Open Access Article
Open Access ArticleA Cu-BOX catalysed enantioselective Mukaiyama-aldol reaction with difluorinated silyl enol ethers and acylpyridine N-oxides†‡
Amparo
Sanz-Marco
 a,
Daniel
Esperilla
a,
Marc
Montesinos-Magraner
a,
Daniel
Esperilla
a,
Marc
Montesinos-Magraner
 a,
Carlos
Vila
a,
Carlos
Vila
 a,
M. Carmen
Muñoz
a,
M. Carmen
Muñoz
 b,
José R.
Pedro
b,
José R.
Pedro
 *a and
Gonzalo
Blay
*a and
Gonzalo
Blay
 *a
*a
aDepartament de Química Orgànica-Facultat de Química, Universitat de València, C/Dr. Moliner 50, 46100-Burjassot, València, Spain. E-mail: gonzalo.blay@uv.es; jose.r.pedro@uv.es
bDepartament de Física Aplicada, Universitat Politècnica de València, C/Cami de Vera s/n, 46022-València, Spain
First published on 9th December 2022
Abstract
A Cu(II)/BOX complex catalyses the enantioselective addition of difluorinated silyl enol ethers to acylpyridine N-oxides. The reaction provides difluorinated chiral tertiary alcohols of great interest in medicinal chemistry. These compounds are obtained in moderate to excellent yields and with high enantioselectivities. The stereochemical outcome of the reaction has been explained by DFT calculations.
Introduction
The introduction of fluoroalkyl substituents in organic molecules has an important effect on their physical and chemical properties, including those relevant to biomedical applications such as biodisponibility and metabolic stability.1 The importance of these properties in pharmacological and agrochemical applications has inspired the organic chemistry community during the last few decades and a huge effort has been dedicated to the development of synthetic methods for organofluorine compounds,2 even in an enantioselective fashion.3 From the synthetic point of view, the synthesis of carbonyl compounds decorated with fluoroalkyl substituents is especially appealing, as they allow for further modification in virtually every functional group. Specifically, the synthesis of difluorinated carbonyl compounds has attracted attention due to the oxygen bioisosteric properties of the –CF2– group4 and the biological activity of some of these compounds (Fig. 1).5 α,α-Difluorocarbonyl compounds can also serve as versatile building blocks for the synthesis of other fluorinated moieties.6 On the other hand, the difluoromethylene group attached to a stereogenic centre can be found as a structural motif in drugs and bioactive compounds (Fig. 1).7 However, the enantioselective synthesis of chiral compounds bearing such a structural motif is not a trivial task as the presence of the fluorine atoms may alter the reactivity of the involved functional groups or the interaction of the substrates with the chiral catalyst, hampering the enantiomeric control.8 Thus, while the electronegativity of fluorinated substituents has facilitated their use as electrophilic starting materials,9 the enantioselective introduction of fluorine-containing scaffolds as nucleophiles is a more challenging task that requires the use of special reagents.10 | ||
| Fig. 1 Some examples of bioactive compounds bearing an α,α-difluorocarbonyl moiety or a CF2 attached to a stereogenic centre. | ||
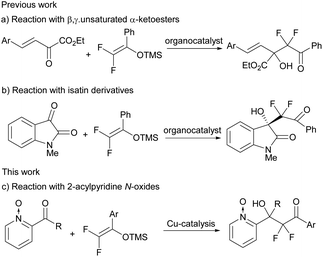
In this context, the employment of difluoroenoxysilanes11 as nucleophiles has been demonstrated to be an efficient tool for the synthesis of difluoromethyl-containing compounds. However, the number of examples involving the nucleophilic addition of difluoroenoxysilanes in an enantioselective fashion is scarce and almost limited to nucleophilic addition to imines,12,13 while the Mukaiyama aldol-type reaction14 remains almost unexplored and only two organocatalytic examples involving reactive α-keto esters15 or isatins16 have been reported by Zhou, to the best of our knowledge (Scheme 1).
2-Acylpyridine N-oxides are interesting substrates for asymmetric catalysis. Their capacity to form a chelate with a chiral metal complex makes them ideal electrophilic substrates for stereoselective transformations, providing pyridine derivatives with chiral pendants. Taking advantage of these properties, these compounds have been widely used in enantioselective transformations. Jørgensen and co-workers described in 2006 the Cu-BOX catalysed addition of ketene silyl–enol ethers to the N-oxides of formylpyridine.17 Since then, a variety of transformations have been reported with these substrates by our group18 and others,19 giving access to enantioenriched compounds containing a widely spread pyridine moiety. Herein, we report our results on the copper-catalysed Mukaiyama aldol reaction of difluoroenoxysilanes and 2-acylpyridine N-oxides (Scheme 1c).
Results and discussion
Optimisation of the reaction conditions
At the onset of our investigation, we chose 2-acetylpyridine N-oxide (1a) and difluoroenoxysilane 2a as reaction partners for the optimization process. Taking into account the precedent reported by our research group,18 we envisioned that Cu(II)/BOX could serve as an effective catalytic system. Therefore, we screened a series of BOX chiral ligands with different substitution patterns, using Cu(OTf)2 as the metal source (Scheme 2 and Table 1). Despite the modest reactivity of the system, promising levels of enantioinduction were obtained with L8 (Table 1, entry 8). Further optimization of the copper(II) source revealed an improvement in enantioselectivity when Cu(BF4)2 was used, albeit still with modest reactivity (Table 1, entry 10). An increase in the equivalents of nucleophile 2a led to the expected improvement in yield, although with a moderate decrease in the enantioselectivity (Table 1, entry 11).§ We next studied the effect of the solvent, observing a further improvement in yield when using THF, keeping the good levels of enantioselectivity (Table 1, entry 14). Finally, we performed the reaction at different temperatures. The reaction virtually shuts down at −20 °C, while an increase to 50 °C has a detrimental effect on the enantioinduction (Table 1, entries 16 and 17). Therefore, we established BOX ligand L8 in combination with Cu(BF4)2 to be our optimal catalytic system, while performing the reaction in THF as the solvent at room temperature.| Entry | Cu salt | L | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a 1a (0.1 mmol), 2a (0.12 or 0.5 mmol), Cu salt (0.01 mmol), L (0.01 mmol), room temperature, 4d. b Isolated yields. c Determined by chiral HPLC analysis. d 2a (0.12 mmol. e 2a (0. 5 mmol). f Reaction performed at −20 °C. g Reaction performed at 50 °C. | |||||
| 1d | Cu(OTf)2 | L1 | CH2Cl2 | 37 | −15 |
| 2d | Cu(OTf)2 | L2 | CH2Cl2 | 43 | 48 |
| 3d | Cu(OTf)2 | L3 | CH2Cl2 | 40 | 10 |
| 4d | Cu(OTf)2 | L4 | CH2Cl2 | 27 | 55 |
| 5d | Cu(OTf)2 | L5 | CH2Cl2 | 24 | 41 |
| 6d | Cu(OTf)2 | L6 | CH2Cl2 | 42 | 67 |
| 7d | Cu(OTf)2 | L7 | CH2Cl2 | 20 | 0 |
| 8d | Cu(OTf)2 | L8 | CH2Cl2 | 32 | 83 |
| 9d | CuBr2 | L8 | CH2Cl2 | 30 | 81 |
| 10d | Cu(BF4)2 | L8 | CH2Cl2 | 24 | 91 |
| 11e | Cu(BF4)2 | L8 | CH2Cl2 | 70 | 85 |
| 12e | Cu(BF4)2 | L8 | DCE | 38 | 80 |
| 13e | Cu(BF4)2 | L8 | CHCl3 | 48 | 84 |
| 14e | Cu(BF4)2 | L8 | THF | 90 | 87 |
| 15e | Cu(BF4)2 | L8 | Toluene | 42 | 66 |
| 16e,f | Cu(BF4)2 | L8 | THF | 9 | — |
| 17e,g | Cu(BF4)2 | L8 | THF | 87 | 73 |
Study of the reaction scope
With the optimized reaction conditions in hand (Table 1, entry 14), we explored the scope of our methodology by reacting enol ether 2a with different 2-acylpyridine N-oxides 1 (Scheme 3). Alkyl substituents are well tolerated at different positions of the pyridine ring of 1. Substrate 1b, bearing a methyl group at position 4 (R1 = 4-Me), delivered the desired product 3ba in fair yield and enantioselectivity (57%, 77% ee). Better results were obtained when using substrate 3c substituted at position 5 (R1 = 5-Me) that provided 3ca in 72% yield and 89% ee. Interestingly, N-oxides 1d (R1 = 6-Me) and 1e (R1 = 6-Br), which have potentially problematic substituents at position 6 of the pyridine ring near the N-oxide coordination site, reacted satisfactorily to provide 3da and 3ea in high yields and still remarkable enantioselectivities (79% and 60% ee, respectively). At this point, we explored other ketones 1f–h bearing a larger ethyl group attached to the carbonyl group. These reacted with 2a to give compounds 3fa, 3ga and 3ha in good yields and with high enantioselectivities (84%, 93% and 83% ee, respectively).Next, the substitution in the difluorosilyl enol ether moiety was evaluated, albeit with diverse success. Unexpectedly, switching from phenyl to the 4-methylphenyl substituent in 2b had a detrimental effect on enantioinduction (3ab, 68% yield, 57% ee). Interestingly, the excellent selectivity was re-established when 2b was reacted with 1c, which afforded compound 3cb in moderate yield but with excellent enantioselectivity (61%, 89% ee). Moreover, phenyl rings substituted at different positions with electron-donating groups 2c (R3 = 4-MeOC6H4), 2d (R3 = 3-MeOC6H4), or halogens 2e (R3 = 4-ClC6H4) and 2f (R3 = 4-FC6H4) were tolerated, giving the expected products 3ac–3af with fair to good enantiomeric excesses. Finally, enol ether 2g bearing a heterocyclic ring (R3 = 2-thienyl) could be employed, yielding aldol 3ag in moderate yield and good enantiomeric excess (83%). Unfortunately, difluoroenoxysilanes 2 bearing an alkyl R3 group (Bn) did not react under the optimised conditions.
At this point, we performed a control experiment to establish the importance of the N-oxide moiety in our method. Upon subjecting 2-acetylpyridine (4) to the optimized reaction conditions with 2a, the corresponding aldol was obtained in less than 10% yield (Scheme 4). This result showcases the importance of the N-oxide scaffold, especially in order to achieve the required coordination of the electrophilic reaction partner with the metal catalyst. Reduction of the N-oxide functional group was achieved by Pd-catalysed hydrogenolysis with concomitant ketone reduction, affording 6aa without a noticeable erosion of enantiomeric excess. On the other hand, treatment of compound 3ac with m-CPBA yielded the corresponding Baeyer–Villiger product 7ac regioselectively. Noticeably, the Baeyer–Villiger reaction did not proceed with compound 3aa and required an electron-richer substituent attached to the carbonyl group. Finally, addition of MeMgBr to the ketone in 3ha allowed us to obtain the corresponding tertiary alcohol 8ha in good yield and with modest diastereoselectivity.
Computational studies
Compound 3ag could be crystallized and subjected to X-ray analysis that allowed us to determine the configuration of the stereogenic centre to be S (Fig. 2).¶ | ||
| Fig. 2 ORTEP plot for the X-ray structure of compound 3ag with thermal ellipsoids drawn at the 50% probability level. Flack parameter 0.01(3). | ||
The stereochemistry of all compounds 3 was assigned by analogy, assuming a common stereochemical pathway. The observed stereochemistry indicates a preferential attack of the silyl enol ether to the Re face of the carbonyl group. To explain this stereochemical outcome, DFT calculations were performed with the UB3LYP-D3 functional,20 as implemented in Gaussian 09,21 with 1a and 2a as model substrates (Fig. 3). Geometries were optimized using the 6-31G(d,p) basis set for H, C, N, O, F, and Si, while the LANL2DZ pseudopotential was employed for Cu.22 Based on these geometries, single-point calculations were performed using functional ωB97XD at the 6-311+G(d,p) level of theory for H, C, N, O, F, and Si and LANL2TZ for Cu.23 As expected, our studies showed that distorted square-planar complexes, with 1a chelating the copper centre with both oxygens, are energetically favoured. Subsequent nucleophilic attack of the silyl enol ether 2a is sterically controlled by the position of the phenyl rings in the ligand, which blocks more efficiently the attack from the Si-face by interaction with the fluorine atoms in 2a. The Re-face approach (TS-Re) presents a barrier of 16.6 kcal mol−1, while the one leading to the attack from the Si face goes up to 18.6 kcal mol−1. This ΔΔG‡ = 2 kcal mol−1 is in good agreement with our experimental results. Finally, the corresponding INT-B will lead to the observed product by TMS-group transfer.
Conclusions
In conclusion, in this communication we present the first enantioselective addition of difluorinated silyl enol ethers to acylpyridine N-oxides, using a Cu(II)/BOX catalytic system. Moderate to excellent yields and high enantioselectivities are usually obtained for a variety of substitution patterns. This methodology provides an innovative access to novel difluorinated chiral tertiary alcohols, which are of great interest in fields such as medicinal chemistry. Finally, we proved the importance of the N-oxide moiety in the success of the method, and we also demonstrated its removal by Pd-catalysed hydrogenolysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Grant PID2020-116944GB-100 funded by MCIN/AEI/ 10.13039/501100011033 and by the “European Union Next Generation EU/PRTR”. Grant CIAICO/2021/147 funded by Conselleria d'Innovació, Universitats, Ciència i Societat Digital. Grant RyC-2016-20187 funded by MCIN/AEI/ 10.13039/501100011033 and by “ESF Investing in your future” to C. V. Access to NMR, MS and X-ray facilities was provided by the Servei Central de Suport a la Investigació Experimental (SCSIE)–UV.References
- (a) K. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006 CrossRef; (b) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (c) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (d) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed; (e) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed.
- (a) J. Rong, C. Ni and J. Hu, Asian J. Org. Chem., 2017, 6, 139 CrossRef CAS; (b) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (c) T. Besset, T. Poisson and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 16830 CrossRef CAS PubMed; (d) Y. Lu, C. Liu and Q.-Y. Chen, Curr. Org. Chem., 2015, 19, 1638 CrossRef CAS; (e) J. Rong, C. F. Ni and J. Hu, Asian J. Org. Chem., 2017, 6, 139 CrossRef CAS; (f) X. Peng, M.-Y. Xiao, J.-L. Zeng, F.-G. Zhang and J.-A. Ma, Org. Lett., 2019, 21, 4808 CrossRef CAS PubMed; (g) Z.-Q. Zhang, M.-M. Zheng, X.-S. Xue, I. Marek, F.-G. Zhang and J.-A. Ma, Angew. Chem., Int. Ed., 2019, 58, 18191 CrossRef CAS PubMed.
- (a) J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed; (b) G. Valero, X. Company and R. Rios, Chem. – Eur. J., 2011, 17, 2018 CrossRef CAS PubMed; (c) D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308 RSC; (d) X.-Y. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed.
- N. A. Meanwell, J. Med. Chem., 2018, 61, 5822 CrossRef CAS PubMed.
- (a) K. Nakayama, H. C. Kawato, H. Inagaki, R. Nakajima, A. Kitamura, K. Someya and T. Ohta, Org. Lett., 2000, 2, 977 CrossRef CAS PubMed; (b) K. Uoto, S. Ohsuki, H. Takenoshita, T. Ishiyama, S. Iimura, Y. Hirota, I. Mitsui, H. Terasawa and T. Soga, Chem. Pharm. Bull., 1997, 45, 1793 CrossRef CAS PubMed.
- (a) Y. Shimada, N. Taniguchi, A. Matsuhisa, K. Sakamoto, T. Yatsu and A. Tanaka, Chem. Pharm. Bull., 2000, 48, 1644 CrossRef CAS PubMed; (b) L. S. Dobson and G. Pattison, Chem. Commun., 2016, 52, 11116 RSC; (c) J. P. Henschke, Y. Liu, X. Huang, Y. Chen, D. Meng, L. Xia, X. Wei, A. Xie, D. Li, Q. Huang, T. Sun, J. Wang, X. Gu, X. Huang, L. Wang, J. Xiao and S. Qiu, Org. Process Res. Dev., 2012, 16, 1905 CrossRef CAS.
- Examples: Lubriprostone; (a) K. McKeage, G. L. Plosker and M. A. A. Siddiqui, Drugs, 2006, 66, 873 CrossRef CAS PubMed; (b) N. J. Carter and L. J. Scott, Drugs, 2009, 69, 1229 CrossRef CAS PubMed Gemcitabine; (c) P. Pourquier, C. Gioffre, G. Kohlhagen, Y. Urasaki, F. Goldwasser, L. W. Hertel, S. Yu, R. T. Pon, W. H. Gmeiner and Y. Pommier, Clin. Cancer Res., 2002, 8, 2499–2504 CAS; (d) T. S. Chou, P. C. Heath, L. E. Patterson, L. M. Poteet, R. E. Lakin and A. H. Hunt, Synthesis, 1992, 565 CrossRef CAS Cedazuridine; (e) D. Ferraris, B. Duvall and G. Delahanty, et al., J. Med. Chem., 2014, 57, 2582 CrossRef CAS PubMed; (f) S. Dhillon, Drugs, 2020, 80, 1373 CrossRef CAS PubMed L-1-Deoxyfuconojirimycin analogue; (g) R.-W. Wang and F.-L. Qing, Org. Lett., 2005, 7, 2189 CrossRef CAS PubMed.
- Y.-L. Liu, J.-S. Yu and J. Zhou, Asian J. Org. Chem., 2013, 2, 194 CrossRef CAS.
- (a) M. Bandini, R. Sinisi and A. Umani-Ronchi, Chem. Commun., 2008, 4360 RSC; (b) J. Nie, G.-W. Zhang, L. Wang, A. Fu, Y. Zheng and J.-A. Ma, Chem. Commun., 2009, 2356 RSC; (c) Y.-L. Liu, T.-D. Shi, F. Zhou, X.-L. Zhao, X. Wang and J. Zhou, Org. Lett., 2011, 13, 3826 CrossRef CAS PubMed; (d) M.-W. Chen, Y. Duan, Q.-A. Chen, D.-S. Wang, C.-B. Yu and Y.-G. Zhou, Org. Lett., 2010, 12, 5075 CrossRef CAS PubMed.
- (a) C. Ni, F. Wang and J. Hu, Beilstein J. Org. Chem., 2008, 4 DOI:10.3762/bjoc.4.21; (b) G. K. S. Prakash, J. Hu, T. Mathew and G. A. Olah, Angew. Chem., Int. Ed., 2003, 42, 5216 CrossRef CAS PubMed; (c) Y. Li and J. Hu, Angew. Chem., Int. Ed., 2007, 46, 2489 CrossRef CAS PubMed; (d) P. V. Ramachandran, A. Tafelska-Kaczmarek and K. Sakavuyi, Org. Lett., 2011, 13, 4044 CrossRef CAS PubMed.
- (a) X.-S. Hu, J.-S. Yu and J. Zhou, Chem. Commun., 2019, 55, 13638 RSC; (b) M. Decostanzi, J. M. Campagne and E. Leclerc, Org. Biomol. Chem., 2015, 13, 7351 RSC.
- (a) W. Kashikura, K. Mori and T. Akiyama, Org. Lett., 2011, 13, 1860–1863 CrossRef CAS PubMed; (b) Z. Yuan, L. Mei, Y. Wei, M. Shi, P. V. Kattamuri, P. McDowell and G. Li, Org. Biomol. Chem., 2012, 10, 2509 RSC; (c) J.-S. Yu and J. Zhou, Org. Chem. Front., 2016, 3, 298 RSC; (d) J.-S. Li, Y.-J. Liu, G.-W. Zhang and J.-A. Ma, Org. Lett., 2017, 19, 6364 CrossRef CAS PubMed; (e) M.-Y. Rong, J.-S. Li, Y. Zhou, F.-G. Zhang and J.-A. Ma, Org. Lett., 2020, 22, 9010 CrossRef CAS PubMed; (f) L. Wang, J. Zhong and X. Lin, Synlett, 2021, 417 CAS; (g) J. Li, H. Chen, R. Zhong, L. Zhu, S. Liu, H. Ding, J. Yang, L. Wang, Y. Lan and Z. Wang, ACS Catal., 2022, 12, 9655 CrossRef CAS; (h) Y.-L. Pan, Y.-B. Shao, Z. Liu, H.-L. Zheng, L. Cai, H.-C. Zhang and X. Li, Org. Chem. Front., 2022, 9, 3990 RSC; (i) Y. Li, N. Gao, G. Cao and D. Teng, New J. Chem., 2022, 46, 6121 RSC.
- For an enantioselective Michael reaction, see: (a) J.-S. Yu, F.-M. Liao, W.-M. Gao, K. Liao, R.-L. Zuo and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 7381 CrossRef CAS PubMed For an enantioselective propargylation, see: (b) X. Gao, R. Cheng, Y.-L. Xiao, X.-L. Wan and X. Zhang, Chem, 2019, 5, 2987 CrossRef CAS.
- For the pioneering work on aldol reactions with difluorenol silyl ethers, see: (a) M. Yamana, T. Ishihara and T. Ando, Tetrahedron Lett., 1983, 24, 507 CrossRef CAS; (b) K. Iseki, Y. Kuroki, D. Asada and Y. Kobayashi, Tetrahedron Lett., 1997, 38, 1447 CrossRef CAS; (c) Y. Jin-Sheng, L. Yun-Lin, X. Jing Tang and Z. Jian, Angew. Chem., Int. Ed., 2014, 53, 9512 CrossRef PubMed; (d) F. Chorki and C. Benoit, J. Org. Chem., 2001, 66, 7858 CrossRef CAS PubMed; (e) O. Kitagawa, T. Taguchi and Y. Kobayashi, Tetrahedron Lett., 1988, 29, 1803 CrossRef CAS.
- Y. Liu and J. Zhou, Acta Chim. Sin., 2012, 70, 1451 CrossRef CAS.
- (a) L. Yun and J. Zhou, Chem. Commun., 2012, 48, 1919 RSC For a related example involving monofluorinated silyl enol ethers, see: J. Zhou, X. Zhao and Y. Liu, Org. Chem. Front., 2014, 1, 742 Search PubMed.
- (a) A. Landa, A. Minnkilä, G. Blay and K. A. Jørgensen, Chem. – Eur. J., 2006, 12, 3472–3483 CrossRef CAS PubMed; (b) A. Landa, B. Richter, R. L. Johansen, A. Minkkilä and K. A. Jørgensen, J. Org. Chem., 2007, 72, 240 CrossRef CAS PubMed.
- (a) M. Holmquist, G. Blay, M. C. Muñoz and J. R. Pedro, Org. Lett., 2014, 16, 1204 CrossRef CAS PubMed; (b) S. Barroso, G. Blay, M. C. Muñoz and J. R. Pedro, Org. Lett., 2011, 13, 402 CrossRef CAS PubMed; (c) S. Barroso, G. Blay, M. C. Muñoz and J. R. Pedro, Adv. Synth. Catal., 2009, 351, 107 CrossRef CAS; (d) S. Barroso, G. Blay and J. R. Pedro, Org. Lett., 2007, 9, 1983 CrossRef CAS PubMed.
- (a) F. He, G. Chen, J. Yang, G. Liang, P. Deng, Y. Xiong and H. Zhou, RSC Adv., 2018, 8, 9414 RSC; (b) P. K. Singh and V. K. Singh, Org. Lett., 2008, 10, 4121 CrossRef CAS PubMed; (c) L. Li, S. Zhang, Y. Hu, Y. Li, C. Li, Z. Zha and Z. Wang, Chem. – Eur. J., 2015, 21, 12885 CrossRef CAS PubMed; (d) J. Sun, Y. Gui, Y. Huang, J. Li, Z. Zha, Y. Yang and Z. Wang, ACS Omega, 2020, 5, 11962 CrossRef CAS PubMed; (e) Y. Luan, A. Huang, Y. Cheng, X. Liu, P. Li and W. Li, Adv. Synth. Catal., 2019, 361, 420 CrossRef; (f) S. Rout, S. K. Ray, R. A. Unhale and V. K. Singh, Org. Lett., 2014, 16, 5568 CrossRef CAS PubMed; (g) A. Livieri, M. Boiocchi, G. Desimoni and G. Faita, Chem. – Eur. J., 2012, 18, 11662 CrossRef CAS PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, revision D.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- (a) P. J. Hay and W. R. J. Wadt, Chem. Phys., 1985, 82, 270 CAS; (b) P. J. Hay and W. R. J. Wadt, Chem. Phys., 1985, 82, 299 CAS; (c) W. R. J. Wadt and P. J. Hay, Chem. Phys., 1985, 82, 284 CAS.
- J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
Footnotes |
| † Dedicated to Professor Joan Bosch, Universitat de Barcelona, on the occasion of his 75th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2190657. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ob01763f |
| § The use of 2 equivalents of 2a led to just a modest increase of yield (35%). On the other hand, the triethylsilyl protected difluoroenoxysilane (TES instead of TMS) was not reactive. |
| ¶ CCDC 2190657 contains the supplementary crystallographic data for compound 3ag. |
| This journal is © The Royal Society of Chemistry 2023 |