Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design of non-cytotoxic 6,7-dihydroxycoumarin-5-carboxylates with antibiofilm activity against Staphylococcus aureus and Candida albicans†
Robert
Zscherp‡
ab,
Aishi
Chakrabarti‡
 cd,
Anna P.
Lehmann
cd,
Anna P.
Lehmann
 ab,
Hedda
Schrey
ef,
Hoaxuan
Zeng
ef,
Wera
Collisi
ef and
Philipp
Klahn
ab,
Hedda
Schrey
ef,
Hoaxuan
Zeng
ef,
Wera
Collisi
ef and
Philipp
Klahn
 *bcd
*bcd
aInstitute of Organic Chemistry, Technische Universität Braunschweig, Hagenring 30, 38106 Braunschweig, Germany
bLaboratories of Natural Product and Conjugation Chemistry (naconLabs) – A Technology Transfer Center of iTUBS mbH, Wilhelmsgarten 3, 38100 Braunschweig, Germany. E-mail: Philipp.Klahn@gu.se
cDivision of Organic and Medicinal Chemistry, Department of Chemistry and Molecular Biology, University of Gothenburg, Kemigården 4, 41296 Göteborg, Sweden
dCentre for Antibiotic Resistance Research in Gothenburg (CARe), Sweden
eDepartment of Microbial Drugs, Helmholtz Centre for Infection Research (HZI), Inhoffenstrasse 7, 38124 Braunschweig, Germany
fGerman Centre for Infection Research (DZIF), Partner Site Hannover-Braunschweig, 38124 Braunschweig, Germany
First published on 6th April 2023
Abstract
The 6,7-dihydroxycoumarin-5-carboxylates DHCou and 4-Me-DHCou have been synthesized via five-step route including a propargyl-Claisen rearrangement as key step. The compounds show antibiofilm activity against Stapylococcus aureus and Candida albicans but lack the cytotoxic activity of parent 6,7-dihydroxycoumarines such as esculetin and 4-methylesculetin.
The occurrence of resistance of microbial pathogens against market antimicrobial drugs has been continuously rising since 1970s and nowadays we see ourselves confronted with multidrug-resistant microbial pathogens causing a strong need for new drugs and concepts to counter act this threat for public health.1–7 In addition, microbial biofilms provide challenges for the development of antimicrobials as bacterial8–13 and fungal pathogens14–16 tend to hide in biofilms preventing drug penetration and leading to recurring and persistent infections.15 Beyond planktonic cells, biofilms are a principal form of microbial growth on surfaces in which microbes embed themselves in sugar, peptide and lipid containing hydrogels. Biofilms are often critical to development of clinical infections in human host14 and can be found for many microbial pathogens such as Methicillin-resistant Staphylococcus aureus (MRSA),10,17Pseudomonas aeruginosa8,18–21 and Candida albicans15,22,23 causing severe infections.
In biofilms these pathogens can even co-occur during infection.24 Compounds which are able to disrupt biofilms or inhibit their formation are of vast importance to make these pathogens susceptible again against antimicrobial drugs.25 The inhibition of biofilm formation can be achieved via different modes of action of compounds: while targeting of efflux pumps by efflux pump inhibitors disables the secretion of building blocks for the biofilms,26 the inhibition of cell–cell communication by quorum sensing inhibitors is another successful strategy to avoid biofilm formation and impair virulence of the pathogens.25
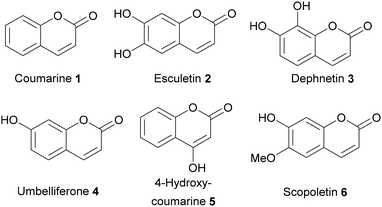
For several coumarins antibiofilm properties have been demonstrated,27–30 such as coumarin (1), esculetin (2), dephnetin (3), umbelliferone (4), 4-hydroxycoumarine (5), and scopoletin (6) (Fig. 1).31–36 The biofilm inhibitory effect of dihydroxycoumarins such as umbelliferone (4) and esculetin (2) has been reported to be mediated by both, efflux pump inhibition as well as impairment of quorum sensing,31,33 making them interesting lead structures for the development of novel biofilm inhibitors. However, both compounds show distinct antiproliferative activities against human cells limiting their potential application.37–40
In this work, we aimed to design novel 6,7-dihydroxycoumarin-5-carboxylate derivatives with reduced cytotoxic activity bearing a molecular handle for attachment and hybridization with further antimicrobial drugs or siderophores moieties to enable future transfer of the antibiofilm activity of 6,7-hydroxycoumarins onto these entities as outlined in Scheme 1. We hypothesized that the 5-position of the coumarin core might be suitable for the incorporation of a carboxylate handle allowing for the conjugation of such compounds to artificial siderophores such as the recently published enterobactin derivative EntKL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41–43 or additional antimicrobial drug moieties3 while retaining the antibiofilm properties. Furthermore, a main metabolic pathway of coumarins mediated by cytochrome P450 monooxygenases.44,45 Leading to cytotoxic intermediates and additionally to depletion of cellular glutathione levels is the 3,4-epoxidation of the core. We expected substituents in 5-position to impair this oxidative metabolization leading to a reduced cytotoxicity of the respective 6,7-dihydroxycoumarines.
41–43 or additional antimicrobial drug moieties3 while retaining the antibiofilm properties. Furthermore, a main metabolic pathway of coumarins mediated by cytochrome P450 monooxygenases.44,45 Leading to cytotoxic intermediates and additionally to depletion of cellular glutathione levels is the 3,4-epoxidation of the core. We expected substituents in 5-position to impair this oxidative metabolization leading to a reduced cytotoxicity of the respective 6,7-dihydroxycoumarines.
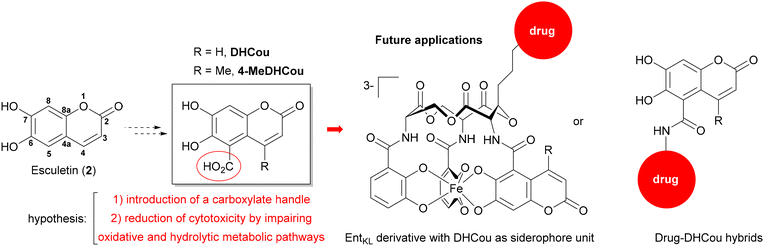 | ||
| Scheme 1 Concept of 6,7-dihydroxcoumarin-5-carboxylates as potential antibiofilm compounds bearing a handle for conjugation to siderophores and antimicrobial drug moieties. | ||
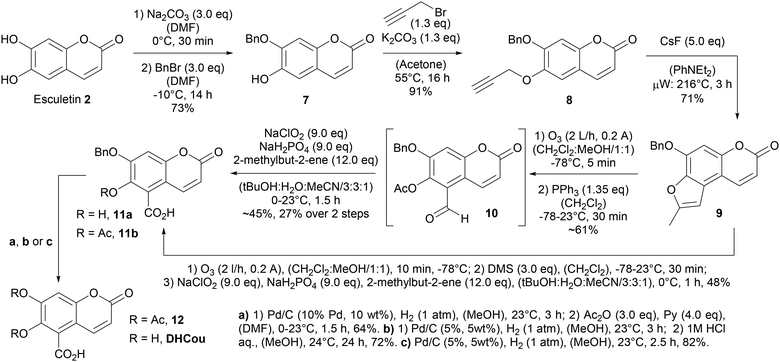
Therefore, we followed a semi-synthetic strategy to generate the 6,7-dihydroxycoumarin-5-carboxylate (DHCou) starting from the natural product esculetin (2) in 5 steps. First, the selective alkylation of the 7-hydroxy function46 with benzyl bromide at −15 °C gave access to the 7-benzyloxy coumarin derivative 7 in 73% yield, which could be further O-alkylated at the 6-hydroxy function in presence of propargyl bromide obtaining the O,O-dialkylated coumarin 8 in 91% yield. Compound 8 was submitted to a cascade-reaction consisting of a thermal [3.3]sigmatropic propargyl-Claisen rearrangement and subsequent CsF-mediated nucleophilic 5-exo-dig cyclization of the intermediate allenylphenolate forming the 2-methylbenzo[d]furan 9 in 71% yield upon heating to 216 °C in the microwave over 3 h. This methodology had been invented by Ishii and co-workers47 and was used earlier for the conversion of O-alkyl derivatives of scopoletin (6) (Scheme 2).48 In order, to enable oxidative cleavage of the furan ring forming the 5-formyl coumarin derivative 10 we explored different oxidative reactions conditions. Most 2-step procedures forming first the corresponding 3,4-epoxide and subsequently furnishing the oxidative cleavage in the presence of sodium periodate or lead tetraacetate failed to give access to the product.
While no conversion to the intermediate 3,4-epoxide was observed in the presence of mCPBA, decomposition occurred when DMDO was applied for epoxide formation. A first success was achieved forming the 3,4-epoxide in the presence of trifluoroacetic acid anhydride (TFAA) and hydrogen peroxide at 0 °C over 5 h and subsequently cleaving the epoxide with sodium periodate in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-mixture of MeOH and water. However, compound 10 was only obtained impure in roughly 26% yield as the reaction occurred with several side reactions. Similarly, 10 was obtained impure in roughly 25% yield, when 2-methylbenzofurane 9 was ozonolyzed at 78 °C in CH2Cl2 followed by reductive workup with dimethyl sulfide. Ozonolysis at 78 °C in a 1
1-mixture of MeOH and water. However, compound 10 was only obtained impure in roughly 26% yield as the reaction occurred with several side reactions. Similarly, 10 was obtained impure in roughly 25% yield, when 2-methylbenzofurane 9 was ozonolyzed at 78 °C in CH2Cl2 followed by reductive workup with dimethyl sulfide. Ozonolysis at 78 °C in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-mixture of CH2Cl2
1-mixture of CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH/1
MeOH/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reductive workup with triphenylphosphine gave access to impure 5-formyl coumarin derivative 10 in roughly 61% yield. Subsequent Pinnick oxidation of the impure aldehyde 10 delivered the O-protected 6,7-dihydroxycoumarin-5-carboxylate 11b in roughly 45% and 27% over two steps from furane 9. We then found that a similar procedure for the direct conversion of compound 9 into compound 11a lacking the acetate was also possible in a one-pot fashion giving 48% yield. The hydrogenolytic cleavage of the O-benzyl moiety in the presence of palladium on charcoal and subsequent O-acetylation with acetic acid anhydride and pyridine gave access to the O,O-diacetate 12 in 64% yield. Furthermore, DHCou was obtained in 72% yield and an overall yield of 16% over 5 steps after hydrogenolytic cleavage of the O-benzyl moiety and subsequent acidic deacetylation in the presence of aqueous HCl.
1 and reductive workup with triphenylphosphine gave access to impure 5-formyl coumarin derivative 10 in roughly 61% yield. Subsequent Pinnick oxidation of the impure aldehyde 10 delivered the O-protected 6,7-dihydroxycoumarin-5-carboxylate 11b in roughly 45% and 27% over two steps from furane 9. We then found that a similar procedure for the direct conversion of compound 9 into compound 11a lacking the acetate was also possible in a one-pot fashion giving 48% yield. The hydrogenolytic cleavage of the O-benzyl moiety in the presence of palladium on charcoal and subsequent O-acetylation with acetic acid anhydride and pyridine gave access to the O,O-diacetate 12 in 64% yield. Furthermore, DHCou was obtained in 72% yield and an overall yield of 16% over 5 steps after hydrogenolytic cleavage of the O-benzyl moiety and subsequent acidic deacetylation in the presence of aqueous HCl.
When DHCou was evaluated by crystal violet staining assay for its effects against formation of S. aureus biofilms no inhibitory activity was observed at the highest concentration of 250 μg mL−1, while esculetin (2) showed inhibition effects of 93% on the formation of S. aureus biofilms at the concentration of 250 μg mL−1 and of 33% at 125 μg mL−1 (Table 1). However, inhibitory effects of 62% could be observed against C. albicans biofilms when DHCou was applied at 250 μg mL−1 in the early stage of biofilm formation (Table 1). Esculetin (2) showed a slightly higher biofilm inhibition against C. albicans of 77% at the same concentration but was not active against preformed biofilms of S. aureus.
| Compound | Biofilm inhibition [% ± SD] | |
|---|---|---|
| S. aureus (DSM 1104) | C. albicans (DSM 11225) | |
| (−): no activity, SD: standard deviation, references [%].a Microporenic acid A (MAA): 93 ± 0.3 (250 μg mL−1), 93 ± 1 (62.5 μg mL−1), 62 ± 6 (7.8 μg mL−1).b MMA: 82 ± 6 (250 μg mL−1), 81 ± 8 (62.5 μg mL−1), 73 ± 17 (7.8 μg mL−1).c Farnesol: 87 ± 3 (250 μg mL−1), 79 ± 14 (31.3 μg mL−1), 67 ± 11 (15.6 μg mL−1).d Farnesol: 75 ± 6 (250 μg mL−1), 58 ± 15 (31.3 μg mL−1), 46 ± 14 (15.6 μg mL−1). | ||
| Esculetin (2) | 93 ± 2 (250 μg mL−1)a | 77 ± 7 (250 μg mL−1)c |
| 33 ± 6 (125 μg mL−1)a | 58 ± 17 (125 μg mL−1)c | |
| 4-Methylesculetin (14) | 94 ± 1 (250 μg mL−1)a | 76 ± 7 (250 μg mL−1)c |
| 48 ± 8 (125 μg mL−1)a | 48 ± 14 (125 μg mL−1)c | |
| DHCou | —b | 62 ± 9 (250 μg mL−1)d |
| 4-MeDHCou | 75 ± 5 (250 μg mL−1)b | 60 ± 2 (250 μg mL−1)d |
| 43 ± 11 (125 μg mL−1)b | ||
| 31 ± 15 (62.5 μg mL−1)b | ||
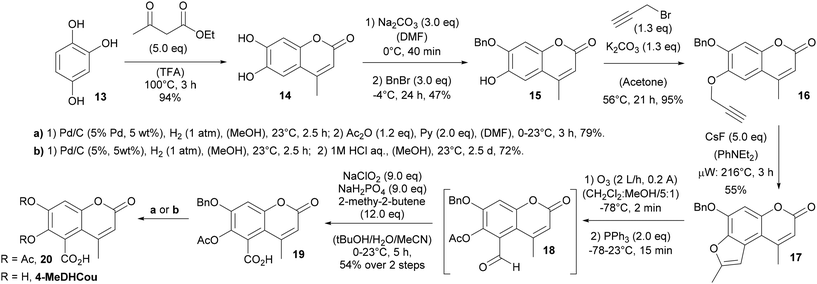
However, due to its cytotoxic effects, these values must be taken with care (Table 2) and a conclusion on the single standing influence of the C5 substitution is not possible. Although, a clear loss in antibiofilm activity against S. aureus was observed, a significant portion of the initial antibiofilm activity against C. albicans could be retained by C5 substitution. Beyond that, validating our initial hypothesis, DHCou showed neither cytotoxicity nor antiproliferative activity against the tested mammalian cell lines (L929 and KB3.1) at the highest concentration tested (1 mg mL−1 = 4.5 mM). Nevertheless, to increase the overall biofilm inhibitory activity of the compounds, we planned to increase the lipophilicity by incorporation of a methyl substituent in the 4-position of the coumarin core. Therefore, we generated 4-methylesculetin (14) via a Pechmann condensation of 1,2,4-trihydroxybenzene (13) with ethylacetoacetate in TFA at 100 °C (Scheme 3). Following the earlier strategy, selective O-benzylation of the 7-hydoxy position and subsequent O-propargylation of the 6-hydroxy position gave access to the O,O-dialkylated precursor for the cascade rearrangement.
| Cell line | Cytotoxicity IC50 [μM] | |||
|---|---|---|---|---|
| DHCou | 4-MeDHCou | Esculetin (2) | 4-Methylesculatin (14) | |
| a For control references epothilon B see Table S4 in the ESI;† (−): no cytotoxicity or changed cells observed (max. concentration 1 mg mL−1 = 4.5 mM for 4-MeDHCou and 4.2 mM for DHCou), n.t.: not tested. | ||||
| KB3.1 (ACC158) | — | — | 29.8 | 30.2 |
| L929 (ACC2) | — | — | 41.5 | 33.8 |
| A549 (ACC107) | n.t. | n.t. | 18.5 | 21.9 |
| A431 (ACC91) | n.t. | n.t. | 41.5 | 38.5 |
| PC-3 (ACC465) | n.t. | n.t. | 46.0 | 38.0 |
| SKOV-3 (ATCC HTB 77) | n.t. | n.t. | 45.5 | 42.7 |
| MCF-7 (A115) | n.t. | n.t. | 19.6 | 27.1 |
The thermal [3.3]sigmatropic propargyl-Claisen rearrangement and subsequent CsF-mediated nucleophilic 5-exo-dig cyclization proceeded with 55% yield and gave access to the 2-methylbenzofurane 17 (Scheme 3). Ozonolysis, followed by reductive workup in the presence of triphenylphosphine and subsequent Pinnick oxidation led to formation of the O-protected 6,7-dihydroxycoumarin-5-carboxylate 19 in 54%. Again, it turned out difficult to isolate the intermediate aldehyde 18, which could only be obtained in small amounts and with certain impurities when the oxidative cleavage was done via a sequence of oxidation with trifluoroperoxoacetic acid and subsequent cleavage in the presence of sodium periodate (see ESI†). From 19 the O,O-diacetate 20 and 4-MeDHCou were obtained following the procedure established before.
Similar to DHCou, 4-MeDHCou showed no cytotoxic activity against the mammalian cervic carcinoma cell line KB3.1 and the mouse fibroblasts cell line L929 when applied at the highest concentration of 1 mg mL−1 (4.2 mM).
In contrast to that and in accordance with the observed cytotoxicity of esculetin (2), 4-methylesculetin (14) exhibited cytotoxic activity against all tested mammalian cell lines (Table 2). Furthermore, while the inhibitory effects against C. albicans biofilms were comparable to that of DHCou, 4-MeDHCou inhibited the formation by 60% at 250 μg mL−1 (Table 1), we could also observe activity against S. aureus biofilms. Thus, 4–MeDHCou showed inhibition effects of 75% on the formation of S. aureus biofilms at the concentration of 250 μg mL−1 and of 43% at 125 μg mL−1. The antibiofilm activity of 4-methylesculetin (14) was observed to be higher compared to 4-MeDHCou with 76% inhibition at 250 μg mL−1 and 48% at 125 μg mL−1 against C. albicans and 94% inhibition at 250 μg mL−1 and 48% at 125 μg mL−1 against S. aureus. Furthermore, no dispersal effects against preformed biofilms of S. aureus were observed for 4-MeDHCou and 4-methylesculetin (14).
However, considering a potential contribution of the observed cytotoxicity of 4-methylesculetin (14) to its antibiofilm activity, a significant portion of the antibiofilm activity could be retained by C5 carboxylate substitution in 4-MeDHCou.
In addition, none of the compounds (DHCou, 4-MeDHCou, esculetin (2) or 4-methylesculetin (14)) showed antimicrobial activity (see Table S1 in the ESI†) against a panel of Gram-positive and Gram-negative bacteria (B. subtilis, S. aureus, M. Smegmatis, A. baumanni, C. violaceum, E. coli, and P. aeruginosa) and different fungi (M. hiemalis, P. anomala, R. glutinis, C. albicans and S. pombe) up to a concentration of 66.7 μg mL−1.
Besides their antibiofilm activity, coumarins are known for their UV fluorescence and use as dyes in chemical biology. In line with that also 4-MeDHCou and its coumarin precursors 15, 16, 17 and 19 showed fluorescence with maximal emission wavelengths between 400–450 nm (Fig. 2). Interestingly 4-MeDHCou was the most bathochromic shifted compound of this series, although fluorescence intensity at normalized concentration decreased along the synthesis route.
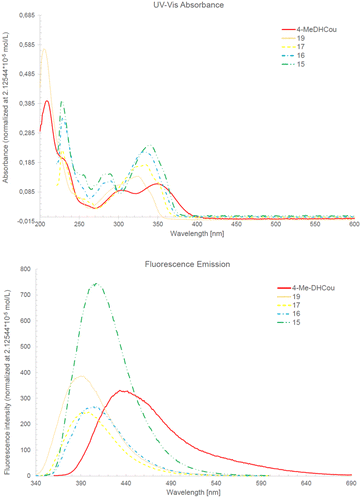 | ||
| Fig. 2 UV/Vis absorption and fluorescence emission of 4-MeDHCou and its precursors 15, 16, 17 and 19 at normalized concentration 2.125 × 10−5 mol L−1. | ||
Currently different approaches to further optimize the structure for higher antibiofilm activity and incorporate 4-MeDHCou to artificial siderophores and antimicrobial drug hybrids are under investigation to explore its potential to serve as mediator for antibiofilm activity (as outlined in Scheme 1).
Conclusions
We synthesized two novel 6,7-dihydroxycoumarin-5-carboxylates, namely DHCou and 4-MeDHCou. In contrast, to their non-carboxylated parent 6,7-dihydroxycoumarins esculetin (2) and 4-methylesculetin (14), these compounds lack any cytotoxic activity towards different mammalian cell lines, while retaining an antibiofilm activity. DHCou displayed inhibitory effects against the early stage of C. albicans biofilm formation but showed no activity against S. aureus biofilms. Furthermore, 4-MeDHCou exhibited antibiofilm activity against both, the formation of S. aureus and C. albicans biofilms. Although the structure activity relationships need to be further investigated to fully understand the observed effects and improve the antibiofilm activity of the compounds, a proof-of-principle for the design of non-cytotoxic hydroxycoumarins retaining antibiofilm activity has been made, holding potential to overcome a major limitation for the application of coumarins as biofilm disruptors. In addition, these moieties might be able to transfer their antibiofilm activity by conjugation to other entities such as antimicrobials drugs or siderophores. Further investigations are ongoing to explore their potential.Author contributions
Conceptualization, funding acquisition, project administration and writing of original draft: PK; supervision: PK, HS; writing – review and editing: PK, RZ, AC, APL, HS and HZ. Investigation: RZ, AC, APL, HS, HZ and WC. Methodology: PK, RZ, AC and APL; resources: PK, AC, RZ, and APL; visualization: PK, HS and APL.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Parts of the work have been carried out within the framework of the Centre of Antimicrobial Resistance Research (CARe) in Gothenburg and the SMART BIOTECS alliance between the Technische Universität Braunschweig and the Leibniz Universität Hannover. This initiative is supported by the Ministry of Science and Culture (MWK) of Lower Saxony (PK), Germany. Financial support by the Deutsche Forschungsgemeinschaft (DFG, grant KL 3012/2-1 (PK)) and the Fonds der Chemischen Industrie (FCI, PK) is gratefully acknowledged. HZ is grateful for a personal PhD stipend from the “Drug Discovery and Cheminformatics for New Anti-Infectives (iCA)” and is financially supported by the Ministry for Science & Culture of the German State of Lower Saxony (MWK no. 21-78904-63-5/19). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.References
- D. J. Krysan, Virulence, 2017, 8, 135–137 CrossRef PubMed.
- E. Ksiezopolska and T. Gabaldón, Genes, 2018, 9, 461 CrossRef PubMed.
- P. Klahn and M. Brönstrup, Nat. Prod. Rep., 2017, 34, 832–885 RSC.
- P. Klahn and M. Brönstrup, Curr. Top. Microbiol. Immunol., 2016, 389, 365–417 Search PubMed.
- M. A. Cook and G. D. Wright, Sci. Transl. Med., 2022, 14, eabo7793 CrossRef CAS PubMed.
- N. Mobarki, B. Almerabi and A. Hattan, Int. J. Med. Dev. Countries, 2019, 40, 561–564 CrossRef.
- T. M. Privalsky, A. M. Soohoo, J. Wang, C. T. Walsh, G. D. Wright, E. M. Gordon, N. S. Gray and C. Khosla, J. Am. Chem. Soc., 2021, 143, 21127–21142 CrossRef CAS PubMed.
- A. Vetrivel, M. Ramasamy, P. Vetrivel, S. Natchimuthu, S. Arunachalam, G.-S. Kim and R. Murugesan, Biologics, 2021, 1, 312–336 CrossRef.
- N. Høiby, T. Bjarnsholt, M. Givskov, S. Molin and O. Ciofu, Int. J. Antimicrob. Agents, 2010, 35, 322–332 CrossRef PubMed.
- V. Silva, L. Almeida, V. Gaio, N. Cerca, V. Manageiro, M. Caniça, J. L. Capelo, G. Igrejas and P. Poeta, Pathogens, 2021, 10, 970 CrossRef CAS PubMed.
- M. H. Muhammad, A. L. Idris, X. Fan, Y. Guo, Y. Yu, X. Jin, J. Qiu, X. Guan and T. Huang, Front. Microbiol., 2020, 11, 1–20 CrossRef PubMed.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS PubMed.
- F. Hemmati, M. A. Rezaee, S. Ebrahimzadeh, L. Yousefi, R. Nouri, H. S. Kafil and P. Gholizadeh, Mol. Biotechnol., 2021, 63, 569–586 CrossRef CAS PubMed.
- S. Fanning and A. P. Mitchell, PLoS Pathog., 2012, 8, e1002585 CrossRef CAS PubMed.
- J. V. Desai, A. P. Mitchell and D. R. Andes, Cold Spring Harbor Perspect. Med., 2014, 4, a019729 CrossRef PubMed.
- M. Gulati and C. J. Nobile, Microbes Infect., 2016, 18, 310–321 CrossRef CAS PubMed.
- A. Boudet, P. Sorlin, C. Pouget, R. Chiron, J.-P. Lavigne, C. Dunyach-Remy and H. Marchandin, Front. Microbiol., 2021, 12, 750489 CrossRef PubMed.
- O. Ciofu and T. Tolker-Nielsen, Front. Microbiol., 2019, 10, 913 CrossRef PubMed.
- M. T. T. Thi, D. Wibowo and B. H. A. Rehm, Int. J. Mol. Sci., 2020, 21, 8671 CrossRef CAS PubMed.
- F. F. Tuon, L. R. Dantas, P. H. Suss and V. S. Tasca Ribeiro, Pathogens, 2022, 11, 300 CrossRef CAS PubMed.
- E. Banin, M. L. Vasil and E. P. Greenberg, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11076–11081 CrossRef CAS PubMed.
- C. Tsui, E. F. Kong and M. A. Jabra-Rizk, Pathog. Dis., 2016, 74, ftw018 CrossRef PubMed.
- T. Atriwal, K. Azeem, F. M. Husain, A. Hussain, M. N. Khan, M. F. Alajmi and M. Abid, Front. Microbiol., 2021, 12, 638309 Search PubMed.
- M. del M. Cendra and E. Torrents, Biotechnol. Adv., 2021, 49, 107734 CrossRef CAS PubMed.
- A. Ghosh, N. Jayaraman and D. Chatterji, ACS Omega, 2020, 5, 3108–3115 CrossRef CAS PubMed.
- A. Reza, J. M. Sutton and K. M. Rahman, Antibiotics, 2019, 8, 229 CrossRef CAS PubMed.
- M. G. da Cunha, J. de Cássia Orlandi Sardi, I. A. Freires, M. Franchin and P. L. Rosalen, Microb. Pathog., 2020, 139, 103855 CrossRef CAS PubMed.
- S. K. Roy, N. Kumari, S. Pahwa, U. C. Agrahari, K. K. Bhutani, S. M. Jachak and H. Nandanwar, Fitoterapia, 2013, 90, 140–150 CrossRef CAS PubMed.
- J. H. Lee, J. H. Park, H. S. Cho, S. W. Joo, M. H. Cho and J. Lee, Biofouling, 2013, 29, 491–499 CrossRef CAS PubMed.
- T. Das, M. C. Das, A. Das, S. Bhowmik, P. Sandhu, Y. Akhter, S. Bhattacharjee and U. C. De, World J. Microbiol. Biotechnol., 2018, 34, 170 CrossRef PubMed.
- J.-H. J. H. Lee, Y. G. Kim, H. S. Cho, S. Y. Ryu, M. H. Cho and J.-H. J. H. Lee, Phytomedicine, 2014, 21, 1037–1042 CrossRef CAS PubMed.
- Y. Zhang, A. Sass, H. Van Acker, J. Wille, B. Verhasselt, F. Van Nieuwerburgh, V. Kaever, A. Crabbé and T. Coenye, Front. Microbiol., 2018, 9, 1–10 CrossRef PubMed.
- F. J. Reen, J. A. Gutiérrez-Barranquero, M. L. Parages and F. O'Gara, Appl. Microbiol. Biotechnol., 2018, 102, 2063–2073 CrossRef CAS PubMed.
- K. Xu, J. L. Wang, M. P. Chu and C. Jia, J. Mycol. Med., 2019, 29, 28–34 CrossRef CAS PubMed.
- F. A. Qais, M. S. Khan, I. Ahmad, F. M. Husain, R. A. Khan, I. Hassan, S. A. Shahzad and W. AlHarbi, ACS Omega, 2021, 6, 18823–18835 CrossRef CAS PubMed.
- Z. He, W. Jiang, Y. Jiang, J. Dong, Z. Song, J. Xu and W. Zhou, J. Oral Microbiol., 2022, 14, 2055523 CrossRef PubMed.
- S.-M. Yu, D.-H. Hu and J.-J. Zhang, Mol. Med. Rep., 2015, 12, 3869–3873 CrossRef CAS PubMed.
- L. Zhang, Q. Xie and X. Li, Phytother. Res., 2022, 36, 279–298 CrossRef CAS PubMed.
- J. Y. Yang, M. A. Della-Fera, D. L. Hartzell, C. Nelson-Dooley, D. B. Hausman and C. A. Baile, Obesity, 2006, 14, 1691–1699 CrossRef CAS PubMed.
- C. Y. Chu, Y. Y. Tsai, C. J. Wang, W. L. Lin and T. H. Tseng, Eur. J. Pharmacol., 2001, 416, 25–32 CrossRef CAS PubMed.
- R. Zscherp, J. Coetzee, J. Vornweg, J. Grunenberg, J. Herrmann, R. Müller and P. Klahn, Chem. Sci., 2021, 12, 10179–10190 RSC.
- P. Klahn, R. Zscherp and C. C. Jimidar, Synthesis, 2022, 54, 3499–3557 CrossRef CAS.
- C. Rohrbacher, R. Zscherp, S. C. Weck, P. Klahn and C. Ducho, Chem. – Eur. J., 2022, e202202408 Search PubMed.
- B. Lake, Food Chem. Toxicol., 1999, 37, 423–453 CrossRef CAS PubMed.
- A. Stefanachi, F. Leonetti, L. Pisani, M. Catto and A. Carotti, Molecules, 2018, 23, 250 CrossRef PubMed.
- M. Kawase, H. Sakagami, N. Motohashi, H. Hauer, S. S. Chatterjee, G. Spengler, A. V. Vigyikanne, A. Molnár and J. Molnár, In Vivo, 2005, 19, 705–711 CAS.
- H. Ishii, T. Ishikawa, S. Takeda, S. Ueki and M. Suzuki, Chem. Pharm. Bull., 1992, 40, 1148–1153 CrossRef CAS.
- H. Ishii, T. Ishikawa, H. Wada, H. Miyazaki, Y. Kaneko and T. Harayama, Chem. Pharm. Bull., 1992, 40, 2614–2619 CrossRef CAS.
- K. T. Yuyama, L. Wendt, F. Surup, R. Kretz, C. Chepkirui, K. Wittstein, C. Boonlarppradab, S. Wongkanoun, J. Luangsa-ard, M. Stadler and W.-R. Abraham, Biomolecules, 2018, 8, 129 CrossRef PubMed.
- K. T. Yuyama, C. Chepkirui, L. Wendt, D. Fortkamp, M. Stadler and W.-R. Abraham, Microorganisms, 2017, 5, 80 CrossRef PubMed.
- C. Chepkirui, K. T. Yuyama, L. A. Wanga, C. Decock, J. C. Matasyoh, W.-R. R. Abraham and M. Stadler, J. Nat. Prod., 2018, 81, 778–784 CrossRef CAS PubMed.
- K. Becker, A. Wessel, J. J. Luangsa-ard and M. Stadler, Biomolecules, 2020, 10, 805 CrossRef CAS PubMed.
- P. Klahn, V. Fetz, A. Ritter, W. Collisi, B. Hinkelmann, T. Arnold, W. Tegge, K. Rox, S. Hüttel, K. I. Mohr, J. Wink, M. Stadler, J. Wissing, L. Jänsch and M. Brönstrup, Chem. Sci., 2019, 10, 5197–5210 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob00303e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |