Open Access Article
Open Access ArticleSulfurized diterpenoids in amber as diagenetic indicators of sulfate-reducing processes in past depositional environments†
Lauriane
Lenen
a,
Alice
Fradet
a,
Philippe
Schaeffer
 a,
Bernard
Gomez
b and
Pierre
Adam
a,
Bernard
Gomez
b and
Pierre
Adam
 *a
*a
aUniversité de Strasbourg, CNRS, Institut de Chimie de Strasbourg UMR 7177, F-67000 Strasbourg, France. E-mail: padam@unistra.fr
bUniversité de Lyon, CNRS, ENS, Laboratoire de Géologie de Lyon, Terre, Planètes, Environnement UMR 5276, F-69622 Villeurbanne, France
First published on 19th December 2022
Abstract
Two novel compounds isolated from an amber sample from the Santonian of Piolenc (Vaucluse, SE France) were identified using nuclear magnetic resonance and high-resolution mass spectrometry as sulfurized analogues of diterpenic acids from the isopimaric series originating from ancient conifers possibly related to the Cupressaceae family. These two compounds are members of a diterpenoid series corresponding to early diagenetic transformation products of resin diterpenoids. They were likely formed once plant resin comes into contact with reduced sulfur species originating from bacterial sulfate reduction occurring in anaerobic settings such as mangroves or marshes. They represent the first evidence of sulfurization processes affecting plant resin prior to diagenetic transformation into amber. Given their mode of formation, these compounds may be used as indicators of sulfate-reducing processes in past depositional environments.
A. Introduction
Amber results from the fossilization of tree resin. Although the most ancient amber identified is dated back to the Carboniferous period (359–299 Ma),1 most of the amber was formed much later in the geological record and the vast majority of amber is found in Cretaceous and younger sedimentary rocks.2,3 The molecular assemblages that constitute amber are generally dominated by terpenic compounds occurring as monomeric entities and polymeric moieties occurring in macromolecular structures.4 These assemblages are inherited from the plants that have produced the resin at the time of origin of the amber. Therefore, molecular investigation of amber using, for instance, gas chromatography coupled with mass spectrometry (GC-MS) or pyrolysis-GC-MS can furnish valuable information concerning the botanical origin of the amber.2,4–11 The molecular signature of amber is, however, generally significantly modified compared to that of the genuine resin by various transformation processes during burial (i.e., diagenesis). As the parent compounds are sometimes absent in amber, a correlation between the molecular signal and a specific biological source is difficult to establish. In this respect, precise identification of molecular biomarkers from amber can provide new molecular tools allowing for a more reliable identification of the botanical sources of amber.11–16 It can also potentially provide useful information on the palaeoenvironmental conditions prevailing in the ecosystems where the resin-producing trees have lived and on the alteration processes associated with these environments.In this context, the molecular analysis of an amber sample from the Santonian (86.3–83.6 Ma) of Piolenc (Vaucluse, SE France) using GC-MS revealed the occurrence of an unreported series of compounds characterized by a molecular mass of 350 Da. Isolation of two members of this series (I and II, Fig. 1) led to their identification as sulfurized analogues of diterpenic acids from the isopimaric series using GC-MS, high resolution mass spectrometry (HR-MS) and comprehensive nuclear magnetic resonance (NMR) investigations.
B. Results and discussion
The gas chromatogram of the apolar fraction isolated from the organic extract of an amber sample from Piolenc after methylation of carboxylic acids16,17 is presented in Fig. 2. The distribution of the lipids is complex and several series of compounds comprising acids from sesquiterpenes (1 and 2) and diterpenes (3–7), as well as sesquiterpenic hydrocarbons (8 and 9), could be detected. | ||
| Fig. 2 Gas chromatogram (GC-MS, EI, 70 eV) of the apolar fraction (FA) isolated from the methylated organic extract of a Piolenc amber sample. The area marked with the red rectangle corresponds to the zone of elution of compounds with a molecular weight of 350 Da (compounds I–VI). *Tentative identification based on the similarity of the EI mass spectra of compounds I and III (cf. ESI Fig. 1S†). | ||
The tricyclic diterpenoid acids comprise, notably, dehydroabietic and callitrisic acids (6 and 7, respectively), and other diterpenic acids (e.g.5) (Fig. 2) related to pimaric, isopimaric and sandaracopimaric acids (10, 11 and 12, Fig. 3). Diterpenoid aromatic hydrocarbons frequently encountered in amber have also been identified in the amber of Piolenc (Fig. 2) based on the comparison of their mass spectra with published spectra.2,11,14,15 The occurrence of specific molecular markers in the organic extract of the Piolenc amber sample suggests that it must originate from resin exuded by conifers of the Cupressaceae family since such molecules occur in the living representatives of this family. In this respect, callitrisic acid 7 (Fig. 2) has only been reported in the resin of the Cupressaceous genus Callitris18 and is therefore considered a specific marker of this conifer family.5,19 Similarly, cuparene 8 specifically occurs in the resin of Cupressaceae.20,21
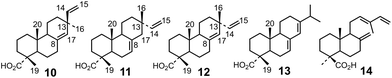 | ||
| Fig. 3 Structures of some common resin acids: pimaric acid 10, isopimaric acid 11, sandaracopimaric acid 12, abietic acid 13, and communic acid 14. | ||
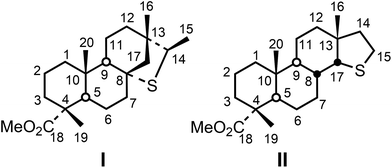
The molecular distribution of the Piolenc amber sample also comprises a quantitatively abundant compound series characterized by a molecular weight of 350 Da (Fig. 2). To the best of our knowledge, the occurrence of the latter has only been reported previously twice in amber samples, either as “unknown” or as “unassigned”.22,23 The electron impact (EI) mass spectra of compounds I–III and three other members of this series are shown in ESI Fig. 1S.† Since it was not possible to obtain more structural information solely based on the interpretation of the EI mass spectra, the isolation of compounds I and II was undertaken to perform detailed NMR structural investigations. These compounds were isolated using a chromatographic sequence comprising fractionation of the methylated organic extract on a silica gel column followed by further HPLC fractionation of the sub-fractions containing compounds I and II using normal phase (silica). Compounds I and II were isolated in sufficient amounts to allow for their identification using detailed NMR studies comprising 1D (1H, 13C, DEPT) NMR spectra as well as homo- (1H–1H; COSY, NOESY) and heteronuclear (1H–13C; HMBC, HSQC) correlation studies. In addition, the elemental composition of compounds I and II was determined using HR-MS.
Identification of compound I
The [M + H]+ ion obtained by HR-MS using positive electrospray ionization (ESI) at m/z 351.3251 corresponds to the molecular formula C21H35O2S (calcd: m/z 351.2352; ESI Fig. 2S†), indicating the contribution of a sulfur atom. This result was rather unexpected since organic sulfur compounds have, to the best of our knowledge, never been reported in amber. It should, however, be mentioned that amber samples from some specific geological sites have been shown to contain sulfur, although in low amounts.24 The molecular formula obtained using HR-MS indicates the occurrence of five degrees of unsaturation as rings or double bonds.According to the NMR data, it appears from the signal at 179.3 ppm in the 13C-NMR spectrum (Table 1) that one degree of unsaturation corresponds to the carbonyl group from a methyl ester functionality. This is corroborated by the occurrence of signals at 3.63 ppm and 52.0 ppm in the 1D 1H- and 13C-NMR spectra (ESI Fig. 4S and 5S†), respectively, likely corresponding to the methyl from a carbomethoxy group. Compound I does not bear any double bond since no signals could be detected in the 110–160 ppm range in the 13C-NMR spectrum (Table 1). The remaining unsaturations, therefore, correspond to rings.
| Positiona | I | II | ||||
|---|---|---|---|---|---|---|
| δ C | δ H | δ H | δ C | δ H | δ H | |
| a cf. Fig. 1 for carbon numbering; a,b: may be interchanged. | ||||||
| 1 | 39.6a | 1.79 | 0.92 | 38.2 | 1.69 | 1.04 |
| 2 | 17.8b | 1.58 | 1.50 | 18.0 | 1.51 | 1.51 |
| 3 | 36.7 | 1.69 | 1.51 | 36.7 | 1.72 | 1.51 |
| 4 | 47.6 | — | — | 47.6 | — | — |
| 5 | 49.9 | 1.72 | — | 49.2 | 1.74 | — |
| 6 | 24.1 | 1.44 | 1.08 | 24.4 | 1.39 | 1.05 |
| 7 | 40.1 | 1.84 | 1.84 | 34.9 | 1.59 | 1.20 |
| 8 | 58.6 | — | — | 34.9 | 1.69 | — |
| 9 | 57.1 | 1.51 | — | 47.1 | 1.38 | — |
| 10 | 39.2 | — | — | 36.0 | — | — |
| 11 | 19.5 | 2.14 | 1.65 | 20.1 | 1.49 | 1.11 |
| 12 | 39.4a | 1.39 | 1.39 | 30.0 | 1.60 | 1.07 |
| 13 | 45.6 | — | — | 43.2 | — | — |
| 14 | 47.4 | 2.97 | — | 45.4 | 1.81 | 1.72 |
| 15 | 21.6 | 1.12 | — | 27.0 | 2.73 | |
| 16 | 24.1 | 0.96 | — | 23.2 | 1.05 | — |
| 17 | 46.9 | 1.92 | 1.49 | 60.8 | 3.15 | — |
| 18 | 179.3 | — | — | 179.4 | — | — |
| 19 | 16.4 | 1.12 | — | 16.9 | 1.15 | — |
| 20 | 17.9b | 0.99 | — | 14.6 | 0.86 | — |
| 1′ | 51.9 | 3.63 | — | 51.9 | 3.62 | — |
One deshielded proton was observed at 2.96 ppm which could correspond to a proton located on a sulfur-bearing carbon atom. This is supported by the chemical shift of the carbon atom bearing this proton deduced from the HSQC experiment (ESI Fig. 6S†): a tertiary C atom at 47.4 ppm. In addition, based on the comparison of the 13C-NMR and the DEPT spectra, it appears that there is one deshielded quaternary carbon atom with a chemical shift at 58.6 ppm, which indicates that it possibly bears a sulfur atom suggesting that the sulfur atom is incorporated into a thioether bridge between a tertiary and a quaternary carbon atom. This hypothesis was confirmed by the interpretation of the long-range (2,3J) 1H–13C correlation pattern (HMBC experiment, ESI Fig. 7S†), and the carbon atom sequence of compound I could finally be established (Fig. 4a).
Connectivities of parts of the structure which could not be correlated based on the long-range (2,3J) 1H–13C correlations could be established using, notably, the 1H–1H COSY and NOESY experiments. The structure of compound I could thus be related to that of diterpenoids from the pimaric or isopimaric acid series (which differ by the configuration at C-13; cf. compounds 10 and 11 in Fig. 3) sulfurized at C-8 and C-14. The relative configuration of the stereocenters and the clear affiliation of compound I to tricyclic resin acids of the isopimaric series could be established based on the interpretation of the chemical shift values of specific protons and on the interpretation of the 1H–1H NOESY correlation pattern (Fig. 4b and ESI Fig. 8S†).
Thus, the 13C chemical shift of the methyl group located at C-4 at 16.4 ppm (Table 1) clearly indicates that this methyl group is axial due to the occurrence of a γ-gauche effect with CH3-20.25 Thus, the chemical shift of the axial methyl group at C-4 is around 16.8 ppm in the case of tricyclic resin acids such as abietic acid 13 (Fig. 3).26 In contrast, the 13C chemical shift of the equatorial methyl groups at C-4 in the case of bicyclic diterpenoids related to communic acid 14 (Fig. 3) is significantly higher (around 28.5 ppm).16,27,28 The relative configuration at C-4 could be confirmed by the detection of spatial interactions between CH3-19 and CH3-20 (NOESY experiment, Fig. 4b and ESI Fig. 8S†), indicating that both methyl groups are located on the same side of the structure. In addition, the occurrence of nuclear Overhauser effects between one proton at C-17 and CH3-20 as well as with one proton of methylene CH2-12 (Fig. 4b) unambiguously shows that compound I is related to diterpenes of the isopimaric 11 acid series. Finally, all the 1H and 13C chemical shifts could be assigned (Table 1).
Identification of compound II
The mass spectrum of compound II obtained by GC-MS (EI) shows the same molecular mass, but a very distinct mode of fragmentation (cf. ESI Fig. 1bS†). The exact mass determined for compound II ([M + H]+: m/z 351.2354) (ESI Fig. 3S†) is compatible with the same molecular formula as for compound I (C21H35O2S). The signal at 3.62 ppm in the 1H-NMR spectrum (ESI Fig. 9S†) and the signals at 179.4 and 51.9 ppm in the 13C-NMR spectrum (ESI Fig. 10S†) show the presence of a carbomethoxy group (Table 1) as observed for compound I. Nevertheless, contrary to compound I, the occurrence of three deshielded proton signals at chemical shifts compatible with those of protons located α to a sulfur atom (one at 3.15 ppm and two others at 2.73 ppm) suggests that compound II bears a thioether bridge located between a secondary and a tertiary carbon atom. The detailed investigation of the one-bond (1J) (ESI Fig. 11S†) and the long range (2,3J) (ESI Fig. 12S†) 1H–13C correlations showed that compound II corresponds to a sulfurized derivative of pimaric, iso- or sandaracopimaric acid (10, 11 and 12, Fig. 3) with a five-membered ring comprising a sulfur atom located between positions C-15 and C-17 (Fig. 5a). The relative configurations of the chiral centers were determined using a NOESY experiment (Fig. 5b and ESI Fig. 13S†).Nuclear Overhauser effects were observed, notably, between H-20/H-19, H-20/H-8, H-8/H-16, H-17/H-16 and H-8/H-17 (Fig. 5b), indicating that all these protons are on the same side of the molecule. This unambiguously shows that the C/D ring junction is cis and that compound II is also related to the series of iso- or sandaracopimaric acid (11 and 12, respectively; Fig. 3), with the left moiety of the carbon skeleton being identical to that of compound I. Finally, all the 1H and 13C chemical shifts could be assigned (Table 1).
Structures of compounds III–VI
Based on the identification of the structures of compounds I and II, we propose that the other compounds with a molecular weight of 350 Da (comprising compounds III–VI) eluting in the range of retention times indicated by the red rectangle in Fig. 2 also correspond to diterpenoid sulfides. These compounds possibly derive from tricyclic diterpenoids from the isopimaric (with the sulfur atom located at other positions than in the case of compounds I and II), pimaric 10, abietic 13 or communic 14 acid series. Among these sulfides, compound III possibly corresponds to an epimer at C-14 of compound I (cf.Fig. 2) given the strong similarities between their EI mass spectra (ESI Fig. 1S†). Only the retention time in gas chromatography and the relative intensities of the main fragments in the EI mass spectra are different.Origin and the mode of formation of compounds I–II
NMR investigation of the structures of I and II clearly showed that these compounds correspond to sulfurized diterpenoids related to isopimaric or sandaracopimaric acids 11 and 12. The occurrence of such compounds in the amber sample from Piolenc is unexpected since the presence of organic sulfur compounds has, to the best of our knowledge, never been reported previously, neither in amber nor in any resin of the extant conifer species. In addition, since we have observed these compounds in amber samples from different geological periods and likely from different botanical origins, the possibility of a direct biosynthetic origin of these compounds is rather improbable. It can therefore be proposed that these compounds may rather correspond to alteration products of isopimaric or sandaracopimaric acids 11 and 12 formed via diagenetic sulfurization processes.Even though organic sulfur compounds have yet not been reported from amber/resin, numerous organic sulfur compounds have been identified in the sedimentary record and their formation has been extensively investigated during the last decades.29–31 They revealed to be formed by abiotic early diagenetic processes involving functionalized biological lipids and inorganic reduced sulfur species resulting from microbial sulfate reduction in anaerobic environments.29–31 Carbonyls and double bonds are the most liable functionalities to be sulfurized in such a context.31–36 In this respect, it appears that isopimaric or sandaracopimaric acids bear double bonds at positions potentially allowing for sulfurization at C-8, C-14, C-15 and C-17 by the addition of H2S or polysulfides of microbial origin. The mechanisms illustrated in Fig. 6 can therefore be proposed to account for the formation of compound I from isopimaric or sandaracopimaric acids 11 and 12 and that of II from sandaracopimaric acid 12.
Compounds I and II present different sites of sulfurization (C-8/C-14 vs. C-15/C-17), indicating a different selectivity in the sulfurization processes. In the case of compound I, the sulfur atom is located at the most substituted positions, which contrasts with the case of compound II, where the sulfur atom is located at the least substituted positions. Therefore, two modes of sulfurization might be considered to account for the formation of I and II. A radical process involving H2S and sandaracopimaric acid 12 can be proposed to explain the formation of compound II since such a mechanism would result in the sulfurization of the least substituted positions of the double bonds (anti-Markovnikov-type selectivity).37 Such a mechanism cannot be considered for the formation of compound I since the sulfur atom is located at the most substituted positions of potential biological precursors as frequently observed in the case of sedimentary organic sulfur compounds formed by abiotic processes.32–35 An acid-catalyzed process involving diterpenic acids and H2S can also be excluded considering the strongly acidic conditions required for such a reaction,38,39 which are not compatible with the conditions prevailing in natural environmental settings. It can therefore be proposed that sulfurization occurred following a concerted reaction involving polysulfides. Thus, it has been shown that sulfurization of isolated alkenes by polysulfides results in the formation of thiols or polysulfides with the sulfur atom located at the most substituted positions (Markovnikov-type selectivity).39 In addition, under these conditions, sulfurization of non-conjugated dienes leads to the formation of thianes or thiolanes with the preferential formation of the smallest sulfur-containing rings.33–35,39 Such a mechanism can be proposed to play a key role in sedimentary sulfurization processes, based on laboratory experiments and on the fact that thiolanes and thianes in sedimentary organic matter are often sulfurized at the most substituted positions.32–35,39 This mechanism might therefore perfectly account for the formation of compound I by sulfurization of isopimaric acid 11 by polysulfides as illustrated in Fig. 7.
 | ||
| Fig. 7 Possible mechanism of sulfurization of isopimaric acid 11 with polysulfides leading to compound I. | ||
This process might comprise first the sulfurization of the vinyl side-chain at the C-14 position due to lower steric hindrance, followed by cyclization at the C-8 position according to a concerted mechanism. The latter would involve a cyclic intermediate, addition of sulfur and hydrogen atoms on the same side of the Δ7 double bond and the formation of the smallest ring as described previously in the case of other substrates.33,35
Sulfurized diterpenoids in amber: geochemical implications
The sulfurized diterpenoids identified in the Piolenc amber sample were possibly formed after the deposition of the resin from Cupressaceae conifer shrubs or trees within sediments. Since the formation of these organo-sulfur compounds required the presence of reduced sulfur species produced by bacterial sulfate reduction, it can be anticipated that the conditions which prevailed in the sediments at the time of deposition were oxygen-depleted, as is the case in environments such as marshes or mangroves. Thus, in such organic-rich environments dominated by plant inputs, the conditions are favorable for the development of anaerobic bacteria, including sulfate-reducing bacteria which will produce H2S or polysulfides able to react with organic compounds. It is unlikely that the sulfurization of the diterpenoids occurred when the resin had already been transformed into amber since amber is a solid material which probably prevents sulfur species from diffusing into it. Therefore, sulfurization most likely occurred at an early stage of burial when the resin is sufficiently fluid to allow the diffusion of reduced sulfur species into the resin drops and their reaction with diterpenoids prior to further diagenetic transformation into amber upon deeper burial.C. Conclusions
Two fossil diterpenoids from a Cretaceous amber sample located in the city of Piolenc (Vaucluse, SE France) were identified as sulfides of diterpenic acids using GC-MS, HR-MS and detailed NMR investigations. These compounds are related to isopimaric and sandaracopimaric acids which can be predominant constituents of conifer resins. A possible diagenetic pathway for their formation is proposed and it involves the sulfurization of isopimaric and sandaracopimaric acids by reduced sulfur species (H2S or polysulfides) originating from bacterial sulfate reduction following two different mechanisms. Sulfurization processes leading to the formation of I and II will be further investigated using laboratory experiments involving resin acids and reduced sulfur species under geochemically relevant conditions. It is likely that these compounds formed at the earliest stages of diagenesis, when the fresh conifer resin entered a sedimentary setting depleted of oxygen (e.g., marsh, mangrove), and came into contact with the reduced sulfur species generated by sulfate-reducing bacteria. Therefore, these novel organo-sulfur compounds represent the first evidence of sulfurization processes affecting plant resin prior to its diagenetic transformation into amber. Thus, these compounds may be used as indicators of sulfate-reducing processes in past depositional environments. In this respect, it should be mentioned that compounds I–II and related structures with the same molecular weight have also been detected in variable amounts, but not systematically, in other amber samples from different origins or ages. Investigation of their presence and significance in terms of palaeoenvironments of deposition is currently underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Estelle Motsch and Stéphanie Coutin (University of Strasbourg) for the mass spectrometry analyses, and Maurice Coppe (University of Strasbourg) for the NMR analyses. The “Centre National de la Recherche Scientifique” and the “Université de Strasbourg” financially supported this research.References
- P. S. Bray and K. B. Anderson, Science, 2009, 326, 132–134 CrossRef CAS PubMed.
- Y. A. Nohra, V. Perrichot, L. Jeanneau, L. Le Pollès and D. Azar, J. Nat. Prod., 2015, 78, 1284–1293 CrossRef CAS PubMed.
- G. Pastorelli, Archaeological Baltic amber: Degradation mechanisms and conservation measures, PhD thesis, Universita di Bologna, 2009 Search PubMed.
- J. B. Lambert, J. A. Santiago-Blay and K. B. Anderson, Angew. Chem., Int. Ed., 2008, 47, 9608–9616 CrossRef CAS.
- K. B. Anderson, Geochem. Trans., 2006, 7, 2, DOI:10.1186/1467-4866-7-2.
- O. O. Sonibare, T. Hoffmann and S. F. Foley, Org. Geochem., 2012, 51, 55–62 CrossRef CAS.
- R. Pereira, I. S. Carvalho, B. R. T. Simoneit and D. A. Azevedo, Org. Geochem., 2009, 40, 863–875 CrossRef CAS.
- J. S. Mills, R. White and L. J. Gough, Chem. Geol., 1984, 47, 15–39 CrossRef CAS.
- B. R. T. Simoneit, P. G. Hatcher and A. Nissenbaum, Org. Geochem., 1988, 13, 677–690 CrossRef.
- O. O. Sonibare, R. J. Huang, D. E. Jacob, Y. Nie, E. Kleine-Benne, T. Hoffmann and S. F. Foley, J. Anal. Appl. Pyrol., 2014, 105, 100–107 CrossRef CAS.
- C. Menor-Salvan, B. R. T. Simoneit, M. Ruiz-Bermejo and J. Alonso, Org. Geochem., 2016, 93, 7–21 CrossRef CAS.
- Y. Wang, W. Jiang, Q. Feng, H. Lu, Y. Zhou, J. Liao, Q. Wang and G. Sheng, Org. Geochem., 2017, 113, 90–96 CrossRef CAS.
- J. Jossang, H. Bel-Kassaoui, A. Jossang, M. Seuleiman and A. Nel, J. Org. Chem., 2008, 73, 412–417 CrossRef CAS.
- K. Kimura, Y. Minamikawa, Y. Ogasawara, Y. Yoshida, K. Saitoh, H. Shinden, Q. Y. Yue, S. Takahashi, T. Miyakawa and H. Koshino, Fitoterapia, 2012, 83, 907–912 CrossRef CAS.
- T. Kawamura, H. Koshino, T. Nakamura, Y. Nagasawa, H. Nanao, M. Shirai, S. Uesugi, M. Ohno and K. Kimura, Org. Geochem., 2018, 120, 12–18 CrossRef CAS.
- N. de Lama Valderrama, P. Schaeffer, A. Leprince, S. Schmitt and P. Adam, Org. Geochem., 2022, 167, 104372 CrossRef CAS.
- H. Brechbühler, H. Büchi, E. Hatz, J. Schreiber and A. Eschenmoser, Angew. Chem., Int. Ed. Engl., 1963, 2, 212–213 CrossRef.
- B. R. T. Simoneit, R. E. Cox, D. R. Oros and A. Otto, Molecules, 2018, 23, 3384, DOI:10.3390/molecules23123384.
- K. Knight, P. Bingham, D. Gimaldi, K. Anderson, R. Lewis and C. Savrda, Cretaceous Res., 2010, 31, 85–93 CrossRef.
- A. Otto and V. Wilde, Bot. Rev., 2001, 67, 141–238 CrossRef.
- P. J. Grantham and A. G. Douglas, Geochim. Cosmochim. Acta, 1980, 44, 1801–1810 CrossRef CAS.
- T. C. Fischer, O. O. Sonibare, B. Aschauer, E. Kleine-Benne, P. Braun and B. Meller, Palaeontology, 2017, 60, 743–759 CrossRef.
- E. C. Stout, C. W. Beck and K. B. Anderson, Phys. Chem. Miner., 2000, 27, 665–678 CrossRef CAS.
- F. Riquelme, P. Northrup, J. L. Ruvalbaca-Sil, V. Stajonoff, D. P. Siddons and J. Alvarado-Ortega, Appl. Phys. A, 2014, 116, 97–109 CrossRef CAS.
- H. Beierbeck, J. K. Saunders and J. W. Apsimon, Can. J. Chem., 1977, 55, 2813–2828 CrossRef CAS.
- B. Gigante, L. Santos and M. J. Marcelo-Curto, Magn. Reson. Chem., 1995, 33, 318–321 CrossRef CAS.
- J. M. Fang, Y. C. Sou, Y. H. Chiu and Y. S. Cheng, Phytochemistry, 1993, 34, 1581–1584 CrossRef CAS.
- D. Zinkel and W. B. Clarke, Phytochemistry, 1985, 24, 1267–1271 CrossRef CAS.
- J. S. Sinninghe Damsté and J. W. de Leeuw, Org. Geochem., 1990, 16, 1077–1101 CrossRef.
- P. Schaeffer, C. Reiss and P. Albrecht, Org. Geochem., 1995, 23, 567–581 CrossRef CAS.
- P. Adam, P. Schneckenburger, P. Schaeffer and P. Albrecht, Geochim. Cosmochim. Acta, 2000, 64, 3485–3503 CrossRef CAS.
- A. Behrens, P. Schaeffer and P. Albrecht, Tetrahedron Lett., 1997, 38, 4921–4924 CrossRef CAS.
- J. Poinsot, P. Schneckenburger, P. Adam, P. Schaeffer, J. Trendel and P. Albrecht, J. Chem. Soc., Chem. Commun., 1997, 2191–2192 RSC.
- S. Gug, P. Schaeffer, P. Adam, S. Klein, E. Motsch and P. Albrecht, Org. Geochem., 2009, 40, 876–884 CrossRef CAS.
- P. Adam, P. Schaeffer, S. Gug, E. Motsch and P. Albrecht, Org. Geochem., 2009, 40, 885–894 CrossRef CAS.
- Y. Hebting, P. Schaeffer, A. Behrens, P. Adam, G. Schmitt, P. Schneckenburger, S. Bernasconi and P. Albrecht, Science, 2006, 312, 1627–1631 CrossRef CAS PubMed.
- F. Dénès, M. Pichowicz, G. Povie and P. Renaud, Chem. Rev., 2014, 114, 2587–2693 CrossRef PubMed.
- W. C. van Zijll Langhout and H. I. Waterman, J. Appl. Chem., 1954, 4, 285–288 CrossRef.
- W. De Graaf, J. S. Sinninghe Damsté and J. W. de Leeuw, J. Chem. Soc., Perkin Trans. 1, 1995, 635–640 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ob02017c |
| This journal is © The Royal Society of Chemistry 2023 |