Etching suppression as a means to Pt dendritic ultrathin nanosheets by seeded growth†
Deliang
Yi
ab,
Cécile
Marcelot
c,
Idaline
Romana
b,
Marine
Tassé
d,
Pier-Francesco
Fazzini
 a,
Laurent
Peres
a,
Nicolas
Ratel-Ramond
c,
Philippe
Decorse
e,
Bénédicte
Warot-Fonrose
c,
Guillaume
Viau
a,
Laurent
Peres
a,
Nicolas
Ratel-Ramond
c,
Philippe
Decorse
e,
Bénédicte
Warot-Fonrose
c,
Guillaume
Viau
 a,
Philippe
Serp
a,
Philippe
Serp
 *b and
Katerina
Soulantica
*b and
Katerina
Soulantica
 *a
*a
aLaboratoire de Physique et Chimie des Nano-Objets, UMR 5215 INSA, CNRS, UPS, Université de Toulouse, F-31077 Toulouse, France. E-mail: ksoulant@insa-toulouse.fr
bLCC, CNRS-UPR 8241, ENSIACET, Université de Toulouse, 31030 Toulouse, France
cCEMES-CNRS, Université de Toulouse, CNRS, 29 rue Jeanne Marvig, 31055 Toulouse, France
dLaboratoire de Chimie de Coordination du CNRS, 205 route de Narbonne, F-31077 Toulouse, France
eITODYS, UMR 7086, CNRS, Université de Paris, F-75013 Paris, France
First published on 21st December 2022
Abstract
2D ultrathin metal nanostructures are emerging materials displaying distinct physical and chemical properties compared to their analogues of different dimensionalities. Nanosheets of fcc metals are intriguing, as their crystal structure does not favour a 2D configuration. Thanks to their increased surface-to-volume ratios and the optimal exposure of low-coordinated sites, 2D metal nanostructures can be advantageously exploited in catalysis. Synthesis approaches to ultrathin nanosheets of pure platinum are scarce compared to other noble metals and to Pt-based alloys. Here, we present the selective synthesis of Pt ultrathin nansosheets by a simple seeded-growth method. The most crucial point in our approach is the selective synthesis of Pt seeds comprising planar defects, a main driving force for the 2D growth of metals with fcc structure. Defect engineering is employed here, not in order to disintegrate, but for conserving the defect comprising seeds. This is achieved by in situ elimination of the principal etching agent, chloride, which is present in the PtCl2 precursor. As a result of etching suppression, twinned nuclei, that are selectively formed during the early stage of nucleation, survive and grow to multipods comprising planar defects. Using the twinned multipods as seeds for the subsequent 2D overgrowth of Pt from Pt(acac)2 yields ultrathin dendritic nanosheets, in which the planar defects are conserved. Using phenylacetylene hydrogenation as a model reaction of selective hydrogenation, we compared the performance of Pt nanosheets to that of a commercial Pt/C catalyst. The Pt nanosheets show better stability and much higher selectivity to styrene than the commercial Pt/C catalyst for comparable activity.
Introduction
The unique structural features of two dimensional (2D) ultrathin nanomaterials afford outstanding properties, exploitable in condensed matter physics, materials science, and chemistry. Excellent mechanic properties, optical transparency and the possibility to control the electronic properties by external stimuli are also characteristics of the 2D configuration. Finally, their high surface area makes them ideal candidates for many applications in which surface activity is crucial, such as catalysis and supercapacitors.1 Among 2D nanomaterials, 2D ultrathin metal nanosheets (UMNS) have recently attracted the attention of the scientific community.2–5 Compared to lamellar materials for which the adoption of a 2D configuration is favored, the 2D morphology in metals is rare and intriguing, as the non-directional metal bonds and the isotropic crystal structures, in which the majority of metals crystallize, do not favor the adoption of 2D shapes. The 2D morphology in metals is thermodynamically unfavorable due to its high surface energy. Despite their instability, free-standing monometallic,6–8 as well as multi-metallic alloys and heterostructured UMNS,8,9 have been synthesized by top-down and bottom-up strategies,5 the latter constituting the most usually employed ones. The 2D morphology is achieved by employment of: (i) soft or hard templates to guide the 2D growth, (ii) shape directing capping agents that kinetically control the growth in 2D, and/or thermodynamically stabilize the extended exposed surfaces, (iii) 2D nanoparticles as seeds on the surface of which subsequent growth takes place with conservation of the 2D shape.2–4,8Physical and chemical properties associated to the 2D ultrathin morphology,2,7 which are not present in their 3D or 1D and 0D counterparts, make 2D-UMNS excellent candidates in the domains of sensing,10 photothermal therapy,11,12 and catalysis. While in the domain of electrocatalysis UMNS are being extensively studied,9,11,13,14 their use in thermal heterogeneous catalysis is much less explored,6,15–19 despite the fact that atom utilisation efficiency and improved mass activity of 2D-UMNS could be important advantages, particularly in the case of reactions catalyzed by noble metals for which cost-efficiency is mandatory.4,20
Defects, such as twin planes in fcc metals, can break the crystal structure symmetry, giving the possibility to expand the morphology repertoire beyond the isotropic shapes imposed by the fcc symmetry.21,22 The origin of the 2D morphology in fcc metals (Ag, Au, Pd), is often associated to planar defects such as twin planes23–26 appearing during nucleation or at later growth stages.27 Grooves of plate-like seeds comprising {111} twin planes, constitute preferential sites for the addition of adatoms, thus leading to in-plane growth.24,28,29 Planar defects giving rise to nanoprisms or nanoplates are thus considered as a major driving force for 2D growth. The internal structure of the seeds formed during nucleation is dictated both by thermodynamics and kinetics, and is determining for the morphology of the final nanocrystal.21,30,31 However, small metal nanocrystals (≤5 nm) are subjected to structural fluctuations rendering the efficient control of the seed microstructure problematic. Consequently, controlling nanocrystal morphology through defect engineering is difficult, even if in some cases, manipulation of the precursor reduction kinetics allows seed structure control, through etching of the nuclei comprising defects.30,32–35 It is well substantiated that twin boundaries are prone to etching, and oxidative etching is regularly employed in initial nucleation stages to control the shape of nanoparticles by selectively eliminating less stable, defect comprising nuclei, in syntheses that otherwise give rise to nanoparticles of mixed morphologies.34,36 The opposite, that is, allowing the survival of only defect comprising nuclei is less common.37,38 Defect engineering is not only a means to control nanocrystal morphology, but also their physical properties and chemical reactivity,39–42 which is crucial for their implementation in catalysis. For example, it was recently shown, both theoretically and experimentally, that oxygen binds strongly to defect sites, and induces site-selective oxide formation in Ag and Pd nanocrystals containing planar defects.43 Interestingly, the easier oxidation of defect-site atoms was also shown to play a critical role in the electrocatalytic oxidation of 5-hydroxylmethylfurfural by Ni-based multipods possessing branches of controllable thickness. In that case, an increase in the branch thickness led to an increase of the density of easily oxidizable defect sites, which in turn led to an improvement of the catalytic activity.44
Pt, one of the most widely used noble metals in catalysis, is a representative example for which the 2D configuration could be an efficient strategy toward improved catalytic performances and cost-efficiency, considering the high surface area and efficient utilization of metal active sites of the 2D nanostructures. Compared to other fcc metals such as Au, Ag and Pd, twinned Pt nanocrystals are less numerous.45–48 This is likely due to the high twin boundary energies of Pt that does not favor twin nucleation.34,49–51 The scarcity of Pt 2D nanocrystals comprising planar defects is even more striking. In fact, a small number of triangular Pt nanoplates with planar defects mixed with isotropic nanoparticles has been reported some years ago,25 and more recently, twinned planar tripods have been formed as the major, albeit not the unique product, from H2PtCl6, poly(vinylpyrrolidone) (PVP) and KBr.52 Interestingly the authors show that the controlled addition of HCl in their mixtures allows manipulation of the nanocrystal shapes, among which Pt platelets.
While several Pt-based UMNS have been synthesized by bottom-up methods,13,53–55 pure Pt UMNS are rather scarce. Pt nanosheets have been obtained by intercalation and subsequent reduction by H2 of PtCl4 between graphite layers, which played the role of a hard template.56 Very recently, a method employing silica-based hollow nanoreactors has also been employed in order to produce 2D Pt UMNS exposing {110} type facets, which have shown excellent performances in the hydrogen evolution reaction (HER).57 Other bottom-up methods employ elaborated or home-made soft templates. For instance, lyotropic liquid crystals form 2D micelles, in which single-crystalline dendritic Pt UMNS have been synthesized at room temperature.58–60 Recently, dendritic Pt UMNS of {110} type facets were prepared by using an amphiphilic surfactant, which acted as the structure directing agent.61 Multilamellar liposomes have also been employed as templates for the interfacially directed formation of dendritic Pt-sheets,62–64 and Pt-2D nanowheels have been prepared from bicelles.65 In another work, it was proposed that the presence of a peptide and AlCl3 induces the 2D assembly of isotropic nanocrystals to give polycrystalline Pt UMNS at room temperature.66 In a different strategy, Liu et al. prepared Pt nanoplates exposing {111} facets by employing as a hard template Ag nanoplates comprising twin defects, which were used as seeds on which Pt was epitaxially grown. The Ag templates were subsequently etched away by HNO3 from Ag@Pt core@shell nanoplates, leaving behind hollow Pt nanoplates.67
Here, we show that the selective synthesis of multipods comprising {111} planar defects from the reduction at room temperature of PtCl2 by H2 in the presence of octadecylamine (ODA) and sodium acetylacetonate (Na(acac)) is a key for the synthesis of Pt UMNS. We had previously shown that in the absence of Na(acac) the reaction yields single crystalline concave Pt nanocubes.68 The presence of Na(acac) radically modifies the reaction outcome by enabling etching suppression via chloride elimination, and through this, the survival of twinned nuclei. These nuclei can thus grow to twinned multipods conserving their planar defects. The selective synthesis of twinned multipods and their use as seeds favors the formation of dentritic Pt UMNS by slow reduction of Pt(acac)2 under H2 in the presence of ODA, thanks to a preferential growth along the {111} twin planes of the seeds. This is the simplest approach reported so far, since it does not demand specifically designed templates as it is the case for the pure Pt UMNS presented in previous works. It is also fundamentally different from the seeded growth methods that have been employed for the synthesis of M@Pt core@shell 2D-UMNS.53,67,69 The dendritic UMNS thus obtained present {111} type basal planes, in contrast to the so far existing Pt nanosheets of similar morphology which present {110} type basal planes. Finally, as metal Pt UMNS are underexplored in selective hydrogenation reactions, we have tested them as catalysts in the selective hydrogenation of phenylacetylene (PhA) into styrene (ST). The selective hydrogenation of alkynes into alkenes is an important process from both the industrial and the academic points of view.70 In industry, the removal of PhA, an impurity in ST, by selective hydrogenation, is crucial, because PhA poisons and deactivates polymerization catalysts in polystyrene production plants. The advantage of this process is that the undesired alkyne is directly converted into the target alkene.71 Achieving high selectivity to the targeted alkene at high alkyne conversions without over-hydrogenation is a key step in the polymer and fine chemical industries.70,71 For this reaction, the catalytic performances of the transition metal strongly depend on its electronic structure, and unpromoted Pt catalysts are not selective because they bind strongly, both C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C,72 provoking overhydrogenation of alkynes to alkanes.70
C,72 provoking overhydrogenation of alkynes to alkanes.70
Experimental
In order to exclude the influence of oxygen and of ambient humidity, the syntheses of the seeds were performed under inert conditions, using vacuum-line and glove-box techniques. Toluene (99%) was purified by a solvent purifier (Innovative Technology Purification System), degassed by Ar bubbling, and then kept in the glovebox. Thus, the presence of moisture and air was strictly excluded from the system during the seed synthesis. Seed purification was performed under ambient conditions. The platinum chloride (PtCl2, 98%) was purchased from Alfa Aesar. PtCl2 only from freshly opened containers should be used, as formation of Pt nanoparticles has been detected in PtCl2 vials opened for a long time. Platinum acetylacetonate (Pt(acac)2, 98%) was purchased from Strem, sodium acetylacetonate (Na(acac), 95%) from Alfa Aesar, octadecylamine (97%) from Sigma Aldrich, and a commercial Pt/C catalyst was purchased from Aldrich (206931-10G), 5 wt% loading, activated carbon support.Synthesis of Pt seeds
For a typical synthesis of Pt seeds in a glove box, 16 mg (0.06 mmol) of PtCl2 (98%), 60 mg (0.5 mmol) of Na(acac) (sodium 2,4-pentanedionate hydrate), 400 mg ODA (octadecylamine, 1.5 mmol) and 7 mL of toluene were mixed in a Fischer–Porter bottle. The bottle was closed, removed from the glove box and sonicated for 10 min, giving rise to a yellow-brown turbid suspension. The Ar was evacuated and the Fischer–Porter bottle was charged with H2 (3 bar) under stirring. The mixture was let to react for 24 hours under stirring (400 rpm) in a double-wall support, thermostated at 20 °C. After the end of the reaction, the Fischer–Porter reactor was evacuated and the suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and the supernatant was removed. The product was washed twice with 20 mL ethanol (96%) and three times with 20 mL toluene. After each solvent addition, the mixture was sonicated for 10 min. The final product was dispersed in 10 mL toluene giving rise to a suspension of 1 mg mL−1 (Pt = 34.1 wt% as determined by TGA analysis of a 10 mg dried sample). After sonication of this dispersion for 10 min, aliquots were used as seeds for the subsequent growth of Pt UMNS.
000 rpm for 10 min and the supernatant was removed. The product was washed twice with 20 mL ethanol (96%) and three times with 20 mL toluene. After each solvent addition, the mixture was sonicated for 10 min. The final product was dispersed in 10 mL toluene giving rise to a suspension of 1 mg mL−1 (Pt = 34.1 wt% as determined by TGA analysis of a 10 mg dried sample). After sonication of this dispersion for 10 min, aliquots were used as seeds for the subsequent growth of Pt UMNS.
Synthesis of Pt nanosheets
For a typical synthesis of Pt UMNS in a glove box, in a Fischer–Porter bottle, Pt(acac)2 (24 mg, 0.06 mmol), and octadecylamine (400 mg, 1.5 mmol) were introduced in toluene (5.8 mL), giving rise to a bright yellow solution. A Pt seed aliquot (1.2 mL, 1 mg mL−1, 2.1 × 10−3 mmol of Pt) was added to the mixture, which was then sonicated for 10 minutes. The Fischer–Porter bottle was evacuated and then charged with 3 bars H2 under stirring. After 7 minutes, the reactor was closed and the mixture was let to react for 4 days under stirring (400 rpm) in a double-wall support, thermostated at 20 °C. Once the reaction completed, the Fischer–Porter bottle was evacuated and the mixture was centrifuged for 10 minutes at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was removed, and 20 mL toluene were added, followed by centrifugation for 10 minutes at 10
000 rpm. The supernatant was removed, and 20 mL toluene were added, followed by centrifugation for 10 minutes at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. This step was repeated once. Then, 20 mL of toluene were added and the mixture was sonicated for 10 min, and the supernatant was removed after letting the mixture to decant. This process was repeated for 3 times. This last step allowed discarding the few small nanocubes that are produced as a byproduct. The precipitate was washed 1 time with pentane and vacuum dried and stored under ambient conditions. The Pt content was analyzed by ICP to be 81.2 wt%.
000 rpm. This step was repeated once. Then, 20 mL of toluene were added and the mixture was sonicated for 10 min, and the supernatant was removed after letting the mixture to decant. This process was repeated for 3 times. This last step allowed discarding the few small nanocubes that are produced as a byproduct. The precipitate was washed 1 time with pentane and vacuum dried and stored under ambient conditions. The Pt content was analyzed by ICP to be 81.2 wt%.
Characterization
Conventional transmission electron microscopy (TEM) characterizations were performed on a JEOL JEM 1011 CX-T electron microscope operating at 100 kV with a point resolution of 4.5 Å. The particle size and morphology were evaluated through TEM by measurement of at least 200 objects. High-resolution transmission electron microscopy (HRTEM) and energy-filtered transmission electron microscopy (EFTEM) were performed on a Cs corrected Hitachi HF3300 microscope (I2TEM) operated at 300 kV with a Gatan imaging filter, and on a JEOL cold FEG ARM microscope operated at 200 kV.Atomic force microscopy (AFM) was performed on a SmartsSPM-1000, AIST-NT microscope, which was used to explore the surface morphology of the samples. Topography was measured in tapping mode with a silicon tip of 15 μm length and 8 nm radius of curvature. AFM topography images of the objects are obtained by a dispersion of UMNS in a mixture of toluene/pentane and deposited on glass substrates. The objects thickness were estimated by measuring their profile with the help of Gwyddion software. The measurements were taken at different locations on the surface, and the scan areas were between 10 and 0.5 μm.
ICP analyses were performed by Kolbe. TGA analyses were performed on a METTLER ATG-DSC3+ instrument.
X-Ray diffraction experiments have been performed on a Malvern Panalytical Empyrean diffractometer, equipped with a Co anticathode X-ray source, a Bragg–Brentano HD mirror, and a PIXCel1D detector. The sample consists in a powder dropcasted on a zero-background Si support. Data have been acquired between 2θ values of 40° and 110° with an angular step of 0.02° and 1 s exposure per step in continuous mode.
X-ray photoelectron spectroscopy (XPS) spectra were recorded from samples prepared by depositing on a Si wafer a few drops of the suspended solids followed by solvent evaporation. The spectra were recorded using a K-alpha plus system (Thermo Fisher Scientific, East-Grinstead, UK) fitted with a micro-focused and monochromatic Al Kα X-ray source (1486.6 eV, spot size of 400 μm). The spectrometer pass energy was set to 150 and 40 eV for the survey and the narrow high-resolution regions, respectively.
Catalytic tests
For the catalytic tests, exactly weighted amounts of dried Pt UMNS were dispersed in 10 mL of solvent to prepare a mother suspension from which aliquots of exactly measured volumes are used in each catalytic run. The metal content of the weighted samples was known from ICP analysis of the dried UMNS. The catalytic reactions were performed in a high-pressure stainless steel autoclave connected to a gas ballast and working at constant pressure. After introduction of the reactants, the autoclave was sealed up and purged with H2 3 times. The hydrogenation experiments were carried out at a stirring rate of 1200 rpm under 5 bar H2 at 25 °C.In a typical experiment, Pt UMNS (0.62 mg, Pt = 81.2 wt%), 410 mg of phenylacetylene, 70 mg decane as internal standard and 20 mL of THF were added in the autoclave. The mixture was sonicated for 10 min. The autoclave was closed and purged with 3 bar H2 3 times, and finally filled with 5 bar H2. The temperature was controlled at 25 °C, and the stirring speed was kept at 1200 rpm. Samples were removed from the autoclave at regular intervals and were analyzed on a PerkinElmer (Clarus 580) gas chromatograph equipped with an Elite-5MS capillary column (30 m × 0.32 × 0.25 μm) and with a flame ionization detector. The same procedure was applied for the tests with 10 mg of the commercial 5 wt% Pt/C catalyst.
The conversion and the selectivity were calculated from the following equations:
| Conv% = (ninitial − nfinal)/ninitial × 100%, |
| Sx% = nx/nproduct × 100%, |
The activity was calculated from the following relationship:
| Activity = molPhA molmetal−1 h−1 |
Results and discussion
Synthesis and characterization
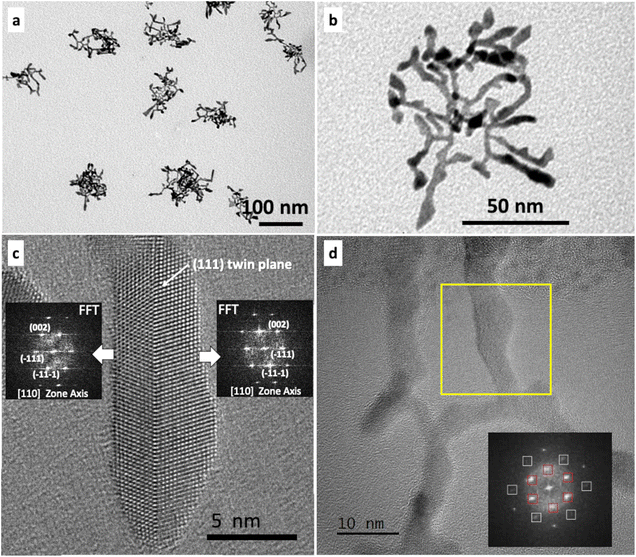
In a previous work,68 we showed that the reduction of PtCl2 under H2 at 20 °C in the presence of octadecylamine (ODA) results in the slow formation of single crystalline concave Pt nanocubes exposing {110} type facets, which grow from cubic seeds through deposition of Pt adatoms on the nanocube corners and their subsequent diffusion along the nanocube edges (Fig. S1†). The simple replacement of PtCl2 by Pt(acac)2 (acac = acetylacetonate) leeds to the slow formation of nano-objects of mixed morphology, among which, few dendritic 2D nanostructures (Fig. 1). High resolution transmission electron microscopy (HRTEM) observation of the multibranched nano-objects shows that they correspond to fcc Pt nanosheets generally observed along a [111] zone axis. In this orientation, fast Fourier transform (FFT) of a large area shows a small angular enlargement of the {220} reflection spots, due to a slight misalignment of the crystallographic orientation between different sections of the nanosheet, as confirmed by the FFT of small areas on different pods (Fig. 1c and d). The FFT also reveal the presence of 1/3 {422} reflexions, which are forbidden in a perfect fcc crystal, and are characteristic of the presence of at least one twin plane running parallel to {111} faces of platelet-shaped nano-objects.46,73 Therefore, under conditions in which PtCl2 forms single crystals exclusively,68 Pt(acac)2 induces the formation of nanostructures dominated by twin defects.The striking difference between the results obtained with the two precursors, incited us to examine the influence of acac in the formation of twinned nanostructures by using PtCl2 as a precursor, but in the presence of sodium acetylacetonate (Na(acac)) as a source of acetylacetonate. The reaction under H2 of PtCl2, ODA and Na(acac) (PtCl2/Na(acac)/ODA = 1/8/25) at 20 °C gives rise to dendritic Pt multipods after 24 hours of reaction (Fig. 2a and b). HRTEM observations confirmed the expected fcc crystal structure of Pt. Interestingly, whenever the branch orientation with respect to the beam direction was favorable, a single {111} twin plane was evidenced (Fig. 2c). Branches oriented along the [111] zone axis were also frequently observed (Fig. 2d). These observations point toward the formation of dentrites composed of thin branches comprising {111} twin planes.
The time dependent morphological evolution of these multipods shows the initial formation of nanostructures presenting one, two or three wormlike branches of about 10 nm length, 1.5–2.0 nm diameter, terminating to enlarged tips (Fig. S2a–c†). Judging from their increased contrast, the tips seem to be thicker. Letting the reaction proceed for several days (Fig. S3†), induces aggregation, which makes subsequent dispersion of the seeds difficult. After 24 hours, these nanostructures evolve into dendritic nano-objects, most likely through multiple branching events. The predominance of three-branched specimens at early reaction times (Fig. S2†) suggests that three-fold symmetry twinned seeds grow by addition of Pt along the {111} twin planes, either on one, two or three of their tips. Outgrown branches are then subjected to additional branching events to give the final multipods, with planar defect conservation. HRTEM analysis of an appropriately oriented nano-object obtained after 8 hours of reaction shows the presence of a twin plane (Fig. S4†).
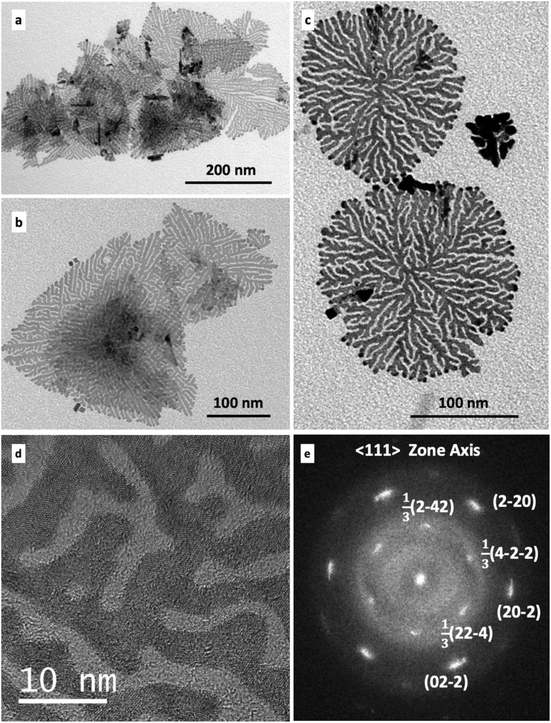
Since in the absence of seeds, the reduction of Pt(acac)2 by H2 in the presence of ODA forms only few UMNS as a minor morphology in a mixture of cuboidal nano-objects (nanocubes and concave nanocubes) (Fig. 1), we expected that, adding the multipods as seeds already including twin defects in the reaction medium, would enhance the shape selectivity to Pt UMNS. Combined to the mild reaction conditions employed in order to limit new nucleation events, the presence of these seeds should favor the growth by heterogeneous nucleation, which could proceed with retention of the planar defects of the seeds. Indeed, the introduction of the multipod seeds in a growth solution containing Pt(acac)2 and ODA gives rise to dendritic Pt UMNS after 4 days reaction under H2 at 20 °C (Fig. 3a–c). The majority of the UMNS have diameters that extend over 100–500 nm and speciments of either triangular or round contours are formed at the same time (Fig. S5†). Most of the times, several UMNS form layers of overlapping individual sheets and folded UMNS are often observed.
X-Ray diffraction of the isolated powder showed that the particles crystallize with the fcc structure (Fig. S6†), and HRTEM observations showed that the Pt UMNS are generally observed along a [111] zone axis (Fig. 3d and e). Fig. 3e shows the FFT recorded from the large area of the UMNS shown in Fig. 3d. This pattern, displaying distinct spots, corresponds to a single crystalline configuration and not to a polycrystalline nano-object, even if it involves several branches of the particle. This proves a very good coherence of the crystalline orientation from one branch to another with only a slight misorientation in the plane perpendicular to the zone axis. This is indicated by the small angular enlargement of the spots, as in the case of the scarce nanosheets formed in the absence of preformed sheets by Pt(acac)2 and ODA (Fig. 1c and d). Additionally, the FFT displays six 1/3 {422} forbidden reflexions, indicating the presence of {111} type twin planes running parallel to the basal UMNS planes (Fig. 3e).
TEM observation of samples obtained after stopping the reaction at various time intervals demonstrates that the dentrites gradually evolve toward UMNS (Fig. S7†). As the UMNS grow from the center toward the periphery, multiple branching events in the growing plane fill the 2D space between the branches, which however do not fuse together, thus forming channels. After 4 days, the UMNS are fully grown and extending the reaction time to 7 days seems to slightly thicken the extremities of the branches. Evaluation of the relative thickness by energy filtered transmission electron microscopy (EFTEM) indicates that it is about 1 nm, that is, about 4–5 Pt layers thick (Fig. S8a and b†). Atomic force microscopy (AFM) measurements on whole nanosheets corroborate the EFTEM result (Fig. S8c†).
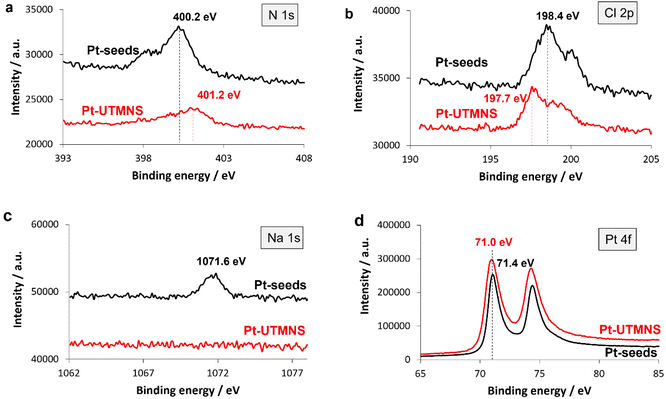
XPS analyses (Fig. 4), relevant both for rationalizing the formation of the nanostructures and the catalysis results,74 have been performed to obtain information on the surface chemistry of the multipod seeds and Pt UMNS. The Pt UMNS present a broad N 1s peak (Fig. 4a) that can be resolved into three components (deconvolution in Fig. S9a and fitting results in Table S1†). The major N 1s signal at 401.2 eV corresponds to an ammonium moiety (–NH3+, 401.3–401.7 eV).75,76 The peak at 399.6 eV corresponds well to what might be expected for a nitrogen atom donating electron density to the Pt surface via its lone pair (399.5–399.6 eV),77,78 but could also correspond to free ODA (399.2–399.4 eV),76 which points toward a moderate charge transfer from the ligand to the metal. The peak at 398.0 eV could correspond to an imine,77 formed by the condensation between the ODA and acac.79 The N 1s spectrum of the Pt seeds is shown on Fig. 4a (deconvolutions in Fig. S10a and fitting results listed in Table S1†). The major species is located at 400.2 eV (coordinated amine), a minor peak at 398.2 eV corresponds to electron rich N species, and the absence of any contribution at 401.2 eV suggests that the nitrogen species are not protonated on the Pt seeds.
Despite the fact that the Pt UNMS growth solution was composed only of [Pt(acac)2] and ODA, Cl was detected by XPS (Fig. 4b). The only explanation for the presence of Cl on the Pt UNMS is its presence on the multipod seeds, which are prepared from the PtCl2 precursor. Indeed, the XPS analysis of the Pt seeds shows the presence of Cl (Fig. 4b). The Cl 2p spectrum of the Pt UMNS) was deconvoluted by fitting two spin orbit split peaks (2p1/2 and 2p3/2) for each of the different chemical states of chlorine (deconvolution in Fig. S9b and fitting results in Table S2†). The major peak (83 at%), appears at a 2p3/2 binding energy (BE) of 197.7 eV, indicating the presence of ionic chloride species, which could be present both as a counter ion of the ammonium (BE = 196.6 eV)80 and coordinated on the Pt surface (BE at 198.2–198.6 eV).81 The second peak with a 2p3/2 BE at 200 eV (17 at%) indicates the presence of more covalent chlorine species. Since Cl species on Pt (metal or oxide) have been reported at BE as high as 199.2–200 eV,82–85 we have attributed this peak to Cl adsorbed on PtO, which has been formed by exposure of the sample to air. The Cl 2p spectrum of the Pt seeds (Fig. 4b) shows the presence of a single ionic chloride contribution with 2p3/2 BE peak at 198.4 eV (deconvolutions are shown in Fig. S10b and fitting results listed in Table S2†), consistent with the presence of a single bonding environment for the Cl atoms.82,86 Since no ammonium was detected for the Pt seeds, we can exclude any contribution from ammonium chloride. NaCl (BE at 198.7 eV) should contribute to this peak, since Na (BE at 1071.6 eV) was detected in the case of the Pt seeds (Fig. 4c). It is also possible that Cl associated to Pt contributes to this peak (BE at 198.3–198.5 eV for 1–3 nm Pt nanoparticles).81
The C 1s spectrum of the UMNS is shown on Fig. S11a and b (fitting results listed in Table S3†). The more intense peak (CA) at 284.8 eV is related to aliphatic sp3 C (C–C/C–H from amine or acac). The second peak (CB) at 286.2 eV can be attributed to contributions of C–O, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N species (from acac,87,88 amine,89,90 and imine).91 Finally, the peak at 288 eV (CC) is attributed to the C
N species (from acac,87,88 amine,89,90 and imine).91 Finally, the peak at 288 eV (CC) is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group from acac.87 The C 1s spectrum of the Pt seeds is shown on Fig. S11c and d (fitting results listed in Table S3†). As for the UMNS aliphatic sp3 carbon (CA) is present. The main difference arises from a shift of the CB and CC peaks from 286.2 to 286.4 eV (CB) and from 288.0 to 288.5 eV (CC). For the CB peak, this shift can be attributed to different contributions of the three C–O, C–N and C
O group from acac.87 The C 1s spectrum of the Pt seeds is shown on Fig. S11c and d (fitting results listed in Table S3†). As for the UMNS aliphatic sp3 carbon (CA) is present. The main difference arises from a shift of the CB and CC peaks from 286.2 to 286.4 eV (CB) and from 288.0 to 288.5 eV (CC). For the CB peak, this shift can be attributed to different contributions of the three C–O, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N species, whereas for the CC peak it could be related to a different environment of the acetylacetonate ion. The BE of the carbonyl group of the acac ligand coordinated on Pt has been measured at 288.5 eV,88 which corresponds well to what we found for the seeds. The lower BE measured for the UMNS could be related to the presence of the acac as anion of the ammonium salt, which is not present on Pt seeds.
N species, whereas for the CC peak it could be related to a different environment of the acetylacetonate ion. The BE of the carbonyl group of the acac ligand coordinated on Pt has been measured at 288.5 eV,88 which corresponds well to what we found for the seeds. The lower BE measured for the UMNS could be related to the presence of the acac as anion of the ammonium salt, which is not present on Pt seeds.
The Pt 4f spectrum of the UMNS is shown on Fig. 4d (see Fig. S9c for deconvolution and Table S4† for fitting results). The peaks at 70.9 eV (61 at%), 71.9 (28 at%), and 73.4 eV (11 at%) correspond to the Pt 4f7/2 peaks of Pt0, Ptδ+ (Pt–Cl species or Pt-acac),78,82 and Pt2+ (PtO) respectively.92 The Pt 4f spectrum of the Pt seeds (deconvolution are shown in Fig. S10c and fitting results listed in Table S4†) is similar to the one of the UMNS (Fig. 4d), pointing to a similar electronic effect of the surrounding ligands in both samples. We noticed only the absence of surface oxidation due to prolonged air exposure for the Pt seeds (no PtO peak at 73.4 eV).
From these XPS analyses, some important information is obtained: first, Cl ions coming from the Pt seeds are present on the UMNS. Second, octadecylamonium ions are absent from the seeds but present on the UMNS. Third, Na is absent from the UMNS but present on the seeds, which points toward NaCl being present on the seeds as an impurity. Fourth, it is clear that, even at room temperature, side reactions between the acac and the amine can take place as it has already been reported for nanoparticle syntheses employing metal acetylacetonates with primary amines.79 Even if the reaction conditions were not the same as in the present work, several organic products have been detected in the case of reactions of oleylamine with Pt(acac)2,93 or Ni(acac)2.79 Schiff condensation of NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was also reported during the grafting of various M(acac)2 precursors on amine functionalized silica.94 Finally, a moderate charge transfer from the amine to the metal has been evidenced.
O was also reported during the grafting of various M(acac)2 precursors on amine functionalized silica.94 Finally, a moderate charge transfer from the amine to the metal has been evidenced.
Mechanistic considerations
The information obtained from our data allows an insight into the processes taking place during the two steps leading to the UMNS: (i) the formation of the twinned multipods, and (ii) the growth of UMNS using the multipods as seeds. As we have seen, employing the twinned multipods as seeds is crucial for greatly improving the shape selectivity to UMNS. Therefore, the synthesis of the dendritic twinned seeds merits further attention, as it is a key for increasing the UMNS shape yield in the second step.In order to better rationalize our results, it is important to identify the essential conditions that allow planar defects to dominate in the multipod seeds. The origin of the striking difference between the nano-objects formed in the presence of Na(acac) (twinned multipods) and the ones in it absence (single crystalline concave nanocubes)68 from the reduction of PtCl2 by H2 in the presence of ODA is the base for determining the most important driving force for the selective formation of twinned multipods, which are crucial for the development of the UMNS.
Due to the very limited solubility of PtCl2 and Na(acac) in toluene, in a first step, PtCl2 should slowly provide soluble Pt species through Cl-bridge splitting by ODA. We presume that PtCl2(ODA)2 is the most probable initially formed Pt species. Complexes involving acac and mixed ODA-acac compounds can be formed,95 however the poor solubility of Na(acac) most likely prevents acac coordination, at least at the beginning of the reaction. Reduction of the soluble intermediate(s) by H2, necessarily involves elimination of the chloride, as HCl, and it is well substantiated in the literature that HCl can dissolve nuclei incorporating defects.22,34,35,96 This is in fact what happens in the case of the reaction between PtCl2 and HDA where only single crystalline concave cubes are produced: chloride-induced etching disintegrates metastable twinned nuclei, and allows formation of more stable single crystalline ones that evolve to concave nanocubes (Fig. S1†).68 However, when Na(acac) is present, the released HCl is efficiently put out of circulation through formation of insoluble NaCl, which is corroborated by the XPS analysis of the multipods, and acacH. Efficient scavenging of the Cl ions switches off the possibility of etching, and allows the survival of the twinned nuclei and their growth beyond a critical size, because the reaction pathway that ripens twinned nuclei to single crystals is blocked. Twinned nuclei subsequently grow to multipods, which conserve the twin defects.
While switching etching off explains the persistence of twinned nuclei that then grow to multipods, it cannot explain the complete absence of single crystalline specimen in the reaction product. The birth of defects during nanocrystal nucleation is poorly understood and mixtures of different nuclei can coexist in the early nucleation stage.97–99 However, our results suggest that nuclei with planar defects is the exclusive product during the early nucleation stage. If this hypothesis is correct, then oxidative etching should be the unique pathway to more stable shapes, such as cuboctahedrons, which then evolve to cuboids. Thus, increasing the temperature should not affect the reaction outcome.31,34 In order to verify this, we performed at 100 °C the standard reaction that gives rise to multibranched twinned seeds at 20 °C. As shown in Fig. S12,† small multipods resembling to the wormlike seeds obtained at early reaction times at 20 °C are the only products of the reaction. Therefore, provided that etching is the main mechanism by which evolution of the nuclei takes place during the nucleation step, in situ suppression of the etching process offers also the opportunity to obtain information on the internal structure of the initially formed metastable nuclei.
The presence of Na(acac) in excess with respect to PtCl2 is necessary for the twinned multipods to be selectively formed. Control experiments performed in the presence of different PtCl2/Na(acac) ratios are shown in Fig. S13a–c.† Reducing the Na(acac)/PtCl2 ratio increases the number of nano-objects with cuboidal symmetry at the expense of dendritic nano-objects, and when a stoichiometric ratio (PtCl2/Na(acac) = 2) is employed (Fig. S13c†), very few multipods are formed. These results point towards a competition between Na(acac) and ODA for chloride. ODA, which is in large excess in solution, could form ODAH+Cl−, which is acidic enough to etch away twinned nuclei produced during the early nucleation stages.100 We assume that, in the absence of a high excess of Na(acac), the Cl ions of ODAH+Cl− have the possibility to etch away twinned nuclei giving the opportunity to the system to attain the thermodynamically favored shapes, before elimination from the solution of the Cl− as NaCl is completed.
Considering the second step of the reaction, that is, the UMNS formation in the presence of twinned seeds, it is worth considering first what is happening with Pt(acac)2 and ODA in the absence of seeds (Fig. 1). Interestingly, the formation of a mixture of cubes and scarce UMNS indicates that under these conditions, twinned nuclei are indeed formed, but only few overpass the critical size after which they cannot be completely dissolved. This indicates that etching is operating in this case also, however it is less efficient than the one induced by Cl. When only Pt(acac)2 and ODA are used, the only possible etchant is the acac ions that may be present as the anion of octadecylammonium.101 Thus, while a few twinned nuclei survive to grow to UMNS, the majority of them does not attain the critical size. Even if it is mild, the etching induced by acac− allows the energy barrier to the stable nuclei to be overpassed with time, yielding cuboidal crystals as the major product. Indeed, the fact that the UMNS produced from the seeded growth with multipods seem to have more homogeneous thickness and they are thinner than the tips of the seeds from which they grow (Fig. 1c), is in agreement with a mild etching process by acac ions.
Scheme 1 summarizes the proposed nucleation pathways, and the different types of seeds favored in each case. When only PtCl2 and ODA are present (Scheme 1a), the Cl ions liberated remain in the reaction medium and etch away the kinetically favored twinned nuclei as soon as they appear. As the reaction proceeds, the thermodynamically favored defect-free nuclei, represented here as cubes, are formed, and after subsequent growth, they evolve to concave cubes as described by Peres et al.68 Addition of Na(acac) in sufficient amounts in the reaction medium (Scheme 1b) traps the Cl ions as NaCl. Due to the insolubility of NaCl, there is no possibility of etching. Only the kinetic product (twinned nuclei) survives and evolves to the twinned multipods. Scheme 1c illustrates the situation where only Pt(acac)2 and ODA are used. In this case, only acac ions of mild etching ability are present. A competition between the kinetic and the thermodynamic product is possible. Only few twinned nuclei have the possibility to attain the critical size beyond which complete dissolution is not possible anymore, and grow to UMNS. The majority of the seeds is transformed to the most stable product that after growth are present as nanoparticles of cubic symmetry.
The last nucleation pathway (Pt(acac)2/ODA) is important for understanding the seeded growth of UMNS by introduction of seeds in the system. Here also, some cubes are still produced due to the mild etching process by the acac ions and probably due to the presence of chloride residues on the seeds and as ODAH+Cl−, as indicated by the XPS of the UMNS. However, when the reaction is performed at low temperature, heterogeneous nucleation on the seeds is favored, etching is limited and the kinetic product (UMNS) is favored. The importance of employing very mild temperature in this second step is reflected in the dramatic modification of the resulting nanoobjects morphology upon slightly increasing the temperature. Indeed, 16 h reaction at 40 °C results in the formation of a high number of thermodynamically more stable nanocrystals of diverse shapes (Fig. S14a†). On the other hand, reducing the temperature to 10 °C, does not allow the reaction to proceed at an appreciable rate (Fig. S14b†).
We have evidenced the determining role of the chloride on the first step of the reaction, that is the seed growth. However, Cl, acac and ODA and their derivatives are also present on the surface of the seeds and the UMNS formed from these seeds. The multiple roles that halides may play have been the subject of several studies.96,102–104 Apart from controlling the reduction kinetics, the ligands, as well as their reaction products detected by XPS, may also play important roles in the stabilization of the exposed facets. For instance, the length of the amine affects the shape of both the seeds and the UMNS (Fig. S15†), with the shorter ligands not being well adapted to produce UMNS. We speculate that long chain amines can be organized parallel to each other on the surface of the UMNS, thus avoiding extensive etching of the UMNS by acac.
To summarize, we have shown that: (i) Cl− is detrimental to the survival of the twinned nuclei, which are formed as the exclusive product in the early nucleation step from PtCl2 and ODA, (ii) the elimination of Cl− as insoluble NaCl is the key for the selective formation of twinned seeds. (iii) Etching suppression allows an insight to the internal structure of the early nucleation products, (iv) acac− as a mild etching agent is responsible for the reduced thickness of the UMNS as compared to the multipod seeds, but also for the formation of some cubes during the seeded growth.
In a work by Xiong et al., 2D nanocrystals of Pt have been formed under different experimental conditions than the ones employed here.52 In that work, despite Cl being present in the precursor, 2D nanocrystals of three-fold symmetry comprising twin planes were formed. Addition of HCl in the system resulted in the formation of single crystalline nanoparticles. Despite the fact that no etching suppression was attempted, the results are in agreement with a transition from twinned to single crystalline nanocrystals upon Cl-induced etching. In general, limitation of oxidative etching can be achieved by eliminating O2 from the reaction medium.102,103 On the other hand the complete absence of etching possibility has been rarely exploited. Thanks to the use of halide-free precursors Rh starfish shaped nanoparticles have been obtained, while addition of HCl to the system or the use of a Cl-comprising precursor led to irregular nanocrystals.38 Etching suppression by in situ removal of the Cl (or other halides), is interesting because the vast majority of precursors employed in noble metal nanoparticle syntheses contain chloride (and less often other halides). In addition, halide containing stabilizing agents such as hexadecyltrimethylammonium chloride (CTAC) or hexadecyltrimethylammonium bromide (CTAB) are routinely used, which can also afford halides in the reaction medium. Both precursor and stabilizers of this kind may induce etching effects, especially in the presence of O2. If this possibility is not considered, the interpretation of the results can be biased.96 A possibility of etching-free nucleation is offered by in situ elimination of precursor ligands that could efficiently ripen the twinned nuclei away, or by using organometallic precursors comprising non-coordinating ligands.
Selective hydrogenation of phenylacetylene
Although UMNS show high surface-to-volume ratios and thus, an increased exposure of low-coordinated sites that can provide high reactivity,105 very few studies exist on the use of Pt nanosheets for selective hydrogenation of alkynes. Graphite intercalated Pt nanosheets were found to be active for hydrogenation of phenylacetylene (PhA), but produce as the major product ethylbenzene (EB) and not the targeted styrene (ST).106,107 It was proposed that the active sites were the edges of the 2D metal nanosheets. Free standing, ligand-free Pt nanosheets exposing the (111) and (200) facets of fcc Pt were reported to be very active for the hydrogenation of ST to EB at 1 bar and room temperature,108 confirming possible selectivity issues due to the strong over-hydrogenation activity of Pt. Since the use of metal Pt nanosheets in selective hydrogenation catalysis is still in its infancy, this incited us to test the Pt UMNS in the selective hydrogenation of PhA, and to benchmark them with a commercial highly dispersed catalyst 5% Pt/C (Pt nanoparticles = 1.1 nm, Fig. S16a†).The selective hydrogenation of PhA was performed at 25 °C under 5 bar of H2 in THF. The results of catalyst activity and selectivity for the two catalysts are presented in Fig. 5, and the TEM analyses of the fresh and spent catalysts are shown in Fig. S16.† The highly dispersed Pt/C catalyst shows slightly higher activity (Fig. 5d), but significantly lower selectivity (Fig. 5c) and stability (Fig. S16†) than the UMNS during the hydrogenation reaction. Considering the geometrical features of Pt particles in Pt/C and those of the Pt UMNS (Fig. S17†), the Pt/C catalyst exhibits a much higher proportion of surface Pt atoms. Thus, the activities measured for these two catalysts indicate that the Pt UMNS surface sites is higher. Notably, the kinetically favorable over-hydrogenation to produce EB is dominant on the Pt/C catalyst, as already reported in the literature for various carbon-supported Pt catalysts.106,109–112 Indeed, the selectivity toward ST on Pt/C decreases dramatically from 60% at 40% conversion to 15% at 97% conversion of PhA, while for Pt UMNS, over hydrogenation is much more limited, the selectivity toward ST being 75% at 97% conversion of PhA. The high ST selectivity obtained with Pt UMNS is surprising considering the reported high activity of Pt nanosheets for ST hydrogenation.108 However, these nanosheets were ligand-free and have different crystallographic orientation that the UMNS presented here. TEM observations performed on the spent catalysts show that severe sintering does occur for the Pt/C catalyst (final mean Pt nanoparticles size = 5.2 nm), while the structure of Pt UMNS is not affected by the reaction (Fig. S16b and d†).
The different performances of these two catalysts could be related to the presence of the ODA as the principal ligand on the Pt UMNS. Indeed, organic ligands, able to induce both steric and electronic effects on metal nanoparticles, can contribute to the tuning of the hydrogenation pathways.113 We thus investigated the influence of the ODA ligand on the Pt/C catalyst using two different ODA concentrations. Knowing that Pt UMNS contains around 20% w/w ligands, we first added 20% w/w ODA with respect to Pt on the Pt/C catalyst (catalyst Pt-ODA1/C). As the ODA ligand can also be adsorbed on the carbon support,114,115 we also prepared a second catalyst (Pt-ODA2/C), containing a significantly higher amount of ODA: 20% w/w ODA with respect to the Pt/C catalyst. The influence of ODA ligand on the performances of the Pt/C catalyst is shown on Fig. S18† and Fig. 5d. The addition of increasing amounts of ligand induces a decrease of activity, which could be assigned to a site blocking effect due to the N-containing ligand,116 and an increase of selectivity towards styrene. The increase in ST selectivity upon ODA addition could arise from an electronic effect induced by the donating amine ligand, which contributes to the formation of electron-richer Pt species. Even if the XPS data of Pt UMNS indicate a moderate charge transfer from the amine to the metal, the Pt 4f7/2 peak of Pt0 at 70.9 eV shows that Pt should be more electron rich in Pt UMNS than in Pt/C before ODA addition. Indeed, small Pt nanoparticles as the ones present in Pt/C are subjected to significant charge transfer from Pt to the carbon support, giving rise to positively charged particles.117,118 On ODA-modified Pt/C, the dissociation of Pt–H bonds to release H2 gas could be accelerated, due to weakened Pt–H bonds on more electron-rich Pt species.119 We thus presume that the weakened H adsorption strength is the main reason for the observed selectivity, since ST selectivity is known to decrease with increasing hydrogen coverage.120 Such electronic effect, which could be related to a downshift of the Pt d-band center72 thanks to the amine ligand,121 has already been reported for selective hydrogenation of PhA on electron rich, phosphine-modified Pd nanoparticles,122 and N-graphene encapsulated Pt nanoparticles.110 While this electronic ligand effect can contribute to the high selectivity obtained with the Pt UMNS catalyst, it cannot by itself justify the better performances of Pt UMNS, since this catalyst outperformed also the Pt-ODA2/C catalyst, both in terms of activity and selectivity (Fig. 5d). Although a direct comparison of catalytic performances is always difficult because of different experimental conditions and reactor configuration, the Pt UMNS catalyst also shows interesting performances compared to other Pt catalysts reported in the literature (Table S5†).
Further studies are needed to determine the exact nature of active sites of Pt UMNS, however, some hypotheses can be made on the basis of our results and from the analysis of the literature. First, carbon supported Pt nanoparticles are active for PhA hydrogenation but not selective for ST formation. These small particles should present, according to their size, different proportion of facets and edges. For 1.1 nm particles, a cubo-octahedron model predicts 75% of edge sites and 25% of (111) facets.123 Second, it has been shown, that in PhA hydrogenation, ligand-free Pt nanosheets exposing the {111} and {200} type facets are very active for ST hydrogenation to EB, suggesting a low selectivity to ST.108 Third, ligand-free Pt nanosheets intercalated between graphene layers, presenting mainly edges, were also poorly selective for PhA hydrogenation.106,107 Finally, our results suggest that the presence of ODA, in itself, is not sufficient to explain the catalytic performance. From these observations, we believe that specific sites of the Pt UMNS surface, such as ODA-decorated lateral sites could be at the origin of the high activity and selectivity of Pt UMNS. The Pt UMNS are highly dendritic and the number of lateral sites is not negligible. These electron-rich sites should be effective to transfer electrons to the π* molecular orbitals of PhA, promoting its activation and improving catalytic activity. These sites should favor a weak π adsorption of the ST molecule,111 rather than a strong di-σ adsorption as observed on Pt(111),124,125 explaining the better selectivity obtained on the Pt UMNS catalyst. Finally, the role of the amino ligands is not limited to activity and selectivity enhancement, but most likely also contributes to the catalyst stability.
Conclusions
Here we have shown that crystalline UMNS of pure Pt presenting {111} basal planes can be selectively synthesized by a seed mediated approach. The most crucial point is the selective synthesis of seeds comprising at least a twin plane, which depends on the possibility to suppress the etching process taking place during the early nucleation step, which is detrimental for the stabilization of twinned seeds. Upon introduction of an excess of Na(acac) in the reaction medium, and due to the formation of NaCl, the chloride present on the PtCl2 precursor is efficiently removed from the reaction, switching off the possibility of efficient oxidative etching, which would otherwise favor the formation of thermodynamically stable defect-free nuclei that evolve to cuboids. These results suggest that when twinned seeds are pursued, chloride containing precursors, which are widely employed for the synthesis of metal nanostructures, should be avoided. For this step, for which preservation of the initial defect comprising nuclei is crucial, in situ halide elimination or the use of organometallic precursors bearing ligands that upon hydrogenation/decomposition are converted to non-coordinating species, could offer an advantage. The use of Pt(acac)2 as the growth precursor allows a mild ripening that accounts for the final shape of the UMNS. The UMNS synthesized here have been studied in the selective hydrogenation of phenylacetylene exhibiting high activity and high selectivity to styrene, even at high conversion.Author contributions
D. Yi: investigation, conceptualization, writing – original draft, visualization; C. Marcelot, I. Romana, M. Tassé, L. Peres, N. Ratel Ramond, P. Decorse: investigation, visualization, writing – review and editing; P.-F. Fazzini: supervision, visualization; B. Warot Fonrose: supervision, resources, funding acquisition, writing – review and editing; G. Viau: supervision, visualization, writing – review and editing, P. Serp, K. Soulantica: conceptualization, supervision, funding acquisition, writing – review and editing.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors thank the EUR grant NanoX ANR-17-EURE-0009 for financial support in the framework of the “Programme des Investissements d'Avenir” through the project CaSh. L. Peres thanks the University of Toulouse for financial support. The authors thank Angélique Gillet, Simon Cayez and Adeline Pham for technical support.References
- H. Zhang, ACS Nano, 2015, 9, 9451–9469 CrossRef CAS PubMed.
- Z. Fan, X. Huang, C. Tan and H. Zhang, Chem. Sci., 2015, 6, 95–111 RSC.
- Y. Chen, Z. Fan, Z. Zhang, W. Niu, C. Li, N. Yang, B. Chen and H. Zhang, Chem. Rev., 2018, 118, 6409–6455 CrossRef CAS PubMed.
- Y. Pei, L. Huang, J. Wang, L. Han, S. Li, S. Zhang and H. Zhang, Nanotechnology, 2019, 30, 222001 CrossRef CAS PubMed.
- T. Wang, M. Park, Q. Yu, J. Zhang and Y. Yang, Mater. Today Adv., 2020, 8, 100092 CrossRef.
- H. Duan, N. Yan, R. Yu, C.-R. Chang, G. Zhou, H.-S. Hu, H. Rong, Z. Niu, J. Mao, H. Asakura, T. Tanaka, P. J. Dyson, J. Li and Y. Li, Nat. Commun., 2014, 5, 3093 CrossRef PubMed.
- A.-X. Yin, W.-C. Liu, J. Ke, W. Zhu, J. Gu, Y.-W. Zhang and C.-H. Yan, J. Am. Chem. Soc., 2012, 134, 20479–20489 CrossRef CAS PubMed.
- Q. Yu and Y. Yang, ChemNanoMat, 2020, 6, 1683–1711 CrossRef CAS.
- M. A. Z. G. Sial, M. A. U. Din and X. Wang, Chem. Soc. Rev., 2018, 47, 6175–6200 RSC.
- S. R. Beeram and F. P. Zamborini, J. Am. Chem. Soc., 2009, 131, 11689–11691 CrossRef CAS PubMed.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28–32 CrossRef CAS PubMed.
- S. Tang, M. Chen and N. Zheng, Small, 2014, 10, 3139–3144 CrossRef CAS PubMed.
- Y. Feng, Q. Shao, F. Lv, L. Bu, J. Guo, S. Guo and X. Huang, Adv. Sci., 2020, 7, 1800178 CrossRef CAS.
- H. Tao, Y. Gao, N. Talreja, F. Guo, J. Texter, C. Yan and Z. Sun, J. Mater. Chem. A, 2017, 5, 7257–7284 RSC.
- L. Zhao, C. Xu, H. Su, J. Liang, S. Lin, L. Gu, X. Wang, M. Chen and N. Zheng, Adv. Sci., 2015, 2, 1500100 CrossRef PubMed.
- Y. Dai, S. Liu and N. Zheng, J. Am. Chem. Soc., 2014, 136, 5583–5586 CrossRef CAS PubMed.
- L. Dai, Y. Zhao, Q. Qin, X. Zhao, C. Xu and N. Zheng, ChemNanoMat, 2016, 2, 776–780 CrossRef CAS.
- N. Yang, H. Cheng, X. Liu, Q. Yun, Y. Chen, B. Li, B. Chen, Z. Zhang, X. Chen, Q. Lu, J. Huang, Y. Huang, Y. Zong, Y. Yang, L. Gu and H. Zhang, Adv. Mater., 2018, 30, 1803234 CrossRef PubMed.
- Z. Huang, S. Li, B. Xu, F. Yan, G. Yuan and H. Liu, Small, 2021, 17, 2006624 CrossRef CAS PubMed.
- M. Luo, Mater. Today, 2019, 23, 45–56 CrossRef CAS.
- S. E. Skrabalak, Acc. Mater. Res., 2021, 2, 621–629 CrossRef CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- V. Germain, J. Li, D. Ingert, Z. L. Wang and M. P. Pileni, J. Phys. Chem. B, 2003, 107, 8717–8720 CrossRef CAS.
- C. Lofton and W. Sigmund, Adv. Funct. Mater., 2005, 15, 1197–1208 CrossRef CAS.
- Y. Xiong, I. Washio, J. Chen, H. Cai, Z.-Y. Li and Y. Xia, Langmuir, 2006, 22, 8563–8570 CrossRef CAS PubMed.
- A. I. Kirkland, D. A. Jefferson, D. G. Duff, P. P. Edwards, I. Gameson, B. F. G. Johnson and D. J. Smith, Proc. R. Soc. London, Ser. A, 1993, 440, 589–609 CrossRef CAS.
- P. Hartman, Z. Kristallogr. – Cryst. Mater., 1956, 107, 225–237 CrossRef CAS.
- R. Jagannathan, R. V. Mehta, J. A. Timmons and D. L. Black, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 13261–13265 CrossRef CAS PubMed.
- J. E. Millstone, S. J. Hurst, G. S. Métraux, J. I. Cutler and C. A. Mirkin, Small, 2009, 5, 646–664 CrossRef CAS PubMed.
- Y. Xia, K. D. Gilroy, H.-C. Peng and X. Xia, Angew. Chem., Int. Ed., 2017, 56, 60–95 CrossRef CAS PubMed.
- Y. Wang, J. He, C. Liu, W. H. Chong and H. Chen, Angew. Chem., Int. Ed., 2015, 54, 2022–2051 CrossRef CAS PubMed.
- Y. Wang, H.-C. Peng, J. Liu, C. Z. Huang and Y. Xia, Nano Lett., 2015, 15, 1445–1450 CrossRef CAS PubMed.
- Q. N. Nguyen, R. Chen, Z. Lyu and Y. Xia, Inorg. Chem., 2021, 60, 4182–4197 CrossRef CAS PubMed.
- R. Long, S. Zhou, B. J. Wiley and Y. Xiong, Chem. Soc. Rev., 2014, 43, 6288–6310 RSC.
- Y. Zheng, J. Zeng, A. Ruditskiy, M. Liu and Y. Xia, Chem. Mater., 2014, 26, 22–33 CrossRef CAS.
- M. N. O'Brien, M. R. Jones, K. A. Brown and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 7603–7606 CrossRef PubMed.
- Y. Xiong and Y. Xia, Adv. Mater., 2007, 19, 3385–3391 CrossRef CAS.
- H. Zhang, X. Xia, W. Li, J. Zeng, Y. Dai, D. Yang and Y. Xia, Angew. Chem., Int. Ed., 2010, 49, 5296–5300 CrossRef CAS PubMed.
- Y. Tang and M. Ouyang, Nat. Mater., 2007, 6, 754–759 CrossRef CAS PubMed.
- Z. Li, J.-Y. Fu, Y. Feng, C.-K. Dong, H. Liu and X.-W. Du, Nat. Catal., 2019, 2, 1107–1114 CrossRef CAS.
- X. Sun, K. Jiang, N. Zhang, S. Guo and X. Huang, ACS Nano, 2015, 9, 7634–7640 CrossRef CAS PubMed.
- Y. Yang, J. Wu, X. Wang, Q. Guo, X. Liu, W. Sun, Y. Wei, Y. Huang, Z. Lan, M. Huang, J. Lin, H. Chen and Z. Wei, Adv. Mater., 2020, 32, 1904347 CrossRef CAS PubMed.
- Q. Zhu, Z. Pan, Z. Zhao, G. Cao, L. Luo, C. Ni, H. Wei, Z. Zhang, F. Sansoz and J. Wang, Nat. Commun., 2021, 12, 558 CrossRef CAS PubMed.
- Z. R. Ramadhan, A. R. Poerwoprajitno, S. Cheong, R. F. Webster, P. V. Kumar, S. Cychy, L. Gloag, T. M. Benedetti, C. E. Marjo, M. Muhler, D.-W. Wang, J. J. Gooding, W. Schuhmann and R. D. Tilley, J. Am. Chem. Soc., 2022, 144, 11094–11098 CrossRef CAS PubMed.
- S. Maksimuk, X. Teng and H. Yang, J. Phys. Chem. C, 2007, 111, 14312–14319 CrossRef CAS.
- S. Maksimuk, X. Teng and H. Yang, Phys. Chem. Chem. Phys., 2006, 8, 4660–4663 RSC.
- L. Ruan, C.-Y. Chiu, Y. Li and Y. Huang, Nano Lett., 2011, 11, 3040–3046 CrossRef CAS PubMed.
- J. L. Elechiguerra, L. Larios-Lopez and M. Jose-Yacaman, Appl. Phys. A, 2006, 84, 11–19 CrossRef CAS.
- J. Chen, T. Herricks and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2589–2592 CrossRef CAS PubMed.
- T. Herricks, J. Chen and Y. Xia, Nano Lett., 2004, 4, 2367–2371 CrossRef CAS.
- S. Kibey, J. B. Liu, D. D. Johnson and H. Sehitoglu, Acta Mater., 2007, 55, 6843–6851 CrossRef CAS.
- L. Ma, C. Wang, M. Gong, L. Liao, R. Long, J. Wang, D. Wu, W. Zhong, M. J. Kim, Y. Chen, Y. Xie and Y. Xiong, ACS Nano, 2012, 6, 9797–9806 CrossRef CAS PubMed.
- L. Bu, N. Zhang, S. Guo, X. Zhang, J. Li, J. Yao, T. Wu, G. Lu, J.-Y. Ma, D. Su and X. Huang, Science, 2016, 354, 1410–1414 CrossRef CAS PubMed.
- W. Wang, X. Zhang, Y. Zhang, X. Chen, J. Ye, J. Chen, Z. Lyu, X. Chen, Q. Kuang, S. Xie and Z. Xie, Nano Lett., 2020, 20, 5458–5464 CrossRef CAS PubMed.
- F. Saleem, Z. Zhang, B. Xu, X. Xu, P. He and X. Wang, J. Am. Chem. Soc., 2013, 135, 18304–18307 CrossRef CAS PubMed.
- M. Shirai, K. Igeta and M. Arai, Chem. Commun., 2000, 623–624 RSC.
- Y.-R. Hong, S. Dutta, S. W. Jang, O. F. Ngome Okello, H. Im, S.-Y. Choi, J. W. Han and I. S. Lee, J. Am. Chem. Soc., 2022, 144, 9033–9043 CrossRef CAS PubMed.
- H. Kawasaki, M. Uota, T. Yoshimura, D. Fujikawa, G. Sakai, M. Annaka and T. Kijima, Langmuir, 2005, 21, 11468–11473 CrossRef CAS PubMed.
- G. Sakai, T. Yoshimura, S. Isohata, M. Uota, H. Kawasaki, T. Kuwahara, D. Fujikawa and T. Kijima, Adv. Mater., 2007, 19, 237–241 CrossRef CAS.
- T. Kijima, Y. Nagatomo, H. Takemoto, M. Uota, D. Fujikawa, Y. Sekiya, T. Kishishita, M. Shimoda, T. Yoshimura, H. Kawasaki and G. Sakai, Adv. Funct. Mater., 2009, 19, 545–553 CrossRef CAS.
- D. Xu, H. Lv, H. Jin, Y. Liu, Y. Ma, M. Han and B. Liu, J. Phys. Chem. Lett., 2019, 10, 663–671 CrossRef PubMed.
- Y. Song, Y. Yang, C. J. Medforth, E. Pereira, A. K. Singh, H. Xu, Y. Jiang, C. J. Brinker, F. van Swol and J. A. Shelnutt, J. Am. Chem. Soc., 2004, 126, 635–645 CrossRef CAS PubMed.
- Y. Song, W. A. Steen, D. Peña, Y.-B. Jiang, C. J. Medforth, Q. Huo, J. L. Pincus, Y. Qiu, D. Y. Sasaki, J. E. Miller and J. A. Shelnutt, Chem. Mater., 2006, 18, 2335–2346 CrossRef CAS.
- Y. Song, M. A. Hickner, S. R. Challa, R. M. Dorin, R. M. Garcia, H. Wang, Y.-B. Jiang, P. Li, Y. Qiu, F. van Swol, C. J. Medforth, J. E. Miller, T. Nwoga, K. Kawahara, W. Li and J. A. Shelnutt, Nano Lett., 2009, 9, 1534–1539 CrossRef CAS PubMed.
- Y. Song, R. M. Dorin, R. M. Garcia, Y.-B. Jiang, H. Wang, P. Li, Y. Qiu, F. van Swol, J. E. Miller and J. A. Shelnutt, J. Am. Chem. Soc., 2008, 130, 12602–12603 CrossRef CAS PubMed.
- E. Zhu, X. Yan, S. Wang, M. Xu, C. Wang, H. Liu, J. Huang, W. Xue, J. Cai, H. Heinz, Y. Li and Y. Huang, Nano Lett., 2019, 19, 3730–3736 CrossRef CAS PubMed.
- H. Liu, P. Zhong, K. Liu, L. Han, H. Zheng, Y. Yin and C. Gao, Chem. Sci., 2018, 9, 398–404 RSC.
- L. Peres, D. Yi, S. Bustos-Rodriguez, C. Marcelot, A. Pierrot, P.-F. Fazzini, I. Florea, R. Arenal, L.-M. Lacroix, B. Warot-Fonrose, T. Blon and K. Soulantica, Nanoscale, 2018, 10, 22730–22736 RSC.
- B. Lim, J. Wang, P. H. C. Camargo, M. Jiang, M. J. Kim and Y. Xia, Nano Lett., 2008, 8, 2535–2540 CrossRef CAS PubMed.
- J. A. Delgado, O. Benkirane, C. Claver, D. Curulla-Ferré and C. Godard, Dalton Trans., 2017, 46, 12381–12403 RSC.
- S. A. Nikolaev, L. N. Zanaveskin, V. V. Smirnov, V. A. Averyanov and K. L. Zanaveskin, Russ. Chem. Rev., 2009, 78, 231–247 CrossRef CAS.
- Z. Wang, A. Garg, L. Wang, H. He, A. Dasgupta, D. Zanchet, M. J. Janik, R. M. Rioux and Y. Román-Leshkov, ACS Catal., 2020, 10, 6763–6770 CrossRef CAS.
- C. Salzemann, J. Urban, I. Lisiecki and M.-P. Pileni, Adv. Funct. Mater., 2005, 15, 1277–1284 CrossRef CAS.
- P. Liu, R. Qin, G. Fu and N. Zheng, J. Am. Chem. Soc., 2017, 139, 2122–2131 CrossRef CAS PubMed.
- J. J. Benítez, M. A. San-Miguel, S. Domínguez-Meister, J. A. Heredia-Guerrero and M. Salmeron, J. Phys. Chem. C, 2011, 115, 19716–19723 CrossRef.
- J. Oviedo, M. A. San-Miguel, J. A. Heredia-Guerrero and J. J. Benítez, J. Phys. Chem. C, 2012, 116, 7099–7105 CrossRef CAS.
- M. Siemer, G. Tomaschun, T. Klüner, P. Christopher and K. Al-Shamery, ACS Appl. Mater. Interfaces, 2020, 12, 27765–27776 CrossRef CAS PubMed.
- X. Fu, Y. Wang, N. Wu, L. Gui and Y. Tang, J. Colloid Interface Sci., 2001, 243, 326–330 CrossRef CAS.
- S. Carenco, S. Labouille, S. Bouchonnet, C. Boissière, X.-F. Le Goff, C. Sanchez and N. Mézailles, Chem. – Eur. J., 2012, 18, 14165–14173 CrossRef CAS PubMed.
- R. K. Blundell and P. Licence, Phys. Chem. Chem. Phys., 2014, 16, 15278–15288 RSC.
- H. Karhu, A. Kalantar, I. J. Väyrynen, T. Salmi and D. Y. Murzin, Appl. Catal., A, 2003, 247, 283–294 CrossRef CAS.
- J. A. Spencer, Y.-C. Wu, L. McElwee-White and D. H. Fairbrother, J. Am. Chem. Soc., 2016, 138, 9172–9182 CrossRef CAS PubMed.
- X. Zhu, B. Cheng, J. Yu and W. Ho, Appl. Surf. Sci., 2016, 364, 808–814 CrossRef CAS.
- N. Job, M. Chatenet, S. Berthon-Fabry, S. Hermans and F. Maillard, J. Power Sources, 2013, 240, 294–305 CrossRef CAS.
- M. Hara, K. Asami, K. Hashimoto and T. Masumoto, Electrochim. Acta, 1983, 28, 1073–1081 CrossRef CAS.
- B. C. Beard, Surf. Sci. Spectra, 1993, 2, 91–96 CrossRef CAS.
- T. Weiss, J. Warneke, V. Zielasek, P. Swiderek and M. Bäumer, J. Vac. Sci. Technol., A, 2016, 34, 041515 CrossRef.
- N. T. Xuyen, H. K. Jeong, G. Kim, K. P. So, K. H. An and Y. H. Lee, J. Mater. Chem., 2009, 19, 1283–1288 RSC.
- J. Ederer, P. Janoš, P. Ecorchard, J. Tolasz, V. Štengl, H. Beneš, M. Perchacz and O. Pop-Georgievski, RSC Adv., 2017, 7, 12464–12473 RSC.
- A. Artemenko, A. Shchukarev, P. Štenclová, T. Wågberg, J. Segervald, X. Jia and A. Kromka, IOP Conf. Ser.: Mater. Sci. Eng., 2021, 1050, 012001 CrossRef CAS.
- M. Kehrer, J. Duchoslav, A. Hinterreiter, M. Cobet, A. Mehic, T. Stehrer and D. Stifter, Plasma Processes Polym., 2019, 16, 1800160 CrossRef.
- A. Funatsu, H. Tateishi, K. Hatakeyama, Y. Fukunaga, T. Taniguchi, M. Koinuma, H. Matsuura and Y. Matsumoto, Chem. Commun., 2014, 50, 8503–8506 RSC.
- X. Yin, M. Shi, J. Wu, Y.-T. Pan, D. L. Gray, J. A. Bertke and H. Yang, Nano Lett., 2017, 17, 6146–6150 CrossRef CAS PubMed.
- R. K. Sodhi, A. Changotra and S. Paul, Catal. Lett., 2014, 144, 1819–1831 CrossRef CAS.
- S. A. De Pascali, P. Papadia, S. Capoccia, L. Marchiò, M. Lanfranchi, A. Ciccarese and F. P. Fanizzi, Dalton Trans., 2009, 7786–7795 RSC.
- S. E. Lohse, N. D. Burrows, L. Scarabelli, L. M. Liz-Marzán and C. J. Murphy, Chem. Mater., 2014, 26, 34–43 CrossRef CAS.
- K. D. Gilroy, J. Puibasset, M. Vara and Y. Xia, Angew. Chem., Int. Ed., 2017, 56, 8647–8651 CrossRef CAS PubMed.
- C. R. Laramy, L.-K. Fong, M. R. Jones, M. N. O'Brien, G. C. Schatz and C. A. Mirkin, Chem. Phys. Lett., 2017, 683, 389–392 CrossRef CAS.
- J. S. Du, W. Zhou, S. M. Rupich and C. A. Mirkin, Angew. Chem., Int. Ed., 2021, 60, 6858–6863 CrossRef CAS PubMed.
- R. R. Grinstead and J. C. Davis, J. Phys. Chem., 1968, 72, 1630–1638 CrossRef CAS.
- S. Cheong, J. Watt, B. Ingham, M. F. Toney and R. D. Tilley, J. Am. Chem. Soc., 2009, 131, 14590–14595 CrossRef CAS PubMed.
- C. J. Wang, E. F. Shapiro and M. L. Personick, in Reference Module in Materials Science and Materials Engineering, Elsevier, 2022, p. B9780128224250000000 Search PubMed.
- N. Zettsu, J. M. McLellan, B. Wiley, Y. Yin, Z.-Y. Li and Y. Xia, Angew. Chem., Int. Ed., 2006, 45, 1288–1292 CrossRef CAS PubMed.
- S. Ghosh and L. Manna, Chem. Rev., 2018, 118, 7804–7864 CrossRef CAS PubMed.
- C. Cao, Q. Xu and Q.-L. Zhu, Chem. Catal., 2022, 2, 693–723 CrossRef.
- M. Shirai, B. M. Bhanage, H. Senboku, S. Fujita and M. Arai, J. Jpn. Pet. Inst., 2002, 45, 420–421 CrossRef CAS.
- M. Shirai, Chem. Rec., 2019, 19, 1263–1271 CrossRef CAS PubMed.
- T.-W. Chen, D.-W. Pang, J.-X. Kang, D.-F. Zhang and L. Guo, Front. Chem., 2022, 9, 818900 CrossRef PubMed.
- S. Galvagno, Z. Poltarzewski, A. Donato, G. Neri and R. Pietropaolo, J. Mol. Catal., 1986, 35, 365–375 CrossRef CAS.
- L. Xia, D. Li, J. Long, F. Huang, L. Yang, Y. Guo, Z. Jia, J. Xiao and H. Liu, Carbon, 2019, 145, 47–52 CrossRef CAS.
- M. Hu, L. Jin, Y. Dang, S. L. Suib, J. He and B. Liu, Front. Chem., 2020, 8, 581512 CrossRef CAS PubMed.
- Y. Liu, W. Guo, X. Li, P. Jiang, N. Zhang and M. Liang, ACS Appl. Nano Mater., 2021, 4, 5292–5300 CrossRef CAS.
- K. Liu, R. Qin and N. Zheng, J. Am. Chem. Soc., 2021, 143, 4483–4499 CrossRef CAS PubMed.
- L. Peres, M. R. Axet, D. Yi, P. Serp and K. Soulantica, Catal. Today, 2020, 357, 166–175 CrossRef CAS.
- D. Das and B. C. Meikap, Indian Chem. Eng., 2021, 63, 435–447 CrossRef CAS.
- M. Crespo-Quesada, R. R. Dykeman, G. Laurenczy, P. J. Dyson and L. Kiwi-Minsker, J. Catal., 2011, 279, 66–74 CrossRef CAS.
- Y. Peng, B. Lu, N. Wang, L. Li and S. Chen, Phys. Chem. Chem. Phys., 2017, 19, 9336–9348 RSC.
- I. C. Gerber and P. Serp, Chem. Rev., 2020, 120, 1250–1349 CrossRef CAS PubMed.
- L.-H. Sun, Q.-Y. Li, S.-N. Zhang, D. Xu, Z.-H. Xue, H. Su, X. Lin, G.-Y. Zhai, P. Gao, S.-I. Hirano, J.-S. Chen and X.-H. Li, Angew. Chem., Int. Ed., 2021, 60, 25766–25770 CrossRef CAS PubMed.
- B. A. Wilhite, M. J. McCready and A. Varma, Ind. Eng. Chem. Res., 2002, 41, 3345–3350 CrossRef CAS.
- Y.-H. Chung, D. Y. Chung, N. Jung and Y.-E. Sung, J. Phys. Chem. Lett., 2013, 4, 1304–1309 CrossRef CAS PubMed.
- M. Guo, H. Li, Y. Ren, X. Ren, Q. Yang and C. Li, ACS Catal., 2018, 8, 6476–6485 CrossRef CAS.
- C. M. Zalitis, A. R. Kucernak, J. Sharman and E. Wright, J. Mater. Chem. A, 2017, 5, 23328–23338 RSC.
- G. Polzonetti, V. Carravetta, M. V. Russo, G. Contini, P. Parent and C. Laffon, J. Electron Spectrosc. Relat. Phenom., 1999, 98–99, 175–187 CrossRef CAS.
- Y. Jin, P. Wang, X. Mao, S. Liu, L. Li, L. Wang, Q. Shao, Y. Xu and X. Huang, Angew. Chem., Int. Ed., 2021, 60, 17430–17434 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Complementary TEM, HRTEM, EFTEM, AFM, catalytic results, XRD pattern, XPS deconvolution and associated tables. See DOI: https://doi.org/10.1039/d2nr05105b |
| This journal is © The Royal Society of Chemistry 2023 |