 Open Access Article
Open Access ArticleTuning the carrier injection barrier of hybrid metal–organic interfaces on rare earth-gold surface compounds†
R.
Castrillo-Bodero
 a,
M.
Blanco-Rey
a,
M.
Blanco-Rey
 bc,
K.
Ali
bc,
K.
Ali
 ace,
J. E.
Ortega
ace,
J. E.
Ortega
 dac,
F.
Schiller
dac,
F.
Schiller
 ac and
L.
Fernández
ac and
L.
Fernández
 *a
*a
aCentro de Física de Materiales CSIC-UPV/EHU-Materials Physics Center, 20018 San Sebastián, Spain. E-mail: lauraisabel.fernandez@ehu.es
bUniversidad del País Vasco UPV/EHU, Dpto. de Polímeros y Materiales Avanzados: Física, Química y Tecnología, 20018 San Sebastián, Spain
cDonostia International Physics Center, 20018 Donostia-San Sebastián, Spain
dUniversidad del País Vasco UPV/EHU, Dpto. Física Aplicada I, 20018 San Sebastián, Spain
eChalmers University of Technology, Chalmersplatsen 4, Götenborg, 41296, Sweden
First published on 1st February 2023
Abstract
Magnetic hybrid metal–organic interfaces possess a great potential in areas such as organic spintronics and quantum information processing. However, tuning their carrier injection barriers on-demand is fundamental for the implementation in technological devices. We have prepared hybrid metal–organic interfaces by the adsorption of copper phthalocyanine CuPc on REAu2 surfaces (RE = Gd, Ho and Yb) and studied their growth, electrostatics and electronic structure. CuPc exhibits a long-range commensurability and a vacuum level pinning of the molecular energy levels. We observe a significant effect of the RE valence of the substrate on the carrier injection barrier of the hybrid metal–organic interface. CuPc adsorbed on trivalent RE-based surfaces (HoAu2 and GdAu2) exhibits molecular level energies that may allow injection carriers significantly closer to an ambipolar injection behavior than in the divalent case (YbAu2).
1. Introduction
Organic semiconducting molecules are an excellent and promising alternative for the development of molecular spintronic based devices.1 They show unique properties, such as strong responses to electrical and optical stimuli, and intrinsic physical and chemical functionality,2–4 which are not usually present in inorganic materials. Organic spintronics pave the way for the development of cleaner, cheaper and efficient technological devices with novel and versatile capabilities. The functionality of such devices is based on the generation, transport and detection of spin-polarized carriers across the hybrid interface between a ferromagnetic metal and an organic semiconductor. That is the so-called spinterface, which is key for the spin injection into the organic molecules. There are two possible scenarios of ferromagnetic metal–organic interaction at the interface. On the one hand, a strong electron hybridization can produce a new interfacial spin-polarized hybrid electronic state that facilitates spin selectivity. 3d transition metal phthalocyanines (Pc),5–7 Alq3,8 as well as C60 on 3d ferromagnetic surfaces9,10 are representative systems featuring such type of spinterface. On the other hand, a weak interaction between the organic and ferromagnetic components, namely physisorption driven by van der Waals forces, can leave the electronic properties of the molecule almost intact. This latter case is an advantageous scenario for more versatile devices that integrate spintronics and optoelectronics functionalities, where optimized injection barriers and carrier mobilities need to be taken into consideration during the design process.11,12In this work, we study the interfacial electronic structure between a long-range ordered copper phthalocyanine (CuPc) monolayer (ML) and the family of two-dimensional (2D) REAu2 surface compounds, where RE is a rare earth species.13–15 The adsorbed molecule CuPc is a chemically stable prototypical p-type semiconductor. It is known to have an unpaired electron leading to an open-shell electronic structure on many different surfaces, such as noble metals,16–21 graphene,22 topological insulators21,23 and some semimetals.21,24 Over the recent years, REAu2 and REAg2 surface alloys have become highly valued for their rich magnetic behavior, which originates from the interplay of 4f magnetism, substrate-mediated ferromagnetism, spin–orbit interaction and valence state effects on their characteristic 2D electron band structure.25–32 Moreover, REAu2 surfaces have been probed as excellent templates of several materials from monodomain ferromagnetic nanodots25,26,28 to molecules,27,33 including on-surface polymerized organic complexes.34,35 Nevertheless, their performance in spinterfaces remains unknown.
We adsorb CuPc monolayers on YbAu2, GdAu2 and HoAu2 surfaces as case studies and analyze the structural properties by scanning tunneling microscopy (STM) and low energy electron diffraction (LEED). Furthermore, by means of photoemission techniques we obtain direct information on the metal-band/molecular orbital alignment, work functions and surface dipoles. These properties turn out to non-trivially depend on the RE species of the substrate. Particularly, the RE valence (divalent Yb, trivalent Gd and Ho31) seems to be key for the energy level alignment of metal and organic molecule. This situation opens up the design of hybrid interfaces on demand using the RE valence as tuning factor. Indeed the valence may influence in the type of carriers injected (electrons/holes) or even approximate to an ambipolar carrier injection, i.e. the simultaneous injection of electrons and holes, which facilitates an effective recombination of the carriers in the active organic layer and the further production of light.36–38
In order to characterize comprehensively our interfaces and the effect of the RE atoms on their electronic structure, we resort to density-functional theory (DFT) calculations. This method confirms the physisorption of CuPc on REAu2, despite the relatively high amount of RE atoms in these surface compounds. Usually, the energy alignment of the organic molecular orbitals with the metal bands at the interface is subject to charge transfer, hybridization and metal substrate polarization effects. However, in the absence of a net charge transfer, like in the present studied case, the surface dipole developed upon adsorption is ascribed to the so-called push-back effect,39–41 which is related to polarization and can be interpreted as the Pauli repulsion between the electron clouds of the metallic surface and the molecule.42–45 The interaction with the substrate, even if it is weak, leads to the renormalization of the molecular levels, inducing a reduction of the ionization potential, electron affinity, and the HOMO–LUMO gap.46,47 While such electronic structure changes are detectable by photoemission, they are a challenge for DFT-based theoretical methods. In the latter case, corrections are required to overcome self-interaction errors (SIE)48 to approximately account for the many-body dynamical polarization effects49 that take place at the CuPc/REAu2 interfaces.
2. Experimental and theory section
2.1. Experimental
Samples have been prepared in an ultra-high vacuum (UHV) chamber at a base pressure of 2 × 10−10 mbar. A Au(111) single crystal was used as substrate, that was cleaned by cycles of Ar+ ion sputtering (Ekin = 1 keV) and annealing to 500 °C. The different REAu2 surface compounds were grown in situ by evaporation of small amounts of RE atoms on the Au(111) surface, held at a fixed temperature. The optimal growth temperature of the substrate is of 300 °C for YbAu2 and varies between 400–450 °C for GdAu2 and HoAu2. Below these temperatures the characteristic moiré pattern was not well formed, and above them the RE metals diffuse into the bulk or re-evaporate from the surface. CuPc molecules have been evaporated on the REAu2 surface with deposition rates of 0.05 ML min−1. The completion of one monolayer (ML) is probed carefully using LEED and STM. The substrate temperature during CuPc evaporation was set to room temperature.Preparation and sample analysis was mainly performed in the laboratories of the Material Physics Center of San Sebastian. X-ray photoelectron spectroscopy measurements were carried out at room temperature, illuminating the sample with monochromatized Al Kα light from a microfocus setup (SPECS Focus 600). The excited photelectrons were collected with a SPECS 150 analyzer at an emission angle of 40°. The overall experimental resolution was extracted from a fit of the Fermi edge and resulted in 0.4 eV. UPS laboratory data were acquired using Helium Iα and IIα excitation lines (hν = 21.2 eV and 40.8 eV, respectively). Complementary ultra-violet photoemission (UPS) measurements were performed at the VUV Photoemission beamline of the Elettra Synchrotron in Trieste. In San Sebastian and Trieste a channel plate-based display type hemispherical analyzer was used with angular and energy resolution set to 0.1° and 40 meV, respectively. At the synchrotron, p-polarized light was used and the sample temperature during measurements was 17 K. In San Sebastian the work function Φ was evaluated by UPS in the laboratory, by measuring the difference in energy between the Fermi level EF and the photoemission cutoff for several extracting voltages (5 V, 10 V and 15 V).50 Scanning tunneling microscopy (STM) experiments were done at 300 K using an Omicron VT Setup.
2.2. DFT calculations
Density functional theory (DFT) calculations were carried out with the projector augmented waves formalism for the ion cores and a basis of plane waves, as implemented in VASP.51,52 The surface was modelled in the supercell approach with up to three atomic planes in the substrate, leaving a vaccum distance of 12.5 Å between slab replicas. The experimental lattice constant and superperiodicty was used for the CuPc layer. REAu2, with RE = Yb and Gd, free-standing flat monolayers as well as relaxed ones on 2 ML Au(111) were used. In this case, the moiré pattern is disregarded and instead a fcc stacked (√3 × √3)R30° structure is used as in previous works. The plane-wave basis was set from a Brillouin zone sampling53 3 × 3 × 1 and an energy cut-off of 400 eV (a finer grid 6 × 6 × 1 was used to obtain densities of states). Tolerances were 10−5 eV in the total energies and 10−4 eV in the ionic relaxation, together with forces on the atoms smaller than 0.05 eV Å−1. First, the atomic positions of the isolated layer of CuPc were allowed to relax. This structure was then rigidly placed on the flat REAu2 monolayers at three different registries (top RE, top Au and bridge between Au atoms), with molecules oriented as indicated by the STM images (see Fig. 1), and adsorption heights z to obtain a first approximation of the adsorption potential Vads(z). The same procedure was applied to REAu2/Au(111) slabs, where the alloy monolayer had been previously optimized. Finally, at the obtained minima, the CuPc were allowed to relax.In the geometry optimization and charge density distribution analyses, the generalized gradient approximation PBE functional54 was used, together with the DFT+U approach55 for electron correlations in the Cu(d) and RE(f) orbitals. Here the Dudarev parameters56U–J = 7.5, 3.5, and 3.5 eV for Gd, Yb and Cu were selected based on previous experience,18,25,27 and occupation matrix control57 was used to initialize the electron distributions with the actual CuPc configuration. The 4f electrons were considered both as valence and core states in the calculations with free-standing REAu2 monolayers. After confirmation that this does not significantly alter the main results (adsorption heights and charge density distributions), the calculations with an Au(111)-2 ML substrate were carried out with the 4f orbital in the ion cores. Additionally, van der Waals (vdW) dispersion forces were accounted for with the cx-13 functional.58,59 At the so-obtained equilibrium geometries, the z-dependence of the carrier density difference (CDD) at the interface upon physisorption is calculated according to the definition
 | (1) |
Here, the ρS(z) terms are the plane-integrated charge densities of each system S, where atomic coordinates are fixed at the optimized positions in the CuPc/REAu2/Au(111) structures found in the PBE+U+vdW calculations.
The effect of self-interaction corrections on the densities of states of these structures was examined using range-separated hybrid functionals (HSE,60,61 as implemented in VASP62), perturbatively: the eigenenergies of a PBE calculation were corrected to first order by applying the exact Hartree–Fock contribution non-selfconsistently to the corresponding wavefunctions. In addition, the DFT+∑axc method63,64 was used to determine the HOMO level alignment in CuPc/REAu2/Au(111). Further details65–70 can be found in the ESI.†
3. Results and discussion
3.1. Structure and adsorption energetics
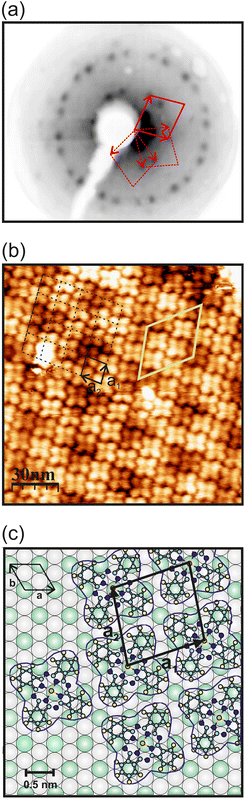
The growth of the monolayer CuPc adsorbed on GdAu2, HoAu2 and YbAu2 surfaces was studied with low-energy electron diffraction (LEED) and scanning tunneling microscopy (STM) at room temperature. It is worth noting that REAu2 surfaces are characterized by a hexagonal surface structure and a long-range moiré periodicity that extends over the whole surface.13,15 Depending on the RE ion, the lattice parameters of the compounds show tiny differences, but these become amplified in the moiré periodicities.31 However, the growth of CuPc shows identical adsorption geometries with analogous LEED patterns on all three REAu2 substrates studied here. Fig. 1(a) displays the LEED pattern of 1 ML of CuPc on HoAu2. CuPc grows with three different rotational domains, as many Pc overlayers on hexagonal surfaces.19,71,72 The surface unit cell of CuPc in this particular case results to be rhomboidal with a lattice parameter a = 1.43 nm and θ = 80°, and it appears marked by a solid red line in Fig. 1(a). The dashed red lines represent the unit cells of the other two rotational domains rotated at 60° with respect to each other. Similar LEED patterns have been measured on 1 ML of CuPc/GdAu2 and CuPc/YbAu2 (Fig. S1 in the ESI†). The complex epitaxy of CuPc (four-fold symmetry) on hexagonal REAu2 surfaces can be expressed in matrix notation73 as , which points out the commensurability of CuPc on these substrates. Considering this arrangement, the packing density of the CuPc overlayer is 0.49 molecule per nm2, very close to the value obtained in CuPc on Au(111) (0.5 molecule per nm2).19 The high degree of commensurability between the molecules and the surface is confirmed by STM measurements. Fig. 1(b) displays a STM micrograph of CuPc grown on HoAu2 and shows one of the three rotational domains of the CuPc ML. The surface unit cells of CuPc monolayer is marked in black, while the moiré periodicity of the substrates underneath is marked in yellow. Fig. 1(c) illustrates the structural model extracted from LEED pattern analysis. In this particular case, it is assumed that CuPc molecules are adsorbed with the Cu ion centered on top of a RE atom of the substrate. Nevertheless, the coincidence between the molecular lattice and the REAu2 surface alloys allows other adsorption sites on the substrate that preserve the same molecular arrangement and LEED pattern. From the LEED measurements one can only evaluate the lattice but not the surface adsorption site of the molecule (Cu atom). In order to further investigate the structure and energetics of the system, we resort to first-principles calculations in the DFT+U approach, including a van der Waals (vdW) functional (see Methods section). The vdW correction is needed to obtain meaningful adsorption wells. CuPc molecular arrays on REAu2 MLs with divalent Yb2+ and trivalent Gd3+ were studied with this approach. Fig. 2(a) shows the adsorption potential energies as a function of the height zCu of the central Cu atom inside the CuPc molecule landing rigidly on free-standing REAu2 MLs. For the three studied adsorption sites (top RE, top Au and bridge site between two Au atoms), the adsorption minima are located at around 3 and 3.25 Å for YbAu2 and GdAu2, respectively, which are characteristic of vdW physisorbed systems. The main observation is that the interaction of CuPc with GdAu2 is stronger than with YbAu2. The adsorption minima on GdAu2 are deeper (Eads ≃ 4 eV) than on YbAu2 (≃3 eV) and there are larger differences between the Eads at the different adsorption sites on GdAu2. Moreover, a larger Eads on GdAu2 is also obtained in the absence of vdW corrections (pink curves), with a shallow adsorption well at 3.25 Å, while interaction of CuPc on YbAu2 results to be much weaker. The Cu–Gd magnetic exchange interaction, absent in YbAu2, is another factor to be considered in the adsorption stability. The largest magnetic effect is found on free-standing GdAu2 at the top Gd site, where antiferromagnetic (AFM) interaction is favored by 0.18 eV over the ferromagnetic (FM) one. At the other sites, the AFM–FM energy differences are one order of magnitude smaller. The same calculation performed on CuPc on supported REAu2 on a Au(111)-2 ML thick layer shows important vdW dispersion forces, which are introduced by the Au(111) layers, dominating the adsorption energetics on GdAu2, as it is shown in Fig. 2(b). Here, Eads values are reduced by up to 2 eV and adsorption becomes slightly more stable on YbAu2. In this case a further energy reduction of ≃0.2 eV is achieved by allowing the CuPc geometry to relax, specially at the Au top and bridge sites.
, which points out the commensurability of CuPc on these substrates. Considering this arrangement, the packing density of the CuPc overlayer is 0.49 molecule per nm2, very close to the value obtained in CuPc on Au(111) (0.5 molecule per nm2).19 The high degree of commensurability between the molecules and the surface is confirmed by STM measurements. Fig. 1(b) displays a STM micrograph of CuPc grown on HoAu2 and shows one of the three rotational domains of the CuPc ML. The surface unit cells of CuPc monolayer is marked in black, while the moiré periodicity of the substrates underneath is marked in yellow. Fig. 1(c) illustrates the structural model extracted from LEED pattern analysis. In this particular case, it is assumed that CuPc molecules are adsorbed with the Cu ion centered on top of a RE atom of the substrate. Nevertheless, the coincidence between the molecular lattice and the REAu2 surface alloys allows other adsorption sites on the substrate that preserve the same molecular arrangement and LEED pattern. From the LEED measurements one can only evaluate the lattice but not the surface adsorption site of the molecule (Cu atom). In order to further investigate the structure and energetics of the system, we resort to first-principles calculations in the DFT+U approach, including a van der Waals (vdW) functional (see Methods section). The vdW correction is needed to obtain meaningful adsorption wells. CuPc molecular arrays on REAu2 MLs with divalent Yb2+ and trivalent Gd3+ were studied with this approach. Fig. 2(a) shows the adsorption potential energies as a function of the height zCu of the central Cu atom inside the CuPc molecule landing rigidly on free-standing REAu2 MLs. For the three studied adsorption sites (top RE, top Au and bridge site between two Au atoms), the adsorption minima are located at around 3 and 3.25 Å for YbAu2 and GdAu2, respectively, which are characteristic of vdW physisorbed systems. The main observation is that the interaction of CuPc with GdAu2 is stronger than with YbAu2. The adsorption minima on GdAu2 are deeper (Eads ≃ 4 eV) than on YbAu2 (≃3 eV) and there are larger differences between the Eads at the different adsorption sites on GdAu2. Moreover, a larger Eads on GdAu2 is also obtained in the absence of vdW corrections (pink curves), with a shallow adsorption well at 3.25 Å, while interaction of CuPc on YbAu2 results to be much weaker. The Cu–Gd magnetic exchange interaction, absent in YbAu2, is another factor to be considered in the adsorption stability. The largest magnetic effect is found on free-standing GdAu2 at the top Gd site, where antiferromagnetic (AFM) interaction is favored by 0.18 eV over the ferromagnetic (FM) one. At the other sites, the AFM–FM energy differences are one order of magnitude smaller. The same calculation performed on CuPc on supported REAu2 on a Au(111)-2 ML thick layer shows important vdW dispersion forces, which are introduced by the Au(111) layers, dominating the adsorption energetics on GdAu2, as it is shown in Fig. 2(b). Here, Eads values are reduced by up to 2 eV and adsorption becomes slightly more stable on YbAu2. In this case a further energy reduction of ≃0.2 eV is achieved by allowing the CuPc geometry to relax, specially at the Au top and bridge sites.
The adsorption geometry at top RE and top Au configurations implies a coincidence of five RE atoms with CuPc, while at bridge site configuration the coincidence is reduced to four atoms. This is shown by the spatial dependence of carrier density difference (CDD) Δρ(r) (see Methods section). The mapping of Δρ(x,y) discloses clear electron accumulation regions that are localized at the coincidence points between the RE atoms of the substrate and the organic ligand, as shown in Fig. 3(a). The charge accumulation areas, represented in red, are clearly localized underneath the Cu and C atoms. These points are hotspots that act as anchoring points for CuPc on both free-standing and supported REAu2 MLs. Δρ(x,y) for all three adsorption sites are represented in Fig. S2 in the ESI.† This larger coincidence would explain the slightly stronger interaction observed in the Vads(z) curves upon CuPc adsorption on top RE and top Au sites compared with Au bridge sites of Fig. 2.
The CDD in the side-view representation of Fig. 3(b) reveals that these areas of charge accumulation (red) are clearly localized at the interface near the CuPc layer. Moreover, such effect induces a certain defect of charge (blue) at the CuPc layer and a slight polarization of the corresponding RE atoms. The CDD topography at the CuPc/REAu2/Au(111) interface qualitatively differs from those at CuPc/Au(111) and CuPc/Ag(111). For CuPc on Au(111) the Δρ(r) distribution is uniform, whereas it is very corrugated throughout the interface with Ag(111), rapidly shifting between positive and negative values.74 In contrast, here Δρ(r) is markedly localized above the RE atomic positions.
3.2. Electrostatics at the interface
Experimental work function (Φ) measurements were performed by photoemission spectroscopy from the cutoff of the secondary emitted electrons (see Methods section) on pristine REAu2 substrates and upon adsorption of 1 ML CuPc on top. Both results were systematically compared, detecting a change in the work function ΔΦ that is related with the surface dipole induced by the evaporation of CuPc (see Table 1). This effect can be assigned to the Pauli push-back effect, which is commonly observed in metal–organic interfaces.39–41 The highest ΔΦ values are found in the samples of CuPc/HoAu2 and CuPc/GdAu2. On the pristine alloys Φ displays similar values for both GdAu2 (4.89 eV) and HoAu2 (4.88 eV) surfaces. However, the highest Φ is measured in YbAu2 (4.98 eV). This is an unexpected result, considering that Yb as pure element is divalent and exhibits smaller values than the trivalent Ho and Gd.42,43| Φ (eV) | ΔΦ (eV) | ΔVvac (eV) | pz (e Å) | ||
|---|---|---|---|---|---|
| CuPc/YbAu2 | UPS(exp) | 4.54 | −0.44 | ||
| Top-Yb | 3.08 | −0.31 | −0.84 | 0.54 | |
| Top-Au | 2.99 | −0.40 | −0.61 | 0.33 | |
| Bridge-Au | 3.12 | −0.28 | −0.66 | 0.33 | |
| CuPc/GdAu2 | UPS(exp) | 4.35 | −0.54 | ||
| Top-Gd | 2.96 | −0.79 | −1.24 | 0.85 | |
| Top-Au | 3.20 | −0.56 | −0.90 | 0.64 | |
| Bridge-Au | 3.15 | −0.61 | −0.98 | 0.69 | |
| CuPc/HoAu2 | UPS(exp) | 4.35 | −0.53 | ||
| CuPc/Au(111) | Exp.75 | 4.81 | −0.99 |
Furthermore, DFT+U has been used to calculate Φ from the electrostatic potentials along the perpendicular direction Z in the supercells. This calculation was carried out for the CuPc/GdAu2 and CuPc/YbAu2 systems. The corresponding Φ and ΔΦ values upon CuPc adsorption are included in Table 1. The values are reasonably close to the measured ones and, in particular, the trend of a stronger work function reduction in GdAu2 is confirmed. However, a disagreement with the experimental Φ values of the pristine surfaces exists. This discrepancy, of around 1.5 eV, can be attributed in part to the finite slab size effect, which adds to a loss in accuracy at the surface electrostatic potential tail. In any case, an error of 0.4 eV is estimated for the calculated Φ,‡ which is smaller than the obtained Φ values and vacuum level downward shifts ΔVvac upon CuPc adsorption shown in Table 1. The plane-integrated CDD curves Δρ(z) (eqn (1) in the Methods section), disclosed in Fig. 3(c), shows a similar sharp-peaked profile across the interface for all adsorption sites on both substrates GdAu2 and YbAu2. The main features are a clear electron depletion in the CuPc and REAu2 planes and a strong accumulation at intermediate, slightly closer to the CuPc plane. The peaks in the YbAu2 case are more intense. The fact that the peaks in the Δρ(z) curves are placed at the same z value for the three sites reflects that the physisorption is mostly governed by the molecule height, rather than by the Cu atom registry with the surface (see Fig. S4 in the ESI†). Table 1 shows the dipoles pz at the CuPc/REAu2 interface, obtained by integration of Δρ(z). The larger induced surface dipole on GdAu2 is consistent with larger ΔVvac and ΔΦ values on this substrate.76 The curve of the plane-averaged accumulated dipole pz across the interface of CuPc/GdAu2 and CuPc/YbAu2 is shown in Fig. S3 in the ESI.†
3.3. Electronic structure
The valence band of adsorbed CuPc MLs on REAu2 surfaces has been investigated by angle resolved photoemission spectroscopy (ARPES). The characteristic dispersing bands of the REAu2 surfaces, already discussed in previous publications14,15,31 can still be observed in the CuPc-covered surface, although with a strongly reduced intensity due to the high surface sensitivity of the photoemission measurements (hν = 21.2 eV, 30 eV). The growth of CuPc introduces new non-dispersing bands that are assigned to the highest occupied orbital (HOMO) levels of the adsorbed molecule. Fig. 4(a) displays in the top panel the photoemission intensity mapping of electronic bands of YbAu2 and at the bottom the same measurement after 1 ML CuPc adsorption (hν = 30 eV). One can distinguish the YbAu2 dispersing bands and two strong non-dispersive emission peaks that arise from the spin–orbit split Yb 4f levels. These features are related with the divalent character of Yb in the YbAu2 surface compound. However, the RE 4f levels are not observed in the band structure measured by ARPES on Ho and Gd alloys (Fig. S6 in the ESI†), because their 4f core levels are detected at much higher binding energies with photoemission.31At first glance, upon CuPc adsorption the band structure of REAu2 surfaces generally appears unaffected. However, an exception is found in the A band. This band, common to all REAu2 surfaces, is characterized by a free-electron-like parabolic dispersion around the ![[capital Gamma, Greek, macron]](https://www.rsc.org/images/entities/char_e0ba.gif) -point of the surface Brillouin zone. After CuPc deposition an upward shift toward the Fermi level (smaller binding energy EB) is detected at
-point of the surface Brillouin zone. After CuPc deposition an upward shift toward the Fermi level (smaller binding energy EB) is detected at ![[capital Gamma, Greek, macron]](https://www.rsc.org/images/entities/char_e0ba.gif) . This effect reveals the surface-state character of the A band and its sensitivity to the presence of adlayers. Fig. 4(b) shows the energy dispersion curves at an emission angle of 0° (
. This effect reveals the surface-state character of the A band and its sensitivity to the presence of adlayers. Fig. 4(b) shows the energy dispersion curves at an emission angle of 0° (![[capital Gamma, Greek, macron]](https://www.rsc.org/images/entities/char_e0ba.gif) -point). In the case of YbAu2, EB shifts from binding energy values of 0.92 eV to 0.84 eV upon CuPc adsorption (δEB = 80 meV). However, for HoAu2 and GdAu2EB is shifted from 1.03 eV to 0.99 eV (δEB = 35 meV). ARPES studies performed on noble-gas/Au(111) and organic molecules/Au(111) interfaces have ascribed a similar shift of the surface-state band to the pushback effect.77 Moreover, models have been developed to account for the surface state energy shift in the cases of graphene/metal78,79 and noble-gas/metal80 interfaces. Thus, it has been established an universal curve that relates the shift with the adlayer height above the metal surface, while the substrate work function plays a minor role.81 For the case studied here, the A band is formed upon REAu2 interaction with the Au(111) support.27,31 Therefore, it is expected to interact with CuPc more weakly than the usual Shockley-like surface states in noble metals, which are localized at the outer regions of the surface. Indeed, the observed δEB values are lower than other typical shifts. In comparison, the Shockley state shift caused by PTCDA physisorption ranges from 0.164 eV on Au(111)82 to 0.95 eV on Ag(100).83
-point). In the case of YbAu2, EB shifts from binding energy values of 0.92 eV to 0.84 eV upon CuPc adsorption (δEB = 80 meV). However, for HoAu2 and GdAu2EB is shifted from 1.03 eV to 0.99 eV (δEB = 35 meV). ARPES studies performed on noble-gas/Au(111) and organic molecules/Au(111) interfaces have ascribed a similar shift of the surface-state band to the pushback effect.77 Moreover, models have been developed to account for the surface state energy shift in the cases of graphene/metal78,79 and noble-gas/metal80 interfaces. Thus, it has been established an universal curve that relates the shift with the adlayer height above the metal surface, while the substrate work function plays a minor role.81 For the case studied here, the A band is formed upon REAu2 interaction with the Au(111) support.27,31 Therefore, it is expected to interact with CuPc more weakly than the usual Shockley-like surface states in noble metals, which are localized at the outer regions of the surface. Indeed, the observed δEB values are lower than other typical shifts. In comparison, the Shockley state shift caused by PTCDA physisorption ranges from 0.164 eV on Au(111)82 to 0.95 eV on Ag(100).83
Fig. 5(a) shows the photoemission results at higher emission angles θ of 1 ML of CuPc on YbAu2, HoAu2 and GdAu2 as well as on pure Au(111). Higher emission angles are usually better suited for the detection of the molecular levels like the HOMO levels. This is related to the symmetry and band character of the molecules and is explored in detail in molecular orbital tomography.84–87 Here, the inclusion of CuPc/Au(111) is used as a reference to estimate and compare energy shifts of the valence band structures among the CuPc/REAu2 samples. The emissions from a possible former lowest unoccupied molecular level (F-LUMO) below the Fermi energy is not detected in any of the four systems, which is in contrast to some chemisorbed systems like CuPc/Ag(111)71 or TiOPc/Ag(111),88,89 where the F-LUMO gets occupied. However, the HOMO related features are readily seen, as previously mentioned above, and present different shifts with respect to the reference CuPc/Au(111) system. In the case of YbAu2, this shift amounts to 0.26 eV, while in the other two cases this value increases to approximately 0.5 eV. The exact HOMO positions have been obtained after peak fit analysis using Lorentzian curves and taking into account the vibrational couplings of CuPc.90–92 These values are presented in Table 2.
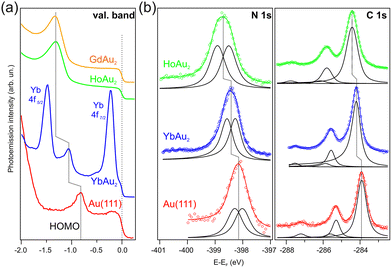 | ||
| Fig. 5 Photoemission measurements for CuPc/REAu2 samples in comparison to CuPc/Au(111).75 (a) Valence bands measured on CuPc ML grown on GdAu2, HoAu2 and YbAu2 MLs. For comparison the valence band of 1 ML of CuPc/Au(111) is given. (hν = 44 eV and 50 eV, θ = (20 ± 7)° for Au(111) and YbAu2 and hν = 21.22 eV and θ = (50 ± 7)° for HoAu2 and GdAu2 substrates, respectively.) (b) N 1s and C 1s core levels of 1 ML CuPc grown on Au(111), HoAu2 and YbAu2 (hν = 520 eV and 390 eV, θ = (0 ± 7)°). | ||
| HOMO/ΔEH | E B N 1s/ΔEN 1s | E B C 1s/ΔEC1s | |
|---|---|---|---|
| CuPc/YbAu2 | 1.08/0.26 | 398.42/0.20 | 284.18/0.24 |
| CuPc/GdAu2 | 1.32/0.50 | 398.74/0.50 | 284.47/0.53 |
| CuPc/HoAu2 | 1.32/0.50 | 398.74/0.52 | 284.42/0.48 |
| CuPc/Au(111) | 0.82/— | 398.22/— | 283.94/— |
In addition, the interaction strength of CuPc with the different REAu2 surfaces was studied by X-ray photoelectron spectroscopy (XPS). Fig. 5(b) shows N 1s and C 1s core levels measured on CuPc/HoAu2 and CuPc/YbAu2. For both N 1s and C 1s, core level shifts to higher binding energies with respect to the CuPc/Au(111) emissions (see Table 2) were detected. In order to disentangle both contributions, a peak fit analysis was carried out using individual Doniach-Sunjic peaks93 of the same width, asymmetry, and Shirley background.94 As in other works, the broad N 1s peak was explained by the contribution of two non-equivalent nitrogen atoms within the Pc ligand that cannot be resolved due to peak width.95 For the fits, constraints for identical width, asymmetry and background were set for both features. The C 1s spectrum of CuPc is composed of three visible peaks, in CuPc/YbAu2 at approximately 284.2 eV, 285.6 eV, and 287.5 eV with approximate area ratios of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, respectively. For the latter case, the width (FWHM) associated with these positions is quite similar (0.4 ± 0.05) eV and there is a small asymmetry of 0.11 in all cases. These values, including the asymmetry, are very similar to those of CuPc/Au(100) results.96 The asymmetry partially results from molecular vibrations96,97 as well as from the reduced molecular layer thickness associated with a metallic substrate interaction. The highest emission peak was originally associated to C–C interaction of the carbon benzene ring atoms, the second peak to the C–N contribution from the pyrole structure and the high binding energy peak to a shake-up satellite, originally associated to the main C 1s peak originating from the π → π* transition.96,98 Later works on H2Pc, CuPc and FePc97,99–102 have shown, however, that there should be two shake-up's with identical shake-up/main peak distances. As a result, the shake-up of the C–C emission overlaps with the C–N emission and is not distinguishable by eyes. With a peak fitting procedure taking into account these four emissions, the intensity relation of the carbon atoms of C–C
0.15, respectively. For the latter case, the width (FWHM) associated with these positions is quite similar (0.4 ± 0.05) eV and there is a small asymmetry of 0.11 in all cases. These values, including the asymmetry, are very similar to those of CuPc/Au(100) results.96 The asymmetry partially results from molecular vibrations96,97 as well as from the reduced molecular layer thickness associated with a metallic substrate interaction. The highest emission peak was originally associated to C–C interaction of the carbon benzene ring atoms, the second peak to the C–N contribution from the pyrole structure and the high binding energy peak to a shake-up satellite, originally associated to the main C 1s peak originating from the π → π* transition.96,98 Later works on H2Pc, CuPc and FePc97,99–102 have shown, however, that there should be two shake-up's with identical shake-up/main peak distances. As a result, the shake-up of the C–C emission overlaps with the C–N emission and is not distinguishable by eyes. With a peak fitting procedure taking into account these four emissions, the intensity relation of the carbon atoms of C–C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C–N = 24
C–N = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 including both shake-ups was then correctly fullfilled for the phthalocyanine molecules. Also in our cases here, the peak fitting results confirm the correct intensity relation (see ESI for details, Fig. S7†). More importantly, however, a rigid multiplet shift from CuPc/Au(111) to CuPc/YbAu2 by approximately 0.25 eV and from CuPc/Au(111) to HoAu2 by 0.5 eV is obtained. These values are very similar to the N 1s core level shifts and, as explained before, to the HOMO level shifts. These results indicate that YbAu2 surfaces show a slightly weaker interaction than GdAu2 and HoAu2 with the CuPc molecules, taking Au(111) as reference. The exact positions of the core levels are again included in Table 2.
8 including both shake-ups was then correctly fullfilled for the phthalocyanine molecules. Also in our cases here, the peak fitting results confirm the correct intensity relation (see ESI for details, Fig. S7†). More importantly, however, a rigid multiplet shift from CuPc/Au(111) to CuPc/YbAu2 by approximately 0.25 eV and from CuPc/Au(111) to HoAu2 by 0.5 eV is obtained. These values are very similar to the N 1s core level shifts and, as explained before, to the HOMO level shifts. These results indicate that YbAu2 surfaces show a slightly weaker interaction than GdAu2 and HoAu2 with the CuPc molecules, taking Au(111) as reference. The exact positions of the core levels are again included in Table 2.
From all the photoemission results we extract that the Fermi level is located well inside the HOMO–LUMO molecular gap, far from the edges. In such scenario we do not expect a significant amount of charge flowing to align EF to the molecular levels. In fact, we observe an almost rigid shift of the spectrum in the three cases, i.e. a very similar energy shift for all HOMO, core and vacuum levels at the three interfaces. This demonstrates a weak molecule/metal surface interaction, with vacuum level pinning of molecular electronic states.45,75 This indicates that the behavior of the metal–organic interfaces is close to the Schottky–Mott limit103 with a notable interface dipole, as it has been previously discussed.
The experimetal HOMO values of Table 2 obtained by photoemision are equivalent to the hole injection barrier (HIB) of the metal–organic interfaces. Likewise, the electron injection barrier (EIB) is given by the LUMO level. In order to estimate a possible LUMO energy and hence an EIB for our samples, we consider a molecular gap of 3 eV, obtained from a combination of normal and inverse photoemission measured on CuPc/Au(111)104 CuPc on both REAu2 and Au(111) is physisorbed, though on REAu2 it reveals a slightly stronger electrostatic interaction. Therefore, the predicted bandgap for CuPc/REAu2 should be close to, or even somewhat smaller than 3 eV. This argument is based on the fact that the bandgap of molecules adsorbed on surfaces becomes smaller in case of stronger interactions.45,105 Hence, we extract for our samples the following LUMO/EIB energies (LUMO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bandgap-HOMO) of 1.67 eV (HoAu2), 1.73 eV (GdAu2), 1.92 eV (YbAu2) and 2.18 eV for Au(111). It is worth to note that the smallest difference EIB-HIB is found in GdAu2 and HoAu2 (approx. 0.4 eV), while it doubles in YbAu2 (0.84 eV). This means that in the interface CuPc/GdAu2 and CuPc/HoAu2, EF is located much closer to the middle of the molecular gap, allowing an ambipolar injection of electrons and holes into the active organic layer.36–38
bandgap-HOMO) of 1.67 eV (HoAu2), 1.73 eV (GdAu2), 1.92 eV (YbAu2) and 2.18 eV for Au(111). It is worth to note that the smallest difference EIB-HIB is found in GdAu2 and HoAu2 (approx. 0.4 eV), while it doubles in YbAu2 (0.84 eV). This means that in the interface CuPc/GdAu2 and CuPc/HoAu2, EF is located much closer to the middle of the molecular gap, allowing an ambipolar injection of electrons and holes into the active organic layer.36–38
Fig. 6 shows the projected densities of states (PDOS) on the molecular orbitals (MO) calculated by DFT+U+vdW (see Methods section) for CuPc adsorbed at the top Yb and top Gd geometries. The a1u and eg MOs corresponds to the HOMO and LUMO of CuPc, respectively and have a marginal contribution from the Cu 3d states. b1g MO is the characteristic singly-occupied molecular orbital (SOMO) of CuPc, which has a strong d-character due to the hybridization between a Cu dx2−y2 state with the N px,y orbital.18 Two features in the PDOS of both substrates are not consistent with the experimental observations, pointing to the so-called self-interaction error (SIE)48 effect in the calculation: (i) the theoretical HOMO levels (a1u) lie 0.2–0.4 eV deeper than the experimental ones (see Table 2) and (ii) the LUMO levels lie at the Fermi level, while workfunctions and photoemission indicate that they should lie above. A better agreement with the experimental HOMO binding energies is obtained by means of the DFT+∑axc approach,63,64 which provides renormalized eigenvalues using an electrostatic image potential model for the substrate. This procedure, described in the ESI,† results in 1.1 and 1.2 eV for the HOMO levels of CuPc on YbAu2 and GdAu2, close to the values of Table 2. However, the LUMO pinning at the Fermi energy is a persistent feature even when using hybrid functionals, which partially correct the SIE. This is shown in the ESI and in Fig. S11.† The performance of these functionals is, nevertheless, conditioned by the occupation of the PBE reference calculation.106 Therefore, the LUMO pinning may be interpreted as an artifact of the calculation and it is manifested by the highly localized interfacial charge density above the RE atoms (anchoring points), as shown by the spectral analysis of the CDDs. This means that the SIE is highly inhomogeneous in CuPc/REAu2 systems, a qualitatively different scenario to that of CuPc physisorbed on pure noble metals.
4. Conclusions
Hybrid metal–organic interfaces have been prepared by adsorption of CuPc on 2D REAu2 surfaces (RE = Gd, Ho and Yb). We observe that CuPc forms a commensurate superstructure with densely packed molecules that differs from the clean CuPc/Au(111) case. Moreover, we see that there is not a clear preference for adsorption sites concerning the central Cu atom of CuPc. By means of photoemission experiments and DFT calculations, we have performed an extensive study on the electrostatics and electronic structure of the interface. Our study clearly reveals that CuPc is physisorbed on all the REAu2 surfaces tried here. The electronic structure of these hybrid interfaces displays characteristic features that are not seen in other physisorbed molecules on pure metals. The interfacial charge density distribution is strongly corrugated and mainly localized on the RE atoms of the substrate.Importantly, photoemission experiments show quantitative differences depending on the CuPc adsorption on divalent or trivalent REAu2 substrates. In all cases the LUMO level of CuPc/REAu2 remains empty as on other noble metals, but the HOMO binding energy values are significantly larger (≃0.3 eV) on trivalent substrates (HoAu2 and GdAu2) than on a divalent one (YbAu2). The DFT+∑axc approach allows to explain the renormalization of the CuPc HOMO level upon adsorption as an interaction with the substrate Coulomb-like potential. This is, in essence, the Pauli push-back effect. Furthermore, it is found that the physisorption of CuPc induces an upward shift of the distinctive surface state of the REAu2 substrate, which is larger in YbAu2 (80 meV) than in HoAu2 and GdAu2 (35 meV). Interestingly, unlike in other noble metals, this state is confined below the REAu2 atomic plane and appears upon its interaction with the Au(111) substrate underneath.
All in all, the REAu2 substrates preserve the CuPc properties, as it occurs on the widely used noble metal substrates, but with the fingerprints of a singular interaction with the RE atoms and the REAu2 band structure. The significant dependence of the HOMO binding energy on the RE valence opens the door to a feasible fine tuning of the carrier injection barriers for organic electronic devices. Remarkably, it is found that hybrid interfaces prepared with trivalent substrates (HoAu2 and GdAu2) exhibit molecular level energies that may allow carrier injection barriers closer to an ambipolar behavior. Such results and the unique ferromagnetic properties of the subtrates reveal a large potential for the design of new organic spintronic devices with extensive functionalities that combine the use of light with electric and magnetic fields.
Author contributions
L. F., F. S. and M. B.-R. conceptualized the work. R. C.-B., K. A., L. F. and F. S. carried out experimental photoemission investigations. The formal analysis of the photoemission measurements was carried out by F. S. STM and LEED investigations were carried out by R. C.-B. and L. F. The experimental investigations were supervised by L. F. and F. S. DFT investigations were performed by M. B.-R. The article was original drafted by L. F. and M. B.-R., and reviewed and edited by all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financial supported by Spanish Ministerio de Ciencia e Innovación (grants No. MAT-2017-88374-P, PID2020-116093RB-C44, PID2019-103910GB-I00 and PTA2019-018134-I funded by MCIN/AEI/10.13039/501100011033/ and by “ESF investing in your future”), and the Basque Government (grants No. IT-1591-22, IT-1527-22). L. F. acknowledges funding from the European Union's Horizon 2020 research and innovation programme through the Marie Skłodowska-Curie Grant Agreement MagicFACE No. 797109. Computational resources were provided by DIPC. Part of the research leading to this results has been supported by the project CALIPSOplus under Grant Agreement 730872 from the EU Framework Programme for Research and Innovation HORIZON 2020. We further aknowledge Polina M. Sheverdyaeva and Paolo Moras for the assistance in the VUV-Photoemission beam line of Elettra Synchrotron in Trieste.References
- M. Urdampilleta, S. Klyatskaya, J.-P. Cleuziou, M. Ruben and W. Wernsdorfer, Nat. Mater., 2011, 10, 502 CrossRef CAS PubMed.
- M. J. Comstock, N. Levy, A. Kirakosian, J. Cho, F. Lauterwasser, J. H. Harvey, D. A. Strubbe, J. M. J. Fréchet, D. Trauner, S. G. Louie and M. F. Crommie, Phys. Rev. Lett., 2007, 99, 038301 CrossRef PubMed.
- T. D. Nguyen, E. Ehrenfreund and Z. V. Vardeny, Science, 2012, 337, 204–209 CrossRef CAS PubMed.
- X. Sun, S. Vélez, A. Atxabal, A. Bedoya-Pinto, S. Parui, X. Zhu, R. Llopis, F. Casanova and L. E. Hueso, Science, 2017, 357, 677–680 CrossRef CAS PubMed.
- M. Cinchetti, K. Heimer, J.-P. Wüstenberg, O. Andreyev, M. Bauer, S. Lach, C. Ziegler, Y. Gao and M. Aeschlimann, Nat. Mater., 2009, 8, 115–119 CrossRef CAS PubMed.
- S. Lach, A. Altenhof, K. Tarafder, F. Schmitt, M. E. Ali, M. Vogel, J. Sauther, P. M. Oppeneer and C. Ziegler, Adv. Funct. Mater., 2012, 22, 989–997 CrossRef CAS.
- S. Javaid, M. Bowen, S. Boukari, L. Joly, J.-B. Beaufrand, X. Chen, Y. J. Dappe, F. Scheurer, J.-P. Kappler, J. Arabski, W. Wulfhekel, M. Alouani and E. Beaurepaire, Phys. Rev. Lett., 2010, 105, 077201 CrossRef CAS PubMed.
- A. Droghetti, P. Thielen, I. Rungger, N. Haag, N. Großmann, J. Stöckl, B. Stadtmüller, M. Aeschlimann, S. Sanvito and M. Cinchetti, Nat. Commun., 2016, 7, 12668 CrossRef CAS PubMed.
- F. Al Ma'Mari, T. Moorsom, G. Teobaldi, W. Deacon, T. Prokscha, H. Luetkens, S. Lee, G. E. Sterbinsky, D. A. Arena, D. A. MacLaren, M. Flokstra, M. Ali, M. C. Wheeler and B. Gavin Burnell, Nature, 2015, 524, 69–74 CrossRef PubMed.
- T. Moorsom, M. Wheeler, T. Mohd Khan, F. Al Ma'Mari, C. Kinane, S. Langridge, D. Ciudad, A. Bedoya-Pinto, L. Hueso, G. Teobaldi, V. K. Lazarov, D. Gilks, G. Burnell, B. J. Hickey and O. Cespedes, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 125311 CrossRef.
- R. Geng, T. T. Daugherty, K. Do, H. M. Luong and T. D. Nguyen, J. Sci.: Adv. Mater. Devices, 2016, 1, 128–140 Search PubMed.
- T. Arnold, A. Atxabal, S. Parui, L. E. Hueso and F. Ortmann, Adv. Funct. Mater., 2018, 28, 1706105 CrossRef.
- M. Corso, L. Fernández, F. Schiller and J. E. Ortega, ACS Nano, 2010, 4, 1603–1611 CrossRef CAS PubMed.
- M. Corso, M. J. Verstraete, F. Schiller, M. Ormaza, L. Fernández, T. Greber, M. Torrent, A. Rubio and J. E. Ortega, Phys. Rev. Lett., 2010, 105, 016101 CrossRef CAS PubMed.
- M. Ormaza, L. Fernández, S. Lafuente, M. Corso, F. Schiller, B. Xu, M. Diakhate, M. J. Verstraete and J. E. Ortega, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 125405 CrossRef.
- S. Stepanow, A. Mugarza, G. Ceballos, P. Moras, J. C. Cezar, C. Carbone and P. Gambardella, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 014405 CrossRef.
- A. Mugarza, N. Lorente, P. Ordejón, C. K. S. Stepanow, M.-L. Bocquet, J. Fraxedas, G. Ceballos and P. Gambardella, Phys. Rev. Lett., 2019, 105, 115702–115705 CrossRef PubMed.
- A. Mugarza, R. Robles, C. Krull, R. Korytár, N. Lorente and P. Gambardella, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 155437 CrossRef.
- B. Stadtmüller, I. Kröger, F. Reinert and C. Kumpf, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 085416–085425 CrossRef.
- D. G. de Oteyza, A. El-Sayed, J. M. Garcia-Lastra, E. Goiri, T. N. Krauss, A. Turak, E. Barrena, H. Dosch, J. Zegenhagen, A. Rubio, Y. Wakayama and J. E. Ortega, J. Chem. Phys., 2010, 133, 214703–214708 CrossRef CAS PubMed.
- R. Sk and A. Deshpande, Mol. Syst. Des. Eng., 2019, 4, 471–483 RSC.
- P. Järvinen, S. K. Hämäläinen, M. Ijäs, A. Harju and P. Liljeroth, J. Phys. Chem. C, 2014, 118, 13320–13325 CrossRef.
- T. Bathon, P. Sessi, K. A. Kokh, O. E. Tereshchenko and M. Bode, Nano Lett., 2015, 15, 2442–2447 CrossRef CAS PubMed.
- K. Sun, M. L. Tao, Y. B. Tu and J. Z. Wang, Molecules, 2017, 22, 740–747 CrossRef PubMed.
- L. Fernández, M. Blanco-Rey, M. Ilyn, L. Vitali, A. Magaña, A. Correa, P. Ohresser, J. E. Ortega, A. Ayuela and F. Schiller, Nano Lett., 2014, 14, 2977–2981 CrossRef PubMed.
- L. Fernández, M. Ilyn, A. Magaña, L. Vitali, J. Ortega and F. Schiller, Adv. Sci., 2016, 14, 1600187–1600192 CrossRef PubMed.
- M. Ormaza, L. Fernández, M. Ilyn, A. Magaña, B. Xu, M. J. Verstraete, M. Gastaldo, M. A. Valbuena, P. Gargiani, A. Mugarza, A. Ayuela, L. Vitali, M. Blanco-Rey, F. Schiller and J. E. Ortega, Nano Lett., 2016, 16, 4230–4235 CrossRef CAS PubMed.
- L. Fernández, M. Corso, F. Schiller, M. Ilyn, M. Holder and J. E. Ortega, Appl. Phys. Lett., 2010, 96, 013107 CrossRef.
- B. Feng, R.-W. Zhang, Y. Feng, B. Fu, S. Wu, K. Miyamoto, S. He, L. Chen, K. Wu, K. Shimada, T. Okuda and Y. Yao, Phys. Rev. Lett., 2019, 123, 116401 CrossRef CAS PubMed.
- Y. Que, Y. Zhuang, Z. Liu, C. Xu, B. Liu, K. Wang, S. Du and X. Xiao, J. Phys. Chem. Lett., 2020, 11, 4107–4112 CrossRef CAS PubMed.
- L. Fernandez, M. Blanco-Rey, R. Castrillo-Bodero, M. Ilyn, K. Ali, E. Turco, M. Corso, M. Ormaza, P. Gargiani, M. A. Valbuena, A. Mugarza, P. Moras, P. M. Sheverdyaeva, A. K. Kundu, M. Jugovac, C. Laubschat, J. E. Ortega and F. Schiller, Nanoscale, 2020, 12, 22258–22267 RSC.
- M. Blanco-Rey, R. Castrillo-Bodero, K. Ali, P. Gargiani, F. Bertran, P. M. Sheverdyaeva, J. E. Ortega, L. Fernandez and F. Schiller, Phys. Rev. Res., 2022, 4, 013237 CrossRef CAS.
- M. Farnesi Camellone, A. Correa, A. Barragán, M. Pedio, S. Fabris, C. Cepek and L. Vitali, J. Phys. Chem. C, 2019, 123, 6496–6501 CrossRef CAS.
- M. Abadía, M. Ilyn, I. Piquero-Zulaica, P. Gargiani, C. Rogero, J. E. Ortega and J. Brede, ACS Nano, 2017, 11, 12392–12401 CrossRef PubMed.
- M. Elsebach, Ph.D. thesis, Fakultät für Mathematik, Informatik und Naturwissenschaften im Fachbereich Physik der Universität Hamburg, 2021.
- A. Risteska and D. Knipp, Handbook of Visual Display Technology, 2016, pp. 1–21 Search PubMed.
- S. Z. Bisri, T. Takenobu, Y. Yomogida, H. Shimotani, T. Yamao, S. Hotta and Y. Iwasa, Adv. Funct. Mater., 2009, 19, 1728–1735 CrossRef CAS.
- T. Kanagasekaran, H. Shimotani, R. Shimizu, T. Hitosugi and K. Tanigaki, Nat. Commun., 2017, 8, 999 CrossRef PubMed.
- N. Koch, J. Phys.: Condens. Matter, 2008, 20, 184008 CrossRef.
- L. Yan, N. J. Watkins, S. Zorba, Y. Gao and C. W. Tang, Appl. Phys. Lett., 2002, 81, 2752–2754 CrossRef CAS.
- G. Witte, S. Lukas, P. S. Bagus and C. Wöll, Appl. Phys. Lett., 2005, 87, 263502 CrossRef.
- J. Ziroff, P. Gold, A. Bendounan, F. Forster and F. Reinert, Surf. Sci., 2009, 603, 354–358 CrossRef CAS.
- H. Yamane and N. Kosugi, J. Phys. Chem. C, 2016, 120, 24307–24313 CrossRef CAS.
- H. Ishii, K. Sugiyama, E. Ito and K. Seki, Adv. Mater., 1999, 11, 605 CrossRef CAS.
- E. Goiri, P. Borghetti, A. El-Sayed, J. E. Ortega and D. G. de Oteyza, Adv. Mater., 2016, 28, 1340–1368 CrossRef CAS PubMed.
- K. S. Thygesen and A. Rubio, Phys. Rev. Lett., 2009, 102, 046802 CrossRef PubMed.
- L. Kronik and Y. Morikawa, in Understanding the Metal-Molecule Interface from First Principles, John Wiley & Sons, Ltd, 2013, ch. 3, pp. 51–89 Search PubMed.
- J. P. Perdew and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 23, 5048–5079 CrossRef CAS.
- L. Hedin, Phys. Rev., 1965, 139, A796–A823 CrossRef.
- J. W. Kim and A. Kim, Curr. Appl. Phys., 2021, 31, 52–59 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- V. I. Anisimov, F. Aryasetiawan and A. I. Lichtenstein, J. Phys.: Condens. Matter, 1997, 9, 767–808 CrossRef CAS.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- J. P. Allen and G. W. Watson, Phys. Chem. Chem. Phys., 2014, 16, 21016–21031 RSC.
- K. Berland and P. Hyldgaard, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 035412 CrossRef.
- T. Björkman, J. Chem. Phys., 2014, 141, 074708 CrossRef PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2006, 124, 219906 CrossRef.
- J. Paier, M. Marsman, K. Hummer and G. Kresse, J. Chem. Phys., 2006, 124, 154709 CrossRef CAS PubMed.
- J. B. Neaton, M. S. Hybertsen and S. G. Louie, Phys. Rev. Lett., 2006, 97, 216405 CrossRef CAS PubMed.
- D. A. Egger, Z.-F. Liu, J. B. Neaton and L. Kronik, Nano Lett., 2015, 15, 2448–2455 CrossRef CAS PubMed.
- N. Marom, O. Hod, G. E. Scuseria and L. Kronik, J. Chem. Phys., 2008, 128, 164107 CrossRef PubMed.
- N. Marom, X. Ren, J. E. Moussa, J. R. Chelikowsky and L. Kronik, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 195143 CrossRef.
- D. A. Egger, S. Weissman, S. Refaely-Abramson, S. Sharifzadeh, M. Dauth, R. Baer, S. Kümmel, J. B. Neaton, E. Zojer and L. Kronik, J. Chem. Theory Comput., 2014, 10, 1934–1952 CrossRef CAS PubMed.
- Q. Zhou, Z.-F. Liu, T. J. Marks and P. Darancet, J. Phys. Chem. A, 2021, 125, 4055–4061 CrossRef CAS PubMed.
- L. Kronik, T. Stein, S. Refaely-Abramson and R. Baer, J. Chem. Theory Comput., 2012, 8, 1515–1531 CrossRef CAS PubMed.
- S. Refaely-Abramson, S. Sharifzadeh, N. Govind, J. Autschbach, J. B. Neaton, R. Baer and L. Kronik, Phys. Rev. Lett., 2012, 109, 226405 CrossRef PubMed.
- I. Kröger, B. Stadtmüller, C. Stadler, J. Ziroff, M. Kochler, A. Stahl, F. Pollinger, T.-L. Lee, J. Zegenhagen, F. Reinert and C. Kumpf, New J. Phys., 2010, 12, 083038 CrossRef.
- L. Fernandez, S. Thussing, A. Mänz, G. Witte, A. X. Brion-Rios, P. Cabrera-Sanfelix, D. Sanchez-Portal and P. Jakob, J. Phys. Chem. C, 2017, 121, 1608–1617 CrossRef CAS.
- D. E. Hooks, T. Fritz and M. D. Ward, Adv. Mater., 2001, 13, 227–241 CrossRef CAS.
- Y. L. Huang, E. Wruss, D. A. Egger, S. Kera, N. Ueno, W. A. Saidi, T. Bucko, A. T. Wee and E. Zojer, Molecules, 2014, 19, 2969 CrossRef PubMed.
- P. Borghetti, A. El-Sayed, E. Goiri, C. Rogero, J. Lobo-Checa, L. Floreano, J. E. Ortega and D. G. de Oteyza, ACS Nano, 2014, 8, 12786–12795 CrossRef CAS PubMed.
- T. C. Leung, C. L. Kao, W. S. Su, Y. J. Feng and C. T. Chan, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 195408 CrossRef.
- T. Andreev, I. Barke and H. Hövel, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 205426 CrossRef.
- P. L. de Andres, P. M. Echenique, D. Niesner, T. Fauster and A. Rivacoba, New J. Phys., 2014, 16, 023012 CrossRef.
- N. Armbrust, J. Güdde and U. Höfer, New J. Phys., 2015, 17, 103043 CrossRef.
- T. Andreev, I. Barke and H. Hövel, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 205426 CrossRef.
- N. Armbrust, F. Schiller, J. Güdde and U. Höfer, Sci. Rep., 2017, 7, 46561 CrossRef CAS PubMed.
- N. Nicoara, J. Méndez and J. M. Gómez-Rodríguez, Nanotechnology, 2016, 27, 475707 CrossRef CAS PubMed.
- M. C. E. Galbraith, M. Marks, R. Tonner and U. Höfer, J. Phys. Chem. Lett., 2014, 5, 50–55 CrossRef CAS PubMed.
- P. Puschnig, S. Berkebile, A. J. Fleming, G. Koller, K. Emtsev, T. Seyller, J. D. Riley, C. Ambrosch-Draxl, F. P. Netzer and M. G. Ramsey, Science, 2009, 326, 702–706 CrossRef CAS PubMed.
- P. Puschnig, E.-M. Reinisch, T. Ules, G. Koller, S. Soubatch, M. Ostler, L. Romaner, F. S. Tautz, C. Ambrosch-Draxl and M. G. Ramsey, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 235427 CrossRef.
- M. Dauth, T. Körzdörfer, S. Kümmel, J. Ziroff, M. Wiessner, A. Schöll, F. Reinert, M. Arita and K. Shimada, Phys. Rev. Lett., 2011, 107, 193002 CrossRef CAS PubMed.
- S. Weiß, D. Lüftner, T. Ules, E. M. Reinisch, H. Kaser, A. Gottwald, M. Richter, S. Soubatch, G. Koller, M. G. Ramsey, F. S. Tautz and P. Puschnig, Nat. Commun., 2015, 6, 8287 CrossRef PubMed.
- I. Kröger, B. Stadtmüller and C. Kumpf, New J. Phys., 2016, 18, 113022 CrossRef.
- A. Lerch, L. Fernandez, M. Ilyn, M. Gastaldo, M. Paradinas, M. A. Valbuena, A. Mugarza, A. B. M. Ibrahim, J. Sundermeyer, U. Höfer and F. Schiller, J. Phys. Chem. C, 2017, 121, 25353–25363 CrossRef CAS.
- N. Ueno, S. Kera, K. Sakamoto and K. K. Okudaira, Appl. Phys. A, 2008, 92, 495–504 CrossRef CAS.
- S. Kera, H. Yamane, I. Sakuragi, K. K. Okudaira and N. Ueno, Chem. Phys. Lett., 2002, 364, 93–98 CrossRef CAS.
- S. Kera, H. Yamane, I. Sakuragi, K. K. Okudaira and N. Ueno, J. Electron Spectrosc. Relat. Phenom., 2007, 156–158, 135–138 CrossRef CAS.
- S. Doniach and M. Šunjic, J. Phys. C: Solid State Phys., 1970, 3, 285 CrossRef CAS.
- D. A. Shirley, Phys. Rev. B: Solid State, 1972, 5, 4709–4714 CrossRef.
- Y. Zhang, T. Learmonth, S. Wang, A. Y. Matsuura, J. Downes, L. Plucinski, S. Bernardis, C. O'Donnell and K. E. Smith, J. Mater. Chem., 2007, 17, 1276–1283 RSC.
- K. T. Park, A. Miller, K. Klier, R. L. Opila and J. E. Rowe, Surf. Sci., 2003, 529, L285–L292 CrossRef CAS.
- B. Brena, Y. Luo, M. Nyberg, S. Carniato, K. Nilson, Y. Alfredsson, J. Åhlund, N. Mårtensson, H. Siegbahn and C. Puglia, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 195214 CrossRef.
- Y. Niwa, H. Kobayashi and T. Tsuchiya, J. Chem. Phys., 1974, 60, 799–807 CrossRef CAS.
- L. Ottaviano, S. Di Nardo, L. Lozzi, M. Passacantando, P. Picozzi and S. Santucci, Surf. Sci., 1997, 373, 318–332 CrossRef CAS.
- H. Peisert, M. Knupfer and J. Fink, Surf. Sci., 2002, 515, 491–498 CrossRef CAS.
- T. Schwieger, H. Peisert, M. S. Golden, M. Knupfer and J. Fink, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 155207 CrossRef.
- K. Greulich, A. Belser, T. Basova, T. Chassé and H. Peisert, J. Phys. Chem. C, 2022, 126, 716–727 CrossRef CAS.
- Y. Liu, J. Guo, E. Zhu, L. Liao, S.-J. Lee, M. Ding, I. Shakir, V. Gambin, Y. Huang and X. Duan, Nature, 2018, 557, 696–700 CrossRef CAS PubMed.
- J. Xiao and P. A. Dowben, J. Phys.: Condens. Matter, 2008, 21, 052001 CrossRef PubMed.
- I. F. Torrente, K. J. Franke and J. I. Pascual, J. Phys.: Condens. Matter, 2008, 20, 184001 CrossRef.
- O. T. Hofmann, V. Atalla, N. Moll, P. Rinke and M. Scheffler, New J. Phys., 2013, 15, 123028 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Further experimental and theoretical details. See DOI: https://doi.org/10.1039/d2nr06440e |
| ‡ The error in the calculated work function Φ can be estimated from the clean Au(111) bottom faces of the model slabs, which shows work function differences up to 0.4 eV (Fig. S5 in the ESI†). |
| This journal is © The Royal Society of Chemistry 2023 |




![[M with combining macron]](https://www.rsc.org/images/entities/char_004d_0304.gif) directions of YbAu2 (top) and 1 ML CuPc on YbAu2. The non-dispersing HOMO levels of CuPc add to the substrate bands after adsorption (
directions of YbAu2 (top) and 1 ML CuPc on YbAu2. The non-dispersing HOMO levels of CuPc add to the substrate bands after adsorption (