 Open Access Article
Open Access ArticleFriedel–Crafts arylation of aldehydes with indoles utilizing arylazo sulfones as the photoacid generator†
Eirini M.
Galathri
a,
Lorenzo
Di Terlizzi
 b,
Maurizio
Fagnoni
b,
Maurizio
Fagnoni
 b,
Stefano
Protti
b,
Stefano
Protti
 b and
Christoforos G.
Kokotos
b and
Christoforos G.
Kokotos
 *a
*a
aLaboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece. E-mail: ckokotos@chem.uoa.gr
bPhotoGreen Lab, Department of Chemistry, University of Pavia, Viale Taramelli 12, Pavia 27100, Italy
First published on 9th December 2022
Abstract
A versatile, inexpensive and sustainable protocol for the preparation of valuable bis-indolyl methanes via visible light-mediated, metal-free Friedel–Crafts arylation has been developed. The procedure, that exploits the peculiar behavior of arylazo sulfones as non-ionic photoacid generators (PAGs), was applied to the conversion of a variety of aliphatic and aromatic aldehydes into diarylmethanes in good to highly satisfactory yields, employing a low-catalyst loading (0.5 mol%) and irradiation at 456 nm.
Introduction
Friedel–Crafts processes, introduced in 1877 by Friedel and Crafts,1 are the most known and applied strategies to functionalize an aromatic ring via an Ar–H bond activation, and nowadays constitutes a textbook reaction for undergraduate studies.2 Among the several targets that can be achieved via Friedel–Crafts alkylation, the synthesis of bis-indolyl methanes (BIMs) and their analogues is considered a significant goal, in view of the biological and pharmacological properties of these compounds, including anti-cancer, anti-oxidant, anti-bacterial, anti-inflammatory, and anti-proliferative activity.3 Furthermore, an indole-3-carbinol metabolite, the dimer 3,3′-bis-indolyl-methane, plays a key-role in the prevention of breast cancer.4 The interest for BIMs is evidenced by the impressive variety of synthetic routes proposed for their preparation, being the Friedel–Crafts-type coupling between aldehydes and indoles the most common one (Scheme 1). In such traditional approach, an acid, either Lewis or Brønsted, is usually required and the use of high temperature or toxic reagents (Scheme 1a) represents the main limitation.5 To improve the sustainability of the synthesis of BIMs, neat reaction conditions, infrared6a sonochemistry or microwave irradiations,6 as well as the use of solid-supported acid catalysts7 have been proposed. More recently, different approaches have been applied with success to this target, including, among others, organocatalysis (Scheme 1b),8 electrochemistry (Scheme 1c)9 and even photochemistry,10a the potential of which in terms of sustainability has been pointed out in the last decades.11–13 In this context, the peculiar behavior of Schreiner's thiourea was exploited to promote a photoacidic process under blue light irradiation (Scheme 1d).10a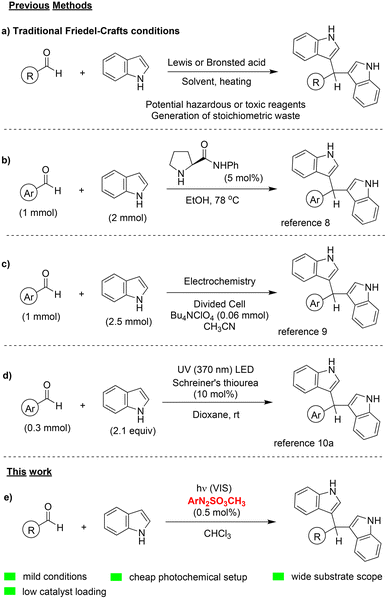 | ||
| Scheme 1 (a-d) Common synthetic pathways for the Friedel–Crafts-type reaction between indoles and aldehydes; (e) our work. | ||
Since 2014, the group of Kokotos is actively involved in the design of innovative organic photocatalysts and photoinitiators and their use in sustainable photocatalyzed processes.14 Furthermore, in the last decade the research group of Fagnoni and Protti has investigated in detail the photoreactivity of arylazo sulfones.15 Such bench stable compounds contained a photolabile dyedauxiliary substituent (CH3SO2N2) that, upon visible light irradiation, undergoes N–S bond homolysis and releases an aryl/methanesulfonyl radical pair.15a Among the different applications of arylazo sulfones in synthesis15 and in chemistry of materials,16 their use as non-ionic photoacid generators (PAGs) able to generate methanesulfonic acid in oxygen-saturated or air equilibrated solution has been recently investigated.17 We thus decided to merge the experience of our groups to propose a fast, versatile and efficient procedure for the visible-light driven synthesis of diarylmethanes via Friedel–Crafts type synthesis of coupling of aldehydes and (hetero)arenes (Scheme 1e).
Results and discussion
We began our investigations by studying the reaction between 3-phenyl propanal (1a) and 1H-indole (2a) to form 3,3′-(3-phenylpropane-1,1-diyl)bis(1H-indole) (4). A detailed optimization of the synthetic procedure considering the irradiation time and wavelengths, catalyst loading, substrates concentration (see Tables S1–5 in the ESI†) has been pursued.18 In particular, we screened a number of common organic solvents (Table S2†), but despite the different green media tested, chloroform proved to be the best (Table S2,† entry 3). We then tested differently substituted arylazo sulfones (3a–h) as PAGs with p-tert-butylphenylazo sulfone (3d) affording the most satisfactory yield (96%) with a very low catalyst loading (0.5 mol%, Table 1).18 Finally, control experiments pointed out that the simultaneous presence of light and the arylazo sulfone is mandatory for the positive outcome of the reaction (Table S8†).18 We thus optimized the reaction conditions as follows: aldehyde 1 (0.5 mmol, 0.5 M), indole 2 (1.1 mmol) and arylazo sulfone 3d (0.5 mol%) in chloroform (1 mL), irradiated at room temperature for 6 h at 456 nm (40 W Kessil lamp).Having in hand the optimized-reaction conditions, we turned our attention in exploring the substrate scope (Schemes 2 and 3). Initially, we employed indole (2a) as representative heteroaromatic and a variety of aliphatic aldehydes were tested (Scheme 2). We began our investigation with aliphatic derivatives, since in literature, to the best of our knowledge, there is a single example concerning the photochemical conversion of an aliphatic aldehyde (1a), that led to 4 in 61% yield.10a Moving to linear substrates, bis-indoles 5 and 6 were isolated in good to high yields. Also, we explored three different cases in the class of α,α-disubstituted aldehydes, and in all cases, the desired products 7–9 were obtained in good yields (66–70%, Scheme 2). We then explored the scope of the aromatic aldehydes. Benzaldehyde provided the double addition product 10 in a good yield. Bromine incorporation either in the para-position or the ortho-position of the aromatic ring was well tolerated, leading to products 11 or 12 in good to excellent yields. Both electron-withdrawing and electron-donating groups were well tolerated, and derivatives 12–18 were isolated in good to excellent yields, with the only exception being that of 4-methoxy derivative 17 (41%). Finally, heteroaromatic thiophene-2-carboxaldehyde (1q) afforded diarylmethane 19 in 79% yield. To further extend the scope of this synthetic proposal, we also envisaged the use of aliphatic aldehydes having double and triple bonds, as well as ketones. Oleyl aldehyde 1q was employed successfully, leading to 20 in 40% yield. Another naturally occurring compound, cinnamaldehyde, led to 21 only in discrete yield, whereas aldehyde 1s that contains a triple bond was also efficiently converted to the corresponding arylated compound 22. In the cases where low yields were obtained, the starting aldehyde (or its corresponding acid via photooxidation) were identified in the NMR of the crude reaction mixture. Unfortunately, ketones did not prove as good substrates. Cyclohexanone required prolonged reaction time (18 h), affording 23 in 40% yield, while acetophenone only gave 17% yield of the isolated product 24 even after 18 h of reaction and an increase of the catalyst loading up to 5 mol%. The latter result comes as no surprise, since when we attempted the same reaction (acetophenone with indole in CHCl3) for 18 h under dark in the presence of 5 mol% MeSO3H, an extremely low yield of 6% of 24 was obtained.
Once the substrate scope of the carbonyl counterpart was investigated, we moved in testing the scope of indoles in photochemical reaction (Scheme 3). A variety of N-substituted indoles were functionalized making use of 3-phenyl propanal (1a) as the reaction partner. Sterically hindered 2-methyl indole was proved to act as a competent nucleophile, providing access to 25 in 95% yield. Then, the substitution pattern on the nitrogen of the indole was probed. Simple N-alkyl substituents, like methyl, butyl or benzyl, secondary alkyl substituents, like cyclopentyl, aryl substituents, like phenyl or allyl substituent were well tolerated, leading to the desired products 26–31 in good to excellent yields.
The photochemical behavior of the arylazo sulfones as visible light PAGs has already been studied in literature17 and a proposed mechanism is described in Scheme 4. Visible-light excitation of 3d results in the homolytic cleavage of the N–S bond to release nitrogen and an aryl (Ar˙)/methane sulfonyl (CH3SO2˙) radical pair (Scheme 4a, path a).15 The CH3SO2˙ is then trapped by oxygen (path b) and generates methanesulfonic acid CH3SO3H. Protonation enhances the electrophilic character of the carbonyl moiety, making it susceptible to nucleophilic attack from the indole (Scheme 4b, paths a and b), leading to intermediate I. Aromatization to II (path c) and protonation thus leads to iminium derivative III (path d), which after indole's nucleophilic addition (path e) and aromatization of the so obtained cation IV (path f) affords the desired product 4–31. In order to verify the reaction mechanism, aldehyde 1a reacted with indole (2a) in the presence of 0.5 mol% of MeSO3H under dark and a lower 63% yield of isolated 4 was obtained after 6 h.
 | ||
| Scheme 4 Proposed reaction mechanism for (a) methanesulfonic acid generation and (b) synthesis of BIMs. | ||
Conclusions
In conclusion, a simple, cheap and efficient photochemical protocol was developed for the activation of aldehydes in their reaction with indoles, leading to triarylmethanes. This method relies on a small organic molecule, an arylazo sulfone, belonging to an intriguing class of shelf-stable and colored compounds for the visible-light photochemical catalytic release of acid under blue LED lamp irradiation. This slow-released acid activates efficiently both aliphatic and aromatic aldehydes to react, leading to diarylmethanes in good to high yields by adopting only a very low catalyst loading (0.5 mol%). We believe this activation mode will find more applications in the near future.Experimental
General procedure for the photochemical reaction between indoles and aldehydes
In a glass vial, catalyst 3d (0.9 mg, 0.0025 mmol 0.5 mol%) in CHCl3 (1 mL), aldehyde 1 (0.50 mmol, 0.5 M) and indole 2 (1.10 mmol) were added consecutively. The vial was left stirring under blue LED bulb irradiation (456 nm) for 6 h (except where otherwise noticed, see ESI† for further details). The desired product was isolated after purification by column chromatography.Author contributions
Conceptualization: C. G. K. and S. P.; reaction optimization, substrate scope and compound characterization: E. G.; synthesis of catalysts: L. T. and S. P.; writing original draft: E. G. and C. G. K.; writing, reviewing and editing: S. P., M. F. and C. G. K.; supervision and project administration: C. G. K.; funding acquisition: E. G. and C. G. K.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Hellenic Foundation for Research and Innovation (HFRI) for financial support through a grant, which is financed by 1st Call for H.F.R.I. Research Projects to Support Faculty Members & Researchers and the procurement of high-cost research equipment grant (grant number 655).References
- (a) C. Friedel and J. M. Crafts, Compt. Rend., 1877, 84, 1392–1395 Search PubMed; (b) C. Friedel and J. M. Crafts, Compt. Rend., 1877, 84, 1450–1454 Search PubMed.
- For selected and recent reviews on Friedel–Crafts reactions, see: (a) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6(6) DOI:10.3762/bjoc.6.6; (b) R. Sunke, S. B. Nallapati, J. S. Kumar, K. S. Kumar and M. Pal, Org. Biomol. Chem., 2017, 15, 4042–4057 RSC.
- (a) M. Kobayashi, S. Aoki, K. Gato, K. Matsunami, M. Kurosu and I. Kitagawa, Chem. Pharm. Bull., 1994, 42, 2449–2451 CrossRef CAS PubMed; (b) G. Sivaprasad, P. T. Perumal, V. R. Prabavathy and N. Mathivanan, Bioorg. Med. Chem. Lett., 2006, 16, 6302–6305 CrossRef CAS PubMed; (c) M. Damodiran, D. Muralidharan and P. T. Perumal, Bioorg. Med. Chem. Lett., 2009, 19, 3611–3614 CrossRef CAS PubMed; (d) A. Kamal, M. N. A. Khan, K. S. Reddy, Y. V. V. Srikanth, S. K. Ahmed, K. P. Kumar and U. S. N. Murthy, J. Enzyme Inhib. Med. Chem., 2009, 24, 559–565 CrossRef CAS PubMed; (e) K. Abdelbaqi, N. Lack, E. T. Guns, L. Kotha, S. Safe and T. Sanderson, Prostate, 2011, 71, 1401–1412 CrossRef CAS PubMed; (f) K. Yoon, S. Lee, S. Cho, K. Kim, S. Khan and S. Safe, Carcinogenesis, 2011, 32, 836–842 CrossRef CAS PubMed; (g) G. S. S. Kumar, S. Kumaresan, A. A. M. Prabhu, N. Bhuvanesh and P. G. Seethalakshmi, Spectrochim. Acta, Part A, 2013, 101, 255–263 CrossRef PubMed.
- (a) Y. S. Kim and J. A. Milner, J. Nutr. Biochem., 2005, 16, 65–73 CrossRef CAS PubMed; (b) E. G. Rogan, In Vivo, 2006, 20, 221–228 CAS.
- For a review, see: (a) M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed For selected examples, see: (b) J. S. Yadav, B. V. S. Reddy, C. V. S. R. Murthy, G. M. Kumar and C. Madan, Synthesis, 2001, 783–787 CrossRef CAS; (c) R. Nagarajan and P. T. Perumal, Tetrahedron, 2002, 58, 1229–1232 CrossRef CAS; (d) G. Gupta, G. Chaudhari, P. Tomar, Y. Gaikwad, R. Azad, G. Pandya, G. Waghulde and K. Patil, Eur. J. Chem., 2012, 3, 475–479 CrossRef CAS; (e) J. Beltrá, M. C. Gimeno and R. P. Herrera, Beilstein J. Org. Chem., 2014, 10, 2206–2214 CrossRef PubMed; (f) H. Veisi, B. Maleki, F. H. Eshbala, H. Veisi, R. Masti, S. S. Ashrafi and M. Baghayeri, RSC Adv., 2014, 4, 30683–30688 RSC.
- For selected examples, see: (a) G. Penieres-Carrillo, J. G. García-Estrada, J. L. Gutiérrez-Ramirez and C. Alvarez-Toledano, Green Chem., 2003, 5, 337–339 RSC; (b) J. Li, H. Dai, W. Xu and T. Li, Ultrason. Sonochem., 2006, 13, 24–27 CrossRef CAS PubMed; (c) N. Azizi, L. Torkian and M. R. Saidi, J. Mol. Catal. A: Chem., 2007, 275, 109–112 CrossRef CAS; (d) M. Zahran, Y. Abdin and H. Salama, ARKIVOC, 2008, 256–265 Search PubMed; (e) A. K. Chakraborti, S. R. Roy, D. Kumar and P. Chopra, Green Chem., 2008, 10, 1111–1118 RSC; (f) J. Li, M. Sun, G. He and X. Xu, Ultrason. Sonochem., 2011, 18, 412–414 CrossRef CAS PubMed; (g) S. Handy and N. M. Westbrook, Tetrahedron Lett., 2014, 55, 4969–4971 CrossRef CAS; (h) U. N. Yadav and G. S. Shankarling, J. Mol. Liq., 2014, 191, 137–141 CrossRef CAS.
- S. B. Kamble, R. K. Swami, S. S. Sakate and C. V. Rode, ChemPlusChem, 2013, 78, 1393–1399 CrossRef CAS PubMed.
- G. Basumatary, R. Mohanta, S. D. Baruah, R. C. Deka and G. Bez, Catal. Lett., 2020, 150, 106–111 CrossRef CAS.
- C. Liu, Z. Xiao, S. Wu, Y. Shen, K. Yuan and Y. Ding, ChemSusChem, 2020, 13, 1997–2001 CrossRef CAS PubMed.
- (a) Z. M. Salem, J. Saway and J. J. Badillo, Org. Lett., 2019, 21, 8528–8532 CrossRef CAS PubMed For a similar use of Schreiner's thiourea for the acetalization of aldehydes, see: (b) N. Spiliopoulou, N. F. Nikitas and C. G. Kokotos, Green Chem., 2020, 22, 3539–3545 RSC.
- For selected reviews, see: (a) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed; (b) D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed; (c) M. N. Hopkinson, A. Tlahuext-Aca and F. Glorius, Acc. Chem. Res., 2016, 49, 2261–2272 CrossRef CAS PubMed; (d) J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS; (e) Visible Light Photocatalysis in Organic Chemistry, ed. C. R. J. Stephenson, T. P. Yoon and D. W. C. MacMillan, Wiley, Weinheim, 2018 Search PubMed; (f) J. Schwarz and B. König, Green Chem., 2018, 20, 323–361 RSC; (g) M. Silvi and P. Melchiorre, Nature, 2018, 554, 41–49 CrossRef CAS PubMed; (h) S. Reischauer and B. Pieber, iScience, 2021, 24, 102209 CrossRef CAS PubMed.
- For selected examples, see: (a) D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed; (b) T. P. Yoon, M. A. Ischay and J. Du, Nature, 2010, 2, 527–532 CAS; (c) R. Brimioulle and T. Bach, Science, 2013, 342, 840–843 CrossRef CAS PubMed; (d) Y. Y. Loh, K. Nagao, A. J. Hoover, D. Hesk, N. R. Rivera, S. L. Colletti, I. W. Davies and D. W. C. MacMillan, Science, 2017, 358, 1182–1187 CrossRef CAS PubMed; (e) A. Fawcett, J. Pradeilles, Y. Wang, T. Mutsuga, E. L. Myers and V. K. Aggarwal, Science, 2017, 357, 283–286 CrossRef CAS PubMed; (f) A. Trowbridge, D. Reich and M. J. Gaunt, Nature, 2018, 561, 522–527 CrossRef CAS PubMed; (g) E. Speckmeier, T. Fische and K. Zeitler, J. Am. Chem. Soc., 2018, 140, 15353–15365 CrossRef CAS PubMed; (h) S. P. Morcillo, E. D. Dauncey, J. H. Kim, J. J. Douglas, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2018, 57, 12945–12949 CrossRef CAS PubMed; (i) J. Wu, P. S. Grant, X. Li, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2019, 58, 5697–5701 CrossRef CAS PubMed; (j) T. Patra, S. Mukherjee, J. Ma, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 10514–10520 CrossRef CAS PubMed; (k) A. Ruffoni, F. Julia, T. D. Svejstrup, A. McMillan, J. J. Douglas and D. Leonori, Nat. Chem., 2019, 11, 426–433 CrossRef CAS PubMed.
- For selected reviews, see: (a) M. Fagnoni, D. Dondi, D. Ravelli and A. Albini, Chem. Rev., 2007, 107, 2725–2756 CrossRef CAS PubMed; (b) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (c) D. Ravelli, S. Protti and M. Fagnoni, Chem. Rev., 2016, 116, 9850–9913 CrossRef CAS PubMed; (d) C. Raviola, S. Protti, D. Ravelli and M. Fagnoni, Chem. Soc. Rev., 2016, 45, 4364–4390 RSC; (e) S. Garbarino, D. Ravelli, S. Protti and A. Basso, Angew. Chem., Int. Ed., 2016, 55, 15476–15484 CrossRef PubMed; (f) S. Protti, A. Albini, R. Viswanathan and A. Greer, Photochem. Photobiol., 2017, 93, 1139–1153 CrossRef CAS PubMed; (g) M. Silvi and P. Melchiorre, Nature, 2018, 554, 41–49 CrossRef CAS PubMed; (h) I. K. Sideri, E. Voutyritsa and C. G. Kokotos, Org. Biomol. Chem., 2018, 16, 4596–4614 RSC; (i) C. Raviola, S. Protti, D. Ravelli and M. Fagnoni, Green Chem., 2019, 21, 748–764 RSC; (j) M. A. Theodoropoulou, N. F. Nikitas and C. G. Kokotos, Beilstein J. Org. Chem., 2020, 16, 833–857 CrossRef CAS PubMed; (k) C. Raviola and S. Protti, Eur. J. Org. Chem., 2020, 5292–5304 CrossRef CAS; (l) N. F. Nikitas, P. L. Gkizis and C. G. Kokotos, Org. Biomol. Chem., 2021, 19, 5237–5253 RSC; (m) S. Protti, D. Ravelli and M. Fagnoni, Trends Chem., 2022, 4, 305–317 CrossRef CAS.
- For contributions employing metal complexes in photoredox reactions, see: (a) I. Triandafillidi, M. G. Kokotou and C. G. Kokotos, Org. Lett., 2018, 20, 36–39 CrossRef CAS PubMed For contributions employing organic molecules in photochemical reactions, see: (b) G. N. Papadopoulos, D. Limnios and C. G. Kokotos, Chem. – Eur. J., 2014, 20, 13811–13814 CrossRef CAS PubMed; (c) N. Kaplaneris, A. Bisticha, G. N. Papadopoulos, D. Limnios and C. G. Kokotos, Green Chem., 2017, 19, 4451–4456 RSC; (d) E. Voutyritsa and C. G. Kokotos, Angew. Chem., Int. Ed., 2020, 59, 1735–1741 CrossRef CAS PubMed; (e) G. N. Papadopoulos, M. G. Kokotou, N. Spiliopoulou, N. F. Nikitas, E. Voutyritsa, D. I. Tzaras, N. Kaplaneris and C. G. Kokotos, ChemSusChem, 2020, 13, 5934–5944 CrossRef CAS PubMed; (f) E. Voutyritsa, M. Garreau, M. G. Kokotou, I. Triandafillidi, J. Waser and C. G. Kokotos, Chem. – Eur. J., 2020, 26, 14453–14460 CrossRef CAS PubMed; (g) N. Spiliopoulou and C. G. Kokotos, Green Chem., 2021, 23, 546–551 RSC; (h) C. S. Batsika, C. Mantzourani, D. Gkikas, M. G. Kokotou, O. G. Mountanea, C. G. Kokotos, P. K. Politis and G. Kokotos, J. Med. Chem., 2021, 64, 5654–5666 CrossRef CAS PubMed; (i) C. S. Batsika, C. Koutsilieris, G. S. Koutoulogenis, M. G. Kokotou, C. G. Kokotos and G. Kokotos, Green Chem., 2022, 24, 6224–6231 RSC.
- (a) S. Crespi, S. Protti and M. Fagnoni, J. Org. Chem., 2016, 81, 9612–9619 CrossRef CAS PubMed; (b) M. Malacarne, S. Protti and M. Fagnoni, Adv. Synth. Catal., 2017, 359, 3826–3830 CrossRef CAS; (c) L. Onuigbo, C. Raviola, A. D. Fonzo, S. Protti and M. Fagnoni, Eur. J. Org. Chem., 2018, 5297–5303 CrossRef CAS; (d) H. O. Abdulla, A. A. Amin, C. Raviola, T. Opatz, S. Protti and M. Fagnoni, Eur. J. Org. Chem., 2020, 1448–1452 CrossRef CAS; (e) D. Qui, C. Lion, J. Mao, Y. Ding, Z. Liu, L. Wei, M. Fagnoni and S. Protti, Adv. Synth. Catal., 2019, 361, 5239–5244 CrossRef; (f) C. Lian, G. Yue, J. Mao, D. Liu, Y. Ding, Z. Liu, D. Qiu, X. Zhao, K. Lu, M. Fagnoni and S. Protti, Org. Lett., 2019, 21, 5187–5191 CrossRef CAS PubMed; (g) L. Di Terlizzi, S. Scaringi, C. Raviola, R. Pedrazzani, M. Bandini, M. Fagnoni and S. Protti, J. Org. Chem., 2022, 87, 4863–4872 CrossRef CAS PubMed; (h) D. Qiu, C. Lian, J. Mao, M. Fagnoni and S. Protti, J. Org. Chem., 2020, 85, 12813–12822 CrossRef CAS PubMed; (i) L. Blank, M. Fagnoni, S. Protti and M. Rueping, Synthesis, 2019, 51, 1243–1252 CrossRef CAS; (j) A. Li, Y. Li, J. Liu, J. Chen, K. Lu, D. Qiu, M. Fagnoni, S. Protti and X. Zhao, J. Org. Chem., 2021, 86, 1292–1299 CrossRef CAS PubMed.
- (a) J. Médard, P. Decorse, C. Mangeney, J. Pinson, M. Fagnoni and S. Protti, Langmuir, 2020, 36, 2786–2793 CrossRef PubMed; (b) L. Lombardi, A. Kovtun, S. Mantovani, G. Bertuzzi, L. Favaretto, C. Bettini, V. Palermo, M. Melucci and M. Bandini, Chem. – Eur. J., 2022, 28, e202200333 CrossRef CAS PubMed.
- L. D. Terlizzi, A. Martinelli, D. Merli, S. Protti and M. Fagnoni, J. Org. Chem., 2022 DOI:10.1021/acs.joc.2c01248.
- For detailed experimental procedures, optimization studies and mechanistic experiments, see ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data, optimization studies, 1H and 13C NMR. See DOI: https://doi.org/10.1039/d2ob02214a |
| This journal is © The Royal Society of Chemistry 2023 |



