Open-circuit voltage loss in perovskite quantum dot solar cells
Abstract
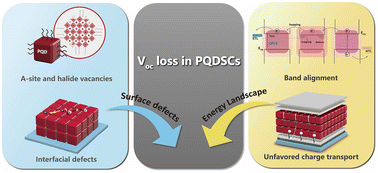
Perovskite quantum dots are a competitive candidate for next-generation solar cells owing to their superior phase stability and multiple exciton generation effects. However, given the voltage loss in perovskite quantum dot solar cells (PQDSCs) is mainly caused by various surface and interfacial defects and the energy band mismatch in the devices, tremendous achievements have been made to mitigate the Voc loss of PQDSCs. Herein, we elucidate the potential threats that hinder the high Voc of PQDSCs. Then, we summarize recent progress in minimizing open-circuit voltage (Voc) loss, including defect manipulation and device optimization, based on band-alignment engineering. Finally, we attempt to shed light on the methodologies used to further improve the performance of PQDSCs.
- This article is part of the themed collections: Recent Review Articles and Halide Perovskite Optoelectronics


 Please wait while we load your content...
Please wait while we load your content...