Protecting-group-free glycosylation of phosphatidic acid in aqueous media†
Abstract
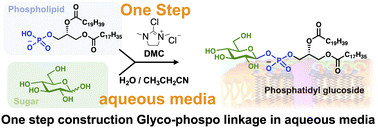
The glycosylation of unprotected carbohydrates has emerged as an area of significant interest because it obviates the need for long reaction sequences involving protecting-group manipulations. Herein, we report the one-pot synthesis of anomeric glycosyl phosphates through the condensation of unprotected carbohydrates with phospholipid derivatives while retaining high stereo- and regioselective control. The anomeric center was activated using 2-chloro-1,3-dimethylimidazolinium chloride to facilitate condensation with glycerol-3-phosphate derivatives in an aqueous solution. A water/propionitrile mixture provided superior stereoselectivity while maintaining good yields. Under these optimized conditions, the condensation of stable isotope-labeled glucose with phosphatidic acid provided efficient access to labeled glycophospholipids as an internal standard for mass spectrometry.


 Please wait while we load your content...
Please wait while we load your content...