Open Access Article
Open Access ArticleSynthesis and characterization of enantiopure chiral NH2/SO palladium complexes†
Nazaret
Moreno-Rodriguez
 a,
L. Gabriel
Borrego
a,
L. Gabriel
Borrego
 a,
Rocío
Recio
a,
Rocío
Recio
 *a,
Victoria
Valdivia
*a,
Victoria
Valdivia
 a,
M. Carmen
Nicasio
a,
M. Carmen
Nicasio
 b,
Eleuterio
Álvarez
b,
Eleuterio
Álvarez
 c,
Noureddine
Khiar
c,
Noureddine
Khiar
 c and
Inmaculada
Fernández
c and
Inmaculada
Fernández
 *a
*a
aDepartamento de Química Orgánica y Farmacéutica, Facultad de Farmacia, Universidad de Sevilla, C/Profesor García González, 2, 41012 Sevilla, Spain. E-mail: rrecioj@us.es
bDepartamento de Química Inorgánica, Universidad de Sevilla, Aptdo 1203, Sevilla, 41071, Spain
cInstituto de Investigaciones Químicas (IIQ), CSIC-Universidad de Sevilla, Avda. Américo Vespucio, 49, 41092 Sevilla, Spain
First published on 27th June 2023
Abstract
A series of enantiopure chiral NH2/SO palladium complexes have been synthesised with high yields by treating the corresponding tert-butylsulfinamide/sulfoxide derivatives with Pd(CH3CN)2Cl2. The enantiopure chiral ligands were prepared by stereoselective addition of tert-butyl or phenyl methylsulfinyl carbanions to different tert-butylsulfinylimines. In all cases, coordination occurs with concomitant desulfinylation. X-ray studies of the Pd complexes showed a higher trans influence of the phenylsulfinyl group in comparison to that of the tert-butylsulfinyl group. Furthermore, we have obtained and characterised two possible palladium amine/sulfonyl complexes, epimers at sulfur, resulting from N-desulfinylation and coordination of palladium with both oxygens of the prochiral sulfonyl group. The catalytic activity and enantioselectivity of the new Pd(II) complexes of acetylated amine/tert-butyl- and phenylsulfoxides in the carboxylated cyclopropanes arylation reaction has been studied, obtaining the best results with the phenylsulfoxide ligand 25(SC,SS) that produced the final arylated product in a 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :7 enantiomeric ratio.
:7 enantiomeric ratio.
Introduction
Homogeneous transition-metal catalysis reactions are essential in various areas of modern synthetic chemistry. Proof of this is the high number of asymmetric catalytic reactions for industrial applications, such as the Takasago isomerization,1 Sumitomo cyclopropanation,2 or Sharpless epoxidation,3 together with the now classic Monsanto asymmetric hydrogenation to produce L-DOPA.4However, for much of the early 20th century, the reactivity and selectivity of all known homogeneous metal catalysts were lower compared to their heterogeneous and biological counterparts.5 Despite advances made in this field to date, finding a catalyst that combines high catalytic capacity and high enantioselectivity at the same time for a wide range of chemical processes continues to represent a significant synthetic challenge.
Nowadays, palladium catalysts are an important tool in many syntheses because of the enormous versatility of this metal. Specifically, in asymmetric catalysis, palladium-catalyzed reactions have gained a prominent place in the synthesis of products of pharmacological and industrial interest.6
A large number of examples can be found in the literature concerning the application of Pd complexes in asymmetric catalysis, such as the reaction of arylboronic acids with N-benzylisatin derivatives7 or cyclic N-sulfonyl imines,8 the Carroll rearrangement,9 C–H activation,10 allylic etherification and amination,11 or decarboxylative [4 + 2] cycloaddition.12
For this purpose, a plethora of different chiral ligands have been developed in asymmetric Pd-catalyzed reactions, such as the well-known binaphtyl phosphine, phosphine dioxide and phosphite ligands,13 or the BINOL-phosphoric acid ligands,14 chiral mono-N-protected aminoacids,15 the bis-oxazoline ligands16 or the tridentate P/C/P or N/C/N ligands.17
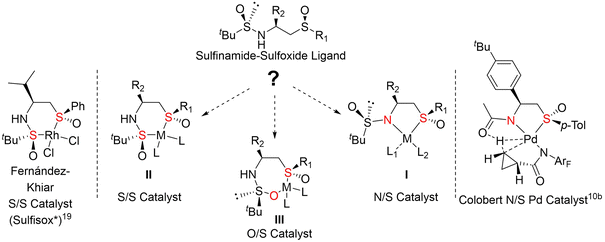
Our contribution in this field was originally focused on the development of carbohydrate-based ligands, such as C2-symmetric bis-thioglycoside-type ligands and sulfur–phosphorous mixed ligands, as precursors of chiral Pd complexes, and their applications in Pd-catalyzed allylic substitution reactions (with ee up to 90% and 96%, respectively) (Fig. 1).18 More recently, in light of the good results obtained with the Sulfisox* ligand, a bidentate sulfinamide/sulfoxide-type ligand (Fig. 1), in the Rh(I) catalyzed enantioselective arylation reactions,19 we considered the possibility of expanding its range of application in the asymmetric catalysis mediated by other metals such as Pd. Even if sulfinamide/phosphine20 and sulfinamide/alcohol derivatives21 have been described as bidentate ligand precursors of chiral Pd complexes, to the best of our knowledge, no sulfinamide/sulfur type bidentate chiral Pd complex, including sulfinamide/sulfoxide, sulfinamide/sulfone or sulfinamide/thioether derivatives, has previously been described, so it is interesting to approach their study.
Other types of bidentate sulfinamide/heteroatom ligands have been developed in Pd catalysis. Thus, among the sulfinamide/phosphine ligands represented in Fig. 2, it is worth highlighting N-Me-XuPhos20a and its sterically hindered analog N-Me-TY-Phos22 in the enantioselective reductive Heck reaction and in the palladium-catalyzed asymmetric fluoroarylation of gem-difluoroalkenes, respectively, or PC-Phos23 and its analog (PC-Phos analog) in the Pd-mediated enantioselective arylation of aryl and alkyl sulfenates24 and in the chiral dearomatization of indole,25 respectively. The Xiao-Phos ligand, previously used as an organocatalyst,26 has provided high enantioselectivities in the asymmetric P–C cross-coupling reaction of secondary phosphine oxides and aryl bromides.27 Interestingly, the sulfinamide/phosphanyl Riera–Verdaguer ligand28 can behave as a P-monodentate or P/O or P/S bidentate ligand, depending on the nature of the Pd precursor and the reaction conditions used.20d Regarding the sulfinamide/alcohol derivatives, to our knowledge, there is only one example in the literature as a bidentate ligand, in the asymmetric allylic alkylation of ethyl 2-fluoroacetoacetate catalyzed by Pd, with high ee (94%) and chemical yield (90%).21a,b
Of special interest is the acetamide/sulfoxide ligand successfully developed by Colobert for the asymmetric C–H activation Pd catalyzed reaction, through a N/S coordination.10b This ligand can be considered structurally related to the aminosulfoxides previously developed by Pettinari as bidentate N/S ligands in Pd complexes.29
Results and discussion
Stereoselective synthesis of sulfinamide/sulfoxide ligands
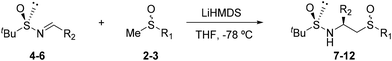
In a previous work, we developed a series of sulfinamide/sulfoxide derivatives as chiral ligands in the Rh-catalyzed addition of arylboronic acids to activated ketones.19 These ligands were obtained, by stereoselective additions of the corresponding racemic methylsulfinyl carbanions to N-tert-butylsulfinylimines, as pair of epimers at the sulfinyl sulfur, which were subsequently separated by column chromatography. In order to avoid this last chromatographic separation step, which in some cases is complicated and ineffective, in this work we have approached the preparation of this type of bidentate ligands in enantiopure form, by adding the corresponding R or S enantiopure methyl sulfoxides, previously obtained by applying the DAG methodology (Scheme 1),30 instead of racemic.The addition of the corresponding methylsulfinyl carbanions, generated by treating the enantiopure sulfoxides with LHMDS at low temperature in the presence of the sulfinylimines 4–6, yielded the corresponding sulfinamide/sulfoxides (7–12) with good to excellent yields as a single diastereoisomer (Table 1). In order to determine the influence of the different nature of the substituents of the ligand on the formation of its palladium complexes, a series of aryl and alkyl derivatives differently substituted both in the chiral carbon (Ph, 2-Napht and iPr) and in the sulfoxide function (Ph and tBu) were easily prepared (Table 1).
| Entry | Sulfinylimine | R2 | Sulfoxide | R1 | Ligand (SON/SO) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 4(RS) | 2-Naph | 2(RS) | Ph | 7(RS,SC,RS) | 90 |
| 2 | 4(RS) | 2-Naph | 2(SS) | Ph | 7(RS,SC,SS) | 94 |
| 3 | 5(RS) | Ph | 2(RS) | Ph | 8(RS,SC,RS) | 91 |
| 4 | 5(RS) | Ph | 2(SS) | Ph | 8(RS,SC,SS) | 89 |
| 5 | 6(RS) | iPr | 2(RS) | Ph | 9(RS,SC,RS) | 81 |
| 6 | 6(RS) | iPr | 2(SS) | Ph | 9(RS,SC,SS) | 91 |
| 7 | 4(RS) | 2-Naph | 3(RS) | t Bu | 10(RS,SC,RS) | 78 |
| 8 | 4(RS) | 2-Naph | 3(SS) | t Bu | 10(RS,SC,SS) | 68 |
| 9 | 5(RS) | Ph | 3(RS) | t Bu | 11(RS,SC,RS) | 77 |
| 10 | 5(RS) | Ph | 3(SS) | t Bu | 11(RS,SC,SS) | 65 |
| 11 | 6(RS) | iPr | 3(RS) | t Bu | 12(RS,SC,RS) | 68 |
| 12 | 6(RS) | iPr | 3(SS) | t Bu | 12(RS,SC,SS) | 66 |
In the obtained ligands, the configurations at both sulfurs (sulfinamide and sulfoxide) were unequivocally assigned based on the known configuration of both starting chiral products, (R)-N-tert-butylsulfinamide and the added (R)- or (S)-methyl sulfoxide. The configuration at the new chiral carbon can be predicted as S based on the known stereochemical control of the tert-butylsulfinyl group of the chiral N-tert-butylsulfinylimines in the nucleophilic additions to the imino double bond. This configurational assignment was confirmed by X-ray.
Synthesis of the complexes
Once the ligands were synthesized, we studied their capacity and mode of coordination with Pd(II). Assuming that the sulfoxide function is coordinated through sulfur,10b,29,31 and taking into account the ambidentate character of the sulfinamido group, the coordination mode of these ligands with the metal in the catalyst, in principle, could take place by one of the three heteroatoms, S, O or N, thus generating bidentate N/S, S/S or O/S type ligands, the ring size of the chelate being 5, 6 or 7 members, respectively (Fig. 3).In a previous work, we demonstrated that the Rh(I) catalysts obtained with these sulfinamide/sulfoxide ligands are S/S 6-membered type II chelates. However, a N/S coordination with a 5-membered type I chelates, similar to that described by Colobert group with the carboxamide/sulfoxide ligands, can also be considered in the case of the Pd(II) complexes with our sulfinamide/sulfoxide ligands, which would therefore imply two different coordination modes for them, S/S or N/S, depending on the nature of the metal.
To evaluate the ability of sulfinamide/sulfoxides 7–12 to form palladium complexes, they were treated with PdCl2(CH3CN)2 as palladium precursor in methylene chloride as solvent. In all cases, the starting material was consumed after 48 h, and the 1H-NMR spectra of the corresponding palladium complexes formed, which precipitated by adding ether, showed the disappearance of the singlet corresponding to the tert-butylsulfinyl group, indicating that the coordination occurs with concomitant N-desulfinylation (Table 2).
| Entry | Ligand (SON/SO) | R2 | R1 | Pd complex | Yield (%) |
|---|---|---|---|---|---|
| 1 | 7(RS,SC,RS) | 2-Naph | Ph | 13(SC,SS) | 94 |
| 2 | 7(RS,SC,SS) | 13(SC,RS) | Quant. | ||
| 3 | 8(RS,SC,RS) | Ph | Ph | 14(SC,SS) | 96 |
| 4 | 8(RS,SC,SS) | 14(SC,RS) | Quant. | ||
| 5 | 9(RS,SC,RS) | iPr | Ph | 15(SC,SS) | 88 |
| 6 | 9(RS,SC,SS) | 15(SC,RS) | 96 | ||
| 7 | 10(RS,SC,RS) | 2-Naph | t Bu | 16(SC,SS) | 92 |
| 8 | 10(RS,SC,SS) | 16(SC,RS) | 97 | ||
| 9 | 11(RS,SC,RS) | Ph | t Bu | 17(SC,SS) | 78 |
| 10 | 11(RS,SC,SS) | 17(SC,RS) | Quant. | ||
| 11 | 12(RS,SC,RS) | iPr | t Bu | 18(SC,SS) | 95 |
| 12 | 12(RS,SC,SS) | 18(SC,RS) | 80 | ||
All 13–18 complexes were obtained in good purity with high yields, without the need of purification, by simple filtration. Complex formation also takes place in other solvents such as chloroform, THF and trifluoromethylbenzene, although in the latter case with a decrease in yield. On the contrary, the nature of the palladium precursor turns out to be decisive for the formation of the complexes, in such a way that these were only obtained by treating the ligands with Pd(CH3CN)2Cl2, but the coordination does not take place with other palladium precursors such as Pd(OAc)2, Pd(cod)Cl2 or Pd[(γ-C3H5)Cl2]. Finally, it was confirmed that the reaction is not affected by the presence of traces of moisture, acid or oxygen.
The reaction was also monitored in an NMR tube, observing in all cases the disappearance of the signal at 1.3 ppm of the tert-butyl of the sulfinamide, at the same time that two new singlets appeared at 2.0 and 1.6 ppm, corresponding to acetonitrile and to the product of desulfinylation, respectively. These last signals disappear when the solvent is removed in vacuo.
In order to confirm the structure proposed for the new palladium complexes, complex 14(SC,RS) was also synthesized from the aminosulfoxide 19(SC,SS) with Pd(CH3CN)2Cl2 (Scheme 2). The amine ligand was previously obtained by N-desulfinylation of 8(RS,SC,SS) with TFA in methanol. The obtained complex presented identical spectroscopic characteristics to those of the complex 14(SC,RS), obtained directly from the sulfinamide/sulfoxide (Scheme 2).
The X-rays of several of the complexes obtained confirmed the desulfinylation, obtaining in all cases 5-membered chelates where Pd is coordinated to the nitrogen of an amino group NH2 and to the sulfur of the sulfoxide SOR (R: Ph, tBu) (Fig. 4). While the NH signal of starting sulfinamide appears as a doublet at 4.8–4.9 ppm in the C-aryl-substituted ligands (7, 8, 10 and 11(RS,SC,RS) and (RS,SC,SS)) and above 4.0–4.2 ppm in the C-isopropyl-substituted ligands (9 and 12(RS,SC,RS) and (RS,SC,SS)), in the corresponding complexes two non-equivalent NH2 proton signals are observed with a significant displacement difference, around 100–150 Hz, due to the conformational stiffness imposed by the chelate (Fig. 4).
In the five-membered chelate ring S1–C1–C2–N1–Pd1, the atom C2 occupies a more marked out-of-plane position. In the case of the phenylsulfoxides (13–15), regardless of the sulfur configuration, S or R, the carbon C2 is placed out of the plane in order to leave the substituent, Ph, 2-Napth or iPr in a pseudo-equatorial position. However, in the case of the tert-butylsulfinyl derivatives (16–18), the C2 carbon is located out of the plane depending on the configuration of the sulfoxide, moving towards the opposite plane where the tert-butyl group attached to the sulfur is placed to avoid their interaction. This forces the naphthyl group at C2 to adopt a pseudoaxial arrangement in the case of the 16(SC,RS) complex, but allows the tert-butyl group to always be pseudoequatorial (Fig. 5).
A common feature of these chelates of Pd with the amine/sulfoxide bidentate ligands is to form pseudo-1D-polymeric structures in the crystalline solid state (Fig. 6). Pseudo-polymeric structures are formed through intermolecular hydrogen bonds between the –NH2 of a Pd complex and the chlorine atoms of neighboring complexes, such that each complex binds to its two contiguous neighbors by means of a hydrogen donor group (–NH2) on the one hand and a hydrogen acceptor group (–Cl) on the other. Other interatomic bonding forces likely intervene in crystal packing besides hydrogen bonds, but the latter must be the ones that mainly contribute to the linear pseudo-polymeric structure ordering.
 | ||
| Fig. 6 Pseudo-polymeric structures formed by 16(SC,RS) (A) and 15(SC,RS) (B) complexes in the crystalline state. | ||
Regarding the IR spectra of the complexes, the SO absorption associated to the sulfinamido group, which is present in the ligand, disappears in all of them and a shift of the absorption associated with the S–O vibration of the sulfoxide towards higher frequencies is observed, due to the coordination of the sulfinyl sulfur with the metal (Table 3). Concerning the 1H-NMR shift of the ABX system, in contrast to the methylene protons which, as expected, are deshielded in all the complexes, methinic protons exhibit a different spectroscopic behavior depending on the sulfinyl configuration. Thus, no change in the chemical shift of methinic proton is observed in the complexes of (SC,RS) configuration but it appears significantly shielded in the epimeric (SC,SS) complexes. Regarding the non-equivalence of the methylene protons, the same trend is observed in the protons of the AB system of the 1-aryl-substituted derivatives, both in the tert-butyl and in the phenyl sulfoxides, decreasing the non-equivalence in the complexes with S configuration in the sulfur, 13(SC,SS), 14(SC,SS), and 18(SC,SS), (entries 1, 3 and 11, Table 3) and increasing in complexes with R configuration at the sulfinyl group, (13(SC,RS), 14(SC,RS), 16(SC,RS) and 17(SC,RS), (entries 2, 4, 8, and 10, Table 3).
| Entry | R2 | R1 | Ligand (SON/SO) | Pd complex | Δν (Hz) in 1H-NMR | ΔνSO (cm−1) in IR | |||
|---|---|---|---|---|---|---|---|---|---|
| Ligand | Complex | Ligand | Complex | ||||||
| ΔνSON | ΔνSOX | ΔνSOX | |||||||
| 1 | 2-Naph | Ph | 7(RS,SC,RS) | 13(SC,SS) | 157 | 77 | 1065 | 1020 | 1133 |
| 2 | 7(RS,SC,SS) | 13(SC,RS) | 66 | 139 | 1064 | 1016 | 1133 | ||
| 3 | Ph | Ph | 8(RS,SC,RS) | 14(SC,SS) | 150 | 61 | 1054 | 1016 | 1138 |
| 4 | 8(RS,SC,SS) | 14(SC,RS) | 79 | 144 | 1074 | 1020 | 1125 | ||
| 5 | iPr | Ph | 9(RS,SC,RS) | 15(SC,SS) | 56 | 128 | 1062 | 1017 | 1141 |
| 6 | 9(RS,SC,SS) | 15(SC,RS) | 40 | 0 | 1066 | 1032 | 1129 | ||
| 7 | 2-Naph | t Bu | 10(RS,SC,RS) | 16(SC,SS) | 171 | 169 | 1066 | 1002 | 1124 |
| 8 | 10(RS,SC,SS) | 16(SC,RS) | 33 | 115 | 1071 | 1024 | 1102 | ||
| 9 | Ph | t Bu | 11(RS,SC,RS) | 17(SC,SS) | 172 | 156 | 1067 | 1026 | 1124 |
| 10 | 11(RS,SC,SS) | 17(SC,RS) | 26 | 132 | 1056 | 1004 | 1115 | ||
| 11 | iPr | t Bu | 12(RS,SC,RS) | 18(SC,SS) | 60 | 0 | 1061 | 1026 | 1121 |
| 12 | 12(RS,SC,SS) | 18(SC,RS) | 169 | 88 | 1064 | 1025 | 1121 | ||
It can also be observed in these complexes the greater “trans influence” of the phenylsulfoxide on the chlorine in trans, with a slightly longer Pd–Cl(1) bond length (less strong bond), than that exerted by the amine on its chlorine in trans Pd–Cl(2) (stronger bond) (entries 1–4, Table 4).
| Entry | R2 | R1 | Pd complex | Bond lengths (Å) | ||||
|---|---|---|---|---|---|---|---|---|
| Pd–N | Pd–S | d1 | d2 | d1 − d2 | ||||
| Pd–Cl(1) | Pd–Cl(2) | |||||||
| 1 | 2-Naph | Ph | 13(SC,SS) | 2.0780 | 2.1929 | 2.3380 | 2.2989 | 0.0391 |
| 2 | 13(SC,RS) | 2.0700 | 2.2020 | 2.3070 | 2.2820 | 0.0250 | ||
| 3 | Ph | Ph | 14(SC,RS) | 2.0450 | 2.2060 | 2.3260 | 2.2870 | 0.0390 |
| 4 | iPr | Ph | 15(SC,RS) | 2.0470 | 2.1993 | 2.3177 | 2.2953 | 0.0224 |
| 5 | 2-Naph | t Bu | 16(SC,RS) | 2.0340 | 2.2265 | 2.2965 | 2.3042 | −0.0077 |
| 6 | Ph | t Bu | 17(SC,SS) | 2.0426 | 2.2333 | 2.3090 | 2.3014 | 0.0076 |
| 7 | iPr | t Bu | 18(SC,SS) | 2.0441 | 2.2336 | 2.3086 | 2.3046 | 0.004 |
It's important to note that the trans influence of the phenylsulfinyl group is just one factor among many that influence the catalytic activity and enantioselectivity of Pd(II) complexes, but it can have significant implications. In general, by understanding and leveraging the trans influence, you can design catalysts that promote specific reactions with high selectivity for a desired enantiomer. In our case, the trans influence of the phenylsulfinyl group can affect the ligand coordination and stability of the complex. The phenylsulfinyl ligand coordinates to the Pd(II) center forming a coordination complex where the trans influence of the phenylsulfinyl group can strengthen the metal–ligand bond, enhancing the stability of the complex.
This effect is not maintained in the case of the sterically hindered tert-butylsulfinyl group, where both Pd–Cl bond lengths present more similar values (entries 5–7, Table 4). As expected, the Pd–S bond lengths are shorter in the phenylsulfinyl derivatives than in the tert-butylsulfinyl analogs (entries 1–4 vs. 5–7, Table 4). It can be explained by considering the electronic and steric effects of the ligands on the metal center.
The observed higher trans influence of the phenylsulfinyl group could be attributed to its stronger electron-donating ability compared to the tert-butylsulfinyl group. The electron-donating nature of the phenyl group can result in increased electron density around the metal center, stabilizing the metal–ligand bond and enhancing the trans influence. Thus, the Pd–Cl bond anti to the PhSO group is longer than the other one in the complex.
It's important to consider that the trans influence can also be affected by various factors, including steric factors. The bulky tert-butylsulfinyl group may hinder the approach of sulfur to the metal center, generating a weaker long Pd–S bond consequently. Thus, the trans influence of the tBuSO group turns out to be like that of the NH2 group and therefore the Pd–Cl bond lengths for both Cl are also similar.
Sulfinamide/sulfone: ability and coordination mode
In order to determine the influence of the ligand structure on the N-desulfinylation process observed during the coordination of the sulfinamide/sulfoxide ligands with the Pd(II) precursor, we prepared other sulfinamide/sulfur derivatives as an analog bidentate ligand with a sulfur function in different oxidation state (sulfone, 21(RS,SC)) as the second coordinating element to the metal, as indicated in Scheme 3.The addition of the methyl sulfonyl carbanion was carried out under identical conditions to those previously described for the sulfinyl carbanion, by dissolving the imine 4(RS) and the methyl tert-butyl sulfone 20 in THF in the presence of LHMDS at −78 °C. The reaction also occurs in this case in a completely stereoselective manner, obtaining the product of the addition of the nucleophile through the least hindered side of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond of the N-sulfinylimine (Scheme 3).
N double bond of the N-sulfinylimine (Scheme 3).
Treatment of the sulfinamide/sulfone ligand 21(RS,SC) with Pd(CH3CN)2Cl2 yielded, after stirring for 3 days, a 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 mixture of two diastereoisomeric palladium complexes, epimers in the sulfur, 22(RS,SC) and 22(SS,SC) (Scheme 4), resulting from the coordination by each one of the sulfonylic oxygens. The coordination was found to proceed again with concomitant desulfinylation, as previously confirmed by monitoring the course of the reaction by NMR, thus eliminating the possibility of N-desulfinylation because of the process of treating the reaction and isolating the complex. The high-resolution mass spectrum confirmed the structure of both complexes.
35 mixture of two diastereoisomeric palladium complexes, epimers in the sulfur, 22(RS,SC) and 22(SS,SC) (Scheme 4), resulting from the coordination by each one of the sulfonylic oxygens. The coordination was found to proceed again with concomitant desulfinylation, as previously confirmed by monitoring the course of the reaction by NMR, thus eliminating the possibility of N-desulfinylation because of the process of treating the reaction and isolating the complex. The high-resolution mass spectrum confirmed the structure of both complexes.
In contrast to the well-known donor properties of the sulfinyl group, sulfones are very weak donors and show very limited coordination chemistry. In this sense, only a few examples of sulfone complexes have been described and structurally characterized.32 Interestingly, the preferential coordination of one of the sulfonyl oxygens from sulfonamides to magnesium and copper has also been described (Fig. 7).33 This coordination proved to be not strong enough to allow the isolation of the corresponding complexes.
 | ||
| Fig. 7 Proposed structures for magnesium sulfonamide (left) and copper sulfonamide complexes (right).32 | ||
In our case, due to the low coordination ability of the sulfonyl group, the complexes obtained from sulfone 21(RS,SC) were relatively labile and readily hydrolyzed by moisture with release of the free ligand. Thus, attempts to crystallize the complex were unsuccessful. Instead, crystals of the ammonium salt of the free ligand were obtained (Fig. 8). It should be noted that the counterion of this ammonium salt is the tert-butane sulfonate, which implies a sulfinamide sulfur oxidation and not a simple hydrolysis during the desulfinylation process.
In view of the observed desulfinylation promoted by Pd in the coordination of our ligands, the possibility of applying these conditions as a N-desulfinylation method of sulfinamides aroused our interest. A comparative study between the classic desulfinylation conditions by treatment with TFA in methanol (method A) and by treatment with PdCl2(CH3CN)2 followed by TMEDA (method B) was achieved, and the results are collected in Table 5.
In the so-called “method A”, after stirring for several days, the corresponding amines were obtained in moderate to good yields (46 to 88% yield, Table 5). The results obtained show the influence of steric effects of the R2 substituent on the reaction yield, obtaining the lowest yield in the case of the bulkier alkyl substituent iPr, with a longer reaction time (entry 4, Table 5).
Regarding “method B”, it consists of the treatment with TMEDA of the desulfinylated palladium complex, obtained in a first stage, to release the corresponding amino sulfoxide through a ligand exchange. Even though it is a two-step process, the overall yields are higher in all cases than those obtained with the classical method A, ranging between good (78%, entry 1, Table 5) and quantitative (entry 3, Table 5) yields.
Asymmetric Pd-catalyzed arylation of cyclopropane carboxylic acid derivative
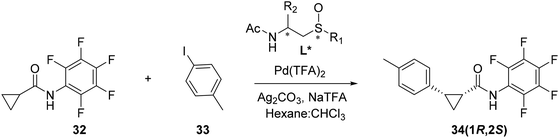
Given the versatility of our synthetic approach, it has been possible to prepare a wide range of amino/sulfoxides with different steric and electronic characteristics. These, through simple acetylation, lead us to the corresponding acetamido/sulfoxides whose catalytic capacity in the reaction of arylation of cyclopropane carboxylic acid derivative mediated by Pd has been previously demonstrated (Table 6).| Entrya | Ligand (AcNH/SO) | R2 | R1 | T (°C) | Conversion (%) | er (1R,2S)/(1S,2R) |
|---|---|---|---|---|---|---|
| a Entries 1–5: data extracted from ref. 10b. | ||||||
| 1 | 28(SC,RS) | Ph | p-Tol | 110 | 80 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 2 | 29(RC,RS) | Ph | p-Tol | 110 | 85 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85 |
| 3 | 30(SC,RS) | p-tBu-C6H4 | p-Tol | 80 | 75 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 4 | 31(SC,RS) | 2-Naph | p-Tol | 110 | 80 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 5 | 31(SC,RS) | Ph | t Bu | 110 | 15 | nd |
| 6 | 25(SC,SS) | Ph | Ph | 80 | 42 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 7 | 110 | 54 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
|||
| 9 | 26(SC,RS) | iPr | Ph | 80 | 28 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 10 | 110 | 46 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
|||
| 11 | 27(SC,RS) | 2-Naph | t Bu | 110 | 36 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
| 12 | 27(SC,SS) | 2-Naph | t Bu | 80 | nc | — |
| 13 | 110 | 34 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
|||
Regrettably, the precursor sulfinamide/sulfoxides ligands have not shown significant catalytic activity in these reactions, obtaining the cyclopropane arylation product in racemic form, with low conversion percentage.
According to the previous published results,10b the stereochemical course of the reaction is controlled by the configuration of the chiral carbon present in these ligands (compare entries 1 and 2, Table 6), and the best stereoselectivity is obtained with the ligand being substituted on said carbon with a p-tert-butylphenyl group (entry 3, Table 6). Regarding the influence of the substituent on sulfur, the enantiomer ratio obtained with different p-tolylsulfinyl derivatives as chiral ligands has been described and a single example of tert-butylsulfoxide is mentioned but the stereoselectivity of the process, which occurs with low conversion percentage (15%, entry 5, Table 6), is not indicated. Therefore, in order to determine the influence of sterically hindered sulfinyl groups, as the tert-butyl sulfinyl group, on the stereochemical course of the process, we decided to explore our chiral ligands on the enantioselectivity of the Pd-catalyzed arylation and optimize the conversion percentage as much as possible. The results obtained using the acetamide/sulfoxides 25(SC,SS), 26(SC,RS), 27(SC,SS) and 27(SC,RS) as chiral ligands, obtained by acetylation of the amino/sulfoxides 19(SC,RS), 23(SC,SS), 24(SC,SS) and 24(SC,RS) respectively, are indicated in Table 6.
Given that in our ligands, the configuration of the stereogenic carbon is maintained as S, the enantioselectivity obtained in the catalytic arylation process always runs in the same sense, in favor of the (1R,2S) stereoisomer, as confirmed by HPLC.
An increase in temperature, from 80 to 110 °C, leads to an increase in conversion and the enantioselectivity is not significantly modified (compare entries 6 vs. 7, Table 6) or slightly increases (compare entries 9 vs. 10, Table 6). The Pd(II) complexes with the phenylsulfinyl ligand can activate substrates for catalytic reactions. The trans influence can affect the activation process by modulating the electron density and electronic properties of the metal center. This activation step is crucial for facilitating the desired chemical transformation and, in our case, a π-stacking interaction of the phenyl group with the aromatic ring of the substrate must also be considered. Moreover, the trans influence of the phenylsulfinyl ligand can bias the orientation and reactivity of the substrates, favoring the cyclopropane ring to be arranged in anti with respect to the PhSO group in the Pd(II) complex. Thus, the cyclopropane ring of the substrate is located in the vicinity of the carbonyl of the ligand amide that should exert its effect as a base. This arrangement is necessary for activation of the CH bond of the cyclopropane ring to take place.
Thus, yields and enantioselectivities obtained with the complexes derived from tert-butyl sulfoxides are lower than those previously described with the p-tolyl sulfoxides, but the reaction proceeds with higher stereoselectivity in the case of the phenylsulfinyl ligand 25(SC,SS) (compare entries 6 and 7 vs. entry 1, Table 6).
With regard to the influence of the chiral carbon, an increase in steric volume associated with changing the Ph by an iPr group in ligand 26(SC,RS) leads to a decrease in enantioselectivity (compare entries 9 and 10 vs. 6 and 7, respectively, Table 6). Finally, it should be noted that er very similar are obtained with ligands 30(SC,RS) and 25(SC,SS), (entries 3 and 6 respectively in Table 6), so the substitution at position 4 of the aromatic ring (tBu vs. H, respectively) does not seem to be a determining factor in the enantioselectivity of the process.
Conclusions
This paper describes the preparation and structural characterizations of a series of enantiopure amine/sulfoxide palladium complexes obtained by treatment of sulfinamide/sulfoxides with PdCl2(CH3CN)2. In all cases coordination took place with concomitant desulfinylation. A similar behavior was observed in the case of the sulfone precursor, obtaining the two possible amine/sulfone palladium complexes, epimers at sulfur. These were spectroscopically characterized, and the structure of both complexes confirmed by the high-resolution mass spectrum.Starting from the N-sulfinamide/sulfoxide, a simple ligand exchange of its N-desulfinylated palladium complex enables us to obtain the corresponding free aminosulfoxide, thus offering a potentially interesting choice for performing N-desulfinylation when sensitive functionalities are present in the substrates.
The acetylated ligands were applied to the Pd-catalyzed arylation reaction, and the influence of temperature and steric factors were studied. The best results were obtained with the phenylsulfoxide ligand 25(SC,SS) which yielded the final arylated product in a 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 enantiomeric ratio.
7 enantiomeric ratio.
X-ray crystallographic studies have shown a higher trans influence of the phenylsulfinyl group compared to that of the tert-butylsulfinyl group which is very similar to that of the amine group. This finding is important for our ongoing work aimed at designing effective and selective catalysts, where the modulation of the trans influence can be decisive for achieve an enantioselective catalytic process. Finally, a common feature of these chelates of Pd with the amine/sulfoxide bidentate ligands is to form pseudo-1D-polymeric structures in the crystalline solid state.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministerio de Ciencia, Innovación y Universidades (grant number PID2019-104767RB-I00) cofinanced by the European Regional Development Fund (ERDF) from FEDER, and by the Consejería de Economía, Conocimiento, Empresas y Universidad, Junta de Andalucía (grant number: US-1381590). We gratefully thank CITIUS for NMR facilities.References
- (a) T. Okano, H. Konishi, J. Kiji, K. Fukuyama, H. Kumobayashi and S. Akutagawa, to Tagasago Perf. Co. Ltd, JP62.178.594, 1986 (CA: 1988, 108, 204837w); (b) H. Komobayashi and S. Akutagawa, to Takasago Perf. Co. Ltd., EP-B1-156.607, 1988 (CA: 1986, 105, 45202v).
- M. Itagaki, K. Masumoto and Y. Yamamoto, Asymmetric Cyclopropanation of 2,5-Dimethyl-2,4-Hexadiene by Copper Catalysts Bearing new Bisoxazoline Ligands, J. Org. Chem., 2005, 70, 3292–3295 CrossRef CAS PubMed.
- (a) T. Katsuki and K. B. Sharpless, The First Practical Method for Asymmetric Epoxidation, J. Am. Chem. Soc., 1980, 102, 5974–5976 CrossRef CAS; (b) J. G. Hill, K. B. Sharpless, C. M. Exon and R. Regenye, Enantioselective Epoxidation of Allylic Alcohols: (2S,3S)-3-Propyloxiranemethanol, Org. Synth., 1985, 63, 66–72 CrossRef CAS.
- (a) W. S. Knowles, M. J. Sabacky and B. D. Vineyard, to Monsanto Co., US12218571A, 1971; (b) W. S. Knowles, Asymmetric Hydrogenations. The Monsanto L Dopa Process, in Asymmetric Catalysis on Industrial Scale, ed. H.-U. Blaser and E. Schmidt, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004, pp. 21–38 Search PubMed.
- K. M. Engle and J. Q. Yu, Developing Ligands for Palladium(II)-Catalyzed C–H Functionalization: Intimate Dialogue between Ligand and Substrate, J. Org. Chem., 2013, 78, 8927–8955 CrossRef CAS PubMed.
- M. A. Fernández-Ibáñez, B. Maciá, D. A. Alonso and I. M. Pastor, Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis, Molecules, 2013, 18, 10108–10121 CrossRef PubMed.
- H. Lai, Z. Huang, Q. Wu and Y. Qin, Synthesis of Novel Enantiopure Biphenyl P,N-Ligands and Application in Palladium-Catalyzed Asymmetric Addition of Arylboronic Acids to N-Benzylisatin, J. Org. Chem., 2009, 74, 283–284 CrossRef CAS PubMed.
- (a) H. Dai, M. Yang and X. Lu, Palladium(II)-Catalyzed One-Pot Enantioselective Synthesis of Arylglycine Derivatives from Ethyl Glyoxylate, p-Toluenesulfonyl Isocyanate and Arylboronic Acids, Adv. Synth. Catal., 2008, 350, 249–253 CrossRef CAS; (b) H. Dai and X. Lu, Palladium-Catalyzed Allylation of Imines with Allyl Alcohols, Tetrahedron Lett., 2009, 50, 3478–3481 CrossRef CAS; (c) G.-N. Ma, T. Zhang and M. Shi, Catalytic Enantioselective Arylation of N-Tosylarylimines with Arylboronic Acids Using C2-Symmetric Cationic N-Heterocyclic Carbene Pd2+ Diaquo Complexes, Org. Lett., 2009, 11, 875–878 CrossRef CAS PubMed; (d) C. S. Marques and A. J. Burke, Catalytic Enantioselective Addition of Phenylboronic Acid and Phenylboroxine to N-Tosylimines: PdII and RhI Catalysis, Eur. J. Org. Chem., 2010, 1639–1643 CrossRef CAS; (e) J. Chen, X. Lu, W. Lou, Y. Ye, H. Jiang and W. Zeng, Palladium(II)-Catalyzed Enantioselective Arylation of α-Imino Esters, J. Org. Chem., 2012, 77, 8541–8548 CrossRef CAS PubMed; (f) X. Gao, B. Wu, Y. Zhong and Y.-G. Zhou, Enantioselective Palladium-Catalyzed Arylation of N-Tosylarylimines with Arylboronic Acids Using a Chiral 2,2′-Bipyridine Ligand, Org. Biomol. Chem., 2016, 14, 55–58 RSC; (g) Q. He, L. Wu, X. Kou, N. Butt, G. Yang and W. Zhang, Pd(II)-Catalyzed Asymmetric Addition of Arylboronic Acids to Isatin-Derived Ketimines, Org. Lett., 2016, 18, 288–291 CrossRef CAS.
- (a) M. F. Carroll, J. Chem. Soc., 1940, 704, 1266–1268 RSC; (b) T. Tsuda, Y. Chujo, S. I. Nishi, K. Tawara and T. Saegusa, Facile Generation of a Reactive Palladium(II) Enolate Intermediate by the Decarboxylation of Palladium(II). Beta.-Ketocarboxylate and its Utilization in Allylic Acylation, J. Am. Chem. Soc., 1980, 102, 6381–6384 CrossRef CAS; (c) J. A. Tunge and E. C. Burger, Transition Metal Catalyzed Decarboxylative Additions of Enolates, Eur. J. Org. Chem., 2005, 1715–1726 CrossRef CAS; (d) R. Kuwano, N. Ishida and M. Murakami, Asymmetric Carroll Rearrangement of Allyl α-Acetamido-β-Ketocarboxylates Catalysed by a Chiral Palladium Complex, Chem. Commun., 2005, 3951–3952 RSC.
- (a) S. E. Ammann, W. Liu and M. C. White, Enantioselective Allylic C–H Oxidation of Terminal Olefins to Isochromans by Palladium(II)/Chiral Sulfoxide Catalysis, Angew. Chem., Int. Ed., 2016, 55, 9571–9575 CrossRef CAS PubMed; (b) S. Jerhaoui, J. P. Djukic, J. Wencel-Delord and F. Colobert, Asymmetric, Nearly Barrierless C(sp3)–H Activation Promoted by Easily-Accessible N-Protected Aminosulfoxides as New Chiral Ligands, ACS Catal., 2019, 9, 2532–2542 CrossRef CAS.
- B. Feng, H. G. Cheng, J. R. Chen, Q. H. Deng, L. Q. Lu and W. J. Xiao, Palladium/Sulfoxide–Phosphine-Catalyzed Highly Enantioselective Allylic Etherification and Amination, Chem. Commun., 2014, 50, 9550–9553 RSC.
- Y. Wei, L. Q. Lu, T. R. Li, B. Feng, Q. Wang, W. J. Xiao and H. Alper, P,S Ligands for the Asymmetric Construction of Quaternary Stereocenters in Palladium-Catalyzed Decarboxylative [4 + 2] Cycloadditions, Angew. Chem., Int. Ed., 2016, 55, 2200–2204 CrossRef CAS PubMed.
- M. M. Pereira, M. J. F. Calvete, R. M. B. Carrilho and A. R. Abreu, Synthesis of Binaphthyl Based Phosphine and Phosphite Ligands, Chem. Soc. Rev., 2013, 42, 6990–7027 RSC.
- (a) A. P. Smalley, J. D. Cuthbertson and M. J. Gaunt, Palladium-Catalyzed Enantioselective C–H Activation of Aliphatic Amines Using Chiral Anionic BINOL-Phosphoric Acid Ligands, J. Am. Chem. Soc., 2017, 139(4), 1412–1415 CrossRef CAS PubMed; (b) L. Yang, R. Melot, M. Neuburger and O. Baudoin, Palladium(0)-Catalyzed Asymmetric C(sp3)–H Arylation Using a Chiral Binol-Derived Phosphate and an Achiral Ligand, Chem. Sci., 2017, 8, 1344–1349 RSC.
- (a) K. S. L. Chan, H.-Y. Fu and J.-Q. Yu, Palladium(II)-Catalyzed Highly Enantioselective C–H Arylation of Cyclopropylmethylamines, J. Am. Chem. Soc., 2015, 137, 2042–2046 CrossRef CAS PubMed; (b) B.-F. Shi, N. Maugel, Y.-H. Zhang and J.-Q. Yu, Pd(II)-Catalyzed Enantioselective Activation of C(sp2)-H and C(sp3)-H Bonds Using Monoprotected Amino Acids as Chiral Ligands, Angew. Chem., Int. Ed., 2008, 47, 4882–4886 CrossRef CAS PubMed; (c) D.-W. Gao, Y.-C. Shi, Q. Gu, Z.-L. Zhao and S.-L. You, Enantioselective Synthesis of Planar Chiral Ferrocenes via Palladium-Catalyzed Direct Coupling with Arylboronic Acids, J. Am. Chem. Soc., 2013, 135, 86–89 CrossRef CAS PubMed.
- D. Best, S. Kujawa and H. W. Lam, Diastereo- and Enantioselective Pd(II)-Catalyzed Additions of 2-Alkylazaarenes to N-Boc Imines Chiral Palladium Pincer Complexes for Asymmetric Catalytic Reactions and Nitroalkenes, J. Am. Chem. Soc., 2012, 134, 18193–18196 CrossRef CAS PubMed.
- J.-K. Liu, J.-F. Gong and M.-P. Song, Chiral Palladium Pincer Complexes for Asymmetric Catalytic Ractions, Org. Biomol. Chem., 2019, 17, 6069–6098 RSC.
- (a) N. Khiar, C. S. Araújo, E. Álvarez and I. Fernández, C-2-Symmetric bis-Thioglycosides as New Ligands for Palladium-Catalyzed Allylic Substitutions, Tetrahedron Lett., 2003, 44, 3401–3404 CrossRef CAS; (b) N. Khiar, C. S. Araújo, B. Suárez and I. Fernández, Sulfur-Sulfur Based Ligands Derived from D-Sugars: Synthesis of Pd(II) Complexes, Application in Palladium-Catalyzed Allylic Alkylation for the Synthesis of Both Members of Enantiomer Pairs, and Structural Studies, Eur. J. Org. Chem., 2006, 1685–1700 CrossRef CAS; (c) N. Khiar, B. Suárez, V. Valdivia and I. Fernández, Phosphinite Thioglycosides Derived from Natural D-Sugars as Useful P/S Ligands for the Synthesis of Both Enantiomers in Palladium-Catalyzed Asymmetric Substitution, Synlett, 2005, 2963–2967 CrossRef CAS.
- L. G. Borrego, R. Recio, E. Álvarez, A. Sánchez-Coronilla, N. Khiar and I. Fernández, Steric Tuning of Sulfinamide/Sulfoxides as Chiral Ligands with C1, Pseudo-meso, and Pseudo-C2 Symmetries: Application in Rhodium(I)-Mediated Arylation, Org. Lett., 2019, 21, 6513–6518 CrossRef CAS PubMed.
- (a) Z. M. Zhang, B. Xu, Y. Qian, L. Wu, Y. Wu, L. Zhou, Y. Liu and J. Zhang, Palladium-Catalyzed Enantioselective Reductive Heck Reactions: Convenient Access to 3,3-Disubstituted 2,3-Dihydrobenzofuran, Angew. Chem., Int. Ed., 2018, 57, 10373–10377 CrossRef CAS PubMed; (b) L. Wang, M. Chen, P. Zhang, W. Li and J. Zhang, Palladium/PC-Phos-Catalyzed Enantioselective Arylation of General Sulfenate Anions: Scope and Synthetic Applications, J. Am. Chem. Soc., 2018, 140, 3467–3473 CrossRef CAS PubMed; (c) Q. Dai, W. Li, Z. Li and J. Zhang, P-Chiral, Phosphines Enabled by Palladium/Xiao-Phos-Catalyzed Asymmetric P–C Cross-Coupling of Secondary Phosphine Oxides and Aryl Bromides, J. Am. Chem. Soc., 2019, 141, 20556–20564 CrossRef CAS PubMed; (d) T. Achard, J. Benet-Buchholz, E. C. Escudero-Adán, A. Riera and X. Verdaguer, N-Benzyl-N-Phosphino-tert-Butylsulfinamide and its Coordination Modes with Ir(I), Cu(I), Pd(II), and Pt(II): P,S or P,O, Organometallics, 2011, 30, 3119–3130 CrossRef CAS.
- (a) X. Zhao, M. Zhao and Y. Tian, Chiral Sulfinamide Ligand as well as Preparation Method and Application of Chiral Sulfinamide Ligand, CN 106748917A, 2017; (b) M. Zhao, Y. Tian and X. Zhao, Thieme Chemistry Journals Awardees – Where Are They Now? Chiral Sulfinamide Ligands and Pd-Catalyzed Asymmetric Allylic Alkylations of Ethyl 2-Fluoroacetoacetate, Synlett, 2017, 28, 1801–1806 CrossRef CAS.
- T. Y. Lin, Z. Pan, Y. Tu, S. Zhu, H. H. Wu, Y. Liu, Z. Li and J. Zhang, Design and Synthesis of TY-Phos and Application in Palladium-Catalyzed Enantioselective Fluoroarylation of gem-Difluoroalkenes, Angew. Chem., Int. Ed., 2020, 59, 22957–22962 CrossRef CAS PubMed.
- Y. Wang, P. Zhang, X. Di, Q. Dai, Z. M. Zhang and J. Zhang, Gold-Catalyzed Asymmetric Intramolecular Cyclization of N-Allenamides for the Synthesis of Chiral Tetrahydrocarbolines, Angew. Chem., Int. Ed., 2017, 56, 15905–15909 CrossRef CAS.
- L. Wang, M. Chen, P. Zhang, W. Li and J. Zhang, Palladium/PC-Phos-Catalyzed Enantioselective Arylation of General Sulfenate Anions: Scope and Synthetic Applications, J. Am. Chem. Soc., 2018, 140, 3467–3473 CrossRef CAS.
- H. Chu, J. Cheng, J. Yang, Y. L. Guo and J. Zhang, Asymmetric Dearomatization of Indole by Palladium/PC-Phos-Catalyzed Dynamic Kinetic Transformation, Angew. Chem., Int. Ed., 2020, 59, 21991–21996 CrossRef CAS PubMed.
- (a) X. Su, W. Zhou, Y. Li and J. Zhang, Design, Synthesis, and Application of a Chiral Sulfinamide Phosphine Catalyst for the Enantioselective Intramolecular Rauhut-Currier Reaction, Angew. Chem., Int. Ed., 2015, 54, 6874–6877 CrossRef CAS PubMed; (b) P. Chen, Z. Yue, J. Zhang, X. Lv, L. Wang and J. Zhang, Phosphine-Catalyzed Asymmetric Umpolung Addition of Trifluoromethyl Ketimines to Morita-Baylis-Hillman Carbonates, Angew. Chem., Int. Ed., 2016, 55, 13316–13320 CrossRef CAS PubMed.
- Q. Dai, W. Li, Z. Li and J. Zhang, P-Chiral Phosphines Enabled by Palladium/Xiao-Phos-Catalyzed Asymmetric P–C Cross-Coupling of Secondary Phosphine Oxides and Aryl Bromides, J. Am. Chem. Soc., 2019, 141, 20556–20564 CrossRef CAS PubMed.
- (a) X. Verdaguer, A. Moyano, M. A. Pericás, A. Riera, M. A. Maestro and J. Mahia, A New Chiral Bidentate (P,S) Ligand for the Asymmetric Intermolecular Pauson–Khand Reaction, J. Am. Chem. Soc., 2000, 122, 10242–10243 CrossRef CAS; (b) X. Verdaguer, M. A. Pericás, A. Riera, M. A. Maestro and J. Mahia, Design of New Hemilabile (P,S) Ligands for the Highly Diastereoselective Coordination to Alkyne Dicobalt Complexes: Application to the Asymmetric Intermolecular Pauson–Khand Reaction, Organometallics, 2003, 22, 1868–1877 CrossRef CAS; (c) X. Verdaguer, A. Lledó, C. López-Mosquera, M. A. Maestro, M. A. Pericás and A. Riera, PuPHOS: A Synthetically Useful Chiral Bidentate Ligand for the Intermolecular Pauson-Khand Reaction, J. Org. Chem., 2004, 69, 8053–8061 CrossRef CAS PubMed; (d) J. Solá, A. Riera, X. Verdaguer and M. A. Maestro, Phosphine–Substrate Recognition through the C–H⋯O Hydrogen Bond: Application to the Asymmetric Pauson–Khand Reaction, J. Am. Chem. Soc., 2005, 127, 13629–13633 CrossRef PubMed; (e) C. Ferrer, A. Riera and X. Verdaguer, Sulfinylmethyl Phosphines as Chiral Ligands in the Intermolecular Pauson–Khand Reaction, Organometallics, 2009, 28, 4571–4576 CrossRef CAS; (f) Y. N. Ji, A. Riera and X. Verdaguer, Asymmetric Intermolecular Pauson–Khand Reaction of Symmetrically Substituted Alkynes, Org. Lett., 2009, 11, 4346–4349 CrossRef CAS PubMed; (g) M. Reves, A. Riera and X. Verdaguer, PNSO Ligands as a Tool to Study Metal Bonding of Electron-Deficient Sulfinyl Groups, Eur. J. Inorg. Chem., 2009, 29–30, 4446–4453 CrossRef; (h) J. Solá, M. Revés, A. Riera and X. Verdaguer, N-Phosphino Sulfinamide Ligands: an Efficient Manner to Combine Sulfur Chirality and Phosphorus Coordination Behavior, Angew. Chem., Int. Ed., 2007, 46, 5020–5023 CrossRef PubMed; (i) M. Revés, T. Achard, J. Sola, A. Riera and X. Verdaguer, N-Phosphino-p-Tolylsulfinamide Ligands: Synthesis, Stability, and Application to the Intermolecular Pauson–Khand Reaction, J. Org. Chem., 2008, 73, 7080–7087 CrossRef; (j) S. Brun, M. Parera, A. Pla-Quintana, A. Roglans, T. León, T. Achard, J. Solà, X. Verdaguer and A. Riera, Chiral N-Phosphino Sulfinamide Ligands in Rhodium(I)-Catalyzed [2 + 2 + 2] Cycloaddition Reactions, Tetrahedron, 2010, 66, 9032–9040 CrossRef CAS.
- C. Pettinari, M. Pellei, G. Cavicchio, M. Crucianelli, W. Panzeri, M. Colapietro and A. Cassetta, Synthesis and Structural Investigations of Novel Palladium(II) and Rhodium(I) Complexes Containing Chiral Ligands with a Stereogenic Sulfur Donor, such as Î2-Amino Sulfoxides and C2-Symmetric bis-Sulfoxides, Organometallics, 1999, 18, 555–563 CrossRef CAS.
- (a) I. Fernández, N. Khiar, J. M. Llera and F. Alcudia, Asymmetric Synthesis of Alkane- and Arenesulfinates of Diacetone-D-Glucose (DAG): an Improved and General Route to Both Enantiomerically Pure Sulfoxides, J. Org. Chem., 1992, 57, 6789–6796 CrossRef; (b) I. Fernández and N. Khiar, Recent Developments in the Synthesis and Utilization of Chiral Sulfoxides, Chem. Rev., 2003, 103, 3651–3706 CrossRef PubMed; (c) N. Khiar, F. Alcudia, J. L. Espartero, L. Rodríguez and I. Fernández, Dynamic Kinetic Resolution of Bis-Sulfinyl Chlorides: A General Enantiodivergent Synthesis of C2-Symmetric Bis-Sulfinate Esters and Bis-Sulfoxides, J. Am. Chem. Soc., 2000, 122, 7598–7599 CrossRef CAS; (d) D. Balcells, G. Ujaque, I. Fernández, N. Khiar and F. Maseras, How Does the Achiral Base Decide the Stereochemical Outcome in the Dynamic Kinetic Resolution of Sulfinyl Chlorides? A Computational Study, Adv. Synth. Catal., 2007, 249, 2103–2110 CrossRef.
- M.M Yang, S. Wang and Z. B. Dong, Recent Advances for Chiral Sulfoxides in Asymmetric Catalysis, Synthesis, 2022, 23, 5168–5185 Search PubMed.
- (a) E. V. Dikarev, R. Y. Becker, E. Block, Z. Shan, R. C. Haltiwanger and M. A. Petrukhina, The First Coordination Complexes of Selenones: A Structural Comparison with Complexes of Sulfones, Inorg. Chem., 2003, 42, 7098–7105 CrossRef CAS PubMed; (b) P. Biagini, F. Calderazzo, F. Marchetti, G. Pampaloni, S. Ramello, M. Salvalaggip, R. Santi and S. Spera, Mono- and Dinuclear Complexes of Sulfones with the Tetrachlorides of Group 4, Dalton Trans., 2004, 2364–2371 RSC; (c) Y. Niu, S. Feng, Y. Ding, R. Qu, D. Wang and J. Han, Theoretical Investigation on Sulfur-Containing Chelating Resin-Divalent Metal Complexes, Int. J. Quantum Chem., 2010, 110, 1982–1993 CAS; (d) H. Richter, S. Beckendorf and G. García-Mancheño, Modifiable Sulfur Tethers as Directing Groups for Aromatic C-H Acetoxylation Reactions, Adv. Synth. Catal., 2011, 353, 295–302 CrossRef CAS; (e) M. Block, M. Bette, C. Wagner, J. Schmidt and D. Steinborn, Rhodium(I) Complexes with κP Coordinated ω-Phosphinofunctionalized Alkyl Phenyl Sulfide, Sulfoxide and Sulfone Ligands and Their Reactions with Sodium bis(Trimethylsilyl)Amide and Ag[BF4], J. Organomet. Chem., 2011, 696, 1768–1781 CrossRef CAS.
- S. Nakamura, H. Nakashima, H. Sigumoto, H. Sano, M. Hattori, N. Shibata and T. Toru, Enantioselectives C-C Bond Formation to Sulfonylimines Through use of the 2-Pyridinesulfonyl Group as a Novel Stereocontroller, Chem. – Eur. J., 2008, 14, 2145–2152 CrossRef CAS PubMed.
- (a) D. H. Hua, S. W. Miao, J. S. Chen and S. Iguchi, Stereoselective Addition Reactions of Chiral N-Benzylidene-p-Toluenesulfinamides. Asymmetric Syntheses of β- and γ-Amino Acids, J. Org. Chem., 1991, 56, 4–6 CrossRef CAS; (b) F. A. Davis, S. Lee, H. M. Zhang and D. L. Fanelli, Applications of the Sulfinimine-Mediated Asymmetric Strecker Synthesis to the Synthesis of α-Alkyl α-Amino Acids, J. Org. Chem., 2000, 65, 8704–8708 CrossRef CAS PubMed.
- M. Harmata, M. Kahraman, D. E. Jones, N. Pavri and S. E. Weatherwax, The Reaction of N-Phenylsulfonimidoyl Chloride with Trimethylsilylethene. A New Route to 2-Alkenylanilines, Tetrahedron, 1998, 54, 9995–10006 CrossRef CAS.
- M. Mikolajczyk, J. Drabowicz and B. Bujnicki, Acid-Catalysed Conversion of Sulphinamides into Sulphinates: A New Synthesis of Optically Active Sulphinates, J. Chem. Soc., Chem. Commun., 1976, 14, 568–569 RSC.
- (a) J. L. García-Ruano, I. Fernández, M. del Prado Catalina and A. Alcudia, Asymmetric Aziridination by Reaction of Chiral N-Sulfinylimines with Sulfur Ylides: Stereoselectivity Improvement by Use of tert-Butylsulfinyl Group as Chiral Auxiliary, Tetrahedron: Asymmetry, 1996, 7, 3407–3414 CrossRef; (b) F. A. Davis, T. Ramachandar and H. Liu, Asymmetric Synthesis of α-Amino 1,3-Dithioketals from Sulfinimines (N-Sulfinyl Imines). Synthesis of (2S,3R)-(−)-3-Hydroxy-3-methylproline, Org. Lett., 2004, 6, 3393–3395 CrossRef CAS PubMed.
- B. F. Li, K. Yuan, L. X. Dai and X. L. Hou, A Mild and Convenient Procedure for Desulfinylation of p-Toluenesulfinyl Amines, Synlett, 2005, 535–537 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1912692–1912695 and 2261905–2261917. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ob00816a |
| This journal is © The Royal Society of Chemistry 2023 |