Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-activated controlled release of camptothecin by engineering porous materials: the ship in a bottle concept in drug delivery†
Eva
Rivero-Buceta
 a,
Mirela E.
Encheva
a,
Mirela E.
Encheva
 b,
Bradley
Cech
b,
Bradley
Cech
 c,
Eduardo
Fernandez
b,
Germán
Sastre
c,
Eduardo
Fernandez
b,
Germán
Sastre
 a,
Christopher C.
Landry
*c and
Pablo
Botella
a,
Christopher C.
Landry
*c and
Pablo
Botella
 *a
*a
aInstituto de Tecnología Química, Universitat Politècnica de València-Consejo Superior de Investigaciones Científicas, Avenida de los Naranjos s/n, 46022 Valencia, Spain. E-mail: pbotella@itq.upv.es
bInstitute of Bioengineering, Universidad Miguel Hernández, Elche, Spain and Centre for Network Biomedical Research (CIBER-BBN), Avenida de la Universidad s/n, 03202 Elche, Spain
cDepartment of Chemistry, University of Vermont, 82 University Place, Burlington, VT 05405, USA. E-mail: Christopher.Landry@uvm.edu
First published on 31st May 2023
Abstract
Many systems for controlled drug release have been developed using different types of nanoparticles modified with azobenzene moieties. In these systems, drug release is often triggered by UV irradiation (either direct or using a near-infrared photosensitizer). These drug delivery systems often face challenges to their use, such as their lack of stability in physiological environments and concerns about their toxicity and bioavailability, that have hindered their translation from pre-clinical studies to clinical trials. Here, we propose a conceptual change by shifting photoswitching activity from the vehicle (nanoparticle) to the load (drug). In this “ship in a bottle” concept, the molecule to be delivered is trapped within a porous nanoparticle and its release is accomplished through a photoisomerization process. Using molecular dynamics, we designed and synthesized a photoswitchable prodrug of the antitumor drug camptothecin that contains an azobenzene functionality, and we have prepared porous silica nanoparticles with pore diameters designed to limit its release when in the trans form. Molecular modelling was used to show that the cis isomer was smaller and better able to pass through the pores than the trans isomer, which was confirmed by stochastic optical reconstruction microscopy (STORM). Thus, prodrug-loaded nanoparticles were prepared by loading the cis prodrug and then using UV irradiation to convert cis to trans isomers, trapping them, within the pores. Release of the prodrug was then accomplished by using a different UV wavelength to convert trans isomers back to cis. In this way, prodrug encapsulation and release could be achieved “on demand” through controlled cis–trans photoisomerization, which allowed the prodrug to be delivered safely and its release to be triggered precisely at the region of interest. Finally, the intracellular release and cytotoxic activity of this novel drug delivery system has been validated in several human cell lines, confirming the ability of this system to accurately control the release of the camptothecin prodrug.
Introduction
Although nanomedicines are sometimes considered the drugs of the future, few of them have been used clinically in therapy or diagnostics.1 They often face challenges that limit their translation from bench to bedside, including concerns about their stability in physiological media, selective accumulation in the region of interest (ROI), accurate and timely drug release, and elimination from the body.2–5 In terms of nanomedicines designed to deliver chemotherapeutic drugs within tumors, efficient nanoparticle penetration into the tumor is a major issue. This is because insufficient blood supply, high-density cells and extracellular matrix, and increasing of interstitial fluid pressure can hinder the diffusion of the therapeutic load within the tumor. To overcome these limitations, several strategies have been proposed, including modulation of tumor microenvironment and optimization of nanoparticle physical properties.6,7 In particular, improving the control of drug release is key to maximizing the performance of nanomedicines. To address this need, stimuli-responsive systems (SRSs) have been developed that provide accurate spatial and temporal control of molecular release, limiting undesired effects on healthy tissue and maximizing therapeutic activity in the ROIs.8,9Controlled molecular release has been accomplished in a variety of ways. For example, physical stimuli, such as magnetism, ultrasound irradiation, or light irradiation, have been used in SRSs to avoid any invasive effect over the surrounding tissue.10,11 In particular, light-driven drug delivery systems (DDSs) can provide excellent local temporal control of drug release.12 They are usually categorized into three groups: photothermal, photochemical, or photoisomerization-triggered systems.13 The last category presents the clear advantage of reversibility, potentially allowing pulsed drug release, which is highly desirable from a clinical perspective. Among the light-driven DDSs, different chemical functional groups have been used in the design of light-switchable supramolecular structures, including spiropyran derivatives,14 ligands containing 1,2-diethienylethene,15 and azobenzene derivatives.16 Indeed, azobenzene is the most frequently used photoisomerizable moiety for the development of DDSs due to its photochemical properties and ease of molecular construction. Azobenzene has two photoisomers, e.g., trans and cis isomers, that can reversibly exchange by rotation across the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond. Conversion from trans to cis conformation takes place under irradiation at wavelength of ∼350 nm, while the conversion of cis to trans occurs under irradiation at ∼450 nm. Many systems for controlled drug release have been developed using different types of azobenzene-modified nanoparticles, including polymers,17,18 liposomes,19 DNA,20 metal organic frameworks (MOFs),21 covalent organic frameworks (COFs),22 and mesoporous silica.23–25 These systems used UV irradiation, either of the azobenzene-containing substrate on its own or combined with a near-infrared (NIR) photosensitizer. The addition of a NIR component allows the use of tissue-penetrating NIR light in biological systems.
N– bond. Conversion from trans to cis conformation takes place under irradiation at wavelength of ∼350 nm, while the conversion of cis to trans occurs under irradiation at ∼450 nm. Many systems for controlled drug release have been developed using different types of azobenzene-modified nanoparticles, including polymers,17,18 liposomes,19 DNA,20 metal organic frameworks (MOFs),21 covalent organic frameworks (COFs),22 and mesoporous silica.23–25 These systems used UV irradiation, either of the azobenzene-containing substrate on its own or combined with a near-infrared (NIR) photosensitizer. The addition of a NIR component allows the use of tissue-penetrating NIR light in biological systems.
However, these DDSs are limited by the requirement to modify the nanoparticles’ surfaces with specific functionalities, resulting in complex supramolecular structures that lead to nanoparticle aggregation in protein-containing physiological environments. This in turn decreases photosensitizing efficiency, leading to undesirable drug discharge, and ultimately results in rapid plasma clearance.11 In addition, from a regulatory point of view, such hybrid materials face challenges including toxicity, bioavailability, pharmacokinetics, and others in translation from pre-clinical studies to clinical trials.5
One way to address these challenges is to shift the photoswitching activity from the vehicle (nanoparticle) to the load (drug). This strategy is especially well suited to a system using a porous nanoparticle. By tailoring the nanoparticle's pore diameter, molecular encapsulation and release is achieved “on demand” by activating and controlling cis–trans photoisomerization, provided that the stereoisomers have different molecular diameters. This “ship in a bottle” strategy (Fig. 1)26 is a concept widely used in heterogeneous catalysis27 but has been little used for drug delivery.28–30
 | ||
| Fig. 1 Artistic scheme of the “ship in a bottle” concept and cis–trans photoisomerization as a drug-controlled release mechanism. | ||
In this work, we have extended the principle of “ship in a bottle” to the intracellular delivery and photo-activated release of an antitumor drug (camptothecin, CPT) by combining it with an azobenzene group and adsorbing it within mesoporous silica nanoparticles with tailored pore diameters. First, the design of this pro-drug was optimized using molecular dynamics, and the cis and trans isomers were adsorbed into custom-made cavities of silica nanoparticles. Then, the uptake of the isomers was examined by stochastic optical reconstruction microscopy (STORM), and their release was studied using cis ← → trans isomerization under UV irradiation. Finally, the intracellular release and cytotoxic activity of this novel DDS was evaluated in several human cell lines to confirm that the photoisomerization mechanism successfully controlled CPT release.
Experimental section
Molecular dynamics
A detailed description of the methods used for molecular dynamics calculations is given in the ESI.† Animations were recorded and are also available.
Preparation of bis(camptothecin glycinate)-4,4′-dicarboxylAzoBEnzene (CAABE)
The synthesis of camptothecin-20-O-glycinate was performed according to published procedures.31 To a mixture of azobenzene-4,4′-dicarbonyl dichloride (I: 125 mg, 0.41 mmol) in anhydrous methylene chloride and dimethylformamide (48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 50 mL), camptothecin-20-O-glycinate (II: 373 mg, 0.92 mmol) and TEA (0.13 mL) were added. The reaction was stirred overnight at room temperature under inert atmosphere. The resulting suspension was filtered and the supernatant was concentrated in a rotary evaporator to obtain an orange oil. This residue was purified by two or three cycles of trituration with ethyl acetate, methanol and ether (in various combinations) to give CAABE (III) as an orange solid (yield: 32%). The CAABE synthesis scheme and characterization protocols are shown in the ESI.†
2, 50 mL), camptothecin-20-O-glycinate (II: 373 mg, 0.92 mmol) and TEA (0.13 mL) were added. The reaction was stirred overnight at room temperature under inert atmosphere. The resulting suspension was filtered and the supernatant was concentrated in a rotary evaporator to obtain an orange oil. This residue was purified by two or three cycles of trituration with ethyl acetate, methanol and ether (in various combinations) to give CAABE (III) as an orange solid (yield: 32%). The CAABE synthesis scheme and characterization protocols are shown in the ESI.†
Preparation of large pore-mesoporous silica nanoparticles (LSN)
Mesoporous silica nanoparticles with large pores (LSN) were synthesized following the low temperature method previously described by our group.4,32 Briefly, 0.25 g of F127 surfactant and 0.70 g of FC4 surfactant were dissolved in 30 mL of and aqueous solution of HCl (0.02 M). Then, 0.23 mL of 1,3,5-trimethylbenzene was added and the mixture was stirred at 10 °C for 2 h. Tetraethyl orthosilicate (TEOS, 1.6 mL) was then added to the solution and stirring was continued at 10 °C for 24 h. The final molar ratio of the reactants TEOS/F127/TMB/FC4/HCl/H2O was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0028
0.0028![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 024
024![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12
0.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08
0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 232. The reaction mixture was then transferred to an autoclave and hydrothermally treated at 135 °C (LSN-1) or 150 °C (LSN-2 and LSN-3) for 24 h. The resulting suspension was cooled, filtered, and washed with water and ethanol. In the case of LSN-3, a second hydrothermal treatment was performed. For this purpose, 0.50 g of LSN-3 was placed in an autoclave and hydrothermally treated at 140° C for 48 h. Finally, the template was removed by digestion in a microwave unit (microwave irradiation over MARS-5 at 800 W, 2245 MHz, and 220 V).33 Methods for materials characterization are described at the ESI.†
232. The reaction mixture was then transferred to an autoclave and hydrothermally treated at 135 °C (LSN-1) or 150 °C (LSN-2 and LSN-3) for 24 h. The resulting suspension was cooled, filtered, and washed with water and ethanol. In the case of LSN-3, a second hydrothermal treatment was performed. For this purpose, 0.50 g of LSN-3 was placed in an autoclave and hydrothermally treated at 140° C for 48 h. Finally, the template was removed by digestion in a microwave unit (microwave irradiation over MARS-5 at 800 W, 2245 MHz, and 220 V).33 Methods for materials characterization are described at the ESI.†
Photoisomerization, drug loading and drug release
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) was irradiated at 350 nm. Absorption spectra were recorded immediately after irradiation in a Cary 50 UV-Vis (Varian) spectrophotometer. When the trans → cis photoswitching reached equilibrium, the solution was irradiated at 450 nm and the absorption spectra corresponding to the cis → trans transformation were recorded immediately after irradiation. The photoisomerization quantum yield (R, also named photoisomerization degree at the photostationary state) was determined using eqn (1):
4 v/v) was irradiated at 350 nm. Absorption spectra were recorded immediately after irradiation in a Cary 50 UV-Vis (Varian) spectrophotometer. When the trans → cis photoswitching reached equilibrium, the solution was irradiated at 450 nm and the absorption spectra corresponding to the cis → trans transformation were recorded immediately after irradiation. The photoisomerization quantum yield (R, also named photoisomerization degree at the photostationary state) was determined using eqn (1):| R = |A0 − At|/|A0 − A∞| × 100 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) was incubated in a 1.5 mL Eppendorf tube with 5 mg of LSN (RT, 1500 rpm, 24 hours). Subsequently, the reaction mixture was centrifuged (16
4 v/v) was incubated in a 1.5 mL Eppendorf tube with 5 mg of LSN (RT, 1500 rpm, 24 hours). Subsequently, the reaction mixture was centrifuged (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100g, 12 min) and the supernatant decanted. The remaining pellet was washed with 1 mL of methanol and 1 mL of water. Adsorption of cis-CAABE into LSN took place in full darkness. A solution of cis-CAABE (1 mg mL−1) in a mixture DMSO
100g, 12 min) and the supernatant decanted. The remaining pellet was washed with 1 mL of methanol and 1 mL of water. Adsorption of cis-CAABE into LSN took place in full darkness. A solution of cis-CAABE (1 mg mL−1) in a mixture DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) was irradiated at 350 nm for two hours to insure the complete conversion of trans to cis isomer prior to adsorption. This mixture was subsequently incubated in a 1.5 mL Eppendorf tube with 5 mg of LSN (RT, 1500 rpm, 24 hours). Then, the mixture was centrifuged (16
4 v/v) was irradiated at 350 nm for two hours to insure the complete conversion of trans to cis isomer prior to adsorption. This mixture was subsequently incubated in a 1.5 mL Eppendorf tube with 5 mg of LSN (RT, 1500 rpm, 24 hours). Then, the mixture was centrifuged (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100g, 12 min) and the supernatant decanted. The remaining pellet was washed with 1 mL of methanol and 1 mL of water.
100g, 12 min) and the supernatant decanted. The remaining pellet was washed with 1 mL of methanol and 1 mL of water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100g, 12 min) and the supernatant was discarded. The solid was then re-suspended in 1 mL of Milli-Q water and incubated at 37 °C for various times. The aliquots from the incubations were centrifuged (16
100g, 12 min) and the supernatant was discarded. The solid was then re-suspended in 1 mL of Milli-Q water and incubated at 37 °C for various times. The aliquots from the incubations were centrifuged (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100g, 15 min) and the supernatants were discarded. Pellets were then incubated in a NaOH solution (0.05 N) and shaken at 1500 rpm for 3 hours at 37 °C. After decanting the supernatant, the remaining pellet was freeze-dried and then reconstituted with 1 mL of a mixture MeOH
100g, 15 min) and the supernatants were discarded. Pellets were then incubated in a NaOH solution (0.05 N) and shaken at 1500 rpm for 3 hours at 37 °C. After decanting the supernatant, the remaining pellet was freeze-dried and then reconstituted with 1 mL of a mixture MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl 95
HCl 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v). Free CPT in the solution was quantified by Reverse-Phase High Performance Liquid Chromatography (RP-HPLC). For comparison, the release of cis-CAABE was also studied, again in full darkness. cis-CAABE/LSN-1 nanoparticles (5 mg) were suspended in 1 mL of Milli-Q water and irradiated at 350 nm for two hours to insure all trans isomer was converted to cis-CAABE. The same procedure described above was then followed. RP-HPLC methods for CPT quantification are described in the ESI.†
5 (v/v). Free CPT in the solution was quantified by Reverse-Phase High Performance Liquid Chromatography (RP-HPLC). For comparison, the release of cis-CAABE was also studied, again in full darkness. cis-CAABE/LSN-1 nanoparticles (5 mg) were suspended in 1 mL of Milli-Q water and irradiated at 350 nm for two hours to insure all trans isomer was converted to cis-CAABE. The same procedure described above was then followed. RP-HPLC methods for CPT quantification are described in the ESI.†
Internal diffusion of CAABE cis/trans isomers studied by STORM
A study by Stochastic Optical Reconstruction Microscopy (STORM) was performed using LSN-1 nanoparticles after adsorption of cis-CAABE or trans-CAABE molecules. Drug loading was performed using the protocols described above. LSN-1 particles loaded with either cis or trans CAABE were excited using a 405 nm laser, and a minimum of 7500 frames were collected for each run. All molecules with a minimum peak height greater than 300 were considered for further analysis in NIS-Elements. For the data analysis and reconstruction several ROIs were created to isolate data specific to individual spheres, from which Cartesian coordinates of the detected molecules were extracted to .txt files for data reconstruction.Penetration depths of each molecule into the sphere were determined by calculating the difference between the distance of each molecule from the sphere center and the calculated radius of the sphere. Averaging the penetration depths for each respective sphere yielded the mean penetration depth. The total number of molecules within a sphere was found by counting the number of Cartesian coordinates detected by STORM. Using the transformed Cartesian coordinates of each molecule and the sphere of best fit, a 3D model of each sphere was reconstructed using Blender modeling software. A Python code was written to import the molecule coordinates and construct a wire-frame sphere to reflect what was imaged via STORM. Movies showing a full 360° view of each sphere were exported as .mp4 files. Additional details on the STORM methods are provided in the ESI.†
Biological validation
 | (2) |
 | ||
| Fig. 3 Hand-crafted irradiation camera used in the in vitro photoisomerization experiments. (a) Scheme of functional components (front view); (b) real experimental device with a 96-well plate. | ||
Finally, additional control experiments testing the biocompatibility of CAABE-free nanoparticles, with and without irradiation, were also performed. Details of these experiments are in the ESI.†
Statistics
Cell viability mean and corresponding standard error of the mean (SEM) values were determined for each experiment, and results were presented as the mean ± SEM. Data were compared by the unpaired Student's t-test. Significant differences among the groups were calculated at p < 0.05 or less. All the analysis and graphs were carried out by using GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA).Results and discussion
Molecular dynamics
Analysis of the structures obtained after 300 ps molecular dynamics simulations (Fig. 4) allowed us to plot the histograms of C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C dihedrals, to determine whether the isomeric configurations were retained during the simulation. While the trans isomer is considerably more stable and should remain structurally intact during simulation, one could expect the cis isomer to isomerize because experimental data showed that it is only stable upon irradiation. Simulation of the cis isomer was performed largely by increasing the dihedral constant to prevent relaxation to the trans isomer. Fig. 4a confirms that the isomer type was retained throughout the simulation. For the trans isomer, the C–N
N–C dihedrals, to determine whether the isomeric configurations were retained during the simulation. While the trans isomer is considerably more stable and should remain structurally intact during simulation, one could expect the cis isomer to isomerize because experimental data showed that it is only stable upon irradiation. Simulation of the cis isomer was performed largely by increasing the dihedral constant to prevent relaxation to the trans isomer. Fig. 4a confirms that the isomer type was retained throughout the simulation. For the trans isomer, the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C dihedral angle showed maxima at 180° and −180°, ±30°. However, the cis isomer did not have the anticipated maximum at a dihedral angle of 0°, due to short-range repulsions between molecule moieties at either side of the C–N
N–C dihedral angle showed maxima at 180° and −180°, ±30°. However, the cis isomer did not have the anticipated maximum at a dihedral angle of 0°, due to short-range repulsions between molecule moieties at either side of the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C dihedral. Instead, maxima at ∼15° and −15° are found with symmetric distributions for each cis-molecule.
N–C dihedral. Instead, maxima at ∼15° and −15° are found with symmetric distributions for each cis-molecule.
Considerable folding was observed for each molecule during the 300 ps of simulation time. A large number of configurations are possible, most of them allowed by the small energetic penalty associated with many of the dihedral angles present in the molecule. This strongly influenced the effective size of the molecule which, in turn, limited its ability to enter the pores of LSN. To study the effective molecular size, several interatomic distances were defined in each molecule using a program to identify the two most distant pairs of atoms in the molecule during the molecular dynamics simulation (Fig. S1, ESI†). The corresponding histograms of maximum distances are shown in Fig. 4b. For all sets of dimers studied (that is, 0, 1, or 2 CH2 groups in the CPT-azobenzene link), the effective molecular size was larger in the trans isomer than in the corresponding cis isomer, and the size difference between isomers became smaller in the series (CH2)n as n increased from 0 to 2. This was due to the fact that the larger molecules, with more degrees of freedom, have a larger capability to fold, so the larger initial size of the trans isomer can be reduced by rotation around an increasing number of dihedrals. When considering molecular design parameters for the ship-in-a-bottle concept, the absolute molecular sizes of the isomers and the difference in size are both important. Fig. 4b shows that the average molecular sizes and differences (in Å), respectively for cis/trans are: 18.6/28.7, Δ = 10.1 (n = 0); 15.8/20.5, Δ = 4.7 (n = 1), and 15.5/18.2, Δ = 2.7 (n = 2).
Movies of molecular structure changes during the simulations (Videos SV2–SV7, ESI†) showed that the molecules were partially unfolded only during the initial stages of the simulations. As the simulations progressed, the conformational energy can be reduced through van der Waals interactions, and folding occurred. Folding was always more effective for the cis isomers, although at n = 2 the difference between cis and trans isomers was almost negligible. As a consequence, the effective molecular size of the cis isomers was always smaller than the trans isomers. Fig. 4c highlights this conclusion, where the molecular size at each conformation was plotted during the simulation time.
Preparation of CAABE and photoisomerization
The key parameters in the selection of the CAABE derivative to be used in our drug delivery system are molecular size and degrees of freedom. In this context, the derivative with (CH2)n where n = 0 appeared to be the best candidate to perform the photoisomerization and loading/release assays with nanoparticles. Unfortunately, all attempts to synthesize the n = 0 CAABE derivative were fruitless, as this compound is quite sterically hindered. However, synthesis of CAABE with n = 1 and n = 2 was successful. The synthetic steps and chemical structure of the n = 1 compound are shown in Fig. S2.† CPT was substituted at 20-OH position with glycine through an ester bond,31 and the resulting molecule was covalently attached to diazobenzene through amide bond formation between the glycine and acid chlorides on the diazobenzene. The 1H-NMR spectrum of trans-CAABE (Fig. S3†) shows the protons of the diazobenzene group as a multiplet along with CPT protons around 7.94 and 8.16 ppm. Peaks in the 13C-NMR spectrum at 167.05 and 165.85 ppm (Fig. S4†) were assigned, respectively, to the ester bond between the glycine and the CPT hydroxyl and to the amide bond between CPT and diazobenzene (Fig. S2†). In the 2D-COSY spectra (Fig. S5†), CAABE was identified by the signal of the glycine methylene protons between 4.14 and 4.47 ppm and their correlation with the proton of the amide group at 9.32 ppm. On the other hand, CAABE HSQC spectrum (Fig. S6†) allowed the chemical shift of each proton to be directly correlated to the chemical displacement of its corresponding carbon. Finally, the molecular weight of CAABE was confirmed by high resolution mass spectrometry (HRMS, Fig. S7†). In addition, for the purpose of comparison, the most relevant structural features of the n = 2 derivative (CAABE-2) are shown at the revised ESI (Fig. S8–S10†).The photoswitching property of diazobenzenes between cis and trans isomers is well known.34–36 In these experiments, the photoisomerization of CAABE was confirmed by UV-Vis spectroscopic studies in solution. The spectrum of trans-CAABE before irradiation (Fig. 5a) featured a band at 347 nm (π–π* transition) and a weak band at 444 nm (n–π* transition). Upon UV light irradiation (350 nm), the absorption band at 344 nm gradually decreased while a band at 450 nm increased, indicating CAABE trans → cis photoisomerization. These spectral changes could be reversed by irradiation at 450 nm, indicating CAABE cis → trans photoisomerization (Fig. 5b) and reversibility. In the isomerization under UV light there were two isosbestic points at 347 and 394 nm, while in the process under visible light the only isosbestic point emerged at 394 nm.37
The photoisomerization quantum yield (R) of the photoisomerization process was determined by irradiating CAABE for various lengths of time and performing the calculation shown in eqn (3). Upon irradiation at 350 nm, the equilibrium absorbance (A∞) corresponding to completion of photoswitching was reached at 116 min; when 450 nm light was used, photoswitching was completed at 10 min (Fig. S11, ESI†). This can be explained by the faster kinetics of cis → trans compared to the trans → cis transformation, which is consistent with the greater stability of the trans isomer.
Preparation of LSN materials, drug loading and drug release
LSN with tailored pore entrances and internal cavities were obtained by a low temperature method and using a dual surfactant system (Pluronic F127 and FC4).4,32,38 The most significant physico-chemical properties of these materials are summarized in Table 1.| Sample | Particle sizea (nm) | Cavity sizeb (nm) | Entrance sizec (nm) | S BET (m2 g−1) | V p (cm3 g−1) |
|---|---|---|---|---|---|
| a Determined from TEM measurements over a minimum of 100 particles. b Calculated from the adsorption curve of the N2 sorption isotherm, based on the BJH method. c Calculated from the desorption curve of the N2 sorption isotherm, based on the BJH method. | |||||
| LSN-1 | 149.6 ± 46.7 | 15.5 | 4.2 | 616.9 | 0.85 |
| LSN-2 | 171.4 ± 61.4 | 13.6 | 7.2 | 429.2 | 0.80 |
| LSN-3 | 160.6 ± 75.9 | 13.8 | 12.0 | 292.6 | 0.81 |
N2 physisorption measurements showed type IV adsorption–desorption isotherms with an H2-type hysteresis loop in LSN-1 and LSN-2. LSN-3 showed an H3-type hysteresis loop as a consequence of the dramatic enlargement of the entrance size (Fig. S12†). As expected, BET surface area decreased with the enlargement of pore diameter. Transmission electron microscopy (TEM) images of LSN materials showed in all cases highly monodisperse nanoparticles (Fig. 6).4 Particle size distribution ranged from 70 to 300 nm, with a similar mean size in the three samples (149–171 nm). The particle diameters measured by dynamic light scattering (DLS) were slightly larger than those observed by TEM, indicating the presence of some particle dimers and oligomers in the suspension (see ESI and Fig. S13†).
 | ||
| Fig. 6 TEM images of large pore mesoporous silica nanoparticles with different pore diameter shown in the insets. (a) LSN-1. (b) LSN-2. (c) LSN-3. | ||
Drug loading is a key step in the development of novel nanomedicines, as it determines both the intrinsic activity of the drug delivery system and its ability to deliver its payload to the target cells while avoiding nonspecific release.2 In the case of porous materials, synthetic control of the pore diameter is crucial to optimizing the loading and release rates of the delivered drug, as has been previously reported for mesoporous silica.39 This is extremely important in the case of CPT, as a variety of negative effects (low water solubility, pronounced loss of activity from lactone-ring hydrolysis, and severe toxicity), strongly limit its clinical use.40 In this study we have designed LSN materials to enhance CAABE loading by modifying the pore entrances of mesoporous silica nanoparticles. As described above, according to molecular dynamics calculations the average molecular size ratio of the cis/trans isomers of CAABE ((CH2)n, n = 1) was 15.8/20.5 (Å). On this basis, both isomers could be expected to diffuse within the internal cavities of the LSN, where the pore diameter was 13 to 15 Å. However, pore entrances should impose a bottleneck (diameters 4–7 nm for LSN-1 and −2, Table 1) that could be used to discriminate cis and trans isomers based on their rates of diffusion. Here, LSN were incubated with a solution of either cis or trans CAABE with (CH2)1 and the amount of adsorbed compound was determined by elemental analysis. The results (Table 2) show that LSN-1, with the smallest pore entrance, adsorbed nearly 7 times as much of the cis isomer as the trans isomer. In contrast, LSN-2 and -3, with larger pore entrances, allowed more diffusion of the trans isomer leading to equal or nearly equal adsorption of both isomers. The smaller mass of both CAABE isomers adsorbed by LSN-3 is related to the lower surface area of this material.
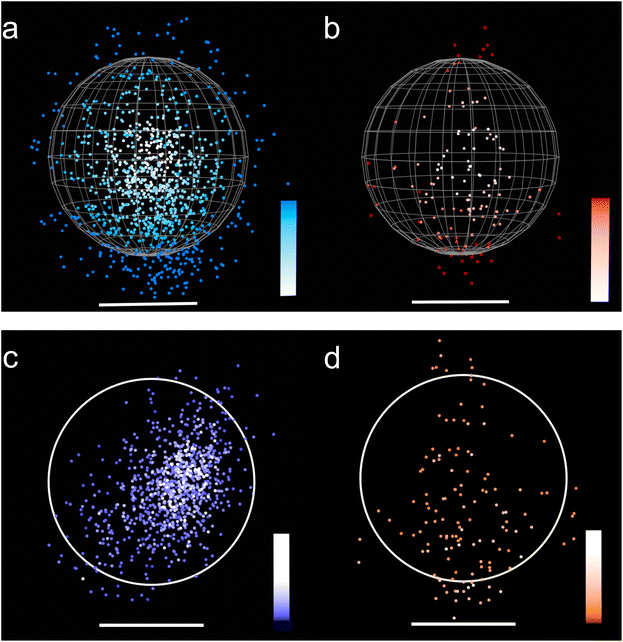
The ability of LSN-1 particles to adsorb more of the cis-CAABE isomer with (CH2)n (n = 1) was also confirmed by stochastic optical reconstruction microscopy (STORM). In this technique, photoswitchable fluorophores are manipulated to stochastically turn on and off, and the STORM instrument records these “blinks” through numerous cycles. Because the position information of each fluorophore is collected in a database, reconstruction of the image and calculations based on the position information are possible. We recently demonstrated the utility of STORM in addressing questions about molecular adsorption in porous materials. In that work, we used STORM to compare the penetration depth of fluorescently labelled proteins with different sizes within silica nanoparticles that had pores with the same diameter. Here, we apply STORM to investigate the penetration depth of the cis/trans-CAABE isomers within LSN-1 particles.
LSN-1 particles were loaded with CAABE isomers by stirring the particles in a solution containing 1 mg mL−1 CAABE and 5 mg mL−1 LSN-1 nanoparticles. Upon excitation at 405 nm in the STORM instrument, emission of the isomers was observed and collected. Several particles were selected for analysis and fluorophore information associated with the particles were extracted as Cartesian coordinates. This information was used to calculate the sphere of best fit in each case, by minimizing the function shown in eqn (SE3) in the ESI.† This allowed us to identify the relative locations of each CAABE molecule within the particle. With this information, reconstructed images were produced from the STORM shown in Fig. 7a (cis) and Fig. 7b (trans). It is qualitatively apparent from these images that a larger amount of the cis CAABE isomer was present in the LSN-1 particles than the trans isomer. Another view of the information (Fig. 7c and d) was created by taking an optical slice through the data set, and flattening the information into one layer by plotting as a function of x and y. Both views confirm that significantly more of the cis isomer was found within the nanoparticle than the trans isomer. Quantitative analysis, summarized in Table 3, was also performed by summing the number of molecules identified in the STORM analysis within the calculated perimeter of the sphere. Five times as many fluorophores were identified in LSN-1 particles loaded with the cis isomer compared to the trans isomer. Additional movies showing this fact are provided at the ESI (Videos SV8–SV11†). These results are similar to our previous experiments using proteins of various sizes to examine adsorption within porous silica nanoparticles.41 We found a close relationship between the pore diameters of the nanoparticles and the sizes of the proteins found in their adsorbed protein coronas. In these studies, the smaller diameter of the cis isomer allows it to penetrate deeper into the LSN-1 particles.
| cis-CAABE loaded in LSN-1 | trans-CAABE loaded in LSN-1 | |
|---|---|---|
| Fluorophore count | 984 | 194 |
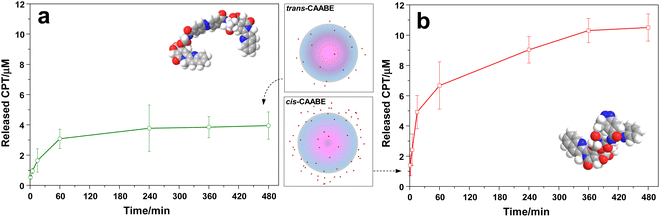
Using drug loading and internal diffusion data from elemental analysis and STORM, we then investigated the controlled release of CAABE from LSN-1 in water, triggered by UV-Visible light irradiation. For these studies, drug-loaded LSN-1 were irradiated at either 350 nm for two hours (for release of the cis isomer) or 450 nm for one hour (for release of the trans isomer), and CPT concentration in solution was measured by RP-HPLC.
Fig. 8 highlights the impact of the LSN-1 pore structure on the diffusion of cis vs. trans CAABE isomers. From the start of the experiment, trans isomer is clearly released at a slower rate than the cis isomer, and the maximum amount that can be released is nearly complete after only one hour. In contrast, the cis isomer showed a faster initial release rate than the trans isomer, and the amount released continued to increase for more than six hours. The total amount of CAABE released was approximately three times higher for the cis isomer. According to STORM data, the release of the cis isomer is expected to occur from the whole mesoporous silica matrix, whereas trans-CAABE should diffuse outside the particle only from the most external layers, with the majority of the drug loading remaining at the core (Fig. 8). This highlights the effectiveness of the “ship in a bottle” mechanism in establishing control over release of the CAABE dimer through photoisomerization, leading to the potential application of this system in biological medium. A former approach to the loading of topotecan into a mesoporous structure by a “ship in a bottle” mechanism was carried out in MIL-100 nanoMOFs, although in this case drug liberation was not subjected to specific stimuli.28 To the best of our knowledge, this is first description of a DDS linking “ship in a bottle” drug loading and cis ← → trans photoisomerization-driven release.
The release patterns of the cis and trans CAABE diastereoisomers from LSN-1 fit very well into Higuchi's square root model shown in eqn (3) (Table 4, and Fig. S14†).
| X = kH·t½ | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
In this equation, X, t, and kH are, respectively, the fraction of discharged drug, release time, and the kinetic constant.42,43 Here, the comparison of kH values for the cis- and trans-CAABE showed the same ratio observed at the end of liberation process (kHcis ∼ 3 fold kHtrans). This means that diffusion plays the dominant role in defining the release rate, with very little contribution from material degradation. This is consistent with the low water solubility of CAABE and the stability of the silica matrix in aqueous conditions.44
Biological validation
The potential therapeutic use of the photo-activated DDS described above was evaluated using three commercial cancer cell lines (SH-SY5Y, U251, HeLa) with similar growth rates (doubling times of 25–30 h),45–47 to avoid inconsistencies related with the overgrowth of an specific cell line after irradiation. CAABE and CPT cytotoxicity was validated using the MTS assay in HeLa cells (Fig. S15†), showing similar IC50 values, which confirms full hydrolysis of the prodrug into the cytosol through enzymatic activity.40 The biocompatibility of LSN-1 was tested in all cell lines in a wide range of concentrations (Fig. S16†), with no significant cytotoxicity observed under our experimental conditions. We also confirmed that CAABE-loaded LSN-1 is not cytotoxic during the first 24 h incubation step (i.e., prior to irradiation) by repeating the MTS assay in each cell line and using CAABE/LSN-1 concentrations up to the maximum amount used in the irradiation experiments (0.48 μg mL−1 prodrug equivalent concentration). No significant decrease in cell viability was observed in these control experiments (Fig. S17†). This is because trans-CAABE is trapped within LSN prior to UV irradiation (Fig. 8). Moreover, we previously showed that CPT-loaded silica nanoparticles induce cell cycle arrest in G2/M phase in response to DNA damage comparable to that observed with the naked drug. This cytotoxicity mechanism requires a longer incubation time (about 48–72 h) to initiate cell apoptosis.48 In addition, some long-term in vivo studies have shown that silica nanoparticles may lead to chronic toxicity, mostly liver inflammation.49 Even in those studies, however, mesoporous silica nanoparticles showed a safer toxicological profile than solid, Stöber-like, silica nanoparticles due to their higher hydrolytic susceptibility and faster plasma clearance. Our data confirmed their potential use in clinical translation as drug delivery vehicles.Fig. 9 shows quantitative measurements of cell viability using the MTS assay after 24 h incubation with CAABE-loaded LSN-1 at two different prodrug equivalent concentrations (0.16 or 0.48 μg mL−1), illumination at 350 nm (2.2 mW cm−2) for 30 or 60 min, and a subsequent rest in the incubator for an additional 72 h.
The neuroblastoma SH-SY5Y cell line showed a significant drop in cell viability at all conditions, consistent with its high sensitivity to CPT cytotoxicity. SH-SY5Y is a noradrenergic cell line and CPT-induced DNA damage is dramatically high; it also exhibits severe repair deficiency.50 Irradiation with UV light activated CAABE trans → cis photoswitching, promoting prodrug release and increasing CPT release within cells, but in SH-SY5Y cells only a long exposure time (60 min) and high prodrug loading (0.48 μg mL−1) showed a statistically significant effect. Conversely, in the case of U251 and HeLa cells, cell death induced by photocontrolled release of CAABE was dramatic, and statistically a significant decrease in cell survival was observed after 30 min of photoexcitation even at a low prodrug concentration (0.16 μg mL−1). In the most noticeable case, HeLa cells exposed to CAABE-loaded LSN-1 at a prodrug dose of 0.48 μg mL−1 showed reduced viability of approximately 50% after 60 min of irradiation, with a final cell survival of ∼30%.
Conclusion
In this study, we have applied the “ship in a bottle” principle to a drug delivery system. By synthesizing photoswitchable prodrugs and precisely tailoring the pore structures of mesoporous silica nanoparticles, drug loading and release was controlled through photoisomerization. This proof-of-concept study was developed in three stages: (i) molecular design and synthesis of a CPT prodrug; (ii) tailoring the pore structure of porous silica nanoparticles for controlled prodrug loading and release; and (iii) in vitro validation of the DDS in cancer cells. The azobenzene moiety requires the use of UV light, with lower penetration in tissue efficiency, which has potential at in vivo applications over skin cancer. However, irradiation in the UVA-I spectrum has a good safety profile, and it may be possible to treat internal tumors using an endoscope particularly considering the high degree of specificity for this method.Author contributions
Eva Rivero-Buceta: investigation, writing original-draft, methodology, validation. Mirela E. Encheva: methodology, validation. Bradley Cech: methodology, validation. Eduardo Fernandez: supervision. Germán Sastre: supervision, investigation, writing original-draft, methodology, validation. Christopher C. Landry: supervision, investigation, writing – original draft, writing – review and editing. Pablo Botella: project administration, supervision, investigation, writing – original draft, writing – review and editing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation (grant number PID2019-111436RB-C21). The authors also acknowledge the Microscopy Imaging Center at the University of Vermont and Dr Victoria Moreno (Centro de Investigación Príncipe Felipe, Valencia, Spain) for her technical support in the in vitro studies.References
- X. Shan, X. Gong, J. Li, J. Wen, Y. Li and Z. Zhang, Acta Pharm. Sin. B, 2022, 12, 3028–3048 CrossRef CAS PubMed.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed.
- J. Cao, D. Huang and N. A. Peppas, Adv. Drug Delivery Rev., 2020, 167, 170–188 CrossRef CAS PubMed.
- F. Gao, P. Botella, A. Corma, J. Blesa and L. Dong, J. Phys. Chem. B, 2009, 113, 1796–1804 CrossRef CAS PubMed.
- A. Corma, P. Botella and E. Rivero-Buceta, Pharmaceutics, 2022, 14, 110 CrossRef CAS PubMed.
- J. Ding, J. Chen, L. Gao, Z. Jiang, Y. Zhang, M. Li, Q. Xiao, S. S. Lee and X. Chen, Nano Today, 2019, 29, 100800 CrossRef CAS.
- J. Chen, Z. Jiang, Y. S. Zhang, J. Ding and X. Chen, Appl. Phys. Rev., 2021, 8, 041321 CAS.
- M. Vallet-Regí, F. Schüth, D. Lozano, M. Colilla and M. Manzano, Chem. Soc. Rev., 2022, 51, 5365–5451 RSC.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2014, 13, 813–827 CrossRef CAS PubMed.
- F. C. Lin, Y. Xie, T. Deng and J. I. Zink, J. Am. Chem. Soc., 2021, 143, 6025–6036 CrossRef CAS PubMed.
- P. Pan, D. Svirskis, S. W. P. Rees, D. Barker, G. I. N. Waterhouse and Z. Wu, J. Controlled Release, 2021, 338, 446–461 CrossRef CAS PubMed.
- N. K. Mal, M. Fujiwara and Y. Tanaka, Nature, 2003, 421, 350–353 CrossRef CAS PubMed.
- Y. Tao, H. F. Chan, B. Shi, M. Li and K. W. Leong, Adv. Funct. Mater., 2020, 30, 1–28 Search PubMed.
- N. Aibani, P. F. da Costa, J. Masterson, N. Marino, F. M. Raymo, J. Callan and B. Callan, J. Controlled Release, 2017, 264, 136–144 CrossRef CAS PubMed.
- A. Presa, R. F. Brissos, A. B. Caballero, I. Borilovic, L. Korrodi-Gregório, R. Pérez-Tomás, O. Roubeau and P. Gamez, Angew. Chem., Int. Ed., 2015, 54, 4561–4565 CrossRef CAS PubMed.
- D. F. Costa, L. P. Mendes and V. P. Torchilin, Adv. Drug Delivery Rev., 2019, 138, 105–116 CrossRef CAS PubMed.
- T. Zhao, P. Wang, Q. Li, A. A. Al-Khalaf, W. N. Hozzein, F. Zhang, X. Li and D. Zhao, Angew. Chem., Int. Ed., 2018, 57, 2611–2615 CrossRef CAS PubMed.
- H. Deng, L. Lin, S. Wang, G. Yu, Z. Zhou, Y. Liu, G. Niu, J. Song and X. Chen, Adv. Mater., 2019, 31, 1–8 Search PubMed.
- C. Yao, P. Wang, X. Li, X. Hu, J. Hou, L. Wang and F. Zhang, Adv. Mater., 2016, 28, 9341–9348 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, G. Song, Y. He, X. Zhang, Y. Liu and H. Ju, Angew. Chem., Int. Ed., 2019, 58, 18207–18211 CrossRef CAS PubMed.
- J. W. Brown, B. L. Henderson, M. D. Kiesz, A. C. Whalley, W. Morris, S. Grunder, H. Deng, H. Furukawa, J. I. Zink, J. Fraser and O. M. Yaghi, Chem. Sci., 2013, 4, 2858–2864 RSC.
- G. Das, T. Prakasam, M. A. Addicoat, S. K. Sharma, F. Ravaux, R. Mathew, M. Baias, R. Jagannathan, M. A. Olson and A. Trabolsi, J. Am. Chem. Soc., 2019, 141, 19078–19087 CrossRef CAS PubMed.
- D. Tarn, D. P. Ferris, J. C. Barnes, M. W. Ambrogio, J. F. Stoddart and J. I. Zink, Nanoscale, 2014, 6, 3335–3343 RSC.
- S. Angelos, Y. W. Yang, N. M. Khashab, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 11344–11346 CrossRef CAS PubMed.
- J. Lu, E. Choi, F. Tamanoi and J. I. Zink, Small, 2008, 4, 421–426 CrossRef CAS PubMed.
- T. Kusukawa and M. Fujita, J. Am. Chem. Soc., 1999, 121(6), 1397–1398 CrossRef CAS.
- A. Corma and H. Garcia, Eur. J. Inorg. Chem., 2004, 6, 1143–1164 CrossRef.
- M. R. Di Nunzio, V. Agostoni, B. Cohen, R. Gref and A. Douhal, J. Med. Chem., 2014, 57, 411–420 CrossRef CAS PubMed.
- J. Xu, L. Wu, T. Guo, G. Zhang, C. Wang, H. Li, X. Li, V. Singh, W. Chen, R. Gref and J. Zhang, Int. J. Pharm., 2019, 556, 89–96 CrossRef CAS PubMed.
- S. Lu, D. Tu, X. Li, R. Li and X. Chen, Nano Res., 2016, 9, 187–197 CrossRef CAS.
- R. B. Greenwald, A. Pendri, C. D. Conover, C. Lee, Y. H. Choe, C. Gilbert, A. Martinez, J. Xia, D. Wu and M.-M. Hsue, Bioorg. Med. Chem., 1998, 6, 551–562 CrossRef CAS PubMed.
- J. Fan, C. Yu, J. Lei, Q. Zhang, T. Li, B. Tu, W. Zhou and D. Zhao, J. Am. Chem. Soc., 2005, 127, 10794–10795 CrossRef CAS PubMed.
- B. Tian, X. Liu, C. Yu, F. Gao, Q. Luo, S. Xie, B. Tu and D. Zhao, Chem. Commun., 2002, 2, 1186–1187 RSC.
- E. Merino and M. Ribagorda, Beilstein J. Org. Chem., 2012, 8, 1071–1090 CrossRef CAS PubMed.
- A. Koteja, M. Szczerba and J. Matusik, J. Phys. Chem. Solids, 2017, 111, 294–303 CrossRef CAS.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- K. Ichimura, Phys. Chem. Chem. Phys., 2015, 17, 2722–2733 RSC.
- L. Huang, X. Yan and M. Kruk, Langmuir, 2010, 26, 14871–14878 CrossRef CAS PubMed.
- P. Horcajada, A. Rámila, J. Pérez-Pariente and M. Vallet-Regí, Microporous Mesoporous Mater., 2004, 68, 105–109 CrossRef CAS.
- P. Botella and E. Rivero-Buceta, J. Controlled Release, 2017, 247, 28–54 CrossRef CAS PubMed.
- A. M. Clemments, P. Botella and C. C. Landry, J. Am. Chem. Soc., 2017, 139, 3978–3981 CrossRef CAS PubMed.
- T. Higuchi, J. Pharm. Sci., 1963, 52, 1145–1149 CrossRef CAS PubMed.
- F. Li, L. Jin, J. Han, M. Wei and C. Li, Ind. Eng. Chem. Res., 2009, 48, 5590–5597 CrossRef CAS.
- E. B. Lim, T. A. Vy and S. W. Lee, J. Mater. Chem. B, 2020, 8, 2096–2106 RSC.
- S. Feles, C. Overath, S. Reichardt, S. Diegeler, C. Schmitz, J. Kronenberg, C. Baumstark-Khan, R. Hemmersbach, C. E. Hellweg and C. Liemersdorf, Methods Protoc., 2022, 5, 1–14 Search PubMed.
- A. M. Luciani, A. Rosi, P. Matarrese, G. Arancia, L. Guidoni and V. Viti, Eur. J. Cell Biol., 2001, 80, 187–195 CrossRef CAS PubMed.
- D. W. Lee, M. Y. Lee, B. Ku and D. H. Nam, J. Biomol. Screening, 2015, 20, 1178–1184 CrossRef PubMed.
- P. Botella, I. Abasolo, Y. Fernández, C. Muniesa, S. Miranda, M. Quesada, J. Ruiz, S. Schwartz and A. Corma, J. Controlled Release, 2011, 156, 246–257 CrossRef CAS PubMed.
- R. Mohammadpour, D. L. Cheney, J. W. Grunberger, M. Yazdimamaghani, J. Jedrzkiewicz, K. J. Isaacson, H. Dobrovolskaia and M. A. Ghandehari, J. Controlled Release, 2020, 324, 471–481 CrossRef CAS PubMed.
- Y. Wang, P. R. Musich, K. Cui, Y. Zou and M. Y. Zhu, Neurotoxic. Res., 2015, 27, 368–383 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, including molecular dynamics methodology, prodrug synthesis and characterization, materials synthesis and characterization, drug loading and release, STORM methods, irradiation camera technology and biological validation. See DOI: https://doi.org/10.1039/d3nr00642e |
| This journal is © The Royal Society of Chemistry 2023 |