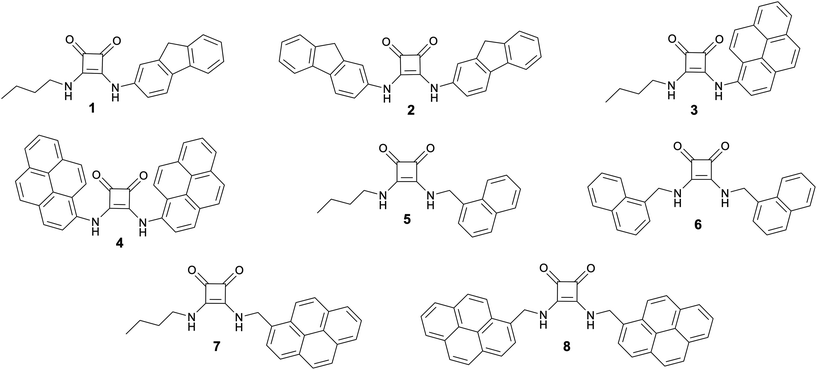
Squaramides for colorimetric and fluorescent anion sensing†
Jakob D. E.
Lane
a and
Katrina A.
Jolliffe
 *abc
*abc
aSchool of Chemistry, The University of Sydney, NSW 2006, Australia. E-mail: kate.jolliffe@sydney.edu.au
bAustralian Research Council Centre of Excellence for Innovations in Peptide and Protein Science, The University of Sydney, 2006, NSW, Australia
cThe University of Sydney Nano Institute (Sydney Nano), The University of Sydney, NSW 2006, Australia
First published on 15th March 2023
Abstract
A series of fluorophore-containing squaramides were synthesised to investigate the fluorescent and colorimetric response to anions of squaramides containing large aromatic substituents. Squaramides in which the fluorophores were conjugated directed to the squaramide motif were found to have highly acidic NH protons, giving rise to complicated fluorescence behaviour and colour changes upon addition of anions. In contrast, squaramides incorporating a methylene spacer to electronically insulate the fluorophore from the squaramide gave a relatively simple fluorescence response upon addition of anions, which was attributed to a decrease in excimer emission relative to monomer emission intensity, likely due to a disaggregation mechanism. The ratio of excimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer emission was used to provide a ratiometric fluorescent response to anions, with the most selective molecules demonstrating a preference for binding to sulfate over other anions.
monomer emission was used to provide a ratiometric fluorescent response to anions, with the most selective molecules demonstrating a preference for binding to sulfate over other anions.
Introduction
Anions play a vital role in many industrial and biological processes and many are common pollutants.1–5 The development of anion receptors, especially those that can sense anions via a fluorescence response, is an increasingly active area of research due to potential applications in industry, medicine and the environment.6–19Dual H-bond donors are widely used for anion recognition due to their ability to form two H-bonds to the anionic guest and their geometric match for Y-shaped anions such as carboxylates.20–25 Early examples of fluorescent anion sensors containing dual H-bond donors, developed by Gunnlaugsson and co-workers, were based on the thiourea motif.26,27 These simple receptors were developed based on the receptor–spacer–fluorophore design principle and displayed photoinduced electron transfer (PET) quenching upon interaction with certain anions.28 They show a simple turn-off response to anions, with some selectivity for more basic anions.17,26,27,29 An alternative dual H-bond fluorescent receptor design involves the direct conjugation of the fluorophore to the receptor, which relies upon internal charge transfer (ICT) to provide a fluorescent response to anions.30,31 When the fluorophore and binding units are in conjugation, electronic changes to the fluorophore upon anion binding influence the wavelength and intensity of fluorescence output, which leads to a more complex response than PET receptors but may give rise to improved selectivity or discrimination between anions, dependent upon whether binding or deprotonation occur in both the ground and the excited state.
Squaramides have emerged as an important dual H-bond donor motif for anion recognition and have been shown to bind to anions with higher affinity than analogous (thio)urea dual H-bond donors, due to the increased acidity of the squaramide NH protons.24,25,32 Squaramides bearing alkyl and aryl substituents have been extensively reported for anion recognition and have been shown to bind anions via H-bonds from the NH protons of the squaramide. This has been confirmed in solution by 1H NMR and UV-Vis titrations, and in the solid state from crystal structure analyses.32–36 Squaramides bearing fluorophores have been previously reported to give a selective response to anions, either as a result of binding or deprotonation. Similar design principles to those used in earlier work on thioureas have been used for the development of fluorescent squaramides for anion sensing, with reported examples of both receptor–spacer–fluorophore squaramide based receptors,18,37,38 and squaramides directly conjugated to the fluorophores.11,39
The most effective fluorescent probes are those which give a ratiometric response to the analyte of interest.40 Previously reported squaramide-based (and simple urea and thiourea-based receptors) receptors have been demonstrated to give a fluorescent response to anions, however most simply reply upon a simple turn-on or turn-off change in fluorescence intensity.12,17 In receptors for both cations and anions, the incorporation of multiple fluorophores into the same receptor has provided a means of ratiometric sensing via monitoring the ratio of monomer and intramolecular excimer emission.41–43 Investigations into dual H-bond donors, specifically squaramides, bearing multiple fluorophores are limited, and the fluorescent response with regard to selective or ratiometric sensing of anions has not been widely investigated.11,34 Therefore, we decided to investigate the anion sensing capabilities of a series of squaramides 1–8 (Fig. 1), bearing both conjugated and methylene-spaced fluorophores to determine the suitability of these simple receptors for anion sensing.
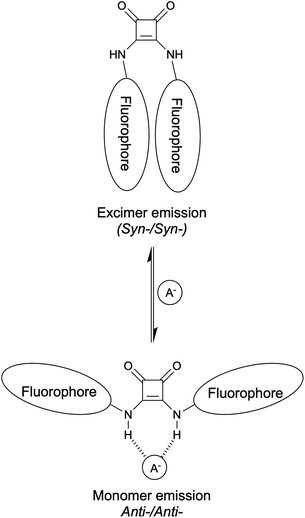
We hypothesised that a squaramide containing two large substituents would adopt the syn-,syn- conformation, with stabilisation of this conformer obtained from π-stacking interactions of aromatic groups, leading to intramolecular excimer emission in the unbound state. Anion binding by the squaramide would require adoption of an anti-,anti- conformation, which was expected to reduce intramolecular excimer emission, and give an increase in monomer emission. Therefore, it was expected that a ratiometric response could be achieved by the desired conformational change shown in Fig. 2. In testing this hypothesis we discovered that direct conjugation of the fluorophores to the squaramides generally results in compounds too acidic for anion binding, whilst introduction of a methylene spacer lead to intermolecular aggregation. This was exploited for sensing anion binding through changes in the fluorescence spectra upon disaggregation.
Results and discussion
Squaramides 1–8 feature fluorophores either conjugated directly to the squaramide dual H-bonding motif (1–4), or separated from this via methylene spacers (5–8). Compounds 1, 3, 5 and 7 each contain a single fluorophore whereas compounds 2, 4, 6 and 8 all bear two identical fluorophore substituents since it was expected that a different response to anions may arise from the symmetric squaramides compared to their asymmetric analogues, due to the possibility of intramolecular excimer emission that we predicted would be altered upon anion binding. Of these receptors, N,N′-difluorenyl squaramide, 2, has previously been reported as a potential cell imaging agent; however, anion recognition was not investigated in this study.44N,N′-Dipyrenyl squaramide, 4, was recently reported by Caltagirone and co-workers, and found to show quantifiable chloride binding by 1H NMR titration in DMSO-d6 (0.5% H2O) with only small changes observed in the UV-vis and fluorescence spectra of 4 upon chloride addition in MeCN.11 Squaramides 1, 3 and 5–8 represent novel compounds.Synthesis
Fluorescent squaramides 1–8 were synthesised following adapted literature procedures (Scheme 1).11,44,45 Squaramides 5–8 were synthesised in single-pot reactions in ethanol, without isolation of the squaramate intermediate. For asymmetric squaramides 5 and 7, diethyl squarate was initially reacted with one equivalent of the relevant amine, followed by addition of butylamine. For symmetric squaramides 6 and 8, diethyl squarate was treated with two equivalents of the respective methylene spaced fluorescent amine.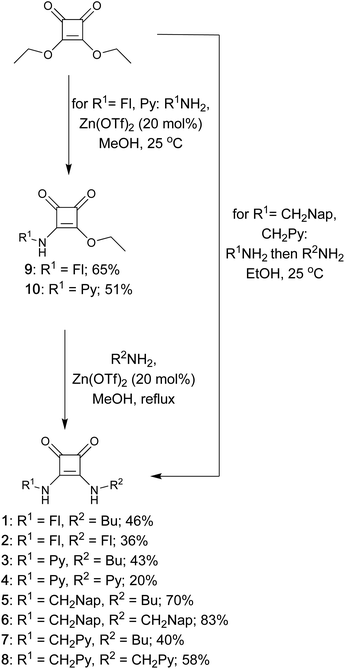 | ||
| Scheme 1 Synthesis of squaramides 1–8 from diethyl squarate (Fl = fluorenyl; Py = pyrenyl; Nap = naphthyl; Bu = butyl). | ||
For the synthesis of aryl squaramides 1–4, addition of either 2-aminofluorene or 1-aminopyrene to diethyl squarate in methanol in the presence of catalytic Zn(OTf)2 at room temperature gave only the monosubstituted squaramate products, 9 and 10, which were isolated in moderate yields (65% and 51% respectively). Heating the mixture to reflux in the presence of either a second equivalent of aminofluorophore or butylamine and Zn(OTf)2 gave the corresponding disubstituted squaramides 1–4.45
pKa determination
Squaramides bearing electron withdrawing substituents can have highly acidic NH protons and may be partially deprotonated in DMSO.45 Furthermore, dual H-bond donors that contain particularly acidic NH protons are liable to deprotonate upon addition of mildly basic anions, meaning that accurate anion binding responses cannot be determined via spectroscopic titration as deprotonation also causes a change in the absorbance spectrum. Therefore, the pKas of squaramides 1–8 were investigated via spectrophotometric pH titrations in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v DMSO-water in the presence of TBAPF6 (0.1 M), following a similar method to that described by Fabbrizzi and coworkers.46 This technique has previously been used for the determination of the pKa of dual H-bond donors (squaramides, thiosquaramides, ureas, thioureas, deltamides and croconamides) in 9
1 v/v DMSO-water in the presence of TBAPF6 (0.1 M), following a similar method to that described by Fabbrizzi and coworkers.46 This technique has previously been used for the determination of the pKa of dual H-bond donors (squaramides, thiosquaramides, ureas, thioureas, deltamides and croconamides) in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v MeCN–water; the low solubility of 1–4 necessitated use of a DMSO-water (9
1 v/v MeCN–water; the low solubility of 1–4 necessitated use of a DMSO-water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution.24,33
1 v/v) solution.24,33
The experimentally determined pKa values for squaramides 1–8 are shown in Table 1. Diaryl squaramides 2 and 4 were observed to have highly acidic NH protons, comparable to squaramides containing strongly electron withdrawing groups, e.g. 3,5-bis(trifluoromethyl)phenyl and 4-nitrophenyl substituents.24,25,33 A single deprotonation event was observed for squaramides 1–3, indicating that the pKa of the second NH proton lies outside the range covered by this technique (pKa >14).24,46 Two deprotonation events were observed for dipyrenyl squaramide, 4, indicating that both NH protons were highly acidic (pKa = 7.5 and 9.6). The pKa of the methylene spaced squaramides (5–8) could not be determined by this method, indicating that they are significantly less acidic than the conjugated squaramides 1–4. This is consistent with previous reports in which squaramides with aryl substituents have been found to be considerably more acidic than those bearing alkyl or benzyl substituents. For example, the pKa of N,N′-dibenzylsquaramide has been reported to be 14.6 in DMSO.24,25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v DMSO–water, TBAPF6 (0.1 M), pKa1 = first deprotonation, pKa2 = second deprotonation. Solution was initially adjusted to pH 4 with aqueous perchloric acid (1 M) and the pH increased gradually by addition of aliquots of aqueous NaOH (0.1 M). Errors estimated at ± 1 pKa units
1 v/v DMSO–water, TBAPF6 (0.1 M), pKa1 = first deprotonation, pKa2 = second deprotonation. Solution was initially adjusted to pH 4 with aqueous perchloric acid (1 M) and the pH increased gradually by addition of aliquots of aqueous NaOH (0.1 M). Errors estimated at ± 1 pKa units
Given the difficulty in obtaining experimental pKas for squaramides of this type in appropriate solvents, we also explored an alternative method to allow approximation of pKa after noting that the signals attributable to the NH protons of 2 and 4 are shifted downfield in comparison to those of 6 and 8. Plotting the 1H NMR chemical shifts of the squaramide NH proton signals in d6-DMSO solution against experimentally determined or calculated pKas24,25,33 for a series of known N,N′-disubstituted squaramides demonstrated an approximate linear relationship between chemical shift (ppm) and pKa (Fig. S5 and Table S1†). Using the trendline from the known compounds, the relatively high chemical shifts of the signals attributable to the NH protons of 2 and 4 (10.0 and 12.0 ppm, respectively) suggest pKas of 11.3 for 2 and 8.2 for 4, providing further evidence for the acidity of these protons, whereas the analogous chemical shifts observed for 6 (7.8 ppm) and 8 (7.9 ppm) correlate with pKas of 15–16.
Anion binding by UV-Visible spectroscopy
The response of conjugated squaramides 1–4 upon addition of anions was next investigated. In DMSO (both 1% and 10% water), in the absence of perchloric acid, squaramides 2–4 each showed a red shifted wavelength of maximum absorbance in the absorbance spectra compared to after the addition of perchloric acid, indicating that, in the absence of acid, the squaramides were partially deprotonated in DMSO. This partial deprotonation is consistent with colour changes upon acidification of the solutions and with previous observations regarding deprotonation of conjugated squaramides in DMSO by Taylor.45 The low pKa values observed for 2–4 (Table 1) suggested that quantitative anion binding titrations would not be possible for these squaramides. However, the vivid colour changes observed upon addition of anions to 4 allowed for qualitative colorimetric anion sensing. Addition of 50 equivalents of a range of anions as their tetrabutylammonium salts to a solution of 4 (25 μM) in DMSO gave varied colour changes depending on the basicity of the anion (Fig. 3), which were consistent with the colour changes observed upon deprotonation of 4. With no anion present, or in the presence of non-basic anions (Cl−, Br− and HSO4−), the solution is yellow; addition of H2PO4−, AcO− and SO42− resulted in a colour change to orange indicative of single deprotonation whereas addition of the highly basic fluoride and hydroxide anions gave a deep blue solution attributable to double deprotonation of the squaramide. Therefore, the colour changes upon addition of anions to 4 offer a simple colorimetric method of anion sensing via a deprotonation sensing mechanism. Discrimination between anions was achieved based on their relative basicity, however this method of anion sensing cannot discriminate between anions of similar basicity (e.g., Cl− and Br−). | ||
| Fig. 3 Colour changes upon addition of different anions (50 equivalents) to dipyrenyl squaramide 4 (25 μM) in DMSO (1% H2O). | ||
In contrast to squaramides 2–4, no changes in the UV-Vis spectrum of 1 were observed upon addition of acid, indicating that it remained protonated in DMSO (in agreement with the greater experimental pKa of 10.4, compared with the lower pKa values obtained for squaramides 2–4). Therefore, changes in the absorbance of 1 upon addition of 50 equivalents of different anions as their tetrabutylammonium (TBA) salts were investigated (Fig. 4) and whereas no significant changes were observed upon addition of Br−, NO3− and HSO4− and only a very small shift was observed upon addition of Cl−, addition of AcO−, H2PO4− and SO42− induced a red shift in the absorbance maximum in the absorbance spectrum, with the greatest shift observed for SO42− (Fig. 4). Importantly, the changes were distinct from those observed for deprotonation during the pH spectrophotometric titration (see Fig. S1†).
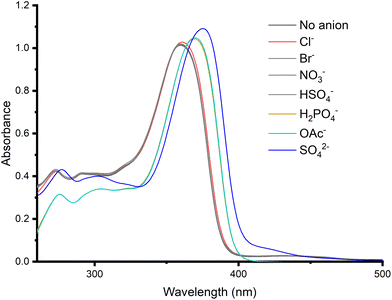 | ||
| Fig. 4 UV-Vis spectra recorded from screen of 1 (25 μM) with 50 equivalents of anions, added as their TBA salts in DMSO (1% H2O) at 298 K. | ||
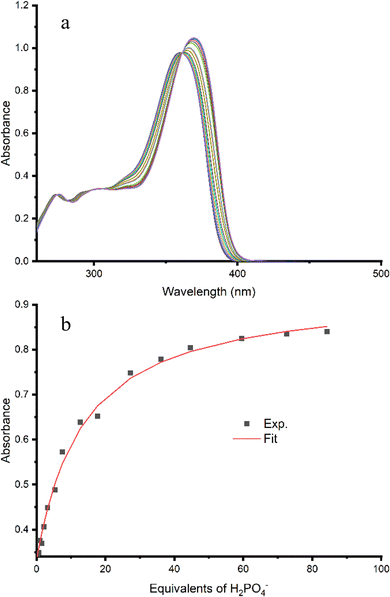
Subsequently, titrations were carried out by addition of Cl−, H2PO4−, AcO− and SO42− to 1 and the resulting binding data fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using Bindfit v0.5 to give the binding affinities shown in Table 2.47,48 A representative titration of 1 with H2PO4− is shown in Fig. 5. The presence of a clear isosbestic point at 365 nm confirms the 1
1 binding model using Bindfit v0.5 to give the binding affinities shown in Table 2.47,48 A representative titration of 1 with H2PO4− is shown in Fig. 5. The presence of a clear isosbestic point at 365 nm confirms the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. For the monovalent anions, higher binding affinity was observed for oxoanions acetate and dihydrogen phosphate (5200 and 3900 M−1 respectively) compared to chloride (400 M−1). This binding trend correlates with anion basicity and has been previously observed for other squaramides.24 Apparent strong binding to divalent sulfate was observed, which is consistent with binding behaviour previously documented for squaramides whereby higher binding affinity for sulfate selectivity is often observed,13,35,36,49–52 however the data could not be fit to a 1
1 binding stoichiometry. For the monovalent anions, higher binding affinity was observed for oxoanions acetate and dihydrogen phosphate (5200 and 3900 M−1 respectively) compared to chloride (400 M−1). This binding trend correlates with anion basicity and has been previously observed for other squaramides.24 Apparent strong binding to divalent sulfate was observed, which is consistent with binding behaviour previously documented for squaramides whereby higher binding affinity for sulfate selectivity is often observed,13,35,36,49–52 however the data could not be fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, indicating the presence of more complex equilibria. No change was observed in the UV-Vis spectra for squaramides 5–8 upon addition of anions. This was likely due to the methylene spacer electronically insulating the aromatic substituents from the squaramide moiety, rather than a lack of binding to the anions.
1 binding model, indicating the presence of more complex equilibria. No change was observed in the UV-Vis spectra for squaramides 5–8 upon addition of anions. This was likely due to the methylene spacer electronically insulating the aromatic substituents from the squaramide moiety, rather than a lack of binding to the anions.
Anion binding by fluorescence spectroscopy
Having investigated anion sensing by 1–8 in the ground state, we next investigated whether anion binding would affect the excited state properties of these molecules. Given the issues encountered above with deprotonation of 2–4, we initially focussed our attention on investigating the impact of anion binding on the fluorescence spectra of compounds 1 and 5–8. However, for 1, the observed shift in absorbance wavelength upon binding to anions did not allow excitation at a consistent wavelength to avoid significant self-absorption at the concentration used in these studies.Therefore, we turned our attention to the fluorescence response of compounds 5–8 upon addition of anions. Surprisingly, in the absence of anions, the fluorescence spectra of 5 and 6 both contained two emission bands, at 325 and 495 nm (Fig. 6), whereas the spectrum of reference amine, 2-naphthalenylmethylamine, displayed only a single emission band at 334 nm at the same concentration. These two bands are consistent with monomer and excimer emission wavelengths respectively,53,54 and suggested that 5 and 6 may be aggregating in solution, with excimer emission a result of intermolecular rather than the desired intramolecular interactions. This was confirmed by dilution experiments, which showed a small decrease in excimer emission with decreasing concentration (Fig. S10†). In contrast, squaramide 7 had relatively low emission intensity (Fig. S9†) and squaramide 8 did not exhibit significant excimer emission.
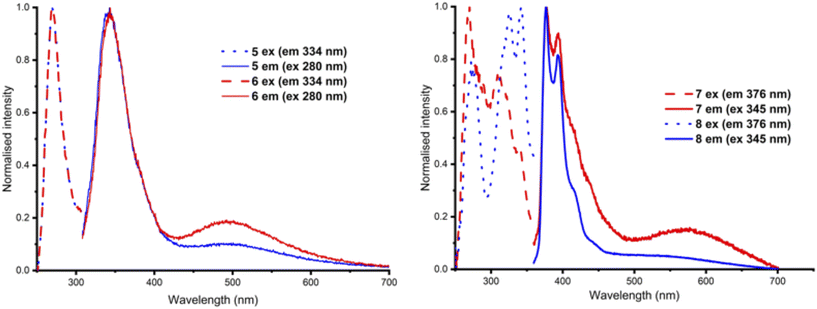 | ||
| Fig. 6 Normalised excitation and emission spectra of (left) 5 and 6 (excitation at 280 nm) and (right) 7 and 8 (excitation at 345 nm) recorded at 25 μM in DMSO (1% H2O) at 298 K. | ||
The lack of intramolecular excimer emission for compounds 5–8 suggests that these compounds do not adopt a syn-,syn- conformation in DMSO solution. Previous computational work has determined that the conformational landscape of squaramides and other dual hydrogen bond donors is complex and impacted by a range of factors including solvent and the steric bulk of the substituents.25,33,55 While DMSO is predicted to lower the energy of the anti-,anti- conformer and so does not provide the best solvent for testing our original hypothesis, the low solubility of these compounds in alternative solvents prevented further investigation. Nevertheless, given recent work highlighting the viability of a sensing mechanism based on aggregation or disaggregation of receptors in solution,15,37 we evaluated changes to the fluorescence spectra of 5–8 upon addition of anions.
Addition of anions to solutions containing 7 or 8 did not appear to induce significant changes in the emission spectra. In contrast, addition of 50 equivalents of a range of anions as the TBA salts to solutions of 5 in DMSO (1% water) at a concentration (25 μM) where the excimer peak was observed showed that addition of acetate, dihydrogen phosphate and sulfate all lead to a small decrease in the excimer emission band at 490 nm. However, addition of increasing amounts of sulfate also leads to a measurable increase in monomer emission together with a small red shift from 334 to 345 nm alongside a small decrease in excimer emission (Fig. 7). This shift was not observed upon addition of acetate or dihydrogenphosphate. Titrations of 5 with chloride, acetate, dihydrogen phosphate and sulfate were performed (Fig. S11†), but the data could not be fit to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, presumably because of multiple factors (anion binding, aggregation) contributing to the overall fluorescence output.
1 binding model, presumably because of multiple factors (anion binding, aggregation) contributing to the overall fluorescence output.
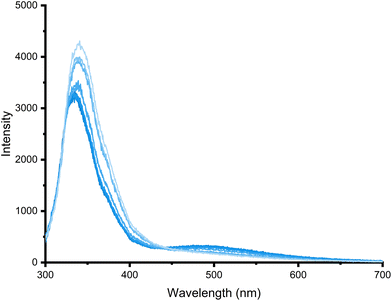 | ||
| Fig. 7 Emission spectra recorded over the course of a titration of 5 (20 μM) with TBA2SO4 (0 to 60 equivalents) in DMSO (1% H2O) (λex 280 nm) at 298 K. | ||
Addition of 50 equivalents of a range of anions as the TBA salts to 25 μM solutions of 6 in DMSO (1% water) showed that addition of acetate, dihydrogen phosphate and sulfate to 6 all lead to a significant decrease in the excimer emission band at 490 nm (Fig. 8), with sulfate giving the greatest change. The reduction in excimer emission relative to monomer emission suggests that binding to these anions possibly results in disaggregation of the receptor in solution.
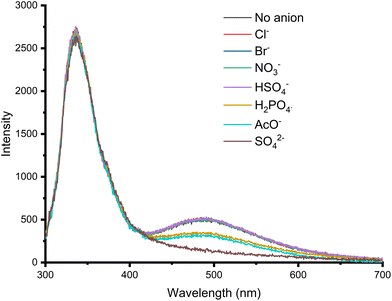 | ||
| Fig. 8 Emission spectra from the anion screen of 6 (20 μM) with anions as their TBA salts (50 equivalents) in DMSO (1% H2O) (λex 280 nm) at 298 K. | ||
Anion titrations were subsequently carried out to further understand the binding of 6 and determine if the relative changes in emission intensities could provide a ratiometric response to these anions upon binding in DMSO at this concentration. A representative fluorescence titration of 6 with sulfate is shown in Fig. 9. This shows the relative excimer emission intensity reduced compared with the relative monomer emission intensity. In contrast to the red shift of the monomer emission band observed for 5 above, for 6 the addition of sulfate leads only to changes in emission intensity and there is a greater ratiometric response to sulfate for 6 than for 5, owing to the greater decrease in the excimer emission intensity at this concentration.
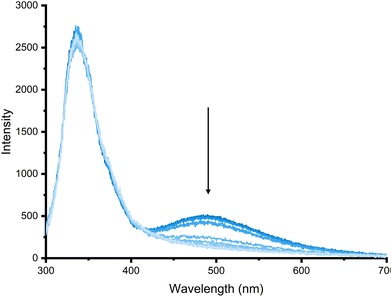 | ||
| Fig. 9 Emission spectra recorded over the course of a titration of 6 (20 μM) with TBA2SO4 (0 to 80 equivalents) in DMSO (1% H2O) (λex 280 nm) at 298 K. | ||
Titrations of other anions (chloride, acetate and dihydrogen phosphate) were also carried out for 6 (Fig. S12†). As the anion screen indicated, a similar profile was observed in the fluorescence spectra upon addition of increasing equivalents of AcO− and H2PO4−, with a decrease in the excimer emission intensity (I490 nm) at higher equivalents of anions. Sulfate caused the greatest decrease in excimer emission intensity. The ratio of I325 nm/I490 nm was taken for each titration, and selectivity and ratiometricity upon anion binding were determined by calculating the ratio of monomer and excimer emission (325 nm and 490 nm respectively). I325 nm/I490 nm was determined for each titration at 10 equivalents of anion (Fig. 10). This reveals that an increased ratiometric response is achieved for interaction of both 5 and 6 with sulfate, with N,N′-dinaphthalenyl squaramide 6 giving a substantially larger ratiometric change for sulfate compared with other anions.
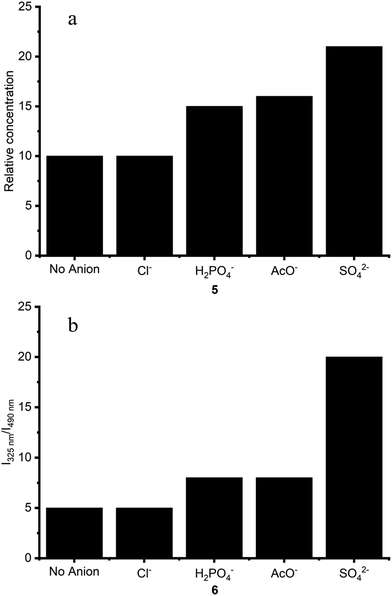 | ||
| Fig. 10 Ratio of I325/I490 for receptors (a) 5 and (b) 6 (25 μM) upon addition of 10 equivalents of anion in DMSO (1% water) at 298 K. | ||
Conclusions
A series of fluorescent squaramides have been synthesised and their anion binding properties investigated via UV-Vis and fluorescence spectroscopy. Fluorophores conjugated directly to the squaramide moiety give rise to relatively acidic NH protons, which were determined to partially deprotonate in DMSO, meaning binding affinities could not be obtained. However, the vivid colour changes upon deprotonation did allow for colorimetric anion sensing in DMSO by N,N′-dipyrenyl squaramide, 4, which showed selectivity for more basic anions. UV-Vis anion binding titrations of N-fluorenyl, N′-butyl squaramide 1 revealed a preference for binding to acetate, dihydrogen phosphate and sulfate over halides and nitrate in DMSO.Squaramides 5–8 did not show changes in the UV-Vis spectra upon addition of anions due to the methylene spacer separating the squaramide from the aromatic substituent. The fluorescence spectra revealed that naphthylmethyl appended squaramides 5 and 6 gave both monomer and excimer emission in the absence of anions, and dilution studies indicated that this was likely due to intermolecular excimer formation, as a consequence of the receptors aggregating in DMSO. Subsequent fluorescence titrations showed that addition of acetate, dihydrogen phosphate and sulfate all lead to an increase in the relative monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) excimer emission intensity, and the ratio clearly indicated the greatest selective response to sulfate.
excimer emission intensity, and the ratio clearly indicated the greatest selective response to sulfate.
Therefore, squaramides 5 and 6 function as fluorescent anions receptors in DMSO that preferentially bind to acetate, dihydrogen phosphate and sulfate, with the greatest response in each case for sulfate, as determined by red shift in monomer emission wavelength (5), or a change in the monomer/excimer emission ratio (5 and 6).
While our initial hypothesis that anion binding would lead to a change in conformation resulting in disruption of intramolecular excimer formation proved false, small structural changes in the squaramide design, i.e., having the fluorophore directly conjugated to the squaramide or separated from the squaramide via a methylene spacer, were determined to have a significant impact upon the squaramide's anion sensing ability, and the mechanism of fluorescent response obtained. By adding a methylene spacer between the squaramide and the fluorophore, the propensity for the squaramides to be deprotonated is significantly reduced and this may be a useful approach for the development of future anion sensors based on the squaramide motif.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Australian Research Council (DP170100118 to K. A. J.). J. L. acknowledges the University of Sydney for an IPRS scholarship. This research was facilitated by access to Sydney Analytical, a core research facility at the University of Sydney.References
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS PubMed
.
- P. A. Gale, E. N. W. Howe and X. Wu, Chem, 2016, 1, 351–422 CAS
.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed
.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS PubMed
.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648–3663 RSC
.
- G. T. Williams, C. J. E. Haynes, M. Fares, C. Caltagirone, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2021, 50, 2737–2763 RSC
.
- J. T. Davis, P. A. Gale and R. Quesada, Chem. Soc. Rev., 2020, 49, 6056–6086 RSC
.
- R. Hein, P. D. Beer and J. J. Davis, Chem. Rev., 2020, 120, 1888–1935 CrossRef CAS PubMed
.
- P. Molina, F. Zapata and A. Caballero, Chem. Rev., 2017, 117, 9907–9972 CrossRef CAS PubMed
.
- P. A. Gale, J. T. Davis and R. Quesada, Chem. Soc. Rev., 2017, 46, 2497–2519 RSC
.
- G. Picci, J. Milia, M. C. Aragoni, M. Arca, S. J. Coles, A. Garau, V. Lippolis, R. Montis, J. B. Orton and C. Caltagirone, Molecules, 2021, 26, 1301 CrossRef CAS PubMed
.
- D. A. McNaughton, M. Fares, G. Picci, P. A. Gale and C. Caltagirone, Coord. Chem. Rev., 2021, 427, 213573 CrossRef CAS
.
- M. Zaleskaya, D. Jagleniec and J. Romański, Dalton Trans., 2021, 50, 3904–3915 RSC
.
- H. Singh, K. Tiwari, R. Tiwari, S. K. Pramanik and A. Das, Chem. Rev., 2019, 119, 11718–11760 CrossRef CAS PubMed
.
- J. Yang, C.-C. Dong, X.-L. Chen, X. Sun, J.-Y. Wei, J.-F. Xiang, J. L. Sessler and H.-Y. Gong, J. Am. Chem. Soc., 2019, 141, 4597–4612 CrossRef CAS PubMed
.
- M. Denis, L. Qin, P. Turner, K. A. Jolliffe and S. M. Goldup, Angew. Chem., Int. Ed., 2018, 57, 5315–5319 CrossRef CAS PubMed
.
- P. A. Gale and C. Caltagirone, Coord. Chem. Rev., 2018, 354, 2–27 CrossRef CAS
.
- X. Bao, X. Wu, S. N. Berry, E. N. W. Howe, Y.-T. Chang and P. A. Gale, Chem. Commun., 2018, 54, 1363–1366 RSC
.
- K. M. Bąk, K. Masłowska and M. J. Chmielewski, Org. Biomol. Chem., 2017, 15, 5968–5975 RSC
.
- L. A. Marchetti, L. K. Kumawat, N. Mao, J. C. Stephens and R. B. P. Elmes, Chem, 2019, 5, 1398–1485 CAS
.
- V. Blažek Bregović, N. Basarić and K. Mlinarić-Majerski, Coord. Chem. Rev., 2015, 295, 80–124 CrossRef
.
- D. E. Gómez, L. Fabbrizzi, M. Licchelli and E. Monzani, Org. Biomol. Chem., 2005, 3, 1495–1500 RSC
.
- J. D. E. Lane, S. N. Berry, W. Lewis, J. Ho and K. A. Jolliffe, J. Org. Chem., 2021, 86, 4957–4964 CrossRef CAS PubMed
.
- V. E. Zwicker, K. K. Y. Yuen, D. G. Smith, J. Ho, L. Qin, P. Turner and K. A. Jolliffe, Chem. – Eur. J., 2018, 24, 1140–1150 CrossRef CAS PubMed
.
- J. Ho, V. E. Zwicker, K. K. Y. Yuen and K. A. Jolliffe, J. Org. Chem., 2017, 82, 10732–10736 CrossRef CAS PubMed
.
- T. Gunnlaugsson, A. P. Davis, G. M. Hussey, J. Tierney and M. Glynn, Org. Biomol. Chem., 2004, 2, 1856–1863 RSC
.
- T. Gunnlaugsson, P. E. Kruger, T. C. Lee, R. Parkesh, F. M. Pfeffer and G. M. Hussey, Tetrahedron Lett., 2003, 44, 6575–6578 CrossRef CAS
.
- L. Fabbrizzi, M. Licchelli, G. Rabaioli and A. Taglietti, Coord. Chem. Rev., 2000, 205, 85–108 CrossRef CAS
.
- T. Gunnlaugsson, A. P. Davis, J. E. O'Brien and M. Glynn, Org. Biomol. Chem., 2005, 3, 48–56 RSC
.
- I. Ohshiro, M. Ikegami, Y. Shinohara, Y. Nishimura and T. Arai, Bull. Chem. Soc. Jpn., 2007, 80, 747–751 CrossRef CAS
.
- V. Amendola, G. Bergamaschi, M. Boiocchi, L. Fabbrizzi and L. Mosca, J. Am. Chem. Soc., 2013, 135, 6345–6355 CrossRef CAS PubMed
.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed
.
- N. Busschaert, R. B. P. Elmes, D. D. Czech, X. Wu, I. L. Kirby, E. M. Peck, K. D. Hendzel, S. K. Shaw, B. Chan, B. D. Smith, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 3617–3626 RSC
.
- G. Picci, M. Kubicki, A. Garau, V. Lippolis, R. Mocci, A. Porcheddu, R. Quesada, P. C. Ricci, M. A. Scorciapino and C. Caltagirone, Chem. Commun., 2020, 56, 11066–11069 RSC
.
- L. Qin, J. R. Wright, J. D. E. Lane, S. N. Berry, R. B. P. Elmes and K. A. Jolliffe, Chem. Commun., 2019, 55, 12312–12315 RSC
.
- L. Qin, A. Hartley, P. Turner, R. B. P. Elmes and K. A. Jolliffe, Chem. Sci., 2016, 7, 4563–4572 RSC
.
- L. K. Kumawat, A. A. Abogunrin, M. Kickham, J. Pardeshi, O. Fenelon, M. Schroeder and R. B. P. Elmes, Front. Chem., 2019, 7, 354 CrossRef CAS PubMed
.
- R. B. P. Elmes, P. Turner and K. A. Jolliffe, Org. Lett., 2013, 15, 5638–5641 CrossRef CAS PubMed
.
- A. Danao, V. Ramalingam, V. Ramamurthy and R. S. Muthyala, J. Photochem. Photobiol., A, 2017, 344, 108–113 CrossRef CAS
.
- S.-H. Park, N. Kwon, J.-H. Lee, J. Yoon and I. Shin, Chem. Soc. Rev., 2020, 49, 143–179 RSC
.
- A. Homberg, E. Brun, F. Zinna, S. Pascal, M. Górecki, L. Monnier, C. Besnard, G. Pescitelli, L. Di Bari and J. Lacour, Chem. Sci., 2018, 9, 7043–7052 RSC
.
- S.-i. Kondo and Y. Matsuta, Tetrahedron Lett., 2016, 57, 1113–1116 CrossRef CAS
.
- S.-i. Kondo, S.-i. Nakajima and M. Unno, Bull. Chem. Soc. Jpn., 2012, 85, 698–700 CrossRef CAS
.
- V. Fernández-Moreira, J. V. Alegre-Requena, R. P. Herrera, I. Marzo and M. C. Gimeno, RSC Adv., 2016, 6, 14171–14177 RSC
.
- A. Rostami, A. Colin, X. Y. Li, M. G. Chudzinski, A. J. Lough and M. S. Taylor, J. Org. Chem., 2010, 75, 3983–3992 CrossRef CAS PubMed
.
- V. Amendola, L. Fabbrizzi, L. Mosca and F. P. Schmidtchen, Chem. Eur. J., 2011, 17, 5972–5981 CrossRef CAS PubMed
.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC
.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC
.
- D. Jagleniec, M. Wilczek and J. Romański, Molecules, 2021, 26, 2751 CrossRef CAS PubMed
.
- L. Qin, S. J. N. Vervuurt, R. B. P. Elmes, S. N. Berry, N. Proschogo and K. A. Jolliffe, Chem. Sci., 2020, 11, 201–207 RSC
.
- D. Jagleniec, Ł. Dobrzycki, M. Karbarz and J. Romański, Chem. Sci., 2019, 10, 9542–9547 RSC
.
- C. Jin, M. Zhang, L. Wu, Y. Guan, Y. Pan, J. Jiang, C. Lin and L. Wang, Chem. Commun., 2013, 49, 2025–2027 RSC
.
- A. Banerjee, A. Sahana, S. Guha, S. Lohar, I. Hauli, S. K. Mukhopadhyay, J. Sanmartín Matalobos and D. Das, Inorg. Chem., 2012, 51, 5699–5704 CrossRef CAS PubMed
.
- K. Morley and J. A. Pincock, J. Org. Chem., 2001, 66, 2995–3003 CrossRef CAS PubMed
.
- I. Sandler, F. A. Larik, N. Mallo, J. E. Beves and J. Ho, J. Org. Chem., 2020, 85, 8074–8084 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob00069a |
| This journal is © The Royal Society of Chemistry 2023 |