High-efficiency visible-light-driven oxidation of primary C–H bonds in toluene over a CsPbBr3 perovskite supported by hierarchical TiO2 nanoflakes†
Jiayu
Yi
a,
Sunzai
Ke
a,
Suwei
Lu
a,
Bo
Weng
 b,
Lijuan
Shen
a,
Xuhui
Yang
b,
Lijuan
Shen
a,
Xuhui
Yang
 a,
Hun
Xue
a,
Min-Quan
Yang
a,
Hun
Xue
a,
Min-Quan
Yang
 *a and
Qingrong
Qian
*a and
Qingrong
Qian
 *a
*a
aCollege of Environmental and Resource Sciences, College of Carbon Neutral Modern Industry, Fujian Key Laboratory of Pollution Control & Resource Reuse, Fujian Normal University, Fuzhou 350117, P.R. China. E-mail: yangmq@fjnu.edu; cnqrqian@fjnu.edu.cn
bcMACS, Department of Microbial and Molecular Systems, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium
First published on 10th August 2023
Abstract
Photocatalytic oxidation of toluene to valuable fine chemicals is of great significance, yet faces challenges in the development of advanced catalysts with both high activity and selectivity for the activation of inert C(sp3)–H bonds. Halide perovskites with remarkable optoelectronic properties have shown to be prospective photoactive materials, but the bulky structure with a small surface area and severe recombination of photogenerated electron–hole pairs are obstacles to application. Here, we fabricate a hierarchical nanoflower-shaped CsPbBr3/TiO2 heterojunction by assembling CsPbBr3 nanoparticles on 2D TiO2 nanoflake subunits. The design significantly downsizes the size of CsPbBr3 from micrometers to nanometers, and forms a type II heterojunction with intimate interfacial contact between CsPbBr3 and TiO2 nanoflakes, thereby accelerating the separation and transfer of photogenerated charges. Moreover, the formed hierarchical heterojunction increaseslight absorption by refraction and scattering, offers a large surface area and enhances the adsorption of toluene molecules. Consequently, the optimized CsPbBr3/TiO2 exhibits a high performance (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μmol g−1 h−1) for photocatalytic toluene oxidation with high selectivity (85%) for benzaldehyde generation under visible light. The photoactivity is about 20 times higher than that of blank CsPbBr3, and is among the best photocatalytic performances reported for selective oxidation of toluene under visible light irradiation.
200 μmol g−1 h−1) for photocatalytic toluene oxidation with high selectivity (85%) for benzaldehyde generation under visible light. The photoactivity is about 20 times higher than that of blank CsPbBr3, and is among the best photocatalytic performances reported for selective oxidation of toluene under visible light irradiation.
Introduction
The direct oxidation of toluene to high-added-value fine chemicals, such as alcohols and aldehydes, is one of the most important conversions in the chemical industry.1–4 However, due to the inertness and high bond dissociation energy (89.8 kcal mol−1) of the C(sp3)–H bond,5 the activation of toluene generally needs to be performed under harsh reaction conditions (high pressure and temperature) and with the assistance of specific metal complexes or aggressive oxidants, which is expensive, energy intensive and causes negative impacts on the environment.6–8 Solar-driven photocatalytic aerobic oxidation using molecular O2 as an oxidant represents a promising methodology to overcome these challenges, but the overall photocatalytic performance reported so far is still unsatisfactory due to the lack of desired photocatalysts with broad optical absorption windows, efficient charge separation, and high product selectivity.9–11Over the past years, halide perovskite materials have triggered wide attention in photocatalysis because of their outstanding optical and electronic properties.12–16 High charge carrier mobility, visible light absorption, and tunable band structures enable them to be potential competitors for photocatalytic oxidation of toluene.17,18 Nevertheless, single-component halide perovskites typically evolve into bulky aggregates with a large particle size (several to tens of micrometers), which leads to severe bulk recombination of charge carriers and restricts the photoactivity.19,20 To tackle this problem, the construction of a heterojunction is an effective strategy. Hybridization of the halide perovskite with preferred substrates not only can prevent the aggregation of the halide perovskite to mediate the bulk recombination of photoexcited electron–hole pairs, but also can establish an interfacial built-in electric field to further facilitate the spatial charge transfer and separation.21
Moreover, the catalytic functions of photocatalysts also strongly depend on the nanostructure.22,23 To realize high photocatalytic performance, the delicate design of a well-defined nanostructure for heterojunction composites is also critical.24–27 For diverse structural configurations, 3D hierarchical flower-like nanostructures constructed using 2D nanoflake subunits have demonstrated intrinsic advantages for photocatalysis. Such hierarchical structures can strengthen the light absorption of photocatalysts by multi-light reflection and scattering within the nanoflake subunits, and prohibit the aggregation of 2D nanoflakes to afford a large surface area with abundant active sites.28,29 In addition, the nanoflake subunits will greatly shorten the diffusion path of charges, thereby facilitating the separation and transfer of photogenerated charges to the surface for redox reactions.30,31 Thus, the development of advanced 2D subunit-assembled hierarchical structures with a heterogeneous junction would be promising for high-efficiency photocatalytic toluene oxidation.
Here, inspired by the above considerations, we design and fabricate a hierarchical nanoflower-shaped CsPbBr3/TiO2 (abbreviated as CPB/TiO2) heterojunction composed of TiO2 nanoflake-supported all-inorganic CsPbBr3 perovskite nanoparticles. Benefiting from the composition and nanostructure engineering, the CPB/TiO2 heterojunction displays excellent performance for photocatalytic toluene oxidation under the irradiation of visible light. The optimized 20%CPB/TiO2 heterojunction composite delivers a toluene conversion rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μmol g−1 h−1 and a high selectivity of 85% towards benzaldehyde (benzyl alcohol as the main by-product). This performance outperforms most of the state-of the-art catalysts in the literature for visible-light-driven oxidation of toluene. This work provides a new idea by constructing a hierarchical heterojunction structure over halide perovskite materials for high-efficiency photocatalytic toluene conversion.
200 μmol g−1 h−1 and a high selectivity of 85% towards benzaldehyde (benzyl alcohol as the main by-product). This performance outperforms most of the state-of the-art catalysts in the literature for visible-light-driven oxidation of toluene. This work provides a new idea by constructing a hierarchical heterojunction structure over halide perovskite materials for high-efficiency photocatalytic toluene conversion.
Experimental section
Materials
Cesium bromide (CsBr, 99.999%), lead bromide (PbBr2, 99.0%) and tetrahydrofuran (THF) were purchased from Macklin (Shanghai, China). N,N-Dimethylformamide (DMF), toluene, tetrabutyl titanate (TBOT), ethanol (EtOH), concentrated hydrochloric acid (HCl, 36%), acetic acid and glycerol were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All chemicals and solvents were used without further purification.Synthesis of mesoporous hierarchical TiO2 nanoflowers
Typically, 1.5 g of Pluronic F127 was dispersed in a mixed solvent of 2.4 mL of acetic acid, 3.2 mL of concentrated hydrochloric acid (HCl) and 30 mL of tetrahydrofuran (THF). After vigorously stirring for 20 min, 3.4 mL of tetrabutyl titanate (TBOT) and 0.2 mL of H2O were added subsequently to obtain a clear primrose yellow solution. After that, the solution was dried in an oven at 45 °C for 24 h to form a light-yellow gel. Then, 1.0 g of the gel was added into 25 mL of ethanol (EtOH) and 5 mL of glycerin under vigorous stirring. After 20 min, the transparent solution was transferred to a 50 mL Teflon-lined autoclave, heated at 150 °C for 15 h, and then cooled naturally to room temperature. The precipitate was washed with ethanol and dried in an oven at 60 °C.32 Finally, the precipitate was calcined in a muffle furnace at 400 °C for 5 h at a heating rate of 1 °C min−1 to obtain the hierarchical TiO2 nanoflowers. To synthesize the collapsed TiO2 without the hierarchical nanoflower structure, the precipitate was calcined in the same muffle furnace at 400 °C for 5 h, but at a heating rate of 5 °C min−1.Synthesis of CsPbBr3/TiO2 composites and blank CsPbBr3
CsPbBr3/TiO2 composites were synthesized according to an antisolvent precipitation method at room temperature.33 Typically, 1 mmol CsBr and 1 mmol PbBr2 were dissolved in 10 mL of N,N-dimethylformamide (DMF) by ultrasonication to form a precursor solution. Meanwhile, 100 mg of TiO2 was dispersed in 10 mL of DMF and stirred to form a uniform white dispersion. Then a certain amount of the CsPbBr3 precursor solution was added into the TiO2 dispersion under ultrasonication. Then, the mixture was added dropwise into 50 ml of toluene and stirred vigorously for 30 min. After that, the solution was centrifuged at 8000 rpm for 3 min. The precipitate was collected and dried in a vacuum oven. A series of x% CsPbBr3/TiO2 composites were synthesized by changing the volume of the added CsPbBr3 precursor solution, where x is the mass ratio of CsPbBr3 in the composite (x = 10, 20, 30, and 40). Blank CsPbBr3 was prepared by the same procedure without the addition of TiO2.Characterization
Scanning electron microscopy (SEM) images were captured using a Hitachi 8100. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were recorded using a JEM-2100F EX microscope at an accelerating voltage of 200 kV. The Brunauer–Emmett–Teller (BET) specific surface area was measured using an N2 adsorption–desorption instrument (BELSORP-mini II). The X-ray diffraction (XRD) patterns of the catalysts were characterized on a Bruker D8 Advance X-ray diffractometer operated at 40 kV and 40 mA with Cu Kα radiation and 2θ ranging from 10° to 80°. The UV-vis diffuse reflectance spectra (DRS) were obtained on a CARY-100 spectrophotometer (Agilent) using 100% BaSO4 powder as a standard sample. X-ray photoelectron spectroscopy (XPS) spectra were recorded using an X-ray photoelectron spectrometer (Thermo Escalab 250 electron spectrometer) with Al-Kα radiation. Raman spectra were obtained using a laser at a wavelength of 785 nm (Thermo Fisher Scientific, DXR 2xi). Photoluminescence (PL) spectra of solid samples were obtained using an RF-5301PC spectrophotometer (Shimadzu, Japan) with a 435 nm excitation wavelength.Electron paramagnetic resonance (EPR) measurements were performed at room temperature using a Magnettech ESR5000X spectrometer. In general, 10 mg samples were dispersed in a mixed solution of 2 mL of CH3CN containing 1 mL of toluene and 2 μL of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO). The suspension was then transferred into a glass capillary. The sealed glass tube was then placed in the microwave cavity of an EPR spectrometer and exposed to a 300 W Xe lamp source (CEL-PF300-T8, Beijing China Education Au-light Co., Ltd) at room temperature.
Photoelectrochemical measurements
The photoelectrochemical analysis was carried out in a conventional three-electrode cell using a Pt plate and an Ag/AgCl electrode as the counter electrode and the reference electrode, respectively. The working electrode was prepared on fluorine-doped tin oxide (FTO) glass that was cleaned by ultrasonication in ethanol for 1 h. Typically, 5 mg of the catalyst was dispersed in 0.5 mL of isopropanol to obtain a slurry. After that, 20 μL of the slurry was dropped onto FTO with a size of 1 cm2 and dried in air. The electrochemical impedance spectroscopy (EIS) measurement was performed in an electrolyte of tetrabutylammonium hexafluorophosphate (TBAPF6) in ethyl acetate (EA). The transient photocurrent was tested in the same electrolyte and a 300 W xenon lamp (CEL-PF300-T8, Beijing China Education Au-light Co., Ltd) equipped with a 420 nm cutoff filter (λ ≥ 420 nm) was used as the light source. Mott–Schottky measurement was performed in an electrolyte of 0.1 M TBAPF6 in CH2Cl2.Density functional theory (DFT) calculations
DFT calculations were carried out using the Vienna ab initio simulation package (VASP) with the projector-augmented-wave (PAW) method.34 The Perdew–Burke–Ernzerhof (PBE) scheme was used for the exchange correlation function, employing the generalized gradient approximation (GGA).35 The electronic wave function was expanded using a plane wave basis with an energy cutoff of 500 eV. The energy convergence criterion was set to 1.0 × 10−5 eV, and the force convergence criterion was set to 0.03 eV Å−1 for each atom. For the CsPbBr3 (110) and TiO2 (101) slabs, a 6 × 3 × 1 and 3 × 9 × 1 k-point mesh was used, respectively. A 30 Å vacuum layer along the z-direction was used to prevent unintended periodic interactions between adjacent slabs. The CsPbBr3/TiO2 heterostructure model consists of two CsPbBr3 layers and one TiO2 layer, with a 20 Å vacuum space along the z-direction. To save computing resources, a 1 × 1 × 1 k-point mesh was employed for the CsPbBr3/TiO2 heterostructure. The van der Waals interactions between TiO2 and CsPbBr3 were taken into account using the DFT-D3 correction method.Photocatalytic activity measurements
All photocatalytic reactions were conducted in a quartz reactor. Typically, 10 mg of the photocatalyst and 1 mL of toluene (9.4 mmol) were dispersed in a reactor containing 2 mL of acetonitrile. The reactor was purged with O2 for 10 min, and then irradiated with visible light (λ > 420 nm) using a 300 W xenon lamp (CEL-PF300-T8, Beijing China Education Au-light Co., Ltd). After light irradiation for 2 h, the liquid product was analyzed by gas chromatography (GC-2030, Shimadzu, Japan, FID detector, nitrogen (N2) as the carrier gas) after centrifuging the suspension at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min.
000 rpm for 3 min.
Results and discussion
Characterization of catalysts
The 3D hierarchical CPB/TiO2 heterostructure was prepared by a simple anti-solvent precipitation method with the addition of TiO2 nanoflowers into the precursor solution of CsPbBr3, as schematically illustrated in Fig. 1a. X-ray diffraction (XRD) was exploited to characterize the crystal structure of blank CsPbBr3, CPB/TiO2 composites and TiO2. As shown in Fig. 1b, the diffraction peaks at 15.1°, 21.5°, 30.7°, 34.4°, and 43.8° can be ascribed to the monoclinic CsPbBr3 (100), (110), (200), (210) and (220) planes (JCPDS no. 18-0364).36–38 In addition, the peaks at 25.3°, 37.7°, 48.0°, 53.8°, 55.0°, and 62.6° can be attributed to the (101), (004), (200), (105), (211), and (204) planes of anatase TiO2, respectively.32 The XRD patterns of CPB/TiO2 composites indicate both characteristic peaks of CsPbBr3 and TiO2, and the peak intensity of CsPbBr3 increases as the CsPbBr3 content increases (Fig. S1†).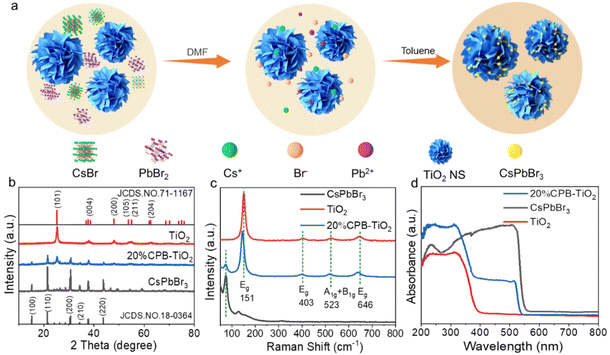 | ||
| Fig. 1 (a) Schematic diagram of the synthesis of 3D nanoflower-shaped CPB/TiO2 heterostructure. (b) XRD patterns, (c) Raman spectra, and (d) UV-vis DRS spectra of CsPbBr3, CPB/TiO2 and TiO2. | ||
Raman spectra were used to further confirm the composition of the synthesized catalysts. As shown in Fig. 1c, the peaks at 151 cm−1 (Eg), 403 cm−1 (Eg), 523 cm−1 (A1g + B1g), and 646 cm−1 (Eg) belong to the anatase TiO2 vibration modes. A new peak of 74 cm−1 assigned to the vibrational mode of the [PbBr6]4− octahedron of CsPbBr3 is observed in the CPB/TiO2 composite.39–41 The characteristic peak of CsPbBr3 is also enhanced with the increment of the loading amount of CsPbBr3 (Fig. S2†). This coincides with the XRD analysis, validating the successful preparation of the CPB/TiO2 composites. Notably, after the growth of CsPbBr3 on the TiO2 nanosheets, the Raman peaks of TiO2 shift to a lower wavenumber compared to that of blank TiO2. The shift indicates an increase in the bond length and a decrease in the bond energy of the Ti–O bond, which can be attributed to the strong electrostatic interaction between CsPbBr3 and TiO2.42–45Fig. 1d shows the UV-vis diffuse reflectance spectroscopy (DRS) analysis of the samples, which is employed to assess the optical absorption properties. It can be seen that the light absorption edge of blank TiO2 is around 390 nm, denoting its UV light response nature. After integrating with CsPbBr3, the light absorption of CPB/TiO2 is extended to approximately 540 nm. Meanwhile, the absorption edge of TiO2 at 390 nm can also be detected. This should be ascribed to the hybridization of the high weight content of CsPbBr3 with the TiO2 support. As such, both absorption features are present in the composite. The greatly extended light absorption enables the visible light excitation of the CPB/TiO2 composites, which is beneficial for promoting solar energy utilization. With the increase of the CsPbBr3 content, the visible light absorption of CPB/TiO2 is also gradually enhanced (Fig. S3†). Moreover, in comparison with blank CsPbBr3, it is notable that the visible light absorption edge of the hybrid CPB/TiO2 composite is slightly blue shifted (ca. 14 nm). This can be attributed to the quantum size effect resulting from the smaller size of CsPbBr3 in the composite. The inference has been demonstrated by the following scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analyses.
As shown in Fig. 2, Fig. S4 and S5,† SEM is firstly utilized to characterize the morphology and microstructure of the CPB/TiO2, blank TiO2 and CsPbBr3 samples. The blank TiO2 presents a nanoflower shape, which is assembled by vertical nanoflakes (Fig. S4†). The surface of the nanoflake subunits is clean and smooth. For blank CsPbBr3, it shows a large particle morphology and the particle sizes range from 0.9 to 2.0 μm (Fig. S5†). After the construction of the CPB/TiO2 heterojunction, the TiO2 nanoflower-shaped structure is maintained (Fig. 2), while the surface of the nanoflake subunits becomes rough due to the in situ growth of CsPbBr3. From Fig. 2b–i, it can be clearly seen that for all the CPB/TiO2 samples, the sizes of the CsPbBr3 particles in the composites are much smaller than that of blank CsPbBr3, indicating that the formation of a heterojunction is beneficial for preventing the aggregation and reducing the size of the CsPbBr3 halide perovskite. This can be ascribed to the fact that the presence of TiO2 nanoflowers with nanoflake subunits can act as heterogeneous nucleus sites to facilitate the nucleation and growth of CsPbBr3, which generates more seeds and leads to the smaller size of the CsPbBr3 particles.20 The significantly decreased particle size could greatly promote the exposure of surface active sites and inhibit the charge recombination of CsPbBr3 under light irradiation. Moreover, it is notable that with the increase of the CsPbBr3 contents from 10% to 40% in the composites, the particle sizes of CsPbBr3 are increased, as schematically illustrated in Fig. 2a. This can be attributed to the further growth of CsPbBr3 at high concentrations.
 | ||
| Fig. 2 (a) Schematic and (b–i) SEM images showing the morphology evolution of x% CPB/TiO2 (where x = 10, 20, 30, and 40) composites. | ||
To further obtain the microscopic structural information of the samples, transmission electron microscopy (TEM) analysis is carried out. Taking 20%CPB/TiO2 as an example, the TEM image of the sample (Fig. 3a and b) shows a nanoflake structure of TiO2. The surface is rough and densely covered by small CsPbBr3 nanoparticles. Since the sample for TEM analysis is prepared by strong ultrasonication, the well-defined CsPbBr3 nanoparticles on the 2D nanoflake surface indicate intimate interfacial contact between the two components. Meanwhile, the intensive coverage of CsPbBr3 forms a large contact interface with TiO2 nanoflakes, which is conducive to the migration of charge carriers across the CPB/TiO2 heterojunction.46 Moreover, nanopores can be observed on the CPB/TiO2, which results from the mesoporous structure of the TiO2 nanoflakes, as demonstrated by the N2 adsorption–desorption analysis (Fig. S6†). The high-resolution TEM (HRTEM) image (Fig. 3c) shows distinct lattice fringes of 0.35 nm and 0.41 nm, corresponding to the (101) planes and (110) planes of anatase TiO2 and monoclinic CsPbBr3, respectively. Furthermore, Fig. 3d shows the corresponding energy dispersive X-ray spectroscopy (EDS) elemental mapping analysis of 20%CPB/TiO2. Cs, Pb, Br, Ti and O elements are uniformly distributed on the nanoflake structure, indicating that CsPbBr3 and TiO2 are well combined. The results further verify the formation of CPB/TiO2 hybrid composite with intimate interfacial contact. Moreover, in comparison with blank TiO2, the 20%CPB/TiO2 composite displays a smaller specific surface area (Table S1†), which can be attributed to the fact that the growth of CsPbBr3 nanoparticles on the surface of TiO2 may aggregate in the TiO2 pores. Correspondingly, the total pore volume of 20%CPB/TiO2 is also slightly lower than that of blank TiO2. The result matches with the SEM measurement.
 | ||
| Fig. 3 (a and b) TEM images, (c) HRTEM image, and (d) corresponding EDS elemental mapping images of 20%CPB/TiO2. | ||
To study the surface chemical states of the samples, X-ray photoelectron spectroscopy (XPS) has been performed. As shown in Fig. 4a, the presence of Cs, Pb, Br, O and Ti elements can be detected from the XPS survey spectrum of CPB/TiO2. The high-resolution spectrum of Ti 2p shows two peaks of Ti 2p3/2 and Ti 2p1/2 at 458.5 eV and 464.2 eV (Fig. 4b), which indicates that Ti element mainly exists as Ti4+ ions. For the O 1s pattern (Fig. 4c), it can be divided into two peaks, which are attributed to the lattice O (Ti–O) and hydroxyl O (–OH). Fig. S7† shows the fine-scanned Cs 3d XPS spectrum, and the two peaks with binding energies at 724.2 eV and 738.1 eV are assigned to Cs 3d5/2 and Cs 3d3/2, indicating that the chemical state of the Cs in the composite is +1. The Pb 4f spectrum in Fig. 4d shows characteristic peaks at 138.0 eV and 142.9 eV, corresponding to Pb 4f7/2 and Pb 4f5/2 orbitals of Pb2+ ion. Moreover, the Br 3d is decomposed into two peaks at 67.9 eV and 68.9 eV, corresponding to the binding energies of Br 3d5/2 and 3d3/2, attributed to the presence of Br− (Fig. 4e). It is worth noting that for the CPB/TiO2 composite, the Ti 2p and O 1s peaks show a negative shift of 0.2 eV in comparison with blank TiO2 (Fig. 4b and c). In contrast, the binding energies of Cs 4d, Pb 4f, and Br 3d in the CPB/TiO2 show a positive shift (Fig. 4d and e and S7†), which are higher than those of blank CsPbBr3 by 0.4 eV. The results verify the strongly interacted interface with electron transfer from the CsPbBr3 to the TiO2 nanosheets in the heterostructure, which is in accordance with the Raman analysis. To further clarify the interaction and electron transfer at the interface, density functional theory (DFT) calculations have been carried out. Fig. 4g and h shows the work functions of the TiO2 (101) and CsPbBr3 (110) slabs, which are 7.09 eV and 4.63 eV, respectively, corresponding to the Fermi levels (Ef) of −3.61 eV and −2.51 eV (vs. the vacuum level). Due to the lower Ef value of TiO2 than that of blank CsPbBr3, the electrons would be migrated from CsPbBr3 to TiO2 at the heterojunction interface until their Ef are aligned. A similar result is obtained from the calculated planar-averaged charge density difference of CPB/TiO2. As shown in Fig. 4f, the light cyan and yellow regions indicate the charge depletion and accumulation area, respectively. The calculation results reveal that the cyan region is mainly on the CsPbBr3 side, while the yellow region is on the TiO2 side, further validating the transfer of electrons from CsPbBr3 to TiO2. This is consistent with the XPS analysis results.
Photocatalytic performances
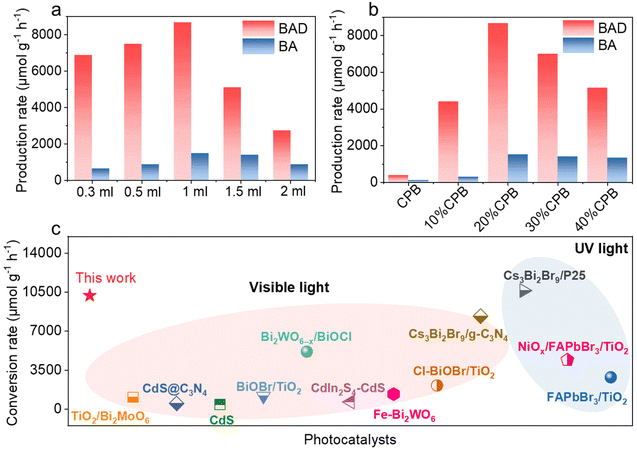
Collectively, the above characterization faithfully verifies the successful fabrication of CPB/TiO2 hybrid composites with intimate interfacial contact and strong chemical interaction. The presence of TiO2 nanoflower with 2D nanoflake subunits greatly inhibits the agglomeration of the halide perovskites, while CsPbBr3 extends the light absorption range of TiO2. In view of the desired structural and optical properties, the photocatalytic oxidation of toluene over the hybrid CPB/TiO2 composites has been tested. A 300 W xenon lamp equipped with a 420 nm cutoff filter is utilized as a visible light source. It is well known that the solvent/reactant ratio has great influence on the oxidation of toluene,2,47 and thus, the reaction condition of different acetonitrile/toluene ratio is firstly optimized using 20%CPB/TiO2 as an example. As shown in Fig. 5a, as the ratio of toluene increases from 0.3 to 1 mL, the photoactivity of the 20%CPB/TiO2 composite gradually increases. The catalyst shows the highest toluene conversion rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μmol g−1 h−1 when the volume ratio of acetonitrile solvent and toluene reactant is 2
200 μmol g−1 h−1 when the volume ratio of acetonitrile solvent and toluene reactant is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The enhanced photoactivity can be attributed to the fact that the increased toluene concentration enhances the reaction possibility between the photogenerated charge carriers and the toluene molecules. However, further increase of toluene to 1.5 mL and 2 mL decreases the conversion rate. This might be ascribed to the excess adsorption of the toluene reactant on the catalyst surface, which would slow down the desorption of the BA and BAD products and suppress the oxidation activity.48 Based on the result, the following photoactivity tests of all the other samples are carried out under the optimized condition with a acetonitrile/toluene ratio of 2
1. The enhanced photoactivity can be attributed to the fact that the increased toluene concentration enhances the reaction possibility between the photogenerated charge carriers and the toluene molecules. However, further increase of toluene to 1.5 mL and 2 mL decreases the conversion rate. This might be ascribed to the excess adsorption of the toluene reactant on the catalyst surface, which would slow down the desorption of the BA and BAD products and suppress the oxidation activity.48 Based on the result, the following photoactivity tests of all the other samples are carried out under the optimized condition with a acetonitrile/toluene ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
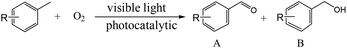
Fig. 5b shows the visible-light-driven photocatalytic performances of the blank CsPbBr3 and CPB/TiO2 composites with different ratios of CPB for the selective oxidation of toluene. The blank CsPbBr3 exhibits a low activity with generation rates of 390 μmol g−1 h−1 for BAD and 130 μmol g−1 h−1 for BA. The unsatisfactory photocatalytic performance should be due to the severe charge recombination caused by the large particle size of CsPbBr3. For blank TiO2, it presents no activity. This is reasonable since the TiO2 has no visible light absorption and cannot be excited by visible light. In contrast, after the hybridization of CsPbBr3 with TiO2, the photocatalytic performances of all the hierarchical CPB/TiO2 heterojunctions are significantly improved. The best photocatalytic toluene oxidation activity is achieved over the 20%CPB/TiO2 composite, which shows a toluene conversion rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μmol g−1 h−1 (with a BAD production rate of 8670 μmol g−1 h−1 and a BA generation rate of 1530 μmol g−1 h−1). The photoactivity is about 20 times higher than that of blank CsPbBr3, which is also among the top photocatalytic performances reported so far for UV and visible-light-driven selective oxidation of toluene (Fig. 5c and Table S2†). The apparent quantum efficiency (AQE) of the 20%CsPbBr3/TiO2 composite reaches 2.9% at λ = 420 nm for the transformation of toluene into BA and BAD (Fig. S14†). The gas chromatography-mass spectrometry (GC-MS) analysis of the reaction solution after light irradiation reveals four peaks, which are ascribed to the acetonitrile solvent, toluene reactant, BAD and BA products (Fig. S8†). No other product or intermediate is detected, validating the high selectivity of the reaction. With the increase of CsPbBr3 loading to higher contests (30% and 40%), it results in a performance degradation. This may be ascribed to the fact that: (1) an excess of halide perovskite tends to aggregate into large particles, which will lead to longer migration distances of charge carriers and increase charge recombination; (2) the coverage of excessive and oversized CsPbBr3 on the TiO2 surface will lead to the reduction of the exposed surface area, inhibiting the adsorption of toluene molecules.
200 μmol g−1 h−1 (with a BAD production rate of 8670 μmol g−1 h−1 and a BA generation rate of 1530 μmol g−1 h−1). The photoactivity is about 20 times higher than that of blank CsPbBr3, which is also among the top photocatalytic performances reported so far for UV and visible-light-driven selective oxidation of toluene (Fig. 5c and Table S2†). The apparent quantum efficiency (AQE) of the 20%CsPbBr3/TiO2 composite reaches 2.9% at λ = 420 nm for the transformation of toluene into BA and BAD (Fig. S14†). The gas chromatography-mass spectrometry (GC-MS) analysis of the reaction solution after light irradiation reveals four peaks, which are ascribed to the acetonitrile solvent, toluene reactant, BAD and BA products (Fig. S8†). No other product or intermediate is detected, validating the high selectivity of the reaction. With the increase of CsPbBr3 loading to higher contests (30% and 40%), it results in a performance degradation. This may be ascribed to the fact that: (1) an excess of halide perovskite tends to aggregate into large particles, which will lead to longer migration distances of charge carriers and increase charge recombination; (2) the coverage of excessive and oversized CsPbBr3 on the TiO2 surface will lead to the reduction of the exposed surface area, inhibiting the adsorption of toluene molecules.
Furthermore, the broad applicability of the CPB/TiO2 photocatalyst is investigated for the photocatalytic selective oxidation of C(sp3)–H of toluene derivatives with different substituents. As shown in Table 1, these aromatics are efficiently converted into the corresponding aldehydes and alcohols. The selectivity for the aldehyde product is higher than 70%. Notably, the substrate with electron-withdrawing substituents (–F, –Cl, and –Br) displays lower photocatalytic activity. This can be attributed to the fact that the electron-withdrawing groups on the benzene ring inhibit the abstraction of hydrogen from the methyl group.49,50 In addition, p-xylene shows a higher toluene conversion rate (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 μmol g−1 h−1) than its isomers of o-xylene (9900 μmol g−1 h−1) and m-xylene (10
746 μmol g−1 h−1) than its isomers of o-xylene (9900 μmol g−1 h−1) and m-xylene (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 μmol g−1 h−1), which can be attributed to the steric hindrance of the methyl groups hindering the reactant adsorption and activation on the catalyst surface.51,52
930 μmol g−1 h−1), which can be attributed to the steric hindrance of the methyl groups hindering the reactant adsorption and activation on the catalyst surface.51,52
| Entry | Substrates | Conversion rate (μmol g−1 h−1) | Selectivity (%) | |
|---|---|---|---|---|
| A | B | |||
| a Reaction conditions: catalyst (10 mg 20%CPB/TiO2), substrate (1 mL), MeCN (2 mL), 1 atm of O2, 2 h irradiation with visible light (λ > 420 nm). | ||||
| 1 |

|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
85 | 15 |
| 2 |

|
5950 | 83 | 17 |
| 3 |

|
9040 | 88 | 12 |
| 4 |

|
9770 | 87 | 13 |
| 5 |

|
9900 | 76 | 24 |
| 6 |

|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
72 | 28 |
| 7 |

|
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 746 |
71 | 29 |
Moreover, the cycle test of the 20%CsPbBr3/TiO2 has been carried out. After repeating the photoactivity test for 4 cycles (Fig. S16†), the catalyst still maintains a high activity with negligible decrease in the conversion rate of toluene. In addition, the used 20%CsPbBr3/TiO2 has been further characterized by XRD and XPS (Fig. S17 and S18†). No obvious change in the crystal structure and surface chemical state is observed for the composite before and after the reaction. These results indicate the high stability of the CsPbBr3/TiO2 composite.
Photoelectric properties
In order to better understand the reasons for the improved photocatalytic performance, the band structures of the CsPbBr3 and the TiO2 are investigated using Mott–Schottky (M–S) measurements and the DRS analysis. As shown in Fig. 6a and b, the positive slopes indicate that CsPbBr3 and TiO2 are typical n-type semiconductors.53 The flat band potentials (EFB) are calculated to be −0.86 V and −0.34 V (vs. NHE) for CsPbBr3 and TiO2, respectively. Usually, the conduction band potential (ECB) for an n-type semiconductor is more negative of about 0.1 eV than that of EFB.54 Thus, the ECB values of CsPbBr3 and TiO2 are determined to be −0.96 V and −0.44 V (vs. NHE), respectively. In addition, the corresponding bandgaps of TiO2 and CsPbBr3 are estimated by Tauc plots transformed from the DRS spectra (Fig. S9†), which are 3.20 eV and 2.28 eV, respectively. Based on these, the valence band positions (EVB) of CsPbBr3 and TiO2 are calculated to be 1.32 V and 2.76 V. As a result, a type-II heterojunction is formed between CsPbBr3 and TiO2 because of the staggered band structures. | ||
| Fig. 6 (a and b) M–S plots of CsPbBr3 and TiO2. (c) Transient photocurrent responses, and (d) EIS Nyquist plots of blank TiO2, CsPbBr3 and the CPB/TiO2 composite. | ||
To study the influence of the heterojunction structure on the transfer behavior of photogenerated charge carriers, transient photocurrent and electrochemical impedance spectra (EIS) tests are performed. As shown in Fig. 6c, under visible light irradiation, both CsPbBr3 and CPB/TiO2 show rapid and stable photocurrent responses, while TiO2 does not. This can be attributed to the inability of TiO2 to be excited by visible light (Fig. 1d). The photocurrent intensity of blank CsPbBr3 is markedly lower than that of 20% CPB/TiO2. This should be due to the agglomerated large particle size of the blank CsPbBr3, which leads to a severe recombination of photogenerated charge carriers.54 Moreover, Fig. 6d shows the Nyquist plots of the samples, which is employed to investigate the interfacial charge transfer efficiency.55 In general, the smaller diameter of the semicircular arc in the Nyquist plots reflects the lower electron transfer resistance. It can be found that the 20%CPB/TiO2 sample has the minimum arc radius, which means the best electronic conductivity and charge separation ability.56,57 Furthermore, the steady-state photoluminescence (PL) spectra are also used to examine the charge separation efficiency. As shown in Fig. S19,† the 20%CsPbBr3/TiO2 composite shows a much lower PL intensity than that of CsPbBr3, revealing that the charge recombination is significantly suppressed in the composite. In addition, the TiO2 shows no obvious PL signal. This is in accordance with the photocurrent analysis that the TiO2 is unable to be excited to generate charge carriers by visible light. These photoelectrochemical findings highlight that the construction of the type-II heterojunction between CsPbBr3 and TiO2 could effectively accelerate charge transfer, thus contributing to improving the photocatalytic performance.
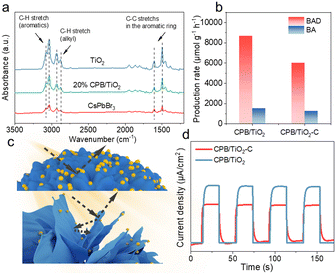
Furthermore, for heterogeneous photocatalytic reactions, the effective adsorption of the reactant on the catalyst surface is another requisite for the ignition of the redox reaction.23,58–60 The BET surface area and porous structure analyses of the TiO2, CsPbBr3 and 20%CPB/TiO2 samples have shown that the introduction of TiO2 nanoflower significantly improves the specific surface area and pore volume of 20%CPB/TiO2 in comparison with that of blank TiO2 (Fig. S6 and Table S1†). Generally, a larger specific surface area and a porous structure enable better adsorption capacity, and exposure of more active sites of catalysts. To further reveal the contribution of the heterostructure for the reactant adsorption, Fourier transform infrared (FTIR) spectroscopy is used to identify the adsorbed toluene species on the CsPbBr3, TiO2 and 20%CPB/TiO2 surface. As shown in Fig. 7a, for all three samples after adsorption equilibrium, the typical absorption peaks of toluene are observed, including the C–H stretching vibration of the aromatic ring at 3083 and 3038 cm−1, the skeleton vibration of the aromatic ring at 1606 and 1495 cm−1, and the C–H symmetric and asymmetric stretching of the methylene group at 2934 and 2875 cm−1.61,62 Notably, the signal of toluene molecules of 20%CPB/TiO2 is obviously higher than that of blank CsPbBr3, yet is lower than that of blank TiO2. The result indicates that the introduction of TiO2 can promote the toluene adsorption capacity of CPB/TiO2, which is conducive to the catalytic conversion.
Finally, to investigate the influence of the hierarchical flower-like nanostructure constructed using 2D nanoflake subunits on the photocatalytic performance, a collapsed 20%CPB/TiO2 catalyst (marked as 20%CPB/TiO2–C) without such structure has been fabricated for comparison. As shown in Fig. 7b, the photoactivity test reveals that the 20%CPB/TiO2–C has a BAD generation rate of 6000 μmol g−1 h−1 and a BA production rate of 1250 μmol g−1 h−1, which is notably lower than the CPB/TiO2 catalyst. Fig. S10† shows the morphology analysis of the 20%CPB/TiO2–C catalyst, which reveals a bulky grain structure with a rough surface. CsPbBr3 nanoparticles are dispersed on the surface of the massive TiO2. The size of the CsPbBr3 particles in the composite is also much smaller than that of blank CsPbBr3, which once again proves that TiO2 plays a critical role in inhibiting the growth of CsPbBr3. Nevertheless, compared with 20%CPB/TiO2, the size of the CsPbBr3 particles is much larger in the 20%CPB/TiO2–C catalyst. The result indicates that the TiO2 nanoflakes are more beneficial for preventing the agglomeration of the CsPbBr3 particles.
In addition, UV-vis DRS is performed to study the optical absorption property of the 20%CPB/TiO2–C catalyst. The sample shows a similar absorption edge as that of 20%CPB/TiO2, but the light absorption intensity in the range of 200–550 nm is lower (Fig. S11†). The result indicates that the 3D hierarchical CPB/TiO2 structure is more efficient for solar energy collection, which can be attributed to the multiple light reflection and refraction caused by the vertical nanoflakes, as schematically illustrated in Fig. 7c. Meanwhile, the transient photocurrent response test shows that the 20%CPB/TiO2 catalyst exhibits a higher photocurrent density than that of CPB/TiO2–C under visible light irradiation, indicating more efficient charge transfer (Fig. 7d). This can be related to the fact that TiO2 nanoflakes facilitate the formation of smaller CsPbBr3 particle, which could shorten the charge transfer distance to the surface and promote the interfacial charge separation. Hence, to sum up, the construction of the unique hierarchical CPB/TiO2 structure greatly enhances the photocatalytic performance due to the existence of TiO2 nanoflakes, which (1) greatly prohibits the aggregation of CsPbBr3, forming much smaller perovskite particles that shorten the charge transfer distance; (2) increases light absorption by multiple reflection and scattering between the nanoflakes structure that benefits the solar energy harvesting and conversion; (3) enhances the adsorption of toluene molecules and promotes the surface reaction rates.
Photocatalytic mechanism
To elucidate the photocatalytic mechanism of toluene oxidation over the CPB/TiO2 photocatalyst, a series of control experiments have been conducted in the absence of O2 or with the addition of specific radical scavengers. As shown in Fig. 8a, toluene oxidation is almost completely suppressed when O2 is replaced by Ar, indicating that O2 is indispensable. When isopropanol (IPA) is added into the reaction system as a scavenger of hydroxyl radicals (˙OH), it has almost no effect on toluene conversion, suggesting that ˙OH is rarely involved in the reaction. In contrast, whether adding ammonium oxalate (AO) to eliminate holes (h+) or butylated hydroxytoluene (BHT) to capture carbon-centered radicals, the reaction rate significantly reduces. Besides, the toluene conversion is also significantly decreased when BQ is added as a superoxide free radical (˙O2−) scavenger. The above results clearly indicate that ˙O2−, h+, and carbon-centered radicals play key roles in the photocatalytic selective oxidation of toluene.Subsequently, to further identify the existence of ˙O2−, electron paramagnetic resonance (EPR) spectroscopy was employed using 5,5-dimethyl-pyrroline-N-oxide (DMPO) as a trapping agent. As shown in Fig. S12† and Fig. 8b, no DMPO-˙O2− signal is detected under dark conditions. After visible light irradiation, distinct signals of DMPO-˙O2− are observed for both CsPbBr3 and CPB/TiO2, while no signals are detected from TiO2. The CPB/TiO2 catalyst exhibits a higher ability to generate more ˙O2− radicals than that of pure CsPbBr3, which is important to promote the oxidation of toluene. Moreover, no DMPO-˙OH signal is observed under the same conditions, demonstrating that ˙OH is absent in the reaction process (Fig. S13 and S15†).
Based on the above analyses, a possible photocatalytic reaction mechanism over the hierarchical CPB/TiO2 heterojunction is proposed. As schematically illustrated in Fig. 8c, upon photoexcitation, electrons and holes are generated in the conduction band (CB) and the valence band (VB) of CsPbBr3. Due to the staggered energy bands, the photogenerated electrons in the CB of CsPbBr3 can efficiently migrate towards the CB of TiO2 and left holes on the VB of CsPbBr3, which prolongs the lifetime of the photogenerated charge carriers. After that, molecular O2 can be activated by electrons on the CB of TiO2. Meanwhile, photogenerated holes oxidize the toluene to produce benzyl radicals (˙CH2–R) on the VB of CsPbBr3. Then, the ˙O2− radical reacts with the benzyl radicals to produce benzaldehyde.
Conclusions
In summary, we have designed and synthesized a hierarchical CPB/TiO2 heterojunction as an advanced photocatalyst for efficient visible-light-driven toluene oxidation by assembling CsPbBr3 nanoparticles onto nanoflower-shaped TiO2 with 2D nanoflake subunits. The formed CPB/TiO2 composite integrates the structural and electronical merits of the nanoflower-shaped TiO2 and halide perovskite CsPbBr3, which not only enhances solar light utilization, but also enhances the adsorption of the toluene reactant, and boosts the separation and transportation of photogenerated carriers. A high toluene oxidation rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 μmol g−1 h−1 is achieved over the optimal CPB/TiO2 composite, which is about 20-fold higher than that of blank CsPbBr3. The study demonstrates the significance of rational design of well-defined hierarchical heterojunction catalysts over metal halide perovskites for efficient photocatalytic redox reactions.
200 μmol g−1 h−1 is achieved over the optimal CPB/TiO2 composite, which is about 20-fold higher than that of blank CsPbBr3. The study demonstrates the significance of rational design of well-defined hierarchical heterojunction catalysts over metal halide perovskites for efficient photocatalytic redox reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2019YFC1908203), the National Natural Science Foundation of China (22178057 and 21905049), the Natural Science Foundation of Fujian Province (2020J01201 and 2021J01197), the Research Foundation of the Academy of Carbon Neutrality of Fujian Normal University (TZH2022-07), and the Award Program for Minjiang Scholar Professorship.References
- W. Wu, G. Zhang, J. Zhang, G. Wang, C.-H. Tung and Y. Wang, Chem. Eng. J., 2021, 404, 126433 CrossRef CAS.
- X. Li, T. Wang, X. Tao, G. Qiu, C. Li and B. Li, J. Mater. Chem. A, 2020, 8, 17657–17669 RSC.
- S. Okunaka, H. Tokudome and Y. Hitomi, J. Catal., 2020, 391, 480–484 CrossRef CAS.
- S. Zhang, W. Huang, X. Fu, X. Zheng, S. Meng, X. Ye and S. Chen, Appl. Catal., B, 2018, 233, 1–10 CrossRef CAS.
- J. Li, B. Ren, X. Yan, P. Li, S. Gao and R. Cao, J. Catal., 2021, 395, 227–235 CrossRef CAS.
- C. Xu, F. Yang, B. Deng, Y. Zhuang, D. Li, B. Liu, W. Yang and Y. Li, J. Catal., 2020, 383, 1–12 CrossRef CAS.
- J. Li, S. Zhang, S. Gui, G. Chen, Y. Wang, Z. Wang, X. Zheng, S. Meng, C. Ruan and S. Chen, Appl. Surf. Sci., 2023, 611, 155616 CrossRef CAS.
- Q. Zhang, J. Wang, X. Ye, Z. Hui, L. Ye, X. Wang and S. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 46735–46745 CrossRef CAS PubMed.
- Y. Liu, L. Chen, Q. Yuan, J. He, C. T. Au and S. F. Yin, Chem. Commun., 2016, 52, 1274–1277 RSC.
- M. Zhou, S. Li, S. Wang, Z. Jiang, C. Yang, F. Guo, X. Wang and W.-K. Ho, Appl. Surf. Sci., 2022, 599, 153985 CrossRef CAS.
- M. Zhang, W. Sun, H. Lv and Z.-H. Zhang, Curr. Opin. Green Sustainable Chem., 2021, 27, 100390 CrossRef CAS.
- Z. Zhang, Y. Liang, H. Huang, X. Liu, Q. Li, L. Chen and D. Xu, Angew. Chem., Int. Ed., 2019, 58, 7263–7267 CrossRef CAS PubMed.
- M. Zhang, Z. Li, X. Xin, J. Zhang, Y. Feng and H. Lv, ACS Catal., 2020, 10, 14793–14800 CrossRef CAS.
- T. Chen, B. Weng, S. Lu, H. Zhu, Z. Chen, L. Shen, M. B. J. Roeffaers and M. Q. Yang, J. Phys. Chem. Lett., 2022, 13, 6559–6565 CrossRef CAS PubMed.
- R. Cheng, J. A. Steele, M. B. J. Roeffaers, J. Hofkens and E. Debroye, ACS Appl. Energy Mater., 2021, 4, 3460–3468 CrossRef CAS.
- H. Huang, J. Zhao, Y. Du, C. Zhou, M. Zhang, Z. Wang, Y. Weng, J. Long, J. Hofkens, J. A. Steele and M. B. J. Roeffaers, ACS Nano, 2020, 14, 16689–16697 CrossRef CAS PubMed.
- H. Huang, H. Yuan, J. Zhao, G. Solís-Fernández, C. Zhou, J. W. Seo, J. Hendrix, E. Debroye, J. A. Steele, J. Hofkens, J. Long and M. B. J. Roeffaers, ACS Energy Lett., 2018, 4, 203–208 CrossRef.
- H. Huang, H. Yuan, K. P. F. Janssen, G. Solís-Fernández, Y. Wang, C. Y. X. Tan, D. Jonckheere, E. Debroye, J. Long, J. Hendrix, J. Hofkens, J. A. Steele and M. B. J. Roeffaers, ACS Energy Lett., 2018, 3, 755–759 CrossRef CAS.
- C. Chen, J. Zhou, J. Geng, R. Bao, Z. Wang, J. Xia and H. Li, Appl. Surf. Sci., 2020, 503, 144287 CrossRef CAS.
- Y. Dai, C. Poidevin, C. Ochoa-Hernandez, A. A. Auer and H. Tuysuz, Angew. Chem., Int. Ed., 2020, 59, 5788–5796 CrossRef CAS PubMed.
- F. Xu, K. Meng, B. Cheng, S. Wang, J. Xu and J. Yu, Nat. Commun., 2020, 11, 4613 CrossRef CAS PubMed.
- M.-Q. Yang, J. Dan, S. J. Pennycook, X. Lu, H. Zhu, Q.-H. Xu, H. J. Fan and G. W. Ho, Mater. Horiz., 2017, 4, 885–894 RSC.
- X. Lin, Z. Xie, B. Su, M. Zheng, W. Dai, Y. Hou, Z. Ding, W. Lin, Y. Fang and S. Wang, Nanoscale, 2021, 13, 18070–18076 RSC.
- S. Wang, B. Y. Guan and X. W. Lou, Energy Environ. Sci., 2018, 11, 306–310 RSC.
- L. Zhu, M. Hong and G. W. Ho, Nano Energy, 2015, 11, 28–37 CrossRef CAS.
- L. He, W. Zhang, K. Zhao, S. Liu and Y. Zhao, J. Mater. Chem. A, 2022, 10, 4758–4769 RSC.
- Q. Shen, S. Zhou, F.-L. Yang, X. Wang and X. Han, J. Mater. Chem. A, 2022, 10, 4974–4980 RSC.
- S. Wang, B. Y. Guan, X. Wang and X. W. D. Lou, J. Am. Chem. Soc., 2018, 140, 15145–15148 CrossRef CAS PubMed.
- S. Wang, B. Y. Guan and X. W. D. Lou, J. Am. Chem. Soc., 2018, 140, 5037–5040 CrossRef CAS PubMed.
- T. Zhu, L. Zhu, J. Wang and G. W. Ho, J. Mater. Chem. A, 2016, 4, 13916–13922 RSC.
- Z. Chen, F. Guo, H. Sun, Y. Shi and W. Shi, J. Colloid Interface Sci., 2022, 607, 1391–1401 CrossRef CAS PubMed.
- K. Lan, Y. Liu, W. Zhang, Y. Liu, A. Elzatahry, R. Wang, Y. Xia, D. Al-Dhayan, N. Zheng and D. Zhao, J. Am. Chem. Soc., 2018, 140, 4135–4143 CrossRef CAS PubMed.
- Q. Sun, W. Ye, J. Wei, L. Li, J. Wang, J.-H. He and J.-M. Lu, J. Alloys Compd., 2022, 893, 162326 CrossRef CAS.
- J. Hafner, J. Comput. Chem., 2008, 29, 2044–2078 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 13115–13118 CrossRef CAS PubMed.
- L. Rao, Y. Tang, C. Yan, J. Li, G. Zhong, K. Tang, B. Yu, Z. Li and J. Z. Zhang, J. Mater. Chem. C, 2018, 6, 5375–5383 RSC.
- J. Liu, L. Zhu, S. Xiang, Y. Wei, M. Xie, H. Liu, W. Li and H. Chen, Sustainable Energy Fuels, 2019, 3, 184–194 RSC.
- G. Gao, Q. Xi, H. Zhou, Y. Zhao, C. Wu, L. Wang, P. Guo and J. Xu, Nanoscale, 2017, 9, 12032–12038 RSC.
- L. Zhang, Q. Zeng and K. Wang, J. Phys. Chem. Lett., 2017, 8, 3752–3758 CrossRef CAS PubMed.
- X. Mo, X. Li, G. Dai, P. He, J. Sun, H. Huang and J. Yang, Nanoscale, 2019, 11, 21386–21393 RSC.
- D. M. Calistru, L. Mihut, S. Lefrant and I. Baltog, J. Appl. Phys., 1997, 82, 5391–5395 CrossRef CAS.
- H. J. Kong, D. H. Won, J. Kim and S. I. Woo, Chem. Mater., 2016, 28, 1318–1324 CrossRef CAS.
- I.-S. Cho, D. W. Kim, S. Lee, C. H. Kwak, S.-T. Bae, J. H. Noh, S. H. Yoon, H. S. Jung, D.-W. Kim and K. S. Hong, Adv. Funct. Mater., 2008, 18, 2154–2162 CrossRef CAS.
- S. K. Lakhera, H. Y. Hafeez, R. Venkataramana, P. Veluswamy, H. Choi and B. Neppolian, Appl. Surf. Sci., 2019, 487, 1289–1300 CrossRef CAS.
- J. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163–2169 CrossRef CAS.
- C. Q. Li, X. Du, S. Jiang, Y. Liu, Z. L. Niu, Z. Y. Liu, S. S. Yi and X. Z. Yue, Adv. Sci., 2022, 9, e2201773 CrossRef PubMed.
- J. Kou, C. Lu, J. Wang, Y. Chen, Z. Xu and R. S. Varma, Chem. Rev., 2017, 117, 1445–1514 CrossRef CAS PubMed.
- J. Wu, Y. Wang, S. Zhang, Y. Liu and F. Wang, Appl. Catal., B, 2023, 332, 122741 CrossRef CAS.
- H. Han, X. Zheng, C. Qiao, Z. Xia, Q. Yang, L. Di, Y. Xing, G. Xie, C. Zhou, W. Wang and S. Chen, ACS Catal., 2022, 12, 10668–10679 CrossRef CAS.
- R. Yuan, S. Fan, H. Zhou, Z. Ding, S. Lin, Z. Li, Z. Zhang, C. Xu, L. Wu, X. Wang and X. Fu, Angew. Chem., Int. Ed., 2013, 52, 1035–1039 CrossRef CAS PubMed.
- W. Sheng, X. Wang, Y. Wang, S. Chen and X. Lang, ACS Catal., 2022, 12, 11078–11088 CrossRef CAS.
- X. Li, S. Lyu and X. Lang, Environ. Res., 2021, 195, 110851 CrossRef CAS PubMed.
- Z. Dong, Z. Zhang, Y. Jiang, Y. Chu and J. Xu, Chem. Eng. J., 2022, 433, 133762 CrossRef CAS.
- X. Li, S. Lu, J. Yi, L. Shen, Z. Chen, H. Xue, Q. Qian and M. Q. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 25297–25307 CrossRef CAS PubMed.
- W. Liao, W. Chen, S. Lu, S. Zhu, Y. Xia, L. Qi, M. Q. Yang and S. Liang, ACS Appl. Mater. Interfaces, 2021, 13, 38239–38247 CrossRef CAS PubMed.
- T. Shan, L. Luo, T. Chen, L. Deng, M. Li, X. Yang, L. Shen and M.-Q. Yang, Green Chem., 2023, 25, 2745–2756 RSC.
- M. Q. Yang, C. F. Tan, W. Lu, K. Zeng and G. W. Ho, Adv. Funct. Mater., 2020, 30, 2004460 CrossRef CAS.
- J. Wang, Z. Wang, J. Zhang, S. P. Chai, K. Dai and J. Low, Nanoscale, 2022, 14, 18087–18093 RSC.
- L. Liu, Z. Wang, J. Zhang, O. Ruzimuradov, K. Dai and J. Low, Adv. Mater., 2023, 35, e2300643 CrossRef PubMed.
- S. Lu, B. Weng, A. Chen, X. Li, H. Huang, X. Sun, W. Feng, Y. Lei, Q. Qian and M.-Q. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 13044–13054 CrossRef CAS PubMed.
- J. Wang, J. Li, W. Yang, Y. Liu, H. Wang, Q. Geng and F. Dong, Appl. Catal., B, 2021, 297, 120489 CrossRef CAS.
- X. Zhao, B. Deng, F. Li, M. Huang, Y. Sun, J. Li and F. Dong, J. Hazard. Mater., 2021, 420, 126577 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr03282e |
| This journal is © The Royal Society of Chemistry 2023 |