Open Access Article
Open Access ArticleAlkylation of NH-sulfoximines under Mitsunobu-type conditions†
Cayden J.
Dodd
 ,
Daniel C.
Schultz
,
Daniel C.
Schultz
 ,
Jinming
Li
,
Jinming
Li
 ,
Craig W.
Lindsley
,
Craig W.
Lindsley
 and
Aaron M.
Bender
and
Aaron M.
Bender
 *
*
Warren Center for Neuroscience Drug Discovery, Department of Pharmacology, Vanderbilt University, 393 Nichol Mill Lane, Franklin, TN 37067, USA. E-mail: aaron.bender@vanderbilt.edu
First published on 6th June 2023
Abstract
Previously described approaches for the alkylation of NH-sulfoximines typically rely either on transition metal catalysis, or the use of traditional alkylation reagents and strong bases. Herein, we report a straightforward alkylation of diverse NH-sulfoximines under simple Mitsunobu-type conditions, despite the unusually high pKa of the NH center.
Sulfoximines, the mono-aza analogues of sulfones, have increasingly emerged as a useful isostere for sulfur-containing, biologically-active molecules.1 Although the first sulfoximine-containing molecule (methionine sulfoximine (1), Fig. 1) was characterized in the 1940s,2 reports of sulfoximine chemistry in the literature have grown particularly quickly over the past twenty years. Indeed, the total reactions per decade involving a sulfoximine substructure increased nearly 100-fold from the 1990s to the 2010s.1a The physicochemical advantages offered to medicinal chemists by the sulfoximine substructure are plentiful and include the potential for chemistry on a basic, nucleophilic nitrogen center (via NH-sulfoximines), increased solubility in protic solvents compared to sulfones, and the addition of a stereocenter.3 A number of sulfoximine-containing drug-like small molecules have been reported in the literature, including the rofecoxib analog 2,4 and the ataxia telangiectasia and Rad3-related kinase inhibitor AZD6738 (3), which has recently entered clinical trials.5 Sulfoximines have also found use in insecticides; the insect neurotoxin sulfoxaflor (4) was approved by the EPA in 2013
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (see Fig. 1 for the chemical structures of selected sulfoximine-containing compounds).1a
6 (see Fig. 1 for the chemical structures of selected sulfoximine-containing compounds).1a
Among other methods, NH-sulfoximines are readily synthesized from the corresponding thioethers using PhI(OAc)2 and an ammonia source,7 and their recent, widespread commercial availability has facilitated explorations around the chemistry available to the nucleophilic NH center. Many groups have described N-sulfonylation, sulfenylation, phosphorylation, acylation, halogenation, trifluoromethylation, and arylation, among other transformations.8 It is therefore perhaps surprising that there are comparatively few reports describing the simple N-alkylation of NH-sulfoximines. To date, such reports rely either on the use of transition metal and/or visible light catalysis,9 or alkylation conditions involving toxic or strongly basic reagents (which are often limited to methylations and other simple alkyl groups)4,10 to effect this kind of transformation (Scheme 1). Although many of these studies are encouraging with respect to the high yields with which alkylated NH-sulfoximines can be isolated, there exists an unmet need to develop a simple, economical approach that is of greater utility to chemists working in the drug discovery space. These historical limitations of NH-sulfoximine alkylations prompted us to consider the following: could this type of functionalization be effected using a simpler, classical approach, specifically Mitsunobu-type chemistry?
The pKa of the sulfoximine NH center is many orders of magnitude beyond the traditionally operable range for Mitsunobu-type chemistry, and, accordingly, such an approach would not obviously be applicable for the functionalization of NH-sulfoximines (pKa's < 11 traditionally tolerated, sulfoximine NH pKa = 24 in DMSO11). However, the advent of next-generation, electron-rich azodicarboxylates including 1,1′-(azodicarbonyl)dipiperidine (ADDP) and tetramethylazodicarboxamide (TMAD or diamide)12 has allowed for select poorly acidic nucleophiles to participate in this type of alkylation reaction. Additionally, reports describing the “all in one” phosphorane reagents (cyanomethylene)trimethylphosphorane (CMMP) and (cyanomethylene)tributylphosphorane (CMBP, Scheme 1),13 detail the alkylations of numerous unconventional and low-acidity nucleophiles including pyrazoles, carbon nucleophiles, amines, and alcohols.14 CMBP, or the Tsunoda reagent, is particularly attractive due to its commercial availability, high thermal stability compared to the azodicarboxylates, and relative stability compared to CMMP.13 We therefore wondered if this reagent might affect the alkylation of racemic NH-sulfoximine 5 using cyclopropylmethanol (Scheme 1).
While an initial reaction using ADDP and P(n-Bu)3 proved unsuccessful toward this end (Table 1, entry 1), we were gratified to observe the desired sulfoximine 6 in relatively low yield using CMBP under similar thermal conditions originally described by Tsunoda and others (entry 2, 30%).13b,14c A survey of lower temperatures (entries 3–6) revealed 70 °C to be optimal (entry 5, 72% isolated yield). Replacement of toluene with alternative solvents (entries 7–9) proved detrimental; no desired product was observed when the reaction was run in DCE under identical conditions (entry 8). Additionally, shortened reaction times were also counterproductive (entry 10), as were changes in reaction concentration (entries 11 and 12). We therefore selected the conditions described in entry 5 to probe the substrate scope of this reaction. Overall, we were encouraged by the operational simplicity of these conditions (minimal reagents without the need for an aqueous workup) as well as the clean conversion (primary isolated byproduct is typically starting material 5, Scheme 1).
| Entrya | Reagent(s) | Solvent | Temp. (°C) | Time (h) | M | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise stated, reactions run under an N2 atmosphere in a sealed vessel with cyclopropylmethanol (2 eq.) and CMBP (2 eq.). b Isolated yield. c Reaction was monitored by LCMS, and no isolation was attempted. d Average of 2 independent experiments. | ||||||
| 1 | P(n-Bu)3 (1.5 eq.), ADDP (1.5 eq.) | THF | r.t. | 2 | 0.2 | 0c |
| 2 | CMBP | Toluene | 120 | 24 | 0.2 | 30 |
| 3 | CMBP | Toluene | r.t. | 24 | 0.2 | 37 |
| 4 | CMBP | Toluene | 50 | 24 | 0.2 | 56 |
| 5 | CMBP | Toluene | 70 | 24 | 0.2 | 72 ± 1 |
| 6 | CMBP | Toluene | 90 | 24 | 0.2 | 49 |
| 7 | CMBP | THF | 70 | 24 | 0.2 | 40 |
| 8 | CMBP | DCE | 70 | 24 | 0.2 | 0c |
| 9 | CMBP | MeCN | 70 | 24 | 0.2 | 46 |
| 10 | CMBP | Toluene | 70 | 3 | 0.2 | 55 |
| 11 | CMBP | Toluene | 70 | 24 | 0.1 | 52 |
| 12 | CMBP | Toluene | 70 | 24 | 0.4 | 64 |
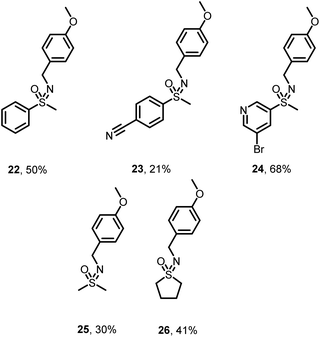
NH-Sulfoximine 5 can be alkylated with a wide variety of alcohols to give substituted sulfoximines 7–21 in moderate to high yields (Scheme 2, 23–79%). Simple aliphatic alcohols (7–11) were well tolerated under the reaction conditions, furnishing the corresponding substituted sulfoximines in high yields. Of note is deuterated sulfoximine 8, which is readily synthesized from methanol-d4 in a comparable yield to the other short chain aliphatic substrates (70%). More complex aliphatic systems (12–16) were also tolerated under the reaction conditions, including strongly basic amines (12), internal alkynes (13), and a variety of common protecting groups (14–16). Benzylic alcohols also readily yielded the corresponding N-benzyl sulfoximines, although these systems were observed to be sensitive to ring electronics. Whereas electron-withdrawing groups generally led to lower isolated yields (no desired NH-alkylation product was observed using (4-nitrophenyl)methanol), electron rich systems were readily isolated in moderate yields (17–19). Both 5- and 6-membered heteroaromatic systems, including pyrazole 20 and furan 21, were compatible with these conditions, and we were encouraged by the amenability of this reaction to these useful drug-like motifs. In parallel with our survey of compatible alcohols, we also explored variations on NH-sulfoximine 5 (Scheme 3) using (4-methoxyphenyl)methanol as a coupling partner. We found that a variety of NH-sulfoximines were well tolerated under the reaction conditions including bromopyridine 24 (68% isolated yield), and simple aliphatic NH-sulfoximines 25 and 26 (30% and 41% isolated yields, respectively). As observed with the alcohol substrate scope, electron-withdrawing modifications on the aryl NH-sulfoximine tended to diminish isolated yields (21% for benzonitrile 23). Although yields remained modest in some cases of alcohols with higher complexity, we were encouraged by the structural diversity of the NH-sulfoximines obtainable under these conditions, with many drug-like motifs tolerated.
As shown in Scheme 4, secondary alcohols were not tolerated under these reaction conditions, and only trace desired N-alkylated sulfoximine products were observed in such cases. Although there is some precedent in the literature for Mitsunobu-type reactions using tertiary alcohols,15 an attempted reaction with tert-butanol was also unsuccessful. This robust chemoselectivity for primary alcohols was successfully leveraged to generate N-alkylated sulfoximine 27, in which 5 was cleanly reacted with (R)-butane-1,3-diol without the need for additional protection steps (Scheme 4). No additional reactivity of the secondary alcohol was observed under these conditions.
In order to further understand the potential applications of this chemistry to additional drug-like motifs, NH-sulfoximine 29 was first generated from known erythropoietin-mimetic 28,7,16 which was subsequently subjected to the optimized alkylation conditions (Scheme 4). Intriguingly, compound 30 was isolated as the major product of the Mitsunobu alkylation for this specific substrate. Presumably, this occurs through α-deprotonation, potentially mediated by the phosphorane species, to form a vinyl sulfoximine with liberation of a phenolate anion. Nucleophilic aromatic substitution (SNAr) at the 2-position of the dimethyl-[1,2,4]triazolo[1,5-a]pyrimidine bicycle would then generate 30 after elimination of a vinylsulfinamide.17 This reactivity raises unique possibilities for the development of new aromatic substitution chemistry via controlled sulfoximine eliminations. For example, although β-eliminations of N-substituted sulfoximines have been previously disclosed,18 the one-pot aromatic substitution reaction described here could allow for the development of new sulfoximine-deletion chemistry within a given substrate (whether occurring through β-elimination or a different pathway).
In summary, we have described an operationally simple alkylation of diverse NH-sulfoximines using the versatile and underutilized Tsunoda reagent. These conditions allow for alkylations of NH-sulfoximines that are otherwise challenging under standard base-mediated SN2-type conditions, and can be applied to the straightforward alkylation of diverse systems. The key advantages of this method include operational simplicity, high functional group tolerance compared to previous reports, and use of easily handled and widely available reagents. Further studies on the reactivity of additional drug-like molecules under similar conditions, with an emphasis on the further characterization of competing sulfoximine elimination pathways, are ongoing in our laboratory and will be reported in due course.
Author contributions
A. M. B. conceived this work and performed synthetic chemistry with C. J. D., D. C. S., and J. L. A. M. B. wrote the manuscript with input and revisions from all authors.Conflicts of interest
There are no conflicts to declare.References
- For recent reviews on the chemistry and biological applications of sulfoximines, see: (a) P. Mäder and L. Kattner, J. Med. Chem., 2020, 63, 14243 CrossRef PubMed; (b) M. Andresini, A. Tota, L. Degennaro, J. A. Bull and R. Luisi, Chem. – Eur. J., 2021, 27, 17293 CrossRef CAS PubMed; (c) Y. Han, K. Xing, J. Zhang, T. Tong, Y. Shi, H. Cao, H. Yu, Y. Zhang, D. Liu and L. Zhao, Eur. J. Med. Chem., 2021, 209, 112885 CrossRef CAS PubMed.
- H. R. Bentley, E. E. McDermott, J. Pace, J. K. Whitehead and T. Moran, Nature, 1949, 163, 675 CrossRef CAS PubMed.
- U. Lücking, Angew. Chem., Int. Ed., 2013, 52, 9399 CrossRef PubMed.
- S. J. Park, H. Buschmann and C. Bolm, Bioorg. Med. Chem. Lett., 2011, 21, 4888 CrossRef CAS PubMed.
- K. M. Foote, J. W. M. Nissink, T. McGuire, P. Turner, S. Guichard, J. W. T. Yates, A. Lau, K. Blades, D. Heathcote, R. Odedra, G. Wilkinson, Z. Wilson, C. M. Wood and P. J. Jewsbury, J. Med. Chem., 2018, 61, 9889 CrossRef CAS PubMed.
- (a) Y. Zhu, M. R. Loso, G. B. Watson, T. C. Sparks, R. B. Rogers, J. X. Huang, B. C. Gerwick, J. M. Babcock, D. Kelley, V. B. Hegde, B. M. Nugent, J. M. Renga, I. Denholm, K. Gorman, G. J. DeBoer, J. Hasler, T. Meade and J. D. Thomas, J. Agric. Food Chem., 2011, 59, 2950 CrossRef CAS PubMed; (b) T. C. Sparks, G. B. Watson, M. R. Loso, C. Geng, J. M. Babcock and J. D. Thomas, Pestic. Biochem. Physiol., 2013, 107, 1 CrossRef CAS PubMed.
- J.-F. Lohier, T. Glachet, H. Marzag, A.-C. Gaumont and V. Reboul, Chem. Commun., 2017, 53, 2064 RSC.
- Selected examples include: (a) W. Zheng, M. Tan, L. Yang, R. R. Kuchukulla, L. Zhou and Q. Zeng, Eur. J. Org. Chem., 2020, 1764 CrossRef CAS; (b) L. Yang, J. Feng, M. Qiao and Q. Zeng, Org. Chem. Front., 2018, 5, 24 RSC; (c) M. Muneeswara, S. S. Kotha and G. Sekar, Synthesis, 2016, 48, 1541 CrossRef CAS; (d) D. L. Priebbenow and C. Bolm, Org. Lett., 2014, 16, 1650 CrossRef CAS PubMed; (e) A. Wimmer and B. König, Adv. Synth. Catal., 2018, 360, 3277 CrossRef CAS PubMed; (f) G. Y. Cho and C. Bolm, Org. Lett., 2005, 7, 1351 CrossRef CAS PubMed; (g) F. Teng, J. Cheng and C. Bolm, Org. Lett., 2015, 17, 3166 CrossRef CAS PubMed.
- (a) S. Gupta, P. Chaudhary, N. Muniyappan, S. Sabiah and J. Kandasamy, Org. Biomol. Chem., 2017, 15, 8493 RSC; (b) F. Teng, S. Sun, Y. Jiang, J.-T. Yu and J. Cheng, Chem. Commun., 2015, 51, 5902 RSC; (c) D. Ma, D. Kong, P. Wu, Y. Tu, P. Shi, X. Wang and C. Bolm, Org. Lett., 2022, 24, 2238 CrossRef CAS PubMed; (d) Y.-Y. Li and B. Gao, Org. Lett., 2023, 25, 2756 CrossRef CAS PubMed; (e) T. Feng, X. Luo, J. Dong and J. Mo, Synth. Commun., 2021, 51, 1284 CAS.
- (a) H. Zhao, H. Han, H. Yang and L. Wang, Mol. Catal., 2018, 455, 210 CrossRef CAS; (b) N. J. Peraino, H.-J. Ho, M. Mondal and N. J. Kerrigan, Tetrahedron Lett., 2014, 55, 4260 CrossRef CAS; (c) C. R. Johnson and O. M. Lavergne, J. Org. Chem., 1993, 58, 1922 CrossRef CAS; (d) S. Gaillard, C. Papamicaël, G. Dupas, F. Marsais and V. Levacher, Tetrahedron, 2005, 61, 8138 CrossRef CAS; (e) F. Lemasson, H.-J. Gais, J. Runsink and G. Raabe, Eur. J. Org. Chem., 2010, 2157 CrossRef CAS; (f) C. M. M. Hendriks, R. A. Bohmann, M. Bohlem and C. Bolm, Adv. Synth. Catal., 2014, 356, 1847 CrossRef CAS.
- M. Reggelin and C. Zur, Synthesis, 2000, 1 CrossRef CAS.
- (a) S. Fletcher, Org. Chem. Front., 2015, 2, 739 RSC; (b) H. Huang and J. Y. Kang, J. Org. Chem., 2017, 82, 6604 CrossRef CAS PubMed.
- (a) T. Tsunoda, F. Ozaki and S. Itô, Tetrahedron Lett., 1994, 35, 5081 CrossRef CAS; (b) T. Tsunoda, C. Nagino, M. Oguri and S. Itô, Tetrahedron Lett., 1996, 37, 2459 CrossRef CAS; (c) I. Sakamoto, H. Kaku and T. Tsunoda, Chem. Pharm. Bull., 2003, 51, 474 CrossRef CAS PubMed.
- (a) A. Mosallanejad and O. Lorthioir, Tetrahedron Lett., 2018, 59, 1708 CrossRef CAS; (b) A. F. M. Noisier, C. S. Harris and M. A. Brimble, Chem. Commun., 2013, 49, 7744 RSC; (c) T. Tonoi, Y. Yoshinaga, M. Fujishiro, K. Mameda, T. Kato, K. Shibamoto and I. Shiina, J. Nat. Prod., 2017, 80, 2335 CrossRef CAS PubMed; (d) F. Zaragoza and H. Stephensen, J. Org. Chem., 2001, 66, 2518 CrossRef CAS PubMed.
- J. E. Green, D. M. Bender, S. Jackson, M. J. O'Donnell and J. R. McCarthy, Org. Lett., 2009, 11, 807 CrossRef CAS PubMed.
- J. Punnonen, J. R. Spencer, T. J. Church, C. S. Tettenborn, K. Lariosa-Willingham, D. Leonoudakis and J. L. Miller, International Pub. No.WO2014081878A2, 2014 Search PubMed.
- For a review of common degradation pathways of sulfoximines, see: S. Wiezorek, P. Lamers and C. Bolm, Chem. Soc. Rev., 2019, 48, 5408 RSC.
- (a) M. Reggelin, S. Slavik and P. Bühle, Org. Lett., 2008, 10, 4081 CrossRef CAS PubMed; (b) M. Harmata and B. F. Herron, Tetrahedron, 1991, 47, 8855 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob00810j |
| This journal is © The Royal Society of Chemistry 2023 |