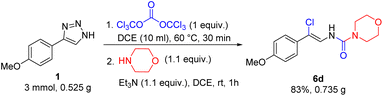Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of N-vinyl isothiocyanates and carbamates by the cleavage of NH-1,2,3-triazoles with one-carbon electrophiles†
Vladimir
Motornov
 * and
Petr
Beier
* and
Petr
Beier
 *
*
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 166 10 Prague, Czech Republic. E-mail: vladimir.motornov@uochb.cas.cz; beier@uochb.cas.cz
First published on 12th January 2023
Abstract
Metal-free cascade reaction of NH-1,2,3-triazoles with one-carbon electrophiles, such as thiophosgene and triphosgene, led to N-vinylated ring cleavage products. Using this approach the synthesis of N-vinylisothiocyanates from NH-triazoles and thiophosgene was achieved. A variety of multifunctional compounds, such as N-vinylcarbamates, unsymmetrical vinylureas, carbamothioates, etc. was prepared by a one-pot method from NH-triazoles, triphosgene and nucleophiles.
1,2,3-Triazoles are versatile building blocks for the synthesis of functionalized heterocycles.1 For example, metal-catalyzed transannulation of N-sulfonyl2 and N-fluoroalkyl-3 1,2,3-triazoles is a useful and well-established methodology, providing access to various, often bioactive, nitrogen-containing heterocycles and carbocycles. Furthermore, cleavage of N-substituted 1,2,3-triazoles with Brønsted4 and Lewis5 acids was studied as an alternative to metal-catalyzed transformations. However, much less attention was devoted to the ring cleavage chemistry of NH-1,2,3-triazoles.6 In comparison to N-sulfonyl and N-fluoroalkyl triazoles, which require multiple synthetic steps, NH-1,2,3-triazoles are easily accessible in one step from simple azide sources (NaN3 or TMSN3) and commercial alkynes,7 or aldehydes and nitroalkanes.8 It is known that the ring cleavage of 1,2,3-triazole is synthetically useful in the presence of an electron-withdrawing group at N1,2 fused triazole derivatives9 and specific substrates with more reactive centres.10 Thus, for a long time NH-1,2,3-triazoles were not considered as building blocks in ring cleavage chemistry. Only very recently, NH-1,2,3-triazoles were successfully used in ring transformations, which was made possible by N-acylation followed by in situ cleavage of unstable N-acyltriazoles.11,12 Cleavage of NH-1,2,3-triazoles with acyl halides led to β-haloenamides,11 while reactions with fluoroalkylated acid anhydrides provides routes to fluoroalkylated oxazoles or 2-acylaminoketones (Scheme 1).12 Thus, utilization of NH-1,2,3-triazoles as building blocks is a highly promising area in organic chemistry.
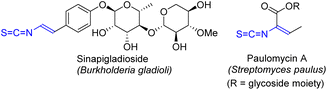
N-Alkenyl compounds are attractive synthetic targets, which are widely used in polymer chemistry, including the synthesis of biocompatible copolymers used in pharmaceutical industry.13 While unsubstituted vinylamides can be prepared by direct vinylation of amides with acetylene,14 the synthesis of substituted vinylamides and more complex N-vinyl compounds (vinyl carbamates, vinylureas, vinylazoles) is a more challenging task. In the recent two decades, the most common methods to access complex N-alkenyl compounds were based on Ru-catalyzed addition of amides to alkynes,15 as well as Cu-16 and Pd-catalyzed17 cross-coupling reactions. Among the variety of N-alkenyl compounds, vinyl isothiocyanates deserve a special attention.18 The vinyl isothiocyanate moiety is present in natural products, such as in Sinapigladioside, which exhibits high antifungal and antibacterial activity (Fig. 1).18,19 Despite very promising biological activities, vinyl isothiocyanates are difficult to obtain synthetically. While reactions of amines and anilines with thiophosgene is an established route to prepare alkyl and aryl isothiocyanates, analogous synthesis of vinyl isothiocyanates is apparently limited.20 Thus, a new method to access complex N-alkenyl compounds, including isothiocyanates, is highly desirable. Herein, we report the metal-free synthesis of vinyl isothiocyanates and other N-alkenyl compounds from NH-1,2,3-triazoles and easily available one-carbon electrophiles, such as thiophosgene and triphosgene.
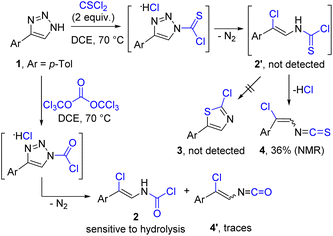
Our study was initiated by the investigation of reactions between NH-1,2,3-triazoles and one-carbon dielectrophiles such as triphosgene and thiophosgene (Scheme 2). We envisioned the formation of N-vinylcarbamoyl chloride 2 or N-vinylthiocarbamoyl chloride 2′via acylated triazole intermediates and further transformations. In the case of 2′ we expected the formation of 2-chlorothiazole 3 due to a high nucleophilicity of the sulfur atom, which could facilitate intramolecular cyclization. To our surprise, heating the mixture of model NH-1,2,3-triazole 1 and thiophosgene at 70 °C led to the formation of vinyl isothiocyanate 4 in moderate yield as E/Z mixture, while no intermediate 2′ or cyclization product 3 were detected. In contrast to the reaction with thiophosgene, the application of triphosgene led to the exclusive formation of N-vinylcarbamoyl chloride 2 with small amount (<10%) of vinyl isocyanate 4′.
Screening of reaction conditions for the synthesis of vinyl isothiocyanate 4a was performed (Table 1). We found that the reaction proceeds more efficiently with electron-rich triazoles, thus, 4-methoxyphenyl-substituted triazole 1a was chosen as a model substrate. Survey of solvents revealed that non-polar toluene (entry 2) afforded slower reaction than chlorinated solvents DCE and CHCl3 (entries 1 and 3). On the contrary, the reaction proceeded quickly in more polar acetonitrile (entry 4). The yields in different solvents were similar. Decrease of CSCl2 amount to 1.5 equiv. led to slight improvement of the yield (entry 5), while increase to 3 equiv. afforded lower yield (entry 6). However, further decrease of CSCl2 amount to 1.0 equiv. resulted in drop of yield (entry 7). Studying the effect of concentration revealed that in more diluted (0.05 M) solution the yield was increased (entry 8), while in more concentrated (0.2 M) solution the reaction was less efficient (entry 9). Further dilution to 0.033 M afforded good 70% NMR yield of the target isothiocyanate 4a (entry 10). Thus, performing the reaction in diluted solution with 1.5 equiv. of CSCl2 was considered optimal (entry 11). Neither reducing nor increasing the temperature did not improve the yield (entries 12 and 13).
| Entry | Solvent | Conc. (M) | Time (h) | Yield of 4ab (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol) and CSCl2 (0.2 mmol) in solvent heated to 70 °C. b Yields were determined by 1H NMR with methyl 4-nitrobenzoate as an internal standard. c CSCl2 (1.5 equiv.). d CSCl2 (3 equiv.). e CSCl2 (1.0 equiv.). f Isolated yield in parentheses. g 50 °C. h 80 °C. | ||||
| 1 | DCE | 0.1 | 12 | 46 |
| 2 | CHCl3 | 0.1 | 12 | 45 |
| 3 | PhMe | 0.1 | 42 | 45 |
| 4 | MeCN | 0.1 | 0.5 | 40 |
| 5c | DCE | 0.1 | 12 | 52 |
| 6d | DCE | 0.1 | 12 | 41 |
| 7e | DCE | 0.1 | 18 | 34 |
| 8 | DCE | 0.05 | 12 | 51 |
| 9 | DCE | 0.2 | 12 | 42 |
| 10 | DCE | 0.033 | 12 | 70 |
| 11c | DCE | 0.033 | 18 | 70 (65)f |
| 12g | DCE | 0.033 | 30 | 49 |
| 13h | DCE | 0.033 | 5 | 61 |
Next, the reaction scope was investigated applying optimized conditions (Scheme 3). 4-Substituted triazoles with various aryl groups were tested. Importantly, the reaction proceeded efficiently with triazoles bearing electron-donating groups. Thus, good yields were obtained for 4-methoxyphenyl- (4a), 3,4,5-trimethoxyphenyl (4c) and 4-phenoxyphenyl- (4d) substituted isothiocyanates. In the case of alkyl-substituted aromatic ring, moderate yield of 4b was obtained. Trifluoromethoxy-substituted triazole also afforded the product 4e in moderate yield. However, in the case of phenyl group without electron-donating substituents, only 22% yield of isothiocyanate was obtained. This is attributed to the formation of side-products, arising from low stability of vinyl isothiocyanates without electron-rich groups on the aryl ring. Moderate yields could be obtained for sterically demanding α-naphthyl- (4g) and 2-thienyl-substituted examples (4h), when milder conditions (45 °C, 24 h) were applied to avoid side reactions. Apart from monosubstituted 1,2,3-NH-triazoles, 4,5-disubstituted substrates bearing one electron-rich aryl group and alkyl group were also found applicable for isothiocyanate synthesis. High yield was obtained for methyl-substituted product 4i, while the reaction was less efficient for ethyl-substituted 4j. Unfortunately, triazoles with electron-deficient aryl substituents were not applicable for this transformation due to their low reactivity under reaction conditions and propensity of forming products of side reactions. Unsubstituted 1,2,3-NH-triazole underwent cleavage with CSCl2 to form 1,2-dichloroethyl isothiocyanate 5 in good yield. Compound 5 is clearly a product of HCl addition to intermediately formed vinyl isothiocyanate.
The developed method was found to be easily scalable as exemplified by the synthesis of isothiocyanate 4a on 1 mmol (175 mg) scale in 71% yield (Scheme 3). The investigation of reactivity of vinyl isothiocyanates, as well as biological activity and applications are attractive future tasks for organic and medicinal chemists.
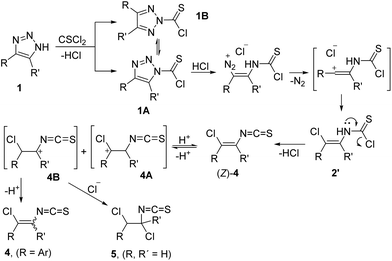
According to 1H–1H ROESY NMR analysis, products 4 were obtained as mixtures of E- and Z-isomers, with the preference for E-isomers. This contrasts with literature reports on 1,2,3-triazole cleavage with Brønsted and Lewis acids,4,5 where the stereoselective formation of Z-isomers was confirmed. To explain the above-mentioned observations, the following mechanism of CSCl2-mediated triazole ring cleavage was proposed (Scheme 4). Thioacylation of 1,2,3-NH-triazole with CSCl2 can lead to both N1- and N2-thioacyl triazoles. Reversible interconversion between regioisomers is possible in the presence of acid, which can explain successful ring cleavage reactions even for 4,5-disubstituted triazoles. N1-Thioacyl-1,2,3-triazole 1A undergoes ring cleavage in the presence of HCl to form diazo compound followed by nitrogen elimination to vinyl cation, which recombines with chloride anion to form thiocarbamoyl chloride 2′. It undergoes elimination of HCl to Z-isomer of vinyl isothiocyanate 4. However, in the presence of HCl protonation of the double bond can take place to form cations 4A and 4B. Their deprotonation (for R = Ar) leads to isomeric mixtures of vinyl isothiocyanates 4, while recombination of cation 4B with chloride anion affords 1,2-dichloroethyl isothiocyanate 5.
Next, cleavage of NH-1,2,3-triazoles with triphosgene was investigated. Since almost quantitative formation of N-vinylcarbamoyl chloride 2 was obtained under similar conditions (60 °C, 30 min), further transformations of 2 were studied without optimization of the first step. Moreover, preliminary results revealed the reaction with triphosgene to be insensitive to concentration, in comparison to synthesis of vinyl isothiocyanates. Thus, 0.2 M concentration was used. Despite low stability of N-vinylcarbamoyl chlorides 2, their electrophilic nature makes them attractive precursors of N-vinylated compounds by the treatment with various nucleophiles. Thus, a one-pot synthesis of N-vinyl compounds starting directly from NH-triazoles and triphosgene was developed (Scheme 5).
 | ||
| Scheme 5 One-pot three-component assembly of N-alkenyl compounds from NH-1,2,3-triazoles, triphosgene, and nucleophiles. Reaction conditions: 1 (0.1 mmol), triphosgene (0.1 mmol), DCE (0.5 ml), 60 °C, 30 min, then nucleophile was added (see ESI† for detailed procedures). An = 4-methoxyphenyl. aIsolated yields. b70 °C, 30 min for the first step. c70 °C, 20 h for the first step. | ||
Firstly, when methanol or ethanol were used as nucleophiles, N-vinylcarbamates 6a–6b were obtained in good yields. Using butylamine unsymmetrical vinylurea 6c was prepared. The method also worked efficiently for a secondary amine (morpholine) as the nucleophile, leading to product 6d in high yield. Sulfur nucleophiles, such as methanthiolate and 4-chlorothiophenol, proved to be competent in this transformation to give carbamothioates 6e and 6f, respectively. In the case of sodium azide the corresponding carbamoyl azide 6g was prepared, albeit in 32% yield, probably, due to its low stability and propensity to decomposition via carbamoyl nitrene.
Regarding the scope of 1,2,3-triazoles, the triphosgene method was successfully applied to 4-phenoxysubstituted triazole, as well as to 4-tolyl, phenyl and 1-naphthyl-substituted triazoles to afford products 6h–6k. Importantly, alkenyl-substituted moiety on the triazole part was also tolerated to give product 6l in 73% yield. For 4,5-disubstituted triazole (Scheme 5, bottom) alcoholysis and hydrolysis of C–N bond rather than only C–Cl bond took place, which led to known α-methoxyketone 7. Importantly, in the case of triphosgene, intermediates vinylcarbamoyl chlorides 2 did not undergo HCl elimination, thus, their treatment with nucleophiles resulted in complete retention of Z-configuration in compounds 6 with the only exception of carbamoyl azide 6g.
To investigate the scalability of the three-component synthesis of N-alkenyl compounds 5, a 3 mmol scale reaction to synthesize vinylurea 5d was attempted. Indeed, the process was found to be easily scalable as the product was obtained without significant loss of the yield (Scheme 6). Inexpensive triethylamine was used as a sacrificial base to remove HCl demonstrating that using excess of nucleophile is not necessary. Thus, complex N-vinylated compounds can be accessed with high atom-economy.
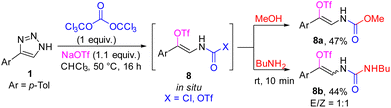
When sodium triflate was added to the reaction mixture in the first step, the present methodology allowed a one-pot four-component assembly of carbamate 8a or unsymmetrical vinylurea 8b with the triflate group (Scheme 7). Changing the solvent to chloroform was crucial for efficient transformation. It is interesting to note that the compound 8b was obtained as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E- and Z-isomers, in contrast to stereoselective formation of Z-isomer of carbamoyl triflate 8a. This difference in configurational stability might be caused by lower double bond isomerization barrier for more electron-rich vinylurea derivative, which makes spontaneous isomerization possible. Since β-enamido triflates are bench stable and synthetically useful building blocks amenable to cross-coupling reactions,21 the development of new routes for their synthesis is sought after.
1 mixture of E- and Z-isomers, in contrast to stereoselective formation of Z-isomer of carbamoyl triflate 8a. This difference in configurational stability might be caused by lower double bond isomerization barrier for more electron-rich vinylurea derivative, which makes spontaneous isomerization possible. Since β-enamido triflates are bench stable and synthetically useful building blocks amenable to cross-coupling reactions,21 the development of new routes for their synthesis is sought after.
In conclusion, N-vinyl isothiocyanates were prepared by the cleavage of NH-1,2,3-triazoles with thiophosgene. A one-pot method to access multifunctionalized N-alkenyl compounds from NH-1,2,3-triazoles, triphosgene and various nucleophiles was developed. The main advantages of the methodology are readily available starting materials, atom economy and short reaction times. The methods can be useful for the preparation of natural products with vinyl isothiocyanate moieties, as well as for the synthesis of complex and multifunctional N-alkenyl compounds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Czech Academy of Sciences (RVO: 61388963) and by the Ministry of Education, Youth and Sports of the Czech Republic (LTAUSA18037). The authors thank Radek Pohl (IOCB Prague) for measurement of 1H–1H ROESY NMR spectra.References
-
(a) M. Akter, K. Rupa and P. Anbarasan, Chem. Rev., 2022, 122, 13108 CrossRef
; (b) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef
; (c) M. Zibinsky and V. V. Fokin, Org. Lett., 2011, 13, 4870 CrossRef PubMed
.
-
(a) H. M. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC
; (b) Chemistry of 1,2,3-triazoles, in Topics in Heterocyclic Chemistry, ed. W. Dehaen and V. A. Bakulev (eds), vol. 40, Springer, 2015 Search PubMed
.
-
(a) V. Motornov, A. Markos and P. Beier, Chem. Commun., 2018, 54, 3258 RSC
; (b) V. Motornov and P. Beier, J. Org. Chem., 2018, 83, 15195 CrossRef
; (c) V. Motornov, V. Košťál, A. Markos, D. Taffner and P. Beier, Org. Chem. Front., 2019, 6, 3776 RSC
; (d) O. Bakhanovich, V. Khutorianskyi, V. Motornov and P. Beier, Beilstein J. Org. Chem., 2021, 17, 504 CrossRef CAS
.
- A. Markos, S. Voltrová, V. Motornov, D. Tichý, B. Klepetářová and P. Beier, Chem. – Eur. J., 2019, 25, 7640 CrossRef CAS
.
-
(a) A. Markos, L. Janecký, T. Chvojka, T. Martinek, H. Martinez-Seara, B. Klepetářová and P. Beier, Adv. Synth. Catal., 2021, 363, 3258 CrossRef CAS
; (b) Z.-F. Xu, H. C. Dai, L. H. Shan and C.-Y. Li, Org. Lett., 2018, 20, 1054 CrossRef CAS PubMed
; (c) D. Yang, L. Shan, Z.-F. Xu and C.-Y. Li, Org. Biomol. Chem., 2018, 16, 1461 RSC
.
- T. Opsomer and W. Dehaen, Chem. Commun., 2021, 57, 1568 RSC
.
-
(a) D. Jankovic, M. Virant and M. Gazvoda, J. Org. Chem., 2022, 87, 4018 CrossRef CAS PubMed
; (b) T. Jin, S. Kamijo and Y. Yamamoto, Eur. J. Org. Chem., 2004, 3789 CrossRef CAS
; (c) S. Payra, A. Saha and S. Banerjee, ChemCatChem, 2018, 10, 5468 CrossRef CAS
.
-
(a) R. Hui, M. Zhao, M. Chen, Z. Ren and Z. Guan, Chin. J. Chem., 2017, 35, 1808 CrossRef CAS
; (b) L. Wu, X. Wang, Y. Chen, Q. Huang, Q. Lin and M. Wu, Synlett, 2016, 27, 437 CAS
; (c) Q. Hu, Y. Liu, X. Deng, Y. Li and Y. Chen, Adv. Synth. Catal., 2016, 358, 1689 CrossRef CAS
; (d) C. P. Parambil, S. P. Veethil and W. Dehaen, Synthesis, 2022, 54, 910 CrossRef
; (e) V. A. Motornov, S. L. Ioffe and A. A. Tabolin, Targets Heterocycl. Syst., 2019, 23, 237 CAS
.
-
(a) D. Yadagiri, M. Rivas and V. Gevorgyan, J. Org. Chem., 2020, 85, 11030 CrossRef CAS PubMed
; (b) V. Helan, A. V. Gulevich and V. Gevorgyan, Chem. Sci., 2015, 6, 1928 RSC
; (c) Y. Shi and V. Gevorgyan, Chem. Commun., 2015, 51, 17166 RSC
; (d) Z. Zhang and V. Gevorgyan, Org. Lett., 2020, 22, 8500 CrossRef CAS PubMed
; (e) A. Joshi, C. Chandra Mohan and S. Adimurthy, Org. Lett., 2016, 18, 464 CrossRef CAS
.
-
(a) Yu. N. Kotovshchikov, G. V. Latyshev, M. A. Navasardyan, D. A. Erzunov, I. P. Beletskaya and N. V. Lukashev, Org. Lett., 2018, 20, 4467 CrossRef
; (b) Yu. N. Kotovshchikov, G. V. Latyshev, E. A. Kirillova, U. D. Moskalenko, N. V. Lukashev and I. P. Beletskaya, J. Org. Chem., 2020, 85, 9015 CrossRef PubMed
; (c) V. A. Voloshkin, Yu. N. Kotovshchikov, G. V. Latyshev, N. V. Lukashev and I. P. Beletskaya, J. Org. Chem., 2022, 87, 7064 CrossRef
.
-
(a) T. Wang, Z. Tang, H. Luo, Y. Tian, M. Xu, Q. Lu and B. Li, Org. Lett., 2021, 23, 6293 CrossRef PubMed
; (b) M. Xu, L. Liu, T. Wang, H. Luo, M. Hou, L. Du, X. Xin, Q. Lu and B. Li, Org. Chem. Front., 2022, 9, 1065 RSC
.
-
(a) V. Motornov and P. Beier, Org. Lett., 2022, 24, 1958 CrossRef PubMed
; (b) V. Motornov and P. Beier, New J. Chem., 2022, 46, 14318 RSC
.
-
(a) L. B. Huang, M. Arndt, K. Goossen, H. Heydt and L. J. Goossen, Chem. Rev., 2015, 115, 2596 CrossRef CAS
; (b) N. A. Cortez-Lemus and A. Licea-Claverie, Prog. Polym. Sci., 2016, 53, 1 CrossRef CAS
.
-
(a) E. Semina, P. Tuzina, F. Bienewald, A. S. K. Hashmi and T. Schaub, Chem. Commun., 2020, 56, 5977 RSC
; (b) K. S. Rodygin, A. S. Bogachenkov and V. P. Ananikov, Molecules, 2018, 23, 648 CrossRef PubMed
.
-
(a) L. Gooßen, K. S. M. Salih and M. Blanchot, Angew. Chem., Int. Ed., 2008, 47, 8492 CrossRef
; (b) L. Gooßen, J. E. Rauhaus and G. Deng, Angew. Chem., Int. Ed., 2005, 44, 4042 CrossRef
; (c) M. Arndt, K. S. M. Salih, A. Fromm, L. J. Goossen, F. Menges and G. Niedner-Schatteburg, J. Am. Chem. Soc., 2011, 133, 7428 CrossRef CAS
.
-
(a) Y. Bolshan and R. A. Batey, Angew. Chem., Int. Ed., 2008, 47, 2109 CrossRef CAS
; (b) Y. Bolshan and R. A. Batey, Tetrahedron, 2010, 66, 5283 CrossRef CAS
; (c) R. A. Bohmann and C. Bolm, Org. Lett., 2013, 15, 4277 CrossRef CAS PubMed
; (d) V. Motornov, G. V. Latyshev, Yu. N. Kotovschikov, N. V. Lukashev and I. P. Beletskaya, Adv. Synth. Catal., 2019, 361, 3306 CrossRef CAS
.
- J. P. Dalvadi, P. K. Patel and K. H. Chikhalia, RSC Adv., 2013, 3, 22972 RSC
.
-
(a) J. Li, Z. Xie, M. Wang, G. Ai and Y. Chen, PLoS One, 2015, 10, e0120542 CrossRef PubMed
; (b) A. D. Argoudelis, T. A. Brinkley, T. F. Brodasky, J. A. Buege, H. F. Meyer and S. A. Mizsak, J. Antibiot., 1982, 35, 285 CrossRef
; (c) L. V. Flórez and M. Kaltenpoth, Environ. Microbiol., 2017, 19, 3674 CrossRef
; (d) L. V. Flórez, K. Scherlach, P. Gaube, C. Ross, E. Sitte, C. Hermes, A. Rodrigues, C. Hertweck and M. Kaltenpoth, Nat. Commun., 2017, 8, 15172 CrossRef PubMed
; (e) L. V. Flórez, K. Scherlach, I. J. Miller, A. Rodrigues, J. C. Kwan, C. Hertweck and M. Kaltenpoth, Nat. Commun., 2018, 9, 2478 CrossRef
.
- B. Dose, S. P. Niehs, K. Scherlach, S. Shahda, L. V. Flórez, M. Kaltenpoth and C. Hertweck, ChemBioChem, 2021, 22, 1920 CrossRef
.
- For rare examples of vinyl isothiocyanate synthesis, see:
(a) N. A. Nedolya, B. A. Trofimov and A. Senning, Sulfur Rep., 1996, 17, 183 CrossRef
; (b) Z. Rappoport and A. Topol, J. Am. Chem. Soc., 1980, 102, 406 CrossRef CAS
; (c) M.-W. Ding, B.-Q. Fu and L. Cheng, Synthesis, 2004, 1067 CrossRef CAS
.
-
(a) T. Chvojka, A. Markos, S. Voltrová, R. Pohl and P. Beier, Beilstein J. Org. Chem., 2021, 17, 2657 CrossRef CAS PubMed
; (b) Q. Lu, T. Wang, Q. Wu, L. Cheng, H. Luo, L. Liu, G. Chu, L. Wang and B. Li, Green Chem., 2022, 24, 4399 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob02115c |
| This journal is © The Royal Society of Chemistry 2023 |