Open Access Article
Open Access ArticleLong-term retention of gold nanoparticles in the liver is not affected by their physicochemical characteristics†
Jennifer
Fernandez Alarcon‡
 a,
Mahmoud
Soliman‡
a,
Mahmoud
Soliman‡
 bc,
Tanja Ursula
Lüdtke
d,
Eva
Clemente
bc,
Tanja Ursula
Lüdtke
d,
Eva
Clemente
 b,
Marko
Dobricic
b,
Martina B.
Violatto
b,
Marko
Dobricic
b,
Martina B.
Violatto
 a,
Alessandro
Corbelli
a,
Fabio
Fiordaliso
a,
Alessandro
Corbelli
a,
Fabio
Fiordaliso
 a,
Chiara
Cordiglieri
a,
Chiara
Cordiglieri
 e,
Laura
Talamini
e,
Laura
Talamini
 a,
Giovanni
Sitia
a,
Giovanni
Sitia
 f,
Sergio
Moya
f,
Sergio
Moya
 d,
Paolo
Bigini
d,
Paolo
Bigini
 *a and
Marco P.
Monopoli
*a and
Marco P.
Monopoli
 *b
*b
aDepartment of Molecular Biochemistry and Pharmacology, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Via Mario Negri 2, 20156 Milano, Italy. E-mail: paolo.bigini@marionegri.it
bDepartment of Chemistry, Royal College of Surgeons of Ireland RCSI, St Stephens Green 123, Dublin, Ireland. E-mail: marcomonopoli@rcsi.ie
cPhysics Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
dDepartment of Soft Matter Nanotechnology, CIC Biomagune, Paseo Miramon 182, 20014 San Sebastian-Donostia, Spain
eINGM Imaging Facility, Istituto Nazionale Genetica Molecolare, Via Francesco Sforza 35, 20122 Milano, Italy
fExperimental Hepatology Unit, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Via Olgettina 58, 20132 Milano, Italy
First published on 25th April 2023
Abstract
Gold nanoparticles (GNPs) are considered promising candidates for healthcare applications, however, their toxicity after long-term exposure to the material remains uncertain. Since the liver is the main filter organ for nanomaterials, this work was aimed at evaluating hepatic accumulation, internalisation and overall safety of well-characterised and endotoxin-free GNPs in healthy mice from 15 minutes to 7 weeks after a single administration. Our data demonstrate that GNPs were rapidly segregated into lysosomes of endothelial cells (LSEC) or Kupffer cells regardless of coating or shape but with different kinetics. Despite the long-lasting accumulation in tissues, the safety of GNPs was confirmed by liver enzymatic levels, as they were rapidly eliminated from the blood circulation and accumulated in the liver without inducing hepatic toxicity. Our results demonstrate that GNPs have a safe and biocompatibile profile despite their long-term accumulation.
Introduction
The use of nanomaterials for drug delivery and healthcare diagnostics represents a rapidly growing opportunity for precision medicine.1 Nanoparticle (NP)-conjugated drugs often show a better pharmacokinetic profile than free drugs controlling drug biodistribution and accumulation in specific organs with an improved therapeutic outcome and reduced off-targeting and side effects.1 To this end, gold nanoparticles (GNPs) have been developed in recent years and their high precision and well-established synthesis methods allow them to be tuned in size, shape, and a wide range of surface modifications.2–5 The GNPs surface can also be easily modified with a wide range of small molecules such as nucleic acids, peptides, small interfering RNA, antibodies, polymers such as polyethylene glycol (PEG), and several drugs,6 making them a very attractive material for nanomedicine. In addition, as an inert metal, gold is not as sensitive to pH variations and is less reactive than silicon dioxide7,8 or metal–oxide NPs,9 thus, maintaining their biological characteristics.Physicochemical properties of NPs, such as the geometry, size, or surface curvature may play a key role in the interactions between NP and cells and tissues, affecting transport and NPs fate. While size plays a fundamental role in retention in filtering organs such as the liver and kidneys, curvature and size affect the rate of internalisation of NPs into cells.4,5,10 NPs less than 10 nm in diameter have been shown to be energetically unsuitable for uptake into cells11,12 and, because of their small size, they are excreted through the kidneys via urine.11,12 In contrast, larger NPs with diameters between 10 and 100 nm are preferentially taken up into cells of the liver and excreted from the organism through the hepatobiliary system, with a slower rate of excretion.11,12 Furthermore, the particle geometry and surface chemistry can drastically alter the fate of GNPs in biological fluids, cells, tissues and organs.13–16 This modification leads to their uptake in filtering organs, especially in cells of the hepatic reticuloendothelial system (RES), which is mainly composed of resident intravascular macrophages (i.e., Kupffer cells)17 and liver sinusoidal endothelial cells (LSECs), a highly specialised endothelial cell layer with an efficient endocytosis capacity that is among the highest of all cell types in the body.18 Emerging studies have started to evaluate the NP biological response after chronic exposure,19,20 but additional studies are required to evaluate the in vivo long-term exposure and their translocations in organs.
Herein, we report the biological distribution of GNPs in healthy and immunocompetent mice following a long-term exposition over 47 days and the influence of shape, size, and polymer surface functionalisation on cellular uptake in the liver, subcellular distribution in various liver resident cells, and long-term accumulation in the hepatobiliary system. Our results show that the percentage of GNPs reaching the liver was strongly dependent on size, with small spherical GNPs remaining longer in the bloodstream, whereas up to 70% of nanorods rapidly landed in the liver, the long-term persistence of GNPs in this organ remains unchanged over 47 days after intravenous administration, with GNPs keeping their shape and size even within subcellular compartments of LSECs and Kupffer cells, regardless of geometry and surface functionalisation. Despite the prolonged accumulation in tissues, the overall safety of GNPs was confirmed by the absence of hepatic necroinflammation but should prompt caution in chronic treatments.
Experimental
Materials
Before use, all glassware was washed with aqua regia and rinsed thoroughly with Milli-Q water. All chemicals were used as received. Hydrogen tetrachloroaurate(III) hydrate (HAuCl4), hexadecyltrimethylammonium chloride (CTAC), hexadecyltrimethylammonium bromide (CTAB), sodium borohydride (NaBH4), ascorbic acid (AA), ethyl-3-(3-dimethyl aminopropyl)carbodiimide (EDC), poly(isobutylene-alt-maleic-anhydride), hydrochloric acid, 5-bromosalicylic acid, silver nitrate (AgNO3), phosphine buffered saline (PBS), ethylenediaminetetraacetic acid (EDTA), resazurin, and dodecylamine (DDA), α-methoxy-ω-thiol-poly(ethylene glycol) (MPEG-SH, Mw = 2 kDa (PEG 2k) and Mw = 5 kDa (PEG 5k)), poly(sodium 4-styrenesulfonate) (PSS, Mw = 70 kDa) were purchased from Sigma-Aldrich (UK). Most buffers were prepared manually using Milli-Q water (resistivity of 18.2 MΩ cm at ca. 25 °C).Synthesis, functionalisation and NPs characterisation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), was added to the NPs solution (100 mL) and the mixture was further stirred for 1–2 min and then left without shaking at RT for 24 h. As a result, PSS molecules were adsorbed to the CTAC/CTAB-coated NPs forming a PSS–CTAC/CTAB complex. After that, the NPs solution was subjected to one-time cleaning by centrifugation (see Table S1† for centrifugation parameters). Thereafter, the supernatant was discarded, and the pellet was dispersed in Milli-Q water. To exchange the PSS–CTAC/CTAB complex, a specific amount of PEG 5k in Milli-Q water (see Table S1†) was added to the solution of PSS-coated NPs and further stirred at RT for 24 h. After that, The PEGylated NPs were subjected to one-time cleaning by centrifugation (see Table S1† for centrifugation parameters), the supernatant was discarded, and the pellet was dispersed in Milli-Q water and stored for further use.
000), was added to the NPs solution (100 mL) and the mixture was further stirred for 1–2 min and then left without shaking at RT for 24 h. As a result, PSS molecules were adsorbed to the CTAC/CTAB-coated NPs forming a PSS–CTAC/CTAB complex. After that, the NPs solution was subjected to one-time cleaning by centrifugation (see Table S1† for centrifugation parameters). Thereafter, the supernatant was discarded, and the pellet was dispersed in Milli-Q water. To exchange the PSS–CTAC/CTAB complex, a specific amount of PEG 5k in Milli-Q water (see Table S1†) was added to the solution of PSS-coated NPs and further stirred at RT for 24 h. After that, The PEGylated NPs were subjected to one-time cleaning by centrifugation (see Table S1† for centrifugation parameters), the supernatant was discarded, and the pellet was dispersed in Milli-Q water and stored for further use.
The DDA capped NPs were transferred back to Milli-Q water using the polymer coating technique as described by Lin et al.24 The amphiphilic polymer (poly(isobutylene-alt-maleic anhydride)) was synthesised by grafting DDA onto its hydrophilic backbone through a spontaneous amide linkage, which converts one maleic anhydride into one corresponding amide and one free carboxylic acid. 75% of the polymer's anhydride rings were grafted by using DDA in THF at 70 °C in order to produce free hydrophobic sides, which will interact later with the hydrophobic sides (hydrophobic chains) of the particles’ surface by hydrophobic interaction. The remaining 25% of the anhydride rings will offer the colloidal stability of the particles by electrostatic repulsion of the surface's free carboxylic acids. For details about the synthesis of the amphiphilic polymer used here, we refer to the previous work of Lin et al.24 The resulting dodecyl-grafted-poly(isobutylene-alt-maleic anhydride) (in the following referred to as PMA) was dissolved in chloroform as a stock solution (0.5 M). The amount of added PMA per NP was calculated according to eqn (S1)–(S4) in the ESI (Appendix).† The polymer coating was then carried out according to the standard protocol already described.24
A transmission electron microscope (TEM) was used to characterize the quality of GNPs. The TEM sample was prepared by placing a drop (4 μL) of diluted NPs solution on top of a copper grid coated with a layer of carbon and left to dry at RT. The images obtained by TEM were analysed and the core size of at least 100 NPs was manually calculated using the free software ImageJ.
The hydrodynamic diameter (dh) and zeta potential (ζ-potential) of a suspension of GNPs (1 mL) were measured by dynamic light scattering (DLS) and laser Doppler anemometry (LDA), respectively. All samples were equilibrated for 5 min at ca. 25 °C to ensure NP motion was due to Brownian motion and not due to any thermal gradients. The NPs were measured in Milli-Q water or PBS at 173° backscatter settings, using a 633 nm laser. The determined dh [nm] and ζ-potentials [mV] were the averages of at least three independent measurements and all data are summarised in Table S2.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600g at RT for 30 min. The supernatants were collected and tested for LPS content following the manufacturer's protocol.30 The detected level of LPS in each NP stock solution was presented in Table S3.†
600g at RT for 30 min. The supernatants were collected and tested for LPS content following the manufacturer's protocol.30 The detected level of LPS in each NP stock solution was presented in Table S3.†
Animals and treatments
Six-week-old female CD1 mice were purchased from Charles River (Italy) and were maintained under specific pathogen-free conditions (SPF) in the Institute's Animal Care Facilities. Animals were bred in rooms at a constant temperature of 21 ± 1 °C, humidity of 55 ± 10% with a 12 h light/dark cycle and ad libitum access to food and water. All mice were regularly checked by a veterinarian who is responsible for animal welfare supervision and experimental protocol review. None of the animals died during the study. Mice were randomly divided into eight groups receiving rods and spheres with two different sizes (large and small) and two different polymer coatings (PMA and PEG) (n = 5 for each experimental group). Briefly, all animals received by intravenous injection the same dose of NPs preparation (200 μL, 2 × 1011 NPs per mouse). At the selected time points (pre-injection, 15 min, 1 h, 8 h, 24 h, 168 h) mice were anesthetised and blood was taken from retro-orbital bleeding and serum was analysed for markers of toxicity. Furthermore, at 15 min, 1, 24, 168, 672 and 1128 hours after NPs injection, 5 mice for each group were sacrificed and the organs were collected for histological analysis. Mice perfused were sacrificed by an overdose of ketamine (150 mg kg−1) and metedomidine (2 mg kg−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The digestion vessels were closed and inserted into the speedwave XPERT microwave from Berghof Products + Instruments GmbH. After finishing the digestion, the resulting solution (250 μL) was added to diluted aqua regia (2.5 mL, approx. 2% HNO3 and 0.5% HCl) and stored until analysis.
3. The digestion vessels were closed and inserted into the speedwave XPERT microwave from Berghof Products + Instruments GmbH. After finishing the digestion, the resulting solution (250 μL) was added to diluted aqua regia (2.5 mL, approx. 2% HNO3 and 0.5% HCl) and stored until analysis.
The samples were measured by means of the ICP-MS. Before the measurement, ICP-MS set up was calibrated with a freshly prepared serial dilution of gold (Roth, Au-Standard (1000 mg mL−1)). The used calibration curve was constructed using gold concentrations from 2 to 2500 parts per billion (ppb). The measurements were carried out with the iCAP-Q ICP-MS (Thermo Scientific, Bremen, Germany) with autosampler ASX-500 (CETAC Technologies, Omaha, USA).
Each analysis was validated by a certified biochemical chemist and haematologist specialist using quality control serums (CQI), in the San Raffaele Mouse Clinic.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, Wako Chemicals, USA) was used to label macrophage calcium binding protein, the labelling system ABC kit (Vectastain Elite) and for chromogenic reaction DAB (Sigma-Aldrich). Nuclei were stained with Mayer's hematoxylin solution (Bio-Optica, Italy) for 30 s, and then washed with water until it became incolor. Samples were dehydrated and mount with xylene-based mounting medium (DPX, Sigma). All images were acquired using Olympus BX61VS.
200, Wako Chemicals, USA) was used to label macrophage calcium binding protein, the labelling system ABC kit (Vectastain Elite) and for chromogenic reaction DAB (Sigma-Aldrich). Nuclei were stained with Mayer's hematoxylin solution (Bio-Optica, Italy) for 30 s, and then washed with water until it became incolor. Samples were dehydrated and mount with xylene-based mounting medium (DPX, Sigma). All images were acquired using Olympus BX61VS.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, Serotec, Kidlington, UK) was used to label lysosomes and endosome membranes of macrophages, Lyve-1 (1
200, Serotec, Kidlington, UK) was used to label lysosomes and endosome membranes of macrophages, Lyve-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, Biotechne) was used to label lysosomal endothelial cells and Hoechst-33258 (1 μg mL−1 in PBS, Thermo Fisher Scientific) to label nuclei.
300, Biotechne) was used to label lysosomal endothelial cells and Hoechst-33258 (1 μg mL−1 in PBS, Thermo Fisher Scientific) to label nuclei.
For each liver section, a minimum of 15 independent fields of view were acquired to achieve proper quantifications. In order to increase confocal resolution of the reflecting signal, Richardson–Lucy deconvolution algorithm was used, applying the specific module in NIS-Elements software v5.30 (Nikon Instruments/Lim Instruments). Moreover, for quality representation, images were further processed using rolling-ball background suppression and median filtering algorithms (using NIS-Elements v5.30). For image quantifications, raw images without processing were used. Signal thresholding, binarisation, object classification and segmentation, to detect tissue and cell primary and secondary objects, were performed using an ad hoc computed pipeline of analysis within the General Analysis module in NIS-Elements v5.30.
Results and discussion
GNPs synthesis and characterisation
In this study, we designed a series of GNPs with different sizes, shapes and surface coating in order to define their biological compatibility in vivo and define how their physicochemical characteristics shape hepatic cellular targeting and hepatobiliary clearance. We synthesised four different types of endotoxin-free GNPs, spherical NPs with a diameter of 25 nm (25-GNPs) and 55 nm (55-GNPs) respectively, and rods of 15 × 55 nm (55-GNRs) and 15 × 90 nm (90-GNRs), respectively. Each GNP was functionalised with two different surface coating strategies, either polyethylene glycol (PEG) or poly(isobutylene-alt-maleic) anhydride (PMA), both negatively charged, to ensure a higher biosafety profile and high colloidal stability (see Tables S1 and S2†).3,23,24,32,33 PEG is a well-known antifouling polymer which conjugates to the gold surface covalently after CTAB displacement. Meanwhile, PMA can be used as a universal platform for functionalisation. All NPs were synthesised under a laminar flow hood to avoid bacteria contamination (i.e., endotoxins), whose presence was tested by using a modified Limulus Amebocyte Lysate (LAL) assay the golden standard assay for detection of LPS (see Methods and Table S3†).4,25,34The characterisation of the NPs is shown in Fig. 1 and Fig. S1–S4,† transmission electron microscopy (TEM) images confirmed a homogeneous size distribution and shape (Fig. 1a). By UV-Vis spectrum a peak absorbance at 524 nm and 532 nm were observed for 25-GNPs and 55-GNPs respectively, meanwhile, rods of 55-GNRs and 90-GNRs have an absorption peak near 510 nm and a second absorption peak of gold nanorods was observed at 820 nm and 1000 nm, respectively (Fig. 1b and Fig. S1†). Following surface coating with PEG and PMA, an increase in size was seen by dynamic light scattering (DLS) (Fig. 1c and Fig. S2, S3†) and a change in the zeta potential (ζ) to an overall negative net charge as a result of new polymer coatings, PEG and PMA (Fig. 1d and Fig. S4†). To evaluate the influence of the NP surface coating on the biomolecular corona formation, we carried out SDS-PAGE in blood sample media.15,35 As expected, CTAC/CTAB capped NPs formed a stronger protein corona, particularly 55-GNPs, while the corona formation becomes attenuated for the same NPs coated with PEG or PMA, indicating that both polymers have an antifouling effect4,35,36 (Fig. S5†).
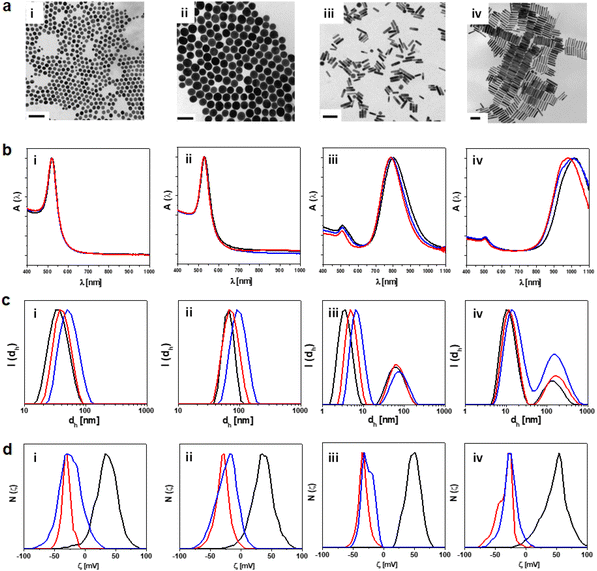 | ||
| Fig. 1 Characterisation of GNPs. (a) Representative TEM images of GNPs (with gold core diameters/lengths of (i) 24.9 ± 2.66 nm (25-GNPs), (ii) 53.97 ± 4.47 (55-GNPs), (iii) 56.2 ± 4.63 nm (55-GNRs), and (iv) 91.3 ± 9.55 nm (90-GNRs)). Scale bars = 100 nm. Histograms of TEM images were presented in Fig. S2.† (b) Normalised absorption spectra A(λ). (c) Hydrodynamic diameter dh [nm] in intensity (dh(I)) and (d) ζ-potential distribution N(ζ) of (i) 25-GNPs, (ii) 55-GNPs, (iii) 55-GNRs and (iv) 90-GNRs. The colour codes were assigned to the type of surfactant/polymer coated-NPs, hexadecyltrimethylammonium chloride (CTAC)/hexadecyltrimethylammonium bromide (CTAB) (black), PEG-coated NPs (blue) and PMA-coated NPs (red). Triplicates of each measurement included in (c and d) were presented in Fig. S1–S3.† | ||
Influence of the shape, size and polymer coating on GNPs hepatic biodistribution
RES organs are responsible for removing non-self-bodies from the circulatory system, and it is widely known that in normal physiological conditions, systemically administrated NPs are cleared by this system.4,11,34 In particular, the mononuclear phagocytic system (MPS) and endothelial cells, such as LSECs, are responsible for pathogen and NP clearance from the circulation, causing their accumulation in the liver, spleen, bone marrow or lymph nodes.34 However, the long-term fate of specific GNPs with different shapes and surface coating is still unknown. For this purpose, a longitudinal study was carried out to evaluate the short- and long-term fate and biodistribution of GNPs of different sizes, shapes and surface coatings, where 2 × 1011 NPs were administrated intravenously to each mouse, and the samples were analysed at three different time points: 1 hour, 1 and 47 days after injection (Fig. 2). This dose was chosen because it can be compared to other prior studies realised and did not cause toxicity.4,8 These time points were chosen to exclude the potential toxicity of GNPs after short- and long-term exposure and also study their fate inside the organism. Especially after 1-hour, potential acute toxicity could be detected. After 1 day, nanomaterials’ half-life decreases with possible accumulation into tissues. Meanwhile, 47 days is a long time point to evaluate long-term effects, accumulation and possible degradation.19 In order to monitor the progressive caption, three additional time points were used only with the largest PMA-coated NPs (55-GNPs and 90-GNRs), an earlier time point of 15 minutes, to understand the real contribution of NPs in the circulation and two additional time points after 7 and 28 days to study the progressive accumulation in liver (Fig. 2).The quantification of the gold content in liver and blood was conducted by inductively coupled plasma mass spectrometry (ICP-MS) analysis (Fig. 3a–f). The data collected was expressed as a percentage of injected dose (% ID), which is calculated from the mass of gold found in the organ divided from the total mass of gold injected in each mouse.
Blood levels of gold showed that, in general, the functionalisation with PEG enhances the overall circulation time in the bloodstream of all GNPs, except for 55-GNPs, in comparison with those PMA-coated (Fig. 3a–d). For PEGylated NPs, 1 hour after treatment, GNRs and 25-GNPs were found with similar percentages around 20 to 30% ID, but after 24 hours almost all GNPs were eliminated from circulation, except for 90-GNRs, with levels around 4% ID. This is in line with the expected ability of PEG to prevent the adsorption of nonspecific proteins on the NP surface and the formation of the protein corona.2,37 NPs internalisation inside cells increases with higher adsorption of proteins.14 55-GNPs were cleared out from the circulation very rapidly, with less than 1% ID after 1 hour. As previously mentioned, PMA-coated NPs were rapidly cleared out from the circulation in less than 1 hour apart from 25-GNPs (Fig. 3c). Independently from the polymer coating, 25-GNPs were able to remain for a longer time in the bloodstream, with an increase of a 10% ID for PMA-coated NPs in comparison with PEG-coated NPs. This effect has been observed by previous studies, where nanospheres of 10 nm in size had a higher half-time circulation than 50 nm, showing that size can play an important role.4,38
Rod-like NPs undergo a faster clearance from blood due to their particular shape, which makes it easier to pass through renal pores and be eliminated through the tubular interspaces with the urine.39 As shown in Fig. 3b–d, an increase in the hepatic levels of 25-GNPs was observed, with an increase of a 15–20% ID while blood values decrease, demonstrating a clear uptake mostly by the hepatobiliary system. The gold content analysis in the liver of the treated mice showed a progressive accumulation of NPs since early time points. PEG-coated NPs reached the liver after 1 hour of administration with a % ID between 30 to 40 for GNRs and 55-GNPs (Fig. 3b) and maintained the same level regardless of the NP size and shape after 24 hours of the injection. However, long-term exposure resulted in a different NP accumulation, while 55-GNPs value remained constant over time (35% ID), a progressive increase for the other NPs was tested. The highest increase was observed in both GNRs (55% and 70% of ID), this increase could be due to a possible interaction of rods with endothelial cells (EC) in the vessels. The membrane receptors from EC are likely to allow only partial NP phagocytosis due to the high aspect ratio, and could cause a progressive release back into circulation followed by liver filtration.12 The pharmacokinetic analysis of PMA-coated NPs was found to be influenced by their size, 25-GNPs had a lower penetration inside the liver in comparison with the other GNPs, with just a 10% ID (Fig. 3d). Different studies have observed that large NPs, around 50 nm, were internalised inside cells requiring receptor-mediated endocytosis, through a re-arrangement of the cell cytoskeleton.12 For this reason, 55-GNPs were able to penetrate inside the liver with around 50% ID. Meanwhile, smaller NPs uptake is less efficient, as the process is more energy demanding due to the membrane re-arrengment for the uptake.40 Also, a strong increase of uptake was found in rod-like NPs, with a 60 to 75% ID only after the 1st hour of administration and the levels remain similar over time. When PMA-coated GNPs interact with the liver, they are strongly retained inside.
To understand in higher detail the pharmacokinetics of PMA-coated NPs inside the liver, 55-GNPs and 90-GNRs, were injected into mice and sacrificed 15 minutes after the treatment. As shown in Fig. 3e, ∼20% of ID was found in the liver for 55-GNPs and 30% ID for 90-GNRs, with a significant difference between 15 minutes and 1 hour treated mice showing a clear trend in PMA kinetics. As gold detection by ICP-MS does not allow to distinguish between NPs translocated into the tissues and the ones still in circulation in the blood vessels, we compared the gold content of perfused (where the blood was removed by intracardial perfusion) and not perfused livers. Interestingly, no difference in gold content was observed indicating that after 1 hour, GNPs are firmly translocated into the liver tissue rather than being transient into the circulatory system of the organ (Fig. 3f and Fig. S6†).
Overall, ICP-MS analysis had shown that NP polymer coating type had an influence on the GNPs retention in the liver, where PMA-coated GNPs rapidly accumulate inside the liver while PEG-coating increase the circulation in blood. However, other NPs properties, such as the shape, played an important role, where rod-like NPs had a higher retention into the liver than spherical ones. Regardless of the liver kinetic differences, it is important to underline that, independently of the GNPs properties, gold accumulated in liver remained 7 weeks after injection, suggesting that the accumulation is not followed by a clearance over time. In vivo similar results were found in mice after 15 months of a single administration without showing any toxic effect.41 However, in vitro studies suggested the formation of extracellular reactive oxygen species (ROS) which helped to degrade elemental gold to ionic gold via a Fenton reaction.19
Influence of the shape, size and polymer coating on the cellular uptake
While ICP-MS provided information on the gold content per organ and the fate of the GNPs, it does not bring information on the cellular mechanisms implicated in the GNPs uptake nor the impact of the chemical modifications on the ' fate administrated intravenously. To this aim, qualitative histological studies revealed a marked increase of GNPs inside hepatic cells by autometallography silver staining (AMG) (Fig. 4) and we focused the analysis on PMA-coated NPs of 55-GNPs and 90-GNRs due to their differences in the liver accumulation kinetics.Polarised and elongated black spots were already visible in the liver of mice sacrificed 15 minutes after the treatment (red arrows) due to an interaction of GNPs with liver sinusoidal endothelial cells (LSECs) (Fig. 4a, b and Fig. S7, S8†).
As expected, surrounding areas near the central vein had a lower signal as NPs were circulating from the portal triad (dotted lines in Fig. 4a–d and Fig. S7†) causing NPs penetration inside the sinusoids (Fig. S7†). After 24 hours, it was observed a visible rise in the black spots localised inside elongated bodies due to a possible uptake of GNPs by Kupffer cells (liver macrophages) or LSEC (Fig. 4e and f), with a simultaneous decrease in the NPs levels found in the sinusoids. Over time, NPs signal slowly and progressively decreased in liver parenchyma (Fig. 4g–j), and after 47 days it was possible to detect GNPs internalised inside hepatic cells (Fig. 4k and l). No differences were observed in terms of signal intensity or parenchyma biodistribution between both NPs, the behaviour inside the liver was very similar.
The evaluation of GNPs biodistribution by AMG is extremely important to have a topography of NP accumulation in the liver. To obtain more information regarding their cellular fate, intracellular localisation and stability, we carried out TEM as gold is an optimal material for this type of analysis due to its electron-dense metallic core that provides enough contrast with the surrounding biological environment, being easily identified in tissues. TEM analysis has shown that GNPs could be easily detected in the tissue section already after 15 minutes of injection, with the GNPs shape and morphology well preserved regardless of the NP type and time point of analysis (Fig. 5). Representative TEM images of GNPs were exclusively observed in Kupffer cells and LSECs with no sign of NPs in the hepatocytes or liver parenchyma where the GNPs are rapidly translocated into the cells and accumulate in secondary lysosomes. This is a common terminal step of endocytosis and phagocytosis, formed by the fusion of a primary lysosome with phagosomes or endosomes, and consequent activation of digesting enzymes like acid hydrolases, which are an indicator of a pH change from <6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 to 4.5.43,44 The number of GNPs agglomerated in the cluster after 1 and 28 days was observed to increase over time (Fig. 5) and despite the strong presence of this cluster, the internalisation did not affect the ultrastructural organisation of both Kupffer cells and LSECs showing perfectly preserved organelles such as endoplasmic reticulum, Golgi apparatus, nucleus and a great quantity of primary lysosomes. Interestingly, after 47 days from the single injection, it was still possible to detect GNPs inside Kupffer cells and LSECs (Fig. 5), confirming that GNPs lysosomal degradation into ionic gold and the following reassembling into nanoleaves already observed in vitro after 2 weeks was exclusively taking place for smaller GNPs (up to 22 nm).19,31 This study was important to understand in which cells and organelles GNPs were internalised and if they remain or migrate over time.
42 to 4.5.43,44 The number of GNPs agglomerated in the cluster after 1 and 28 days was observed to increase over time (Fig. 5) and despite the strong presence of this cluster, the internalisation did not affect the ultrastructural organisation of both Kupffer cells and LSECs showing perfectly preserved organelles such as endoplasmic reticulum, Golgi apparatus, nucleus and a great quantity of primary lysosomes. Interestingly, after 47 days from the single injection, it was still possible to detect GNPs inside Kupffer cells and LSECs (Fig. 5), confirming that GNPs lysosomal degradation into ionic gold and the following reassembling into nanoleaves already observed in vitro after 2 weeks was exclusively taking place for smaller GNPs (up to 22 nm).19,31 This study was important to understand in which cells and organelles GNPs were internalised and if they remain or migrate over time.
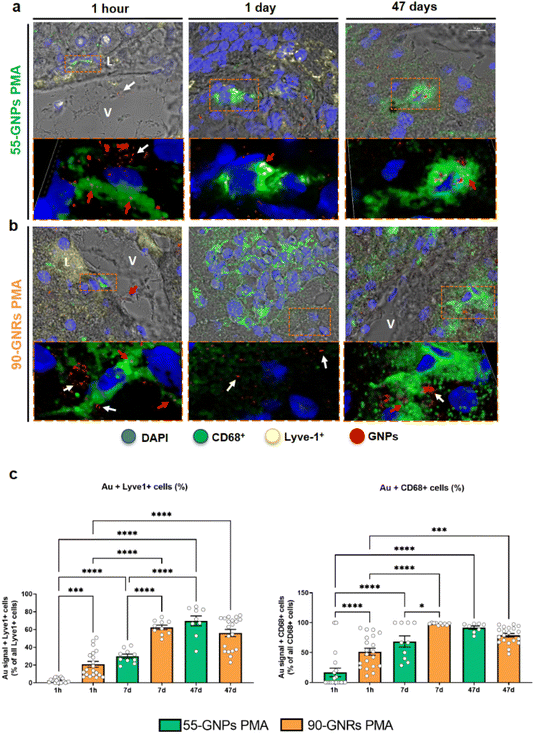
To obtain quantitative data related to the segregation of the NPs inside Kupffer cells and LSECs, a co-localisation study was performed by using structured illumination microscopy (SIM) where the NP presence was detected by measuring the reflected light on the GNP surface.31 Macrophages and ECs were immunostained using fluorescent antibodies against CD68 and Lyve-1, respectively (Fig. 6 and Fig. S9, S10†).
We observed clusters of GNPs reflective signals after 1 hour of treatment (Fig. 6a) where most of the NPs aggregates were found in the peripherical areas of CD68+ cells and parenchymal proximities to the sinusoids, due to a fast interaction between GNPs with LSECs, while only small aggregations of NPs were still visible in the vessels after 1 hour from injection. Aggregates of 55-GNPs reflective structures were already visible intracellularly within CD68+ cells at 24 hours from the injection, where GNPs were retained in the lysosomes, forming little vesicles of clustered laser-reflecting structures as shown in Fig. 6a. Meanwhile, 90-GNRs reflecting structures were visible both in the intercellular spaces surrounding CD68+ cells (white arrowheads) and within intracellular areas (red arrowheads) (Fig. 6b). A similar behaviour was exhibited after 47 days where GNPs were still visible inside the hepatic cells (Fig. 6a and b).
Whereas the total percentage of Lyve-1 and CD68 cells reflection from internalised GNPs was approximately similar (Fig. 6c and Fig. S11†), a relevant difference was observed between sphere-shaped and rod-shaped GNPs. Inside Kupffer cells, 55-GNPs rapidly formed small clusters evidencing an easy passage inside the cell through endocytosis. In contrast, rod-like NPs formed smaller clusters in the surrounding areas of the phagocytic cells, due to a lower internalisation rate despite the fact that they had a faster interaction with hepatic cells. If nanorods are not orientated in the correct direction towards the cell membrane,45 as they are more elongated due to a larger length-to-radius, phagocytosis is slower.21,46 Despite the multivalent cell membrane receptors, rod NPs may be too large, and their uptake may not be possible due to a partial wrapping and resulting in the particle releasement back into the hepatic system, followed by the uptake by another phagocytic cell in the proximity.2
Regardless of the detection and imaging methodologies used, our data strongly suggests that, although the physico-chemical properties play a role in the total amount of NPs filtered by the liver, they do not seem crucial in terms of: (1) kinetics (very fast in the first hours after administration); (2) cellular segregation (they exclusively penetrated in LSECs and macrophages) and; (3) longer lasting aggregation.
Liver nanosafety
Ultrastructural analyses by TEM did not reveal marked cellular alterations in animals treated with GNPs. However, since the liver plays a major role in metabolic processes of so many drugs, to ensure that no toxic effect was caused by the NPs inside the hepatic system, a toxicological study was performed with liver tissue and serum (Fig. 7 and Fig. S12, S13†). The serological levels of alanine transaminase (sALT) and aspartate transaminase (sAST), hepatic enzymes which activities are elevated during liver damage, were quantified to detect possible inflammation or necrosis in tissue. As it is shown in Fig. 7a (Fig. S12†), no value was over the maximum level of normality for sALT, 70 U L−1 (dashed line), the most specific and sensitive biomarker for liver injury. A significant increase of sAST, was observed for spherical NPs after 47 days and after 24 hours for rod-like NPs, overpassing the maximum value of normality for sAST of 83 U per L per mice (Fig. 7b and Fig. S13†). However, the enzyme sAST is not specific for the liver, an increase in its levels could be due to damage to the liver, skeletal muscle or any other cell that suffers lysis such as red blood cells. Histological evaluation with H&E and IBA-1 staining in liver tissues after 24 hours and 47 days (Fig. 7c and Fig. S14–S17†) did not show any sign of inflammation, steatosis, fibrosis or hepatocyte necrosis in comparison with the control mice (Fig. S14–S16†). Also, macroscopic examination of all organs, weight loss, diarrhea, inability to walk or other types of clinical signs were not observed in mice, demonstrating that GNPs even if they accumulate in the liver, they did not cause any type of toxic effect over time.Conclusions
Overall, our data strongly suggest that the hepatic filtering of GNPs, after bloodstream circulation, may be influenced by their geometry and the polymer coating on their surface. However, these parameters almost exclusively influenced the kinetics of penetration in the liver that very often occurs in the first hours after administration for all types of GNPs. On the other hand, once penetrated in the liver, their fate seems independent of the physical–chemical nature of GNPs. By an integrated method of analysis combining ICP-MS, AMG, TEM and SIM, we were able to demonstrate that all type of GNPs remained entrapped in endothelial or, more frequently, macrophagic lysosomes not undergoing to an expected process of biodegradation or clearance by exocytosis of multivesicular bodies. A similar behaviour was also seen in terms of biocompatibility; it is indeed interesting to note that, despite the large presence of GNPs at least up until the 7th week after administration neither acute nor chronic markers of hepatic dysfunction were found. We can conclude here that independently of the NPs size, shape and surface chemistry for the set of NPs under study, spherical and nanorods with sizes between 25 and 90 nm, accumulated in the liver within 1 day and remained in Kupffer cells and LSECs for 47 days. The percentage of GNPs reaching the liver is strongly affected by their size and aspect ratio, being 20% of the given dose for the 25-GNPs and up to 70% for the nanorods. However, in all cases, what reaches the liver remains practically intact there. This is a fundamental finding with large implications for the use of GNPs for nanomedicine and the size and dose of GNPs must be carefully controlled to avoid long-term accumulation in the liver.Author contributions
J. F. A., M. S., P. B., M. M. conceived the idea and planned the experiments. J. F. A., M. S., T. U. L., E. C., M. B. V., M. D., F. F., A. C., C. C., G. S. conducted the experiments. J. F. A. and M. S. prepared the figures. J. F. A. and P. B. wrote the manuscript, and all others contributed to revisions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 814236 - Nanocarb. M. S. acknowledges the H2020-MSCA-IF grant no. 894656. G. S. is supported by the Italian Association for Cancer Research (AIRC) grants 22820 and 22737.References
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed.
- B. Li and L. A. Lane, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1542 Search PubMed.
- M. G. Soliman, B. Pelaz, W. J. Parak and P. del Pino, Chem. Mater., 2015, 27, 990–997 CrossRef CAS.
- L. Talamini, M. B. Violatto, Q. Cai, M. P. Monopoli, K. Kantner, Ž. Krpetić, A. Perez-Potti, J. Cookman, D. Garry, C. P. Silveira, L. Boselli, B. Pelaz, T. Serchi, S. Cambier, A. C. Gutleb, N. Feliu, Y. Yan, M. Salmona, W. J. Parak, K. A. Dawson and P. Bigini, ACS Nano, 2017, 11, 5519–5529 CrossRef CAS PubMed.
- W. G. Kreyling, A. M. Abdelmonem, Z. Ali, F. Alves, M. Geiser, N. Haberl, R. Hartmann, S. Hirn, D. J. de Aberasturi, K. Kantner, G. Khadem-Saba, J.-M. Montenegro, J. Rejman, T. Rojo, I. R. de Larramendi, R. Ufartes, A. Wenk and W. J. Parak, Nat. Nanotechnol., 2015, 10, 619–623 CrossRef CAS PubMed.
- K. Sztandera, M. Gorzkiewicz and B. Klajnert-Maculewicz, Mol. Pharm., 2019, 16, 1–23 CrossRef CAS PubMed.
- P. Picchetti, G. Moreno-Alcántar, L. Talamini, A. Mourgout, A. Aliprandi and L. De Cola, J. Am. Chem. Soc., 2021, 143, 7681–7687 CrossRef CAS PubMed.
- L. Talamini, P. Picchetti, L. M. Ferreira, G. Sitia, L. Russo, M. B. Violatto, L. Travaglini, J. Fernandez Alarcon, L. Righelli, P. Bigini and L. De Cola, ACS Nano, 2021, 15, 9701–9716 CrossRef CAS PubMed.
- J. Kolosnjaj-Tabi, Y. Javed, L. Lartigue, J. Volatron, D. Elgrabli, I. Marangon, G. Pugliese, B. Caron, A. Figuerola, N. Luciani, T. Pellegrino, D. Alloyeau and F. Gazeau, ACS Nano, 2015, 9, 7925–7939 CrossRef CAS PubMed.
- A.-L. Bailly, F. Correard, A. Popov, G. Tselikov, F. Chaspoul, R. Appay, A. Al-Kattan, A. V. Kabashin, D. Braguer and M.-A. Esteve, Sci. Rep., 2019, 9, 12890 CrossRef PubMed.
- Y. Jiang, S. Huo, T. Mizuhara, R. Das, Y.-W. Lee, S. Hou, D. F. Moyano, B. Duncan, X.-J. Liang and V. M. Rotello, ACS Nano, 2015, 9, 9986–9993 CrossRef CAS PubMed.
- P. Decuzzi and M. Ferrari, Biomaterials, 2007, 28, 2915–2922 CrossRef CAS PubMed.
- M. P. Monopoli, C. Åberg, A. Salvati and K. A. Dawson, Nat. Nanotechnol., 2012, 7, 779–786 CrossRef CAS PubMed.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. V. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- K. A. Dawson and Y. Yan, Nat. Nanotechnol., 2021, 16, 229–242 CrossRef CAS PubMed.
- A. Salvati, A. S. Pitek, M. P. Monopoli, K. Prapainop, F. B. Bombelli, D. R. Hristov, P. M. Kelly, C. Åberg, E. Mahon and K. A. Dawson, Nat. Nanotechnol., 2013, 8, 137–143 CrossRef CAS PubMed.
- E. Papini, R. Tavano and F. Mancin, Front. Immunol., 2020, 11, 567365 CrossRef CAS PubMed.
- K. K. Sørensen and B. Smedsrød, The liver: biology and pathobiology, John Wiley & Sons Ltd, New Jersey, 2020 Search PubMed.
- A. Balfourier, N. Luciani, G. Wang, G. Lelong, O. Ersen, A. Khelfa, D. Alloyeau, F. Gazeau and F. Carn, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 103–113 CrossRef CAS PubMed.
- P. Falagan-Lotsch, E. M. Grzincic and C. J. Murphy, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13318–13323 CrossRef CAS PubMed.
- C. Hanske, G. González-Rubio, C. Hamon, P. Formentín, E. Modin, A. Chuvilin, A. Guerrero-Martínez, L. F. Marsal and L. M. Liz-Marzán, J. Phys. Chem. C, 2017, 121, 10899–10906 CrossRef CAS.
- X. Ye, L. Jin, H. Caglayan, J. Chen, G. Xing, C. Zheng, V. Doan-Nguyen, Y. Kang, N. Engheta, C. R. Kagan and C. B. Murray, ACS Nano, 2012, 6, 2804–2817 CrossRef CAS PubMed.
- M. Xu, M. G. Soliman, X. Sun, B. Pelaz, N. Feliu, W. J. Parak and S. Liu, ACS Nano, 2018, 12, 10104–10113 CrossRef CAS PubMed.
- C.-A. J. Lin, R. A. Sperling, J. K. Li, T.-Y. Yang, P.-Y. Li, M. Zanella, W. H. Chang and W. J. Parak, Small, 2008, 4, 334–341 CrossRef CAS PubMed.
- EuNCl Endotoxin Levels Online, https://euncl.org/about-us/assay-cascade/PDFs/Prescreening/EUNCL-STE-001_2_2.pdf?m=1476164492, accessed January 2023.
- N. C. Bell, C. Minelli and A. G. Shard, Anal. Methods, 2013, 5, 4591 RSC.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Anal. Chem., 2007, 79, 4215–4221 CrossRef CAS PubMed.
- N. G. Khlebtsov, Anal. Chem., 2008, 80, 6620–6625 CrossRef CAS PubMed.
- A. Konwar, D. Chowdhury and A. Dan, Mater. Chem. Front., 2019, 3, 716–725 RSC.
- S. Klein, S. Petersen, U. Taylor, D. Rath and S. Barcikowski, J. Biomed. Opt., 2010, 15, 036015 CrossRef PubMed.
- L. Guerrini, R. Alvarez-Puebla and N. Pazos-Perez, Materials, 2018, 11, 1154 CrossRef PubMed.
- L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng and Q. Wang, Nanoscale, 2021, 13, 10748–10764 RSC.
- K. M. Tsoi, S. A. MacParland, X.-Z. Ma, V. N. Spetzler, J. Echeverri, B. Ouyang, S. M. Fadel, E. A. Sykes, N. Goldaracena, J. M. Kaths, J. B. Conneely, B. A. Alman, M. Selzner, M. A. Ostrowski, O. A. Adeyi, A. Zilman, I. D. McGilvray and W. C. W. Chan, Nat. Mater., 2016, 15, 1212–1221 CrossRef CAS PubMed.
- E. Clemente, M. Martinez-Moro, D. N. Trinh, M. G. Soliman, D. I. R. Spencer, R. A. Gardner, M. Kotsias, A. Sánchez Iglesias, S. Moya and M. P. Monopoli, J. Colloid Interface Sci., 2022, 613, 563–574 CrossRef CAS PubMed.
- D. N. Trinh, R. A. Gardner, A. N. Franciosi, C. McCarthy, M. P. Keane, M. G. Soliman, J. S. O'Donnell, P. Meleady, D. I. R. Spencer and M. P. Monopoli, ACS Nano, 2022, 16, 5463–5475 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS PubMed.
- G. Sonavane, K. Tomoda and K. Makino, Colloids Surf., B, 2008, 66, 274–280 CrossRef CAS PubMed.
- C. Ruggiero, L. Pastorino and O. L. Herrera, in 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, IEEE, Buenos Aires, 2010, pp. 3731–3732.
- H. Gao, W. Shi and L. B. Freund, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9469–9474 CrossRef CAS PubMed.
- M. R. K. Ali, M. A. Rahman, Y. Wu, T. Han, X. Peng, M. A. Mackey, D. Wang, H. J. Shin, Z. G. Chen, H. Xiao, R. Wu, Y. Tang, D. M. Shin and M. A. El-Sayed, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E3110–E3118 CAS.
- P. Saftig and J. Klumperman, Nat. Rev. Mol. Cell Biol., 2009, 10, 623–635 CrossRef CAS PubMed.
- P. Lajoie, G. Guay, J. W. Dennis and I. R. Nabi, J. Cell Sci., 2005, 118, 1991–2003 CrossRef CAS PubMed.
- M. Borkowska, M. Siek, D. V. Kolygina, Y. I. Sobolev, S. Lach, S. Kumar, Y.-K. Cho, K. Kandere-Grzybowska and B. A. Grzybowski, Nat. Nanotechnol., 2020, 15, 331–341 CrossRef CAS PubMed.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662–668 CrossRef CAS PubMed.
- R. Augustine, A. Hasan, R. Primavera, R. J. Wilson, A. S. Thakor and B. D. Kevadiya, Mater. Today Commun., 2020, 25, 101692 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr00685a |
| ‡ Contributed equally as first author. |
| This journal is © The Royal Society of Chemistry 2023 |