132.3, 136.6, 139.8, 148.7, 153.8, 158.6. Anal. calc. for (C19H16N6O3): C, 60.63; H, 4.29; N, 22.33. Found: C, 59.03; H, 4.27; N, 21.99.9-(3-Aminophenyl)-N-(4-methoxybenzyl)-9H-purin-6-amine (21)
To a solution of 20 (1 g, 2.61 mmol) in EtOAc (8 mL) and EtOH (10 mL), tin(II) chloride (2.5 g, 13.32 mmol) was added. The mixture was refluxed for 2 hours at 80 °C. Then, it was cooled and the reaction was basified until pH 10 with 10 mL of NaOH 2.5 M in water. The slurry was filtered through a Celite pad, which was washed with 50 mL of MeOH. Volatiles were removed, and the residue was sonicated 15 minutes with 50 mL of EtOAc and 10 mL of brine solution. Then, the aqueous phase was extracted with EtOAc (3 × 20 mL). The organic layers were dried over anhydrous sodium sulfate, filtered, evaporated, and the compound was obtained (21, 809 mg, 88% yield) as a pale brown solid, without further purification. MS (ES, positive mode): m/z 347 (M + H)+. 1H NMR (400 MHz, DMSO-d6) δ: 3.71 (s, 3H), 4.65 (s, 2H), 5.44 (br s, 2H), 6.61 (ddd, J = 8.1, 2.2, 1.0 Hz, 1H), 6.82–6.90 (m, 3H), 7.04 (t, J = 2.1 Hz, 1H), 7.17 (t, J = 8.0 Hz, 1H), 7.30 (d, J = 8.6 Hz, 2H), 8.25 (s, 1H), 8.36 (br s, 1H), 8.45 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 42.8, 55.5, 108.8, 110.5, 113.4, 114.1, 129.0, 130.3, 132.5, 136.2, 140.1, 150.3, 153.4, 155.1, 158.6.tert-Butyl (2-((3-(4-((4-methoxybenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino)ethyl)-(methyl)carbamate (22)
Following the general procedure B, 16 (110 mg, 0.32 mmol) reacted with N-Boc-(methylamino)acetaldehyde (55 mg, 0.32 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (3 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1
4) (3 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The compound was obtained (22, 125 mg, 78% yield) as a white solid. Mp: 56–58 °C. MS (ES, positive mode): m/z 503 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.46 (s, 9H), 2.90 (s, 3H), 3.32 (m, 2H), 3.51 (m, 2H), 3.81 (s, 3H), 4.80 (d, J = 5.6 Hz, 2H), 6.45 (d, J = 3.7 Hz, 1H), 6.58 (dd, J = 8.8, 1.9 Hz, 1H), 6.86–6.95 (m, 4H), 7.22 (d, J = 3.7 Hz, 1H), 7.26 (t, J = 8.2 Hz, 1H), 7.31–7.38 (m, 2H), 8.40 (s, 1H). 13C NMR (126 MHz, CDCl3) δ: 28.5, 34.7, 34.7, 45.1, 41.9, 48.0, 55.4, 80.1, 99.4, 103.6, 108.1, 111.3, 112.7, 114.3, 125.1, 129.1, 130.2, 130.4, 138.8, 149.3, 149.8, 151.7, 155.9, 159.3. Anal. calc. for (C28H34N6O3·0.5H2O): C, 66.12; H, 6.87; N, 16.52. Found: C, 66.07; H, 6.75; N, 16.15.
2). The compound was obtained (22, 125 mg, 78% yield) as a white solid. Mp: 56–58 °C. MS (ES, positive mode): m/z 503 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.46 (s, 9H), 2.90 (s, 3H), 3.32 (m, 2H), 3.51 (m, 2H), 3.81 (s, 3H), 4.80 (d, J = 5.6 Hz, 2H), 6.45 (d, J = 3.7 Hz, 1H), 6.58 (dd, J = 8.8, 1.9 Hz, 1H), 6.86–6.95 (m, 4H), 7.22 (d, J = 3.7 Hz, 1H), 7.26 (t, J = 8.2 Hz, 1H), 7.31–7.38 (m, 2H), 8.40 (s, 1H). 13C NMR (126 MHz, CDCl3) δ: 28.5, 34.7, 34.7, 45.1, 41.9, 48.0, 55.4, 80.1, 99.4, 103.6, 108.1, 111.3, 112.7, 114.3, 125.1, 129.1, 130.2, 130.4, 138.8, 149.3, 149.8, 151.7, 155.9, 159.3. Anal. calc. for (C28H34N6O3·0.5H2O): C, 66.12; H, 6.87; N, 16.52. Found: C, 66.07; H, 6.75; N, 16.15.
tert-Butyl (2-((3-(6-((4-methoxybenzyl)amino)-9H-purin-9-yl)phenyl)amino)ethyl) (methyl) carbamate (23)
Following the general procedure B, 21 (100 mg, 0.29 mmol) reacted with N-Boc-(methylamino)acetaldehyde (50 mg, 0.29 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (6 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1
4) (6 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The compound was obtained (23, 97 mg, 67% yield) as a white solid. Mp: 82–84 °C. MS (ES, positive mode): m/z 504 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.39 (s, 9H), 2.84 (s, 3H), 3.25 (t, J = 5.9 Hz, 1H), 3.28–3.31 (m, 2H), 3.73 (s, 3H), 4.69–4.84 (m, 2H), 6.07 (br s, 1H), 6.57 (d, 7.8 Hz, 1H), 6.83–6.88 (m, 3H), 7.21–7.37 (m, 3H), 7.94 (s, 1H), 8.38 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 28.4, 34.9, 41.8, 42.6, 47.9, 55.3, 80.0, 106.9, 107.0, 111.7, 112.2, 114.1, 120.1, 129.2, 130.5, 135.8, 139.3, 149.2, 149.5, 153.6, 155.7, 157.1, 159.1. Anal. calc. for (C27H33N7O3): C, 64.39; H, 6.61; N, 19.47. Found: C, 63.92 H, 6.75; N, 19.07.
1). The compound was obtained (23, 97 mg, 67% yield) as a white solid. Mp: 82–84 °C. MS (ES, positive mode): m/z 504 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.39 (s, 9H), 2.84 (s, 3H), 3.25 (t, J = 5.9 Hz, 1H), 3.28–3.31 (m, 2H), 3.73 (s, 3H), 4.69–4.84 (m, 2H), 6.07 (br s, 1H), 6.57 (d, 7.8 Hz, 1H), 6.83–6.88 (m, 3H), 7.21–7.37 (m, 3H), 7.94 (s, 1H), 8.38 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 28.4, 34.9, 41.8, 42.6, 47.9, 55.3, 80.0, 106.9, 107.0, 111.7, 112.2, 114.1, 120.1, 129.2, 130.5, 135.8, 139.3, 149.2, 149.5, 153.6, 155.7, 157.1, 159.1. Anal. calc. for (C27H33N7O3): C, 64.39; H, 6.61; N, 19.47. Found: C, 63.92 H, 6.75; N, 19.07.
tert-Butyl 4-(2-((3-(4-((4-methoxybenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino)ethyl)pipe-ridine-1-carboxylate (24)
Following the general method B, 16 (138 mg, 0.4 mmol) reacted with tert-butyl 4-(2-oxoethyl)piperidine-1-carboxylate (91 mg, 0.4 mmol). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (24, 182 mg, 82% yield) as a white solid. Mp: 68–70 °C. MS (ES, positive mode): m/z 557 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.11–1.21 (m, 2H), 1.46 (s, 9H), 1.53–1.63 (m, 3H), 1.69 (m, 2H), 2.69 (m, 2H), 3.18 (t, J = 6.9 Hz, 2H), 3.81 (s, 3H), 4.12 (m, 2H), 4.79 (d, J = 5.5 Hz, 2H), 6.45 (d, J = 3.6 Hz, 1H), 6.57 (dd, J = 8.0, 1.7 Hz, 1H), 6.86–6.95 (m, 4H), 7.21 (d, J = 3.6 Hz, 1H), 7.26 (t, J = 8.0 Hz, 2H), 7.31–7.37 (m, 2H), 8.41 (1H, s). 13C NMR (126 MHz, CDCl3) δ: 28.3, 32.1, 33.8, 36.0, 41.2, 44.1, 45.1, 55.4, 79.4, 99.4, 103.5, 108.6, 111.3, 112.8, 114.4, 125.0, 129.0, 130.1, 138.3, 149.3, 149.5, 150.9, 154.8, 155.6, 158.9, 159.2. Anal. calc. for (C32H40N6O3): C, 69.04; H, 7.24; N, 15.10. Found: C, 68.69; H, 7.01; N, 14.85.
3). The compound was obtained (24, 182 mg, 82% yield) as a white solid. Mp: 68–70 °C. MS (ES, positive mode): m/z 557 (M + H)+. 1H NMR (400 MHz, CDCl3) δ: 1.11–1.21 (m, 2H), 1.46 (s, 9H), 1.53–1.63 (m, 3H), 1.69 (m, 2H), 2.69 (m, 2H), 3.18 (t, J = 6.9 Hz, 2H), 3.81 (s, 3H), 4.12 (m, 2H), 4.79 (d, J = 5.5 Hz, 2H), 6.45 (d, J = 3.6 Hz, 1H), 6.57 (dd, J = 8.0, 1.7 Hz, 1H), 6.86–6.95 (m, 4H), 7.21 (d, J = 3.6 Hz, 1H), 7.26 (t, J = 8.0 Hz, 2H), 7.31–7.37 (m, 2H), 8.41 (1H, s). 13C NMR (126 MHz, CDCl3) δ: 28.3, 32.1, 33.8, 36.0, 41.2, 44.1, 45.1, 55.4, 79.4, 99.4, 103.5, 108.6, 111.3, 112.8, 114.4, 125.0, 129.0, 130.1, 138.3, 149.3, 149.5, 150.9, 154.8, 155.6, 158.9, 159.2. Anal. calc. for (C32H40N6O3): C, 69.04; H, 7.24; N, 15.10. Found: C, 68.69; H, 7.01; N, 14.85.
tert-Butyl 4-(2-((3-(6-((4-methoxybenzyl)amino)-9H-purin-9-yl)phenyl)amino)ethyl)piperidine-1-carboxy-late (25)
Following the general procedure B, 16 (138 mg, 0.4 mmol) reacted with tert-butyl 4-(2-oxoethyl)piperidine-1-carboxylate (91 mg, 0.4 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (8 mL). After 90 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 2
4) (8 mL). After 90 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The compound was obtained (25, 169 mg, 76% yield) as a white solid. Mp: 70–72 °C. MS (ES, positive mode): m/z 558. 1H NMR (400 MHz, DMSO-d6) δ: 1.02 (m, 2H), 1.39 (s, 9H), 1.47–1.60 (m, 3H), 1.68 (d, 2H, J = 12.9 Hz), 2.60–2.76 (m, 2H), 3.08 (q, J = 6.6 Hz, 2H), 3.92 (d, J = 13.0 Hz, 2H), 4.65 (s, 2H), 5.94 (t, J = 5.4 Hz, 1H), 6.62 (dd, J = 8.1, 1.5 Hz, 1H), 6.83–6.88 (m, 2H), 6.91 (dd, J = 7.7, 2.0 Hz, 1H), 7.01 (t, J = 2.1 Hz, 1H), 7.22 (t, J = 8.0 Hz, 1H), 7.26–7.34 (m, 2H), 8.25 (1H, s), 8.37 (br s, 1H), 8.49 (s, 1H). 13C NMR (126 MHz, DMSO-d6) δ: 28.6, 32.1, 33.5, 35.5, 39.9, 43.2, 44.4, 55.5, 78.9, 106.7, 110.3, 111.8, 114.1, 120.1, 129.0, 130.2, 132.3, 136.3, 140.3, 148.9, 150.3, 153.0, 154.3, 154.8, 158.6. Anal. calc. for (C31H39N7O3): C, 66.76; H, 7.05; N, 17.13. Found: C, 66.41; H, 6.99; N, 17.13.
3). The compound was obtained (25, 169 mg, 76% yield) as a white solid. Mp: 70–72 °C. MS (ES, positive mode): m/z 558. 1H NMR (400 MHz, DMSO-d6) δ: 1.02 (m, 2H), 1.39 (s, 9H), 1.47–1.60 (m, 3H), 1.68 (d, 2H, J = 12.9 Hz), 2.60–2.76 (m, 2H), 3.08 (q, J = 6.6 Hz, 2H), 3.92 (d, J = 13.0 Hz, 2H), 4.65 (s, 2H), 5.94 (t, J = 5.4 Hz, 1H), 6.62 (dd, J = 8.1, 1.5 Hz, 1H), 6.83–6.88 (m, 2H), 6.91 (dd, J = 7.7, 2.0 Hz, 1H), 7.01 (t, J = 2.1 Hz, 1H), 7.22 (t, J = 8.0 Hz, 1H), 7.26–7.34 (m, 2H), 8.25 (1H, s), 8.37 (br s, 1H), 8.49 (s, 1H). 13C NMR (126 MHz, DMSO-d6) δ: 28.6, 32.1, 33.5, 35.5, 39.9, 43.2, 44.4, 55.5, 78.9, 106.7, 110.3, 111.8, 114.1, 120.1, 129.0, 130.2, 132.3, 136.3, 140.3, 148.9, 150.3, 153.0, 154.3, 154.8, 158.6. Anal. calc. for (C31H39N7O3): C, 66.76; H, 7.05; N, 17.13. Found: C, 66.41; H, 6.99; N, 17.13.
tert-Butyl (S)-2-((tert-butoxycarbonyl)amino)-4-((3-(4-((4-methoxybenzyl)amino)-7H-pyrrolo[2,3-d]pyrimi-din-7-yl)phenyl)amino)butanoate (26)
Following the general method B, 16 (138 mg, 0.4 mmol) and 8 (109 mg, 0.4 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (8 mL). After 120 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1
4) (8 mL). After 120 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The compound was obtained (26, 139 mg, 58% yield) as a white solid. Mp: 72–74 °C. MS (ES, positive mode): m/z 603. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H), 1.45 (s, 9H), 1.83 (m, 1H), 2.15 (m, 1H), 3.27 (m, 2H), 3.81 (s, 3H), 4.30 (m, 1H), 4.81 (d, J = 5.5 Hz, 2H), 6.47 (d, J = 3.6 Hz, 1H), 6.60 (d, J = 8.2 Hz, 1H), 6.87–6.93 (m, 4H), 7.22 (d, J = 3.7 Hz, 1H), 7.26 (t, J = 8.1 Hz, 1H), 7.31–7.36 (m, 2H), 8.40 (s, 1H). 13C NMR (126 MHz, CDCl3) δ: 27.9, 28.3, 32.6, 40.0, 45.0, 52.0, 55.3, 79.9, 82.3, 99.1, 103.5, 108.6, 111.5, 112.9, 114.2, 125.9, 129.0, 130.1, 130.3, 138.7, 148.9, 149.6, 151.3, 155.7, 155.8, 159.2, 171.7. Anal. calc. for (C33H42N6O5): C, 65.76; H, 7.02; N, 13.94. Found: C, 65.55; H, 6.83; N, 13.67.
1). The compound was obtained (26, 139 mg, 58% yield) as a white solid. Mp: 72–74 °C. MS (ES, positive mode): m/z 603. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H), 1.45 (s, 9H), 1.83 (m, 1H), 2.15 (m, 1H), 3.27 (m, 2H), 3.81 (s, 3H), 4.30 (m, 1H), 4.81 (d, J = 5.5 Hz, 2H), 6.47 (d, J = 3.6 Hz, 1H), 6.60 (d, J = 8.2 Hz, 1H), 6.87–6.93 (m, 4H), 7.22 (d, J = 3.7 Hz, 1H), 7.26 (t, J = 8.1 Hz, 1H), 7.31–7.36 (m, 2H), 8.40 (s, 1H). 13C NMR (126 MHz, CDCl3) δ: 27.9, 28.3, 32.6, 40.0, 45.0, 52.0, 55.3, 79.9, 82.3, 99.1, 103.5, 108.6, 111.5, 112.9, 114.2, 125.9, 129.0, 130.1, 130.3, 138.7, 148.9, 149.6, 151.3, 155.7, 155.8, 159.2, 171.7. Anal. calc. for (C33H42N6O5): C, 65.76; H, 7.02; N, 13.94. Found: C, 65.55; H, 6.83; N, 13.67.
tert-Butyl (S)-2-((tert-butoxycarbonyl)amino)-4-((3-(6-((4-methoxybenzyl)amino)-9H-purin-9-yl)phenyl) amino) butanoate (27)
Following the general procedure B, 21 (138 mg, 0.40 mmol) reacted with 8 (109 mg, 0.40 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (8 mL). After 90 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1
4) (8 mL). After 90 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The compound was obtained (27, 108 mg, 45% yield) as a white solid. Mp: 82–84 °C. MS (ES, positive mode): m/z 604. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H), 1.45 (s, 9H), 1.83 (m, 1H), 2.16 (m, 1H), 3.17–3.46 (m, 2H), 3.80 (s, 3H), 4.29 (d, J = 5.2 Hz, 1H), 4.45 (br s, 1H), 4.72–4.82 (m, 2H), 5.30 (d, J = 8.1 Hz, 1H), 6.21 (br s, 1H), 6.65 (dd, J = 8.0, 2.0 Hz, 1H), 6.86–6.92 (m, 4H), 7.27–7.42 (m, 3H), 7.96 (s, 1H), 8.47 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 28.0, 28.3, 32.6, 39.9, 44.2, 52.0, 55.3, 80.0, 82.4, 107.6, 111.8, 112.4, 114.1, 120.2, 129.2, 130.5, 135.9, 139.2, 149.2, 153.7, 154.8, 155.8, 159.1, 171.7. Anal. calc. for (C32H41N7O5·0.5H2O): C, 62.73; H, 6.91; N, 16.00. Found: C, 63.04; H, 7.04; N, 15.76.
1). The compound was obtained (27, 108 mg, 45% yield) as a white solid. Mp: 82–84 °C. MS (ES, positive mode): m/z 604. 1H NMR (400 MHz, CDCl3) δ: 1.44 (s, 9H), 1.45 (s, 9H), 1.83 (m, 1H), 2.16 (m, 1H), 3.17–3.46 (m, 2H), 3.80 (s, 3H), 4.29 (d, J = 5.2 Hz, 1H), 4.45 (br s, 1H), 4.72–4.82 (m, 2H), 5.30 (d, J = 8.1 Hz, 1H), 6.21 (br s, 1H), 6.65 (dd, J = 8.0, 2.0 Hz, 1H), 6.86–6.92 (m, 4H), 7.27–7.42 (m, 3H), 7.96 (s, 1H), 8.47 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 28.0, 28.3, 32.6, 39.9, 44.2, 52.0, 55.3, 80.0, 82.4, 107.6, 111.8, 112.4, 114.1, 120.2, 129.2, 130.5, 135.9, 139.2, 149.2, 153.7, 154.8, 155.8, 159.1, 171.7. Anal. calc. for (C32H41N7O5·0.5H2O): C, 62.73; H, 6.91; N, 16.00. Found: C, 63.04; H, 7.04; N, 15.76.
Ethyl 4-((3-(4-((4-methoxybenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino)butanoate (28)
Following the general method B, 16 (138 mg, 0.4 mmol) reacted with ethyl-4-oxobut-2-enoate (51 mg, 0.4 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (8 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1
4) (8 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The compound was obtained (28, 97 mg, 53% yield) as a yellow syrup. MS (ES, positive mode): m/z 460. 1H NMR (400 MHz, CDCl3) δ: 1.24 (t, J = 7.2 Hz, 3H), 1.92–1.99 (m, 2H), 2.42 (t, J = 7.2 Hz, 2H), 3.20 (t, J = 6.9 Hz, 2H), 3.80 (s, 3H), 4.13 (q, J = 7.2 Hz, 2H), 4.77 (d, J = 5.5 Hz, 2H), 6.43 (d, J = 3.6 Hz, 1H), 6.57 (ddd, J = 8.2, 2.2, 1.0 Hz, 1H), 6.86–6.93 (m, 4H), 7.19 (d, J = 3.7 Hz, 1H), 7.25 (m, 1H), 7.30–7.35 (m, 2H), 8.42 (s, 1H). 13C NMR (126 MHz, CDCl3) δ: 14.2, 24.5, 32.0, 43.2, 44.9, 55.3, 60.5, 98.9, 103.7, 108.6, 111.2, 112.9, 114.1, 124.7, 129.0, 130.1, 130.6, 138.8, 149.1, 149.7, 151.8, 156.1, 159.1, 173.4. Anal. calc. for (C26H29N5O3): C, 67.95; H, 6.36; N, 15.24. Found: C, 67.91; H, 6.45; N, 14.84.
1). The compound was obtained (28, 97 mg, 53% yield) as a yellow syrup. MS (ES, positive mode): m/z 460. 1H NMR (400 MHz, CDCl3) δ: 1.24 (t, J = 7.2 Hz, 3H), 1.92–1.99 (m, 2H), 2.42 (t, J = 7.2 Hz, 2H), 3.20 (t, J = 6.9 Hz, 2H), 3.80 (s, 3H), 4.13 (q, J = 7.2 Hz, 2H), 4.77 (d, J = 5.5 Hz, 2H), 6.43 (d, J = 3.6 Hz, 1H), 6.57 (ddd, J = 8.2, 2.2, 1.0 Hz, 1H), 6.86–6.93 (m, 4H), 7.19 (d, J = 3.7 Hz, 1H), 7.25 (m, 1H), 7.30–7.35 (m, 2H), 8.42 (s, 1H). 13C NMR (126 MHz, CDCl3) δ: 14.2, 24.5, 32.0, 43.2, 44.9, 55.3, 60.5, 98.9, 103.7, 108.6, 111.2, 112.9, 114.1, 124.7, 129.0, 130.1, 130.6, 138.8, 149.1, 149.7, 151.8, 156.1, 159.1, 173.4. Anal. calc. for (C26H29N5O3): C, 67.95; H, 6.36; N, 15.24. Found: C, 67.91; H, 6.45; N, 14.84.
Ethyl 4-((3-(6-((4-methoxybenzyl)amino)-9H-purin-9-yl)phenyl)amino)butanoate (29)
Following the general procedure B, 21 (138 mg, 0.40 mmol) reacted with ethyl-4-oxobut-2-enoate (51 mg, 0.40 mmol) in DMF/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (8 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1
4) (8 mL). After 60 min, volatiles were removed, and the residue was purified by CCTLC in the Chromatotron (hexane/EtOAc 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The compound was obtained (29, 99 mg, 54% yield) as a colorless syrup. MS (ES, positive mode): m/z 461. 1H NMR (400 MHz, CDCl3) δ: 1.25 (t, J = 7.1 Hz, 3H), 1.85–2.11 (m, 2H), 2.44 (t, J = 7.1 Hz, 2H), 3.22 (t, J = 6.9 Hz, 2H), 3.79 (s, 3H), 4.08 (br s, 1H), 4.14 (q, J = 7.2 Hz, 2H), 4.83 (m, 2H), 6.26 (br s, 1H), 6.64 (ddd, J = 8.4, 2.3, 1.1 Hz, 1H), 6.83–6.97 (m, 4H), 7.27–7.42 (m, 3H), 7.94 (s, 1H), 8.47 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.2, 24.4, 31.9, 43.2, 55.3, 60.6, 107.6, 111.9, 112.2, 114.1, 120.2, 129.2, 130.5, 135.9, 139.2, 149.4, 153.6, 154.8, 159.1, 173.4. Anal. calc. for (C25H28N6O3): C, 65.20; H, 6.13; N, 18.25. Found: C, 65.12; H, 6.32, N, 17.88.
1). The compound was obtained (29, 99 mg, 54% yield) as a colorless syrup. MS (ES, positive mode): m/z 461. 1H NMR (400 MHz, CDCl3) δ: 1.25 (t, J = 7.1 Hz, 3H), 1.85–2.11 (m, 2H), 2.44 (t, J = 7.1 Hz, 2H), 3.22 (t, J = 6.9 Hz, 2H), 3.79 (s, 3H), 4.08 (br s, 1H), 4.14 (q, J = 7.2 Hz, 2H), 4.83 (m, 2H), 6.26 (br s, 1H), 6.64 (ddd, J = 8.4, 2.3, 1.1 Hz, 1H), 6.83–6.97 (m, 4H), 7.27–7.42 (m, 3H), 7.94 (s, 1H), 8.47 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.2, 24.4, 31.9, 43.2, 55.3, 60.6, 107.6, 111.9, 112.2, 114.1, 120.2, 129.2, 130.5, 135.9, 139.2, 149.4, 153.6, 154.8, 159.1, 173.4. Anal. calc. for (C25H28N6O3): C, 65.20; H, 6.13; N, 18.25. Found: C, 65.12; H, 6.32, N, 17.88.
tert-Butyl 4-(2-((3,3-dimethylbutyl)(3-(4-methyl-7H-pyrrolo[2,3-d]pyrimidin-7-yl)phenyl)amino)ethyl)pipe-ridine-1-carboxylate (30)
A solution containing 5 (61 mg, 0.20 mmol) and tert-butyl 4-(2-oxoethyl)piperidine-1-carboxylate (90 mg, 0.40 mmol) in MeOH (8 mL) was recirculated through a 30 mm CatCart™ in the H-cube at 60 Bar, 100% of Hydrogen production, at 150 °C and a flow rate 0.5 mL min−1. The reaction was followed by HPLC and 3 hours later, the pressure was released, and the system was further eluted with 30 mL of MeOH. Volatiles were removed and the crude was purified through a C18 Biotage system using a gradient of water and MeCN as mobile phase (from 20% of MeCN to 100%). The compound was obtained (31, 32 mg, 46% yield) as a white solid. Mp: 85–87 °C. MS (ES, positive mode): m/z 520. 1H NMR (400 MHz, CDCl3) δ: 0.97 (s, 9H), 1.07–1.33 (m, 2H), 1.45 (s, 9H), 1.49–1.64 (m, 5H), 1.71 (d, J = 13.0 Hz, 2H), 2.66–2.72 (m, 2H), 2.78 (s, 3H), 3.17–3.45 (m, 4H), 4.07–4.12 (m, 2H), 6.60 (dd, J = 8.2, 2.5 Hz, 1H), 6.69 (d, J = 3.7 Hz, 1H), 6.82 (dd, J = 7.3, 1.9 Hz, 1H), 7.07 (t, J = 2.2 Hz, 1H), 7.30 (dd, J = 8.5, 7.8 Hz, 1H), 7.47 (d, J = 3.7 Hz, 1H), 8.79 (s, 1H). 13C NMR (100 MHz, CDCl3) δ: 21.6, 28.5, 29.3, 29.4, 29.8, 32.2, 33.8, 34.2, 39.9, 43.9, 47.2, 48.5, 79.3, 100.3, 107.5, 110.1, 110.2, 118.8, 127.9, 130.1, 138.6, 148.8, 150.1, 151.8, 154.9, 159.6. Anal. calc. for (C31H45N5O2): C, 71.64; H, 8.73; N, 13.48. Found: C, 71.28; H, 8.81; N, 13.35.N 1-(3-(6-Amino-9H-purin-9-yl)phenyl)-N2-methylethane-1,2-diamine (31)
A solution containing 22 (150 mg, 0.3 mmol) in trifluoroacetic acid (3 mL) was stirred at 70 °C for 16 h. Then, volatiles were removed, the residue was treated with EtOAc (15 mL) and NaOH 1 M (15 mL), and extracted with isobutanol (3 × 10 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and evaporated. The compound was obtained (30, 75 mg, 89% yield) as a white solid. Mp: 148–150 °C. MS (ES, positive mode): m/z 284. 1H NMR (400 MHz, DMSO-d6) δ: 2.31 (s, 3H), 2.69 (t, J = 6.3 Hz, 2H), 3.14 (q, J = 6.0 Hz, 2H), 5.88 (t, J = 5.5 Hz, 1H), 6.64 (dd, J = 8.2, 2.3 Hz, 1H), 6.94 (dd, J = 7.6, 2.0 Hz, 1H), 7.04 (t, J = 2.2 Hz, 1H), 7.23 (t, J = 8.0 Hz, 1H), 7.32 (br s, 2H), 8.18 (s, 1H), 8.47 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ: 36.5, 42.9, 50.8, 106.8, 110.4, 111.6, 119.8, 130.3, 136.5, 140.3, 149.7, 150.5, 153.6, 156.8. HRMS (ESI): calcd for C14H17N5 [M + H]+ 284.1545, found 284.1615.Author contributions
J.-M. O.: conceptualization, investigation, methodology, visualization, validation, writing original draft; N. d R.: investigation, validation, visualization; M.-J. P.-P.: conceptualization, writing-review and editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation AEI/10.13039/501100011033 grant PID2019-105117RR-C22 (to M.-J. P.-P.), by AECSIC, grant number PIE-202380E090 (to M.-J. P.-P.) and by the European Commission – NextGenerationEU (Regulation EU 2020/2094), through CSIC's Global Health Platform (PTI Salud Global). NdR thanks Fondo Social Europeo (Programa Operativo de Empleo Juvenil 2014–2020) and YEI (Comunidad de Madrid).References
- E. S. Matyugina, S. N. Kochetkov and A. L. Khandazhinskaya, Synthesis and biological activity of aza and deaza analogues of purine nucleosides, Russ. Chem. Rev., 2021, 90, 1454–1491 CrossRef.
- M. Skoreński and M. Sieńczyk, The Fellowship of Privileged Scaffolds—One Structure to Inhibit Them All, Pharmaceuticals, 2021, 14, 1164 CrossRef PubMed.
- P. Perlíková and M. Hocek, Pyrrolo[2,3-d]pyrimidine (7-deazapurine) as a privileged scaffold in design of antitumor and antiviral nucleosides., Med. Res. Rev., 2017, 37, 1429–1460 CrossRef PubMed.
- O. M. Khalil, A. M. Kamal, S. Bua, H. El Sayed Teba, Y. M. Nissan and C. T. Supuran, Pyrrolo and pyrrolopyrimidine sulfonamides act as cytotoxic agents in hypoxia via inhibition of transmembrane carbonic anhydrases, Eur. J. Med. Chem., 2020, 188, 112021 CrossRef CAS PubMed.
- L. Froux, A. Elbahnsi, B. Boucherle, A. Billet, N. Baatallah, B. Hoffmann, J. Alliot, R. Zelli, W. Zeinyeh, R. Haudecoeur, B. Chevalier, A. Fortuné, S. Mirval, C. Simard, P. Lehn, J.-P. Mornon, A. Hinzpeter, F. Becq, I. Callebaut and J.-L. Décout, Targeting different binding sites in the CFTR structures allows to synergistically potentiate channel activity, Eur. J. Med. Chem., 2020, 190, 112116 CrossRef CAS PubMed.
- M. S. Mohamed, A. I. Sayed, M. A. Khedr and S. H. Soror, Design, synthesis, assessment, and molecular docking of novel pyrrolopyrimidine (7-deazapurine) derivatives as non-nucleoside hepatitis C virus NS5B polymerase inhibitors, Bioorg. Med. Chem., 2016, 24, 2146–2157 CrossRef CAS PubMed.
- T.-C. Kuo, L.-W. Li, S.-H. Pan, J.-M. Fang, J.-H. Liu, T.-J. Cheng, C.-J. Wang, P.-F. Hung, H.-Y. Chen, T.-M. Hong, Y.-L. Hsu, C.-H. Wong and P.-C. Yang, Purine-Type Compounds Induce Microtubule Fragmentation and Lung Cancer Cell Death through Interaction with Katanin., J. Med. Chem., 2016, 59, 8521–8534 CrossRef CAS PubMed.
- M.-R. Huang, Y.-L. Hsu, T.-C. Lin, T.-J. Cheng, L.-W. Li, Y.-W. Tseng, Y. Chou, J.-H. Liu, S.-H. Pan, J.-M. Fang and C.-H. Wong, Structure-guided development of purine amide, hydroxamate, and amidoxime for the inhibition of non-small cell lung cancer, Eur. J. Med. Chem., 2019, 181, 111551 CrossRef CAS PubMed.
- M. I. El-Gamal and C.-H. Oh, Diarylureas and Diarylamides with Pyrrolo[2,3-d]pyrimidine Scaffold as Broad-Spectrum Anticancer Agents, Chem. Pharm. Bull., 2014, 62, 25–34 CrossRef CAS PubMed.
- M. Šála, M. Kögler, P. Plačková, I. Mejdrová, H. Hřebabecký, E. Procházková, D. Strunin, G. Lee, G. Birkus, J. Weber, H. Mertlíková-Kaiserová and R. Nencka, Purine analogs as phosphatidylinositol 4-kinase IIIβ inhibitors., Bioorg. Med. Chem. Lett., 2016, 26, 2706–2712 CrossRef PubMed.
- B. García-Reyes, L. Witt, B. Jansen, E. Karasu, T. Gehring, J. Leban, D. Henne-Bruns, C. Pichlo, E. Brunstein, U. Baumann, F. Wesseler, B. Rathmer, D. Schade, C. Peifer and U. Knippschild, Discovery of Inhibitor of Wnt Production 2 (IWP-2) and Related Compounds As Selective ATP-Competitive Inhibitors of Casein Kinase 1 (CK1) δ/ε, J. Med. Chem., 2018, 61, 4087–4102 CrossRef PubMed.
- T. D. Manz, S. C. Sivakumaren, A. Yasgar, M. D. Hall, M. I. Davis, H.-S. Seo, J. D. Card, S. B. Ficarro, H. Shim, J. A. Marto, S. Dhe-Paganon, A. T. Sasaki, M. B. Boxer, A. Simeonov, L. C. Cantley, M. Shen, T. Zhang, F. M. Ferguson and N. S. Gray, Structure-Activity Relationship Study of Covalent Pan-phosphatidylinositol 5-Phosphate 4-Kinase Inhibitors, ACS Med. Chem. Lett., 2020, 11, 346–352 CrossRef CAS PubMed.
- D. Rong, K. Zhou, W. Fang, H. Yang, Y. Zhang, Q. Shi, Y. Huang, J. Li, H. Dong, L. Li, J. Ding, X. Huang and Y. Wang, Structure-Aided Design, Synthesis, and Biological Evaluation of Potent and Selective Non-Nucleoside Inhibitors Targeting Protein Arginine Methyltransferase 5, J. Med. Chem., 2022, 65, 7854–7875 CrossRef CAS PubMed.
- S. Kashyap, J. Sandler, U. Peters, E. J. Martinez and T. M. Kapoor, Using ‘biased-privileged’ scaffolds to identify lysine methyltransferase inhibitors, Bioorg. Med. Chem., 2014, 22, 2253–2260 CrossRef CAS PubMed.
- M. Šála, K. R. Hollinger, A. G. Thomas, R. P. Dash, C. Tallon, V. Veeravalli, L. Lovell, M. Kögler, H. Hřebabecký, E. Procházková, O. Nešuta, A. Donoghue, J. Lam, R. Rais, C. Rojas, B. S. Slusher and R. Nencka, Novel Human Neutral Sphingomyelinase 2 Inhibitors as Potential Therapeutics for Alzheimer's Disease, J. Med. Chem., 2020, 63, 6028–6056 CrossRef PubMed.
- G. Amato, A. Manke, R. Wiethe, V. Vasukuttan, R. Snyder, Y. L. Yueh, A. Decker, S. Runyon and R. Maitra, Functionalized 6-(Piperidin-1-yl)-8,9-Diphenyl Purines as Peripherally Restricted Inverse Agonists of the CB1 Receptor, J. Med. Chem., 2019, 62, 6330–6345 CrossRef CAS PubMed.
- H. Cao, G. Histand and D. Lin, Selectfluor-Induced Oxidative Amination of N-Heteroaromatics with Purine, J. Org. Chem., 2023, 88, 5687–5695 CrossRef PubMed.
- O. I. Afanasyev, E. Kuchuk, D. L. Usanov and D. Chusov, Reductive Amination in the Synthesis of Pharmaceuticals., Chem. Rev., 2019, 119, 11857–11911 CrossRef CAS PubMed.
- L. Aguado, H. J. Thibaut, E.-M. Priego, M.-L. Jimeno, M. J. Camarasa, J. Neyts and M.-J. Pérez-Pérez, 9-Arylpurines as a Novel Class of Enterovirus Inhibitors, J. Med. Chem., 2010, 53, 316–324 CrossRef CAS PubMed.
- L. Aguado, M.-D. Canela, H. J. Thibaut, E.-M. Priego, M.-J. Camarasa, P. Leyssen, J. Neyts and M.-J. Pérez-Pérez, Efficient synthesis and anti-enteroviral activity of 9-arylpurines, Eur. J. Med. Chem., 2012, 49, 279–288 CrossRef CAS PubMed.
- A. Gigante, M.-D. Canela, L. Delang, E.-M. M. Priego, M.-J. Camarasa, G. Querat, J. Neyts, P. Leyssen and M.-J. Pérez-Pérez, Identification of [1,2,3]Triazolo[4,5-d]pyrimidin-7(6 H)-ones as Novel Inhibitors of Chikungunya Virus Replication, J. Med. Chem., 2014, 57, 4000–4008 CrossRef CAS PubMed.
- A. Gigante, A. Gómez-SanJuan, L. Delang, C. Li, O. Bueno, A.-M. Gamo, E.-M. Priego, M.-J. Camarasa, D. Jochmans, P. Leyssen, E. Decroly, B. Coutard, G. Querat, J. Neyts and M.-J. Pérez-Pérez, Antiviral activity of [1,2,3]triazolo[4,5-d]pyrimidin-7(6 H)-ones against chikungunya virus targeting the viral capping nsP1, Antiviral Res., 2017, 144, 216–222 CrossRef CAS PubMed.
- A. Gómez-Sanjuan, A.-M. Gamo, L. Delang, A. Pérez-Sánchez, S. N. Amrun, R. Abdelnabi, S. Jacobs, E.-M. Priego, M.-J. Camarasa, D. Jochmans, P. Leyssen, L. F. P. Ng, G. Querat, J. Neyts and M.-J. Pérez-Pérez, Inhibition of the Replication of Different Strains of Chikungunya Virus by 3-Aryl-[1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones, ACS Infect. Dis., 2018, 4, 605–619 CrossRef PubMed.
- Accent Therapeutics, Inc., United States, WO2021081211, 2012.
- M. A. T. Blaskovich, Unusual Amino Acids in Medicinal Chemistry, J. Med. Chem., 2016, 59, 10807–10836 CrossRef CAS PubMed.
- I. M. Mándity, S. B. Ötvös, G. Szőlősi and F. Fülöp, Harnessing the Versatility of Continuous-Flow Processes: Selective and Efficient Reactions., Chem. Rec., 2016, 16, 1018–1033 CrossRef PubMed.
- N. S. Suveges, R. O. M. A. de Souza, B. Gutmann and C. O. Kappe, Synthesis of Mepivacaine and Its Analogues by a Continuous-Flow Tandem Hydrogenation/Reductive Amination Strategy, Eur. J. Org. Chem., 2017, 6511–6517 CrossRef CAS.
- L. Aguado, M.-J. Camarasa and M.-J. Pérez-Pérez, Microwave-Assisted Synthesis of 9-Arylpurines, J. Comb. Chem., 2009, 11, 210–212 CrossRef CAS PubMed.
- A. Y. Sukhorukov, Catalytic Reductive Amination of Aldehydes and Ketones With Nitro Compounds: New Light on an Old Reaction, Front. Chem., 2020, 8, 215 CrossRef CAS PubMed.
- A. M. Fiore, G. Romanazzi, M. M. Dell'Anna, M. Latronico, C. Leonelli, M. Mali, A. Rizzuti and P. Mastrorilli, Mild and efficient synthesis of secondary aromatic amines by one-pot stepwise reductive amination of arylaldehydes with nitroarenes promoted by reusable nickel nanoparticles, Mol. Catal., 2019, 476, 110507 CrossRef.
- G. Romanazzi, V. Petrelli, A. M. Fiore, P. Mastrorilli and M. M. Dell'Anna, Metal-based Heterogeneous Catalysts for One-Pot Synthesis of Secondary Anilines from Nitroarenes and Aldehydes, Molecules, 2021, 26, 1120 CrossRef CAS PubMed.
- Hoffmann-La Roche Inc., United States, US20200268762, 2020.
- Effector Therapeutics Inc., WO2017075412, p. 2017.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob00822c |
| This journal is © The Royal Society of Chemistry 2023 |