Pd(II)-catalyzed coupling of C–H bonds of carboxamides with iodoazobenzenes toward modified azobenzenes†
Sonam
Suwasia
,
Sugumar
Venkataramani
 * and
Srinivasarao Arulananda
Babu
* and
Srinivasarao Arulananda
Babu
 *
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Knowledge City, Sector 81, SAS Nagar, Mohali, Manauli P.O., Punjab 140306, India. E-mail: sugumarv@iisermohali.ac.in; sababu@iisermohali.ac.in
First published on 24th January 2023
Abstract
In this paper, we report a synthetic protocol for the construction of biaryl motif-based or π-extended azobenzene and alkylated azobenzene derivatives via the Pd(II)-catalyzed bidentate directing group (DG)-aided C–H activation and functionalization strategy. In the past, the synthesis of biaryl motif-based azobenzenes was accomplished through the traditional cross-coupling reaction involving organometallic reagents and aryl halides or equivalent coupling partners. We have shown the direct coupling of C–H bonds of aromatic/aliphatic carboxamides (possessing a DG) with iodoazobenzenes as the coupling partners through the Pd(II)-catalyzed bidentate DG-aided, site-selective C–H functionalization method. Azobenzene-containing compounds are a versatile class of photo-responsive molecules that have found applications across branches of chemical, biological and materials sciences and are prevalent in medicinally relevant molecules. Accordingly, the synthesis of new and functionalized azobenzene-based scaffolds has been an attractive topic of research. Although the classical methods are efficient, they need pre-functionalized starting materials. This protocol involving the Pd(II)-catalyzed, directing group-aided site-selective C–H arylation of aromatic and aliphatic carboxamides using iodoazobenzene as the coupling partner affording azobenzene-based carboxamides is an additional route and also a contribution towards enriching the library of modified azobenzenes. We have also shown the photoswitching properties of representative compounds synthesized via the Pd(II)-catalyzed directing group-aided site-selective C–H functionalization method.
Introduction
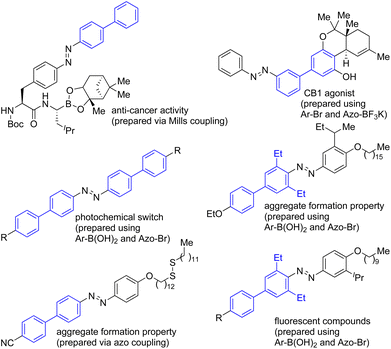
The celebrated transition metal-catalyzed cross-coupling reactions have received overwhelming attention in various branches of chemical sciences.1–3 Although the preparation of organometallic reagents is a prerequisite to accomplish traditional cross-coupling reactions, they are vital features of modern organic synthesis.1–3 In recent years, the concept of transition metal-catalyzed direct functionalization of C–H bonds of small organic molecules has received immense attention.4–8 The functionalization of a C–H bond of an organic compound is an ideal synthetic transformation that helps avoid the organometallic reagent preparation step. The regio- or site-selective C–H functionalization of various classes of organic compounds has been achieved with or without the help of directing groups (DGs).4–8 In particular, we have witnessed tremendous developments in the area pertaining to the Pd(II)-catalyzed, bidentate DG-aided site-selective C–H functionalization of carboxamides.8–10 For example, the site-selective functionalization of the β-, γ-C–H bonds of carboxylic acids has been attained by using the bidentate DG 8-aminoquinoline (8-AQ). On the other hand, the site-selective functionalization of the γ-, δ-C–H bonds of amine compounds has been accomplished by using the bidentate DG picolinamide (PA).8–10Azobenzenes are a distinctive class of organic molecules that have received significant attention in various branches of chemical, materials and biological sciences.7,11–13 Azobenzenes can interconvert between their metastable (E)- and (Z)-geometrical isomers in the presence of light or thermal condition and thus they exhibit the cis–trans isomerization phenomena under photochemical and thermal conditions. Due to these photo- and thermal isomerization processes, various physicochemical properties (viz. geometry, end-to-end molecular distance, electronic properties, color, polarity/dipole moment, etc.) of azobenzene motifs are modulated.11 Azobenzene motifs are used as photoactive molecular switches in various types of small and macromolecules, bio- and chemosensors, and pharmaceuticals, and they also have been used as molecular probes to study the functions of biological molecules including peptides and proteins. Furthermore, many azobenzene motifs have been found to exhibit potent biological activities (e.g., antibacterial and anti-inflammatory activities, Fig. 1) and some of them are currently being used as medicines.11–13
Due to their inherent physicochemical characteristics, application in chemical and biological sciences and prevalence in materials chemistry, various protocols have been developed for the synthesis of azobenzenes.7,11 While classical methods including azo-coupling, Mills and Wallach transformations are popularly used for assembling azobenzenes, sometimes assembling azobenzenes having different functional groups in one or both of the aryl rings is relatively difficult via the classical methods.11 Furthermore, the classical methods need pre-functionalized starting materials. At times, the specific preparation of the π-extended azobenzenes, e.g. biaryl motif-based azobenzenes, needs the employment of traditional cross-coupling reactions involving organometallic reagents (Scheme 1).11,13 Nevertheless, some of the important biologically active and photo-responsive biaryl motif-based azobenzenes have been prepared using organometallic reagents (Fig. 1).12,13
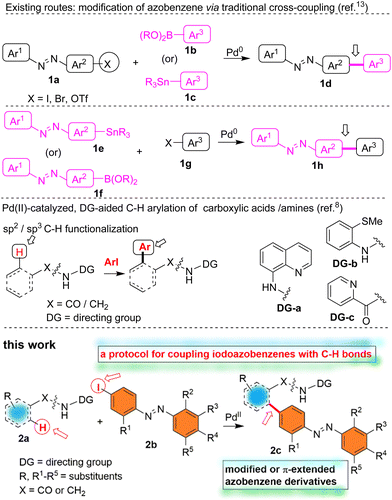 | ||
| Scheme 1 Cross-coupling method towards π-extended azobenzene-molecules. Coupling of the C–H bonds of carboxamides with iodoazobenzenes towards modified azobenzenes. | ||
Inspired by the traditional cross-coupling reaction-based synthesis of biaryl-based π-extended azobenzenes 1d and 1h which involved the use of organometallic reagents,12,13 we became interested in the construction of modified azobenzenes by using the Pd(II)-catalyzed bidentate DG-aided C–H functionalization method.8–10 The azo group-directed transition metal-catalyzed C–H functionalization involving the functionalizing of the ortho-C–H bonds of the azobenzene scaffold has been one of the earliest developed C–H functionalization methods affording modified azobenzenes.4,7 We herein report the construction of biaryl motif-based or π-extended and alkylated azobenzene derivatives via the Pd(II)-catalyzed bidentate directing group-aided direct C–H functionalization tactics involving the coupling of aromatic and aliphatic carboxamides 2a (possessing a DG, e.g., 8-aminoquinoline and picolinamide) with iodoazobenzenes 2b (Scheme 1). Given that azobenzene scaffolds have found several applications in different areas of chemical sciences, there has been a persistent effort in developing new routes for synthesizing functionalized azobenzene-based scaffolds. Along this line, we present our efforts toward enriching the library of modified and especially biaryl-based azobenzenes 2cvia the Pd(II)-catalyzed DG-aided coupling of C–H bonds of carboxamides 2a with iodoazobenzenes 2b (Scheme 1).
Results and discussion
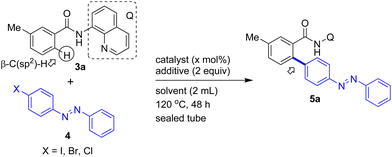
We commenced our investigation on the synthesis of modified azobenzenes via the Pd(II)-catalyzed, bidentate DG-aided direct coupling of the β-C–H bonds of various aromatic carboxamides with iodoazobenzenes. Initially, we assembled 3-methylbenzamide 3a possessing the 8-aminoquinoline bidentate DG. Table 1 shows the optimization reaction comprising the arylation of the ortho-β-C(sp2)–H bond of 3a with 4-iodoazobenzene (4a) under the standard reaction conditions,8–10 involving a Pd catalyst and an iodide ion scavenger (e.g., AgOAc, Ag2CO3, and K2CO3). In concurrence with the literature reports,8–10 the Pd(II)-catalyzed bidentate DG-aided site-selective β-C–H activation/functionalization (e.g. arylation) of an aromatic carboxamide using aryl iodide as an arylating agent is believed to proceed via a well-documented Pd(II)/Pd(IV) redox catalytic cycle.8,9a Moreover, the use of an iodide ion scavenging additive is essential and it is believed that the iodide ion scavenger assists in restoring the Pd(II) catalyst in the Pd(II)/Pd(IV) redox catalytic cycle.8–10| Entry | Catalyst (x mol%) | X | Additive | Solvent | 5a: Yielda (%) |
|---|---|---|---|---|---|
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a: X = I, 4ab: X = Br, 4ac: X = Cl. 3a (0.2 mmol) and 4 (3 equiv.).
b 100 °C.
c 110 °C.
d 4a: X = I, 4ab: X = Br, 4ac: X = Cl. 3a (0.2 mmol) and 4 (3 equiv.).
b 100 °C.
c 110 °C.
d ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a (0.26 mmol) and 24 h.
e 3a (0.26 mmol) and 24 h.
e ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a (0.3 mmol). 3a (0.3 mmol).
|
|||||
| 1 | Pd(OAc)2 (3) | I | AgOAc | p-Xylene | 39 |
| 2 | Pd(OAc)2 (3) | I | Ag2CO3 | p-Xylene | <5 |
| 3 | Pd(OAc)2 (3) | I | Na2CO3 | p-Xylene | 28 |
| 4 | Pd(OAc)2 (3) | I | Cs2CO3 | p-Xylene | <10 |
| 5 | Pd(OAc)2 (3) | I | Ag2CO3 | t-AmylOH | 43 |
| 6 | Pd(OAc)2 (3) | I | K2CO3 | t-AmylOH | <5 |
| 7b | Pd(OAc)2 (3) | I | K2CO3 | t-BuOH | <5 |
| 8c | Pd(OAc)2 (3) | I | K2CO3 | Toluene | 26 |
| 9d | Pd(OAc)2 (3) | I | K2CO3 | p-Xylene | 85 |
| 10e | Pd(OAc)2 (1) | I | K2CO3 | p-Xylene | 47 |
| 11 | PdCl2 (3) | I | K2CO3 | p-Xylene | 42 |
| 12 | Pd(TFA)2 (3) | I | K2CO3 | p-Xylene | 35 |
| 13 | PdCl2(MeCN)2 (3) | I | K2CO3 | p-Xylene | 70 |
| 14 | Pd(OAc)2 (3) | Br | K2CO3 | p-Xylene | 24 |
| 15 | Pd(OAc)2 (5) | Br | K2CO3 | p-Xylene | <10 |
| 16 | Pd(OAc)2 (3) | Cl | K2CO3 | p-Xylene | 0 |
Heating a mixture of 3-methylbenzamide 3a, 4-iodoazobenzene (4a, 3 equiv.), Pd(OAc)2 (3 mol%) and AgOAc (2 equiv.) in p-xylene yielded the expected β-C–H arylated benzamide viz. the biaryl-based azobenzene motif 5a in 39% yield (entry 1, Table 1). Next, we performed the same reaction using Ag2CO3 or Na2CO3 or Cs2CO3 as the additive instead of AgOAc. These trials were not effective and yielded product 5a in <5–28% yields (entries 2–4, Table 1). We then performed the Pd(II)-catalyzed C–H arylation reaction of 3a with 4a in the presence of Ag2CO3 in t-amylOH solvent, which yielded product 5a in a satisfactory yield (43%, entry 5, Table 1). The same reaction when performed using K2CO3 as the additive in t-amylOH or t-BuOH solvent did not yield product 5a (entries 6 and 7, Table 1).
Then, heating a mixture of 3a with 4a in the presence of K2CO3 in toluene yielded product 5a in 26% yield (entry 8, Table 1). Next, we heated 3a with 4a in the presence of Pd(OAc)2 (3 mol%) and K2CO3 in p-xylene solvent instead of toluene solvent, and this attempt yielded the expected β-C–H arylated product (biaryl-based azobenzene motif) 5a in 85% yield (entry 9, Table 1). The same reaction using a minimum catalyst loading (1 mol% of Pd(OAc)2) afforded product 5a in only 47% yield (entry 10, Table 1). Furthermore, the reaction of 3a with 4a in the presence of other palladium catalysts such as PdCl2, Pd(TFA)2 and PdCl2(MeCN)2 instead of Pd(OAc)2 was also found to afford the biaryl-based azobenzene motif 5a in 35–70% yields (entries 11–13, Table 1). We also performed the Pd(II)-catalyzed β-C–H arylation of 3a using 4-bromoazobenzene (4ab) or 4-chloroazobenzene (4ac) instead of 4-iodoazobenzene (4a). These attempts were not fruitful and yielded the biaryl-based azobenzene motif 5a in 0–24% yields (entries 14–16, Table 1).
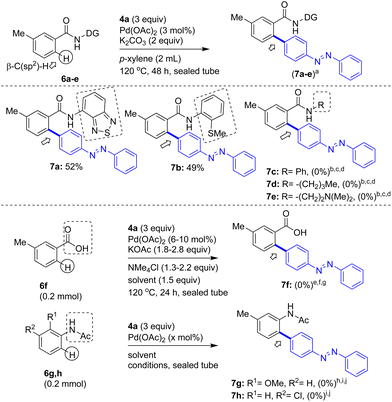
Additionally, to test the efficiency of other bidentate directing groups, we prepared 3-methylbenzamides 6a–e linked with DGs other than 8-aminoquinoline. At first, we performed the Pd(II)-catalyzed β-C–H arylation of 3-methylbenzamide 6a possessing the 4-amino-2,1,3-benzothiadiazole DG with 4a. This reaction gave the azobenzene motif-based carboxamide (β-C–H arylation product) 7a in 52% yield (Table 2). Similarly, the Pd(II)-catalyzed β-C–H arylation of 3-methylbenzamide 6b possessing the 2-(methylthio)aniline DG with 4a gave the azobenzene motif-based carboxamide 7b (β-C–H arylation product) in 49% yield. The C–H arylation of 3-methylbenzamides possessing the simple amide groups (which were assembled from aniline and n-butylamine) with 4a did not yield the expected products 7c and 7d, respectively. Next, the C–H arylation of 3-methylbenzamide possessing the N,N-dimethylethylenediamine DG with 4a failed to afford product 7e (Table 2). Trials comprising the direct C–H arylation of 3-methylbenzoic acid (6f) with 4a did not yield product 7f. Furthermore, we also attempted the C–H arylation of acetanilides (6g and 6h) with 4a and these attempts did not yield the expected products 7g and 7h, respectively. Thus, the reaction of 3a with 4a in the presence of Pd(OAc)2 (3 mol%) and K2CO3 (2 equiv.) in p-xylene at 120 °C for 48 h, which gave 5a in 85% yield, are the optimal reaction conditions (Table 1). Though 3-methylbenzamide 3a contains two ortho-β-C(sp2)–H bonds, the arylation selectively occurred at the least hindered ortho-β-C–H bond of 3a and afforded the mono-β-C–H arylation product 5a. This observation is in concurrence with the literature reports dealing with the C–H arylation of (meta) 3-substituted benzamides.8,9b
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7a–e from the corresponding benzamide substrates 6a–e prepared from 3-methylbenzoic acid and the corresponding amine DGs.
b Substrate (0.19 mmol) and conditions in entry 9 (Table 1), sealed tube.
c Substrate (0.19 mmol) and conditions in entry 13 (Table 1), sealed tube.
d Substrate (0.19 mmol) and conditions in entry 1 (Table 1) in toluene at 110 °C, sealed tube.
e Pd(OAc)2 (6 mol%), NMe4Cl (1.3 equiv.), KOAc (1.8 equiv.) and AcOH.
f Pd(OAc)2 (10 mol%), NMe4Cl (2.2 equiv.), KOAc (2.8 equiv.) and TFA.
g Using PEPPSI-iPr (6 mol%), NMe4Cl (2.2 equiv.), KOAc (2.8 equiv.) and AcOH.
h Pd(OAc)2 (3 mol%), K2CO3 (2 equiv.), p-xylene (2 mL), 120 °C, 48 h.
i Pd(OAc)2 (1 mol%), AgOAc (1.1 equiv.), CF3COOH (1 mL), 100 °C, 12 h.
j 7a–e from the corresponding benzamide substrates 6a–e prepared from 3-methylbenzoic acid and the corresponding amine DGs.
b Substrate (0.19 mmol) and conditions in entry 9 (Table 1), sealed tube.
c Substrate (0.19 mmol) and conditions in entry 13 (Table 1), sealed tube.
d Substrate (0.19 mmol) and conditions in entry 1 (Table 1) in toluene at 110 °C, sealed tube.
e Pd(OAc)2 (6 mol%), NMe4Cl (1.3 equiv.), KOAc (1.8 equiv.) and AcOH.
f Pd(OAc)2 (10 mol%), NMe4Cl (2.2 equiv.), KOAc (2.8 equiv.) and TFA.
g Using PEPPSI-iPr (6 mol%), NMe4Cl (2.2 equiv.), KOAc (2.8 equiv.) and AcOH.
h Pd(OAc)2 (3 mol%), K2CO3 (2 equiv.), p-xylene (2 mL), 120 °C, 48 h.
i Pd(OAc)2 (1 mol%), AgOAc (1.1 equiv.), CF3COOH (1 mL), 100 °C, 12 h.
j ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a (4 equiv.) Pd(OAc)2 (5 mol%), AgOAc (2.3 equiv.), CF3COOH (0.5 mL), 120 °C, 12 h. 4a (4 equiv.) Pd(OAc)2 (5 mol%), AgOAc (2.3 equiv.), CF3COOH (0.5 mL), 120 °C, 12 h.
|
|---|
|
Next, we focused our attention to expand the substrate scope and obtain various biaryl-based azobenzene scaffolds via the Pd(II)-catalyzed 8-aminoquinoline DG-aided direct coupling of C–H bonds of different aromatic carboxamides with iodoazobenzenes. Towards this, we initially performed the Pd(II)-catalyzed 8-aminoquinoline DG-aided β-C–H arylation of various benzamides 3a–i having substituents at the ortho- or meta-positions with 4-iodoazobenzene (4a). Accordingly, various benzamide substrates 3a–f having a substituent (e.g., Me, Cl, OPh, OBn and OEt) at the ortho- or meta-position were reacted with 4a in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. These reactions gave the corresponding biaryl-based azobenzene motifs 5a–f in 29–86% yields (Scheme 2).
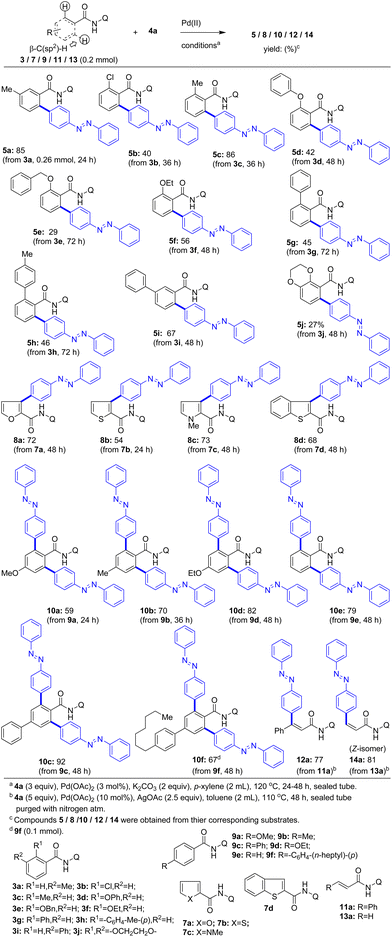 | ||
| Scheme 2 The coupling of the β-C(sp2)–H bond of various carboxamides with iodoazobenzene (4a) towards π-extended modified azobenzene-molecules 5, 8, 10, 12, and 14. | ||
Subsequently, various biaryl-based carboxamides 3g–i and benzo[b][1,4]dioxine carboxamide 3j were reacted with 4a in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. These reactions yielded the corresponding π-extended azobenzene motifs 5g–j in 27–67% yields (Scheme 2). Next, heteroaryl-2-carboxamides including furan-2-carboxamide 7a, thiophene-2-carboxamide 7b, pyrrole-2-carboxamide 7c and benzothiophene-2-carboxamide 7d possessing the 8-aminoquinoline DG were reacted with 4a in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. These reactions gave the corresponding azobenzene motif-connected heteroaryl-2-carboxamides 8a–d in 54–73% yields (Scheme 2).
Having performed the Pd(II)-catalyzed mono-β-C–H arylation of various ortho/meta-substituted benzamides 3a–j and heteroaryl-2-carboxamides 7a–d with 4a, we then wished to perform the double β-C–H arylation of various para-substituted benzamides 9a–f with 4a. Accordingly, benzamides 9a, 9b, and 9d having a substituent (e.g., OMe, Me and OEt) at the para-position and simple benzamide 9e possessing the 8-aminoquinoline DG were reacted with 4a in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. These reactions gave the corresponding π-extended, bis-azobenzene motif-based carboxamides 10a, 10b, 10d, and 10e (59–82% yields) via the double (ortho)β-C–H arylation of the corresponding benzamides 9a, 9b, 9d, and 9e. Then, the biaryl-based carboxamides 9c and 9f possessing the 8-aminoquinoline DG were reacted with 4a in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. These reactions gave the corresponding π-extended, bis-azobenzene motif-based carboxamides 10c and 10f (67–92% yields) via the double (ortho)β-C–H arylation of the corresponding benzamides 9c and 9f.
Then the Pd(II)-catalyzed C–H arylation of cinnamamide 11a possessing the 8-aminoquinoline DG with 4a afforded the azobenzene motif-based cinnamamide derivative 12a in 77% yield. Similarly, acrylamide 13a possessing the 8-aminoquinoline DG was treated with 4a in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. This reaction afforded the azobenzene motif-based cinnamamide 14a possessing Z stereochemistry (in 81% yield). The stereochemistry of products 12a and 14a is proposed based on our earlier report10a dealing with the Z selective β-C–H arylation of their parent substrates 11a and 13a, respectively.
Having explored the C–H arylation of various carboxamides with 4-iodoazobenzene (4a), we then wished to further expand the generality and substrate scope of this protocol comprising the direct C–H coupling of carboxamides with iodoazobenzenes. Towards this, the assembly of various substituted iodoazobenzenes was planned to use them as arylating agents in the Pd(II)-catalyzed C–H arylation of various carboxamides to obtain a wide range of π-extended, modified azobenzene derivatives. Accordingly, various 4-iodoazobenzene derivatives 4b–h and 4k (Scheme 3) were prepared via the standard azobenzene synthesis method. We then performed the Pd(II)-catalyzed 8-aminoquinoline DG-aided β-C–H arylation of 3-methylbenzamide 3a with 4-iodoazobenzenes 4b–e having a substituent (e.g. OMe, Me and Et) at the ortho- or para-position in the aryl ring ‘b’ of 4 (Scheme 3). These reactions afforded the corresponding biaryl-based azobenzene motifs 15a–d in 61–99% yields (Scheme 3).
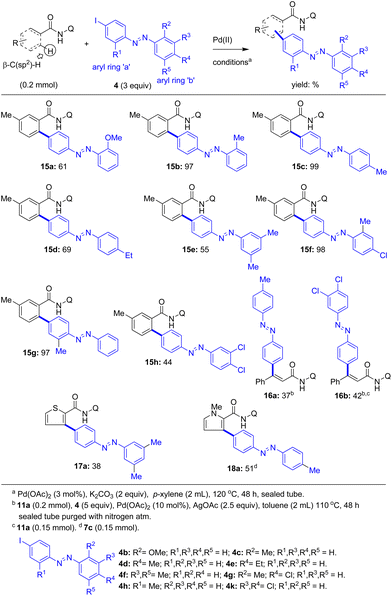 | ||
| Scheme 3 The coupling of the β-C(sp2)–H bond of carboxamides with various iodoazobenzenes towards biaryl-based modified azobenzene molecules 15–18. | ||
Next we carried out the Pd(II)-catalyzed β-C–H arylation of 3a with 4-iodoazobenzenes 4f or 4g or 4k having two substituents (e.g. Me and Cl) in the aryl ring ‘b’ of 4. These reactions afforded the corresponding azobenzene motif-based carboxamides 15e (55%), 15f (98%), and 15h (44%). Then the Pd(II)-catalyzed β-C–H arylation of 3a with 4-iodoazobenzene 4h having a methyl substituent in the aryl ring ‘a’ of 4 yielded the azobenzene motif-based carboxamide 15g in 97% yield. Then the cinnamamide substrate 11a was subjected to the β-C–H arylation with iodoazobenzenes 4d or 4k in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. These reactions afforded the corresponding azobenzene motif-based cinnamamide derivatives 16a and 16b in 37–42% yields. Additionally, heteroaryl-2-carboxamides including thiophene-2-carboxamide 7b and pyrrole-2-carboxamide 7c were subjected to the Pd(II)-catalyzed β-C–H arylation with iodoazobenzenes 4d or 4f in p-xylene at 120 °C. These reactions gave the corresponding π-extended, azobenzene motif-based heteroaryl-2-carboxamides 17a and 18a in 38–51% yields (Scheme 3). Furthermore, we assembled the 3-iodoazobenzene (4i) and then performed the Pd(II)-catalyzed β-C–H arylation of 3-methylbenzamide 3a using 4i, which successfully yielded the biaryl-based azobenzene motif 19a in 68% yield (Scheme 4).
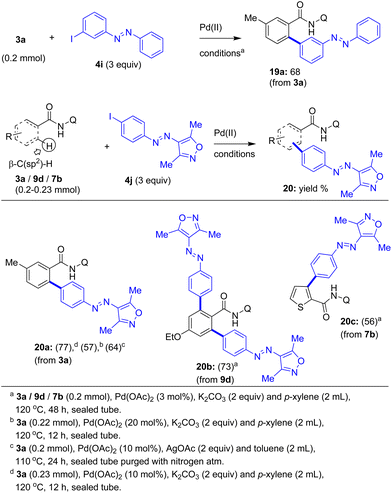 | ||
| Scheme 4 The coupling of the β-C(sp2)–H bond of carboxamides with iodoazobenzenes 4i and j towards the biaryl-based modified azobenzene molecules 19a and 20a–c. | ||
Additionally, we assembled the aryl-heteroaryl (isoxazole ring)-based azobenzene derivative 4j having an iodo substituent. Then 3-methylbenzamide 3a was subjected to the β-C–H arylation with 4j in the presence 20 mol% of the Pd(OAc)2 catalyst and K2CO3 (2 equiv.) in p-xylene at 120 °C for 12 h. This reaction gave the aryl-isoxazole ring-based azobenzene motif 20a in a moderate yield (57% yield, Scheme 4). The treatment of benzamide 3a with 4j in the presence of 10 mol% of the Pd(OAc)2 catalyst and AgOAc (2 equiv.) in toluene at 110 °C for 24 h gave product 20a in a slightly improved yield (64%, Scheme 4). The treatment of benzamide 3a with 4j in the presence of only 10 mol% of the Pd(OAc)2 catalyst and K2CO3 (2 equiv.) in p-xylene at 120 °C for 12 h afforded product 20a in a good yield (77%, Scheme 4). Subsequently, benzamide 9d having a substituent at the para-position possessing the 8-aminoquinoline DG was reacted with 4j in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. This reaction gave the corresponding π-extended, bis-azobenzene motif-based carboxamide 20b in 73% yield via the double (ortho)β-C–H arylation of 9d. Next, thiophene-2-carboxamide 7b possessing the 8-aminoquinoline DG was reacted with 4j in the presence of the Pd(OAc)2 catalyst and K2CO3 in p-xylene at 120 °C. This reaction yielded the corresponding π-extended, azobenzene motif-based thiophene-2-carboxamide 20c in 56% yield (Scheme 4).
We then wished to explore the installation of the azobenzene motif in aliphatic chains via the Pd(II)-catalyzed arylation of inert C(sp3)–H bonds of aliphatic carboxamides by using iodoazobenzenes as the arylating agents (Scheme 5). We assembled various aliphatic carboxamides 21a–i from their corresponding carboxylic acids and 8-aminoquinoline. Then the aliphatic carboxamides 21a–h were treated with 4-iodoazobenzene (4a) under the standard Pd(II)-catalyzed 8-aminoquinoline-aided β-C(sp3)–H arylation reaction conditions. Accordingly, a mixture of the corresponding aliphatic carboxamides 21a–h and 4a was reacted in presence of the Pd(OAc)2 catalyst (3 mol%) and K2CO3 (2 equiv.) in p-xylene at 120 °C for 48 h. These reactions successfully afforded the corresponding azobenzene motif installed aliphatic carboxamides 22a–h in 55–97% yields (Scheme 5). Additionally, the Pd(II)-catalyzed 8-aminoquinoline-aided β-C(sp3)–H arylation of cyclobutanecarboxamide (21i) with 4-iodoazobenzene (4a) yielded the bis-azobenzene motif-based cyclobutanecarboxamide (having an all cis-stereochemistry, 51% yield) via the bis-β-C–H arylation of 21i. The installation of the azobenzene motif at the β position in 21a–h and cyclobutanecarboxamide 21i is proposed based on the literature reports,8 which have documented the occurrence of the arylation at the β-C(sp3)–H bonds of substrates with the help of the 8-aminoquinoline DG.
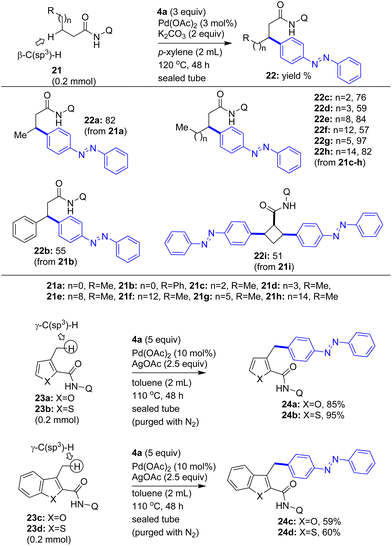 | ||
| Scheme 5 The coupling of the β- and γ-C(sp3)–H bonds of carboxamides with iodoazobenzene 4a towards the modified azobenzene molecules 22a–i and 24a–d. | ||
Having obtained various azobenzene motif installed aromatic and aliphatic carboxamides via the Pd(II)-catalyzed arylation of the β-C–H bonds of aromatic/aliphatic carboxamides, we then intended to attempt the arylation of the remote sp2/sp3 γ-C–H bonds8i,10b of appropriate carboxamides with iodoazobenzenes. Toward this, we assembled 3-methyl heteroaryl-2-carboxamides 23a–d. Then we subjected 3-methyl furan-2-carboxamide 23a and 3-methyl thiophene-2-carboxamide 23b to the Pd(II)-catalyzed 8-AQ DG-aided arylation of the methyl γ-C(sp3)–H bond with 4-iodoazobenzene (4a). These reactions yielded the corresponding azobenzene motif-based diarylmethane derivatives 24a and 24b in 85–95% yields (Scheme 5). Similarly, we subjected 3-methyl benzofuran-2-carboxamide 23c and 3-methyl benzothiophene-2-carboxamide 23d to the Pd(II)-catalyzed arylation of the methyl γ-C(sp3)–H bond with 4-iodoazobenzene (4a). These reactions yielded the corresponding azobenzene motif-based diarylmethane derivatives 24c and 24d in 59–60% yields.
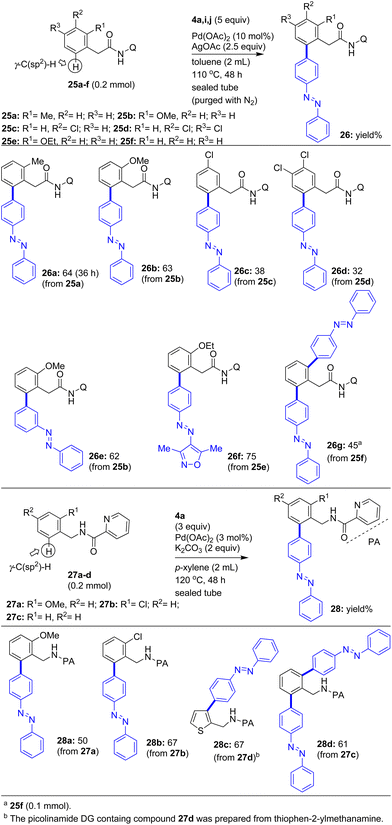
Subsequently, in order to attempt the arylation of the ortho-γ-C(sp2)–H bonds of appropriate carboxamides with iodoazobenzenes, we assembled various arylacetamides 25a–f possessing the 8-aminoquinoline DG. Additionally, we assembled various benzylamine derivatives 27a–d possessing the bidentate directing group picolinamide (PA) (Scheme 6). We then attempted the Pd(II)-catalyzed 8-AQ-aided arylation of the ortho-γ-C(sp2)–H bond of arylacetamides 25a–d possessing substituents at the ortho/meta/para positions with 4a in the presence of the Pd(OAc)2 catalyst and the AgOAc additive in toluene at 110 °C. These reactions yielded the corresponding biaryl-based azobenzene motifs 25a–d in 32–64% yields (Scheme 6).
Next, we performed the Pd(II)-catalyzed 8-AQ-aided arylation of the ortho-γ-C(sp2)–H bond of the arylacetamides 25b and 25e possessing the 8-aminoquinoline DG with iodoazobenzenes 4i or 4j in the presence of the Pd(OAc)2 catalyst and the AgOAc additive in toluene at 110 °C. These reactions yielded the corresponding biaryl-based, azobenzene motifs 26e and 26f in 62–75% yields (Scheme 6). Next we performed the bis-γ-C(sp2)–H arylation of phenylacetamide 25f possessing the 8-aminoquinoline DG with 4a. This reaction gave the π-extended, bis-azobenzene motif-based phenylacetamide derivative 26g in 45% yield via the double γ-C(sp2)–H arylation of 25f. Successively, we intended to attempt the Pd(II)-catalyzed picolinamide DG-aided γ-C(sp2)–H arylation of benzylamine derivatives 27a–d. Accordingly, we subjected the ortho-substituted benzylamine derivatives 27a and 27b possessing the picolinamide DG to the Pd(II)-catalyzed γ-C(sp2)–H arylation with 4a in the presence of the Pd(OAc)2 catalyst and the K2CO3 additive in p-xylene at 120 °C. These reactions yielded the corresponding biaryl-based, azobenzene motifs 28a and 28b in 50–67% yields (Scheme 6).
Next the 2-thiophenemethylamine derivative 27d possessing the picolinamide DG was subjected to the Pd(II)-catalyzed γ-C(sp2)–H arylation with 4a. This reaction yielded the π-extended, azobenzene motif-based 2-thiophenemethylamine derivative 28c in 67% yield. Finally, we performed the bis-γ-C(sp2)–H arylation of the benzylamine derivative 27c possessing the picolinamide DG with 4a in the presence of Pd(OAc)2 catalyst and K2CO3 additive in p-xylene at 120 °C. These reactions gave the corresponding π-extended, bis-azobenzene motif-based benzylamine derivative 28d in 61% yield via the double γ-C(sp2)–H arylation of 27d (Scheme 6). The installation of the azobenzene motif at the methyl γ-C(sp3)–H bonds of substrates 23a–d and the ortho-γ-C(sp2)–H bonds of 25a–f and 27a–d is proposed based on earlier literature reports which have reported the arylation of sp2/sp3 γ-C–H bonds of the corresponding substrates possessing the 8-aminoquinoline and picolinamide directing groups, respectively.8–10
To show the scalability of this protocol, we also attempted the Pd(II)-catalyzed, 8-AQ DG-aided C–H arylation of a benzamide with iodoazobenzene on a gram scale. Accordingly, substrate 3a was subjected to the ortho-γ-C(sp2)–H bond arylation with 4a, which afforded the biaryl-based azobenzene motif 5a in 77% yield (Scheme 7). Then, to show the utility of this protocol, we attempted the removal of the 8-aminoquinoline DG after performing the Pd(II)-catalyzed, 8-AQ DG-aided ortho-C–H arylation of the carboxamides with iodoazobenzenes. Towards this, initially, the biaryl-based azobenzene carboxamide derivative 5a was subjected to the standard amide hydrolysis conditions. From various trials, the treatment of biaryl-based azobenzene carboxamide derivative 5a with excess amounts of KOH in an alcohol solvent at 100–120 °C was found to afford the 8-aminoquinoline DG removed biaryl-based azobenzene carboxylic acid 29a. To determine the suitable conditions for obtaining the DG-removed azobenzene motif-based carboxylic acid 29a in a good yield, the amide hydrolysis of 5a was attempted by using different alcohol solvents. Accordingly, the KOH-mediated amide hydrolysis of 5a was performed in MeOH or 2-propanol or 1-octanol or EtOH, which afforded the azobenzene motif-based carboxylic acid 29a in 30–73% yields (Scheme 7). Product 29a was obtained in a good yield when the hydrolysis of 5a was performed in EtOH solvent. Similarly, azobenzene carboxamide derivatives 22b and 8b were subjected to the amide hydrolysis conditions to afford the corresponding 8-aminoquinoline DG removed azobenzene carboxylic acids 29b and 29c in 58–67% yields (Scheme 7).
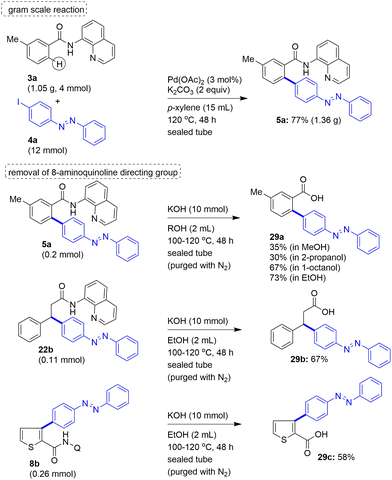 | ||
| Scheme 7 The gram scale C–H arylation of carboxamide 3a with 4a affording 5a. The removal of the 8-aminoquinoline DG from 5a/22b/8b. | ||
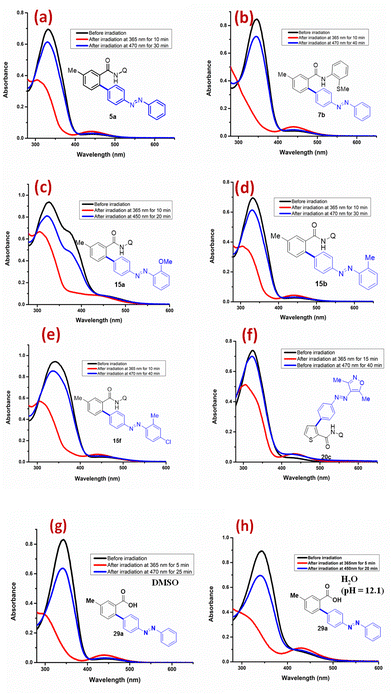
We obtained the UV-Vis absorption data λmax (nm) of all the compounds prepared in this work14 (the corresponding UV-Vis absorption spectral data are given in the ESI†). The primary results of the photoswitching studies and the related kinetic measurements of the representative compounds prepared in this work are shown (Table 3 and Fig. 2, see the ESI† for additional data). The thermodynamically stable and the native trans-isomers of the azoarene derivatives 5a, 7b, 15a, 15b, 15f, 20c and 29a were irradiated at 365 nm to convert them into their corresponding cis-isomers. In each case, the solutions were continuously irradiated to ensure that a photostationary state (PSS) was reached. Based on the spectral data, the PSS composition has been estimated.15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Sample | UV-Vis absorption data λmax (nm) | Photoisomerization and PSS composition | Conc. (μM) | Z to E thermal isomerizationd | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| E-Isomer π–π*/n–π* | Z-Isomer π–π*/n–π* | λ forward |
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zb (%) Zb (%) |
λ reverse |
Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ec (%) Ec (%) |
k (×10−3) min−1 | t 1/2 (min) | |||
| a DMSO. b Forward E–Z isomerization was done using a 365 nm LED (5–15 min). c The reverse Z–E isomerization was done using 470 or 450 nm LEDs (20–40 min). d All kinetics experiments were carried out at 60 °C. | ||||||||||
| 1 | 5a | 331/445 | 304/445 | 365 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 58 |
470 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
6.8 | 16.23 | 43 |
| 2 | 7b | 346/431 | —/440 | 365 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 87 |
470 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
18.2 | 15.01 | 46 |
| 3 | 15a | 327/451 | 306/— | 365 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
450 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
33.9 | 13.31 | 52 |
| 4 | 15b | 338/445 | 304/443 | 365 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
470 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
21.9 | 15.8 | 44 |
| 5 | 15f | 346/452 | 306/441 | 365 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
470 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
24.4 | 24.6 | 28 |
| 6 | 20c | 326/441 | 306/440 | 365 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
470 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
15.4 | 5.57 | 124 |
| 7 | 29a | 345/444 | 297/438 | 365 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89 89 |
470 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
12.6 | 20.6 | 34 |
The conversion of the corresponding cis-isomers of 5a, 7b, 15a, 15b, 15f, 20c and 29a into their trans-compounds was realized either at 470 or 450 nm. In about 20–40 min, such a reverse isomerization step afforded the corresponding trans-isomers of 5a, 7b, 15a, 15b, 15f, 20c and 29a (Fig. 2). Since compound 29a has a free carboxylic acid, we also attempted to isomerize it between the trans- and cis-isomers in water. In this regard, we have performed the photoswitching experiments under different pH conditions (7.5, 9.5, and 12.1) in phosphate buffer solution. Notably, we observed very good photoswitching even under aqueous conditions. Indeed, under each pH condition, compound 29a exhibited consistent photoisomerization in both directions up to five cycles.
Apart from that, these selected candidates, after enriching their Z-isomers, were subjected to thermal reverse isomerization kinetics in DMSO at 60 °C. Based on the exponential growth and first-order fit, we estimated the rate constant and half-life. As expected, the azoisoxazole derivative 20c showed the maximum half-life.15 Furthermore, we performed the thermal reverse isomerization kinetics of 29a under various pH conditions, and excellent half-lives were observed (pH 7.5: 114 min; pH 9.5: 101 min; pH 12.1: 114 min) indicating that it is a better photoswitch even under aqueous conditions.
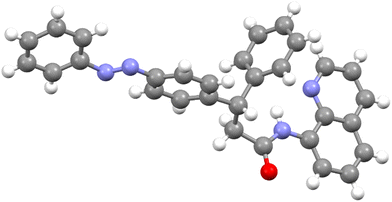
All the biaryl motif-based azobenzene and alkylated azobenzene derivatives prepared using the Pd(II)-catalyzed DG-aided β-C–H functionalization of aromatic and aliphatic carboxamides with iodoazobenzenes as arylating agents were characterized by NMR and HRMS analysis. Additionally, the structure of compound 22b which was obtained from the β-C–H arylation of the corresponding aliphatic carboxamide 21b with iodoazobenzene 4a was unequivocally ascertained by X-ray structural analysis (Fig. 3).
Conclusions
In summary, we have shown the application of the Pd(II)-catalyzed bidentate directing group-aided site-selective C–H functionalization strategy for the construction of biaryl motif-based or π-extended azobenzene and alkylated azobenzene derivatives. The various aromatic/aliphatic carboxamides possessing the bidentate directing group 8-aminoquinoline or picolinamide were subjected to the Pd(II)-catalyzed site-selective arylation of the β-, γ-C–H bonds using iodoazobenzenes as the arylating agents. These reactions have led to the assembly of the corresponding biaryl motif-based or π-extended azobenzene and alkylated azobenzene derivatives. We have shown that after the C–H arylation of carboxamides with iodoazobenzene, it is possible to remove the directing group, 8-aminoquinoline. We have also shown the initial studies comprising the photoswitching properties of representative compounds synthesized via the Pd(II)-catalyzed directing group-aided site-selective direct C–H functionalization method. In general, the traditional cross-coupling reaction involving organometallic reagents has been one of the popular routes for the synthesis of biaryl motif-based π-extended azobenzenes. This paper shows the direct coupling of the C–H bonds of aromatic and aliphatic carboxamides with iodoazobenzenes as arylating agents without using organometallic agents. Notably, azobenzene motifs are a unique class of photo-responsive compounds that have found applications across various branches of chemical, biological and materials sciences. Furthermore, azobenzene motifs are prevalent in medicinally important molecules. Thus, this protocol involving the Pd(II)-catalyzed, directing group-aided site-selective C–H arylation of carboxamides using iodoazobenzenes affording azobenzene-based carboxamides would enrich the library of modified azobenzenes. Overall, as a primary goal, we have tried to show a direct C–H coupling route as an ancillary method to the existing traditional cross-coupling methods for obtaining the biaryl motif-based or π-extended azobenzene and alkylated azobenzene derivatives by using carboxamides and iodoazobenzene as coupling partners. We have prepared various iodoazobenzenes and the azo groups in iodoazobenzenes have no role in the main concept of C–H coupling (and served as a by-stander) as iodoazobenzenes as a whole served as arylating agents in the coupling with carboxamides to afford biaryl motif-based or π-extended azobenzene and alkylated azobenzene derivatives. Although at present we have not shown any direct application of our method for obtaining molecules similar to the molecules shown in Fig. 1, further studies for finding the properties and possible applications of the present method and prepared compounds will be attempted and the results will be reported in the near future. Furthermore, this work also contributes to the development and application of Pd(II)-catalyzed bidentate directing group-aided site-selective C–H functionalization methods.Experimental
General
1H and 13C NMR spectra of compounds were recorded (using TMS as an internal standard) using 400 and ∼101 MHz (or 500 and ∼126 MHz) NMR spectrometers, respectively. The HRMS analysis data of samples reported here were obtained from QTOF mass analyzer using the electrospray ionization (ESI) method. IR spectra of samples reported here were recorded as neat or thin films. Column chromatography purification was carried out on silica gel (100–200 mesh). Reactions were conducted in anhydrous solvents in sealed tubes (filled with ambient air) or sealed tubes purged with a nitrogen atm. Organic layers obtained after the workup were dried using anhydrous Na2SO4. Thin layer chromatography (TLC) analyses were performed on silica gel or alumina plates and components were visualized by observation under iodine vapor or using a UV lamp. Isolated yields of all the products are reported and yields were not optimized. In all of the cases, after the Pd(II)-catalyzed reactions, the respective crude reaction mixtures were subjected to column chromatographic purification, and we were focused on isolating the corresponding main C–H coupled products shown in the respective tables/schemes and any other by-products were not obtained in characterizable or demonstrable amounts. While in most of the cases, the azobenzenes with a trans-geometry were isolated in pure form, in some cases, the NMR spectra revealed the presence of partial amounts of azobenzenes with a cis-geometry. Most of the aromatic and aliphatic carboxamides possessing the corresponding bidentate directing groups used in this work are known compounds and they were assembled using standard amide coupling methods from their corresponding carboxylic acids/acid chlorides and amines.8–10 Iodoazobenzenes used in this work were assembled via the standard azobenzene preparation methods.11 In some cases, to obtain pure NMR spectra we gently heated the isolated azobenzenes to convert the azobenzenes with a cis-geometry into azobenzenes with a trans-geometry. The absorption spectra and photoswitching studies of samples (concentration = 0.01 g per 100 mL in DMSO) have been carried out in DMSO and analyzed using UV-Vis spectroscopy. Initially, the absorption spectra of appropriate compounds (5a, 7b, 15a, 15b, 15f, 20c and 29a) were recorded to find the p–p* and n–p* absorption bands. By utilizing the π–π* band, the compound was subjected to irradiation at 365 nm light for forward (E to Z) photoisomerization. The absorption intensity of the π–π* of the Z-isomer band decreases and shifts towards the blue region, whereas the intensity of the n–π* absorption band increases due to the non-planarity of the Z-isomer. The reverse photoisomerization was performed by irradiating at 450 or 470 nm using the n–π* absorption band.General procedure for the preparation of 5a–j, 7a–b, 8a–d, 10a–f, 15a–h, 17a, 18a, 19a, 20a–c, 22a–i, and 28a–dvia the Pd(II) catalyzed C–H arylation of the corresponding carboxamides
A mixture of an appropriate carboxamide (0.2–0.26 mmol, 1 equiv.), Pd(OAc)2 (3 mol%), the appropriate azo-based iodoarene compound (3 equiv.), and K2CO3 (2 equiv.) in p-xylene(2–3 mL) was heated at 120 °C for 12–48 h in air. After the reaction period, the reaction mixture was concentrated in vacuo and the resulting crude residue was purified by column chromatography on silica or neutral alumina gel (eluent = EtOAc/hexane) to furnish the corresponding azobenzene-based carboxamide derivative (see the corresponding table/scheme for the specific entry).General procedure for the preparation of 12a, 14a, 16a–b, 24a–d, and 26a–gvia the Pd(II) catalyzed C–H arylation of the corresponding carboxamides
A mixture of an appropriate carboxamide (0.2 mmol), Pd(OAc)2 (10 mol%), the azo-based iodoarene compound (5 equiv.) and AgOAc (2.5 equiv.) in anhydrous toluene (2–3 mL) was heated at 110 °C for 36–48 h under a nitrogen atm. After the reaction period, the reaction mixture was concentrated in vacuo and the resulting crude residue was purified by column chromatography on silica or neutral alumina gel (eluent = EtOAc/hexane) to furnish the corresponding azobenzene-based carboxamide derivative (see the corresponding table/scheme for the specific entry).A typical procedure for the removal of the directing group 8-aminoquinoline and the synthesis of azobenzene carboxylic acid derivatives 29a–c
A solution of azobenzene-based carboxamide (0.2 mmol, 1 equiv.) and KOH (10 mmol, 50 equiv.) in ethanol (2 mL) was heated in a sealed tube at 100 °C for 48 h. After this period, the reaction mixture was diluted with water and extracted with EtOAc (2 × 10 mL). Then the aqueous layer was acidified with 1 N HCl and extracted with EtOAc (2 × 10 mL). The organic layers were dried over Na2SO4 and evaporated under reduced pressure to afford a crude mixture, which was then purified by column chromatography on silica or neutral alumina gel to afford the corresponding azobenzene-based carboxylic acid derivative (see the corresponding table/scheme for the specific entry).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 10
hexane = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) as an orange colored solid (97 mg, 85%, 0.26 mmol scale); Rf (10% EtOAc/hexane) 0.2; mp: 156–158 °C; IR (DCM): 3239, 1669, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.84 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.87–7.84 (m, 4H), 7.78 (s, 1H), 7.69–7.66 (m, 2H), 7.56–7.42 (m, 7H), 7.31–7.28 (m, 1H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.6, 151.8, 147.9, 142.8, 138.4, 138.2, 136.5, 136.0, 136.0, 134.5, 131.4, 131.0, 130.6, 130.0, 129.8, 129.1, 127.7, 127.2, 123.0, 122.8, 121.7, 121.4, 116.4, 21.1. HRMS (ESI) m/z [M + H]+ calcd for C29H23N4O: 443.1872, found 443.1858.
90) as an orange colored solid (97 mg, 85%, 0.26 mmol scale); Rf (10% EtOAc/hexane) 0.2; mp: 156–158 °C; IR (DCM): 3239, 1669, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.84 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.87–7.84 (m, 4H), 7.78 (s, 1H), 7.69–7.66 (m, 2H), 7.56–7.42 (m, 7H), 7.31–7.28 (m, 1H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.6, 151.8, 147.9, 142.8, 138.4, 138.2, 136.5, 136.0, 136.0, 134.5, 131.4, 131.0, 130.6, 130.0, 129.8, 129.1, 127.7, 127.2, 123.0, 122.8, 121.7, 121.4, 116.4, 21.1. HRMS (ESI) m/z [M + H]+ calcd for C29H23N4O: 443.1872, found 443.1858.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 159–160 °C; IR (DCM): 3283, 1658, 1513 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55 (d, 1H, J = 7.0 Hz), 8.45 (s, 1H), 7.90–7.87 (m, 4H), 7.77 (s, 1H), 7.67–7.57 (m, 4H), 7.54–7.48 (m, 3H), 7.47 (s, 2H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 154.5, 152.6, 152.0, 147.5, 142.3, 138.5, 136.4, 134.9, 132.0, 131.1, 131.0, 130.6, 130.1, 129.8, 129.7, 129.1, 123.3, 122.9, 116.0, 114.8, 21.1; HRMS (ESI): m/z [M + H]+ calcd for C26H20N5OS: 450.1389, found 450.1378.
hexane) 0.2; mp: 159–160 °C; IR (DCM): 3283, 1658, 1513 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55 (d, 1H, J = 7.0 Hz), 8.45 (s, 1H), 7.90–7.87 (m, 4H), 7.77 (s, 1H), 7.67–7.57 (m, 4H), 7.54–7.48 (m, 3H), 7.47 (s, 2H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 154.5, 152.6, 152.0, 147.5, 142.3, 138.5, 136.4, 134.9, 132.0, 131.1, 131.0, 130.6, 130.1, 129.8, 129.7, 129.1, 123.3, 122.9, 116.0, 114.8, 21.1; HRMS (ESI): m/z [M + H]+ calcd for C26H20N5OS: 450.1389, found 450.1378.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 122–124 °C; IR (DCM): 3331, 1675, 1508 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.49 (d, 1H, J = 8.2 Hz), 8.37 (s, 1H), 7.97–7.91 (m, 4H), 7.70–7.66 (m, 3H), 7.55–7.49 (m, 3H), 7.46–7.38 (m, 2H), 7.35 (d, 1H, J = 7.8 Hz), 7.31–7.28 (m, 1H), 7.04 (t, 1H, J = 7.6 Hz), 2.50 (3H, s), 2.03 (3H, s); 13C NMR (∼101 MHz, CDCl3): δC 168.0, 152.6, 151.8, 142.5, 138.4, 138.3, 136.0, 135.7, 133.1, 131.5, 131.1, 130.6, 129.8, 129.7, 129.1, 128.9, 125.4, 124.4, 123.3, 123.0, 120.1, 21.1, 19.2; HRMS (ESI) m/z [M + Na]+ calcd for C27H23N3NaOS: 460.1460, found 460.1472.
hexane) 0.2; mp: 122–124 °C; IR (DCM): 3331, 1675, 1508 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.49 (d, 1H, J = 8.2 Hz), 8.37 (s, 1H), 7.97–7.91 (m, 4H), 7.70–7.66 (m, 3H), 7.55–7.49 (m, 3H), 7.46–7.38 (m, 2H), 7.35 (d, 1H, J = 7.8 Hz), 7.31–7.28 (m, 1H), 7.04 (t, 1H, J = 7.6 Hz), 2.50 (3H, s), 2.03 (3H, s); 13C NMR (∼101 MHz, CDCl3): δC 168.0, 152.6, 151.8, 142.5, 138.4, 138.3, 136.0, 135.7, 133.1, 131.5, 131.1, 130.6, 129.8, 129.7, 129.1, 128.9, 125.4, 124.4, 123.3, 123.0, 120.1, 21.1, 19.2; HRMS (ESI) m/z [M + Na]+ calcd for C27H23N3NaOS: 460.1460, found 460.1472.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 154–155 °C; IR (DCM): 3335, 1679, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.86 (s, 1H), 8.79 (dd, 1H, J1 = 6.9, J2 = 2.0 Hz), 8.67 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.83 (d, 4H, J = 8.4 Hz), 7.70 (d, 2H, J = 8.5 Hz), 7.55–7.42 (m, 8H), 7.38 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 165.0, 152.6, 151.9, 148.2, 141.8, 141.0, 138.4, 136.3, 136.1, 134.0, 132.0, 131.1, 130.4, 129.5, 129.2, 129.1, 128.6, 127.9, 127.3, 123.0, 122.8, 122.2, 121.6, 117.0; HRMS (ESI) m/z [M + H]+ calcd for C28H20ClN4O: 463.1326, found 463.1344.
hexane) 0.2; mp: 154–155 °C; IR (DCM): 3335, 1679, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.86 (s, 1H), 8.79 (dd, 1H, J1 = 6.9, J2 = 2.0 Hz), 8.67 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.83 (d, 4H, J = 8.4 Hz), 7.70 (d, 2H, J = 8.5 Hz), 7.55–7.42 (m, 8H), 7.38 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 165.0, 152.6, 151.9, 148.2, 141.8, 141.0, 138.4, 136.3, 136.1, 134.0, 132.0, 131.1, 130.4, 129.5, 129.2, 129.1, 128.6, 127.9, 127.3, 123.0, 122.8, 122.2, 121.6, 117.0; HRMS (ESI) m/z [M + H]+ calcd for C28H20ClN4O: 463.1326, found 463.1344.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 136–137 °C; IR (DCM): 3352, 1674, 1521 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.76 (s, 1H), 8.85 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.64 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.06 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85–7.83 (m, 4H), 7.76–7.74 (m, 2H), 7.53–7.44 (m, 6H), 7.38 (t, 2H, J = 8.2 Hz), 7.34 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 2.60 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 168.1, 152.6, 151.6, 148.1, 143.3, 138.7, 138.4, 136.8, 136.2, 136.1, 134.3, 131.0, 130.1, 129.5, 129.4, 129.0, 127.8, 127.6, 127.2, 122.9, 122.8, 122.0, 121.5, 116.6, 19.9; HRMS (ESI) m/z [M + H]+ calcd for C29H23N4O: 443.1872, found 443.1897.
hexane) 0.2; mp: 136–137 °C; IR (DCM): 3352, 1674, 1521 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.76 (s, 1H), 8.85 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.64 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.06 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85–7.83 (m, 4H), 7.76–7.74 (m, 2H), 7.53–7.44 (m, 6H), 7.38 (t, 2H, J = 8.2 Hz), 7.34 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 2.60 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 168.1, 152.6, 151.6, 148.1, 143.3, 138.7, 138.4, 136.8, 136.2, 136.1, 134.3, 131.0, 130.1, 129.5, 129.4, 129.0, 127.8, 127.6, 127.2, 122.9, 122.8, 122.0, 121.5, 116.6, 19.9; HRMS (ESI) m/z [M + H]+ calcd for C29H23N4O: 443.1872, found 443.1897.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 154–156 °C; IR (DCM): 3350, 1679, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.14 (s, 1H), 8.74 (dd, 1H, J1 = 6.6, J2 = 2.4 Hz), 8.67 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.08 (dd, 1H, J1 = 8.2, J2 = 1.5 Hz), 7.87–7.82 (m, 4H), 7.73 (d, 2H, J = 8.4 Hz), 7.50–7.42 (m, 6H), 7.37 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.33–7.27 (m, 3H), 7.13 (d, 2H, J = 8.1 Hz), 7.09 (t, 1H, J = 7.4 Hz), 6.99 (d, 1H, J = 8.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 164.8, 156.7, 155.0, 152.7, 151.8, 148.1, 142.6, 141.4, 138.4, 136.2, 134.4, 131.0, 130.5, 129.8, 129.4, 129.1, 128.6, 127.8, 127.3, 125.0, 124.0, 123.0, 122.8, 121.8, 121.5, 119.7, 117.6, 116.8; HRMS (ESI) m/z [M + H]+ calcd for C34H25N4O2: 521.1978, found 521.1992.
hexane) 0.2; mp: 154–156 °C; IR (DCM): 3350, 1679, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.14 (s, 1H), 8.74 (dd, 1H, J1 = 6.6, J2 = 2.4 Hz), 8.67 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.08 (dd, 1H, J1 = 8.2, J2 = 1.5 Hz), 7.87–7.82 (m, 4H), 7.73 (d, 2H, J = 8.4 Hz), 7.50–7.42 (m, 6H), 7.37 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.33–7.27 (m, 3H), 7.13 (d, 2H, J = 8.1 Hz), 7.09 (t, 1H, J = 7.4 Hz), 6.99 (d, 1H, J = 8.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 164.8, 156.7, 155.0, 152.7, 151.8, 148.1, 142.6, 141.4, 138.4, 136.2, 134.4, 131.0, 130.5, 129.8, 129.4, 129.1, 128.6, 127.8, 127.3, 125.0, 124.0, 123.0, 122.8, 121.8, 121.5, 119.7, 117.6, 116.8; HRMS (ESI) m/z [M + H]+ calcd for C34H25N4O2: 521.1978, found 521.1992.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 10
hexane = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) as an orange solid (31 mg, 29%, 0.2 mmol scale); Rf (10% EtOAc
90) as an orange solid (31 mg, 29%, 0.2 mmol scale); Rf (10% EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 144–146 °C; IR (DCM): 3328, 1678, 1576 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.14 (s, 1H), 8.83 (dd, 1H, J1 = 7.1, J2 = 1.8 Hz), 8.71 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.14 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.89–7.86 (m, 4H), 7.73 (d, 2H, J2 = 8.5 Hz), 7.54–7.39 (m, 9H), 7.20–7.18 (m, 3H), 7.15 (d, 1H, J = 7.6 Hz), 7.08 (d, 1H, J = 8.2 Hz), 5.26 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 165.6, 155.7, 152.7, 151.7, 148.0, 143.0, 141.1, 138.4, 136.7, 136.2, 134.6, 130.9, 130.5, 129.4, 129.0, 128.4, 127.9, 127.7, 127.4, 127.0, 126.9, 123.0, 122.9, 122.8, 121.7, 121.5, 116.8, 112.3, 70.5; HRMS (ESI) m/z [M + H]+ calcd for C35H27N4O2: 535.2134, found 535.2140.
hexane) 0.2; mp: 144–146 °C; IR (DCM): 3328, 1678, 1576 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.14 (s, 1H), 8.83 (dd, 1H, J1 = 7.1, J2 = 1.8 Hz), 8.71 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.14 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.89–7.86 (m, 4H), 7.73 (d, 2H, J2 = 8.5 Hz), 7.54–7.39 (m, 9H), 7.20–7.18 (m, 3H), 7.15 (d, 1H, J = 7.6 Hz), 7.08 (d, 1H, J = 8.2 Hz), 5.26 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 165.6, 155.7, 152.7, 151.7, 148.0, 143.0, 141.1, 138.4, 136.7, 136.2, 134.6, 130.9, 130.5, 129.4, 129.0, 128.4, 127.9, 127.7, 127.4, 127.0, 126.9, 123.0, 122.9, 122.8, 121.7, 121.5, 116.8, 112.3, 70.5; HRMS (ESI) m/z [M + H]+ calcd for C35H27N4O2: 535.2134, found 535.2140.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; IR (DCM): 3347, 1677, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.15 (s, 1H), 8.81 (dd, 1H, J1 = 7.1, J2 = 1.8 Hz), 8.74 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.14 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.89–7.86 (m, 4H), 7.72–7.69 (m, 2H), 7.53–7.46 (m, 6H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.12 (d, 1H, J = 7.7 Hz), 7.07 (d, 1H, J = 8.1 Hz), 4.21 (q, 2H, J = 7.0 Hz), 1.39 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 165.6, 156.2, 152.7, 151.6, 148.0, 143.3, 141.3, 138.5, 136.2, 134.5, 130.9, 130.5, 129.4, 129.0, 127.9, 127.4, 126.5, 122.9, 122.8, 122.5, 121.5, 121.5, 116.7, 111.7, 64.6, 14.7; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1987.
hexane) 0.2; IR (DCM): 3347, 1677, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.15 (s, 1H), 8.81 (dd, 1H, J1 = 7.1, J2 = 1.8 Hz), 8.74 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.14 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.89–7.86 (m, 4H), 7.72–7.69 (m, 2H), 7.53–7.46 (m, 6H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.12 (d, 1H, J = 7.7 Hz), 7.07 (d, 1H, J = 8.1 Hz), 4.21 (q, 2H, J = 7.0 Hz), 1.39 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 165.6, 156.2, 152.7, 151.6, 148.0, 143.3, 141.3, 138.5, 136.2, 134.5, 130.9, 130.5, 129.4, 129.0, 127.9, 127.4, 126.5, 122.9, 122.8, 122.5, 121.5, 121.5, 116.7, 111.7, 64.6, 14.7; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1987.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 228–230 °C; IR (DCM): 3354, 1680, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.70 (s, 1H), 8.60 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.55 (dd, 1H, J1 = 5.6, J2 = 3.4 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.86–7.83 (m, 4H), 7.74–7.72 (m, 2H), 7.65–7.46 (m, 9H), 7.44–7.42 (m, 2H), 7.34 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 7.30–7.26 (m, 1H), 7.21–7.16 (m, 1H); 13C NMR (∼101 MHz, CDCl3): δC 167.3, 152.7, 151.7, 148.0, 143.3, 140.7, 140.2, 139.7, 138.3, 136.1, 136.0, 134.2, 131.0, 130.0, 129.6, 129.5, 129.4, 129.1, 128.7, 128.3, 127.7, 127.5, 127.2, 122.9, 122.8, 121.6, 121.4, 116.5; HRMS (ESI) m/z [M + H]+ calcd for C34H25N4O: 505.2028, found 505.2035.
hexane) 0.2; mp: 228–230 °C; IR (DCM): 3354, 1680, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.70 (s, 1H), 8.60 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.55 (dd, 1H, J1 = 5.6, J2 = 3.4 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.86–7.83 (m, 4H), 7.74–7.72 (m, 2H), 7.65–7.46 (m, 9H), 7.44–7.42 (m, 2H), 7.34 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 7.30–7.26 (m, 1H), 7.21–7.16 (m, 1H); 13C NMR (∼101 MHz, CDCl3): δC 167.3, 152.7, 151.7, 148.0, 143.3, 140.7, 140.2, 139.7, 138.3, 136.1, 136.0, 134.2, 131.0, 130.0, 129.6, 129.5, 129.4, 129.1, 128.7, 128.3, 127.7, 127.5, 127.2, 122.9, 122.8, 121.6, 121.4, 116.5; HRMS (ESI) m/z [M + H]+ calcd for C34H25N4O: 505.2028, found 505.2035.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 220–222 °C; IR (DCM): 3340, 1680, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.70 (s, 1H), 8.60 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.57 (dd, 1H, J1 = 6.4, J2 = 2.6 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.7 Hz), 7.86–7.83 (m, 4H), 7.74–7.72 (m, 2H), 7.63–7.59 (m, 1H), 7.53–7.41 (m, 9H), 7.34 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.09 (d, 2H, J = 7.9 Hz), 2.24 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.5, 152.6, 151.6, 148.0, 143.3, 140.7, 139.6, 138.3, 137.3, 137.2, 136.1, 136.0, 134.2, 131.0, 130.0, 129.5, 129.4, 129.1, 129.0, 128.5, 127.7, 127.2, 122.8, 122.8, 121.6, 121.4, 116.6, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C35H27N4O: 519.2185, found 519.2194.
hexane) 0.2; mp: 220–222 °C; IR (DCM): 3340, 1680, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.70 (s, 1H), 8.60 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.57 (dd, 1H, J1 = 6.4, J2 = 2.6 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.7 Hz), 7.86–7.83 (m, 4H), 7.74–7.72 (m, 2H), 7.63–7.59 (m, 1H), 7.53–7.41 (m, 9H), 7.34 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.09 (d, 2H, J = 7.9 Hz), 2.24 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.5, 152.6, 151.6, 148.0, 143.3, 140.7, 139.6, 138.3, 137.3, 137.2, 136.1, 136.0, 134.2, 131.0, 130.0, 129.5, 129.4, 129.1, 129.0, 128.5, 127.7, 127.2, 122.8, 122.8, 121.6, 121.4, 116.6, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C35H27N4O: 519.2185, found 519.2194.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 188–190 °C; IR (DCM): 3302, 1674, 1531 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.95 (s, 1H), 8.86 (dd, 1H, J1 = 7.4, J2 = 1.0 Hz), 8.53 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.21 (d, 1H, J = 1.8 Hz), 8.06 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz) 7.91–7.85 (m, 5H), 7.75 (d, 4H, J = 7.9 Hz), 7.66 (d, 1H, J = 8.0 Hz), 7.58–7.41 (m, 8H), 7.31 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.6, 152.7, 152.0, 148.0, 142.5, 141.1, 139.7, 138.4, 138.2, 136.6, 136.1, 134.4, 131.2, 131.0, 129.8, 129.2, 129.1, 129.0, 128.0, 128.0, 127.8, 127.3, 127.2, 123.1, 122.8, 121.8, 121.5, 116.5; HRMS (ESI) m/z [M + H]+ calcd for C34H25N4O: 505.2028, found 505.2041.
hexane) 0.2; mp: 188–190 °C; IR (DCM): 3302, 1674, 1531 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.95 (s, 1H), 8.86 (dd, 1H, J1 = 7.4, J2 = 1.0 Hz), 8.53 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.21 (d, 1H, J = 1.8 Hz), 8.06 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz) 7.91–7.85 (m, 5H), 7.75 (d, 4H, J = 7.9 Hz), 7.66 (d, 1H, J = 8.0 Hz), 7.58–7.41 (m, 8H), 7.31 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.6, 152.7, 152.0, 148.0, 142.5, 141.1, 139.7, 138.4, 138.2, 136.6, 136.1, 134.4, 131.2, 131.0, 129.8, 129.2, 129.1, 129.0, 128.0, 128.0, 127.8, 127.3, 127.2, 123.1, 122.8, 121.8, 121.5, 116.5; HRMS (ESI) m/z [M + H]+ calcd for C34H25N4O: 505.2028, found 505.2041.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 190–192 °C; IR (DCM): 3347, 1679, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.85 (dd, 1H, J1 = 7.4, J2 = 1.5 Hz), 8.67 (dd, 1H, J1 = 4.3, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85–7.80 (m, 4H), 7.69–7.67 (m, 2H), 7.55–7.44 (m, 5H), 7.38 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.09 (d, 1H, J = 8.4 Hz), 7.05 (d, 1H, J = 8.4 Hz), 4.39 (s, 4H); 13C NMR (∼101 MHz, CDCl3): δC 165.1, 152.6, 151.4, 147.8, 143.5, 142.7, 141.3, 138.0, 136.7, 134.2, 132.5, 130.9, 129.4, 129.0, 127.9, 127.5, 126.2, 122.9, 122.9, 122.7, 121.9, 121.4, 118.4, 117.2, 64.7, 64.3; HRMS (ESI) m/z [M + H]+ calcd for C30H23N4O3: 487.1770, found 487.1786.
hexane) 0.2; mp: 190–192 °C; IR (DCM): 3347, 1679, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.85 (dd, 1H, J1 = 7.4, J2 = 1.5 Hz), 8.67 (dd, 1H, J1 = 4.3, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85–7.80 (m, 4H), 7.69–7.67 (m, 2H), 7.55–7.44 (m, 5H), 7.38 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.09 (d, 1H, J = 8.4 Hz), 7.05 (d, 1H, J = 8.4 Hz), 4.39 (s, 4H); 13C NMR (∼101 MHz, CDCl3): δC 165.1, 152.6, 151.4, 147.8, 143.5, 142.7, 141.3, 138.0, 136.7, 134.2, 132.5, 130.9, 129.4, 129.0, 127.9, 127.5, 126.2, 122.9, 122.9, 122.7, 121.9, 121.4, 118.4, 117.2, 64.7, 64.3; HRMS (ESI) m/z [M + H]+ calcd for C30H23N4O3: 487.1770, found 487.1786.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 170–172 °C; IR (DCM): 3338, 1672, 1530 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.86 (s, 1H), 8.88 (dd, 1H, J1 = 6.6, J2 = 2.4 Hz), 8.84 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.18 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 8.03 (d, 2H, J1 = 6.9, J2 = 1.8 Hz), 7.97–7.94 (m, 4H), 7.69 (d, 1H, J = 1.7 Hz), 7.57–7.53 (m, 4H), 7.51–7.49 (m, 1H), 7.47 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 6.78 (d, 1H, J = 1.8 Hz); 13C NMR (∼101 MHz, CDCl3): δC 156.8, 152.8, 152.2, 148.3, 143.5, 142.3, 138.7, 136.3, 134.6, 134.4, 131.5, 131.0, 130.4, 129.1, 128.0, 127.4, 122.9, 122.8, 121.8, 121.7, 116.8, 114.8; HRMS (ESI) m/z [M + H]+ calcd for C26H19N4O2: 419.1508, found 419.1513.
hexane) 0.2; mp: 170–172 °C; IR (DCM): 3338, 1672, 1530 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.86 (s, 1H), 8.88 (dd, 1H, J1 = 6.6, J2 = 2.4 Hz), 8.84 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.18 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 8.03 (d, 2H, J1 = 6.9, J2 = 1.8 Hz), 7.97–7.94 (m, 4H), 7.69 (d, 1H, J = 1.7 Hz), 7.57–7.53 (m, 4H), 7.51–7.49 (m, 1H), 7.47 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 6.78 (d, 1H, J = 1.8 Hz); 13C NMR (∼101 MHz, CDCl3): δC 156.8, 152.8, 152.2, 148.3, 143.5, 142.3, 138.7, 136.3, 134.6, 134.4, 131.5, 131.0, 130.4, 129.1, 128.0, 127.4, 122.9, 122.8, 121.8, 121.7, 116.8, 114.8; HRMS (ESI) m/z [M + H]+ calcd for C26H19N4O2: 419.1508, found 419.1513.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 145–146 °C; IR (DCM): 3308, 1649, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.11 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 1.1 Hz), 8.18 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.02–7.98 (m, 3H), 7.96–7.93 (m, 2H), 7.72 (dd, 2H, J1 = 8.4, J2 = 1.8 Hz), 7.59 (d, 1H, J = 5.0 Hz), 7.56–7.48 (m, 4H), 7.43 (dd, 1H, J1 = 8.2, J2 = 1.1 Hz), 7.19 (d, 1H, J = 5.1 Hz), 7.17 (t, 1H, J = 2.3 Hz); 13C NMR (∼101 MHz, CDCl3): δC 160.4, 152.7, 152.7, 147.7, 142.1, 138.4, 137.9, 136.3, 135.9, 134.4, 131.3, 131.1, 130.5, 129.6, 129.2, 127.7, 127.3, 123.6, 123.0, 121.6, 121.4, 116.4; HRMS (ESI) m/z [M + H]+ calcd for C26H19N4OS: 435.1280, found 435.1266.
hexane) 0.2; mp: 145–146 °C; IR (DCM): 3308, 1649, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.11 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 1.1 Hz), 8.18 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.02–7.98 (m, 3H), 7.96–7.93 (m, 2H), 7.72 (dd, 2H, J1 = 8.4, J2 = 1.8 Hz), 7.59 (d, 1H, J = 5.0 Hz), 7.56–7.48 (m, 4H), 7.43 (dd, 1H, J1 = 8.2, J2 = 1.1 Hz), 7.19 (d, 1H, J = 5.1 Hz), 7.17 (t, 1H, J = 2.3 Hz); 13C NMR (∼101 MHz, CDCl3): δC 160.4, 152.7, 152.7, 147.7, 142.1, 138.4, 137.9, 136.3, 135.9, 134.4, 131.3, 131.1, 130.5, 129.6, 129.2, 127.7, 127.3, 123.6, 123.0, 121.6, 121.4, 116.4; HRMS (ESI) m/z [M + H]+ calcd for C26H19N4OS: 435.1280, found 435.1266.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 144–146 °C; IR (DCM): 3316, 1657, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.96 (s, 1H), 8.88 (dd, 1H, J1 = 7.7, J2 = 1.0 Hz), 8.18 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 7.98 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.95–7.87 (m, 4H), 7.67 (d, 2H, J = 8.4 Hz), 7.58–7.47 (m, 4H), 7.43 (dd, 1H, J1 = 8.2, J2 = 1.0 Hz), 7.15 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 6.86 (d, 1H, J = 2.6 Hz), 6.33 (d, 1H, J = 2.6 Hz), 4.10 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 160.3, 152.8, 151.8, 147.6, 138.7, 138.3, 135.7, 134.9, 130.9, 130.5, 129.1, 128.6, 127.8, 127.3, 127.2, 123.8, 123.2, 122.8, 121.3, 121.2, 115.7, 109.4, 37.2; HRMS (ESI) m/z [M + H]+ calcd for C27H22N5O: 432.1824, found 432.1840.
hexane) 0.2; mp: 144–146 °C; IR (DCM): 3316, 1657, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.96 (s, 1H), 8.88 (dd, 1H, J1 = 7.7, J2 = 1.0 Hz), 8.18 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 7.98 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.95–7.87 (m, 4H), 7.67 (d, 2H, J = 8.4 Hz), 7.58–7.47 (m, 4H), 7.43 (dd, 1H, J1 = 8.2, J2 = 1.0 Hz), 7.15 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 6.86 (d, 1H, J = 2.6 Hz), 6.33 (d, 1H, J = 2.6 Hz), 4.10 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 160.3, 152.8, 151.8, 147.6, 138.7, 138.3, 135.7, 134.9, 130.9, 130.5, 129.1, 128.6, 127.8, 127.3, 127.2, 123.8, 123.2, 122.8, 121.3, 121.2, 115.7, 109.4, 37.2; HRMS (ESI) m/z [M + H]+ calcd for C27H22N5O: 432.1824, found 432.1840.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 173–175 °C; IR (DCM): 3301, 1651, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.24 (s, 1H), 8.89 (d, 1H, J = 7.6 Hz), 8.24 (d, 1H, J = 3.9 Hz), 8.17 (d, 2H, J = 8.2 Hz), 8.03–7.98 (m, 4H), 7.79 (d, 2H, J = 8.2 Hz), 7.63–7.50 (m, 6H), 7.47–7.40 (m, 2H), 7.21 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 160.7, 153.1, 152.7, 147.7, 140.7, 140.2, 138.4, 137.2, 137.0, 136.3, 135.9, 134.3, 131.4, 131.4, 129.3, 127.7, 127.3, 126.7, 125.0, 124.7, 124.0, 123.0, 122.6, 121.8, 121.4, 116.6; HRMS (ESI) m/z [M + H]+ calcd for C30H21N4OS: 485.1436, found 485.1446.
hexane) 0.2; mp: 173–175 °C; IR (DCM): 3301, 1651, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.24 (s, 1H), 8.89 (d, 1H, J = 7.6 Hz), 8.24 (d, 1H, J = 3.9 Hz), 8.17 (d, 2H, J = 8.2 Hz), 8.03–7.98 (m, 4H), 7.79 (d, 2H, J = 8.2 Hz), 7.63–7.50 (m, 6H), 7.47–7.40 (m, 2H), 7.21 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 160.7, 153.1, 152.7, 147.7, 140.7, 140.2, 138.4, 137.2, 137.0, 136.3, 135.9, 134.3, 131.4, 131.4, 129.3, 127.7, 127.3, 126.7, 125.0, 124.7, 124.0, 123.0, 122.6, 121.8, 121.4, 116.6; HRMS (ESI) m/z [M + H]+ calcd for C30H21N4OS: 485.1436, found 485.1446.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 190–192 °C; IR (DCM): 3338, 1673, 1521 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 9.97 (s, 1H), 8.72 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.27–8.25 (m, 2H), 7.84–7.76 (m, 12H), 7.60–7.55 (m, 7H), 7.50–7.44 (m, 2H), 7.17 (s, 2H), 3.98 (s, 3H); 13C NMR (∼101 MHz, DMSO-d6): δC 166.6, 159.5, 151.9, 151.0, 148.8, 143.1, 140.8, 138.1, 136.3, 133.8, 131.6, 129.7, 129.4, 128.8, 127.5, 126.7, 122.5, 122.4, 122.3, 122.0, 116.8, 115.0, 55.7; HRMS (ESI) m/z [M + H]+ calcd for C41H31N6O2: 639.2508, found 639.2532.
hexane) 0.2; mp: 190–192 °C; IR (DCM): 3338, 1673, 1521 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 9.97 (s, 1H), 8.72 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.27–8.25 (m, 2H), 7.84–7.76 (m, 12H), 7.60–7.55 (m, 7H), 7.50–7.44 (m, 2H), 7.17 (s, 2H), 3.98 (s, 3H); 13C NMR (∼101 MHz, DMSO-d6): δC 166.6, 159.5, 151.9, 151.0, 148.8, 143.1, 140.8, 138.1, 136.3, 133.8, 131.6, 129.7, 129.4, 128.8, 127.5, 126.7, 122.5, 122.4, 122.3, 122.0, 116.8, 115.0, 55.7; HRMS (ESI) m/z [M + H]+ calcd for C41H31N6O2: 639.2508, found 639.2532.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 196–198 °C; IR (DCM): 3349, 1675, 1522 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.74 (s, 1H), 8.59–8.56 (m, 2H), 8.02 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.87–7.84 (m, 8H), 7.75–7.72 (m, 4H), 7.53–7.45 (m, 6H), 7.44–7.40 (m, 2H), 7.39 (s, 2H), 7.31 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 2.57 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.3, 152.6, 151.7, 148.0, 143.2, 139.9, 139.6, 138.3, 136.0, 134.1, 133.6, 130.9, 130.5, 129.5, 129.1, 127.7, 127.2, 122.9, 122.8, 121.7, 121.4, 116.6, 21.4; HRMS (ESI) m/z [M + H]+ calcd for C41H31N6O: 623.2559, found 623.2585.
hexane) 0.2; mp: 196–198 °C; IR (DCM): 3349, 1675, 1522 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.74 (s, 1H), 8.59–8.56 (m, 2H), 8.02 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.87–7.84 (m, 8H), 7.75–7.72 (m, 4H), 7.53–7.45 (m, 6H), 7.44–7.40 (m, 2H), 7.39 (s, 2H), 7.31 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 2.57 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.3, 152.6, 151.7, 148.0, 143.2, 139.9, 139.6, 138.3, 136.0, 134.1, 133.6, 130.9, 130.5, 129.5, 129.1, 127.7, 127.2, 122.9, 122.8, 121.7, 121.4, 116.6, 21.4; HRMS (ESI) m/z [M + H]+ calcd for C41H31N6O: 623.2559, found 623.2585.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 210–212 °C; IR (DCM): 3277, 1676, 1521 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.61–8.58 (m, 2H), 8.03 (dd, 1H, J1 = 8.4, J2 = 1.6 Hz), 7.89–7.85 (m, 8H), 7.81–7.72 (m, 8H), 7.56–7.43 (m, 11H), 7.32 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.1, 152.6, 151.8, 148.0, 143.1, 142.6, 140.5, 139.8, 138.3, 136.1, 134.9, 134.1, 131.0, 129.6, 129.1, 129.1, 128.5, 128.2, 127.7, 127.4, 127.2, 123.0, 122.8, 121.9, 121.5, 116.7; HRMS (ESI) m/z [M + H]+ calcd for C46H33N6O: 685.2716, found 685.2720.
hexane) 0.2; mp: 210–212 °C; IR (DCM): 3277, 1676, 1521 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.61–8.58 (m, 2H), 8.03 (dd, 1H, J1 = 8.4, J2 = 1.6 Hz), 7.89–7.85 (m, 8H), 7.81–7.72 (m, 8H), 7.56–7.43 (m, 11H), 7.32 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.1, 152.6, 151.8, 148.0, 143.1, 142.6, 140.5, 139.8, 138.3, 136.1, 134.9, 134.1, 131.0, 129.6, 129.1, 129.1, 128.5, 128.2, 127.7, 127.4, 127.2, 123.0, 122.8, 121.9, 121.5, 116.7; HRMS (ESI) m/z [M + H]+ calcd for C46H33N6O: 685.2716, found 685.2720.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 198–200 °C; IR (DCM): 3339, 1675, 1521 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.79 (s, 1H), 8.60–8.57 (m, 2H), 8.04 (d, 1H, J = 8.3 Hz), 7.86–7.84 (m, 8H), 7.75–7.73 (m, 4H), 7.53–7.39 (m, 8H), 7.32 (dd, 1H, J1 = 8.2, J2 = 4.3 Hz), 7.08 (s, 2H), 4.22 (q, 2H, J = 7.0 Hz), 1.53 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.1, 159.1, 152.5, 151.7, 147.8, 143.1, 141.6, 138.2, 136.0, 136.0, 134.1, 130.9, 129.4, 129.0, 127.6, 127.1, 122.8, 122.7, 121.6, 121.3, 116.5, 115.6, 63.8, 14.8; HRMS (ESI) m/z [M + H]+ calcd for C42H33N6O2: 653.2665, found 653.2669.
hexane) 0.2; mp: 198–200 °C; IR (DCM): 3339, 1675, 1521 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.79 (s, 1H), 8.60–8.57 (m, 2H), 8.04 (d, 1H, J = 8.3 Hz), 7.86–7.84 (m, 8H), 7.75–7.73 (m, 4H), 7.53–7.39 (m, 8H), 7.32 (dd, 1H, J1 = 8.2, J2 = 4.3 Hz), 7.08 (s, 2H), 4.22 (q, 2H, J = 7.0 Hz), 1.53 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.1, 159.1, 152.5, 151.7, 147.8, 143.1, 141.6, 138.2, 136.0, 136.0, 134.1, 130.9, 129.4, 129.0, 127.6, 127.1, 122.8, 122.7, 121.6, 121.3, 116.5, 115.6, 63.8, 14.8; HRMS (ESI) m/z [M + H]+ calcd for C42H33N6O2: 653.2665, found 653.2669.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 226–228 °C; IR (DCM): 3344, 1677, 1522 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.76 (s, 1H), 8.60–8.56 (m, 2H), 8.03 (d, 1H, J = 8.2 Hz), 7.86 (d, 8H, J = 8.1 Hz), 7.75 (d, 4H, J = 8.3 Hz), 7.69–7.65 (m, 1H), 7.59 (d, 2H, J = 7.6 Hz), 7.52–7.41 (m, 8H), 7.32 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.1, 152.6, 151.7, 148.0, 143.0, 139.9, 138.3, 136.1, 134.0, 131.0, 129.8, 129.6, 129.1, 129.0, 127.7, 127.2, 123.0, 122.9, 122.8, 121.9, 121.4, 116.7; HRMS (ESI) m/z [M + H]+ calcd for C40H29N6O: 609.2403, found 609.2409.
hexane) 0.2; mp: 226–228 °C; IR (DCM): 3344, 1677, 1522 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.76 (s, 1H), 8.60–8.56 (m, 2H), 8.03 (d, 1H, J = 8.2 Hz), 7.86 (d, 8H, J = 8.1 Hz), 7.75 (d, 4H, J = 8.3 Hz), 7.69–7.65 (m, 1H), 7.59 (d, 2H, J = 7.6 Hz), 7.52–7.41 (m, 8H), 7.32 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.1, 152.6, 151.7, 148.0, 143.0, 139.9, 138.3, 136.1, 134.0, 131.0, 129.8, 129.6, 129.1, 129.0, 127.7, 127.2, 123.0, 122.9, 122.8, 121.9, 121.4, 116.7; HRMS (ESI) m/z [M + H]+ calcd for C40H29N6O: 609.2403, found 609.2409.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; IR (DCM): 3294, 1735, 1467 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.92 (s, 1H), 8.62–8.59 (m, 2H), 8.09–8.07 (m, 1H), 7.89–7.85 (m, 7H), 7.82–7.79 (m, 5H), 7.68 (d, 2H, J = 8.1 Hz), 7.53–7.45 (m, 8H), 7.35–7.32 (m, 3H), 7.28 (s, 2H), 2.71 (t, 2H, J = 7.8 Hz), 1.73–1.66 (m, 2H), 1.44–1.31 (m, 8H), 0.92 (t, 3H, J = 6.9 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.3, 152.6, 151.7, 143.2, 143.2, 142.5, 140.4, 137.1, 134.5, 131.0, 131.0, 129.7, 129.6, 129.6, 129.5, 129.1, 129.1, 128.3, 127.9, 127.5, 127.2, 127.2, 122.9, 122.8, 121.9, 121.3, 35.7, 31.8, 31.5, 29.3, 29.2, 22.7, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C53H47N6O: 783.3811, found 783.3813.
hexane) 0.2; IR (DCM): 3294, 1735, 1467 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.92 (s, 1H), 8.62–8.59 (m, 2H), 8.09–8.07 (m, 1H), 7.89–7.85 (m, 7H), 7.82–7.79 (m, 5H), 7.68 (d, 2H, J = 8.1 Hz), 7.53–7.45 (m, 8H), 7.35–7.32 (m, 3H), 7.28 (s, 2H), 2.71 (t, 2H, J = 7.8 Hz), 1.73–1.66 (m, 2H), 1.44–1.31 (m, 8H), 0.92 (t, 3H, J = 6.9 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.3, 152.6, 151.7, 143.2, 143.2, 142.5, 140.4, 137.1, 134.5, 131.0, 131.0, 129.7, 129.6, 129.6, 129.5, 129.1, 129.1, 128.3, 127.9, 127.5, 127.2, 127.2, 122.9, 122.8, 121.9, 121.3, 35.7, 31.8, 31.5, 29.3, 29.2, 22.7, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C53H47N6O: 783.3811, found 783.3813.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 172–174 °C; IR (DCM): 3323, 1674, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.83 (s, 1H), 8.78 (dd, 1H, J1 = 7.4, J2 = 1.2 Hz), 8.53 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.95–7.89 (m, 4H), 7.55–7.42 (m, 7H), 7.39 (s, 5H), 7.30 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 6.69 (s, 1H); 13C NMR (∼101 MHz, CDCl3): δC 164.3, 152.7, 152.6, 151.5, 147.9, 141.3, 141.0, 138.3, 136.1, 134.5, 131.1, 130.7, 129.4, 129.1, 128.6, 128.4, 127.8, 127.3, 123.2, 123.1, 122.9, 121.5, 121.4, 116.5; HRMS (ESI) m/z [M + H]+ calcd for C30H23N4O: 455.1872, found 455.1858.
hexane) 0.2; mp: 172–174 °C; IR (DCM): 3323, 1674, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.83 (s, 1H), 8.78 (dd, 1H, J1 = 7.4, J2 = 1.2 Hz), 8.53 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.95–7.89 (m, 4H), 7.55–7.42 (m, 7H), 7.39 (s, 5H), 7.30 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 6.69 (s, 1H); 13C NMR (∼101 MHz, CDCl3): δC 164.3, 152.7, 152.6, 151.5, 147.9, 141.3, 141.0, 138.3, 136.1, 134.5, 131.1, 130.7, 129.4, 129.1, 128.6, 128.4, 127.8, 127.3, 123.2, 123.1, 122.9, 121.5, 121.4, 116.5; HRMS (ESI) m/z [M + H]+ calcd for C30H23N4O: 455.1872, found 455.1858.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 110–112 °C; IR (DCM): 3338, 1674, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.92 (s, 1H), 8.83 (dd, 1H, J1 = 7.4, J2 = 1.1 Hz), 8.58 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.88–7.83 (m, 4H), 7.75 (d, 2H, J = 8.5 Hz), 7.55–7.44 (m, 5H), 7.34 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.00 (d, 1H, J = 12.6 Hz), 6.34 (d, 1H, J = 12.5 Hz); 13C NMR (∼101 MHz, CDCl3): δC 164.6, 152.7, 152.4, 148.1, 138.3, 138.1, 137.6, 136.2, 134.3, 131.1, 130.4, 129.1, 127.9, 127.4, 125.8, 122.9, 121.8, 121.6, 116.7; HRMS (ESI) m/z [M + H]+ calcd for C24H19N4O: 379.1559, found 379.1541.
hexane) 0.2; mp: 110–112 °C; IR (DCM): 3338, 1674, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.92 (s, 1H), 8.83 (dd, 1H, J1 = 7.4, J2 = 1.1 Hz), 8.58 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.88–7.83 (m, 4H), 7.75 (d, 2H, J = 8.5 Hz), 7.55–7.44 (m, 5H), 7.34 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.00 (d, 1H, J = 12.6 Hz), 6.34 (d, 1H, J = 12.5 Hz); 13C NMR (∼101 MHz, CDCl3): δC 164.6, 152.7, 152.4, 148.1, 138.3, 138.1, 137.6, 136.2, 134.3, 131.1, 130.4, 129.1, 127.9, 127.4, 125.8, 122.9, 121.8, 121.6, 116.7; HRMS (ESI) m/z [M + H]+ calcd for C24H19N4O: 379.1559, found 379.1541.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; IR (DCM): 3334, 1669, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.80 (dd, 1H, J1 = 7.6, J2 = 0.8 Hz), 8.50 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.02 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.83 (d, 2H, J = 8.4 Hz), 7.74 (s, 1H), 7.64 (d, 2H, J = 8.4 Hz), 7.55 (dd, 1H, J1 = 8.0, J2 = 1.6 Hz), 7.51 (t, 1H, J = 8.1 Hz), 7.45–7.38 (m, 4H), 7.27 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 7.05 (d, 1H, J = 8.2 Hz), 6.99–6.95 (m, 1H), 3.98 (s, 3H), 2.49 (s, 3H); 13C NMR (∼126 MHz, CDCl3): δC 167.7, 156.9, 152.2, 147.9, 142.5, 142.3, 138.3, 138.1, 136.5, 135.9, 134.4, 132.3, 131.3, 130.5, 129.8, 129.7, 127.7, 127.2, 123.1, 121.6, 121.3, 120.7, 116.8, 116.3, 112.7, 56.3, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1965.
hexane) 0.2; IR (DCM): 3334, 1669, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.80 (dd, 1H, J1 = 7.6, J2 = 0.8 Hz), 8.50 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.02 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.83 (d, 2H, J = 8.4 Hz), 7.74 (s, 1H), 7.64 (d, 2H, J = 8.4 Hz), 7.55 (dd, 1H, J1 = 8.0, J2 = 1.6 Hz), 7.51 (t, 1H, J = 8.1 Hz), 7.45–7.38 (m, 4H), 7.27 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 7.05 (d, 1H, J = 8.2 Hz), 6.99–6.95 (m, 1H), 3.98 (s, 3H), 2.49 (s, 3H); 13C NMR (∼126 MHz, CDCl3): δC 167.7, 156.9, 152.2, 147.9, 142.5, 142.3, 138.3, 138.1, 136.5, 135.9, 134.4, 132.3, 131.3, 130.5, 129.8, 129.7, 127.7, 127.2, 123.1, 121.6, 121.3, 120.7, 116.8, 116.3, 112.7, 56.3, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1965.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 152–153 °C; IR (DCM): 3331, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.86 (s, 1H), 8.84 (dd, 1H, J1 = 7.6, J2 = 1.1 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85 (d, 2H, J = 8.4 Hz), 7.79 (s, 1H), 7.67 (d, 2H, J = 8.4 Hz), 7.56–7.52 (m, 2H), 7.48–7.42 (m, 3H), 7.38–7.29 (m, 3H), 7.27–7.23 (m, 1H), 2.68 (s, 3H), 2.50 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.2, 150.8, 147.9, 142.6, 138.4, 138.2, 138.0, 136.6, 136.0, 135.9, 134.5, 131.4, 131.2, 130.9, 130.5, 130.0, 129.8, 127.7, 127.3, 126.4, 123.1, 121.7, 121.4, 116.4, 115.3, 21.2, 17.5; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2015.
hexane) 0.2; mp: 152–153 °C; IR (DCM): 3331, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.86 (s, 1H), 8.84 (dd, 1H, J1 = 7.6, J2 = 1.1 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.05 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85 (d, 2H, J = 8.4 Hz), 7.79 (s, 1H), 7.67 (d, 2H, J = 8.4 Hz), 7.56–7.52 (m, 2H), 7.48–7.42 (m, 3H), 7.38–7.29 (m, 3H), 7.27–7.23 (m, 1H), 2.68 (s, 3H), 2.50 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.2, 150.8, 147.9, 142.6, 138.4, 138.2, 138.0, 136.6, 136.0, 135.9, 134.5, 131.4, 131.2, 130.9, 130.5, 130.0, 129.8, 127.7, 127.3, 126.4, 123.1, 121.7, 121.4, 116.4, 115.3, 21.2, 17.5; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2015.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 204–206 °C; IR (DCM): 3327, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.84 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 0.9 Hz), 8.51 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.04 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.84 (d, 2H, J = 8.4 Hz), 7.76 (d, 3H, J = 8.4 Hz), 7.67 (d, 2H, J = 8.4 Hz), 7.54 (t, 1H, J = 8.0 Hz), 7.48–7.42 (m, 3H), 7.31–7.27 (m, 3H), 2.52 (s, 3H), 2.44 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 151.9, 150.8, 147.9, 142.5, 141.5, 138.4, 138.1, 136.6, 136.0, 136.0, 134.5, 131.4, 130.5, 130.0, 129.8, 129.7, 127.7, 127.2, 122.9, 122.8, 121.6, 121.4, 116.4, 21.5, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2027.
hexane) 0.2; mp: 204–206 °C; IR (DCM): 3327, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.84 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 0.9 Hz), 8.51 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.04 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.84 (d, 2H, J = 8.4 Hz), 7.76 (d, 3H, J = 8.4 Hz), 7.67 (d, 2H, J = 8.4 Hz), 7.54 (t, 1H, J = 8.0 Hz), 7.48–7.42 (m, 3H), 7.31–7.27 (m, 3H), 2.52 (s, 3H), 2.44 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 151.9, 150.8, 147.9, 142.5, 141.5, 138.4, 138.1, 136.6, 136.0, 136.0, 134.5, 131.4, 130.5, 130.0, 129.8, 129.7, 127.7, 127.2, 122.9, 122.8, 121.6, 121.4, 116.4, 21.5, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2027.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 156–158 °C; IR (DCM): 3327, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.86 (s, 1H), 8.87 (d, 1H, J = 7.4 Hz), 8.51 (s, 1H), 8.04 (d, 1H, J = 8.2 Hz), 7.87 (d, 2H, J = 7.6 Hz), 7.81–7.79 (m, 3H), 7.69 (d, 2H, J = 8.4 Hz), 7.56–7.52 (m, 1H), 7.47–7.41 (m, 3H), 7.34 (d, 2H, J = 7.4 Hz), 7.28–7.26 (m, 1H), 2.76 (q, 2H, J = 7.3 Hz), 2.52 (s, 3H), 1.32 (t, 3H, J = 7.1 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.0, 151.0, 148.0, 147.8, 142.5, 138.4, 138.1, 136.6, 136.0, 135.9, 134.5, 131.4, 130.6, 130.0, 129.8, 128.5, 127.7, 127.2, 123.0, 122.9, 121.7, 121.4, 116.4, 28.8, 21.1, 15.4; HRMS (ESI) m/z [M + H]+ calcd for C31H27N4O: 471.2185, found 471.2170.
hexane) 0.2; mp: 156–158 °C; IR (DCM): 3327, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.86 (s, 1H), 8.87 (d, 1H, J = 7.4 Hz), 8.51 (s, 1H), 8.04 (d, 1H, J = 8.2 Hz), 7.87 (d, 2H, J = 7.6 Hz), 7.81–7.79 (m, 3H), 7.69 (d, 2H, J = 8.4 Hz), 7.56–7.52 (m, 1H), 7.47–7.41 (m, 3H), 7.34 (d, 2H, J = 7.4 Hz), 7.28–7.26 (m, 1H), 2.76 (q, 2H, J = 7.3 Hz), 2.52 (s, 3H), 1.32 (t, 3H, J = 7.1 Hz); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.0, 151.0, 148.0, 147.8, 142.5, 138.4, 138.1, 136.6, 136.0, 135.9, 134.5, 131.4, 130.6, 130.0, 129.8, 128.5, 127.7, 127.2, 123.0, 122.9, 121.7, 121.4, 116.4, 28.8, 21.1, 15.4; HRMS (ESI) m/z [M + H]+ calcd for C31H27N4O: 471.2185, found 471.2170.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 154–156 °C; IR (DCM): 3331, 1671, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.84 (s, 1H), 8.84 (d, 1H, J = 7.5 Hz), 8.51 (dd, 1H, J1 = 4.1, J2 = 1.4 Hz), 8.05 (dd, 1H, J1 = 8.2, J2 = 1.1 Hz), 7.84 (d, 2H, J = 8.3 Hz), 7.78 (s, 1H), 7.67 (d, 2H, J = 8.3 Hz), 7.56–7.51 (m, 1H), 7.47–7.41 (m, 5H), 7.30–7.27 (m, 1H), 7.12 (s, 1H), 2.52 (s, 3H), 2.41 (s, 6H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.9, 151.9, 147.9, 142.6, 138.7, 138.4, 138.1, 136.5, 136.0, 135.9, 134.5, 132.7, 131.4, 130.5, 130.0, 129.8, 127.7, 127.2, 123.0, 121.6, 121.4, 120.6, 116.4, 21.3, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C31H27N4O: 471.2185, found 471.2193.
hexane) 0.2; mp: 154–156 °C; IR (DCM): 3331, 1671, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.84 (s, 1H), 8.84 (d, 1H, J = 7.5 Hz), 8.51 (dd, 1H, J1 = 4.1, J2 = 1.4 Hz), 8.05 (dd, 1H, J1 = 8.2, J2 = 1.1 Hz), 7.84 (d, 2H, J = 8.3 Hz), 7.78 (s, 1H), 7.67 (d, 2H, J = 8.3 Hz), 7.56–7.51 (m, 1H), 7.47–7.41 (m, 5H), 7.30–7.27 (m, 1H), 7.12 (s, 1H), 2.52 (s, 3H), 2.41 (s, 6H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.9, 151.9, 147.9, 142.6, 138.7, 138.4, 138.1, 136.5, 136.0, 135.9, 134.5, 132.7, 131.4, 130.5, 130.0, 129.8, 127.7, 127.2, 123.0, 121.6, 121.4, 120.6, 116.4, 21.3, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C31H27N4O: 471.2185, found 471.2193.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 129–130 °C; IR (DCM): 3331, 1671, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.86 (d, 1H, J = 7.5 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.5 Hz), 8.04 (dd, 1H, J1 = 8.3, J2 = 1.4 Hz), 7.85 (d, 2H, J = 8.4 Hz), 7.78 (s, 1H), 7.68 (d, 2H, J = 8.4 Hz), 7.54–7.51 (m, 2H), 7.47–7.44 (m, 3H), 7.32–7.27 (m, 2H), 7.22–7.20 (m, 1H), 2.64 (s, 3H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.0, 149.0, 147.9, 143.0, 139.8, 138.4, 138.2, 136.6, 136.5, 136.0, 135.9, 134.5, 131.5, 131.0, 130.5, 129.9, 129.8, 127.8, 127.3, 126.7, 123.2, 121.7, 121.4, 116.7, 116.4, 21.2, 17.4; HRMS (ESI) m/z [M + H]+ calcd for C30H24ClN4O: 491.1639, found 491.1663.
hexane) 0.2; mp: 129–130 °C; IR (DCM): 3331, 1671, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.86 (d, 1H, J = 7.5 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.5 Hz), 8.04 (dd, 1H, J1 = 8.3, J2 = 1.4 Hz), 7.85 (d, 2H, J = 8.4 Hz), 7.78 (s, 1H), 7.68 (d, 2H, J = 8.4 Hz), 7.54–7.51 (m, 2H), 7.47–7.44 (m, 3H), 7.32–7.27 (m, 2H), 7.22–7.20 (m, 1H), 2.64 (s, 3H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.8, 152.0, 149.0, 147.9, 143.0, 139.8, 138.4, 138.2, 136.6, 136.5, 136.0, 135.9, 134.5, 131.5, 131.0, 130.5, 129.9, 129.8, 127.8, 127.3, 126.7, 123.2, 121.7, 121.4, 116.7, 116.4, 21.2, 17.4; HRMS (ESI) m/z [M + H]+ calcd for C30H24ClN4O: 491.1639, found 491.1663.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 168–170 °C; IR (DCM): 3330, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.87 (d, 1H, J = 7.6 Hz), 8.53 (dd, 1H, J1 = 4.2, J2 = 1.5 Hz), 8.03 (dd, 1H, J1 = 8.2, J2 = 1.2 Hz), 7.87–7.85 (m, 2H), 7.79 (s, 1H), 7.60 (d, 1H, J = 8.3 Hz), 7.56–7.40 (m, 9H), 7.28 (dd, 1H, J1 = 8.2, J2 = 4.5 Hz), 2.64 (s, 3H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.9, 153.0, 149.8, 147.9, 142.7, 138.5, 138.4, 138.0, 136.7, 136.0, 135.9, 134.6, 131.9, 131.4, 130.7, 130.4, 129.9, 129.0, 127.8, 127.3, 127.2, 122.9, 121.7, 121.4, 116.4, 115.6, 21.2, 17.5; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2043.
hexane) 0.2; mp: 168–170 °C; IR (DCM): 3330, 1670, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.87 (d, 1H, J = 7.6 Hz), 8.53 (dd, 1H, J1 = 4.2, J2 = 1.5 Hz), 8.03 (dd, 1H, J1 = 8.2, J2 = 1.2 Hz), 7.87–7.85 (m, 2H), 7.79 (s, 1H), 7.60 (d, 1H, J = 8.3 Hz), 7.56–7.40 (m, 9H), 7.28 (dd, 1H, J1 = 8.2, J2 = 4.5 Hz), 2.64 (s, 3H), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.9, 153.0, 149.8, 147.9, 142.7, 138.5, 138.4, 138.0, 136.7, 136.0, 135.9, 134.6, 131.9, 131.4, 130.7, 130.4, 129.9, 129.0, 127.8, 127.3, 127.2, 122.9, 121.7, 121.4, 116.4, 115.6, 21.2, 17.5; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2043.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 151–153 °C; IR (DCM): 3313, 1673, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.07 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.96 (d, 1H, J = 2.2 Hz), 7.85 (d, 2H, J = 8.5 Hz), 7.77 (s, 1H), 7.73 (dd, 1H, J1 = 8.5, J2 = 2.2 Hz), 7.68 (d, 2H, J = 8.4 Hz), 7.58 (d, 1H, J = 8.5 Hz), 7.54 (t, 1H, J = 8.1 Hz), 7.49–7.42 (m, 3H), 7.31 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.7, 151.5, 151.4, 147.9, 143.7, 138.4, 138.3, 136.3, 136.1, 136.0, 134.8, 134.4, 133.4, 131.4, 130.8, 130.5, 129.9, 127.8, 127.3, 123.7, 123.3, 122.8, 121.7, 121.4, 116.4, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C29H21Cl2N4O: 511.1092, found 511.1104.
hexane) 0.2; mp: 151–153 °C; IR (DCM): 3313, 1673, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.52 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.07 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.96 (d, 1H, J = 2.2 Hz), 7.85 (d, 2H, J = 8.5 Hz), 7.77 (s, 1H), 7.73 (dd, 1H, J1 = 8.5, J2 = 2.2 Hz), 7.68 (d, 2H, J = 8.4 Hz), 7.58 (d, 1H, J = 8.5 Hz), 7.54 (t, 1H, J = 8.1 Hz), 7.49–7.42 (m, 3H), 7.31 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.7, 151.5, 151.4, 147.9, 143.7, 138.4, 138.3, 136.3, 136.1, 136.0, 134.8, 134.4, 133.4, 131.4, 130.8, 130.5, 129.9, 127.8, 127.3, 123.7, 123.3, 122.8, 121.7, 121.4, 116.4, 21.1; HRMS (ESI) m/z [M + H]+ calcd for C29H21Cl2N4O: 511.1092, found 511.1104.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 184–186 °C; IR (DCM): 3322, 1666, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.80 (dd, 1H, J1 = 7.3, J2 = 1.2 Hz), 8.54 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.07 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.94 (d, 2H, J = 8.4 Hz), 7.83 (d, 2H, J = 8.3 Hz), 7.55 (d, 2H, J = 8.4 Hz), 7.50–7.44 (m, 2H), 7.41 (s, 5H), 7.35–7.30 (m, 3H), 6.71 (s, 1H), 2.47 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 164.3, 152.7, 151.4, 150.8, 147.8, 141.7, 141.0, 140.9, 138.3, 136.1, 134.5, 130.7, 129.8, 129.3, 128.5, 128.4, 127.8, 127.3, 123.2, 123.0, 122.9, 121.5, 121.4, 116.5, 21.6; HRMS (ESI) m/z [M + H]+ calcd for C31H25N4O: 469.2028, found 469.2029.
hexane) 0.2; mp: 184–186 °C; IR (DCM): 3322, 1666, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.80 (dd, 1H, J1 = 7.3, J2 = 1.2 Hz), 8.54 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.07 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.94 (d, 2H, J = 8.4 Hz), 7.83 (d, 2H, J = 8.3 Hz), 7.55 (d, 2H, J = 8.4 Hz), 7.50–7.44 (m, 2H), 7.41 (s, 5H), 7.35–7.30 (m, 3H), 6.71 (s, 1H), 2.47 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 164.3, 152.7, 151.4, 150.8, 147.8, 141.7, 141.0, 140.9, 138.3, 136.1, 134.5, 130.7, 129.8, 129.3, 128.5, 128.4, 127.8, 127.3, 123.2, 123.0, 122.9, 121.5, 121.4, 116.5, 21.6; HRMS (ESI) m/z [M + H]+ calcd for C31H25N4O: 469.2028, found 469.2029.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 140–142 °C; IR (DCM): 3361, 1669, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.78 (dd, 1H, J1 = 7.4, J2 = 2.3 Hz), 8.57 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.10 (dd, 1H, J1 = 8.2, J2 = 1.6 Hz), 8.02 (d, 1H, J = 2.2 Hz), 7.97–7.94 (m, 2H), 7.79 (dd, 1H, J1 = 8.6, J2 = 2.2 Hz), 7.62 (d, 1H, J = 8.5 Hz), 7.58–7.55 (m, 2H), 7.52–7.46 (m, 2H), 7.43–7.39 (m, 5H), 7.35 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 6.73 (s, 1H); 13C NMR (∼101 MHz, CDCl3): δC 164.1, 152.1, 151.6, 151.5, 147.8, 142.2, 140.8, 138.3, 136.2, 135.0, 134.4, 133.5, 131.0, 130.7, 129.4, 128.6, 128.3, 127.8, 127.4, 123.9, 123.2, 123.1, 122.9, 121.6, 121.5, 116.6; HRMS (ESI) m/z [M + H]+ calcd for C30H21Cl2N4O: 523.1092, found 523.1104.
hexane) 0.2; mp: 140–142 °C; IR (DCM): 3361, 1669, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.78 (dd, 1H, J1 = 7.4, J2 = 2.3 Hz), 8.57 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.10 (dd, 1H, J1 = 8.2, J2 = 1.6 Hz), 8.02 (d, 1H, J = 2.2 Hz), 7.97–7.94 (m, 2H), 7.79 (dd, 1H, J1 = 8.6, J2 = 2.2 Hz), 7.62 (d, 1H, J = 8.5 Hz), 7.58–7.55 (m, 2H), 7.52–7.46 (m, 2H), 7.43–7.39 (m, 5H), 7.35 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 6.73 (s, 1H); 13C NMR (∼101 MHz, CDCl3): δC 164.1, 152.1, 151.6, 151.5, 147.8, 142.2, 140.8, 138.3, 136.2, 135.0, 134.4, 133.5, 131.0, 130.7, 129.4, 128.6, 128.3, 127.8, 127.4, 123.9, 123.2, 123.1, 122.9, 121.6, 121.5, 116.6; HRMS (ESI) m/z [M + H]+ calcd for C30H21Cl2N4O: 523.1092, found 523.1104.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 87–90 °C; IR (DCM): 3304, 1653, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.15 (s, 1H), 8.85 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.19 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.03–8.00 (m, 3H), 7.75–7.72 (m, 2H), 7.62 (d, 1H, J = 5.0 Hz), 7.59 (s, 2H), 7.53 (t, 1H, J = 8.1 Hz), 7.45 (dd, 1H, J1 = 8.3, J2 = 1.2 Hz), 7.22–7.19 (m, 3H), 2.47 (s, 6H); 13C NMR (∼101 MHz, CDCl3): δC 160.4, 153.0, 152.8, 147.7, 142.2, 139.0, 138.4, 137.7, 136.3, 135.8, 134.4, 133.0, 131.1, 130.4, 129.6, 127.7, 127.3, 123.5, 121.6, 121.4, 120.7, 116.4, 21.3; HRMS (ESI) m/z [M + H]+ calcd for C28H23N4OS: 463.1593, found 463.1598.
hexane) 0.2; mp: 87–90 °C; IR (DCM): 3304, 1653, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.15 (s, 1H), 8.85 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.19 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.03–8.00 (m, 3H), 7.75–7.72 (m, 2H), 7.62 (d, 1H, J = 5.0 Hz), 7.59 (s, 2H), 7.53 (t, 1H, J = 8.1 Hz), 7.45 (dd, 1H, J1 = 8.3, J2 = 1.2 Hz), 7.22–7.19 (m, 3H), 2.47 (s, 6H); 13C NMR (∼101 MHz, CDCl3): δC 160.4, 153.0, 152.8, 147.7, 142.2, 139.0, 138.4, 137.7, 136.3, 135.8, 134.4, 133.0, 131.1, 130.4, 129.6, 127.7, 127.3, 123.5, 121.6, 121.4, 120.7, 116.4, 21.3; HRMS (ESI) m/z [M + H]+ calcd for C28H23N4OS: 463.1593, found 463.1598.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 185–186 °C; IR (DCM): 3317, 1659, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.85 (dd, 1H, J1 = 7.7, J2 = 1.1 Hz), 8.17 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 7.99 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85–7.80 (m, 4H), 7.64 (dd, 2H, J1 = 6.7, J2 = 1.8 Hz), 7.54–7.50 (m, 1H), 7.43 (dd, 1H, J1 = 8.2, J2 = 1.1 Hz), 7.33 (d, 2H, J = 8.2 Hz), 7.15 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 6.86 (d, 1H, J = 2.6 Hz), 6.32 (d, 1H, J = 2.6 Hz), 4.09 (s, 3H), 2.46 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 160.3, 152.0, 150.9, 147.6, 141.4, 138.4, 138.3, 135.7, 134.9, 130.4, 129.8, 128.7, 127.8, 127.3, 127.1, 123.8, 123.1, 122.8, 121.3, 121.1, 115.7, 109.4, 37.2, 21.5; HRMS (ESI) m/z [M + H]+ calcd for C28H24N5O: 446.1981, found 446.1990.
hexane) 0.2; mp: 185–186 °C; IR (DCM): 3317, 1659, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.85 (dd, 1H, J1 = 7.7, J2 = 1.1 Hz), 8.17 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 7.99 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.85–7.80 (m, 4H), 7.64 (dd, 2H, J1 = 6.7, J2 = 1.8 Hz), 7.54–7.50 (m, 1H), 7.43 (dd, 1H, J1 = 8.2, J2 = 1.1 Hz), 7.33 (d, 2H, J = 8.2 Hz), 7.15 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 6.86 (d, 1H, J = 2.6 Hz), 6.32 (d, 1H, J = 2.6 Hz), 4.09 (s, 3H), 2.46 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 160.3, 152.0, 150.9, 147.6, 141.4, 138.4, 138.3, 135.7, 134.9, 130.4, 129.8, 128.7, 127.8, 127.3, 127.1, 123.8, 123.1, 122.8, 121.3, 121.1, 115.7, 109.4, 37.2, 21.5; HRMS (ESI) m/z [M + H]+ calcd for C28H24N5O: 446.1981, found 446.1990.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 139–140 °C; IR (DCM): 3331, 1667, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.84 (dd, 1H, J1 = 7.6, J2 = 1.0 Hz), 8.41 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.20 (t, 1H, J = 1.6 Hz), 8.04 (dd, 1H, J1 = 8.3, J2 = 1.4 Hz), 7.91 (dd, 2H, J1 = 8.3, J2 = 1.6 Hz), 7.81 (s, 1H), 7.74 (d, 1H, J = 8.1 Hz), 7.59 (d, 1H, J = 7.7 Hz), 7.56–7.49 (m, 5H), 7.44 (d, 2H, J = 8.1 Hz), 7.35 (t, 1H, J = 7.8 Hz), 7.28 (dd, 1H, J1 = 8.6, J2 = 4.2 Hz), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.7, 152.9, 152.6, 147.9, 141.1, 138.4, 138.1, 136.6, 136.0, 134.5, 131.8, 131.5, 131.1, 130.7, 130.0, 129.1, 129.0, 127.7, 127.3, 123.1, 122.9, 122.3, 121.6, 121.4, 116.3, 21.2; HRMS (ESI) m/z [M + H]+ calcd for C29H23N4O: 443.1872, found 443.1855.
hexane) 0.2; mp: 139–140 °C; IR (DCM): 3331, 1667, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.85 (s, 1H), 8.84 (dd, 1H, J1 = 7.6, J2 = 1.0 Hz), 8.41 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.20 (t, 1H, J = 1.6 Hz), 8.04 (dd, 1H, J1 = 8.3, J2 = 1.4 Hz), 7.91 (dd, 2H, J1 = 8.3, J2 = 1.6 Hz), 7.81 (s, 1H), 7.74 (d, 1H, J = 8.1 Hz), 7.59 (d, 1H, J = 7.7 Hz), 7.56–7.49 (m, 5H), 7.44 (d, 2H, J = 8.1 Hz), 7.35 (t, 1H, J = 7.8 Hz), 7.28 (dd, 1H, J1 = 8.6, J2 = 4.2 Hz), 2.52 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 167.7, 152.9, 152.6, 147.9, 141.1, 138.4, 138.1, 136.6, 136.0, 134.5, 131.8, 131.5, 131.1, 130.7, 130.0, 129.1, 129.0, 127.7, 127.3, 123.1, 122.9, 122.3, 121.6, 121.4, 116.3, 21.2; HRMS (ESI) m/z [M + H]+ calcd for C29H23N4O: 443.1872, found 443.1855.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 170–172 °C; IR (DCM) 3333, 1672, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.54 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.75–7.73 (m, 3H), 7.66–7.63 (m, 2H), 7.55 (t, 1H, J = 8.1 Hz), 7.50–7.43 (m, 3H), 7.33 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 2.71 (s, 3H), 2.51 (s, 3H), 2.48 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 169.3, 167.8, 153.7, 152.1, 147.9, 142.6, 138.4, 138.2, 136.5, 136.1, 136.0, 134.5, 132.5, 131.4, 130.5, 129.8, 129.7, 127.8, 127.3, 122.3, 121.7, 121.4, 116.4, 21.1, 12.1, 11.7; HRMS (ESI) m/z [M + H]+ calcd for C28H24N5O2: 462.1930, found 462.1913.
hexane) 0.2; mp: 170–172 °C; IR (DCM) 3333, 1672, 1523 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.83 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.54 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.09 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.75–7.73 (m, 3H), 7.66–7.63 (m, 2H), 7.55 (t, 1H, J = 8.1 Hz), 7.50–7.43 (m, 3H), 7.33 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 2.71 (s, 3H), 2.51 (s, 3H), 2.48 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 169.3, 167.8, 153.7, 152.1, 147.9, 142.6, 138.4, 138.2, 136.5, 136.1, 136.0, 134.5, 132.5, 131.4, 130.5, 129.8, 129.7, 127.8, 127.3, 122.3, 121.7, 121.4, 116.4, 21.1, 12.1, 11.7; HRMS (ESI) m/z [M + H]+ calcd for C28H24N5O2: 462.1930, found 462.1913.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 148–150 °C; IR (DCM): 3366, 1684, 1516 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.80 (s, 1H), 8.61–8.57 (m, 2H), 8.10–8.08 (m, 1H), 8.01 (d, 1H, J = 8.2 Hz), 7.74–7.68 (m, 7H), 7.46–7.41 (m, 2H), 7.37–7.35 (m, 1H), 7.05 (s, 2H), 4.22 (q, 2H, J = 7.0 Hz), 2.70 (s, 6H), 2.48 (s, 6H), 1.52 (t, 3H, J = 6.9 Hz); 13C NMR (∼101 MHz, CDCl3): δC 169.4, 167.4, 159.2, 153.7, 152.0, 147.9, 144.1, 143.0, 141.7, 132.5, 129.7, 129.4, 128.9, 127.9, 127.5, 122.2, 121.8, 121.7, 121.3, 119.0, 115.6, 64.0, 14.8, 12.1, 11.7; HRMS (ESI) m/z [M + H]+ calcd for C40H35N8O4: 691.2781, found 691.2780 (given the presence of two unsymmetrical azo systems, the NMR data indicated the occurrence of rotamers).
hexane) 0.2; mp: 148–150 °C; IR (DCM): 3366, 1684, 1516 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.80 (s, 1H), 8.61–8.57 (m, 2H), 8.10–8.08 (m, 1H), 8.01 (d, 1H, J = 8.2 Hz), 7.74–7.68 (m, 7H), 7.46–7.41 (m, 2H), 7.37–7.35 (m, 1H), 7.05 (s, 2H), 4.22 (q, 2H, J = 7.0 Hz), 2.70 (s, 6H), 2.48 (s, 6H), 1.52 (t, 3H, J = 6.9 Hz); 13C NMR (∼101 MHz, CDCl3): δC 169.4, 167.4, 159.2, 153.7, 152.0, 147.9, 144.1, 143.0, 141.7, 132.5, 129.7, 129.4, 128.9, 127.9, 127.5, 122.2, 121.8, 121.7, 121.3, 119.0, 115.6, 64.0, 14.8, 12.1, 11.7; HRMS (ESI) m/z [M + H]+ calcd for C40H35N8O4: 691.2781, found 691.2780 (given the presence of two unsymmetrical azo systems, the NMR data indicated the occurrence of rotamers).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 160–162 °C; IR (DCM): 3306, 1652, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.12 (s, 1H), 8.85 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.21 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.06 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.91–788 (m, 2H), 7.72–7.02 (m, 2H), 7.62 (d, 1H, J = 5.0 Hz), 7.54 (t, 1H, J = 8.2 Hz), 7.47 (dd, 1H, J1 = 8.5, J2 = 1.5 Hz), 7.24 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.19 (d, 1H, J = 5.0 Hz), 2.79 (s, 3H), 2.47 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 169.8, 160.5, 153.6, 153.0, 147.5, 142.2, 138.3, 137.6, 136.1, 134.4, 132.7, 131.1, 130.4, 129.6, 127.8, 127.4, 123.0, 121.7, 121.3, 116.6, 12.2, 11.7; HRMS (ESI) m/z [M + H]+ calcd for C25H20N5O2S: 454.1338, found 454.1349.
hexane) 0.2; mp: 160–162 °C; IR (DCM): 3306, 1652, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.12 (s, 1H), 8.85 (dd, 1H, J1 = 7.6, J2 = 1.2 Hz), 8.21 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.06 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.91–788 (m, 2H), 7.72–7.02 (m, 2H), 7.62 (d, 1H, J = 5.0 Hz), 7.54 (t, 1H, J = 8.2 Hz), 7.47 (dd, 1H, J1 = 8.5, J2 = 1.5 Hz), 7.24 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.19 (d, 1H, J = 5.0 Hz), 2.79 (s, 3H), 2.47 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 169.8, 160.5, 153.6, 153.0, 147.5, 142.2, 138.3, 137.6, 136.1, 134.4, 132.7, 131.1, 130.4, 129.6, 127.8, 127.4, 123.0, 121.7, 121.3, 116.6, 12.2, 11.7; HRMS (ESI) m/z [M + H]+ calcd for C25H20N5O2S: 454.1338, found 454.1349.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 150–152 °C; IR (DCM): 3351, 1685, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.77 (s, 1H), 8.81–8.76 (m, 2H), 8.14 (d, 1H, J = 8.2 Hz), 7.92 (d, 2H, J = 7.2 Hz), 7.90 (d, 2H, J = 7.6 Hz), 7.56–7.48 (m, 7H), 7.42 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 3.67–3.58 (m, 1H), 2.95 (dd, 1H, J1 = 15.7, J2 = 8.1 Hz), (dd, 1H, J1 = 15.2, J2 = 7.8 Hz), 1.49 (d, 3H, J = 6.9 Hz); 13C NMR (100 MHz, CDCl3): δC 170.0, 152.7, 151.4, 149.3, 148.1, 138.3, 136.3, 134.3, 130.8, 129.1, 127.9, 127.7, 127.4, 123.2, 122.8, 121.6, 121.5, 116.5, 46.7, 36.8, 21.8; HRMS (ESI) m/z [M + H]+ calcd for C25H23N4O: 395.1872, found 395.1858.
hexane) 0.2; mp: 150–152 °C; IR (DCM): 3351, 1685, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.77 (s, 1H), 8.81–8.76 (m, 2H), 8.14 (d, 1H, J = 8.2 Hz), 7.92 (d, 2H, J = 7.2 Hz), 7.90 (d, 2H, J = 7.6 Hz), 7.56–7.48 (m, 7H), 7.42 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 3.67–3.58 (m, 1H), 2.95 (dd, 1H, J1 = 15.7, J2 = 8.1 Hz), (dd, 1H, J1 = 15.2, J2 = 7.8 Hz), 1.49 (d, 3H, J = 6.9 Hz); 13C NMR (100 MHz, CDCl3): δC 170.0, 152.7, 151.4, 149.3, 148.1, 138.3, 136.3, 134.3, 130.8, 129.1, 127.9, 127.7, 127.4, 123.2, 122.8, 121.6, 121.5, 116.5, 46.7, 36.8, 21.8; HRMS (ESI) m/z [M + H]+ calcd for C25H23N4O: 395.1872, found 395.1858.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 138–140 °C; IR (DCM): 3348, 1685, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.77 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.72 (dd, 1H, J1 = 6.6, J2 = 2.4 Hz), 8.15 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.89–7.85 (m, 4H), 7.53–7.48 (m, 7H), 7.47–7.42 (m, 1H), 7.39 (d, 2H, J = 7.3 Hz), 7.33 (t, 2H, J = 7.8 Hz), 7.22 (t, 1H, J = 7.2 Hz), 4.90 (t, 1H, J = 7.7 Hz), 3.39 (d, 2H, J = 7.8 Hz); 13C NMR (∼126 MHz, CDCl3): δC 169.3, 152.7, 151.3, 148.1, 147.0, 143.2, 138.2, 136.3, 134.2, 130.8, 129.1, 128.8, 128.6, 127.9, 127.8, 127.4, 126.8, 123.2, 122.8, 121.6, 121.5, 116.6, 47.0, 44.2; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2009.
hexane) 0.2; mp: 138–140 °C; IR (DCM): 3348, 1685, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.77 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.72 (dd, 1H, J1 = 6.6, J2 = 2.4 Hz), 8.15 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.89–7.85 (m, 4H), 7.53–7.48 (m, 7H), 7.47–7.42 (m, 1H), 7.39 (d, 2H, J = 7.3 Hz), 7.33 (t, 2H, J = 7.8 Hz), 7.22 (t, 1H, J = 7.2 Hz), 4.90 (t, 1H, J = 7.7 Hz), 3.39 (d, 2H, J = 7.8 Hz); 13C NMR (∼126 MHz, CDCl3): δC 169.3, 152.7, 151.3, 148.1, 147.0, 143.2, 138.2, 136.3, 134.2, 130.8, 129.1, 128.8, 128.6, 127.9, 127.8, 127.4, 126.8, 123.2, 122.8, 121.6, 121.5, 116.6, 47.0, 44.2; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2009.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 99–100 °C; IR (DCM): 3351, 1685, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.72 (s, 1H), 8.77–8.74 (m, 2H), 8.12 (d, 1H, J = 8.2 Hz), 7.91 (d, 2H, J = 7.5 Hz), 7.91 (d, 2H, J = 7.6 Hz), 7.54–7.46 (m, 7H), 7.40 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 3.47–3.42 (m, 1H), 2.97–2.86 (m, 2H), 1.88–1.74 (m, 2H), 1.35–1.24 (m, 2H), 0.92 (t, 3H, J = 7.3 Hz); 13C NMR (∼101 MHz, CDCl3): δC 170.1, 152.7, 151.4, 148.1, 148.0, 138.2, 136.3, 134.3, 130.8, 129.1, 128.3, 127.9, 127.4, 123.2, 122.7, 121.6, 121.5, 116.4, 45.7, 42.3, 38.4, 20.6, 14.0; HRMS (ESI) m/z [M + H]+ calcd for C27H27N4O: 423.2185, found 423.2169.
hexane) 0.2; mp: 99–100 °C; IR (DCM): 3351, 1685, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.72 (s, 1H), 8.77–8.74 (m, 2H), 8.12 (d, 1H, J = 8.2 Hz), 7.91 (d, 2H, J = 7.5 Hz), 7.91 (d, 2H, J = 7.6 Hz), 7.54–7.46 (m, 7H), 7.40 (dd, 1H, J1 = 8.2, J2 = 4.2 Hz), 3.47–3.42 (m, 1H), 2.97–2.86 (m, 2H), 1.88–1.74 (m, 2H), 1.35–1.24 (m, 2H), 0.92 (t, 3H, J = 7.3 Hz); 13C NMR (∼101 MHz, CDCl3): δC 170.1, 152.7, 151.4, 148.1, 148.0, 138.2, 136.3, 134.3, 130.8, 129.1, 128.3, 127.9, 127.4, 123.2, 122.7, 121.6, 121.5, 116.4, 45.7, 42.3, 38.4, 20.6, 14.0; HRMS (ESI) m/z [M + H]+ calcd for C27H27N4O: 423.2185, found 423.2169.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 100–102 °C; IR (DCM): 3351, 1685, 1526 cm−1; 1H NMR (500 MHz, CDCl3): δH 9.59 (s, 1H), 8.64–8.61 (m, 2H), 7.98 (dd, 1H, J1 = 6.6, J2 = 1.3 Hz), 7.79–7.75 (m, 4H), 7.41–7.33 (m, 7H), 7.27 (dd, 1H, J1 = 6.6, J2 = 3.4 Hz), 3.32–3.27 (m, 1H), 2.83–2.74 (m, 2H), 1.77–1.62 (m, 2H), 1.26–1.05 (m, 4H), 0.74 (t, 3H, J = 5.8 Hz); 13C NMR (∼126 MHz, CDCl3): δC 170.0, 152.7, 151.4, 148.0, 147.9, 138.2, 136.2, 134.3, 130.7, 129.0, 128.2, 127.8, 127.3, 123.1, 122.7, 121.5, 121.4, 116.4, 45.6, 42.5, 35.8, 29.5, 22.6, 14.0; HRMS (ESI) m/z [M + H]+ calcd for C28H29N4O: 437.2341, found 437.2328.
hexane) 0.2; mp: 100–102 °C; IR (DCM): 3351, 1685, 1526 cm−1; 1H NMR (500 MHz, CDCl3): δH 9.59 (s, 1H), 8.64–8.61 (m, 2H), 7.98 (dd, 1H, J1 = 6.6, J2 = 1.3 Hz), 7.79–7.75 (m, 4H), 7.41–7.33 (m, 7H), 7.27 (dd, 1H, J1 = 6.6, J2 = 3.4 Hz), 3.32–3.27 (m, 1H), 2.83–2.74 (m, 2H), 1.77–1.62 (m, 2H), 1.26–1.05 (m, 4H), 0.74 (t, 3H, J = 5.8 Hz); 13C NMR (∼126 MHz, CDCl3): δC 170.0, 152.7, 151.4, 148.0, 147.9, 138.2, 136.2, 134.3, 130.7, 129.0, 128.2, 127.8, 127.3, 123.1, 122.7, 121.5, 121.4, 116.4, 45.6, 42.5, 35.8, 29.5, 22.6, 14.0; HRMS (ESI) m/z [M + H]+ calcd for C28H29N4O: 437.2341, found 437.2328.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 99–100 °C; IR (DCM): 3351, 1686, 1526 cm−1; 1H NMR (500 MHz, CDCl3): δH 9.59 (s, 1H), 8.64–8.60 (m, 2H), 7.97 (dd, 1H, J1 = 6.6, J2 = 1.3 Hz), 7.78–7.77 (m, 1H), 7.77–7.74 (m, 3H), 7.40–7.31 (m, 7H), 7.26 (dd, 1H, J1 = 6.6, J2 = 3.4 Hz), 3.33–3.37 (m, 1H), 2.82–2.73 (m, 2H), 1.76–1.59 (m, 2H), 1.16–1.09 (m, 14H), 0.75 (t, 3H, J = 5.8 Hz); 13C NMR (∼126 MHz, CDCl3): δC 170.0, 152.7, 151.3, 148.0, 147.9, 138.2, 136.2, 134.3, 130.7, 129.0, 128.2, 127.8, 127.3, 123.1, 122.7, 121.4, 121.3, 116.4, 45.6, 42.5, 36.1, 31.8, 29.5, 29.5, 29.4, 29.2, 27.4, 22.6, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C33H39N4O: 507.3124, found 507.3105.
hexane) 0.2; mp: 99–100 °C; IR (DCM): 3351, 1686, 1526 cm−1; 1H NMR (500 MHz, CDCl3): δH 9.59 (s, 1H), 8.64–8.60 (m, 2H), 7.97 (dd, 1H, J1 = 6.6, J2 = 1.3 Hz), 7.78–7.77 (m, 1H), 7.77–7.74 (m, 3H), 7.40–7.31 (m, 7H), 7.26 (dd, 1H, J1 = 6.6, J2 = 3.4 Hz), 3.33–3.37 (m, 1H), 2.82–2.73 (m, 2H), 1.76–1.59 (m, 2H), 1.16–1.09 (m, 14H), 0.75 (t, 3H, J = 5.8 Hz); 13C NMR (∼126 MHz, CDCl3): δC 170.0, 152.7, 151.3, 148.0, 147.9, 138.2, 136.2, 134.3, 130.7, 129.0, 128.2, 127.8, 127.3, 123.1, 122.7, 121.4, 121.3, 116.4, 45.6, 42.5, 36.1, 31.8, 29.5, 29.5, 29.4, 29.2, 27.4, 22.6, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C33H39N4O: 507.3124, found 507.3105.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 70–72 °C; IR (DCM): 3353, 1688, 1526 cm−1; 1H NMR (500 MHz, CDCl3): δH 9.82 (s, 1H), 8.67–8.65 (m, 2H), 8.10 (d, 1H, J = 6.2 Hz), 7.78–7.75 (m, 4H), 7.45–7.32 (m, 8H), 3.35–3.28 (m, 1H), 2.87 (d, 2H, J = 5.9 Hz), 1.77–1.64 (m, 2H), 1.25–1.11 (m, 22H), 0.78 (t, 3H, J = 5.7 Hz); 13C NMR (∼126 MHz, CDCl3): δC 170.4, 152.6, 151.3, 148.0, 146.8, 138.2, 136.5, 133.5, 130.7, 129.0, 128.3, 128.1, 128.0, 123.0, 122.6, 121.7, 121.3, 118.3, 45.3, 42.5, 36.2, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 27.4, 22.6, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C37H47N4O: 563.3750, found 563.3730.
hexane) 0.2; mp: 70–72 °C; IR (DCM): 3353, 1688, 1526 cm−1; 1H NMR (500 MHz, CDCl3): δH 9.82 (s, 1H), 8.67–8.65 (m, 2H), 8.10 (d, 1H, J = 6.2 Hz), 7.78–7.75 (m, 4H), 7.45–7.32 (m, 8H), 3.35–3.28 (m, 1H), 2.87 (d, 2H, J = 5.9 Hz), 1.77–1.64 (m, 2H), 1.25–1.11 (m, 22H), 0.78 (t, 3H, J = 5.7 Hz); 13C NMR (∼126 MHz, CDCl3): δC 170.4, 152.6, 151.3, 148.0, 146.8, 138.2, 136.5, 133.5, 130.7, 129.0, 128.3, 128.1, 128.0, 123.0, 122.6, 121.7, 121.3, 118.3, 45.3, 42.5, 36.2, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.5, 29.4, 29.3, 27.4, 22.6, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C37H47N4O: 563.3750, found 563.3730.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 79–80 °C; IR (DCM): 3352, 1687, 1531 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.71 (s, 1H), 8.76–8.73 (m, 2H), 8.13 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.91–7.86 (m, 4H), 7.54–7.46 (m, 7H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.44–3.40 (m, 1H), 2.97–2.86 (m, 2H), 1.89–1.75 (m, 2H), 1.34–1.23 (m, 8H), 0.85 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 170.1, 152.7, 151.4, 148.1, 148.0, 138.2, 136.3, 134.3, 130.8, 129.1, 128.3, 127.9, 127.4, 123.2, 122.7, 121.5, 121.4, 116.5, 45.7, 42.6, 36.2, 31.7, 29.2, 27.4, 22.6, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C30H33N4O: 465.2654, found 465.2663.
hexane) 0.2; mp: 79–80 °C; IR (DCM): 3352, 1687, 1531 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.71 (s, 1H), 8.76–8.73 (m, 2H), 8.13 (dd, 1H, J1 = 8.3, J2 = 1.5 Hz), 7.91–7.86 (m, 4H), 7.54–7.46 (m, 7H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.44–3.40 (m, 1H), 2.97–2.86 (m, 2H), 1.89–1.75 (m, 2H), 1.34–1.23 (m, 8H), 0.85 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 170.1, 152.7, 151.4, 148.1, 148.0, 138.2, 136.3, 134.3, 130.8, 129.1, 128.3, 127.9, 127.4, 123.2, 122.7, 121.5, 121.4, 116.5, 45.7, 42.6, 36.2, 31.7, 29.2, 27.4, 22.6, 14.1; HRMS (ESI) m/z [M + H]+ calcd for C30H33N4O: 465.2654, found 465.2663.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 82–84 °C; IR (DCM): 3357, 1688, 1527 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.70 (s, 1H), 8.76–8.73 (m, 2H), 8.13 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.91–7.86 (m, 4H), 7.54–7.45 (m, 7H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.44–3.40 (m, 1H), 2.96–2.85 (m, 2H), 1.86–1.74 (m, 2H), 1.26–1.22 (m, 26H), 0.89 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 170.0, 152.7, 151.4, 148.0, 147.9, 138.2, 136.3, 134.4, 130.8, 129.1, 128.3, 127.9, 127.3, 123.2, 122.8, 121.5, 121.4, 116.5, 45.7, 42.6, 36.2, 32.0, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.4, 27.5, 22.7, 14.2; HRMS (ESI) m/z [M + H]+ calcd for C39H51N4O: 591.4063, found 591.4078.
hexane) 0.2; mp: 82–84 °C; IR (DCM): 3357, 1688, 1527 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.70 (s, 1H), 8.76–8.73 (m, 2H), 8.13 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.91–7.86 (m, 4H), 7.54–7.45 (m, 7H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.44–3.40 (m, 1H), 2.96–2.85 (m, 2H), 1.86–1.74 (m, 2H), 1.26–1.22 (m, 26H), 0.89 (t, 3H, J = 7.0 Hz); 13C NMR (∼101 MHz, CDCl3): δC 170.0, 152.7, 151.4, 148.0, 147.9, 138.2, 136.3, 134.4, 130.8, 129.1, 128.3, 127.9, 127.3, 123.2, 122.8, 121.5, 121.4, 116.5, 45.7, 42.6, 36.2, 32.0, 29.8, 29.7, 29.7, 29.6, 29.6, 29.5, 29.4, 27.5, 22.7, 14.2; HRMS (ESI) m/z [M + H]+ calcd for C39H51N4O: 591.4063, found 591.4078.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 224–226 °C; IR (DCM): 3343, 1675, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.63 (s, 1H), 8.76 (d, 1H, J = 3.0 Hz), 8.32 (d, 1H, J = 7.6 Hz), 8.05 (dd, 1H, J1 = 7.6, J2 = 3.6 Hz), 7.86–7.80 (m, 8H), 7.52–7.42 (m, 10H), 7.40 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 7.34 (d, 1H, J = 7.8 Hz), 7.30–7.26 (m, 1H), 4.32–4.27 (m, 1H), 4.22–4.15 (m, 2H), 3.67 (dd, 1H, J1 = 21.6, J2 = 11.0 Hz), 2.89–2.82 (m, 1H); 13C NMR (∼126 MHz, CDCl3): δC 168.4, 152.7, 151.0, 147.9, 144.1, 138.2, 136.2, 133.9, 130.6, 129.0, 127.7, 127.6, 127.2, 122.7, 122.6, 121.3, 121.2, 116.6, 54.8, 39.0, 30.2; HRMS (ESI) m/z [M + H]+ calcd for C38H31N6O: 587.2559, found 587.2542.
hexane) 0.2; mp: 224–226 °C; IR (DCM): 3343, 1675, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.63 (s, 1H), 8.76 (d, 1H, J = 3.0 Hz), 8.32 (d, 1H, J = 7.6 Hz), 8.05 (dd, 1H, J1 = 7.6, J2 = 3.6 Hz), 7.86–7.80 (m, 8H), 7.52–7.42 (m, 10H), 7.40 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 7.34 (d, 1H, J = 7.8 Hz), 7.30–7.26 (m, 1H), 4.32–4.27 (m, 1H), 4.22–4.15 (m, 2H), 3.67 (dd, 1H, J1 = 21.6, J2 = 11.0 Hz), 2.89–2.82 (m, 1H); 13C NMR (∼126 MHz, CDCl3): δC 168.4, 152.7, 151.0, 147.9, 144.1, 138.2, 136.2, 133.9, 130.6, 129.0, 127.7, 127.6, 127.2, 122.7, 122.6, 121.3, 121.2, 116.6, 54.8, 39.0, 30.2; HRMS (ESI) m/z [M + H]+ calcd for C38H31N6O: 587.2559, found 587.2542.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 117–118 °C; IR (DCM): 3339, 1667, 1530 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.80 (s, 1H), 8.91–8.89 (m, 2H), 8.24 (d, 1H, J = 8.2 Hz), 7.90–7.85 (m, 4H), 7.61–7.56 (m, 2H), 7.53–7.45 (m, 7H), 6.38 (d, 1H, J = 1.5 Hz), 4.47 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 157.6, 152.7, 151.3, 148.4, 143.4, 142.5, 138.7, 136.4, 134.4, 131.5, 130.8, 129.6, 129.1, 128.1, 127.4, 123.1, 122.8, 121.7, 121.7, 116.5, 114.6, 31.4; HRMS (ESI) m/z [M + H]+ calcd for C27H21N4O2: 433.1665, found 433.1658.
hexane) 0.2; mp: 117–118 °C; IR (DCM): 3339, 1667, 1530 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.80 (s, 1H), 8.91–8.89 (m, 2H), 8.24 (d, 1H, J = 8.2 Hz), 7.90–7.85 (m, 4H), 7.61–7.56 (m, 2H), 7.53–7.45 (m, 7H), 6.38 (d, 1H, J = 1.5 Hz), 4.47 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 157.6, 152.7, 151.3, 148.4, 143.4, 142.5, 138.7, 136.4, 134.4, 131.5, 130.8, 129.6, 129.1, 128.1, 127.4, 123.1, 122.8, 121.7, 121.7, 116.5, 114.6, 31.4; HRMS (ESI) m/z [M + H]+ calcd for C27H21N4O2: 433.1665, found 433.1658.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 130–132 °C; IR (DCM): 3049, 1661, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.50 (s, 1H), 8.88 (dd, 1H, J1 = 7.4 J2 = 1.5 Hz), 8.80 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.19 (dd, 1H, J1 = 8.2, J2 = 1.5 Hz), 7.94–7.88 (m, 4H), 7.63–7.46 (m, 8H), 7.43 (d, 1H, J = 5.1 Hz), 6.96 (d, 1H, J = 5.1 Hz), 4.63 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 161.0, 152.7, 151.3, 148.3, 144.6, 143.6, 138.6, 136.4, 134.6, 132.3, 131.5, 130.8, 129.7, 129.1, 128.0, 127.6, 127.4, 123.1, 122.8, 121.8, 121.7, 116.6, 35.2; HRMS (ESI) m/z [M + H]+ calcd for C27H21N4OS: 449.1436, found 449.1424.
hexane) 0.2; mp: 130–132 °C; IR (DCM): 3049, 1661, 1524 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.50 (s, 1H), 8.88 (dd, 1H, J1 = 7.4 J2 = 1.5 Hz), 8.80 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.19 (dd, 1H, J1 = 8.2, J2 = 1.5 Hz), 7.94–7.88 (m, 4H), 7.63–7.46 (m, 8H), 7.43 (d, 1H, J = 5.1 Hz), 6.96 (d, 1H, J = 5.1 Hz), 4.63 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 161.0, 152.7, 151.3, 148.3, 144.6, 143.6, 138.6, 136.4, 134.6, 132.3, 131.5, 130.8, 129.7, 129.1, 128.0, 127.6, 127.4, 123.1, 122.8, 121.8, 121.7, 116.6, 35.2; HRMS (ESI) m/z [M + H]+ calcd for C27H21N4OS: 449.1436, found 449.1424.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 214–215 °C; IR (DCM): 3341, 1671, 1530 cm−1; 1H NMR (400 MHz, CDCl3): δH 11.11 (s, 1H), 8.98–8.95 (m, 2H), 8.24 (d, 1H, J = 8.2 Hz), 7.87 (d, 2H, J = 7.3 Hz), 7.68 (d, 2H, J = 8.3 Hz), 7.68 (d, 1H, J = 8.3 Hz), 7.63–7.52 (m, 6H), 7.50–7.43 (m, 4H), 7.27–7.23 (m, 1H), 4.77 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 158.2, 153.8, 152.7, 151.3, 148.6, 143.4, 142.8, 138.8, 136.4, 134.2, 130.8, 129.5, 129.0, 128.9, 128.1, 127.4, 127.4, 125.6, 123.5, 123.1, 122.8, 122.1, 121.8, 121.6, 117.0, 112.2, 30.0; HRMS (ESI) m/z [M + H]+ calcd for C31H23N4O2: 483.1821, found 483.1813.
hexane) 0.2; mp: 214–215 °C; IR (DCM): 3341, 1671, 1530 cm−1; 1H NMR (400 MHz, CDCl3): δH 11.11 (s, 1H), 8.98–8.95 (m, 2H), 8.24 (d, 1H, J = 8.2 Hz), 7.87 (d, 2H, J = 7.3 Hz), 7.68 (d, 2H, J = 8.3 Hz), 7.68 (d, 1H, J = 8.3 Hz), 7.63–7.52 (m, 6H), 7.50–7.43 (m, 4H), 7.27–7.23 (m, 1H), 4.77 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 158.2, 153.8, 152.7, 151.3, 148.6, 143.4, 142.8, 138.8, 136.4, 134.2, 130.8, 129.5, 129.0, 128.9, 128.1, 127.4, 127.4, 125.6, 123.5, 123.1, 122.8, 122.1, 121.8, 121.6, 117.0, 112.2, 30.0; HRMS (ESI) m/z [M + H]+ calcd for C31H23N4O2: 483.1821, found 483.1813.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; IR (DCM): 3301, 1663, 1529 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.66 (s, 1H), 8.92 (dd, 1H, J1 = 7.0, J2 = 1.9 Hz), 8.75 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.20 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.94 (d, 1H, J = 8.0 Hz), 7.91–7.84 (m, 4H), 7.80 (d, 1H, J = 8.0 Hz), 7.63–7.56 (m, 2H), 7.53–7.46 (m, 7H), 7.43–7.38 (m, 1H), 4.90 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 161.4, 152.7, 151.2, 148.4, 142.7, 140.0, 139.1, 138.6, 138.2, 136.3, 134.4, 133.1, 130.8, 129.2, 129.0, 128.2 128.0, 127.4, 126.8, 125.0, 124.0, 123.1, 122.8, 122.7, 122.1, 121.8, 116.8, 33.0; HRMS (ESI) m/z [M + H]+ calcd for C31H23N4OS: 499.1593, found 499.1605.
hexane) 0.2; IR (DCM): 3301, 1663, 1529 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.66 (s, 1H), 8.92 (dd, 1H, J1 = 7.0, J2 = 1.9 Hz), 8.75 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.20 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.94 (d, 1H, J = 8.0 Hz), 7.91–7.84 (m, 4H), 7.80 (d, 1H, J = 8.0 Hz), 7.63–7.56 (m, 2H), 7.53–7.46 (m, 7H), 7.43–7.38 (m, 1H), 4.90 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 161.4, 152.7, 151.2, 148.4, 142.7, 140.0, 139.1, 138.6, 138.2, 136.3, 134.4, 133.1, 130.8, 129.2, 129.0, 128.2 128.0, 127.4, 126.8, 125.0, 124.0, 123.1, 122.8, 122.7, 122.1, 121.8, 116.8, 33.0; HRMS (ESI) m/z [M + H]+ calcd for C31H23N4OS: 499.1593, found 499.1605.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 144–146 °C; IR (DCM): 3338, 1683, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.79 (s, 1H), 8.76 (d, 1H, J = 7.2 Hz), 8.67 (d, 1H, J = 3.9 Hz), 8.13 (d, 1H, J = 8.2 Hz), 7.94–7.91 (m, 4H), 7.59 (d, 2H, J = 8.1 Hz), 7.56–7.49 (m, 5H), 7.41–7.36 (m, 3H), 7.30 (d, 1H, J = 9.4 Hz), 3.94 (s, 2H), 2.50 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 169.3, 152.6, 151.6, 148.2, 144.7, 142.8, 138.4, 138.4, 136.2, 134.4, 131.0, 131.0, 130.3, 130.1, 129.1, 128.1, 127.9, 127.4, 127.4, 122.9, 122.9, 121.6, 121.6, 116.3, 40.1, 20.5; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2027.
hexane) 0.2; mp: 144–146 °C; IR (DCM): 3338, 1683, 1525 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.79 (s, 1H), 8.76 (d, 1H, J = 7.2 Hz), 8.67 (d, 1H, J = 3.9 Hz), 8.13 (d, 1H, J = 8.2 Hz), 7.94–7.91 (m, 4H), 7.59 (d, 2H, J = 8.1 Hz), 7.56–7.49 (m, 5H), 7.41–7.36 (m, 3H), 7.30 (d, 1H, J = 9.4 Hz), 3.94 (s, 2H), 2.50 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 169.3, 152.6, 151.6, 148.2, 144.7, 142.8, 138.4, 138.4, 136.2, 134.4, 131.0, 131.0, 130.3, 130.1, 129.1, 128.1, 127.9, 127.4, 127.4, 122.9, 122.9, 121.6, 121.6, 116.3, 40.1, 20.5; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O: 457.2028, found 457.2027.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 150–152 °C; IR (DCM): 3300, 1679, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.26 (s, 1H), 8.79 (d, 1H, J = 7.4 Hz), 8.75 (dd, 1H, J1 = 4.1, J2 = 1.2 Hz), 8.16 (d, 1H, J = 8.1 Hz), 7.96 (d, 2H, J = 8.3 Hz), 7.91 (d, 2H, J = 7.3 Hz), 7.63 (d, 2H, J = 8.3 Hz), 7.56–7.45 (m, 5H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 7.38 (t, 1H, J = 8.0 Hz), 7.03–7.00 (m, 2H), 3.97 (s, 3H), 3.89 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 170.0, 157.9, 152.7, 151.7, 148.0, 143.8, 143.6, 138.6, 136.2, 135.0, 131.0, 130.5, 129.1, 128.2, 128.0, 127.4, 122.9, 122.8, 122.7, 121.7, 121.5, 121.3, 116.5, 109.8, 56.0, 37.3; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1964.
hexane) 0.2; mp: 150–152 °C; IR (DCM): 3300, 1679, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.26 (s, 1H), 8.79 (d, 1H, J = 7.4 Hz), 8.75 (dd, 1H, J1 = 4.1, J2 = 1.2 Hz), 8.16 (d, 1H, J = 8.1 Hz), 7.96 (d, 2H, J = 8.3 Hz), 7.91 (d, 2H, J = 7.3 Hz), 7.63 (d, 2H, J = 8.3 Hz), 7.56–7.45 (m, 5H), 7.42 (dd, 1H, J1 = 8.3, J2 = 4.3 Hz), 7.38 (t, 1H, J = 8.0 Hz), 7.03–7.00 (m, 2H), 3.97 (s, 3H), 3.89 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 170.0, 157.9, 152.7, 151.7, 148.0, 143.8, 143.6, 138.6, 136.2, 135.0, 131.0, 130.5, 129.1, 128.2, 128.0, 127.4, 122.9, 122.8, 122.7, 121.7, 121.5, 121.3, 116.5, 109.8, 56.0, 37.3; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1964.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 146–148 °C; IR (DCM): 3338, 1683, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.74–8.71 (m, 2H), 8.14 (d, 1H, J = 8.2 Hz), 7.95 (d, 2H, J = 8.4 Hz), 7.93 (t, 2H, J = 8.7 Hz), 7.60 (d, 1H, J = 1.3 Hz), 7.57–7.51 (m, 7H), 7.42–7.39 (m, 2H), 7.33 (d, 1H, J = 8.2 Hz), 3.89 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 168.8, 152.6, 151.8, 148.0, 142.7, 140.3, 136.6, 134.3, 134.0, 131.4, 131.2, 130.9, 130.1, 129.1, 127.9, 127.7, 127.5, 123.1, 123.0, 121.8, 121.6, 116.7, 42.4; HRMS (ESI) m/z [M + H]+ calcd for C29H22ClN4O: 477.1482, found 477.1473.
hexane) 0.2; mp: 146–148 °C; IR (DCM): 3338, 1683, 1526 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.81 (s, 1H), 8.74–8.71 (m, 2H), 8.14 (d, 1H, J = 8.2 Hz), 7.95 (d, 2H, J = 8.4 Hz), 7.93 (t, 2H, J = 8.7 Hz), 7.60 (d, 1H, J = 1.3 Hz), 7.57–7.51 (m, 7H), 7.42–7.39 (m, 2H), 7.33 (d, 1H, J = 8.2 Hz), 3.89 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 168.8, 152.6, 151.8, 148.0, 142.7, 140.3, 136.6, 134.3, 134.0, 131.4, 131.2, 130.9, 130.1, 129.1, 127.9, 127.7, 127.5, 123.1, 123.0, 121.8, 121.6, 116.7, 42.4; HRMS (ESI) m/z [M + H]+ calcd for C29H22ClN4O: 477.1482, found 477.1473.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 119–120 °C; IR (DCM): 3304, 1732, 1469 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.75 (s, 1H), 8.72–8.69 (2H, m), 8.14 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.98–7.91 (m, 4H), 7.70 (s, 1H), 7.58–7.49 (m, 8H), 7.41 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.84 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 168.3, 152.5, 152.0, 148.3, 141.7, 141.4, 138.2, 136.3, 134.1, 132.7, 132.7, 132.2, 131.7, 131.4, 131.3, 130.0, 129.1, 127.9, 127.3, 123.2, 123.0, 121.9, 121.7, 116.4, 41.8; HRMS (ESI) m/z [M + H]+ calcd for C29H21Cl2N4O: 511.1092, found 511.1112.
hexane) 0.2; mp: 119–120 °C; IR (DCM): 3304, 1732, 1469 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.75 (s, 1H), 8.72–8.69 (2H, m), 8.14 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.98–7.91 (m, 4H), 7.70 (s, 1H), 7.58–7.49 (m, 8H), 7.41 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.84 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 168.3, 152.5, 152.0, 148.3, 141.7, 141.4, 138.2, 136.3, 134.1, 132.7, 132.7, 132.2, 131.7, 131.4, 131.3, 130.0, 129.1, 127.9, 127.3, 123.2, 123.0, 121.9, 121.7, 116.4, 41.8; HRMS (ESI) m/z [M + H]+ calcd for C29H21Cl2N4O: 511.1092, found 511.1112.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 152–154 °C; IR (DCM): 3333, 1681, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.23 (s, 1H), 8.80 (dd, 1H, J1 = 7.5, J2 = 1.3 Hz), 8.75 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.12 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 8.02 (t, 1H, J = 1.5 Hz), 7.95–7.93 (m, 1H), 7.87–7.85 (m, 2H), 7.69–7.66 (m, 1H), 7.59 (t, 1H, J = 7.7 Hz), 7.55–7.46 (m, 5H), 7.43–7.39 (m, 2H), 7.09 (d, 1H, J = 7.7 Hz), 7.04 (d, 1H, J = 8.1 Hz), 4.00 (s, 3H), 3.92 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 170.0, 157.9, 152.6, 152.5, 148.0, 143.7, 142.0, 138.6, 136.2, 135.0, 132.3, 131.0, 129.0, 128.9, 128.2, 128.0, 127.4, 123.4, 122.9, 122.8, 122.2, 121.7, 121.4, 121.2, 116.5, 109.7, 56.0, 37.3; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1961.
hexane) 0.2; mp: 152–154 °C; IR (DCM): 3333, 1681, 1528 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.23 (s, 1H), 8.80 (dd, 1H, J1 = 7.5, J2 = 1.3 Hz), 8.75 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.12 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 8.02 (t, 1H, J = 1.5 Hz), 7.95–7.93 (m, 1H), 7.87–7.85 (m, 2H), 7.69–7.66 (m, 1H), 7.59 (t, 1H, J = 7.7 Hz), 7.55–7.46 (m, 5H), 7.43–7.39 (m, 2H), 7.09 (d, 1H, J = 7.7 Hz), 7.04 (d, 1H, J = 8.1 Hz), 4.00 (s, 3H), 3.92 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 170.0, 157.9, 152.6, 152.5, 148.0, 143.7, 142.0, 138.6, 136.2, 135.0, 132.3, 131.0, 129.0, 128.9, 128.2, 128.0, 127.4, 123.4, 122.9, 122.8, 122.2, 121.7, 121.4, 121.2, 116.5, 109.7, 56.0, 37.3; HRMS (ESI) m/z [M + H]+ calcd for C30H25N4O2: 473.1978, found 473.1961.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 158–160 °C; IR (DCM): 3369, 1684, 1527 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.20 (s, 1H), 8.80–8.79 (m, 1H), 8.74 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.16 (dd, 1H, J1 = 8.2, J2 = 1.6 Hz), 7.88–7.86 (m, 2H), 7.64–7.60 (m, 2H), 7.56–7.48 (m, 2H), 7.43 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.38 (t, 1H, J = 8.0 Hz), 7.03–7.01 (m, 2H), 4.21 (q, 2H, J = 7.0 Hz), 3.88 (s, 2H), 2.77 (s, 3H), 2.55 (s, 3H), 1.41 (t, 3H, J = 7.0); 13C NMR (∼101 MHz, CDCl3): δC 170.2, 169.4, 157.2, 153.8, 152.0, 148.0, 143.6, 138.5, 136.2, 135.0, 132.5, 130.6, 130.3, 128.1, 128.0, 127.4, 122.3, 122.1, 121.8, 121.5, 121.2, 116.4, 110.6, 64.1, 37.4, 14.7, 12.2, 11.6; HRMS (ESI) m/z [M + H]+ calcd for C30H28N5O3: 506.2192, found 506.2211.
hexane) 0.2; mp: 158–160 °C; IR (DCM): 3369, 1684, 1527 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.20 (s, 1H), 8.80–8.79 (m, 1H), 8.74 (dd, 1H, J1 = 4.2, J2 = 1.6 Hz), 8.16 (dd, 1H, J1 = 8.2, J2 = 1.6 Hz), 7.88–7.86 (m, 2H), 7.64–7.60 (m, 2H), 7.56–7.48 (m, 2H), 7.43 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 7.38 (t, 1H, J = 8.0 Hz), 7.03–7.01 (m, 2H), 4.21 (q, 2H, J = 7.0 Hz), 3.88 (s, 2H), 2.77 (s, 3H), 2.55 (s, 3H), 1.41 (t, 3H, J = 7.0); 13C NMR (∼101 MHz, CDCl3): δC 170.2, 169.4, 157.2, 153.8, 152.0, 148.0, 143.6, 138.5, 136.2, 135.0, 132.5, 130.6, 130.3, 128.1, 128.0, 127.4, 122.3, 122.1, 121.8, 121.5, 121.2, 116.4, 110.6, 64.1, 37.4, 14.7, 12.2, 11.6; HRMS (ESI) m/z [M + H]+ calcd for C30H28N5O3: 506.2192, found 506.2211.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; IR (DCM): 3341, 1684, 1527 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.41 (s, 1H), 8.65 (dd, 1H, J1 = 7.4, J2 = 1.3 Hz), 8.57 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.00 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.89–7.84 (m, 8H), 7.64–7.62 (m, 4H), 7.55–7.48 (m, 8H), 7.47–7.43 (m, 3H), 7.21 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.88 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 169.6, 152.6, 151.6, 148.0, 144.3, 143.2, 138.1, 136.0, 134.3, 131.0, 130.6, 130.2, 129.8, 129.1, 129.0, 127.8, 127.3, 127.2, 122.9, 121.4, 121.4, 116.1, 40.3; HRMS (ESI) m/z [M + H]+ calcd for C41H31N6O: 623.2559, found 623.2558.
hexane) 0.2; IR (DCM): 3341, 1684, 1527 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.41 (s, 1H), 8.65 (dd, 1H, J1 = 7.4, J2 = 1.3 Hz), 8.57 (dd, 1H, J1 = 4.2, J2 = 1.7 Hz), 8.00 (dd, 1H, J1 = 8.3, J2 = 1.6 Hz), 7.89–7.84 (m, 8H), 7.64–7.62 (m, 4H), 7.55–7.48 (m, 8H), 7.47–7.43 (m, 3H), 7.21 (dd, 1H, J1 = 8.3, J2 = 4.2 Hz), 3.88 (s, 2H); 13C NMR (∼101 MHz, CDCl3): δC 169.6, 152.6, 151.6, 148.0, 144.3, 143.2, 138.1, 136.0, 134.3, 131.0, 130.6, 130.2, 129.8, 129.1, 129.0, 127.8, 127.3, 127.2, 122.9, 121.4, 121.4, 116.1, 40.3; HRMS (ESI) m/z [M + H]+ calcd for C41H31N6O: 623.2559, found 623.2558.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 99–100 °C; IR (DCM): 3319, 1676, 1520 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55–8.53 (m, 1H), 8.39 (s, 1H), 8.20 (dt, 1H, J1 = 7.9, J2 = 1.0 Hz), 8.00 (dt, 2H, J1 = 8.7, J2 = 2.0 Hz), 7.95–7.93 (m, 2H), 7.83 (td, 1H, J1 = 7.8, J2 = 1.7 Hz), 7.57–7.49 (m, 5H), 7.42–7.57 (m, 2H), 7.02–6.98 (m, 2H), 4.68 (d, 2H, J = 5.5 Hz), 3.99 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 163.4, 158.8, 152.8, 151.7, 150.3, 148.0, 143.4, 143.2, 137.2, 131.0, 130.1, 129.1, 128.6, 126.0, 123.7, 122.9, 122.6, 122.3, 110.0, 55.9, 36.8; HRMS (ESI) m/z [M + H]+ calcd for C26H23N4O2: 423.1821, found 423.1833.
hexane) 0.2; mp: 99–100 °C; IR (DCM): 3319, 1676, 1520 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55–8.53 (m, 1H), 8.39 (s, 1H), 8.20 (dt, 1H, J1 = 7.9, J2 = 1.0 Hz), 8.00 (dt, 2H, J1 = 8.7, J2 = 2.0 Hz), 7.95–7.93 (m, 2H), 7.83 (td, 1H, J1 = 7.8, J2 = 1.7 Hz), 7.57–7.49 (m, 5H), 7.42–7.57 (m, 2H), 7.02–6.98 (m, 2H), 4.68 (d, 2H, J = 5.5 Hz), 3.99 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 163.4, 158.8, 152.8, 151.7, 150.3, 148.0, 143.4, 143.2, 137.2, 131.0, 130.1, 129.1, 128.6, 126.0, 123.7, 122.9, 122.6, 122.3, 110.0, 55.9, 36.8; HRMS (ESI) m/z [M + H]+ calcd for C26H23N4O2: 423.1821, found 423.1833.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 123–125 °C; IR (DCM): 3387, 1677, 1516 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55–8.53 (m, 1H), 8.25 (m, 1H), 8.19–8.17 (m, 1H), 7.99 (dt, 2H, J1 = 8.7, J2 = 2.1 Hz), 7.96–7.93 (m, 2H), 7.84 (td, 1H, J1 = 7.8, J2 = 1.7 Hz), 7.58–7.48 (m, 6H), 7.45–7.41 (m, 1H), 7.36 (t, 1H, J = 7.8 Hz), 7.30 (d, 1H, J = 1.4 Hz), 4.76 (d, 2H, J = 5.3 Hz); 13C NMR (∼101 MHz, CDCl3): δC 163.5, 152.7, 152.0, 149.7, 148.1, 144.4, 142.7, 137.3, 136.1, 133.0, 131.1, 129.9, 129.4, 129.1, 129.0, 128.9, 126.1, 123.0, 122.9, 122.3, 39.6; HRMS (ESI) m/z [M + H]+ calcd for C25H20ClN4O2: 427.1326, found 427.1342.
hexane) 0.2; mp: 123–125 °C; IR (DCM): 3387, 1677, 1516 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55–8.53 (m, 1H), 8.25 (m, 1H), 8.19–8.17 (m, 1H), 7.99 (dt, 2H, J1 = 8.7, J2 = 2.1 Hz), 7.96–7.93 (m, 2H), 7.84 (td, 1H, J1 = 7.8, J2 = 1.7 Hz), 7.58–7.48 (m, 6H), 7.45–7.41 (m, 1H), 7.36 (t, 1H, J = 7.8 Hz), 7.30 (d, 1H, J = 1.4 Hz), 4.76 (d, 2H, J = 5.3 Hz); 13C NMR (∼101 MHz, CDCl3): δC 163.5, 152.7, 152.0, 149.7, 148.1, 144.4, 142.7, 137.3, 136.1, 133.0, 131.1, 129.9, 129.4, 129.1, 129.0, 128.9, 126.1, 123.0, 122.9, 122.3, 39.6; HRMS (ESI) m/z [M + H]+ calcd for C25H20ClN4O2: 427.1326, found 427.1342.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 120–122 °C; IR (DCM): 3377, 1673, 1517 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55 (d, 1H, J = 4.6 Hz), 8.47–8.40 (m, 1H), 8.26 (d, 1H, J = 7.8 Hz), 8.02 (d, 2H, J = 8.1 Hz), 7.97 (d, 2H, J = 8.0 Hz), 7.87 (t, 1H, J = 7.7 Hz), 7.60 (d, 2H, J = 8.1 Hz), 7.58–7.48 (m, 3H), 7.46–7.43 (m, 1H), 7.33 (d, 1H, J = 5.2 Hz), 7.16 (d, 1H, J = 5.2 Hz), 4.95 (d, 2H, J = 5.8 Hz); 13C NMR (∼101 MHz, CDCl3): δC 164.1, 152.7, 151.6, 149.5, 148.2, 139.6, 138.6, 137.4, 136.5, 131.1, 129.5, 129.1, 129.1, 126.4, 124.5, 123.2, 122.9, 122.4, 37.2; HRMS (ESI) m/z [M + Na]+ calcd for C23H18N4NaOS: 421.1099, found 421.1112.
hexane) 0.2; mp: 120–122 °C; IR (DCM): 3377, 1673, 1517 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.55 (d, 1H, J = 4.6 Hz), 8.47–8.40 (m, 1H), 8.26 (d, 1H, J = 7.8 Hz), 8.02 (d, 2H, J = 8.1 Hz), 7.97 (d, 2H, J = 8.0 Hz), 7.87 (t, 1H, J = 7.7 Hz), 7.60 (d, 2H, J = 8.1 Hz), 7.58–7.48 (m, 3H), 7.46–7.43 (m, 1H), 7.33 (d, 1H, J = 5.2 Hz), 7.16 (d, 1H, J = 5.2 Hz), 4.95 (d, 2H, J = 5.8 Hz); 13C NMR (∼101 MHz, CDCl3): δC 164.1, 152.7, 151.6, 149.5, 148.2, 139.6, 138.6, 137.4, 136.5, 131.1, 129.5, 129.1, 129.1, 126.4, 124.5, 123.2, 122.9, 122.4, 37.2; HRMS (ESI) m/z [M + Na]+ calcd for C23H18N4NaOS: 421.1099, found 421.1112.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; IR (DCM): 3386, 1677, 1512 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.44 (d, 1H, J = 4.5 Hz), 7.97–7.93 (m, 9H), 7.91–7.86 (m, 1H), 7.73 (t, 1H, J = 7.7 Hz), 7.61–7.57 (m, 5H), 7.56–7.48 (m, 6H), 7.41 (d, 2H, J = 7.6 Hz), 7.37–7.34 (m, 1H), 4.66 (d, 2H, J = 4.9 Hz); 13C NMR (∼101 MHz, CDCl3): δC 163.0, 152.7, 151.7, 149.5, 147.8, 143.9, 143.0, 137.1, 132.8, 131.0, 130.1, 129.9, 129.1, 127.6, 126.0, 122.9, 122.9, 122.0, 39.4; HRMS (ESI) m/z [M + H]+ calcd for C37H29N6O: 573.2403, found 573.2420.
hexane) 0.2; IR (DCM): 3386, 1677, 1512 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.44 (d, 1H, J = 4.5 Hz), 7.97–7.93 (m, 9H), 7.91–7.86 (m, 1H), 7.73 (t, 1H, J = 7.7 Hz), 7.61–7.57 (m, 5H), 7.56–7.48 (m, 6H), 7.41 (d, 2H, J = 7.6 Hz), 7.37–7.34 (m, 1H), 4.66 (d, 2H, J = 4.9 Hz); 13C NMR (∼101 MHz, CDCl3): δC 163.0, 152.7, 151.7, 149.5, 147.8, 143.9, 143.0, 137.1, 132.8, 131.0, 130.1, 129.9, 129.1, 127.6, 126.0, 122.9, 122.9, 122.0, 39.4; HRMS (ESI) m/z [M + H]+ calcd for C37H29N6O: 573.2403, found 573.2420.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.1; mp: 190–192 °C; IR (DCM): 2918, 1679, 1285 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.96 (d, 2H, J = 8.4 Hz), 7.95 (d, 2H, J = 7.0 Hz), 7.83 (s, 1H), 7.56–7.48 (m, 5H), 7.42 (dd, 1H, J1 = 7.9, J2 = 1.0 Hz), 7.32 (d, 1H, J = 7.8 Hz), 2.46 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 173.4, 152.8, 151.6, 144.0, 139.9, 137.7, 133.1, 131.5, 131.1, 131.0, 129.4, 129.1, 129.0, 122.9, 122.7, 21.0; HRMS (ESI) m/z [M + H]+ calcd for C20H17N2O2: 317.1290, found 317.1274 (in the proton NMR, the COOH signal was not clearly visible).
hexane) 0.1; mp: 190–192 °C; IR (DCM): 2918, 1679, 1285 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.96 (d, 2H, J = 8.4 Hz), 7.95 (d, 2H, J = 7.0 Hz), 7.83 (s, 1H), 7.56–7.48 (m, 5H), 7.42 (dd, 1H, J1 = 7.9, J2 = 1.0 Hz), 7.32 (d, 1H, J = 7.8 Hz), 2.46 (s, 3H); 13C NMR (∼101 MHz, CDCl3): δC 173.4, 152.8, 151.6, 144.0, 139.9, 137.7, 133.1, 131.5, 131.1, 131.0, 129.4, 129.1, 129.0, 122.9, 122.7, 21.0; HRMS (ESI) m/z [M + H]+ calcd for C20H17N2O2: 317.1290, found 317.1274 (in the proton NMR, the COOH signal was not clearly visible).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.2; mp: 170–172 °C; IR (DCM): 2933, 1723, 1276 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.90 (d, 2H, J = 7.4 Hz), 7.86 (d, 2H, J = 7.9 Hz), 7.54–7.46 (m, 3H), 7.40 (d, 2H, J = 7.8 Hz), 7.34–7.21 (m, 5H), 4.67–4.61 (m, 1H), 3.21–3.12 (m, 2H); 13C NMR (∼101 MHz, CDCl3): δC 152.7, 151.4, 146.4, 142.7, 130.9, 129.1 128.8, 128.4, 127.6, 126.9, 123.2, 122.8, 46.5, 38.7; HRMS (ESI) m/z [M + H]+ calcd for C21H19N2O2: 331.1447, found 331.1431 (in the proton NMR, the COOH signal and in the carbon NMR, the carbonyl peak of COOH were not clearly visible).
hexane) 0.2; mp: 170–172 °C; IR (DCM): 2933, 1723, 1276 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.90 (d, 2H, J = 7.4 Hz), 7.86 (d, 2H, J = 7.9 Hz), 7.54–7.46 (m, 3H), 7.40 (d, 2H, J = 7.8 Hz), 7.34–7.21 (m, 5H), 4.67–4.61 (m, 1H), 3.21–3.12 (m, 2H); 13C NMR (∼101 MHz, CDCl3): δC 152.7, 151.4, 146.4, 142.7, 130.9, 129.1 128.8, 128.4, 127.6, 126.9, 123.2, 122.8, 46.5, 38.7; HRMS (ESI) m/z [M + H]+ calcd for C21H19N2O2: 331.1447, found 331.1431 (in the proton NMR, the COOH signal and in the carbon NMR, the carbonyl peak of COOH were not clearly visible).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) 0.1; mp: 252–254 °C; IR (DCM): 2928, 1679, 1294 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 7.92–7.90 (m, 5H), 7.70 (d, 2H, J = 8.4 Hz), 7.64–7.56 (m, 3H), 7.28 (d, 1H, J = 5.1 Hz); 13C NMR (∼101 MHz, DMSO-d6): δC 163.3, 152.5, 151.6, 146.4, 139.1, 132.1, 132.0, 131.8, 131.0, 130.0, 129.5, 123.1, 122.4; HRMS (ESI) m/z [M + H]+ calcd for C17H13N2O2S: 309.0698, found 309.0683 (in the proton NMR, the COOH signal seems to be appearing as a broad signal around 3–3.5 ppm).
hexane) 0.1; mp: 252–254 °C; IR (DCM): 2928, 1679, 1294 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 7.92–7.90 (m, 5H), 7.70 (d, 2H, J = 8.4 Hz), 7.64–7.56 (m, 3H), 7.28 (d, 1H, J = 5.1 Hz); 13C NMR (∼101 MHz, DMSO-d6): δC 163.3, 152.5, 151.6, 146.4, 139.1, 132.1, 132.0, 131.8, 131.0, 130.0, 129.5, 123.1, 122.4; HRMS (ESI) m/z [M + H]+ calcd for C17H13N2O2S: 309.0698, found 309.0683 (in the proton NMR, the COOH signal seems to be appearing as a broad signal around 3–3.5 ppm).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
IISER Mohali is thanked for providing the funding, NMR, HRMS and X-ray facilities. S. S. thanks IISER Mohali for providing the PhD fellowship.References
- (a) J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508 CrossRef; (b) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (c) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed; (d) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS PubMed; (e) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (f) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2013, 53, 74 CrossRef PubMed; (g) E.-i. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738 CrossRef CAS PubMed; (h) R. F. Heck, Acc. Chem. Res., 1979, 12, 146 CrossRef CAS.
- (a) S. P. Stanforth, Tetrahedron, 1998, 54, 263 CrossRef CAS; (b) F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 2419 CAS; (c) A. Biffis, P. Centomo, A. D. Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249 CrossRef CAS PubMed; (d) A. de Meijere, S. Bräse and M. Oestreich, Metal-Catalyzed Cross-Coupling Reactions and More, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2014, vol. 1–3 CrossRef; (e) J. P. Sestelo and L. A. Sarandeses, Molecules, 2020, 25, 4500 CrossRef CAS PubMed; (f) Á. Molnár, Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Developments, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013 CrossRef.
- For selected reviews dealing with the application of biaryls, see: (a) S. Yuan, J. Chang and B. Yu, Top. Curr. Chem., 2020, 378, 23 CrossRef CAS PubMed; (b) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS PubMed; (c) J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177 CrossRef CAS PubMed; (d) C. Liu, C.-L. Ji, Z.-X. Qin, X. Hong and M. Szostak, iScience, 2019, 19, 749 CrossRef CAS PubMed; (e) M. C. Kozlowski, B. J. Morgan and E. C. Linton, Chem. Soc. Rev., 2009, 38, 3193 RSC; (f) F.-X. Felpin and S. Sengupta, Chem. Soc. Rev., 2019, 48, 1150 RSC; (g) Y.-F. Zhang and Z.-J. Shi, Acc. Chem. Res., 2019, 52, 161 CrossRef CAS PubMed; (h) J. E. Anthony, Chem. Rev., 2006, 106, 5028 CrossRef CAS PubMed.
- (a) S. Murahashi, J. Am. Chem. Soc., 1955, 77, 6403 CrossRef CAS; (b) S. Murahashi and S. Horiie, J. Am. Chem. Soc., 1956, 78, 4816 CrossRef CAS; (c) D. R. Fahey, J. Organomet. Chem., 1971, 27, 283 CrossRef CAS; (d) D. R. Fahey, J. Chem. Soc. D, 1970, 417a RSC.
- (a) F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826 CrossRef CAS PubMed; (b) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (c) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (d) P. A. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (e) N. Kuhl, M. H. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236 CrossRef CAS PubMed; (f) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Y. Yu, Chem. Rev., 2017, 117, 8754 CrossRef CAS PubMed; (g) T. Yoshinoa and S. Matsunaga, Adv. Synth. Catal., 2017, 359, 1245 CrossRef; (h) J. J. Topczewski and M. S. Sanford, Chem. Sci., 2015, 6, 70 RSC; (i) T. Besset, T. Poisson and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 16830 CrossRef CAS PubMed; (j) A. Banerjee, S. Sarkar and B. K. Patel, Org. Biomol. Chem., 2017, 15, 505 RSC; (k) M. Maraswami and T.-P. Loh, Synthesis, 2019, 51, 1049 CrossRef CAS; (l) R. Mandal, B. Garai and B. Sundararaju, ACS Catal., 2022, 12, 3452 CrossRef CAS; (m) M. Miura, T. Satoh and K. Hirano, Bull. Chem. Soc. Jpn., 2014, 87, 751 CrossRef CAS; (n) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem. – Eur. J., 2010, 16, 2654 CrossRef CAS PubMed.
- (a) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107 RSC; (b) S. V. Kumar, S. Banerjee and T. Punniyamurthy, Org. Chem. Front., 2020, 7, 1527 RSC; (c) R. Das, G. S. Kumar and M. Kapur, Eur. J. Org. Chem., 2017, 5439 CrossRef CAS; (d) S. M. Ujwaldev, N. A. Harry, M. A. Divakar and G. Anilkumar, Catal. Sci. Technol., 2018, 8, 5983 RSC; (e) T. Yanagi, K. Nogi and H. Yorimitsu, Tetrahedron Lett., 2018, 59, 2951 CrossRef CAS; (f) W. Ali, G. Prakash and D. Maiti, Chem. Sci., 2021, 12, 2735 RSC; (g) S. K. Sinha, S. Guin, S. Maiti, J. P. Biswas, S. Porey and D. Maiti, Chem. Rev., 2022, 122, 5682 CrossRef CAS PubMed; (h) R. Manoharan and M. Jeganmohan, Asian J. Org. Chem., 2019, 8, 1949 CrossRef CAS; (i) J. I. Higham and J. A. Bull, Org. Biomol. Chem., 2020, 18, 7291 RSC.
- (a) T. H. L. Nguyen, N. Gigant and D. Joseph, ACS Catal., 2018, 8, 1546 CrossRef CAS; (b) N. K. Mishra, J. Park, H. Oh, S. H. Han and I. S. Kim, Tetrahedron, 2018, 74, 6769 CrossRef CAS.
- (a) S. Rej, Y. Ano and N. Chatani, Chem. Rev., 2020, 120, 1788 CrossRef CAS PubMed; (b) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053 CrossRef CAS PubMed; (c) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603 RSC; (d) X. Yang, G. Shan, L. Wang and Y. Rao, Tetrahedron Lett., 2016, 57, 819 CrossRef CAS; (e) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B.-F. Shi, Chem. Rev., 2021, 121, 14957 CrossRef CAS PubMed; (f) R. K. Rit, M. R. Yadav, K. Ghosh and A. K. Sahoo, Tetrahedron, 2015, 71, 4450 CrossRef CAS; (g) G. He, B. Wang, W. A. Nack and G. Chen, Acc. Chem. Res., 2016, 49, 635 CrossRef CAS PubMed; (h) S. A. Babu, Y. Aggarwal, P. Patel and R. Tomar, Chem. Commun., 2022, 58, 2612 RSC; (i) S.-F. Ni, G. Huang, Y. Chen, J. S. Wright, M. Li and L. Dang, Coord. Chem. Rev., 2022, 455, 214255 CrossRef CAS.
- For selected papers dealing with the bidentate DG-aided C–H functionalization, see: (a) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS PubMed; (b) E. T. Nadres, G. I. F. Santos, D. Shabashov and O. Daugulis, J. Org. Chem., 2013, 78, 9689 CrossRef CAS PubMed; (c) F.-J. Chen, S. Zhao, F. Hu, K. Chen, Q. Zhang, S.-Q. Zhang and B.-F. Shi, Chem. Sci., 2013, 4, 4187 RSC; (d) A. García-Rubia, B. Urones, R. G. Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2011, 50, 10927 CrossRef PubMed; (e) G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124 CrossRef CAS PubMed; (f) N. Hoshiya, K. Takenaka, S. Shuto and J. Uenishi, Org. Lett., 2016, 18, 48 CrossRef CAS PubMed; (g) K. S. Kanyiva, Y. Kuninobu and M. Kanai, Org. Lett., 2014, 16, 1968 CrossRef CAS PubMed; (h) J. Ghouilem, C. Tran, N. Grimblat, P. Retailleau, M. Alami, V. Gandon and S. Messaoudi, ACS Catal., 2021, 11, 1818 CrossRef CAS; (i) C. Wang, L. Zhang and J. You, Org. Lett., 2017, 19, 1690 CrossRef CAS PubMed; (j) P. Hu and T. Bach, Synlett, 2015, 26, 2853 CrossRef CAS; (k) A. Seki and Y. Takahashi, Tetrahedron Lett., 2021, 74, 153130 CrossRef CAS; (l) J. Bolsakova, L. Lukasevics and L. Grigorjeva, J. Org. Chem., 2020, 85, 4482 CrossRef CAS PubMed; (m) D. A.-P. de León, A. C. Sánchez-Chávez and L. A. Polindara-García, J. Org. Chem., 2019, 84, 1289 Search PubMed; For selected papers on monodentate amide DG-aided C–H functionalization, see: (n) M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3680 CrossRef CAS PubMed; (o) E. J. Yoo, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 17378 CrossRef CAS PubMed; (p) Q. Gu, H. H. A. Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868 CrossRef CAS PubMed.
- For selected papers from our group dealing with bidentate DG-aided C–H functionalization, see: (a) R. Parella and S. A. Babu, J. Org. Chem., 2015, 80, 12379 CrossRef CAS PubMed; (b) R. Parella and S. A. Babu, J. Org. Chem., 2017, 82, 7123 CrossRef CAS PubMed; (c) B. Gopalakrishnan, S. Mohan, R. Parella and S. A. Babu, J. Org. Chem., 2016, 81, 8988 CrossRef CAS PubMed; (d) R. Padmavathi, R. Sankar, B. Gopalakrishnan, R. Parella and S. A. Babu, Eur. J. Org. Chem., 2015, 3727 CrossRef CAS; (e) N. Bisht, P. Singh and S. A. Babu, Synthesis, 2022, 54, 4059 CrossRef CAS; (f) Naveen, V. Rajkumar, S. A. Babu and B. Gopalakrishnan, J. Org. Chem., 2016, 81, 12197 CrossRef CAS PubMed; (g) R. Padmavathi and S. A. Babu, Asian J. Org. Chem., 2019, 8, 899 CrossRef CAS; (h) R. Parella and S. A. Babu, J. Org. Chem., 2017, 82, 6550 CrossRef CAS PubMed; (i) R. Kaur, S. Banga and S. A. Babu, Org. Biomol. Chem., 2022, 20, 4391 RSC; (j) C. Reddy, N. Bisht, R. Parella and S. A. Babu, J. Org. Chem., 2016, 81, 12143 CrossRef CAS PubMed; (k) R. Tomar, S. Suwasia, S. Venkataramani and S. A. Babu, Chem. Commun., 2022, 58, 12967 RSC; (l) R. Padmavathi and S. A. Babu, Org. Biomol. Chem., 2023 10.1039/D2OB02261C.
- Selected reviews dealing with azobenzenes: (a) E. Léonard, F. Mangin, C. Villette, M. Billamboz and C. Len, Catal. Sci. Technol., 2016, 6, 379 RSC; (b) B. Tylkowski, R. Jastrąb and M. Skrobańska, Phys. Sci. Rev., 2016, 20160002 Search PubMed; (c) H.-B. Cheng, S. Zhang, J. Qi, X.-J. Liang and J. Yoon, Adv. Mater., 2021, 33, 2007290 CrossRef CAS PubMed; (d) F. Hamon, F. Djedaini-Pilard, F. Barbot and C. Len, Tetrahedron, 2009, 65, 10105 CrossRef CAS; (e) E. Merino, Chem. Soc. Rev., 2011, 40, 3835 RSC; (f) C. Y. Chang, C. Fedele, A. Priimagi, A. Shishido and C. J. Barrett, Adv. Opt. Mater., 2019, 7, 1900091 CrossRef; (g) X. Pang, J.-a. Lv, C. Zhu, L. Qin and Y. Yu, Adv. Mater., 2019, 31, 1904224 CrossRef CAS PubMed; (h) M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry and G. A. Woolley, Acc. Chem. Res., 2015, 48, 2662 CrossRef CAS PubMed; (i) W.-C. Xu, S. Sun and S. Wu, Angew. Chem., Int. Ed., 2019, 58, 9712 CrossRef CAS PubMed; (j) A. Chevalier, P.-Y. Renard and A. Romieu, Chem. – Asian J., 2017, 12, 2008 CrossRef CAS PubMed; (k) H. M. D. Bandarab and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809 RSC; (l) A. S. Amutha, K. R. S. Kumar and N. Tamaoki, ChemPhotoChem, 2019, 3, 337 CrossRef; (m) E. Wagner-Wysiecka, N. Łukasik, J. F. Biernat and E. Luboch, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 189 CrossRef CAS PubMed; (n) M. Zhu and H. Zhou, Org. Biomol. Chem., 2018, 16, 8434 RSC; (o) S. Crespi, N. A. Simeth and B. König, Nat. Rev., 2019, 3, 133 CAS.
- For selected papers dealing with bioactive azobenzenes, see: (a) M. V. Westphal, M. A. Schafroth, R. C. Sarott, M. A. Imhof, C. P. Bold, P. Leippe, A. Dhopeshwarkar, J. M. Grandner, V. Katritch, K. Mackie, D. Trauner, E. M. Carreira and J. A. Frank, J. Am. Chem. Soc., 2017, 139, 18206 CrossRef CAS PubMed; (b) K. A. Palasis, N. A. Lokman, B. C. Quirk, A. Adwal, L. Scolaro, W. Huang, C. Ricciardelli, M. K. Oehler, R. A. McLaughlin and A. D. Abell, Int. J. Mol. Sci., 2021, 22, 10844 CrossRef CAS PubMed; (c) B. Blanco, K. A. Palasis, A. Adwal, D. F. Callen and A. D. Abell, Bioorg. Med. Chem., 2017, 25, 5050 CrossRef CAS PubMed; (d) E. R. Thapaliya, J. Zhao and G. C. R. Ellis-Davies, ACS Chem. Neurosci., 2019, 10, 2481 CrossRef CAS PubMed; (e) P. Verwilst, J. Han, J. Lee, S. Mun, H.-G. Kang and J. S. Kim, Biomaterials, 2017, 115, 104 CrossRef CAS PubMed; (f) M. Wegener, M. J. Hansen, A. J. M. Driessen, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2017, 139, 17979 CrossRef CAS PubMed; (g) W. Szymanski, M. E. Ourailidou, W. A. Velema, F. J. Dekker and B. L. Feringa, Chem. – Eur. J., 2015, 21, 16517 CrossRef CAS PubMed.
- For selected reviews/papers dealing with the cross-coupling-based synthesis of biaryl-based or modified azobenzenes, see: (a) M. Walther, W. Kipke, S. Schultzke, S. Ghosh and A. Staubitz, Synthesis, 2021, 53, 1213 CrossRef CAS; (b) J. Strueben, P. J. Gates and A. Staubitz, J. Org. Chem., 2014, 79, 1719 CrossRef CAS PubMed; (c) J. H. Harvey, B. K. Butler and D. Trauner, Tetrahedron Lett., 2007, 48, 1661 CrossRef CAS; (d) B.-C. Yu, Y. Shirai and J. M. Tour, Tetrahedron, 2006, 62, 10303 CrossRef CAS; (e) M. Han, D. Ishikawa, E. Muto and M. Hara, J. Lumin., 2009, 129, 1163 CrossRef CAS; (f) M. Han, Y. Norikane, K. Onda, Y. Matsuzawa, M. Yoashida and M. Hara, New J. Chem., 2010, 34, 2892 RSC; (g) M. R. Han, D. Hashizume and M. Hara, New J. Chem., 2007, 31, 1746 RSC; (h) M. Han, M. Hara, K. Fukasawa and M. Shimomura, WO2008111637A1, 2008; (i) I. Köhl and U. Lüning, Synthesis, 2014, 46, 2376 CrossRef; (j) M. Han and M. Hara, J. Am. Chem. Soc., 2005, 127, 10951 CrossRef CAS PubMed; (k) D. Peng and C. Chen, Angew. Chem., Int. Ed., 2021, 60, 22195 CrossRef CAS PubMed , this paper was published during the investigation of the current work and reported an example of picolinamide-aided C–H functionalization of 1-naphthylamine with iodoazobenzene for preparing the required substrate for their study.
- UV-Vis absorption (λmax (nm)) value of compounds, 5a: 331, 7a: 347, 7b: 346, 5b: 330, 5c: 332, 5d: 332, 5e: 334, 5f: 333, 5g: 330, 5h: 330, 5i: 335, 5j: 338, 8a: 328, 8b: 326, 8c: 330, 8d: 330, 10a: 337, 10b: 335, 10c: 341, 10d: 340, 10e: 338, 10f: 337, 12a: 336, 14a: 348, 15a: 327, 15b: 338, 15c: 337, 15d: 335, 15e: 337, 15f: 346, 15g: 334, 15h: 337, 16a: 342, 16b: 346, 17a: 332, 18a: 335, 19a: 322, 20a: 328, 20b: 330, 20c: 326, 22a: 330, 22b: 329, 22c: 328, 22d: 330, 22e: 329, 22f: 329, 22g: 330, 22h: 329, 22i: 330, 24a: 333, 24b: 332, 24c: 332, 24d 331, 26a: 332, 26b: 330, 26c: 331, 26d: 332, 26e: 324, 26f: 329, 26g: 345, 28a: 345, 28b: 336, 28c: 354, 28d: 342, 29a: 345, 29b: 334, 29c: 348 (nm) (the corresponding UV-Vis absorption spectral data are given in the ESI†).
- P. Kumar, A. Srivastava, C. Sah, S. Devi and S. Venkataramani, Chem. – Eur. J., 2019, 25, 11924 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2233013. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ob02322a |
| This journal is © The Royal Society of Chemistry 2023 |