Open Access Article
Open Access ArticleSynthesis and in vitro photodynamic activity of aza-BODIPY-based photosensitizers†
Tamás
Hlogyik‡
a,
Réka
Laczkó-Rigó‡
 b,
Éva
Bakos
b,
Miklós
Poór
c,
Zoltán
Kele
d,
Csilla
Özvegy-Laczka
*b and
Erzsébet
Mernyák
b,
Éva
Bakos
b,
Miklós
Poór
c,
Zoltán
Kele
d,
Csilla
Özvegy-Laczka
*b and
Erzsébet
Mernyák
 *a
*a
aDepartment of Inorganic, Organic and Analytical Chemistry, University of Szeged, Dóm tér 8, H-6720 Szeged, Hungary. E-mail: bobe@chem.u-szeged.hu; Fax: +36 62 544200; Tel: +36 62 544277
bDrug resistance research group, Institute of Enzymology, Research Centre for Natural Sciences, Magyar tudósok körútja 2, H-1117 Budapest, Hungary
cDepartment of Pharmacology, Faculty of Pharmacy, University of Pécs, Rókus u. 2, H-7624 Pécs, Hungary. E-mail: laczka.csilla@ttk.hu; Tel: +3613826789
dDepartment of Medicinal Chemistry, University of Szeged, Dóm tér 8, H-6720 Szeged, Hungary
First published on 12th July 2023
Abstract
Aza-BODIPY dyes have recently come to attention owing to their excellent chemical and photophysical properties. In particular, their absorption and emission maxima can efficiently be shifted to the red or even to the NIR spectral region. On this basis, aza-BODIPY derivatives are widely investigated as fluorescent probes or phototherapeutic agents. Here we report the synthesis of a set of novel aza-BODIPY derivatives as potential photosensitizers for use in photodynamic therapy. Triazolyl derivatives were obtained via Cu(I)-catalyzed azide–alkyne cycloaddition as the key step. In vitro photodynamic activities of the newly synthesized compounds were evaluated on the A431 human epidermoid carcinoma cell line. Structural differences influenced the light-induced toxicity of the test compounds markedly. Compared to the initial tetraphenyl aza-BODIPY derivative, the compound bearing two hydrophilic triethylene glycol side chains showed substantial, more than 250-fold, photodynamic activity with no dark toxicity. Our newly synthesized aza-BODIPY derivative, acting in the nanomolar range, might serve as a promising candidate for the design of more active and selective photosensitizers.
1. Introduction
Organic fluorophores are utilized in several bioapplications; however, they must meet numerous requirements, including easy availability, strong absorption, preferably in the near-infrared region (NIR), and tunable photophysical properties.1–4 Nowadays, rhodamine, cyanine, and boron-dipyrromethene (BODIPY) dyes are the most commonly used organic compounds for bioapplications. Nevertheless, all of them possess several drawbacks, such as limited synthetic availability, low fluorescence quantum yields, and low emission maxima or optical instability. BODIPY dyes stand out with their excellent photophysical properties and post-functionalization capabilities. However, their low absorption maxima limit their applications, especially, when a higher penetration depth is required.5 The favorable properties of BODIPY dyes are retained in their aza counterparts, which are formally available upon replacing the C-8 atom with a nitrogen atom.5,6 Additionally, the absorption and emission maxima of the latter are highly improved, reaching the red or even the NIR region. Owing to their excellent chemical and photophysical properties, aza-BODIPY dyes have recently come to attention. They have been widely investigated not only as fluorescent probes7 but also as promising phototherapeutic agents.8 Special attention has recently been paid to utilize aza-BODIPY dyes as potential photosensitizers in photodynamic therapy (PDT).6 PDT is a minimally invasive approach for cancer therapy and other medical purposes.1 The basis of PDT is the photoexcitation of a photosensitizer (PS) that produces cytotoxic reactive oxygen species, which then induce various cellular processes finally leading to cell death. On the other hand, ideally, the photosensitizer should have no dark toxicity allowing specific treatment by focused illumination of the targeted tissue. The first aza-BODIPY-based photosensitizers were prepared by O'Shea and colleagues in 2002.6 Later, singlet oxygen yields were improved by the introduction of bromine or iodine atoms into certain positions of the dye core.9 An important correlation appeared between the position of the halogen and fluorescence quantum yields obtained.10–13 Heavy-atom substitutions at the pyrrole rings seemed to be more advantageous than those at the peripheral aryl rings. PS1, synthesized by Gallagher and O'Shea et al., was proved to be an exciting PS through Phase 1 clinical trials in tumor models (Fig. 1).14 It was shown that the direct bromo substitution on the aza-BODIPY core results in efficient 1O2 generation. Nevertheless, the optically beneficial heavy-atom effect often results in marked dark cytotoxicity of photosensitizers.15–17 In spite of the several advantages of PDT in clinical treatment, its low selectivity often limits its application.18 A PS which could be activated by tumor-associated stimuli (activatable PS: aPS) could serve as a better alternative.19,20 O'Shea et al. reported recently an aza-BODIPY-based aPS (PS2) bearing a pH-responsive amine receptor. The utilization of PS2 resulted in reversible off/on switching of 1O2 production.21 An acid-enhanced 1O2 rate for PS2 was determined compared to a non-acidic solution. Unwanted side effects might additionally arise from non-selective PS accumulation in both tumorous and adjacent healthy tissues.19 The encapsulation of PSs in ligand-modified liposomes is a powerful method to improve selectivity.22 Ju et al. designed a trifunctional aza-BODIPY-based aPS (PS3) to elaborate the selective tumor imaging methodology.23 The presence of diethylaminophenyl groups facilitated pH-activatable 1O2 generation. Encapsulation into cyclic RGD peptide-poly(ethylene glycol)-block-poly(lactic acid) nanomicelles enabled the formation of water-soluble nanoparticles. The self-monitoring capability of the nanoparticles provided a possibility for tracking PDT efficacy.The introduction of hydrophilic groups into a photosensitizer might provide more favorable bioapplications due to improvements in its water-solubility and biocompatibility. One of the reported strategies includes the introduction of oligo(ethylene glycol) side chains onto the dye core.24 Additionally, the presence of a triazole ring in biologically active compounds might also lead to the improvement of their activity, in particular, concerning anticancer agents.25 Although certain aza-BODIPY derivatives have even reached clinical trials,14 it is highly desired to permanently search for new candidates with improved selectivity and efficacy.
Encouraged by the predominant importance of aza-BODIPY dyes in PDT, here we aimed to synthesize new, heavy-atom-free derivatives as potential photosensitizers. The introduction of (N-benzyltriazolyl) or azido(triethylene glycol) side chains was planned via post-functionalization, utilizing the Cu(I)-catalyzed azide–alkyne cycloaddition reaction (CuAAC) as a key step. The evaluation of in vitro phototoxic activities of the newly synthesized dyes in A431 cancer cells was also conducted.
2. Results and discussion
2.1. Synthesis of aza-BODIPY derivatives
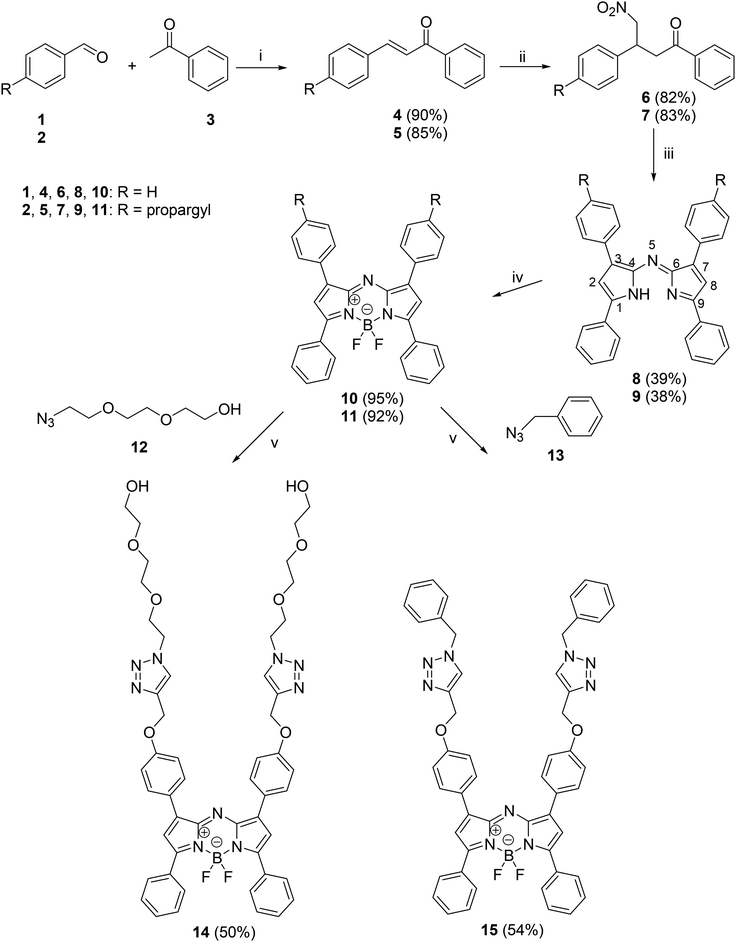
Aza-BODIPY derivatives might be available via different protocols.26,27 Here we have selected a routine methodology starting with the Michael addition of nitromethane to chalcones (4 and 5) (Scheme 1). Two diaryl-α,β-unsaturated ketones (4 and 5), differing in the substitution pattern of the phenyl ring of the aldehyde, were synthesized via aldol condensation between benzaldehyde or its 4-propargyloxy counterpart and acetophenone. The addition of nitromethane afforded compounds 6 and 7 in high yields. The aza-bridge was established using ammonium acetate as the ammonia source in ethanol solvent under reflux conditions. The applied aza-BODIPY synthesis strategy is formally considered to be dimerization, and thus aryl substituents at positions C-1 and C-7 or C-3 and C-5 are identical in pairs. The azadipyrromethenes (8 and 9) formed were subjected to chelation with BF3·OEt2 using triethylamine (TEA), a weak organic base, at room temperature. The establishment of the fused three-ring system is crucial in order to inhibit the rotation around the aza-bridging bonds and to obtain a fluorescent compound. Tetraphenyl derivative 10 was synthesized with the aim of getting a reference PS for biological testing. However, bis-propargyl derivative 11 allowed post-functionalization leading to potentially more promising PSs. In an effort to obtain aza-BODIPY derivatives with high photodynamic activity, compound 11 was subjected to the CuAAC reaction with two different azides as coupling partners. In the first reaction, benzyl azide was chosen as the coupling partner. The click reaction was efficiently achieved via our recently developed, well-established methodology, using CuI as a catalyst and PPh3 as an accelerating ligand.25 The (N-benzyltriazolyl)methyloxy compound (15) was obtained in an excellent yield. Our next click strategy included the introduction of hydrophilic oligo(ethylene glycol) side chains via the CuAAC reaction. Propargyl derivative 11 as a terminal alkyne was reacted with azido-triethylene glycol as an azide partner under the reaction conditions described above. The newly synthesized aza-BODIPY derivative 14, bearing two hydrophilic side chains, might possess improved biological activity in comparison with its counterpart 11.2.2. Photophysical characterization of aza-BODIPY dyes
Due to their good solubility in DMF, UV-Vis and fluorescence spectroscopic properties of compounds 10, 11, 14, and 15 were examined in this solvent. Among the four derivatives examined, compound 10 had the highest absorption signal, showing its maximum at 654 nm (Fig. 2A). The absorption maxima of derivatives 11, 14, and 15 appeared at higher wavelengths, from 667 to 670 nm (Table 1), with the lowest absorbance observed for dye 14 (Fig. 2A). The emission wavelength maxima of the four compounds tested were between 676 and 701 nm (Table 1). Compound 10 showed the highest fluorescence emission signal (Fig. 2B), which was more than two-fold larger than those of the other three derivatives (Fig. 2C and D). It was followed by 11, then by 15 and 14. | ||
| Fig. 2 Absorption spectra of compounds 10, 11, 14, and 15 (A). Fluorescence excitation and emission spectra of 10 (B), 11 (C), 14 (D), and 15 (E). The representative spectra of test compounds (2 μM each) shown here were recorded in DMF at room temperature (see excitation and emission wavelengths collected in Table 1). | ||
| Compound | 10 | 11 | 14 | 15 |
|---|---|---|---|---|
| Solvent | DMF | DMF | DMF | DMF |
| λ abs [nm] | 654 | 667 | 670 | 670 |
| λ em [nm] | 676 | 693 | 701 | 701 |
| Δλ [nm] | 22 | 26 | 31 | 31 |
| ε max [M−1 cm−1] (±SD) | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987 (±7394) 987 (±7394) |
74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435 (±4023) 435 (±4023) |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 (±5206) 505 (±5206) |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 825 (±5586) 825 (±5586) |
2.3. Evaluation of in vitro dark- and light-induced toxicity of aza-BODIPY dyes
To evaluate the potential dark- and light-induced toxic effects of the aza-BODIPY dyes (10, 11, 14, and 15), the epidermoid carcinoma cell line A431, a typical model in toxicology and cancer research, was chosen. A431 cells were incubated with increasing concentrations of the compounds for 24 hours. The next day, the compounds were removed and the cells were illuminated with an LED source of 658 nm for 10 min. Cell viability after 48 hours was determined with PrestoBlue. Dark controls were treated the same way, with the exception that they were kept in the dark throughout the experiment. Fig. 3A shows that all the compounds (10, 11, 14, and 15) exerted only negligible dark toxicity even at 10 μM. Encouraged by the low dark toxicity results, the in vitro photodynamic activity of the compounds was investigated. Fig. 3B shows that compound 15 exhibited only moderate toxicity even at 10 μM. Dye 11 was found to be more active, resulting in 50% cell death already at 5 μM test concentration. Still, since compounds effective in nanomolar concentration were looked for, neither compound 15 nor compound 11 was selected for further experiments.The two triazolyl aza-BODIPY derivatives, namely tetraphenyl compound 10 (IC50: 0.96 μM) and its bis-triazolyl-TEG counterpart 14 (IC50: 3.66 nM), resulted in efficient cell killing when irradiated, with the latter being 268-fold more active (Fig. 4). The significant increase in the phototoxicity results obtained for compound 14 might be assigned to its hydrophilic side chains present at the two phenyl rings possibly resulting in increased membrane permeability. It should be highlighted that structural differences influenced the light-induced toxicity of the test compounds markedly. The test compounds differ only in the para-substituents of the phenyl rings originating from the benzaldehyde starting compound or introduced by post-functionalization. To the best of our knowledge, there are no literature reports for aza-BODIPY derivatives acting on the A431 cell line in such a low nanomolar range.
 | ||
| Fig. 4 Concentration-dependent light-induced toxicity of compounds 10 (A) and 14 (B) with calculated IC50 values. | ||
2.4. Evaluation of in vitro ROS generation
Next, we investigated the mechanism of light-induced toxicity. To this aim, ROS generation by the compounds after illumination was measured using the DCF-DA dye. DCF-DA enters the cells passively, where it is converted to non-fluorescent H2-DCF by esterases. H2-DCF is then oxidized by ROS to fluorescent DCF. Fig. 5 shows that, in harmony with their phototoxicity, 10 and 14 resulted in increased ROS levels, while in cells incubated with non-toxic 11 and 15, we observed only a small increase in ROS levels compared to the cells illuminated in the absence of the dyes.3. Conclusions
New red-emitting aza-BODIPY derivatives (11, 14 and 15) were synthesized. A bis-propargyl tetraphenyl derivative (11) served as a terminal alkyne in CuAAC reactions with two different azide partners. The introduction of a triazole moiety was performed with the aim of increasing the anticancer potential of the compounds. The bis-triazolyl-TEG derivative (14) exhibited outstanding light-induced toxicity against the A431 carcinoma cell line with an excellent photo-to-dark toxicity ratio. We confirmed that post-functionalization of the tetraphenyl aza-BODIPY core might lead to superior photosensitizers without the need for the introduction of halogen atoms. The significance of our work lies in the simplicity and high efficiency of the newly synthesized compounds. Compound 14, acting in the nanomolar range, might serve as a promising candidate for the design of more active and selective photosensitizers.4. Experimental part
4.1. Chemistry
Melting points (Mp) were determined with Kofler hot-stage apparatus and are uncorrected. Elemental analyses were performed with a PerkinElmer CHN analyzer model. Thin-layer chromatography was performed on silica gel 60 F254 (layer thickness 0.2 mm, Merck); eluents: a: 30% ethyl acetate/70% hexanes, b: 25% ethyl acetate/75% hexanes, c: 50% dichloromethane/50% hexanes, d: 60% dichloromethane/40% hexanes, e: 10% methanol/90% dichloromethane, and f: 5% methanol/95% dichloromethane. The spots were detected with I2 or UV (365 nm) after spraying with 5% phosphomolybdic acid in 50% aqueous phosphoric acid and heating at 100–120 °C for 10 min. Flash chromatography was performed on silica gel 60, 40–63 μm (Merck). 1H NMR spectra were recorded in DMSO-d6 or CDCl3 solution with a Bruker DRX-500 instrument at 500 MHz. 13C NMR spectra were recorded with the same instrument at 125 MHz under the same conditions. Mass spectrometry: full scan mass spectra of the newly synthesized compounds were acquired in the range of 100 to 1100 m/z with a Q Exactive Plus quadrupole-orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a heated electrospray (HESI). Analyses were performed in positive ion mode by flow injection mass spectrometry with a mobile phase of 50% aqueous acetonitrile containing 0.1 v/v% formic acid (0.3 ml min−1 flow rate). Aliquots of 5 μl of samples were injected into the flow. The ESI capillary was adjusted to 3.5 kV and N2 was used as a nebulizer gas.4.1.2.1. Synthesis of chalcones 4 and 5. Benzaldehyde (1, 1.06 g, 10 mmol) or 4-(prop-2-ynyloxy)benzaldehyde 2 (1.60 g, 10 mmol) was dissolved in a solution of sodium hydroxide (0.80 g, 20 mmol) in methanol (10 mL), and stirred at rt for 10 minutes. Acetophenone (3, 1.17 mL, 10 mmol) was added, and the stirring was continued at rt for 2 h. The solvent was removed under reduced pressure, and the residue was extracted with dichloromethane (3 × 15 mL). The combined organic layers were dried over sodium sulfate and the solvent was removed under reduced pressure. Compound 4 or 5 was obtained as a yellow solid (4: 1.87 g, 90%; 5: 2.28 g, 85%).
4: Mp = 55.0–57.0 °C, Rf = 0.64a. Compound 4 was identical to the compound described in the literature.28,29
5: Mp = 60.3–62.1 °C, Rf = 0.60b. Compound 5 was identical to the compound described in the literature.30,31
4.1.2.2. Synthesis of 4-nitro-1,3-diphenylbutan-1-one (6) or 4-nitro-1-phenyl-3-(4-prop-2-ynyloxy-phenyl)butan-1-one (7). Chalcone 4 (1.50 g, 7.2 mmol) or 5 (1.89 g, 7.2 mmol) was dissolved in a solution of 0.2 equiv. of sodium hydroxide (58 mg, 1.44 mmol) in methanol (12 mL). This was followed by the addition of nitromethane (1.92 mL, 36 mmol) and the solution was stirred at 60 °C for 3 h. The solvent was removed under reduced pressure, and the residue was extracted with ethyl acetate (3 × 15 mL) and washed with brine (1 × 10 mL). The combined organic layers were dried over sodium sulfate, and the solvent was removed under reduced pressure. Compound 6 was obtained as a pale yellow solid (1.59 g, 82%) and compound 7 as a pale yellow oil (1.93 g, 83%).
6: Mp = 68.5–69.4 °C, Rf = 0.55a. Compound 6 was identical to the compound described in the literature.32
7: oil, Rf = 0.50a. ESI-HRMS: m/z: 346.10547 [M + Na]+ (C19H17NO4 + Na+ requires 346.10498 [M + Na]+). 1H NMR (500 MHz, DMSO-d6) δ 3.45–3.57 (overlapping multiplets, 3H, CH2 and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 4.01 (m, 1H), 4.75 (d, J = 4.7 Hz, 2H, O–CH2), 4.82 (dd, 1H, J = 12.8 Hz, J = 5.7 Hz, 1H), 4.95 (dd, 1H, J = 12.8 Hz, J = 9.7 Hz, 1H), 6.92 (d, J = 7.3 Hz, 2H), 7.31 (d, J = 7.3 Hz, 2H), 7.51 (t, J = 7.6 Hz, 2H), 7.63 (t, J = 7.6 Hz, 1H), 7.93 (d, J = 7.6 Hz, 2H). 13C NMR (125 MHz, DMSO-d6) δ 197.4 (C
CH), 4.01 (m, 1H), 4.75 (d, J = 4.7 Hz, 2H, O–CH2), 4.82 (dd, 1H, J = 12.8 Hz, J = 5.7 Hz, 1H), 4.95 (dd, 1H, J = 12.8 Hz, J = 9.7 Hz, 1H), 6.92 (d, J = 7.3 Hz, 2H), 7.31 (d, J = 7.3 Hz, 2H), 7.51 (t, J = 7.6 Hz, 2H), 7.63 (t, J = 7.6 Hz, 1H), 7.93 (d, J = 7.6 Hz, 2H). 13C NMR (125 MHz, DMSO-d6) δ 197.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 156.2 (C), 136.3 (C), 133.3 (CH), 132.4 (C), 128.7 (2 × CH), 128.6 (2 × CH), 127.8 (2 × CH), 114.6 (2 × CH), 79.7 and 79.2 and 78.0 (CH2 and C
O), 156.2 (C), 136.3 (C), 133.3 (CH), 132.4 (C), 128.7 (2 × CH), 128.6 (2 × CH), 127.8 (2 × CH), 114.6 (2 × CH), 79.7 and 79.2 and 78.0 (CH2 and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 55.2 (CH2), 41.2 (CH2), 38.4 (CH).
CH), 55.2 (CH2), 41.2 (CH2), 38.4 (CH).
4.1.2.3. Synthesis of (3,5-diphenyl-1H-pyrrol-2-yl)-(3,5-diphenyl-pyrrol-2-ylidene)amine 8 and 5-phenyl-3–4-prop-2-ynyloxy-phenyl-1H-pyrrol-2-yl-[5-phenyl-3-(4-prop-2-ynyloxy-phenyl)-pyrrol-2-ylidene]-amine 9. To a solution of compound 6 (1.20 g, 4.5 mmol) or compound 7 (1.45 g, 4.5 mmol) in ethanol (20 mL), ammonium acetate (12.1 g, 158 mmol) was added. The solution was stirred under reflux for 24 h, then cooled to room temperature and three-quarters of the solvent was removed under reduced pressure. The residue was filtered and washed with cold ethanol. The remaining solid was suspended in water and then extracted with dichloromethane (3 × 20 mL). The combined organic layers were dried over sodium sulfate and the solvent was removed under reduced pressure. The residue was purified by flash chromatography with CH2Cl2/hexane = 40/60 as an eluent. Compound 8 was obtained as a dark blue solid, and compound 9 as a dark green solid (390 mg, 39% for compound 8 and 475 mg, 38% for compound 9).
8: Mp = 287.3–290.8 °C, Rf = 0.65c. Compound 8 was identical to the compound described in the literature.32
9: Mp = 234.8–236.4 °C, Rf = 0.38c. ESI-HRMS: m/z: 558.21836 [M + H]+ (C38H27N3O2 + H+ requires 558.21761 [M + H]+). 1H NMR (500 MHz, DMSO-d6) δ 3.61 (s, 2H, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 4.91 (d, J = 2.1 Hz, 2 × OCH2), 7.12 (d, J = 7.1 Hz, 4H), 7.54 (t, J = 7.5 Hz, 2H), 7.58 (s, 2H), 7.62 (t, J = 7.5 Hz, 4H), 8.07 (overlapping multiplets, 8H). 13C NMR (125 MHz, DMSO-d6) δ 157.3 (2 × C), 154.9 (2 × C), 148.7 (2 × C), 141.2 (2 × C), 131.3 (2 × C), 130.4 (2 × CH), 129.8 (4 × CH), 129.3 (4 × CH), 126.5 (4 × CH), 126.4 (2 × C), 114.8 (4 × CH), 114.6 (2 × CH), 79.1 and 78.2 (2 × C
CH), 4.91 (d, J = 2.1 Hz, 2 × OCH2), 7.12 (d, J = 7.1 Hz, 4H), 7.54 (t, J = 7.5 Hz, 2H), 7.58 (s, 2H), 7.62 (t, J = 7.5 Hz, 4H), 8.07 (overlapping multiplets, 8H). 13C NMR (125 MHz, DMSO-d6) δ 157.3 (2 × C), 154.9 (2 × C), 148.7 (2 × C), 141.2 (2 × C), 131.3 (2 × C), 130.4 (2 × CH), 129.8 (4 × CH), 129.3 (4 × CH), 126.5 (4 × CH), 126.4 (2 × C), 114.8 (4 × CH), 114.6 (2 × CH), 79.1 and 78.2 (2 × C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 55.4 (2 × OCH2).
CH), 55.4 (2 × OCH2).
4.1.2.4. Synthesis of aza-BODIPY 10 and 11. A stirred solution of compound 8 (314 mg, 0.69 mmol) or 9 (385 mg, 0.69 mmol) in toluene (10 mL) was treated with triethylamine (0.96 mL, 10 equiv.). The solution was stirred at rt for 15 min, then BF3·OEt2 (1.28 mL, 15 equiv.) was added dropwise and the solution was stirred under reflux for 5 h. After cooling to rt, the reaction was quenched using water (30 mL) and extracted with toluene (3 × 20 mL). The combined organic layers were dried over sodium sulfate and the solvent was removed under reduced pressure. The residue was purified by flash chromatography with CH2Cl2/hexane = 35/65 as an eluent. Compound 10 was obtained as a dark red solid (331 mg, 95%) and compound 11 as a dark greenish-blue solid (390 mg, 92%).
10: Mp = 221.5–224.8 °C, Rf = 0.40c. Compound 10 was identical to the compound described in the literature.32,331H NMR (500 MHz, CDCl3) δ 7.05 (s, 2H), 7.42–7.50 (overlapping multiplets, 12H), 8.04–8.08 (overlapping multiplets, 8H).
11: Mp = 183.5–187.0 °C, Rf = 0.24d. ESI-HRMS: m/z: 606.21601 [M + H]+ (C38H26BF2N3O2 + H+ requires 606.21589 [M + H]+). 1H NMR (500 MHz, CDCl3) δ 2.59 (s, 2H, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 4.78 (d, J = 2.4 Hz, 2 × OCH2), 6.94 (s, 2H), 7.08 (d, J = 7.1 Hz, 4H), 7.47–7.49 (overlapping multiplets, 6H), 8.02–8.07 (overlapping multiplets, 8H). 13C NMR (125 MHz, CDCl3) δ 159.3 (2 × C), 158.9 (2 × C), 145.5 (2 × C), 143.6 (2 × C), 131.8 (2 × C), 130.9 (4 × CH), 130.7 (2 × CH), 129.5 (4 × CH), 128.5 (4 × CH), 126.2 (2 × C), 117.8 (2 × CH), 115.2 (4 × CH), 78.3 and 75.9 (2 × C
CH), 4.78 (d, J = 2.4 Hz, 2 × OCH2), 6.94 (s, 2H), 7.08 (d, J = 7.1 Hz, 4H), 7.47–7.49 (overlapping multiplets, 6H), 8.02–8.07 (overlapping multiplets, 8H). 13C NMR (125 MHz, CDCl3) δ 159.3 (2 × C), 158.9 (2 × C), 145.5 (2 × C), 143.6 (2 × C), 131.8 (2 × C), 130.9 (4 × CH), 130.7 (2 × CH), 129.5 (4 × CH), 128.5 (4 × CH), 126.2 (2 × C), 117.8 (2 × CH), 115.2 (4 × CH), 78.3 and 75.9 (2 × C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 55.9(2 × OCH2).
CH), 55.9(2 × OCH2).
4.1.2.5. Synthesis of 14. To a stirred solution of compound 11 (303 mg, 0.5 mmol) in dry toluene (10 mL), PPh3 (26 mg, 0.1 mmol) and CuI (3.2 mg, 0.05 mmol) were added. The solution was treated with azido-PEG3-alcohol (175 mg, 1.0 mmol) and DIPEA (0.70 mL, 4 mmol). The solution was stirred under reflux for 2 h. After cooling to rt, the solvent was removed under reduced pressure and the residue was purified by flash chromatography with methanol/CH2Cl2 = 1/9 as an eluent. Compound 14 was obtained as a dark blue solid (239 mg, 50%).
14: Mp = 42.6–43.5 °C, Rf = 0.60e. ESI-HRMS: m/z: 978.39006 [M + Na]+ (C50H52BF2N9O8 + Na+ requires 978.38922 [M + Na]+). 1H NMR (500 MHz, DMSO-d6) δ 3.37 (t, J = 5.2 Hz, 4H), 3.45–3.48 (overlapping multiplets, 8H), 3.51–3.53 (overlapping multiplets, 4H), 3.82 (t, J = 5.2 Hz, 4H), 4.54–4.57 (overlapping multiplets, 6H), 5.31 (s, 4H), 7.27 (d, J = 8.8 Hz, 4H), 7.47 (s, 2H), 7.55–7.57 (overlapping multiplets, 6H), 8.06–8.08 (overlapping multiplets, 4H), 8.19 (d, J = 8.8 Hz, 4H), 8.26 (s, 2H). 13C NMR (125 MHz, CDCl3) δ 159.7 (2 × C), 158.4 (2 × C), 144.6 (2 × C), 142.9 (2 × C), 142.2 (2 × C), 131.1 (2 × C), 130.9 (2 × CH), 130.7 (4 × CH), 129.3 (4 × CH), 128.5 (4 × CH), 125.0 (2 × C), 124.6 (2 × C), 118.4 (2 × CH), 115.2 (4 × CH), 72.2 (2 × CH2), 69.5 (2 × CH2), 69.4 (2 × CH2), 68.6 (2 × CH2), 61.2 (2 × CH2), 60.1 (2 × CH2), 49.3 (2 × CH2).
4.1.2.6. Synthesis of 15. To a stirred solution of compound 11 (300 mg, 0.5 mmol) in dry toluene (10 mL), PPh3 (26 mg, 0.1 mmol) and CuI (3.2 mg, 0.05 mmol) were added. The solution was treated with benzyl azide (125 mg, 1.0 mmol) and DIPEA (0.70 mL, 4 mmol). The solution was stirred under reflux for 5 h. After cooling to rt, the solvent was removed under reduced pressure and the residue was purified by flash chromatography with ethyl acetate/CH2Cl2 = 1/9 as an eluent. Compound 15 was obtained as a reddish-dark blue solid (235 mg, 54%).
15: Mp = 220.2–223.8 °C, Rf = 0.69f. ESI-HRMS: m/z: 872.34405 [M + H]+ (C52H40BF2N9O2 + H+ requires 872.34389 [M + H]+). 1H NMR (500 MHz, DMSO-d6) δ 5.29 (s, 4H), 5.62 (s, 4H), 7.26 (d, J = 8.9 Hz, 4H), 7.31–7.38 (overlapping multiplets, 10H), 7.48 (s, 2H), 7.55–7.57 (overlapping multiplets, 6H), 8.07 (m, 4H), 8.19 (d, J = 8.9 Hz, 4H), 8.33 (s, 2H). 13C NMR (125 MHz, CDCl3) δ 159.6 (2 × C), 158.3 (2 × C), 144.5 (2 × C), 142.8 (2 × C), 142.6 (2 × C), 135.7 (2 × C), 131.0 (2 × C), 130.8 (2 × CH), 130.6 (4 × CH), 129.2 (4 × CH), 128.5 (4 × CH), 128.4 (4 × CH), 127.9 (2 × CH), 127.7 (4 × CH), 124.6 (2 × CH), 124.4 (2 × C), 118.3 (2 × CH), 115.1 (4 × CH), 61.2 (2 × CH2), 52.7 (2 × CH2).
4.2. Spectroscopic measurements
Dimethyl formamide (DMF; HPLC grade) was purchased from Merck (Darmstadt, Germany).Absorption spectra were collected by employing a V730 UV-Vis spectrophotometer (Jasco, Tokyo, Japan). Fluorescence excitation and emission spectra were recorded using a Fluorolog τ3 spectrofluorometer (Jobin–Yvon/SPEX, Longjumeau, France). General spectroscopic properties of compounds 10, 11, 14, and 15 were examined in 10 mm path-length quartz cuvettes, in DMF at room temperature.
4.3. Phototoxicity measurements
For the phototoxicity measurements, the A431 epidermoid carcinoma cell line (ATCC) was used. The cells were maintained in DMEM (Gibco, Thermo Fischer Scientific (Waltham, MA, US)) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin at 37 °C with 5% CO2 and 95% humidity.Compounds 10, 11, 14, and 15 were dissolved in DMF (Sigma, Merck, Darmstadt, Germany) at a final concentration of 20 mM. For the irradiation procedure, 7.5 × 103 cells were seeded in 48-well plates (ThermoScientific) and incubated for 24 h. The next day, compounds 10, 11, 14, and 15 with increasing concentrations (0–25 μM diluted in DMEM) were added to the cells and were incubated for another 24 h. Prior to illumination, the medium was removed from the cells and fresh DMEM (300 μl per well) was added. The plates were then irradiated from the bottom for 10 min (with periodical 30/15/30 s illumination) with a 658 nm LED light source (dose of 4 W cm−2, in-house equipment). After illumination, the cells were incubated for 48 h at 37 °C with 5% CO2 and 95% humidity before being assessed for cell viability. As a dark toxicity control, the experiments were performed under the same conditions but without illumination and keeping the plates in the dark throughout the experiment.
Cell viability was determined using PrestoBlue reagent (ThermoFisher Scientific, Waltham, MA, US) according to the manufacturers’ instructions. Briefly, 5% PrestoBlue reagent diluted in phosphate-buffered saline (PBS) was added to the cells, and after 60 min incubation at 37 °C, fluorescence was measured in an Enspire fluorescent microplate reader (PerkinElmer, Waltham MA, US). The results are presented as the average of three independent biological replicates with three technical replicates in each experiment.
4.4. Measurement of ROS generation
The ROS generation potential of the dyes was measured using DCF-DA (2′,7′-dichlorofluorescein diacetate, Sigma, Merck, Darmstadt, Germany). 7.5 × 103 A431 cells were seeded on 48-well plates and incubated with compounds 10, 11, 14, and 15 at 1–10 μM concentration. After 24 hours of incubation with 10, 11, 14, and 15, the cells were washed three times with PBS. Next, A431 cells were incubated with the cell-permeant DCF-DA dye (10 μM in HBSS) for 30 min at 37 °C with 5% CO2 and 95% humidity, and then the cells were washed three times with PBS. Finally, the cells were illuminated for 10 min, as described in section 4.3, and fluorescence was measured in an Enspire fluorescent plate reader at 485/530 nm. The results are presented as the average of three independent biological replicates with three technical replicates in each experiment.4.5. Statistical analysis
Statistical analysis was performed using Graph Prism v8.0.1 software (GraphPad Software Inc., San Diego, California). An unpaired t-test with Welch's correction was performed to compare the results with those of untreated samples. ns: P > 0.05; *: P ≤ 0.05; **: P ≤ 0.01; ***: P ≤ 0.001.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Research, Development, and Innovation Office-NKFIH through projects OTKA SNN 139323, OTKA FK 128751, and OTKA K 138518. The help of Dr Krisztina Németh (Institute of Organic Chemistry, RCNS, Budapest) and Dr Péter Kele (Institute of Organic Chemistry, RCNS, Budapest) for providing the light source for illumination is greatly appreciated.References
- A. M. Smith, M. C. Mancini and S. Nie, Second window for in vivo imaging, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- Y. Cai, Z. Wei, C. Song, C. Tang, W. Han and X. Dong, Optical nano-agents in the second near-infrared window for biomedical applications, Chem. Soc. Rev., 2019, 48, 22–37 RSC.
- K. K. Ng and G. Zheng, Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications, Chem. Rev., 2015, 115, 11012–11042 CrossRef CAS PubMed.
- X. Miao, W. Hu, T. He, H. Tao, Q. Wang, R.-F. Chen, L. Jin, H. Zhao, X. Lu, Q. Fan and W. Huang, Deciphering the intersystem crossing in near-infrared BODIPY photosensitizers for highly efficient photodynamic therapy, Chem. Sci., 2019, 10, 3096–3102 RSC.
- T. Kowada, H. Maeda and K. Kikuchi, BODIPY-based probes for the fluorescence imaging of biomolecules in living cells, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC.
- J. Killoran, L. Allen, J. F. Gallagher, W. M. Gallagher and D. F. O'Shea, Synthesis of BF2 chelates of tetraarylazadipyrromethenes and evidence for their photodynamic therapeutic behaviour, Chem. Commun., 2002, 17, 1862–1863 RSC.
- G. Grossi, M. Morgunova, S. Cheung, D. Scholz, E. Conroy, M. Terrile, A. Panarella, J. C. Simpson, W. M. Gallagher and D. F. O'Shea, Lysosome triggered near-infrared fluorescence imaging of cellular trafficking processes in real time, Nat. Commun., 2016, 7, 10855 CrossRef PubMed.
- S. G. Awuah and Y. You, Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy, RSC Adv., 2012, 2, 11169–11183 RSC.
- A. Gorman, J. Killoran, C. O'Shea, T. Kenna, W. M. Gallagher and D. F. O'Shea, In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy, J. Am. Chem. Soc., 2004, 126, 10619–10631 CrossRef CAS PubMed.
- N. Adarsh, R. R. Avirah and D. Ramaiah, Tuning Photosensitized Singlet Oxygen Generation Efficiency of Novel Aza-BODIPY Dyes, Org. Lett., 2010, 12, 5720–5723 CrossRef CAS PubMed.
- Q. Tang, W. Si, C. Huang, K. Ding, W. Huang, P. Chen, Q. Zhang and X. Dong, An aza-BODIPY photosensitizer for photoacoustic and photothermal imaging guided dual modal cancer phototherapy, J. Matr. Chem., 2017, 5, 1566–1573 RSC.
- Y. Gawale, N. Adarsh, S. K. Kalva, J. Joseph, M. Pramanik, D. Ramaiah and N. Sekar, Carbazole-Linked Near-Infrared Aza-BODIPY Dyes as Triplet Sensitizers and Photoacoustic Contrast Agents for Deep-Tissue Imaging, Chem. – Eur. J., 2017, 23, 6570–6578 CrossRef CAS PubMed.
- H. Ş Çınar, Ş. Özçelik, K. Kaya, Ö. D Kutlu, A. Erdoğmuş and A. Gül, Synthesis and photophysical properties of monomeric and dimeric halogenated aza-BODIPYs, J. Mol. Struct., 2020, 1200, 127108–127117 CrossRef.
- A. T. Byrne, A. E. O'Connor, M. Hall, J. Murtagh, K. O'Neill, K. M. Curran, K. Mongrain, J. A. Rousseau, R. Lecomte, S. McGee, J. J. Callanan, D. F. O'Shea and W. M. Gallagher, Vascular-targeted photodynamic therapy with BF2-chelated Tetraaryl-Azadipyrromethene agents: a multi-modality molecular imaging approach to therapeutic assessment, Br. J. Cancer, 2009, 101, 1565–1573 CrossRef CAS PubMed.
- L. Ming and C. Li, Recent Advances in Activatable Organic Photosensitizers for Specific Photodynamic Therapy, ChemPlusChem, 2020, 85, 948–957 CrossRef PubMed.
- S. H. Lim, C. Thivierge, P. Nowak-Sliwinska, J. Han, H. Van Den Bergh, G. WagniHres, K. Burgess, H. B. Lee and J. Med, Chem, 2010, 53, 2865–2874 CAS.
- M. C. Malacarne, M. B. Gariboldi and E. Caruso, BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years, Int. J. Mol. Sci., 2022, 23, 10198–10220 CrossRef CAS PubMed.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, BODIPY dyes in photodynamic therapy, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- J. F. Lovell, T. W. Liu, J. Chen and G. Zheng, Activatable photosensitizers for imaging and therapy, Chem. Rev., 2010, 110, 2839–2857 CrossRef CAS PubMed.
- X. Li, S. Kolemen, J. Yoon and E. U. Akkaya, Activatable Photosensitizers: Agents for Selective Photodynamic Therapy, Adv. Funct. Mater., 2017, 27, 1604053 CrossRef.
- S. O. McDonnell, M. J. Hall, L. T. Allen, A. Byrne, W. M. Gallagher and D. F. O'Shea, Supramolecular photonic therapeutic agents, J. Am. Chem. Soc., 2005, 127, 16360–16361 CrossRef CAS PubMed.
- G. A. van Dongen, G. W. Visser and M. B. Vrouenraets, Photosensitizer-antibody conjugates for detection and therapy of cancer, Adv. Drug Delivery Rev., 2004, 56, 31–52 CrossRef CAS PubMed.
- J. Tian, J. Zhou, Z. Shen, L. Ding, J.-S. Yu and H. Ju, ApH-activatable and aniline-substituted photosensitizer for near-infrared cancer theranostics, Chem. Sci., 2015, 6, 5969–5977 RSC.
- G. Fan, L. Yang and Z. Chen, Water-soluble BODIPY and aza-BODIPY dyes: synthetic progress and applications, Front. Chem. Sci. Eng., 2014, 8, 405–417 CrossRef CAS.
- J. Szabó, Z. Pataki, J. Wölfling, G. Schneider, N. Bózsity, R. Minorics, I. Zupkó and E. Mernyák, Synthesis and biological evaluation of 13α-estrone derivatives as potential antiproliferative agents, Steroids, 2016, 113, 14–21 CrossRef PubMed.
- Z. Shi, X. Han, W. Hu, H. Bai, B. Peng, L. Ji, Q. Fan, L. Li and W. Huang, Bioapplications of small molecule Aza-BODIPY: from rational structural design to in vivo investigations, Chem. Soc. Rev., 2020, 49, 7533–7567 RSC.
- G. Yuan and D. F. O'Shea, Azadipyrromethenes: from traditional dye chemistry to leading edge applications, Chem. Soc. Rev., 2016, 45, 3846–3864 RSC.
- J. T. Li, W. Z. Yang, S. X. Wang, S. H. Li and T. S. Li, Improved synthesis of chalcones under ultrasound irradiation, Ultrason. Sonochem., 2002, 9, 237–239 CrossRef CAS PubMed.
- C. A. M. Alonso, N. R. Candeias, D. P. Simão, A. F. Trindade, J. A. S. Coelho, B. Tan and R. Franzén, Comprehensive Organic Chemistry Experiments for the Laboratory Classroom, RSC, 2016, 951 Search PubMed.
- C. Niu, G. Li, A. Tuerxuntayi and H. A. Aisa, Synthesis and Bioactivity of New Chalcone Derivatives as Potential Tyrosinase Activator Based on the Click Chemistry, Chin. J. Chem., 2015, 33, 486–494 CrossRef CAS.
- G. Singh, J. Singh, S. S. Mangat and A. Aurora, Synthetic approach towards ‘click’ modified chalcone based organotriethoxysilanes; UV-Vis study, RSC Adv., 2014, 4, 60853–60865 RSC.
- L. P. Jameson and S. V. Dzyuba, Aza-BODIPY: Improved synthesis and interaction with soluble Aβ1–42 oligomers, Bioorg. Med. Chem. Lett., 2013, 23, 1732–1735 CrossRef CAS PubMed.
- Y. Xu, M. Zhao, L. Zou, L. Wu, M. Xie, T. Yang, S. Liu, W. Huang and Q. Zhao, Highly Stable and Multifunctional Aza-BODIPY-Based Phototherapeutic Agent for Anticancer Treatment, ACS Appl. Mater. Interfaces, 2018, 10, 44324–44335 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob00699a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |