All-printed and stretchable organic electrochemical transistors using a hydrogel electrolyte†
Abstract
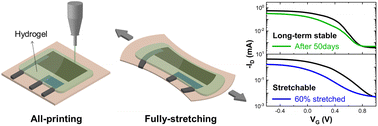
Stretchable electronic devices are expected to play an important role in wearable electronics. Solution-processable conducting materials are desirable because of their versatile processing. Herein, we report the fabrication of fully stretchable organic electrochemical transistors (OECTs) by printing all components of the device. To achieve the stretchability of the whole body of the devices, a printed planar gate electrode and polyvinyl alcohol (PVA) hydrogel electrolyte were employed. Stretchable silver paste provided a soft feature to drain/source, gate and interconnect, without any additional strategies needed to improve the stretchability of the metallic components. The resulting OECTs showed a performance comparable to inkjet or screen-printed OECTs. The maximum transconductance and on/off ratio were 1.04 ± 0.13 mS and 830, respectively. The device was stable for 50 days and stretched up to 110% tensile strain, which makes it suitable for withstanding the mechanical deformation expected in wearable electronics. This work paves the way for all-printed and stretchable transistors in wearable bioelectronics.
- This article is part of the themed collections: Nanoscale Most Popular 2023 Articles and Nanomaterials for printed electronics


 Please wait while we load your content...
Please wait while we load your content...