Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CVD of MoS2 single layer flakes using Na2MoO4 – impact of oxygen and temperature–time-profile†
Romana Alice
Kalt
 a,
Andrea
Arcifa‡
b,
Christian
Wäckerlin
a,
Andrea
Arcifa‡
b,
Christian
Wäckerlin
 cd and
Andreas
Stemmer
*a
cd and
Andreas
Stemmer
*a
aNanotechnology Group, ETH Zürich, Säumerstrasse 4, CH-8803 Rüschlikon, Switzerland. E-mail: kaltro@ethz.ch; astemmer@ethz.ch
bSurface Science & Coating Technologies, Swiss Federal Laboratories for Materials Science and Technology (EMPA), Überlandstrasse 129, CH-8600 Dübendorf, Switzerland
cInstitute of Physics, Swiss Federal Institute of Technology Lausanne (EPFL), Station 3, CH-1015 Lausanne, Switzerland
dLaboratory for X-ray Nanoscience and Technologies, Paul-Scherrer-Institute (PSI), CH-5232 Villigen PSI, Switzerland
First published on 2nd November 2023
Abstract
Two-dimensional (2D) materials are of great interest in many fields due to their astonishing properties at an atomic level thickness. Many fundamentally different methods to synthesize 2D materials, such as exfoliation or chemical vapor deposition (CVD), have been reported. Despite great efforts and progress to investigate and improve each synthesis method, mainly to increase the yield and quality of the synthesized 2D materials, most approaches still involve some compromise. Herein, we systematically investigate a chemical vapor deposition (CVD) process to synthesize molybdenum disulfide (MoS2) single layer flakes using sodium molybdate (Na2MoO4), deposited on a silica (SiO2/Si) substrate by spin-coating its aqueous solution, as the molybdenum source and sulfur powder as sulfur source, respectively. The focus lies on the impact of oxygen (O2) in the gas flow and temperature–time-profile on reaction process and product quality. Atomic force microscopy (AFM), Raman and photoluminescence (PL) spectroscopy, X-ray photoelectron spectroscopy (XPS), and time-of-flight secondary ion mass spectrometry (ToF-SIMS) were used to investigate MoS2 flakes synthesized under different exposure times of O2 and with various temperature–time-profiles. This detailed study shows that the MoS2 flakes are formed within the first few minutes of synthesis and elaborates on the necessity of O2 in the gas flow, as well as drawbacks of its presence. In addition, the applied temperature–time-profile highly affects the ability to detach MoS2 flakes from the growth substrate when immersed in water, but it has no impact on the flake.
Introduction
Crystals with a layered structure, like graphite, can generally be mechanically cleaved into single- or very few-layered flakes, analogous to graphene.1,2 About two-thirds of the transition metal dichalcogenide (TMDC) crystals consist of such a layered structure and allow fabrication of single layered flakes, commonly called two-dimensional (2D) materials.1 These single layered TMDCs possess outstanding chemical and physical properties,3,4 making them interesting 2D materials useful as building blocks for a wide range of devices from electronics5,6 to catalysis,7 and biomedical applications.8 Molybdenum disulfide (molybdenite, MoS2) is a prominent representative of the layered TMDCs and is widely studied due to its substantial direct band gap,9,10 high on/off ratio,11 flexibility,12 strong photoluminescence,13,14 and many more properties.15 Next to the fabrication of MoS2 single layer flakes by mechanical cleavage, other top-down synthesis methods such as electrochemical exfoliation16 and liquid exfoliation in a suitable solvent17 or with a pre-intercalation step18 were developed to increase the production yield of flakes. However, the latter methods lack control over the lateral size and number of layers, and in addition, transitions in the polytype from the trigonal prismatic (2H) to the octahedral phase (1T) cannot be prevented.18 This inhomogeneity in the fabricated flakes gives rise to undesired varying properties of the final product. To tackle these issues, bottom-up approaches such as atomic layer deposition,19 molecular beam epitaxy,20 and chemical vapor deposition (CVD)21,22 have been developed. Among these synthesis methods, CVD is regarded as a very promising technique due to good scalability, low cost, relative simplicity, and better control of the size and thickness of the synthesized MoS2 flakes. Nevertheless, control of the synthesis by CVD remains a challenge, and the underlying growth mechanism is not yet fully understood due to a large number of different parameters such as precursors, temperature–time-profile, composition and rate of gas flow, growth substrate material, and pressure, as well as their interdependence.23,24 Commonly, synthesis of MoS2 flakes by CVD is performed using molybdenum trioxide (MoO3) powder as molybdenum (Mo) source, placed in a crucible and partially covered by the growth substrate, and sulfur (S) powder positioned separately in the upstream heating zone. This set-up leads to spatial nonuniformity of the synthesized MoS2 flakes on the growth substrate and, therefore, reduced control of the growth process due to the significant local differences in vapor pressure, non-homogeneous diffusion of the precursors, and reaction of vaporized sulfur with MoO3 powder, poisoning the Mo-source.25 Enhanced process control was achieved using gaseous precursors such as preheated molybdenum hexacarbonyl (Mo(CO)6) as Mo-source and diethyl sulfide (S(C2H5)2) or hydrogen sulfide (H2S) as S-source.26–28 However, H2S and Mo(CO)6 gases are highly toxic, which limits their wider application, while the organic ligands in S(C2H5)2 get incorporated in the synthesized MoS2, reducing the final flake quality.29,30 To circumvent the toxicity and inhomogeneous diffusion of the Mo-source in the gas phase when using Mo(CO)6 gas or MoO3 powder piled up below the growth substrate, the Mo-source can instead be uniformly distributed over the entire growth substrate prior to the synthesis by spin-coating an aqueous Mo-solution.31,32 This synthesis method provides an effective approach to synthesize single layered MoS2, yet, despite its simplicity, it is seldomly used and lacks detailed description and in-depth investigation of process parameters and their influence on the reaction process and flake quality.In this work, we investigate the CVD process based on sodium molybdate (Na2MoO4), spin-coated as aqueous solution onto a silica (SiO2/Si) growth substrate. The focus lies on the impact of oxygen (O2) in the gas flow and the temperature–time-profile, defined by holding temperature and holding time, on reaction process and product quality. Our synthesis protocol, with O2 present in the gas flow in the early stage of the synthesis process, yields homogeneous distributions of high-quality MoS2 single layer flakes over a wide range of temperature–time-profiles. Interestingly, for low enough holding temperature and short holding time, MoS2 flakes detach from the growth substrate when immersed in water. We propose a reaction process (see Fig. 1), which we validated using atomic force microscopy (AFM), Raman and photoluminescence (PL) spectroscopy as well as X-ray photoemission spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) to determine flake quality, sample topography, and chemical composition.
Synthesis and characterization
Synthesis model
Our synthesis of MoS2 single layer flakes by CVD with sodium molybdate (Na2MoO4) is based on the vapour–liquid–solid (VLS) growth mode.32,33 Therein, vaporized sulfur (S) reacts with liquified Na2MoO4 on the growth substrate to form solid MoS2 when the temperature is raised above the specific melting point of S and Na2MoO4, respectively. Here, we first summarize the synthesis model (Fig. 1 and its subsets identified by roman numerals) and subsequently address key aspects as the requirement of oxygen (O2) in the formation of MoS2 single layer flakes, process duration, influence of the temperature–time-profile (T–t-profile), and the role of sodium.Spin-coating and drying of the aqueous solution of Na2MoO4 on silica (SiO2/Si) lead to formation of small clumps uniformly distributed over the entire substrate, which entails homogeneous distribution of the Mo-source (Ia, Ib). While heating up the tube furnace to a specific temperature (holding temperature, Thold), the O2 in the gas flow burns away any kind of airborne organic contaminants on the growth substrate and enables the growth of triangular flakes (II). When the center of the tube furnace reaches a temperature of 650 °C the sulfur powder, placed in a crucible in the upstream heating zone, starts to melt and evaporates, subsequently being transported to the growth substrate by the gas flow, where it reacts with the liquified Na2MoO4 at around 690 °C to form MoS2 within a very short time frame (III).32 With increasing time, only chemical changes related to sodium (Na) originating from the Mo-source take place (IV). At low Thold and short holding time (thold), which refers to the time frame in which the center of the tube furnace is at Thold, a thin layer of Na-containing and water-soluble by-product remains between the growth substrate and MoS2 flakes (Va). Upon immersion of the growth substrate into water, this layer dissolves, leading to complete detachment of the MoS2 flakes. These detached MoS2 flakes float on the water surface as a greenish carpet and can be collected by immersing a fresh substrate and picking them up. Samples synthesized at high Thold and long thold, however, do not exhibit this water-soluble layer due to enhanced migration of the Na-by-product into the substrate (Vb). Consequently, the MoS2 flakes are in direct contact with the growth substrate rendering them impossible to be detached by water. This Na-by-product might be composed of sodium oxide (Na2O), a possible side-product of the MoS2 flake formation, as described elsewhere.34 Na2O exhibits both temperature-dependent reactivity with SiO2, forming sodium silicate glasses, and migration into SiO2, where it becomes diluted within the silica glass matrix, consonant with our findings.35
Requirement of oxygen
The composition of the gas flow present during CVD plays a crucial role for successful synthesis of triangular MoS2 single layer flakes with Na2MoO4 uniformly distributed on a SiO2/Si substrate by spin-coating its aqueous solution. Kim et al. already reported that a brief exposure of oxygen (O2) to the growth substrate is mandatory in the initial phase of synthesis to etch the carbon atoms introduced by iodixanol, which the authors added to their Mo-solution to improve adhesion to the substrate during spin-coating, and subsequently to promote the formation of large MoS2 flakes.34 Even in the absence of an extra carbon source, such as iodixanol, we found that O2 has a strong effect on the morphology of MoS2, while synthesis of triangular shaped MoS2 single layer flakes is impossible in the absence of O2 (Fig. 2a and Fig. S31†). Yet, O2 exposure throughout the synthesis must be avoided, as MoS2 is susceptible to oxidation (Fig. 2b).36,37 To determine the impact of O2 exposure time on the formation and oxidation of MoS2 flakes, we performed X-ray photoelectron spectroscopy (XPS) on a series of samples synthesized at 710 °C for 6 min with different exposure times of O2 (1.3% in nitrogen, see Experimental and Table S1†). Analysis of the high-resolution X-ray photoelectron (XP) spectra reveals the disappearance of the Mo 3d signal of MoS2 and the concurrent rise of a Mo6+ signal of oxidized molybdenum with increasing exposure time of O2 (Fig. 2c). Oxidation of MoS2 is also confirmed by the disappearance of S 2s and S 2p signals and the concurrent rise of a sulfate signal. The apparent S/Mo-ratio, determined by the formula described in Experimental, continuously decreases with exposure time of O2 longer than 10 min, further corroborating the oxidation of MoS2 flakes (Fig. S32†). The apparent S/Mo-ratio of the sample synthesized without O2 in the gas flow is about 1.9, i.e., close to the stoichiometric value of MoS2. In addition, the high-resolution XP-spectra of such a sample (Fig. S21†) is readily comparable with those of MoS2 powder (Fig. S8†) as well as with those of samples exhibiting large triangular shaped flakes (Fig. S15,† as an example). MoS2 thus forms even in absence of O2, but the latter has a dramatic impact on the morphology of the product. Based on these findings, we propose that O2 burns organic contaminants on the surface of the growth substrate at the beginning of synthesis, enabling lateral growth of MoS2 single layer flakes, just as oxygen exposure in a plasma environment serves to remove organic contaminants from surfaces.38 However, as the synthesis time progresses, O2 becomes detrimental and even has a destructive effect by oxidizing grown MoS2 flakes, so the presence of oxygen in the gas phase late in the synthesis is not conducive.In summary, O2 proves to be a crucial parameter in this type of CVD process and must be carefully adjusted to promote the growth of MoS2 single layer flakes while avoiding any detrimental oxidation. Unless otherwise specified, in the following experiments, the exposure time of O2 is kept constant at about 2.5 min (until the tube furnace reaches a temperature of 200 °C) to determine the impact of temperature and synthesis time on the formation of MoS2.
Formation of MoS2 single layer flakes
Light microscopy of MoS2 flakes synthesized at 710 °C and thold > 4 min shows no discernible differences in size and number with respect to thold, suggesting a short time frame required for the synthesis of MoS2 flakes (Fig. 3a). Analysis of the high-resolution XP-spectra of samples synthesized at 710 °C and various thold confirms the formation of MoS2 by the disappearance of the Mo 3d signal of Na2MoO4 and the concurrent rise of that of MoS2 with increasing thold, as well as the appearance of S 2s and S 2p signals (Fig. 3b and additional samples in ESI† section XPS analysis). Since no significant changes in the apparent S/Mo-ratio were measured for all samples with thold > 4 min, the conversion of Na2MoO4 to MoS2 was found to be completed at very short thold (Fig. 3c). The O 1s signal of Na2MoO4 also becomes undetectable for all samples with a thold > 4 min, corroborating the fast consumption of the Mo-source and conversion to MoS2 (Fig. S33†). However, a minor peak at the binding energy range characteristic of Mo6+ 3d3/2 remained observable even at the highest thold. This spectral feature might indicate the presence of unreacted molybdate, or the occurrence of some minor re-oxidation of MoS2 flakes after exposure to air. Alternatively, the signal could entirely constitute the secondary structure of the Mo 3d signal of MoS2, as found by Wang et al. for the case of nearly pure MoS2.39 In our samples, small amounts of oxidized MoS2 cannot be unambiguously identified by the analysis of the O 1s region, as the latter is dominated by the signal of the SiO2/Si substrate, which partially overlaps with the O 1s signal of oxidized MoS2 (Fig. S33†). Despite the curve fitting model used in this work assumes that the minor peak at the binding energy range characteristic of Mo 3d3/2 of oxidized MoS2 is solely ascribed to such species, the actual interpretation of the signal may be not so straightforward.Overall, the XPS analysis reveals that MoS2 flakes can be synthesized within 2 min at 710 °C and extending beyond 4 min does not affect their size, number, and chemistry. In the following, we explore a range of Thold (710–810 °C) and thold (2–60 min) and study their influence on the MoS2 flakes.
Influence of temperature–time-profile
The temperature–time-profile (T–t-profile) is defined by the maximum temperature (holding temperature, Thold) and the duration of Thold (holding time, thold) and applies to the center of the tube furnace, where the tube furnace thermocouple and growth substrate were placed. Since the synthesis and any other reactions can also occur outside the thold timeframe during heating and cooling, we introduced an additional parameter denoted as synthesis time (tsyn), indicating the time during which the temperature in the center of the tube furnace is higher than 650 °C (see Experimental). By using different T–t-profiles, other synthesis parameters, such as the sulfur (S) content in the gas phase, which is governed by the temperature in the S-evaporating zone (see Experimental), will also be affected. To exclusively investigate the influence of T–t-profile, a single synthesis was conducted at 790 °C for 10 min, using 14 growth substrates placed in a row within the reaction tube (14-sample test). Taking advantage of the temperature gradient within the tube furnace, Thold and tsyn increase and decrease progressively for the growth substrates along the reaction tube, reaching a maximum for those situated in the center, the standard synthesis position. This allows simultaneous synthesis of samples with different T–t-profiles, while keeping the sulfur content as constant as possible. The growth substrates were labeled from I to XIV, starting with the sample located in the upstream heating zone, and further described by their distance from the center of the tube furnace, the position of growth substrate number VII (Fig. S3†). We always refer to these samples by the term “14-sample test” to distinguish them from the results obtained by the standard synthesis conducted with a single growth substrate positioned at the center of the tube furnace.For the holding temperatures and times tested (710–810 °C and 4–60 min, respectively), light microscopy shows no obvious effects on the morphology of the MoS2 flakes, which also applies to flakes synthesized in the 14-sample test (examples shown in Fig. S34 and S35†). All flakes are triangular in shape, evenly distributed over the complete growth substrate and similar in size and number independent on the applied T–t-profile. Furthermore, all flakes show the same color indicating an identical flake thickness,40,41 except for the most upstream and most downstream samples placed in the tube furnace in the 14-sample test (samples I and XIV in S35†). Measurements with an external thermocouple indicate that Thold was about 660 °C at the position of growth substrate number XIV, suggesting unfavorable process conditions. Therefore, samples I and XIV are excluded from further analysis. It is worth noting that the synthesized MoS2 flakes don't exhibit exact triangular shapes but have slightly concave edges. Despite the former has lowest formation energy, various morphologies such as truncated triangles, hexagons, and dendritic (snowflakes) can easily be synthesized with CVD, by varying the growth environment, such as holding temperatures of precursors, position of growth substrate, and synthesis time.24,42,43 In the end, the molybdenum to sulfur (Mosource/Ssource) ratio, which can be tuned by the as mentioned synthesis parameters, determines the shape of MoS2 flakes. In our synthesis method, we assume a similar growth environment during the formation of MoS2 flakes for each T–t-profile investigated, resulting in the same shape of flakes, as first the amount of molybdenum available for the synthesis is constant for each synthesis. Second, each T–t-profile studied had the same heating rate, and the sulfur crucible was placed so that the sulfur melted at the same time during heating, resulting in the same Mosource/Ssource-ratio within the short time frame the MoS2 flakes are formed. The Raman spectra of all samples show two characteristic peaks at around 404 cm−1 and 384 cm−1 representing the out-of-plane vibration mode A1g and the in-plane vibration mode E2g1, respectively (Fig. S36†). The difference of about 20 cm−1 between the two peaks indicates the presence of single layers (Table S5†).44,45 AFM height analysis also results in about 1 nm thickness, thus confirming the presence of single layers (Fig. S37†).45,46 Additionally, AFM measurements reveal prominent particle-like features around and on the flakes. The density of these features decreases with increasing Thold and thold (Fig. 4a). Flakes exposed to water no longer show such particle-like features and, moreover, no detrimental defects, indicating that these features are not defective structures of the MoS2 flakes but rather a water-soluble by-product of the synthesis that is apparently not assimilated into the MoS2 flakes (Fig. S38 and S44†). Due to the fast consumption of the Mo-source (see Fig. 3b and c), this by-product is no leftover of the Mo-salt. This by-product raises no concerns as it is not incorporated into the MoS2 flakes and can be easily removed through water, a process that naturally occurs during transfer of MoS2 flakes using a water-based method. A discussion on the decrease in concentration of the by-product with increasing Thold and thold is provided in Role of sodium.
 | ||
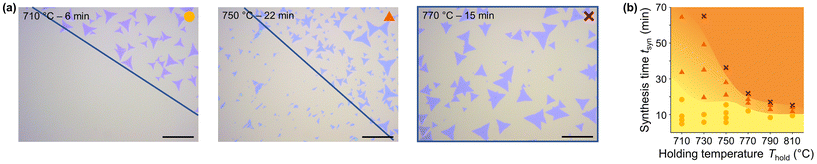
| Fig. 4 (a) AFM measurements of samples synthesized with different T–t-profiles. The scale bars represent 2 μm. (b)–(d) The shape of the markers indicate the degree of detachability, where circles represent 100% detachability, triangles represent partial detachability with varying degrees, and crosses no detachability at all (see Fig. 5). (b) IB/IA-ratio versus synthesis time tsyn of all synthesized samples listed in ESI Table S2,† where different colors represent the corresponding Thold. Low IB/IA-ratio indicates low density of defects and high crystal quality.46 (c) IB/IA-ratio of the samples synthesized in the 14-sample test. The grey vertical line indicates the center of the tube furnace, where sample number VII was placed. The samples I and XIV were excluded, as they did not pass the first quality screening with the light microscope (see Fig. S35†). (d) IB/IA-ratio of various samples before (symbols and colors as in b) and after transfer (stars) with the PMMA-assisted transfer process. All measurements are conducted after a storage time of at least 48 hours, after which no further water adsorption is expected, influencing the measurement. | ||
The crystal quality of MoS2 flakes was also investigated by photoluminescence (PL). The PL spectrum of a MoS2 single layer flake shows a prominent peak at around 1.85 eV and a less dominant one at around 2.0 eV (Fig. S39†). These two resonances are the direct excitonic transitions at the Brillouin zone K point due to the spin–orbit splitting of the valence band and are known as A- and B-exciton, respectively.47,48 The ratio of the A- and B-exciton PL intensity (IB/IA-ratio) reflects the crystal quality of the MoS2 flakes, with a low IB/IA-ratio indicating a low density of defects and high sample quality.49 When analyzed on the growth substrate, flakes synthesized with different T–t-profiles strongly differ in their measured IB/IA-ratios, showing a parabolic trend with increasing tsyn for each Thold (Fig. 4b). The IB/IA-ratio of the samples synthesized in the 14-sample test exhibits an increasing trend the closer the sample was placed to the center of the tube furnace, where it is exposed to a higher Thold and longer tsyn (Fig. 4c). This behavior correlates well with the respective right branches of the parabolas in Fig. 4b, where increasing tsyn leads to a higher IB/IA-ratio. The left branches in Fig. 4b are not discernible in Fig. 4c due to the too high Thold and long thold in the 14-sample test. However, any PL measurement of MoS2 single layer flakes strongly depends on the substrate material and the interactions the flakes establish with their surroundings.50–52 To determine whether the applied T–t-profile affects the crystal quality or alters the growth substrate–flake interactions, which in turn influence the PL, random samples were transferred onto freshly cleaned SiO2/Si substrates using the poly(methyl methacrylate) (PMMA) assisted method (see Experimental). All transferred flakes have a similar IB/IA-ratio of 0.1, regardless of the T–t-profile applied for their synthesis (red stars in Fig. 4d), indicating an equally high quality of the synthesized MoS2 flakes. Therefore, the observed differences in PL for MoS2 flakes on the growth substrate infer different interactions between the flakes and the substrate, potentially resulting from variations in the concentrations of the by-product, which are dependent on the applied T–t-profile and will be discussed in more detail later.
For a range of T–t-profiles, we observed that synthesized MoS2 flakes detach from the growth substrate when immersed in water directly after synthesis (Fig. 5a and Fig. S40, S41†). The detached flakes float on the water surface as a greenish carpet and can be collected by immersing a fresh substrate and lifting them off (Fig. S42†). To determine the influence of the T–t-profile on the detachability, samples were synthesized with different combinations of Thold and thold and analyzed by light microscopy after partial immersion of the growth substrate into water. The detachability was subsequently evaluated qualitatively using a rating system with yellow circles representing full (100%) detachability, orange triangles partial detachability, and red crosses no detachability at all (Fig. 5a). The T–t-profile strongly influences detachability, which generally decreases with increasing Thold and tsyn (Fig. 5b). The transition from full to zero detachability occurs over a wide range of tsyn for low Thold (710–750 °C) and becomes significantly narrower for higher Thold. The same trend is evident for tsyn, where the detachability decreases rapidly with increasing Thold for long tsyn. The samples synthesized in the 14-sample test show a reduction in detachability for the samples placed closer to the center of the tube furnace, where they are exposed to a higher Thold and longer tsyn (Fig. 4c and S41†). This finding corroborates the relationship between the T–t-profile and detachability as it excludes other parameters influenced by the T–t-profile that could potentially account for the variation in detachability. Comparison of the IB/IA-ratio measured by PL on the growth substrate with the degree of detachability shows for all samples, including those synthesized in the 14-sample test, that the IB/IA-ratio minimum is always around the tsyn at which the samples start to lose their detachability (Fig. 4b and c). Further, the observed parabola compresses with increasing Thold (Fig. 4b), just as the transition range between water-detachable and non-detachable decreases. This correlation additionally illustrates the influence of the T–t-profile on the growth substrate–flake interaction and suggests that the observed influence of the T–t-profile on PL and detachability is likely attributable to the same underlying cause.
Besides the T–t-profile, storage of MoS2 flakes in ambient conditions also affects detachability, becoming lower with increasing storage time. Notably, in dry environments, this effect is suppressed, indicating a direct involvement of humidity on the aging process (Fig. S43†). The formation of particle-like features and the IB/IA-ratio measured by PL also depend on the storage time. Samples measured by AFM directly after synthesis show a smooth surface without any significant features, which appear only after about 12 hours of storage in ambient conditions (22 °C, 40–60% RH, Fig. S44†). The IB/IA-ratio of samples with water-detachable flakes strongly changes in the first 40 hours of storage, while samples with non-detachable flakes have a constant IB/IA-ratio (Fig. S45†). These findings confirm that the by-product governs growth substrate–flake interactions, consequently being responsible for variations in PL and detachability. However, the parabolic behavior of the PL measurements cannot be rationalized at this point yet.
In summary, the T–t-profiles tested show no influence on shape, size, number of layers, and quality of the MoS2 flakes, suggesting a wide range of suitable T–t-profiles for simple and fast synthesis of MoS2 single layer flakes in high quality and large quantity. The XPS and AFM measurements unveil the presence of a water-soluble by-product, of which the concentration decreases with increasing Thold and thold and thus depends on the T–t-profile. This by-product, its variation in concentration, and formation of clumps with storage time due to moisture adsorption, are responsible for differences in the growth substrate–flake interaction and thus for the degree of detachability and variances in PL. We expect that this by-product or rather the different growth substrate–flake interaction will also affect the measurements of electrical properties. However, we defer this characterization as it is beyond the scope of this manuscript. Here we investigate the CVD process in more detail by discussing the role of sodium in the synthesis, as we suspect the presence of a sodium-containing by-product.
Role of sodium
Sodium (Na) is known to support the lateral growth of MoS2 flakes and is typically added directly to the Mo-source as sodium chloride (NaCl) or sodium hydroxide (NaOH).34,53 Since our Mo-source (Na2MoO4) already contains Na no additional Na was added. Although the XPS measurements reveal that the conversion of Na2MoO4 to MoS2 is completed after a thold of about 4 min (see Fig. 3b and c), the apparent Na/Mo-ratio decreases continuously with increasing thold. Moreover, the Na 1s signal shifts towards higher binding energy, indicating chemical changes that still take place after synthesis of MoS2 (Fig. 6a and b). If no volatile Na-containing products are formed, the decrease of the apparent Na/Mo-ratio and chemical shift with longer thold indicate a migration of Na into the growth substrate,54 suggesting the involvement of the SiO2/Si growth substrates in the synthesis process. It is worth noting that the apparent Na/Mo-ratio of spin-coated Na2MoO4 of 4.4, as measured by XPS, is substantially larger than the expected stoichiometry for Na2MoO4. The observed deviation is discussed in some detail in the ESI (see ESI† section XPS analysis 2.6). While XPS is a powerful tool to quantitatively analyze the chemical composition of samples, it lacks lateral resolution. Therefore, time-of-flight secondary ion mass spectrometry (ToF-SIMS) measurements were performed to obtain a chemical mapping of the MoS2 flakes and growth substrate to identify possible local features and anomalies. No remarkable variances could be detected in the mapping of S−- and Mo+-ions of samples synthesized with different T–t-profiles (Fig. S46 and S47†). In contrast, a significant change in the mapping of Na+-ions is observed for samples synthesized at varying Thold and thold. Specifically, a rapid decrease in Na intensity is firstly observed as Thold and thold increases, which is in line with the outcomes of the XPS quantitative analysis (Fig. 6c, d and Fig. S48, S49†). Secondly, the areas of the growth substrate that were immersed into water to detach the MoS2 flakes show an increased concentration of Na+-ions at the sites where MoS2 flakes were previously grown (Fig. 6c). These “ghost-flakes” indicate that Na preferentially accumulates between the MoS2 flakes and the growth substrate. Finally, in areas where no MoS2 flakes were grown, a decrease of Na+-ion concentration can be detected in the water-exposed part compared to the non-exposed part (Fig. 6d). These observations indicate the presence of a Na-containing water-soluble by-product and point to a potential key role of Na, not only in supporting the lateral growth of MoS2 flakes, but also in mediating the ability to detach the MoS2 flakes with water. Possible Na-containing water-soluble by-products formed during the conversion of Na2MoO4 to MoS2 could be sodium sulfite (Na2SO3) and its in air easily weathered and oxidized form sodium sulfate (Na2SO4),55 or sodium oxide (Na2O),34 which forms sodium hydrogencarbonate (NaHCO3) and sodium carbonate (Na2CO3) if exposed to air. However, none of these species could be clearly identified in the ToF-SIMS and XPS measurements. Nonetheless, Na2O has a temperature-dependent reactivity with SiO2 forming sodium silicate glasses,35 and can migrate into the substrate (see Fig. 1), diluting in the silica glass matrix, consistent with the decrease in concentration and shift in binding energy of Na with increasing Thold and thold as measured by XPS. | ||
| Fig. 6 (a) Apparent Na/Mo-ratio of samples synthesized at 710 °C with different thold, determined by the formula described in the Experimental. The grey line marks the thold of 4 min after which no changes in the apparent S/Mo-ratio were detected anymore (see Fig. 3b and c). SC marks the sample with spin-coated Na2MoO4 as a reference. (b) High-resolution XP-spectra of Na 1s signal. (c and d) ToF-SIMS elemental maps of N+-ions at a partly washed area of samples synthesized with different T–t-profiles. The blue lines mark the immersion depth of the growth substrate into water to detach the flakes. The scan size was 100 μm. | ||
To search for possible interactions between Na2MoO4, SiO2, and O2 in the gas flow, selected syntheses were repeated in the absence of sulfur (Table S1†). No significant differences were observed in the high-resolution XP-spectra of either the Mo 3d or Na 1s core level peak regions, regardless of the exposure time of O2 (Fig. S50†). Thus, when simply heating Na2MoO4 on SiO2/Si, with or without O2 present in the gas flow, no chemical reactions take place and only minor amounts of Mo and Na are lost by evaporation or migration into the substrate, despite increased mobility due to Na2MoO4 being in the liquid state. The reaction of S with Na2MoO4 to form MoS2 is indispensable for the migration of Na into the substrate, strengthening the assumption that a Na-containing by-product with enhanced mobility in SiO2 such as Na2O, is formed.34,35 These findings reveal an involvement of the SiO2 in the synthesis of MoS2 flakes, and we strongly recommend transferring the synthesized MoS2 flakes onto a fresh substrate for further processing, such as device fabrication and characterization, as the growth substrate and growth substrate–flake interaction are greatly affected by the synthesis by-products and substrate modifications through Na migration. Water-based transfer methods enable mitigation of these risks.
Concluding this section, our experiments reveal the presence of a water-soluble and Na-containing by-product around, on, and beneath the synthesized MoS2 flakes, significantly affecting the behavior of the MoS2 flakes. Synthesis at lower Thold (710–750 °C) and shorter thold (4–10 min) results in a high concentration of this Na-by-product. Due to the water-solubility of such by-products, the MoS2 flakes easily detach from the growth substrate when immersed in water. Moreover, the hygroscopic nature of the Na-by-product facilitates the adsorption of moisture, forming clumps and creating a water-rich environment surrounding the MoS2 flakes. With increasing Thold or thold, the concentration of Na-by-product diminishes due to its migration into the growth substrate. Consequently, the local environment experienced by the MoS2 flakes changes, leading to a shift in the IB/IA-ratio measured by PL. At certain combinations of Thold and thold, the MoS2 flakes begin to lose their water-detachability as the Na-by-product migrates away from the interphase between the growth substrate and flakes, resulting in a direct growth substrate–flake contact. This unmediated connection apparently inhibits the detachment of MoS2 flakes from the growth substrate by water. At this point, the IB/IA-ratio has its minimal value (see Fig. 4b). With further increase in Thold and thold, direct contacts between growth substrate and flake increase, leading to a reduced degree of detachability until it is no longer possible to detach the MoS2 flakes at all. The IB/IA-ratio increases again, which may be attributed to induced strain due to the lattice mismatch between growth substrate and MoS2 flakes, as well as differences in thermal conductivity.56 The transferred MoS2 flakes are free of any Na-by-product, due to its water solubility and the water-based transfer method used. Furthermore, the flakes are not in direct contact with the fresh substrate, as water clusters are expected to be trapped between the transferred flakes and the fresh substrate. Due to the absence of particles and direct substrate–flake contact a low IB/IA-ratio is measured.
Conclusions
We have presented a simple CVD method to synthesize MoS2 single layer flakes that cover the entire growth substrate with high uniformity and good quality, using Na2MoO4 deposited directly on the growth substrate (SiO2/Si) by spin-coating its aqueous solution and sulfur powder as Mo- and S-source, respectively. In-depth studies using Raman, PL, AFM, XPS, and Tof-SIMS measurements firstly showed that the composition of the gas flow must be carefully selected to promote the growth of MoS2 single layer flakes and to avoid any detrimental oxidation of the synthesized flakes. Secondly, the study shows that the reaction to form MoS2 flakes takes place within the first few minutes of synthesis at temperatures higher than 710 °C and long synthesis time is not necessary. Moreover, the range of suitable T–t-profiles for the synthesis of MoS2 single layer flakes is very wide, rendering this CVD method very robust, insensitive to variations during the synthesis process, and adaptable to different types of tube furnaces. However, the T–t-profile exhibited a strong influence on the growth substrate–flake interaction and the detachability of MoS2 flakes from the growth substrate with water. While no apparent flake parameter correlated with the detachability of the MoS2 flakes, the growth substrate–flake interaction was found to be governed by a Na-containing by-product derived from the Mo-source. The presence of this Na-containing by-product, particularly facilitated by low Thold and short thold, and its water-solubility enables the detachment of MoS2 flakes. In contrast, high Thold and long thold promote the migration of this Na-by-product into the growth substrate, in turn decreasing its concentration below the MoS2 flakes and thus hindering detachability.Experimental
Sample fabrication
Sample characterization
All samples were measured after storage for at least 48 hours, if not stated otherwise.Raman and photoluminescence (PL) spectra were recorded in air at ambient conditions by a NT-MDT (Moscow, Russia) Raman system equipped with a green laser (561 nm, 50 mW) and an 100× objective lens (NA = 0.8). The gratings were 150 lines per mm for PL measurements and 1800 lines per mm for Raman measurements. The peak of silica from the substrate was applied as an internal standard for all spectra.
Measurements with the atomic force microscope (AFM) were performed under ambient conditions using a Cypher S Asylum Research AFM (Oxford Instruments, Santa Barbara, USA) in amplitude modulation mode (AM-AFM). The records were analyzed with Gwyddion.
X-ray photoelectron spectroscopy (XPS) was conducted with a PHI Quantera SXM spectrometer (ULVAC-PHI, Chanhassen, USA), operating in constant-analyzer-energy (CAE) mode and equipped with a monochromatic Al Kα X-ray source (1.487 keV). A nominal beam-spot size of 150 μm was used for all spectral acquisitions. High resolution spectra were acquired with a pass energy and an energy step of 55 and 0.1 eV, respectively. For the survey spectra, the pass energy and step size were 280 and 1.0 eV. All spectra were offset by using the O 1s signal of the growth substrate (SiO2/Si) as an internal reference (binding energy of 532.5 eV). A detailed description of the XPS chemical state analysis and curve fittings is provided in the ESI (ESI† section XPS analysis). The atomic ratio of the elements of interest xa was estimated from the high-resolution X-ray photoelectron spectra with
Measurements with the time-of-flight secondary ion mass spectrometry (ToF-SIMS) were performed using an IONTOF, ToF-SIMS 5 instrument (IONTOF, Munster, Germany). A beam of 25 keV Bi+ as primary ions was randomly raster-scanned across the area of interest. Images and spectra of the sputtered positive and negative ions were recorded on separate areas of the sample (extraction voltage 2 kV).
Author contributions
A. Arcifa and R. Kalt conducted the XPS measurements and interpreted the XP-spectra. C. Wäckerlin performed the ToF-SIMS measurements. C. Wäckerlin and R. Kalt analyzed the ToF-SIMS spectra. R. Kalt synthesized all samples and conducted the Raman, PL, and AFM study. R. Kalt devised all experiments and wrote the original draft with input from all authors. A. Stemmer supervised the project, and reviewed, commented, and edited the manuscript drafts.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors gratefully acknowledge the Cleanroom Operations Team of the Binning and Rohrer Nanotechnology Center (BRNC) for their help and support. C. Wäckerlin gratefully acknowledges financial support by the Swiss National Science Foundation (grant PCEFP2_202775) and the University of Zürich Research Priority Program LightChEC. The ToF-SIMS experiments were performed at Empa Dübendorf.References
- J. A. Wilson and A. D. Yoffe, Adv. Phys., 1969, 35, 193 CrossRef.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, ACS Nano, 2014, 8, 1102 CrossRef CAS PubMed.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263 CrossRef PubMed.
- X. Duan, C. Wang, A. Pan, R. Yu and X. Duan, Chem. Soc. Rev., 2015, 44, 8859 RSC.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699 CrossRef CAS PubMed.
- K. F. Mak and J. Shan, Nat. Photonics, 2016, 10, 216 CrossRef CAS.
- G. W. Shim, W. Hong, S. Y. Yang and S.-Y. Choi, J. Mater. Chem. A, 2017, 5, 14950 RSC.
- A. Bolotsky, D. Butler, C. Dong, K. Gerace, N. R. Glavin, C. Muratore, J. A. Robinson and A. Ebrahimi, ACS Nano, 2019, 13, 9781 CrossRef CAS PubMed.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- H. J. Conley, B. Wang, J. I. Ziegler, R. F. Haglund, S. T. Pantelides and K. I. Bolotin, Nano Lett., 2013, 13, 3626 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed.
- K. Liu, Q. Yan, M. Chen, W. Fan, Y. Sun, J. Suh, D. Fu, S. Lee, J. Zhou, S. Tongay, J. Ji, J. B. Neaton and J. Wu, Nano Lett., 2014, 14, 5097 CrossRef CAS PubMed.
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C.-Y. Chim, G. Galli and F. Wang, Nano Lett., 2010, 10, 1271 CrossRef CAS PubMed.
- B. Birmingham, J. Yuan, M. Filez, D. Fu, J. Hu, J. Lou, M. O. Scully, B. M. Weckhuysen and Z. Zhang, ACS Appl. Nano Mater., 2018, 1, 6226 CrossRef CAS.
- R. Ganatra and Q. Zhang, ACS Nano, 2014, 8, 4074 CrossRef CAS PubMed.
- Z. Zeng, Z. Yin, X. Huang, H. Li, Q. He, G. Lu, F. Boey and H. Zhang, Angew. Chem., Int. Ed., 2011, 50, 11093 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568 CrossRef CAS PubMed.
- X. Fan, P. Xu, D. Zhou, Y. Sun, Y. C. Li, M. A. T. Nguyen, M. Terrones and T. E. Mallouk, Nano Lett., 2015, 15, 5956 CrossRef CAS PubMed.
- L. K. Tan, B. Liu, J. H. Teng, S. Guo, H. Y. Low and K. P. Loh, Nanoscale, 2014, 6, 10584 RSC.
- D. Fu, X. Zhao, Y.-Y. Zhang, L. Li, H. Xu, A.-R. Jang, S. I. Yoon, P. Song, S. M. Poh, T. Ren, Z. Ding, W. Fu, T. J. Shin, H. S. Shin, S. T. Pantelides, W. Zhou and K. P. Loh, J. Am. Chem. Soc., 2017, 139, 9392 CrossRef CAS PubMed.
- Y.-H. Lee, X.-Q. Zhang, W. Zhang, M.-T. Chang, C.-T. Lin, K.-D. Chang, Y.-C. Yu, J. T.-W. Wang, C.-S. Chang, L.-J. Li and T.-W. Lin, Adv. Mater., 2012, 24, 2320 CrossRef CAS PubMed.
- Y. Shi, H. Li and L.-J. Li, Chem. Soc. Rev., 2015, 44, 2744 RSC.
- L. Seravalli and M. Bosi, Materials, 2021, 14, 7590 CrossRef CAS PubMed.
- J. Zhang, F. Wang, V. B. Shenoy, M. Tang and J. Lou, Mater. Today, 2020, 40, 132 CrossRef CAS.
- X. Ling, Y.-H. Lee, Y. Lin, W. Fang, L. Yu, M. S. Dresselhaus and J. Kong, Nano Lett., 2014, 14, 464 CrossRef CAS PubMed.
- K. Zhang, B. M. Bersch, F. Zhang, N. C. Briggs, S. Subramanian, K. Xu, M. Chubarov, K. Wang, J. O. Lerach, J. M. Redwing, S. K. Fullerton-Shirey, M. Terrones and J. A. Robinson, ACS Appl. Mater. Interfaces, 2018, 10, 40831 CrossRef CAS PubMed.
- K. Kang, S. Xie, L. Huang, Y. Han, P. Y. Huang, K. F. Mak, C.-J. Kim, D. Muller and J. Park, Nature, 2015, 520, 656 CrossRef CAS PubMed.
- D. Dumcenco, D. Ovchinnikov, O. L. Sanchez, P. Gillet, D. T. L. Alexander, S. Lazar, A. Radenovic and A. Kis, 2D Mater., 2015, 2, 044005 CrossRef.
- J.-G. Song, G. H. Ryu, Y. Kim, W. J. Woo, K. Y. Ko, Y. Kim, C. Lee, I.-K. Oh, J. Park, Z. Lee and H. Kim, Nanotechnology, 2017, 28, 465103 CrossRef PubMed.
- P. J. Whitham, D. P. Strommen, S. Lundell, L. D. Lau and R. Rodriguez, Plasma Chem. Plasma Process., 2014, 34, 755 CrossRef CAS.
- A. Michail, J. Parthenios, D. Anestopoulos, C. Galiotis, M. Christian, L. Ortolani, V. Morandi and K. Papagelis, 2D Mater., 2018, 5, 035035 CrossRef.
- S. Li, Y.-C. Lin, X.-Y. Liu, Z. Hu, J. Wu, H. Nakajima, S. Liu, T. Okazaki, W. Chen, T. Minari, Y. Sakuma, K. Tsukagoshi, K. Suenaga, T. Taniguchi and M. Osada, Nanoscale, 2019, 11, 16122 RSC.
- H. R. Rasouli, N. Mehmood, O. Cakiroglu and T. S. Kasirga, Nanoscale, 2019, 11, 7317 RSC.
- H. Kim, G. H. Han, S. J. Yun, J. Zhao, D. H. Keum, H. Y. Jeong, T. H. Ly, Y. Jin, J.-H. Park, B. H. Moon, S.-W. Kim and Y. H. Lee, Nanotechnology, 2017, 28, 36LT01 CrossRef PubMed.
- S. Kikuchi, N. Koga, H. Seino and S. Ohno, J. Nucl. Sci. Technol., 2016, 53, 682 CrossRef CAS.
- R. Ionescu, A. George, I. Ruiz, Z. Favors, Z. Mutlu, C. Liu, K. Ahmed, R. Wu, J. S. Jeong, L. Zavala, K. A. Mkhoyan, M. Ozkan and C. S. Ozkan, Chem. Commun., 2014, 50, 11226 RSC.
- H. Zhou, F. Yu, Y. Liu, X. Zou, C. Cong, C. Qiu, T. Yu, Z. Yan, X. Shen, L. Sun, B. I. Yakobson and J. M. Tour, Nano Res., 2013, 6, 703 CrossRef CAS.
- A. Belkind and S. Gershman, Vac. Technol. Coat., 2008, 1, 1 CrossRef.
- X. Wang, C. R. Cormier, A. Khosravi, C. M. Smyth, J. R. Shallenberger, R. Addou and R. M. Wallace, Surf. Sci. Spectra, 2020, 27, 014019 CrossRef CAS.
- M. M. Benameur, B. Radisavljevic, J. S. Héron, S. Sahoo, H. Berger and A. Kis, Nanotechnology, 2011, 22, 125706 CrossRef CAS PubMed.
- H. Li, G. Lu, Z. Yin, Q. He, H. Li, Q. Zhang and H. Zhang, Small, 2012, 8, 682 CrossRef CAS PubMed.
- Q. Li, L. Zhang, C. Li, J. He, Y. Wei, J. Zhao, R. Zhang, P. Wang, S. Fu, F. Chen, R. Peng and M. Wang, J. Phys. Chem. C, 2022, 126, 3549 CrossRef CAS.
- W. Xu, S. Li, S. Zhou, J. K. Lee, S. Wang, S. G. Sarwat, X. Wang, H. Bhaskaran, M. Pasta and J. H. Warner, ACS Appl. Mater. Interfaces, 2018, 10, 4630 CrossRef CAS PubMed.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385 CrossRef CAS.
- E. Pollmann, L. Madauss, S. Schumacher, U. Kumar, F. Heuvel, C. vom Ende, S. Yilmaz, S. Güngörmüs and M. Schleberger, Nanotechnology, 2020, 31, 505604 CrossRef CAS PubMed.
- Y. Xiao, W. Zhend, B. Yuan, C. Wen and M. Lanza, Cryst. Res. Technol., 2021, 56, 2100056 CrossRef CAS.
- R. Coehoorn, C. Haas, J. Dijkstra, C. J. F. Flipse, R. A. Groot and A. Wold, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 6195 CrossRef CAS PubMed.
- R. Coehoorn, C. Haas and R. A. de Groot, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 6203 CrossRef CAS PubMed.
- K. M. McCreary, A. T. Hanbicki, S. V. Sivaram and B. T. Jonker, APL Mater., 2018, 6, 111106 CrossRef.
- C. Hou, J. Deng, J. Guan, Q. Yang, Z. Yu, Y. Lu, Z. Xu, Z. Yao and J. Zheng, Phys. Chem. Chem. Phys., 2021, 23, 24579 RSC.
- E. Ji, K. Yang, J.-C. Shin, Y. Kim, J.-W. Park, J. Kim and G.-H. Lee, Nanoscale, 2022, 14, 14106 RSC.
- Y. Yu, Y. Yu, C. Xu, Y.-Q. Cai, L. Su, Y. Zhang, Y.-W. Zhang, K. Gundogdu and L. Cao, Adv. Funct. Mater., 2016, 26, 4733 CrossRef CAS.
- C. Xie, P. Yang, Y. Huan, F. Cui and Y. Zhang, Dalton Trans., 2020, 49, 10319 RSC.
- G. H. Frischat, J. Am. Ceram. Soc., 1968, 51, 528 CrossRef CAS.
- C. Yu, P. Chang, L. Guan and J. Tao, Mater. Chem. Phys., 2020, 240, 122203 CrossRef CAS.
- P. Yan, J. Wang, G. Yang, N. Lu, G. Chu, X. Zhang and X. Shen, Superlattices Microstruct., 2018, 120, 235 CrossRef CAS.
- S. Takeda, M. Fukawa, Y. Hayashi and K. Matsumoto, Thin Solid Films, 1999, 339, 220 CrossRef CAS.
- S. Takeda and M. Fukawa, Thin Solid Films, 2003, 444, 153 CrossRef CAS.
- M. P. Seah, I. S. Gilmore and S. J. Spencer, J. Electron Spectrosc. Relat. Phenom., 2001, 120, 93 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr03907b |
| ‡ Currently at WinGD (Winterthur Gas & Diesel Ltd.), Schützenstrasse 3, CH-8400 Winterthur, Switzerland. |
| This journal is © The Royal Society of Chemistry 2023 |