 Open Access Article
Open Access ArticleAn expedient copper-catalysed asymmetric synthesis of γ-lactones and γ-lactams. Application to the synthesis of lucidulactone A†
O. Stephen
Ojo
,
David L.
Hughes
and
Christopher J.
Richards
 *
*
School of Chemistry, University of East Anglia, Norwich, NR4 7TJ, UK. E-mail: Chris.Richards@uea.ac.uk
First published on 24th April 2023
Abstract
The parent Josiphos ligand gave excellent ee values (95–99%) and good yields (60–97%) in the copper-catalysed asymmetric conjugate reduction of β-aryl α,β-unsaturated lactones and lactams with PMHS. The substrates were obtained from stereospecific copper-catalysed addition of arylboronic acids to alkynoates followed by deprotection and cyclisation. The acyclic lactam precursors also underwent reduction with good ee values (83–85%) and yields (79–95%). Application of this asymmetric reduction methodology included the synthesis of natural product lucidulactone A.
Introduction
The construction of chiral β-substituted lactones and lactams has garnered considerable attention because of their ubiquitous existence as core structures in numerous biologically active natural products and clinical theraupetics.1 For example, a chiral γ-butyrolactone is present in natural products lucidulactone A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a and (3R)-A-factor 2.2b Chiral γ-lactams are found in important pharmaceuticals such as rolipram 3, an antidepressant drug (Fig. 1a). Also, β-GABA derivatives such as baclofen 4, a muscle relaxant, can be accessed via hydrolysis of the corresponding chiral lactams (Fig. 1b).3a–c Consequently, the development of new methods for the synthesis of chiral β-substituted lactones and lactams, particularly in a stereo-controlled fashion, is of significant importance.
2a and (3R)-A-factor 2.2b Chiral γ-lactams are found in important pharmaceuticals such as rolipram 3, an antidepressant drug (Fig. 1a). Also, β-GABA derivatives such as baclofen 4, a muscle relaxant, can be accessed via hydrolysis of the corresponding chiral lactams (Fig. 1b).3a–c Consequently, the development of new methods for the synthesis of chiral β-substituted lactones and lactams, particularly in a stereo-controlled fashion, is of significant importance.
Two methods for the for the enantioselective synthesis of chiral lactams and lactones are transition metal catalysed and involve either: (i) conjugate addition of an organometallic derived nucleophile to an α,β-unsaturated lactone or lactam precursor,4 or (ii) reduction of an unsaturated β-substituted precursor. Although molecular hydrogen may be employed as the reducing agent,5 a key development was the use of a copper catalyst incorporating the P,P-bidentate ligand p-tol-BINAP, and employing polymethylhydrosiloxane (PMHS) as the stoichiometric reductant (Scheme 1).6 This biaryl ligand, which has also been applied to the corresponding reduction of α,β-unsaturated esters7 and enones,8 has been superseded to some extent by other biaryl bisphosphines,9 of which the most notable are the SEGPHOS ligands.10 However, the specific application of the latter to the reduction of β-substituted unsaturated lactones is very limited.11 The only other ligand type to give high product ee values in copper-catalysed conjugate reduction reactions are the ferrocene-based Josiphos ligands as applied to acyclic substrates including enones,12 nitoalkenes13 and unsaturated nitriles.14,15
 | ||
| Scheme 1 Existing methods for the asymmetric synthesis of β-substituted γ-lactones and lactams by copper-catalysed asymmetric reduction.6 | ||
In this Paper we report the highly enantioselective synthesis of β-substituted γ-lactones and lactams by copper-catalysed conjugate reduction employing a readily available Josiphos ligand. Coupled with the accessible synthesis of the unsaturated lactone and lactam precursors by copper-catalysed alkynoate-addition/cyclisation, the overall methodology provides rapid access to the title compounds in high ee.
Results and discussion
Previous methods for the synthesis of β-substituted butenolides include the use of ring-closing metathesis16 and glyoxylic acid condensation followed by reduction.17 For the generation of β-aryl derivatives we were attracted to the simplicity of copper-catalysed conjugate addition of arylboronic acids to appropriately functionalised alkynoates, followed by cyclisation,18 and the potential to extend this methodology to give the corresponding lactams. Commencing with propargyl alcohol 5, TBS-protection gave 6, followed by treatment with n-BuLi and ethyl chloroformate to furnish alkynoate 7 (Scheme 2). The copper-catalysed conjugate syn-addition of aryl boronic acids produced β-aryl-alkenoates (Z)-8a–e exclusively,18a and to ensure good yields the functionalised boronic acids required typically the use of 30 mol% Cu(OAc)2. A stoichiometric quantity of Cu(OAc)2 was required with 2-methoxyphenylboronic acid to achieve conversion within twelve hours. Finally, acid mediated TBS deprotection and cyclisation of (Z)-8a–e furnished 9a–e, such that β-substituted α,β-unsaturated lactones were generated in four steps from commercially available propargyl alcohol (62–81% overall yield).Extension of this methodology to PMP-protected β-substituted α,β-unsaturated lactams required alkynoate 13 generated from Boc protection and propargylation of 10, followed by reaction with ethyl chloroformate (Scheme 3). Conjugate addition of aryl boronic acids were successful using 5–30 mol% of Cu(OAc)2 to generate β-aryl alkenoates 14a–c as exclusively the Z diastereoisomer. As for the generation of (Z)-8a–e, this is a consequence of stereospecific carbocupration of the alkyne followed by rapid protonolysis avoiding possible Z/E isomerisation.18a Subsequent acid-promoted Boc deprotection and cyclisation gave lactams 15a–c in five steps from p-anisidine (ca. 30% overall yield). Addition to 13 of an alkyl cuprate (Gilman reagent) generated from MeLi and CuI gave β-alkyl-alkenoate (Z)-14d in good yield and as a single isomer,19 with subsequent cyclisation providing β-alkyl lactam 15d.
The conjugate addition of boronic acid 18 (obtained in three steps from commercially available 16) to alkynoate 7 didn't generate the desired product. To solve this problem 18 was coupled successfully with 20 (obtained in one step from commercially available tetronic acid 19) to produce 9f (Scheme 4). The highly oxygenated phenolic moiety is present in many lignan natural products such as lucidulactone A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and descurainolide A.20 The former was isolated from Ganoderma lucidum, a medicinal mushroom associated with various health benefits.
2 and descurainolide A.20 The former was isolated from Ganoderma lucidum, a medicinal mushroom associated with various health benefits.
As the exploration of substrates of type 9f in transition-metal catalysed asymmetric reactions are rare, the initial objective was the enantioselective reduction of this substrate as a key step in the synthesis of 1. For this we chose to explore the use of ferrocene ligands as many are known with different chelating functional groups,21 and within several ligand classes it is possible to vary readily substituents and stereochemistry for the purpose of ee optimisation (Table 1).22
| Entry | Cu source (mol%) | L (mol%) | Conv. (time) | Yieldb (%) | eec (%) (config.) |
|---|---|---|---|---|---|
a On a 0.285 mmol scale, THF/t-BuOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
b Isolated yields.
c Determined by HPLC analysis.
d Cu(OAc)2 and Cu(OTf)2 also performed well (93 and 94% ee respectively).
e CuCl also performed well (96% ee). 1).
b Isolated yields.
c Determined by HPLC analysis.
d Cu(OAc)2 and Cu(OTf)2 also performed well (93 and 94% ee respectively).
e CuCl also performed well (96% ee).
|
|||||
| 1 | CuCl2 (5) | R,Sp-L1 (6) | <5% (16 h) | — | — |
| 2 | CuCl2 (5) | S,Sp-L2 (6) | 100% (16 h) | 21 | 81 (R) |
| 3 | (R,Sp)-L3-CuCl2d (3) | — | 100% (22 h) | 59 | 96 (R) |
| 4 | (R,Sp)-L3-CuBre (3) | — | 100% (12 h) | 78 | 99 (R) |
| 5 | (R,Sp)-L3-CuBr (2) | — | 95% (24 h) | 67 | 98 (R) |
In our initial ligand screen P,O ligand L1 performed poorly (entry 1), whereas P,N ligand L2 produced encouraging enantioselectivity (entry 2). Higher enantioselectivity was obtained with the P,P ligand L3, the parent Josiphos ligand,23 as a preformed complex with CuCl2 (entry 3). Switching to the CuBr complex gave lactone 6f in good yield and with essentially complete control of enantioselectivity (entry 4) such that further ligand optimization was not needed. Using less than 3 mol% of this complex led to a longer reaction time and lower yield (entry 5).
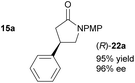
Table 2 shows the substrate scope of this study, which was also extended to acyclic substrates (Z)-14a–c. The asymmetric conjugate reduction of 9a–9e by (R,Sp)-Josiphos complex L3-CuBr produced (R)-21a–21e with excellent enantioselectivities (≥97% ee; entries 1–5). Based on these results, this work proved to be a convenient method for generating β-aryl lactones. Both the (R) and (S) enantiomers of 21f were generated in 99% ee using (R,Sp)-L3-CuBr and (S,Rp)-L3-CuBr, respectively (entry 6). The absolute configuration of (R)-21d and (R)-21f were confirmed by X-ray crystallography.24 The absolute configuration of (R)-21a–c and (R)-21e were confirmed by optical rotation and comparison to the literature data.25 Aryl and methyl β-substituted chiral γ-lactams 22a–d were also generated with excellent enantioselectivities (≥95%; entries 7–10), and the absolute configuration of 22a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 22d
4 and 22d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 were confirmed by optical rotation determination and comparison to the literature data.
26 were confirmed by optical rotation determination and comparison to the literature data.
| Entrya | Substrate | Productb,c | Entrya | Substrate | Productb,c | Entrya | Substrate | Productb,c |
|---|---|---|---|---|---|---|---|---|
a The reactions were carried out on a 0.312 mmol scale using 3 mol% (R,Sp)-L3-CuBr, PMHS (3 eq.), NaOt-Bu (2 eq.) in THF/t-BuOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), room temperature, 12 h.
b Isolated yields.
c Enantiomeric excess determined by chiral HPLC analysis.
d (S)-21f was obtained in 78% yield and 99% ee using (S,Rp)-L3-CuBr. 1), room temperature, 12 h.
b Isolated yields.
c Enantiomeric excess determined by chiral HPLC analysis.
d (S)-21f was obtained in 78% yield and 99% ee using (S,Rp)-L3-CuBr.
|
||||||||
| γ-Lactones | ||||||||
| 1 |

|
6d |

|
10 |

|
|||
| 2 |

|
γ-Lactams | Acyclic γ-lactam precursors | |||||
| 3 |

|
7 |
|
11 |
|
|||
| 4 |

|
8 |

|
12 |
|
|||
| 5 |

|
9 |

|
13 |

|
|||
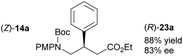
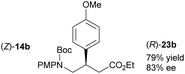
Acyclic products 23a–c were obtained in 83–85% ee (entries 11–13).27 The absolute configuration of 23c was determined as R following cyclisation to (R)-22c. Subsequent treatment with ceric ammonium nitrate generated (R)-24c,28 which was converted to baclofen 4 in a previous study (Scheme 5a).3b Likewise, 23a (78% ee), obtained from a larger scale reduction reaction (1.71 g of (Z)-14a – 1.8 mol% catalyst loading), was cyclised to (R)-22a. Removal of the PMP group produced (R)-24a that was converted previously to high value chiral pyrrolidine (R)-25 (Scheme 5b).3d Treatment of (S)-22d with ceric ammonium nitrate furnished known compound (S)-24a, which has been converted previously to a β-GABA derivative (S)-26 (Scheme 5c).3a As the enantioselectivity of the reduction of the acyclic precursors to the unsaturated lactams is lower than that obtained with the unsaturated lactams themselves, reduction of the latter may of course be used to generate these products in higher ee.
Finally, the total synthesis of lucidulactone A 1 was completed in two steps from (R)-21f (Scheme 6). Treatment with LiHMDS followed by addition to the resulting enolate of oxaziridine (R)-27 resulted in the highly diastereoselective formation of (3S,4R)-28.29 Subsequent treatment with TBAF generated (3S,4R)-1 in 71% over two steps. The specific rotation and NMR data obtained agree with the isolated sample data (see ESI†).2a
Conclusions
In conclusion, the parent Josiphos ligand (L3) in combination with copper bromide gives a catalyst for the highly enantioselective reduction of β-aryl substituted α,β-unsaturated γ-lactones and γ-lactams using PMHS as the hydride source. Coupled with the generation of these reduction substrates by copper-catalysed stereospecific β-arylation of alkynoates, followed by cyclisation, this provides overall a simple and accessible methodology for the single enantiomer synthesis of the title compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Leverhulme Trust (RPG-2018-270) (O. S. O.) is thanked for financial support.References
- (a) B. Mao, M. Fañanás-Mastral and B. L. Feringa, Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones, Chem. Rev., 2017, 117, 10502–10566 CrossRef CAS PubMed; (b) C. You, S. Li, X. Li, H. Lv and X. Zhang, Enantioselective Rh-Catalyzed Anti-Markovnikov Hydroformylation of 1,1-Disubstituted Allylic Alcohols and Amines: An Efficient Route to Chiral Lactones and Lactams, ACS Catal., 2019, 9, 8529–8533 CrossRef CAS; (c) J. Sietmann, M. Ong, C. Mück-Lichtenfeld, C. Daniliuc and J. M. Wahl, Desymmetrization of Prochiral Cyclobutanones via Nitrogen Insertion: A Concise Route to Chiral γ-Lactams, Angew. Chem., Int. Ed., 2021, 60, 9719–9723 CrossRef CAS PubMed.
- (a) X.-F. Wang, Y.-M. Yan, X.-L. Wang, X.-J. Ma, X.-Y. Fu and Y.-X. Cheng, Two new compounds from Ganoderma lucidum, J. Asian Nat. Prod. Res., 2015, 17, 329–332 CrossRef CAS PubMed; (b) K. Mori, Revision of the Absolute Configuration of A-Factor, Tetrahedron, 1983, 39, 3107–3109 CrossRef CAS.
- (a) V. Rodríguez, M. Sánchez, L. Quintero and F. Sartillo-Piscil, The 5-exo-trig radical cyclization reaction under reductive and oxidative conditions in the synthesis of optically pure GABA derivatives, Tetrahedron, 2004, 60, 10809–10815 CrossRef; (b) I. J. Montoya-Balbás, B. Valentín-Guevara, E. López-Mendoza, I. Linzaga-Elizalde, M. Ordoñez and P. Román-Bravo, Efficient Synthesis of β-Aryl-γ-lactams and Their Resolution with (S)-Naproxen: Preparation of (R)- and (S)-Baclofen, Molecules, 2015, 20, 22028–22043 CrossRef PubMed; (c) K. Biswas, R. Gholap, P. Srinivas, S. Kanyal and K. Das Sarma, β-substituted γ-butyrolactams from mucochloric acid: synthesis of (±)-baclofen and other γ-aminobutyric acids and useful building blocks, RSC Adv., 2014, 4, 2538–2545 RSC; (d) X. Li, C. You, Y. Yang, Y. Yang, P. Li, G. Gu, L. W. Chung, H. Lv and X. Zhang, Rhodium-catalyzed asymmetric hydrogenation of β-cyanocinnamic esters with the assistance of a single hydrogen bond in a precise position, Chem. Sci., 2018, 9, 1919–1924 RSC.
- C. Shao, H.-J. Yu, N.-Y. Wu, P. Tian, R. Wang, C.-G. Feng and G.-Q. Lin, Asymmetric Synthesis of β-Substituted γ-Lactams via Rhodium/Diene-Catalyzed 1,4-Additions: Application to the Synthesis of (R)-Baclofen and (R)-Rolipram, Org. Lett., 2011, 13, 788–791 CrossRef CAS PubMed.
- (a) J. J. Verendel, J.-Q. Li, X. Quan, B. Peters, T. Zhou, O. R. Gautun, T. Govender and P. G. Andersson, Chiral Hetero- and Carbocylic Compounds from the Asymmetric Hydrogenation of Cyclic Alkenes, Chem. – Eur. J., 2012, 18, 6507–6513 CrossRef CAS PubMed; (b) Q. Lang, G. Gu, Y. Cheng, Q. Yin and X. Zhang, Highly Enantioselective Synthesis of Chiral γ-Lactams by Rh-Catalyzed Asymmetric Hydrogenation, ACS Catal., 2018, 8, 4824–4828 CrossRef CAS.
- (a) G. Hughes, M. Kimura and S. L. Buchwald, Catalytic Enantioselective Conjugate Reduction of Lactones and Lactams, J. Am. Chem. Soc., 2003, 125, 11253–11258 CrossRef CAS PubMed; (b) G. L. Larson and R. J. Liberatore, Organosilanes in Metal-Catalyzed, Enantioselective Reductions, Org. Process Res. Dev., 2021, 25, 1719–1787 CrossRef CAS.
- D. H. Appella, Y. Moritani, R. Shintani, E. M. Ferreira and S. L. Buchwald, Asymmetric Conjugate Reduction of α,β-Unsaturated Esters Using a Chiral Phosphine-Copper Catalyst, J. Am. Chem. Soc., 1999, 121, 9473–9474 CrossRef CAS.
- (a) Y. Moritani, D. H. Appella, V. Jurkauskas and S. L. Buchwald, Syntheis of β-Alkyl Cyclopentanones in High Enantiomeric Excess via Copper-Catalyzed Asymmetric Conjugate Reduction, J. Am. Chem. Soc., 2000, 122, 6797–6798 CrossRef CAS; (b) V. Jurkauskas and S. L. Buchwald, Dynamic Kinetic Resolution via Asymmetric Conjugate Reduction: Enantio- and Diastereoselective Synthesis of 2,4-Dialkyl Cyclopentanones, J. Am. Chem. Soc., 2002, 124, 2892–2893 CrossRef CAS PubMed.
- M. P. Rainka, J. E. Milne and S. L. Buchwald, Dynamic Kinetic Resolution of α,β-Unsaturated Lactones through Asymmetric Copper-Catalyzed Conjugate Reduction: Application to the Total Synthesis of Eupomatilone, Angew. Chem., Int. Ed., 2005, 44, 6177–6180 CrossRef CAS PubMed.
- (a) B. H. Lipshutz, J. M. Servesko, T. B. Petersen, P. P. Papa and A. A. Lover, Asymmetric 1,4-Reductions of Hindered β-Substituted Cycloalkenones Using Catalytic SEGPHOS-Ligated CuH, Org. Lett., 2004, 6, 1273–1275 CrossRef CAS PubMed; (b) B. H. Lipshutz, J. M. Servesko and B. R. Taft, Asymmetric 1,4-Hydrosilylations of α,β-Unsaturated Esters, J. Am. Chem. Soc., 2004, 126, 8352–8353 CrossRef CAS PubMed.
- Using DTBM-SEGPHOS with (Ph3P)CuH and PMHS, 4-phenylfuran-2(5H)-one is reduced in 99% ee.10b See also: B. H. Lipshutz, B. A. Frieman, J. B. Unger and D. M. Nihan, Thermally Accelerated Asymmetric Hydrosilylations Using Ligated Copper Hydride, Can. J. Chem., 2005, 83, 606–614 CrossRef CAS.
- B. H. Lipshutz and J. M. Servesko, CuH-Catalyzed Asymmetric Conjugate Reductions of Acyclic Enones, Angew. Chem., Int. Ed., 2003, 42, 4789–4792 CrossRef CAS PubMed.
- C. Czekelius and E. M. Carreira, Catalytic Enantioselective Conjugate Reduction of β,β-Disubstituted Nitroalkenes, Angew. Chem., Int. Ed., 2003, 42, 4793–4795 CrossRef CAS PubMed.
- D. Lee, D. Kim and J. Yun, Highly Enantioselective Conjugate Reduction of β,β-Disubstituted σ,β-Unsaturated Nitriles, Angew. Chem., Int. Ed., 2006, 45, 2785–2787 CrossRef CAS PubMed.
- With some of these substrates use of DTBM-SEGPHOS gives poor results highlighting the significance of ligand choice as a function of the substrate. See: B. H. Lipshutz, Rediscovering Organocopper Chemistry Through Copper Hydride. It's All About the Ligand, Synlett, 2009, 509–524 CrossRef CAS.
- (a) A. K. Chatterjee, J. P. Morgan, M. Scholl and R. H. Grubbs, Synthesis of Functionalized Olefins by Cross and Ring-Closing Metatheses, J. Am. Chem. Soc., 2000, 122, 3783–3784 CrossRef CAS; (b) A. Fürstner, O. R. Thiel, L. Ackermann, H.-J. Schanz and S. P. Nolan, Ruthenium Carbene Complexes with N,N-Bis(mesityl)imidazol-2-ylidene Ligands: RCM Catalysts of Extended Scope, J. Org. Chem., 2000, 65, 2204–2207 CrossRef PubMed.
- J. J. Bourguigon and C. G. Wermuth, Synthesis of β-Substituted, γ-Functionalized Butanolides and Butenolides and Succinaldehydic Acids from Glyoxylic Acid, J. Org. Chem., 1981, 46, 4889–4894 CrossRef.
- (a) Y. Yamamoto, N. Kira and Y. Harada, Cu-catalyzed stereoselective conjugate addition of arylboronic acids to alkynoates, Chem. Commun., 2008, 2010–2012 RSC; (b) Y. Yamamoto and N. Kirai, Synthesis of 4-Aryl-Substituted Butenolides and Pentenolides by Copper-Catalysed Hydroarylation, Heterocycles, 2010, 80, 269–279 CrossRef CAS PubMed.
- E. J. Corey and J. A. Katzenellenbogen, A New Stereospecific Synthesis of Trisubstituted and Tetrasubstituted Olefins. The Conjugate Addition of Dialkylcopper-Lithium Reagents to α,β-Acetylenic Esters, J. Am. Chem. Soc., 1969, 91, 1851–1852 CrossRef CAS.
- O. S. Ojo, B. Nardone, S. F. Musolino, A. R. Neal, L. Wilson, T. Lebl, A. M. Z. Slawin, D. B. Cordes, J. E. Taylor, J. H. Naismith, A. D. Smith and N. J. Westwood, Synthesis of the natural product descurainolide and cyclic peptides from lignin-derived aromatics, Org. Biomol. Chem., 2018, 16, 266–273 RSC.
- R. G. Arrayás, J. Adrio, J. Carlos and J. C. Carretero, Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis, Angew. Chem., Int. Ed., 2006, 45, 7674–7715 CrossRef PubMed.
- (a) R. A. Arthurs, D. L. Hughes and C. J. Richards, Stereoselective Synthesis of All Possible Phosferrox Ligand Diastereoisomers Displaying Three Elements of Chirality: Stereochemical Optimization for Asymmetric Catalysis, J. Org. Chem., 2020, 85, 4838–4847 CrossRef CAS PubMed; (b) R. A. Arthurs, A. C. Dean, D. L. Hughes and C. J. Richards, Copper(I) Complexes of P-Stereogenic Josiphos and Related Ligands, Eur. J. Org. Chem., 2021, 2719–2725 CrossRef CAS.
- A. Togni, C. Breutel, A. Schnyder, F. Spindler, H. Landert and A. Tijani, A Novel Easily Accessible Chiral Ferrocenyldiphosphine for Highly Enantioselective Hydrogenation, Allylic Alkylation, and Hydroboration Reactions, J. Am. Chem. Soc., 1994, 116, 4062–4066 CrossRef CAS.
- CCDC 2121166 and 2121167 contains the supplementary crystallographic data for this paper.†.
- (a) A. V. Malkov, F. Friscourt, M. Bell, M. E. Swarbrick and P. Kočovský, Enantioselective Baeyer-Villiger Oxidation Catalyzed by Palladium(II) Complexes with Chiral P,N-Ligands, J. Org. Chem., 2008, 73, 3996–4003 CrossRef CAS PubMed; (b) C. O. Oliveira, R. A. Angnes and C. R. D. Correia, Intermolecular Enantioselective Heck-Matsuda Arylations of Acyclic Olefins: Application to the Synthesis of β-Aryl-γ-Lactones and β-Aryl Aldehydes, J. Org. Chem., 2013, 78, 4373–4385 CrossRef CAS PubMed.
- Determined following deprotection to (S)-24d and comparison to literature data: J. Barluenga, F. Aznar, C. Ribas and C. Valdés, Cycloaddition Reactions of Chiral 2-Amino-1,3-butadienes with Nitroalkenes: Synthesis of Enantiomerically Pure 4-Nitrocyclohexanones, J. Org. Chem., 1997, 62, 6746–6753 CrossRef CAS.
- It is anticipated that the γ-lactone precursors (Z)-8a–e will result in a similar level of enantioselectivity under these conditions.
- Deprotection of the PMP group with ceric ammonium nitrate (CAN) is known to proceed without compromising adjacent stereogenic centres. See, for example: (a) J. J. Fleming and J. Du Bois, A Synthesis of (+)-Saxitoxin, J. Am. Chem. Soc., 2006, 128, 3926–3927 CrossRef CAS PubMed; (b) D. Y. Park, K.-H. Kim and C.-H. Cheon, Enantioselective Synthesis of β-Aminotetralins via Chiral Phosphoric Acid-catalyzed Reductive Amination of β-Tetralones, Adv. Synth. Catal., 2018, 360, 462–467 CrossRef CAS.
- F. A. Davis, A. C. Sheppard, B.-C. Chen and M. S. Haque, Chemistry of Oxaziridines. 14. Asymmetric oxidation of ketone enolates using enantiomerically pure (camphorylsulfonyl)oxaziridine, J. Am. Chem. Soc., 1990, 112, 6679–6690 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, copies of the 1H and 13C spectra, HPLC traces and details of the X-ray crystal structure determinations. CCDC 2121166 and 2121167. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ob00563a |
| This journal is © The Royal Society of Chemistry 2023 |







