Analytical methods for the analysis of volatile natural products
Yue
Li
 *
*
Department of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742, USA. E-mail: yueli@umd.edu
First published on 17th April 2023
Abstract
Covering: 2000 to 2022
This article reviews the latest developments of analytical methods of volatile natural products. Volatile organic compounds (VOCs) released from biological systems correspond to a series of compounds, originating from primary and secondary metabolites. These compounds are important for intra- and interspecies chemical communication and interaction of living organisms. These valuable natural products can find applications in many fields, including foods, human nutrition, pharmaceuticals, perfumes, cosmetics and so on. Therefore, the deciphering of their structures is of increasing importance in many fields of natural product chemistry. Due to the large diversity of these compounds, there is no “single” analytical instrument or method that can be used to study all of them. Furthermore, most of the volatile compounds can be collected only in low concentrations. Therefore, their detection, identification and structural characterization are challenging tasks. The review briefly describes the extraction and preparation methods of samples, then introduces the tools of instrumental analysis utilized to identify or quantify the VOCs of natural products, including spectroscopic and mass spectrometric methods, such as offline GC-MS, multi-dimensional GC-MS, and online approaches including PTR-MS, SIFT-MS, SEMI-MS, DART-MS etc. The current challenges of analytical techniques and future directions are also briefly discussed.
1. Introduction
Natural products are a class of compounds or substance produced by living organisms present in nature. Volatile natural products are a series of volatile compounds of different classes originating from primary and secondary metabolites released from many different natural resources.1–3 Generally, these compounds are lipophilic substances with low molecular weight, low boiling point, and high vapor pressure in natural conditions.4 The natural products have high structure diversity and many unique biological activities due to the natural selection and evolutionary processes that have shaped their utility over thousands of years.5,6 The volatile natural products can be used in many different fields, such as perfumes, cosmetics, foods, human nutrition, and pharmaceuticals.7–10 For example, to date, more than 700 compounds have been reported as aroma or flavor in fruits and vegetables.11 Currently, there is the increasing scientific interest in the biochemistry, physiology, ecology and atmospheric chemistry for these volatile organic compounds (VOCs) because the compounds contain important bio-information related with biological metabolism.12–14 Species and amounts of VOCs of every biological sample are unique and stable with fingerprint characteristics, namely biological VOC characteristics.10,15,16Plants act as a major atmospheric source by releasing different volatile compounds to ambient environment.17,18 These secondary metabolic products protect plants from environmental stresses, mediate in plant–plant or plant-insect communication, and affect our climate globally.19–21 By releasing volatiles, plants can defend themselves from the attack of pathogenics organisms or herbivores, intoxicating, repealing, or even attracting their natural enemies.22,23 These chemical signals are nowadays defined as semiochemicals and can be divided in two classes: those that act as intermediate in the communication of individuals from the same species (intraspecific) and those that act as intermediate in the communication between different species (interspecific).22,24,25 For example, Arroyo et al.26 confirmed the antifungal activity of (E)-hex-2-enal on susceptibility of strawberry fruits to Colletotrichum acutatum. Sekine et al.27 studied the antifungal effects of the volatile compounds from black zira against a soil borne phytopathogenic fungus, Fusarium oxysporum. The knowledge on how the metabolic pathways of the VOCs proceed, how the signal is transmitted between plants and how receiver plants respond is important for agricultural production, environmental chemistry, atmospheric chemistry, and medicine chemistry.28–30
Marine algae were found to release the bioactive volatile compounds into the seawater, which play an important role for the chemical communications in marine ecosystems.31 Many insects can release special VOCs to attract heterosexual insects for copulation, which are called insect sex pheromones.32 The human body can also release volatile compounds.33,34 These compounds will vary at different healthy conditions, thus can potentially indicate occurrences of some diseases.35–37 One of the most promising and challenging applications in analysis of VOCs is the discovery of possible biomarkers for diseases, such as cancer. A few studies were attempted to describe the relationship between the various VOC components of lung cancer patients and healthy persons, which potentially can be applied in lung cancer early diagnosis.38,39 For example, Robroeks et al.40,41 by using only 22 of 1099 VOCs from exhaled breath of a patient with cystic fibrosis (CF), obtained 100% correct identification of patients not only with this disease, but also between CF patients with or without Pseudomonas colonization. Abaffy et al.42–44 compared volatile metabolomic signatures of non-neoplastic skin from the same patient and found increasing levels of lauric acid and palmitic acid in melanoma as a consequence of upregulated de novo lipid synthesis, characteristic of cancer.
Trace gas monitoring always plays a key role in many areas of life sciences such as agrotechnology, microbiology, molecular biology, physiology, or phytopathology.13,45 The deciphering of the VOCs is of increasing importance in many fields of natural product chemistry, including identifications of pheromones, semiochemicals, bioregulators, flavors etc. The interpretation of bioinformation involved in biological VOCs would facilitate relevant fields, such as the insect prevention, fruit quality control, food safety or disease diagnosis.12,46–48 Any VOC research will rely on analytical techniques for trace gas analysis. Currently, many techniques have been developed and successfully used in a wide range of scientific research areas (e.g. atmospheric chemistry, plant biochemistry, hazardous compound detection, and disease biomarkers).49–51 In the traditional way, the volatile compounds are collected in the head space of samples that are enclosed in a collection chamber using absorbing materials.25,52,53 Subsequently, the collected volatiles can be desorbed by thermal desorption or extracted by organic solvents, then analyzed using GC-MS or other analytical techniques. The advantage of the above approach is that the volatile compounds can be concentrated on the surface of the absorbing materials, resulting in increased detection sensitivity.
Fig. 1 represents a typical workflow for the untargeted metabolomics-based study of VOCs in plants or related organisms. Initially, one needs to choose the material of interest for the study. For example, if the main interest is to study the volatile components released from a plant, the researcher will need to choose what kinds (fresh, dry) and which part of plants (stems, flowers, leaves or seeds) will be used for VOC collection. Next step, one will need to determine how to prepare samples. Sample preparation should be efficient (not losing or destroying compounds of interest during extracting as much as possible) and preferably not time consuming. The third step, instrumental analysis is performed through optical spectroscopy or mass spectrometry, and spectral data will be obtained. Ideally, the instrumental analysis process should provide separation of the different components in mixtures, if possible, then introduce the analytes to the instruments for analysis. Usually, the raw data will need to be processed first before extracting any meaningful information, such as baseline and background subtraction, data smoothing and normalization etc. The fifth step will be data analysis, in which qualitative or quantitative results can be obtained, including identifications of metabolites observed in the instrumental measurements. Afterward, appropriate chemometric techniques (for example, univariate or multivariate analysis) can be applied to obtain patterns from the raw data, which help in the biological interpretation with models, pathways etc.54,55
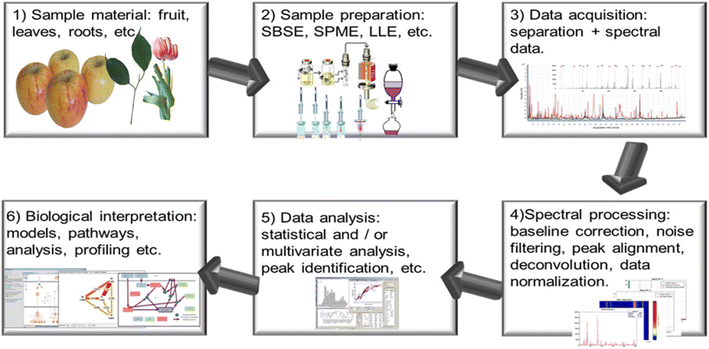 | ||
| Fig. 1 Typical workflow for VOC analysis. (Reprinted with permission from Lubes and Goodarzi.23 Copyright (2017) American Chemical Society). | ||
In this review, I will describe and discuss the methods and analytical techniques, which are commonly used in VOC analysis, including sample preparation, laser spectroscopy methods, mass spectrometry etc. I will list a few typical experimental examples, including for the analysis of VOCs that are emitted by plants, bacteria, insects, animals and human bodies. The detection of the VOCs from human bodies potentially can be applied in early clinical diagnosis of diseases and therapeutic monitoring.
2. Sample preparation methods
In analysis of volatile compounds, the vapor in headspace of samples can be directly injected to instruments using a gas-tight syringe. The method is called static headspace (HS) method.56 The major advantage of the method is that no sample preparation is needed. Thus, it can perform a quick and efficient analysis, and almost real time analysis is possible. However, a basic promise for this method is that the equilibration between vapor and the sample surface should be reached. It is especially critical for quantitative analysis. As a sample preparation technique without extraction, headspace analysis is usually combined with gas chromatography mass spectrometry (GC-MS) to analyze volatile organic compounds in complex matrices.53,57,58 With the development of automation, the HS method is becoming more convenient and effective. Headspace gas chromatography mass spectrometry (HS-GC-MS) now plays an important role in routine analysis of volatile compounds.However, the amounts of biogenic VOCs are usually at trace level from complicated biological samples, and their compositions are usually complex with significantly different structures and polarity.12,59 Thus, it is often a tough task to sample biogenic VOCs entirely without appropriate sample preparation. The sample preparation is critical for successful experiments and measurements of volatile natural products. Ideally, sample preparation must be efficient (not destroying or losing any target compounds during extraction) and preferably not time consuming. Efficient sampling techniques should possess a wide sampling range, high extraction capacity and selectivity, and can be conveniently coupled with subsequent analytical instruments.
Usually, analysis of VOCs is performed on either fresh or freeze-dried samples. In any case, samples need to be (pre)treated to extract and/or preconcentrate VOCs from the complex matrices. One of the main advantages of freeze drying is that samples can be preserved for a longer time before analysis because elimination of water is an efficient way to quench the enzymatic activity.60,61 However, it has been demonstrated that sometimes this process increases the risk of losing volatiles.62,63
If one considers how to make a plan and choose an appropriate method used for sample preparation, one of the important criteria is the physico-chemical property, on which the concentration and purification of these target compounds is based.64 Briefly, one needs to consider the following factors:
(1) The volatility of the target compound: it is important to avoid loss during sample preparation for highly volatile compounds.
(2) (For liquid extraction) solubility of the analyte in different solvents, in order to more efficiently extract the target compounds, meanwhile minimize the concentrations of other compounds, which potentially interfere with analysis of the compounds of interest.
(3) (For solid-phase extraction) the adsorption and absorption of the analyte on a particular material.
In the following sections, four sample extraction methods for volatile compounds will be discussed, including liquid extraction (LE), solid-phase extraction (SPE), solid-phase microextraction (SPME), and most recently stir bar sorptive extraction (SBSE). Table 1 lists the brief descriptions and comparisons of different sample preparation methods.
| Methods | Strengths | Weaknesses | Key examples |
|---|---|---|---|
| Static headspace (HS) | - Simple | - Low sensitivity | - Plants53 |
| - No sample preparation needed | - Food and environmental samples57,58 | ||
| - Real-time analysis possible | |||
| Liquid extraction (LE) | - Simple, efficient | - Low recovery of analytes | - Biological samples69,71 |
| - Economical | - Loss of analytes during sample extraction | - Environmental samples70 | |
| - Longer extraction time | - Food72 | ||
| - Expose to large volumes of organic solvents | |||
| Solid-phase extraction (SPE) | - Simple, efficient | - Loss of analytes during sample extraction | - Environmental samples76,77 |
| - Solvent free | - Possible background signal from SPE material | - Food79–81 | |
| - Selectivity of sorbent and eluent need to be optimized | - Plants88 | ||
| Solid phase microextraction (SPME) | - Solvent free | - Low extraction capacity | - Plants52,92,96–100 |
| - Fast, sensitive and economical | - Extraction conditions need to be optimized | - Bacteria and fungi103 | |
| - Automatic sampling possible | - Human metabolomics104–108 | ||
| - Reduction of sample quantity | |||
| Stir bar sorptive extraction (SBSE) | - Solvent free | - Not fully automatic sampling | - Environmental samples109 |
| - High extraction capacity | - Limited number of coatings are available | - Pharmaceutical samples113 | |
| - High sensitivity | - Plants114–116 |
2.1. Liquid extraction (LE)
Volatile compounds can be efficiently collected by the solvent extraction method before being released to the ambient environment. The selection of the solvent will depend mainly on the chemical properties of those compounds.65,66 In the LE method, solvents with different polarities (such as methanol, ethanol, hexane, dichloromethane) need to be tested in order to determine the most effective one. Generally, for polar metabolites, the extraction process can be performed with polar solvents such as water, methanol, or water–methanol, water–acetonitrile mixture solution. In the case of the weakly polar or nonpolar metabolites, chloroform, methylene chloride, methyl tertiary-butyl ether or hexane is commonly used as the extraction solvent. Extraction can be accelerated with ultrasounds and heating.67 In addition, steam distillation, hydro-distillation and vacuum distillation can be also used to improve extraction efficiency.68The extraction solvents and methods may differ in different experiments; however, in most cases, the final extracts should be either polar, nonpolar, or of intermediate polarity.69 Additional sample preparation procedures are sometimes needed to further enrich a given group of metabolites. These typically are based on liquid–liquid extraction or liquid/solid-phase extraction.70,71 After extraction, centrifugation or decantation steps are often needed for separation of the insoluble material from the liquid phase. Subsequently, a concentration step is performed using a vacuum rotary evaporator or simply by blowing a stream of nitrogen into the vial containing the sample solution. Temperature and extraction time are parameters that can be optimized for more effective extraction. For most cases, room temperature is preferred to avoid thermal degradation of compounds. However, higher temperatures are sometimes required to inactivate the activity of enzymes.72
Compared to other extraction methods, liquid extraction is a relatively simple and efficient way for sample concentration and purification. A main disadvantage of the method is that one will be exposed to large volumes of organic solvents. Another technical issue could be the low recovery of analytes because extraction depends on the partition coefficient between the solvents and matrices of samples. Some of the compounds might not totally be extracted and still remain in the original matrix. Therefore, it is recommendable to perform the extraction in different types of solvents or solvent mixtures, then compare them through instrumental analysis in the process of method developments.
In order to overcome the problem of using a high volume of organic solvents, several miniaturized liquid-extraction techniques have been developed such as liquid–liquid microextraction and dispersive liquid–liquid microextraction.73–75 The main advantage of liquid–liquid microextraction (μ-LLE) is that it does not require special equipment, while the main disadvantage is that its extraction efficiency is lower than conventional liquid extraction. In the dispersive liquid–liquid microextraction method, a mixture of two solvents (extraction solvent and disperser) is injected by syringe into the aqueous sample. This extraction can be assisted by ultrasound to improve the extraction efficacy.
2.2. Solid-phase extraction (SPE)
Solid-phase extraction is also one of the simplest sample preparation techniques for extracting and preconcentrating trace-level compounds from complex matrices.76 It was first introduced in 1970 and has had a great impact in the field of analytical sciences.77 SPE is based on the same principle as liquid chromatography, where the partition coefficient of an analyte between a liquid phase and an adsorbent material depends on its solubility and the interactions produced by the functional groups present in the compound.78,79 Currently, a wide range of chemically modified adsorbent materials in either silica gel or synthetic resins, like reversed-phase (C2, C8, C18) etc., are commercially available.The selectivity of the separation will be determined by the type of sorbent and eluent employed.73,74 The choice of the sorbent depends on the nature of the analyte, of the matrix, and of the possible interferents. Of particular importance are the silica and polymeric sorbents, such as styrene-divinylbenzene copolymers. Silica sorbents have been applied in analysis of volatile compounds of enological products.80,81 The silica sorbents are characterized by presenting a low loading capacity and a high consumption of solvents and time. In addition, in some cases, one can be faced with irreversible sorption and with the degradation of certain analytes.82 Ferreira et al.81 found that silica-based sorbents were suitable for the extraction of analytes that show a Bronsted–Lowry acid character. SPE based on styrene-divinylbenzene polymers is characterized by presenting a higher loading capacity, more stability against extreme pH values, and the capacity for employment in both reversed and normal modes.82
Due to the unique properties of nanostructured materials, numerous efforts have been made to develop facile analytical strategies that integrate metallic and metal oxide nano-particles, magnetic nano-particles, carbon-based nano-particles, and silicon and polymer based nano-particles with SPE techniques.83–85 Azzouz et al. reviewed recent developments on the use of nanostructured materials in different sorptive extraction techniques for the analysis of trace-level organic pollutants from various environmental matrices.76 Trace-level organic pollutants (at the ng L−1 or pg L−1 level) can be effectively trapped through hydrogen bonding and van der Waal, electrostatic, and p-interactions because of the high surface area, functional groups and ordered structural arrangements of the nano-particles, improving the performance of those analytical techniques while minimizing sample preparation steps and reducing solvent volumes.
Purge and trap is one of the solid phase extraction techniques and has been proved suitable for sampling not only VOCs, but also semi-VOCs.86,87 It uses ultra-purified inert gas as the carrier gas to continuously pass samples to carry out VOCs. The VOCs are trapped in the trap that contains the sorbent.88 After trapping VOCs, the temperature will increase with a rapid ramp in order to desorb VOCs trapped. The type of sorbent in the trap can be various, so the method can achieve high selectivity for different volatile compounds. Properly prolonging sampling time could also improve the enrichment effect.
Even though SPE has proven to be an effective method for isolation of VOCs in different types of samples, miniaturized solid-phase extraction techniques, such as solid-phase microextraction and stir bar sorptive extraction, are nowadays the trend for metabolomics analysis. As discussed in the following sections, the main advantage of these miniaturized techniques is the significant reduction in the quantity of sample and using simpler instrumentation for analysis.
2.3. Solid phase microextraction (SPME)
Solid phase microextraction (SPME) is a solvent free sample preparation technique, first described by Arthur and Pawliszyn in 1990.89 The technique is a fast, sensitive and economical method with high sample throughput by combining sampling, analyte isolation, and enrichment into one step,90,91 and has been widely used for the determination of trace levels of different compounds, such as volatile organic compounds emitted from different plant organs and environmental contaminants in plants.92,93As shown in Fig. 2, a typical SPME device consists of a fiber and a fiber holder. The latter is equipped with a spring-loaded plunger, a stainless-steel barrel, and an adjustable depth gauge with a stainless steel needle.95 A fused-silica fiber is coated with a thin film of one or several polymeric stationary phases, such as polydimethylsiloxane (PDMS), carboxen (CAR), or divinylbenezene (DVB), which can absorb or adsorb VOCs from the sample matrixes. Volatile compounds in the samples are trapped and concentrated in the fiber and can be directly desorbed on the injection port of the gas chromatography by thermal desorption. SPME can be coupled to different instrument configurations and has widely been used for the analysis of metabolites in plants, especially VOCs from plants.92,96,97 In experiments, the SPME fiber needs to be properly selected to obtain high selectivity and sensitivity, and wide linear ranges for target compounds. The extraction conditions, such as the extraction time and temperature, desorption time, salinity, pH, and stirring speed, need to be optimized. SPME is easy to use and does not require an experienced operator. Moreover, it can be fully adapted to automatic sampling systems widely employed in different areas, especially in food and drug quality and control, agriculture, plant sciences, clinical chemistry etc.
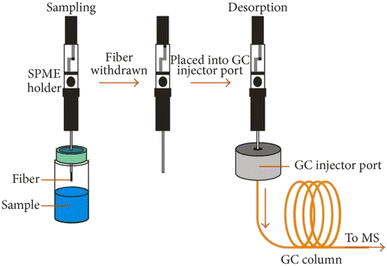 | ||
| Fig. 2 Diagram of analysis with solid phase microextraction gas chromatography mass spectrometry (SPME-GC-MS). (Reprinted with permission from Schmidt and Podmore.94 Copyright (2015) K. Schmidt and I. Podmore). | ||
SPME plays an important role in the analysis of VOCs in plants.53,98,99 As an example, the high-throughput screening of volatiles from 94 different tomato genotypes was demonstrated by HS-SPME coupled to GC-MS, detecting 322 distinct plant derived compounds.100 In a study of different species of Citrus monstruosa, 44 volatile metabolites were detected, in which monoterpenes were found to be the most abundant among them. Conversely, some VOCs were found to be specific for only a few of the samples.101 SPME has been used in a multiplatform metabolomics analysis to determine the biochemical differences in 31 rice varieties from a diverse range of genetic backgrounds and origins. Comparing fragrant rice varieties, Mumm et al. observed differences in the metabolic profiles of jasmine and basmati varieties.102
Arora et al. explored the use of SPME and ambient plasma ionization mass spectrometry to rapidly acquire VOC signatures of bacteria and fungi.103 This study presents a new approach for the identification of pathogens from VOC signatures collected using SPME and ambient ionization MS by training classifiers on just a few samples of data.
The SPME technique was also applied in the area of human metabolomics or human health.104,105 It is known that VOCs present in human feces have a potential to be used in diagnosis of diseases. Dixon et al.106 compared eight different commercially available SPME fibers for human fecal VOC metabolomics. In the analysis of 16 volatiles present in human urine of 19 healthy volunteers, compounds like acetone, 2-butanone, 3-methyl-2-butanone, 2-pentanone and so on were identified and quantified, supporting the concept of hybrid volatolomics improving and complementing the chemical information on the physiological status of an individual.107 In the area of cancer research, recent investigations are aimed at finding potential biomarkers for discrimination of cancer cells from normal ones. For example, the study conducted by Huang et al.108 found potential VOC biomarkers of MCF-7, MDA-MB-231, and CCD-1095Sk cell lines. Fingerprinting analysis showed that each kind of cell line provided a unique chromatographic profile. Applying PCA and partial least-squares data analysis (PLS-DA), four volatiles (2-ethyl-1-hexanol, 2,4-dimethyl-benzaldehyde, cyclohexanol, and p-xylene) were identified as potential biomarkers for discriminating breast cancer cell lines from a normal mammary cell line.
The above examples clearly demonstrate the potential of the SPME technique in different areas, especially for in vivo concentration and sampling, as a powerful tool in analysis of volatile organic compounds.
2.4. Stir bar sorptive extraction (SBSE)
Stir bar sorptive extraction (SBSE) was developed in 1999 and introduced for the trace analysis of organic compounds extracted from biological and environmental samples.109 SBSE has the same technical principle as SPME, but has higher stationary phase extraction capacity and extraction recovery.110 It is also a solvent-free method, which has been widely used for analyte enrichment in environmental, food and pharmaceutical samples.111,112SBSE consists of a bar coated with a sorbent polymer that can be immersed in samples to extract analytes according to their chemical affinities. The analytes can be thermically desorbed in the injection port, then analyzed by GC. In comparison to SPME, a major advantage of SBSE is that it has a higher sensitivity. Typical SBSE is between 50 and 250 times thicker than SPME; therefore, sample enrichment can be significantly improved.113 By coupling SBSE with various analytical techniques, excellent selectivity, sensitivity, and simplicity can be achieved for trace-level assays of volatile compounds from various specimen.
The SBSE technique has been applied in the VOCs composition analysis of raspberry cultivars. 29 volatile compounds, such as α-ionone, β-ionone, geraniol, linalool, and (Z)-3-hexenol, were quantified.114 In the field of plant defense or crop protection, some studies have also been performed with SBSE. For example, in studies conducted on healthy trees and trees infected with the Citrus tristeza virus (CTV) (genus Closterovirus), which is a plant pathogen that infects important citrus crops, a total of 383 VOCs were observed.115 Putative biomarkers of CTV were identified as terpenoid (myrcene, carene, ocimene, bulnesene), alcohols (nundecanol, surfynol), and two acetones (geranyl acetone and neryl acetate), allowing efficient discrimination between trees (infected, healthy, and coinfected by another pathogen). Using SBSE coupled with GC-MS, Errard et al.116 studied the effects of single versus multiple-pest infestation by Tetranychus urticae and Myzus persicae on the tomato fruit (Solanum lycopersicum). They observed that plants had a different response according to the pest infestation: the volatile emission presented differences between the adaxial and the abaxial leaf epidermis being the chemical compounds cyclohexadecane, dodecane, aromadendrene, and β-elemene emitted as indicator of multiple infestation.
So far, SBSE has not been widely accepted as SPME. A major reason is that protocols based on SBSE are still not fully automatized. The other possible reason is that only a limited number of coatings are commercially available.
Based on the above descriptions, it can be clearly seen that every extraction technique has its own characteristic and selectivity. No single approach can be considered as an all-around sampling technique for all kinds of biological VOCs. Conventional methods, such as LE and SPE, are simple and straightforward, but usually require multiple steps, longer extraction time, and larger amounts of solvents. Moreover, many unstable volatiles may be thermally decomposed and degraded during thermal extraction or distillation. SPME has been considered a desired sampling technique for biological VOCs, because it is solvent-free, and easy-handling procedure. It is also non-invasive during sampling and can be easily coupled to the consequent analytical instruments. However, SPME has the limited extraction capacity, and some important biological VOC components could be lost during sampling processes. Therefore, one should choose different sampling techniques depending on biological systems and target compounds in order to achieve the best extraction results.
3. Instrumental analytical methods
3.1. Spectroscopic methods
Spectroscopic techniques created new opportunities for analysis of volatile natural products in recent years.12,117 Although spectroscopic techniques have no separation capability, some techniques such as ultraviolet (UV) and infrared (IR) spectroscopy have been applied for the noninvasive analysis of trace volatile components.118,119 In general, spectroscopic methods are very specific since each gaseous molecule has its specific absorption lines. Moreover, a molecular transition (absorption frequencies of the light) can be determined with very high accuracy. Spectroscopic detection with high sensitivity and analytical speed nowadays offers particular potential for the real-time analysis of trace biological VOCs.120,121 The infrared wavelength region between 2 and 20 mm is called the fingerprint region; the molecule gives a unique, specific absorption pattern that can be clearly discriminated from other components. On the other hand, for the complex mixtures, one needs to be careful to choose a proper absorption line for quantitative analysis, where there should be low or no interference from other components in the sample mixtures.122Next to its specificity, spectroscopic techniques can also achieve a very high sensitivity. Within absorption spectroscopy, this can be done by increasing the path length of the light through the gases. Based on this consideration, most of absorption cells are designed with high reflective mirrors in a multi-pass arrangement, combined with advanced modulation techniques. This type of absorption spectroscopy has evolved in a wide range of methods, such as cavity enhanced spectroscopy,123,124 cavity ring down spectroscopy,125 wavelength modulation spectroscopy126etc. An overview of the above spectroscopic methods can be found in ref. 127 and 128. Table 2 lists the brief descriptions and comparisons of different instrumental analytical methods.
| Methods | Detection mechanisms | Strengths | Weaknesses | Key examples |
|---|---|---|---|---|
| (I) Spectroscopic methods | ||||
| Absorption spectroscopy (UV/IR/FT-IR/TILDAS) | - Measure absorption of light radiation of compounds | - Sensitive | - Low selectivity | - Insects129,130 |
| - Specific | - Identification of unknown compounds difficult | - Atmospheric chemistry131–135 | ||
| - Real-time analysis | - No separation capability | - Clinical applications136 | ||
| - Quick and efficient | - Impropriate for analysis of complex mixtures | |||
| Laser-induced fluorescence (LIF) | - Sample is excited by a laser, and the fluorescence emitted is detected by a photodetector | - Low background | - Work only for molecules fluoresce | - Atmospheric chemistry137–142 |
| - High sensitivity | - Sensitive to interference from changes in sample pH and oxygen level | |||
| - Time-resolved information can be obtained | - No separation capability | |||
| - Two and three dimensional images possible | ||||
| Photoacoustic spectroscopy (PAS) | - Photoacoustic effect caused by laser excitation | - High sensitivity and selectivity | - Strong background signal | - Atmospheric chemistry143,144 |
| - Highly linear response and a wide dynamic measurement range | - No separation capability | - Plants13,145–147 | ||
| - Non-invasive real-time method | - Food148,149 | |||
| - Clinical applications150–158 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (II) Mass spectrometry methods | ||||
| GC-MS | - Separation based on different strengths of interaction between analytes and the stationary phase of a column | - Separation capability | - Time-consuming | - Plants159–162 |
| - Analytes are ionized by EI or CI ion source | - Sensitive | - Labor intensive | - Pharmaceutical sciences163 | |
| - Ionize polar and non-poler molecules | - Thermal degradation possible | - Food164–168 | ||
| - Quantitative analysis possible | - Impossible real time analysis | - Biology169–174 | ||
| - Structural information can be obtained | - Co-elution | |||
| - Good databases and libraries available | ||||
| Multi-dimensional GC-MS | - Combine two or more columns with orthogonal separation characteristics | - Better separation efficiency | - More complicated instrumentation design | - Food and beverage175–179 |
| - Higher detection sensitivity | - Longer time for measurements | - Bacteria and fungi180,181 | ||
| - Clinical applications182 | ||||
| PTR-MS | - Proton-transfer reactions between hydronium ions and analytes | - Sensitive | - Cannot efficiently ionize weakly polar and non-polar compounds | - Atmospheric chemistry183–187 |
| - Real-time analysis possible | - Cannot discriminate isomeric compounds | - Food and beverage188–205 | ||
| - No structural information | - Plants206–213 | |||
| - Insects214,215 | ||||
| - Bacteria and fungi216,217 | ||||
| - Vertebrates218 | ||||
| - Clinical applications219–224 | ||||
| SIFT-MS | - Reagent ions created in a microwave cavity discharge ion source react with analytes | - Rapid | - Lower sensitivity | - Atmospheric chemistry225 |
| - Real-time analysis possible | - No structural information for unknown compounds | - Food226–239 | ||
| - Quantification without need for calibration using internal standards | - Cannot discriminate isomeric compounds | - Bacteria and fungi240,241 | ||
| - Animals242,243 | ||||
| - Clinical applications244–247 | ||||
| SESI-MS | - Ionized via gas-phase reactions with an electrospray | - Rapid, sensitive | - Cannot efficiently ionize weakly polar and non-polar compounds | - Food248 |
| - Real-time analysis possible | - No structural information | - Plant249 | ||
| - Bacteria250 | ||||
| - Clinical applications251–256 | ||||
| DART-MS | - Ionized by ion-molecule reactions between high energy metastable helium or nitrogen beam and analytes | - Rapid, sensitive | - Cannot couple with GC or LC | - Pharmaceutical science257 |
| - Real-time analysis possible | - Impropriate for quantification analysis | - Food258–260 | ||
| - Ionize weakly polar and even non-polar compounds | - Plant261 | |||
| - Insect262 | ||||
| - Clinical applications263,264 | ||||
The absorption techniques have been widely used in analysis of various volatile natural products. For example, Butenandt et al. extracted 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pheromone glands, yielding 280 g of crude extract, from which they obtained 12 mg of the pure pheromone.129 The pheromone was identified by elemental analysis and UV spectroscopy as a hexadecadienol with conjugated double bonds. Based on behavioral data, males of P. cruciferae were found to emit aggregation pheromones that attracts both sexes when the males are feeding on host material.130 The pheromones have been studied by UV spectroscopy, combined with other techniques including mass spectrometry, NMR spectroscopy, chiral and achiral gas chromatography, molecular modeling etc. Eight male-specific compounds were chromatographically isolated, and spectroscopically analyzed. Three dominant compounds were identified as (+)-ar-himachalene; (+)-trans-himachalene; (+)-cadinene, respectively.
000 pheromone glands, yielding 280 g of crude extract, from which they obtained 12 mg of the pure pheromone.129 The pheromone was identified by elemental analysis and UV spectroscopy as a hexadecadienol with conjugated double bonds. Based on behavioral data, males of P. cruciferae were found to emit aggregation pheromones that attracts both sexes when the males are feeding on host material.130 The pheromones have been studied by UV spectroscopy, combined with other techniques including mass spectrometry, NMR spectroscopy, chiral and achiral gas chromatography, molecular modeling etc. Eight male-specific compounds were chromatographically isolated, and spectroscopically analyzed. Three dominant compounds were identified as (+)-ar-himachalene; (+)-trans-himachalene; (+)-cadinene, respectively.
Near-infrared spectroscopy (NIRS) is widely applied in evaluation of food quality because of its non-destructive nature, rapidity, low cost, and environmental friendliness.266,267 A review article focused on a rapid and real-time analysis using infrared laser spectroscopy, which can be used in situ, and does not require sampling. The technique offers opportunities for online monitoring of food processing operations and in vivo sensing of food properties during consumption from their volatile components.268 Fourier transform infrared spectroscopy (FT-IR) is another effective method for gaseous measurement.269,270 FT-IR is a multiplex instrument with an ability to simultaneously scan over a wide wavelength range. This technique has advantages over conventional IR spectroscopy including higher throughput and wavelength accuracy. Moreover, FT-IR has the possibility to obtain molecular specific information for virtually any sample in any state with no treatment or minimal sample preparation. Currently, the technique has made possible the analysis of contaminants at parts per billion (ppb) levels in different matrices; such as water and soils.271,272 FT-IR has been widely used to rapid and real time measure gaseous samples in various applications.273,274 The applications of the technique in atmospheric chemistry have been thoroughly reviewed by Tuazon et al.,131 Marshall et al.,132 and Hanst and Hanst.133
Infrared spectroscopy can deliver additional information on functional groups and C–C double bonds, which cannot be obtained from other techniques such as GC-MS (discussed in Section 3.2.1).275 Therefore, the combination of GC and IR spectroscopy has led to the development of different kinds of GC-IR instruments to obtain more information about target compounds.275,276
Tunable diode laser absorption spectroscopy (TDLAS) is a combination of the traditional laser absorption techniques with tunable diode lasers, which measures the absorption characteristics of the molecules by scanning across its particular central absorption peak.277,278 TDLAS uses a laser light source that can be tunable over a small wavenumber range with a very narrow line width. One of the representative TDLAS applications for biological VOCs related research is the quantification of formaldehyde (CH2O).134 Formaldehyde is a ubiquitous component of the troposphere and the most abundant carbonyl compound found in the boundary layer atmosphere. The CH2O measurements based on TDLAS have yielded several important insights about terminal alkene emissions and photochemistry.134 Methane is another important greenhouse gas and tropospheric ozone precursor. The Aerodyne Ethane-Mini spectrometers based on the TILDAS technique has been developed to quantify methane in sampled ambient air.135
The optical sensor of formaldehyde based on multi-pass absorption spectroscopy has been developed to search for the possible cancer biomarker in air exhaled from human lungs.136 The selection of the spectral range (3595.77 → 3596.20 nm), and the use of sample pressure reduced to 0.01 atm, enabled to immunize the detection against typical interferents present in breath at high concentration including water vapor, carbon dioxide and methane.
The LIF technique has been widely used in analysis of the atmospheric radicals, such as online in situ characterization of volatiles in the close vicinity of wood areas and forests.137,138 The biogenic volatile organic compounds (BVOCs) emitted by plants to the atmosphere play a significant role in tropospheric photochemistry especially in ozone and secondary organic aerosol productions as a result of interplays between the compounds and atmospheric radicals, such as hydroxyl radical (OH), ozone (O3) or NOn (NO + NO2).139 The chemical species emitted by forests create complex atmospheric oxidation chemistry and influence global atmospheric oxidation capacity and climate. The Southern Oxidant and Aerosol Study (SOAS) tested the oxidation chemistry in a forest, where isoprene was the dominant volatile compound.138 OH was measured by laser-induced fluorescence and by taking the difference in signals without and with an OH scavenger that was added just outside the instrument's pinhole inlet. The SOAS results provided strong evidence that the current isoprene mechanisms are consistent with measured OH and HO2; thus, capture significant aspects of the atmospheric oxidation chemistry in the isoprene-rich forest. In another study, the eddy covariance method was applied to estimate fluxes of OH and HO2 together with fluxes of isoprene, the sum of methyl vinyl ketone and methacrolein, and the sum of monoterpenes above a mixed deciduous forest.141 Highly sensitive measurements of OH and HO2 were performed by LIF, and the BVOCs were also measured by PTR-MS (discussed in Section 3.2.3) at a time resolution of 5 s each.
Spectroscopic techniques (LIF and IRLAS) were also combined with mass and temperature measurements to gain further insights into the complex pyrolysis mechanisms of thermally thick wood particles.280 In a similar study, the volatiles in the pyrolysis product gas were characterized by means of in situ LIF.281 The measurements in close vicinity to the surface of a pyrolyzing beech wood particle indicated that the composition of the condensable gas species strongly depended on the particle size and heating rate.
The LPAS technique has been used to monitor trace gas concentrations under atmospheric conditions with high sensitivity within a small volume of gas, non-invasively and on-line under dynamic conditions.45 Over the last decade, laser-based infrared photoacoustic spectroscopy has been developed as a versatile tool for sensitive and continuous trace gas detection.284,286 The detection limits of LPAS for most gases can reach ppb or even ppt levels, which are much lower than those of conventional FT-IR. The technique was also implemented in plant physiological studies and has gained remarkable impact.145,146 In 2005, a laser-based photoacoustic system was used for the first online, in planta, monitoring of the NO production from pathogen-infected tobacco leaves.287 This study indicated that NO influenced the kinetics of cell death and resistance to both avirulent and virulent bacteria in tobacco, and suggested that NO is integral to the elicitation of cell death associated with the two bacterial pathogens in tobacco.
The LPAS was used to investigate the on-line evolution of ethanol and acetaldehyde from the imbibed nonaged and aged seeds of cabbage.147 The results show that the performance of LPAS was superior to that of FT-IR and of GC. The sensitivity of the LPAS method allows measurements on seeds to be performed 3 hours after the onset of imbibition and even sooner.
Gases produced inside harvested fruit can sensitively influence the continuing quality of the stored fruit and its maximum time of storability. The evolution of gaseous volatiles inside “Golden Delicious” apples were studied using the CO2 laser photoacoustic spectroscopy.148 The CO2 laser photoacoustic spectroscopy could determine the low concentration of ethylene, ethanol and ammonia. In a similar study, several new methods based on PAS were developed to detect ethylene and ethanol compounds from the internal atmosphere of apples.149 The non-invasive, real-time inspection methods can reveal when the degradation process begins, and can be used to improve the quality and storability of fruits.
During several decades, the potential of the PAS technique applied in the medical clinic has been recognized.150,151 The detection of VOCs from the exhaled breath represents an attractive non-invasive tool for monitoring and diagnosing disease.152,153 Human exhaled breath consists of more than 3000 volatile organic compounds, many of which are relevant biomarkers for various diseases. Thus, breath analysis has promise as non-invasive, simple and point-of-care clinical measurements to reduce the medical burden caused by invasive, time-consuming and expensive diagnostic devices. Spectroscopic techniques can offer information to correlate its signals to exhaled components for molecular identification and quantification to provide the pathophysiological status of a patient. Exhaled breath analysis based on PAS can allow rapid progress in the area of clinical applications because the procedure is non-invasive and breath can be sampled as often as necessary, continuously even during sleep154–156 as opposed to blood, which cannot be sampled continuously. Recently, analytical methods for searching biomarkers in exhaled air have been extensively developed.157,158 For example, researchers have been applying PAS into human breath analyses for different exhaled biomarkers such as ammonia for renal disease.288 In a recent review paper, the evolution of mid-infrared sensing technologies has been discussed with a special focus on photoacoustic spectroscopy, and its application in exhaled breath biomarker detection.153 With laser-based photoacoustic spectroscopy, volatile compounds can be identified with high sensitivity, at a high rate, and with good selectivity.
As discussed above, the spectroscopic techniques can perform sensitive analysis of volatile natural products. However, compared to other instrumental analytical techniques such as mass spectrometry, the techniques have lower selectivity and cannot be efficiently used in identifications of unknown compounds in complex matrices. Thus, the spectroscopic techniques are often combined with other techniques, such as mass spectrometry, in the practical applications of studies of VOCs.289
3.2. Mass spectrometry (MS)
Mass spectrometry is currently playing an indispensable role in biological analysis, due to its inherent sensitivity and rapid identification capabilities of different compounds.290–292 MS can detect various compounds in the wide mass range, obtain mass spectrum at each time point, and can provide information of molecular structure of a compound. The MS techniques can perform real-time and instant quantitative analysis, and are especially suitable for trace, unstable and short-lived biological VOCs generated during metabolism.293,294In this review, I will briefly discuss the mass spectrometry techniques currently used in analysis of volatile natural products, including offline GC-MS and multi-dimensional GC-MS, and several online MS techniques including PTR-MS, SESI-MS, SIFT-MS and DART-MS.
In a typical GC-MS measurement, volatile compounds are collected from a headspace, trapped on an adsorptive surface or solvent extracts, followed by direct injection or thermal desorption in the GC port for separation. Electron ionization (EI) is usually used as the ion source of the GC-MS instruments. As a typical hard ionization technique, EI will generate a series of fragments peaks of ions in mass spectra. The VOCs in complex mixtures can be identified by comparisons of the standard mass spectra with databases, or internal standards based on the matching of fragmentation patterns and chromatographic retention indices.300–303
Besides mass spectrometer, several different detection systems can be also coupled to GC in order to obtain a better qualitative and quantitative analytical result, such as flame ionization detector (FID)304 or Fourier transform infrared (FT-IR).305 The main advantage of GC-MS is that it has the high sensitivity and the low limit of detection. The advantage makes GC-MS one of the most widespread analytical techniques in many scientific fields including food chemistry, metabolomics, forensics, environmental sciences etc.306–308 The GC-MS method is currently often referred as the “gold standard” for studies of volatile compounds.309,310
As discussed in the previous section, headspace SMPE is often coupled with GC-MS and used in analysis of VOCs.311,312 An example is the analysis of ground carob pods from three different European countries to decode their released aroma.159 The chromatographic results highlighted 54 common VOCs, in which the most abundant ions corresponded to be propanoic acid 2-methyl (isobutyric acid), acetic acid, butanoic acid, hexanoic acid, and propanoic acid 2-methyl-methyl ester. Kaixin San is a prescription traditional Chinese medicine with the effects of “tonifying the kidney and brain” and “improving memory”. HS-GC-MS and HS-GC-ion mobility spectrometry have been used in the comprehensive characterization of VOCs in Kaixin San and the quantitative analysis of the main pharmacodynamic substances.163 A total of 117 VOCs were identified and 10 components (isocalamenediol, a-asarone, β-asarone, methyl eugenol, isoeugenol methyl ether, camphor, anethol, 2,4-di-tert-butylphol, linalool, asarylaldehyde) were suggested as the quality markers.
Volatile compounds in mango peel and seed were chemically characterized and compared with those in mango pulp based on HS-SPME/GC-MS.166 More than 60 volatile compounds were detected in mango by-products. The results indicated that mango peel was a valuable matrix of odor-active compounds, which were found in even larger quantities than in edible mango fractions. 3-Carene was the predominant compound, although other compounds such as decanal, 1-octen-3-one, nonanal, limonene, damascenone, and 2-nonenal were the most odor-active compounds.
Garrido et al. reported the chemical profiling of VOCs of gametophyte and sporophyte life stages of Leiosporoceros dussii, from Panama using HS-SPME-GC-MS in order to assess distinguishing chemical markers between the male and female gametophytes, and sporophytes of this hornwort.162 A total of 27 VOCs were identified, and the gametophyte and sporophyte showed clear differences in the type and amount of VOCs.
SPME coupled to GC-MS was also employed for the headspace determination of the volatile organic fraction emitted by two of the most common Mediterranean demosponges, Ircinia variabilis and Sarcotragus spinosulus.169 A different composition in sulfur compounds was detected between volatile compounds, indicating a different ability of the two species in producing such chemicals. Another study reported a survey of the volatiles released by males and females of Nicrophorus vespilloides Herbst in nonbreeding status and at different stages of breeding based on headspace analyses by using SPME-GC-MS.170 The volatiles included phenolic compounds, alcohols, aldehydes and ketones and were similar in both sexes. With the onset of breeding, the volatile profiles of males and females become distinct, with a number of female-specific compounds occurring. Tabata et al. determined the structure of a sex pheromone of P. solenopsis in order to develop an effective lure for monitoring this pest.171 From volatiles emitted by virgin adult females, they isolated a compound, which was attractive to males. By means of coupled GC-MS and nuclear magnetic resonance spectroscopy, they identified the compound as (2,2-dimethyl-3-isopropylidenecyclobutyl) methyl 3-methylbut-2-enoate.
Hayes et al. investigated changes through time in the attractiveness of natural honeybee hive products to the small hive beetle as the hive products were altered by the action of beetle larvae and fermentation by K. ohmeri.172 They used GC-MS and choice-test behavioral assays to investigate the changes using products sampled from three apiaries, and found that attractiveness of the fermenting hive products increased as fermentation progressed, and volatile profiles became more complex.
GC-MS was also used to identify 103 organic compounds from urine, feces, anal glands, and preputial glands of free-ranging African wild dogs, Lycaon pictus.174 Aliphatic acids were found to be the dominant class of compound in all materials. In addition, urine contained dimethyl sulfone, 1,3-propanediol, benzoic acid, 1-methyl-2,4-imidazolidinedione, and squalene as major components; feces contained indole and cholesterol; and both contained 2-piperidone, phenol, 4-methyl phenol, benzene acetic acid, and benzenepropanoic acid and other compounds.
To facilitate the screening of volatile active compounds in natural products, Li et al. developed a new biochromatography method that used rat vascular smooth muscle cells (VSMC), which are rich in L-type calcium channels (LCC), to prepare the stationary phase.313 This integrated method, which coupled cell membrane chromatography (CMC) with GC-MS via microextraction by packed sorbent (MEPS) technology, was termed VSMC/CMC-MEPS-GC-MS. As a biomembrane chromatography method, CMC maintains the biological characteristics of the cell membrane. The chromatographic stationary phase that contains live cell membranes can mimic the physiological process and selectively bind active compounds in samples, making CMC a suitable technique for the identification of active components in complex sample matrices.314,315 The VSMC/CMC-MEPS-GC-MS method has been shown to be a highly efficient, reliable, environmentally friendly, qualitative bioanalytical method, especially for compounds that are volatile, thermally unstable or difficult to purify.
In the aspect of data analysis, a study was focused on the use of integrated chemometric methods as complementary tools for GC-MS analysis, in order to achieve comprehensive analysis of the chromatographic fingerprint of Iranian C. aurantium L. peel extract.316 The study showed that the application of the MCR-ALS method with the pre-processed data could efficiently assist GC-MS in trace analysis through improvement of the analysis of overlapped or embedded peaks. The proposed methodology not only significantly enhanced the separation ability of the hyphenated system to achieve accurate qualitative identification, but also elegantly enhanced its ability in quantitative analysis.
The above application examples clearly demonstrate that the GC-MS technique is a powerful tool in analysis of volatile organic compounds. However, a drawback of the technique is that the measurements are time-consuming and labor-intensive, which limits the number of samples that can be analyzed in practice. Moreover, the compounds need to be converted to gas-phase in the GC injection port. It can cause thermal degradation or reactions to take place, especially for compounds unstable under high temperature.317
The above co-elution problem can be solved by adding another dimension of separation based on gas chromatography.320 The two dimensional gas chromatography (GC × GC) technique was developed by Liu and Phillips in 1991.321 This technique combines two columns with orthogonal separation characteristics installed in the same GC oven. The complete effluent from the first dimension is focused (mainly by a thermal-based modulator) and transferred to the second column in small concentrated segments, being therefore separated in slices. This focusing effect creates narrow second-dimension peaks, which can efficiently enhance the detection sensitivity. Currently, most metabolomics-related 2D-GC studies apply a conventional nonpolar stationary phase column and a second column with a semipolar or polar stationary phase (e.g., polyethylene glycol).
The developments of multi-dimensional GC have greatly improved the separation capacity for complex biological VOCs.322 In a review, the basic principles of the two-dimensional chromatographic systems have been discussed whether for the heart-cuts or comprehensive modes.323 Moreover, recent trends in higher integrated dimensions have been presented, as well as fundamentals of multidimensional stationary phase orthogonality and compositions to guide users select the most optimum combinations for different classes of compounds.
Mitrevski et al. developed a novel hybrid comprehensive 2D-GC method for precise and high-resolution characterization of multiple volatile components from a coffee sample.175 In a similar study, the comprehensive 2D GC-MS was used in a study of the difference of volatile components in green, oolong and black teas.176 A total of 450 compounds were tentatively identified. This study demonstrated the power of the GC × GC-MS method combined with multivariate data analysis to investigate natural products with high complexity.
Comprehensive 2D GC-MS metabolite profiling methods were also applied for nontargeted detection of volatile and nonvolatile compounds to determine changes that occurred during anaerobic fermented cucumber spoilage by L. buchneri LA1147 and during reproduction of spoilage with natural microbiota.178 Univariate analysis of variance combined with hierarchial clustering analysis revealed 92 metabolites that changed during spoilage. In another study, the investigation of the volatile compounds of dried rhizomes of Coptis chinensis Franch, C. deltoidei C. Y. Cheng et Hsiao, and C. teeta Wall was carried out to determine the chemical composition of the valuable natural products.179 Volatile profiles were established and compared based on the measurements using HS-SPME coupled to comprehensive 2D GC × GC-MS. The majority of the identified compounds eluted as well-separated (pure) components as a result of high-resolution capability of the GC × GC method. More than 290 volatile and semivolatile organic compounds were tentatively characterized. These compounds are distributed over the chemical groups of hydrocarbons, acids, alkenes, alkynes, aldehydes, ketones, alcohols, esters, furans, and terpenoids.
A valve-based comprehensive 2D GC coupled with a time-of-flight mass spectrometer (GC × GC TOF-MS) was developed.324 The valve that interfaced two columns injected aliquots of the first column effluent onto the second column at rapid intervals. The appropriate sampling rate was used to ensure that all the components in the sample were analyzed by the second separation. The performance characteristics of the instrument were evaluated using a complex sample containing a mixture of fuel components, natural products, and organo-phosphorous compounds. The valve-based GC × GC, designed to function with an extended temperature of operation range, was shown to have high chromatographic resolution, high separation efficiency and low detection limits. Typical peak widths at base were nominally from 100 to 300 ms on column 2 and nominally 10 s on column 1.
A potential problem with the 2D GC method is that for some samples, the chromatograms often contain broad, tailing analyte bands. This results in difficulties with quantitative analysis. In a study, Edwards et al. investigated the inlet and the modulator as the potential sources of these tailing bands.325 A simple inlet backflushing device was developed to isolate the inlet from the primary column after the injection. Briefly, a three-way switching valve was activated after predetermined time following the injection to allow carrier gas bypass the inlet and the primary column. In this way, analytes retained within the inlet were prevented from entering the column and were subsequently removed via the carrier gas split line. The inlet backflushing approach was demonstrated to be an effective tool for improving the chromatography of problematic GC × GC analyses of samples containing complicated matrices.
Furthermore, a multi-capillary column (MCC) has been developed to improve separation efficiency of traditional GC columns.182 The MCC was fabricated by combining a bundle of some 1000 parallel thin capillaries inside a stainless-steel tube, and the inner surface of each capillary in the MCC was covered by film of a stationary liquid phase. Sacrificing some chromatographic resolution, the separation time could be effectively shortened by utilizing the MCC, as the length of the commonly used straight MCC is only 40–250 mm permitting ultra-fast GC separation for near real-time analysis. The hyphenation of the MCC with ion mobility spectrometry (IMS) or proton transfer reaction mass spectrometry (discussed in the next section) have already been successfully implemented for fast analysis of VOCs.326–328 Chen et al. described a first attempt to develop a two-dimensional hyphenated instrument by coupling of a multi-capillary column with a high-pressure photon ionization source (HPPI).182 The MCC HPPI-TOF-MS system was preliminarily applied for rapid and online analysis of flavor compounds in the exhaled gas of a volunteer after mouth rinsing with a gargle product. The rapid changes of the three flavor compounds (eucalyptol, L-menthone and linalool), as well as the steady endogenous metabolite acetone, in the exhaled gas were successfully determined with a time resolution of only 1.5 min.
As a chromatographic method, one needs a certain period of time for sample collection (minutes to hours) in a typical GC experiment. It excludes the possibility of real-time analysis. The need for real-time measurements of VOCs has led to considerable interest in non-chromatographic methods. In this review, several non-separative methods based on mass spectrometry are discussed in the following sections, including proton transfer reaction mass spectrometry (PTR-MS), selected ion flow tube mass spectrometry (SIFT-MS), secondary electrospray ionization mass spectrometry (SESI-MS) and direct analysis in real time (DART-MS). These techniques have been established as an attractive option in analysis of volatile natural products.
A typical PTR-MS instrument usually consists of four different parts, ion source, drift tube, ion interfaces, and mass analyzer, as shown in Fig. 3. The ion source produces high-purity hydronium ions, which are introduced to the drift tube for proton-transfer reactions with analytes. In the drift tube, a volatile compound (R) with higher proton affinity than H2O (691 kJ mol−1) can be ionized via the proton transfer reaction with H3O+ to produce the product ions, RH+. The product ions are then transmitted through the ion interfaces to a mass analyzer for detection. As an excellent technique to measure a broad spectrum of volatile compounds, PTR-MS has been extensively used in many different fields of biogenic volatile organic compounds, ranging from laboratory experiments to field studies.333–342 The ability of PTR-MS to simultaneously monitor and quantify compounds from very diverse chemical groups with high sensitivity and non-invasively makes it an excellent method to monitor aroma, flavor and fermentation related trace gases during growing, ripening or fruit storage.190,343–345
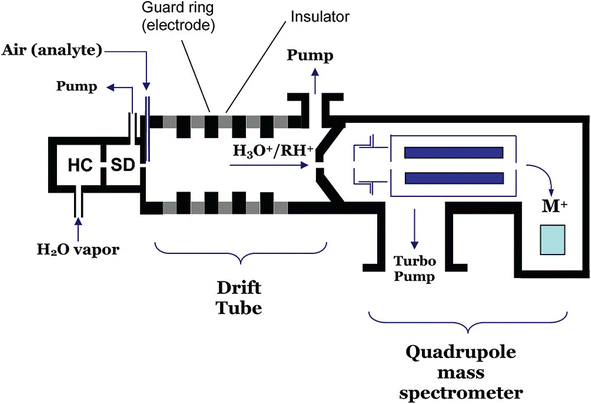 | ||
| Fig. 3 Simplified representation of a proton-transfer reaction mass spectrometer utilizing a quadrupole mass filter: HC = hollow cathode discharge source; SD = source drift region. (Reprinted with permission from Blake et al.332 Copyright (2009) American Chemical Society). | ||
The applications of PTR-MS have enabled important advances in understanding sources of many VOCs, as well as their environmental effects in the atmosphere. The PTR source used to be coupled with a quadrupole mass spectrometer. The major disadvantage of the setup is its limited mass resolution due to the nature of the quadrupole mass spectrometer. In the past few decades, many new mass spectrometric techniques were coupled to the PTR ion source, and the performance of the PTR-MS instruments has been significantly improved. Time-of-flight mass spectrometers have currently become the preferred mass analyzers for PTR-MS instruments, which can provide higher sensitivity and higher mass resolution measurements. Improvements of the ion interface in recent years have also enhanced instrument sensitivities and achieved better detection limits. Moreover, utilizing reagent ions other than H3O+ further expands the detection capability of the PTR-MS instruments.
The emissions of BVOCs in the Mediterranean region were monitored with an on-line PTR-TOF-MS.183 Anabolic and catabolic BVOC emissions were observed to be strongly seasonal dependent. Amplified drought decreased all BVOC emission rates in spring and summer by around 40–50%, especially through stomatal closure, with no effect in autumn. In temperate forests, nearly all tree species associate with arbuscular mycorrhizal (AM) or ectomycorrhizal (ECM) fungi. Trowbridge et al. measured BVOC fluxes at the soil-atmosphere interface in plots dominated by AM- and ECM-associated trees in a deciduous forest in south-central Indiana, USA during the early and late vegetative growing season using the PTR-MS.185 Their results demonstrated the importance of soil dynamics characterized by mycorrhizal associations to BVOC dynamics in forested ecosystems. PTR-MS was also used to study online VOC emissions of tree species.186,187 Of special interest was a change in the emission behavior under changing environmental conditions such as flooding or fast light/dark changes. Flooding of the root system caused an up to 20 times increase in several VOCs, dominated by the emission of ethanol and acetaldehyde, which can be explained by the production of ethanol under anoxic conditions of the root system, and subsequent transport and partial oxidation to acetaldehyde within the green leaves.187
Yanez-Serrano et al. reported the first atmospheric BVOC measurements at the Amazonian Tall Tower Observatory (ATTO) site, located in central Amazonia.346 A quadrupole PTR-MS with seven ambient air inlets, positioned from near ground to about 80 m, was deployed for BVOC monitoring. They reported diel and seasonal ambient mixing ratios for isoprene, monoterpenes, isoprene oxidation products, acetaldehyde, acetone, methyl ethyl ketone, methanol and acetonitrile. Yanez-Serrano et al. also characterized forest below-canopy VOC mixing ratios, monitored by PTR-MS, at Montseny Natural Park, a Mediterranean forest 50 km from the Barcelona urban area.347 Measurements were taken every 2 min during six months around the maximum emission period of summer. They found that all VOCs had diel cycles with higher mixing ratios during the day, but different patterns over time. Even though the biogenic source was the strongest source profile at the site, they found a strong influence of anthropogenic air masses infiltrating the forest canopy and altering the biogenic air masses at the site.
Kreuzwieser et al. tested the hypothesis that Dionaea releases VOCs to allure prey insects.207 Olfactory choice bioassays were performed to elucidate if Dionaea attracts Drosophila melanogaster. The VOCs emitted by the plant were analyzed by PTR-MS and GC-MS. Over 60 VOCs, including terpenes, benzenoids, and aliphatics, were emitted by Dionaea, predominantly in the light. From these results, they concluded that Dionaea attracts insects on the basis of food smell mimicry because the scent released has strong similarity to the bouquet of fruits and plant flowers. Such a volatile blend is emitted to attract insects searching for food to visit the deadly capture organ of the Venus flytrap.
Van Dam et al. used PTR-MS for online analysis of herbivore-induced VOCs from six different species of Brassica over time.209 The roots were either artificially damaged or infested with cabbage root fly larvae. Brassica nigra, B. juncea and B. napus primarily emitted m/z 60 directly after artificial damage or root fly infestation. Undamaged plants could be clearly distinguished from damaged plants on the basis of one or two marker VOCs. They found that the type of sulfur containing VOC emitted was species specific and independent of sinigrin being present in the roots. Crespo et al. analyzed on-line VOC emissions by roots of Brassica nigra plants under attack by cabbage root fly larvae, Delia radicum.210 The VOCs were detected using PTR-MS and GC-MS. Their analyses showed that several sulfur containing compounds, such as methanethiol, dimethyl sulfide, dimethyl disulfide, dimethyl trisulfide and glucosinolate breakdown products, such as thiocyanates and isothiocyanates, were emitted by the roots in response to infestation. The PTR-MS analyses showed that infested plants with actively feeding herbivores could clearly be distinguished from uninfested plants within hours after infestation until the feeding had stopped. Above-ground VOC emissions of Scots pine (Pinus sylvestris) saplings were measured in the laboratory using a PTR-TOF-MS before, during and after a 48 hour exposure to bark-feeding pine weevils.211 Monoterpenes dominated the emissions during and after herbivore feeding. The maximum monoterpene emission response was linearly proportional to the damaged bark area, demonstrating a dose-dependent response.
Thirty-five VOCs were tested on B. cinerea in vitro and volatile emission was analyzed in strawberry harvested at four ripening stages by HS-SPME-GC-MS and PTR-TOF-MS.191 The authors concluded that key strawberry aroma compounds stimulate B. cinerea conidial germination and some typical wound-volatiles can stimulate pathogen conidial germination or mycelial growth. PTR-TOF-MS was also utilized to obtain the mass-resolved fingerprints of VOCs of three species of citrus fruit (grapefruit, pummelo, and sweetie).192 The application of the PCA allowed to select volatile organic compounds characteristic for a studied citrus species. Based on the obtained results, they concluded that esters and acetic acid were associated with pummelo samples, while limonene and linalool were related to grapefruit and sweetie samples, respectively.
Giacomuzzi et al. investigated the diel emission of VOCs from intact apple (Malusx domestica Borkh., cv. Golden Delicious) and grape (Vitis vinifera L., cv. Pinot Noir) foliage.196 Volatiles were monitored continuously for 48 h by PTR-TOF-MS and identified by GC-MS. The majority of the VOCs were terpenes, followed by green leaf volatiles, and aromatic compounds. In another study, the analysis of volatile compounds was used for the discrimination of fire blight (Erwinia amylovora) and blossom blight (Pseudomonas syringae pv. syringae) on apple propagation material.197 Possible marker compounds were identified by GC-MS and PTR-TOF-MS. After a preliminary validation in vitro, a diagnostic protocol was successfully developed to scale up to real nursery conditions on cold stored, asymptomatic dormant plants.
Badra et al. compared the volatile profile of apple trees infested with two aphid species, the green apple aphid Aphis pomi, and the rosy apple aphid Dysaphis plantaginea, by CLSA-GC-MS complemented by PTR-TOF-MS.212 They found that benzaldehyde and (E)-β-farnesene were exclusively associated with A. pomi, whereas linalool, (E)-4,8-dimethyl-1,3,7-nonatriene were only associated with D. plantag inea. VOCs emitted during the infection of apple (Malus pumila var. domestica) plants by Erwinia amylovora or Pseudomonas syringae pv. syringae were studied by GC-MS and PTR-MS.213 The authors found that infected plants showed a disease-specific emission of volatile organic compounds, including several bio-active compounds, such as hexenal isomers and 2,3-butanediol.
Real-time profiling of VOCs of mango ripening based on PTR-TOF-MS was demonstrated using headspace measurements of ‘Tommy Atkins’ mangoes.198 In this study, volatile metabolites produced during the ripening process were sampled directly, which enabled simultaneous and rapid detection of a wide range of compounds. A paper studied the effect of bruising on the volatilome released by pears by using PTR-MS.199 The release of m/z 45 and 47 were significantly higher in bruised samples, indicating that the bruising event accelerated the natural ripening process.
As tropical fruits produce a wide range of VOCs, PTR-TOF-MS was used to fingerprint the volatile profile of four tropical fruits (avocado, banana, mango and mangosteen) and determine whether the instrument could be used to assess fruit ripening stages.203 Data were subsequently subjected to partial least squares discriminant analysis. Given the rapidity and the potential to use this analysis method on a large scale, the results showed that PTR-TOF-MS has a high potential to become a commercial standard tool for monitoring food quality from entering the storage chain up to the ‘ready to eat’ labeling. The aromatic profile and the pungency of 21 different Capsicum varieties, belonging to four different species, have been investigated to evaluate a possible correlation between different VOC emission and their spiciness.204 The measurement of capsaicinoid concentration was performed by a HPLC method, while the VOCs from fresh samples were analyzed using PTR-MS. VOCs detected at m/z 103.08 and 43.05 showed a higher contribution in the correlation with the capsaicin content. This study provided promising results, showing a real possibility to use these tools in routine operations for predicting the spiciness of fresh peppers useful for breeding programs and consumers.
Root herbivores are usually difficult to study, as they usually are hidden in the soil. However, root herbivores may be traced by analyzing specific VOCs produced by damaged roots. These VOCs not only support parasitoids in the localization of their host, but also may help scientists study belowground plant-herbivore interactions. In a review paper, Danner et al. provided a brief overview of PTR-MS and illustrated how this technology can be applied to detect specific root-herbivore induced VOCs from Brassica plants.214
Fungi colonizing aboveground (AG) or belowground (BG) plant structures can modify VOC patterns, thereby altering the information content for AG insects. A study by combining GC-MS and PTR-MS revealed that endophyte-infected roots emitted less VOCs and more CO2.216 Their results demonstrated that symbiotic fungi in plants may influence soil insect distribution by changing their behavior towards root volatiles. This study provided the first evidence of an AG endophytic microorganism affecting BG processes by changing plant volatile emission. Several analytical techniques including GC-MS, PTR-MS and laser photoacoustic detection, were used to characterize the volatiles emitted by Erwinia amylovora and other plant-pathogenic bacteria.217 Diverse volatiles were found to be emitted by the different bacterial species examined.
In a study, Kilpinen et al. presented a combination of PTR-MS and video analysis for real-time measurement of semiochemicals emitted by isolated groups of bed bugs during specific behavioral activities.215 The most distinct peaks observed in the PTR-MS measurements were always observed close to the termination of mating attempts, corresponding to the defensive emissions that bed bugs have been suspected to exploit for prevention of unwanted copulations. The main components of these emissions were identified as (E)-2-hexenal and (E)-2-octenal recorded in ratios between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 3
3 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Several mycobacterial species can produce serious infections in humans, and the treatment required depends on the infecting species. Crespo et al. proposed a method potentially allowing cultures to be identified by headspace analysis and screened for differences between mycobacterial species based on the volatiles released during growth.219 Short-chain volatile compound emissions from two non-tuberculosis slow growing mycobacterial species, Mycobacterium avium and Mycobacterium kansasii, and a non-pathogenic fast growing species, Mycobacterium smegmatis, in Middlebrook M7H9 culturing media were analyzed with an online PTR-MS instrument. Measurable differences between the headspace of the two slow growing mycobacteria were found, as well as differences with respect to the faster growing mycobacteria. Three compounds, attributed to sulfur-containing volatiles dimethyl sulfide, propanethiol and dimethyl disulfide, were found to be specific to M. avium.
In a pilot study, PTR-MS was utilized for online analysis of the exhaled breath of 13 cervical cancer patients and 34 female healthy volunteers.220 On the basis of the statistical analysis, four characteristic ions at m/z 76, 87, 93, and 121 were found for discriminating cervical cancer. The sensitivity and specificity were calculated to be 92.3% and 88.2%, respectively, using the stepwise discriminant analysis.
Heart failure (HF) is a preeminent cause of cardiovascular morbidity and mortality. Shaltaeva et al. found out that there was a significant difference in PTR-MS spectra of exhaled breath between patients with HF-PEF and without HF.221 In compare with the control group, the concentrations of acetone, acetic acid, ethanol, propylene biomarkers were found to be significantly higher in the HF-PEF group. A study investigated whether there were different concentrations of VOCs in the exhaled breath between patients with MDD (Major depressive disorder) and healthy controls using PTR-MS.222 The authors observed that the concentrations of the ions at m/z 88, 89, and 90 were significantly decreased in patients with MDD compared with the healthy controls. Moreover, changes during the time in concentrations of m/z 93 and 69 significantly differed between the groups.
Early diagnosis of COVID-19 is of the utmost importance but remains challenging. The objective of a study was to characterize exhaled breath from mechanically ventilated adults with COVID-19.223 In this prospective observational study, they used a real-time online PTR-MS instrument to perform a metabolomic analysis of exhaled air from adults undergoing invasive mechanical ventilation in the intensive care unit due to severe COVID-19 or non-COVID-19 acute respiratory distress syndrome (ARDS). Through multivariate analysis, they identified a characteristic breathprint for COVID-19, and could differentiate between COVID-19 and non-COVID-19 ARDS with accuracy of 93%. The four most prominent volatile compounds in COVID-19 patients were tentatively identified as methylpent-2-enal, 2,4-octadiene, 1-chloroheptane, and nonanal, respectively. In another study, a high sensitivity mass spectrometer combined with artificial intelligence was used to develop a method for the identification of COVID-19 in human breath within seconds.224 A set of 1137 positive and negative subjects from different age groups, collected in two periods from two hospitals in the USA, was used for the method development. The subjects exhaled into a Tedlar bag, and the exhaled breath samples were subsequently analyzed using PTR-TOF-MS. The results showed that this method for the identification of COVID-19 infection is a promising tool, which can give fast and accurate results.
As a non-chromatographic technique, a main drawback of PTR-MS is that it cannot discriminate isomeric compounds. Furthermore, due to the nature of soft ionization, only protonated ions or water clusters of analytes can be usually observed in mass spectra.348 Thus, the PTR-MS technique cannot be efficiently applied in identifications of unknown compounds. A technical note reported a number of experiments carried out with a PTR-TOF-MS equipped with a prototype fast-GC system, which allowed a fast separation of the isobaric isoprenoid compounds that otherwise could not be identified by a conventional PTR-TOF-MS analysis.349 The potential of this fast-GC system to adequately complement the information provided by PTR-TOF-MS was investigated by using the BVOC emissions of Quercus ilex and Eucalyptus camaldulensis. The results showed that this new instrument was a quick and handy tool to determine the contribution of isoprene and eucalyptol to m/z 69.07 and monoterpenes and (Z)-3-hexenal to m/z 81.07, integrating well the on-line information provided by PTR-TOF-MS. In another study, Ruzsanyi et al. successfully implemented the coupling of a multi-capillary column (MCC) with a PTR-TOF-MS system.328 They demonstrated that the approach could discriminate the ketone isomers, 3-heptanone and 2-methyl-3-hexanone, and different aldehydes. The characteristics of the fast GC separation, i.e. comparatively fast flow, high sample volume, small dimensions, present an ideal combination with a PTR-TOF-MS. This provides an additional dimension to the data, which adds valuable information with only a moderate sacrifice to the real-time capability of the PTR-MS instrument.
Another limitation of the PTR-MS instruments results from its ionization mechanisms. Only compounds with a proton affinity higher than that of the water molecule can be ionized and detected. Therefore, it cannot efficiently ionize weakly polar and non-polar compounds, such as key air components (e.g. CO, CO2), methane or other short-chain alkanes.332,350 Recently, a novel dipolar proton transfer reaction mass spectrometer (DP-PTR-MS) for real-time and on-line monitoring of atmospheric VOCs has been developed.351 Compared with conventional PTR-MS with one kind of reagent ion, DP-PTR-MS had three kinds of reagent ions, H3O+, OH− and (CH3)2COH+, which could be switched according to the actual measurement need. DP-PTR-MS improved the qualitative ability and expanded the detection range effectively.
The principle of the SIFT-MS technique has been fully described in ref. 352, 353, 356 and 357. As shown Fig. 4, a typical SIFT-MS instrument consists of four regions, ion source, mass filter, flow tube and ion detection system. In short, a mixture of reagent ions is created in a microwave cavity discharge ion source. The reagent ions (H3O+, NO+ or O2+ ions) are injected into a fast-flowing inert carrier gas, usually pure helium, through a Venturi-type inlet. A swarm of thermalized ions are created and convected along a flow tube. A gaseous sample is introduced into the ion swarm via a heated sampling line coupled directly to a sample inlet port. In the flow tube, the reagent ions react with the volatile analytes in the sample during a well-defined reaction time. The remaining of the reagent ions together with the analyte ions are sampled from the flowing swarm via a pinhole orifice located at the downstream end of the flow tube and are directed into the detector, usually a differentially pumped quadrupole mass spectrometer. The ions are detected and counted by an electron multiplier/pulse counting system. An on-board computer can directly calculate the concentrations of analytes with the aid of a kinetics library compiled from numerous studies of ion-molecule reactions.358 The unique analytical capability of the SIFT-MS technique allows quantification of volatile compounds directly from the measurement of physical parameters without the need for regular instrumental calibration using internal or external standards. Typically, the dynamic ranges of real-time analyses of BVOCs by SIFT-MS can be achieved from sub ppbv to tens of ppmv.
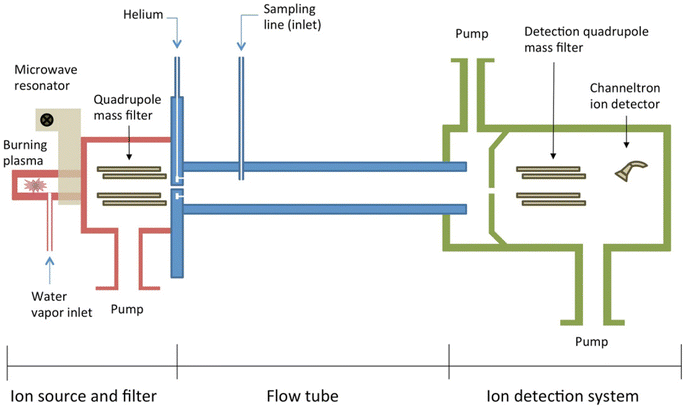 | ||
| Fig. 4 Selected ion flow tube mass spectrometer. VOCs are introduced into the flow tube of the instrument via the inlet, where they are ionized by a beam of precursor ions (H3O+, NO+, or O2+) generated by the ion source. Ionized VOCs, the fragments, and water clusters are separated by the quadrupole mass filter and detected by a channeltron ion detector. (Reprinted with permission from Materic et al.19 Copyright (2015) John Wiley and Sons). | ||
The SIFT-MS technique has been widely used by atmospheric chemists, tree physiologists and forest entomologists. Lehnert et al. developed a method based on SIFT-MS, which allowed simultaneous on-line measurement of isoprene and MBO (2-methyl-3-buten-2-ol) by employing different reagent ions.225 They tested the suitability of the method by measuring the emission of young trees of Populus, Picea, and Pinus. The results confirmed that Populus nigra, Picea glauca, and Picea abies emit isoprene while Pinus ponderosa emits MBO.
SIFT-MS has also found application in the analysis of formation and release of odorants in fruits, vegetables, nuts and seeds.226–230 Guerrini et al. tested a transportable mass spectrometry system based on SIFT-MS for the characterization and discrimination of natural resins by analysis of their VOC profiles.359 They chose diterpenoid, triterpenoid, and aromatic resins as reference components, focusing on the most identified in archeological artifacts. This work created a SIFT-MS database of mass spectra suitable for characterizing archeological samples.
During storage of shredded cabbage, characteristic sulfurous volatile compounds can be formed affecting cabbage aroma. SIFT-MS was used to measure the concentration of cabbage volatiles during storage.232 The results showed that low pH salad dressings were more suitable for storage of ready-to-eat cabbage products in terms of sensory quality while high temperature was not suitable for cabbage storage, since undesirable aroma compound formation was high, desirable aroma loss occurred quickly, and microbial growth occurred at a very early stage of storage. Changes in furan and other volatile compounds in the headspace of sliced carrot (Daucus carota ssp. Sativus) during air-drying were also studied using SIFT-MS.233 The authors found that aldehydes, alcohols and terpenes decreased 60–90% during the early stages of drying; however, significant increases in (E)-2-octenal, 2,3-butanedione, 2-methylbutanal, acetone, bornyl acetate, butanal, butanone, furfural, furaneol and sotolon, hexanal, methyl acetate, monoterpenes, norfuraneol, undecane, β-caryophyllene, β-ionone, p-cymene, and p-cymenene were found at the end of drying.
A review summarized the applications of SIFT-MS to recent deodorization studies of garlic-derived volatile organosulphur compounds.354 In a similar study, different varieties of garlic were examined for their volatile sulfur compound concentration, which may be used for purposes of quality control as well as for authentication and classification of garlic varieties.234
To determine volatile formation during storage and thawing, whole, pureed, blanched, and raw green and red bell peppers (Capsicum annuum) were frozen quickly or slowly, then stored at −18 °C for up to 7 months, with and without SnCl2 addition during thawing. Headspace analysis was performed by SIFT-MS.235 The authors found that after blanching, (Z)-3-hexenal had a large significant decrease in concentration since it is a heat labile compound while most other volatiles did not change in concentration.
The SIFT-MS has also been adapted for the detection of volatile traces from medically important fungi such as species of Aspergillus, Candida, Cryptococcus, Fusarium and Mucor.240 Preliminary measurements were made of the volatile compounds emitted by the bacterium E. coli JM109 using SIFT-MS as a step towards the real time, non-invasive monitoring of accidental infections of mammalian cell cultures.241 The results of this study suggested that monitoring volatile compounds might assist in the early detection of bacterial infection in large-scale bioreactors.
Spooner et al.243 applied multivariate analysis and SIFT-MS to evaluate serum headspace analysis as a faster screening tool for M. bovis infection in badgers, obtaining a much faster diagnosis. However, the insufficient accuracy (88% of true positive and 38% of false positive) made this approach unsuitable as an alternative for conventional diagnostic techniques.
A protocol has been developed to provide a comprehensive workflow for online and offline breath analysis based on SIFT-MS.244 Following the protocol, 50 human breath samples could be analyzed and interpreted in less than 3 h. Key advantages of SIFT-MS were exploited in this study, including the acquisition of real-time results and direct compound quantification without need for calibration curves. For the metabolites investigated, this technique proved to be reliable and repeatable. Direct on-line analysis of single or multiple breath exhalations or off-line analysis of breath samples collected into bags were performed using SIFT-MS.245 Several potential biomarkers have been quantified by SIFT-MS, including ammonia, acetone, hydrogen cyanide, alcohols, pentane, acetic acid, methane, and sulfur compounds. SIFT-MS was also used to determine the repeatability of the analysis of volatile metabolites within the breath of healthy volunteers, with emphasis on the influence of sampling methodology.246 This study presented new evidence of the short-term repeatability of SIFT-MS analysis of prominent breath metabolites detected over a wide concentration range.
A study investigated the feasibility of the SIFT-MS technology to identify breath VOCs for the detection of head and neck squamous cell carcinoma (HNSCC).247 Exhaled alveolarbreath samples were collected into sampling bags from newly diagnosed, histologically confirmed, untreated patients with HNSCC and from non-cancer participants. Breath samples were analyzed by SIFT-MS that probed for 91 specific VOCs that had been previously reported as breath biomarkers of HNSCC and other malignancies. Their results show that the median concentration of hydrogen cyanide (HCN) was significantly higher in the HNSCC group compared to the non-cancer group. A receiver operating curve analysis suggested moderate accuracy of HCN in distinguishing HNSCC from non-cancer individuals. There were no statistically significant differences in the concentrations of the other compounds of interest. This study demonstrated the feasibility of breath testing in a clinical environment using SIFT-MS. However, the small sample size and patient heterogeneity, particularly in smoking habits, tumor subsite, and stages of HNSCC, were acknowledged as limitations of this study.
As a non-separation method, currently, the SIFT-MS technique has two major weaknesses: (1) the limit of quantification is at a higher-than desirable level of ppbv for real-time analyses;360 (2) the identification of isobaric compounds is also a problem, as a non-chromatographic method and soft ionization technique.
Farrell et al. presented a rapid and sensitive method based on SESI-MS for profiling volatile emissions from the intact berries of non-Muscat grape cultivars (Pinot Noir, Chardonnay and Sauvignon Blanc).248 Approximately 300 peaks were detected in positive ion mode, and fewer (70–100) in negative ion mode. They monitored changes in grape berry volatile composition during ripening to screen for potential ripeness markers and observed 10 [M + H]+ peaks and two [M−H]− peaks that evolved in a significant linear trend (R2 ≥ 0.80, p < 0.05) for the combined data across all cultivars either increasing or decreasing in the final four weeks of ripening. Their results implied that SESI-MS in combination with portable MS instrumentation has potential for real-time field analysis. SESI-MS was also used to investigate the emissions of a Begonia semperflorens in real time and in vivo.249 To illustrate the capabilities of the system for VOC analysis, they subjected the plant to mechanical damage and monitored its response. As a result, ∼1200 VOCs were monitored displaying different kinetics. They observed significant VOC changes on the time scale of minutes, implying that these details would not be detected by off-line methods. They concluded that the capability of SESI-MS to capture highly dynamic VOC emissions and wide analyte coverage made it an attractive tool to complement GC/MS in plant VOC studies.
Bean et al. expanded the use of SESI-MS to the detection of bacterial volatiles as a method for bacterial identification.250 In their study, E. coli K12 and P. aeruginosa PAO1 were cultured aerobically for 24 hours in 50 mL LB-Lennox at 37 °C and the SESI-MS spectra of the headspace volatiles were collected in 2 minutes. Carbon dioxide was used as the carrier gas for volatile delivery to the reaction chamber. They demonstrated that SESI-MS volatile fingerprinting, combined with a statistical analysis method, could be used to differentiate bacterial genera, species, and mixed cultures in a variety of growth media.
Vapors released by the skin in the hand of one human subject were detected in real time by sampling directly from the ambient gas surrounding the hand, then ionizing by SESI.251 A dominating peak of lactic acid and a complete series of saturated and singly unsaturated fatty acids (C12 to C18) were observed. In another study, SESI was used to detect the headspace above the hand skin of two subjects, several peaks (63 for one subject and 37 for the other) arise above the background with masses reaching up to 348 Da.252 In spite of the different patterns, they shared 30 common peaks. The compounds have been assigned by collision-induced dissociation, most of them as amines.
In a work, Li et al. presented a protocol to characterize the exhaled VOCs in real time by using secondary nanoelectrospray ionization coupled to high resolution mass spectrometry (Sec-nanoESI-HRMS).253 Hundreds of peaks were observed in the background-subtracted mass spectra of exhaled breath. Less than 30 s was used for one exhalation measurement, and it took approximately 7 min for six replicated measurements.
Martinez-Lozano et al. have quantified a series of short-chain fatty acids (C3–C6) based on their standard vapors.254 They concluded that the concentration in the breath of a fasting individual was in the order of 100 ppt, in agreement with what it would be expected based on typical plasma concentrations. Using the SESI-MS technique, several respiratory diseases, including chronic obstructive pulmonary disease, obstructive sleep apnea, idiopathic pulmonary fibrosis, asthma, and lung cancer have been investigated over the past years. Several new potential biomarkers for these diseases were identified and new metabolic insights into their pathophysiology were obtained.255,256
A two-stage thermal desorption/secondary electrospray ionization/time-of-flight mass spectrometry (TD-SESI-TOFMS) for faster targeted breath profiling has been developed.371 A new ionization source was devised to constrain the thermal desorption plume and promote efficient mixing in the ionization region. Seventeen 2.5 dm3 distal breath samples were collected from asthma patients and healthy controls respectively, and subjected to comparative high-throughput screening. Eleven metabolites were selected and successfully targeted. This technique was demonstrated to have potential as a rapid screening method for biomarkers in breath in the pptv to ppbv range with analysis times of ca. 12 min.
Bruderer et al. systematically compared the analysis of volatile organic compounds with SESI-MS and PTR-MS.372 Exhaled breath from 14 healthy adults were analyzed simultaneously using both methods. A clear mass dependence was observed for each method with the highest number of detected m/z features for SESI in the high mass region (150–250 Da) and for PTR-MS in the low mass region (50–150 Da). SESI yielded a significantly higher numbers of peaks (828) compared to PTR (491) among a total of 1304 unique breath features. In another study of trace vapor analyses of three ketones (2-pentanone, 2-hexanone and 2-heptanone), the parallel quantitative analysis by SIFT-MS and SESI-MS was performed to improve the understanding of the quantitative aspects of SESI.373 The results showed that the sensitivity of SESI-MS was much higher than that of SIFT-MS and thus it can be used for real-time monitoring of much lower concentrations of volatile organic compounds in various areas.
A work illustrated that DART-MS can be used to track organosulfur defense compound chemistry under mild conditions.261Petiveria alliacea was used as a model plant that exploits the enzyme alliinase to generate induced organosulfur compounds in response to herbivory. The results showed that the downstream thiosulfinate products, petivericin and pyruvate, inhibited alliinase activity by 60% and 29%, respectively, after 1 h, and a mixture of the two inhibited alliinase activity by 65%. By 2 h, alliinase activity in the presence of these alliinase-derived products ceased completely.
A work tried to improve the SPMESH (solid-phase mesh-enhanced sorption from headspace) approach for use in food analyses based on DART-MS.381 The strong correlations between SPME-GC-MS and SPMESH-DART-MS suggest that the latter can be used as a rapid alternative (∼30 s per sample) to conventional GC-MS analyses (∼30 min per sample). In another study, the headspace volatiles of several species were analyzed by DART-MS.382 It was demonstrated that several of these products have unique but consistent headspace chemical profiles and that multivariate statistical analysis processing of their chemical signatures could be used to accurately identify the species of plants from which the materials are derived. DART-MS was applied for non-invasive VOC fingerprinting of Fusarium isolates growing under standardized conditions.383 The observed VOCs could readily be associated with the fungal species producing them in the headspace above developing fungal colonies.
A major limit to wider application of the DART source in the analysis of gases is its poor detection sensitivity caused by open-air sampling. Thus, preconcentration methods, such as SPME, have been coupled with the DART source to improve the detection sensitivity. Yuan et al. introduced a simple approach to collect and analyze human exhaled breath aerosol (EBA) by combining a face mask with a SPME fiber.263 The SPME fiber was inserted into a face mask to form SPME-in-mask that covered nose and mouth for in vivo sampling of EBA, and the SPME fiber was then coupled with DART-MS to directly analyze the molecular compositions of EBA under ambient conditions. A trace amount of analytes could be detected owing to the enrichment of SPME fibers and high-efficient desorption and ionization processes.
In a study, a confined interface between the DART ion source outlet and mass spectrometer sampling orifice was developed.260 The schematic diagram of the DART-MS interface and the experimental setup for the studies of VOCs are shown in Fig. 5.
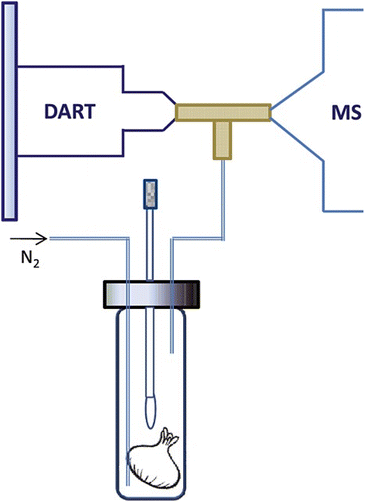 | ||
| Fig. 5 Schematic diagram of the confined DART ion source and the experimental setup for VOC studies. The tee-shaped tube was held on the position by the cone-shaped DART source outlet and mass spectrometer orifice without any other mounting devices. (Reprinted with permission from Li.260 Copyright (2012) John Wiley and Sons). | ||
A Tee-shaped PEEK flow tube was placed between the DART ion source outlet and the orifice of the mass spectrometer. In the confined DART (cDART) ion source, the plasma generated by the atmospheric pressure glow discharge collides and ionizes gas-phase molecules in a Tee-shaped flow tube instead of in open air. It can lead to significant increase of collision reaction probability between high energy metastable molecules and analytes. The experimental results showed that the ionization efficiency was increased at least by two orders of magnitude. This technique has been applied in the real time analysis of VOCs of Citrus Limon (lemon) and wounded Allium Cepa (onion).260 The cDART has been also used in real-time analysis of insect pheromone release262 and human's breath.264
4. Current challenges and future direction
The techniques discussed above have been demonstrated that they can be efficiently applied in analysis of volatile natural products. However, there are still many challenges to tackle for all analytical techniques. Due to the large diversity of volatile natural products, there is no “one” analytical technique, which can be used to study them all. Volatile compounds can be collected often only in low quantities and occur mostly in complex mixtures. Their detection, identification and structural characterization are challenging tasks. Therefore, special sample preparation steps are usually required, along with multiple analytical techniques and methods to enrich and separate different chemical classes of compounds.GC-MS is a reliable method for the identification and quantification of volatile compounds. However, this method is time-consuming and cannot perform real-time analysis. With the optical spectroscopy or mass spectrometry techniques, the dynamics of low concentrations of volatiles can be detected in a time scale of seconds. An exquisite advantage of the PTR-MS, SIFT-MS, SESI-MS and DART-MS online approaches is their relative simplicity, where molecules can be directly detected from the samples. Therefore, the volatile emissions can be easily monitored in real-time and can continuously collect data for compounds of interest. However, online approaches have two major disadvantages compared with offline methods (e.g., GC-MS; HPLC-MS): (i) a lack of resolution of isobaric compounds and (ii) sensitivity loss due to matrix effects, which will significantly affect the quantitative analysis.384 The developments of the novel analytical methodology including new sampling techniques with high selectivity and capacity, and more sensitive analytical instruments with high separation efficiency, detection resolution and sensitivity will be crucial for the study of biological VOCs.
New methods for online and real-time monitoring of trace amount of gases are available nowadays and have demonstrated their ability in many applications. Currently, mass spectrometry has improved the identification of volatile metabolites through measurements of accurate masses, GC or LC retention time, and fragmentation patterns of target analytes. However, the identification of unknown metabolites observed in mass spectra is still a challenging task. The challenge is mainly caused by the limited number of libraries and databases containing MS data of specialized metabolites.
Ion mobility coupled to mass spectrometry is an alternative separation method, which can be used in analysis of isobaric compounds.385,386 Ion mobility separation has a different principle than the one used in GC or LC. It refers to the differential speeds, at which ions migrate through a gas under the influence of an electric field. The physical property associated with ion mobility is an ion's collisional cross section (CCS) which, for a given mobility gas (usually helium or nitrogen), provides a fixed value that can be used to characterize a compound. Hence it can resolve isobaric species and provide the rapid, sensitive and broad-spectrum detection of trace organic compounds in gas mixtures. With coupling of ion mobility with MS, improvement in signal to noise enhancement, charge-state identification, structure classification, and isomer separation can be obtained. An overview of IMS-based applications for the identification of metabolites (volatile and non-volatile) over the last few decades has been discussed, followed by future assumptions for creating IM-based databases.385 These novel approaches can significantly improve our understanding of the vast chemical diversity found in natural products.
5. Summary
Volatile organic compounds released from biological systems are important for intra- and interspecies chemical communication and interaction of living organisms. The deciphering of the structures of the volatile compounds is of increasing importance in many fields of natural product chemistry. Due to the large diversity of these compounds, there is no “single” analytical instrument or method that can be used to study all of them. Furthermore, most of the volatile compounds can be collected only in low concentrations. It makes their detection and identification challenging. Newer technologies are now emerging, including mass spectrometers with high resolution and sensitivity, online metabolomic databases, as well as improved algorithms for annotation and identification of metabolites. In order to perform a comprehensive analysis of volatile natural products, it will be necessary that analytical scientists, instrumentation engineers and bioinformaticians work closely together to develop a more efficient, reliable and automated platform for analysis of volatile natural products.6. Conflicts of interest
There are no conflicts of interest to declare.7. References
- S. A. Goff and H. J. Klee, Plant volatile compounds: Sensory cues for health and nutritional value?, Science, 2006, 311, 815–819 CrossRef CAS PubMed.
- J. D. Blande, J. K. Holopainen and U. Niinemets, Plant volatiles in polluted atmospheres: stress responses and signal degradation, Plant, Cell Environ., 2014, 37, 1892–1904 CrossRef CAS PubMed.
- O. Tyc, C. X. Song, J. S. Dickschat, M. Vos and P. Garbeva, The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria, Trends Microbiol., 2017, 25, 280–292 CrossRef CAS PubMed.
- E. Pichersky, J. P. Noel and N. Dudareva, Biosynthesis of plant volatiles: Nature's diversity and ingenuity, Science, 2006, 311, 808–811 CrossRef CAS PubMed.
- J. Y. Hong, Role of natural product diversity in chemical biology, Curr. Opin. Chem. Biol., 2011, 15, 350–354 CrossRef CAS PubMed.
- M. Grigalunas, S. Brakmann and H. Waldmann, Chemical Evolution of Natural Product Structure, J. Am. Chem. Soc., 2022, 144, 3314–3329 CrossRef CAS PubMed.
- R. G. Berger, Biotechnology of flavours-the next generation, Biotechnol. Lett., 2009, 31, 1651–1659 CrossRef CAS PubMed.
- K. Saeed, I. Pasha, M. F. J. Chughtai, Z. Ali, H. Bukhari and M. Zuhair, Application of essential oils in food industry: challenges and innovation, J. Essent. Oil Res., 2022, 34, 97–110 CrossRef CAS.
- M. S. Brewer, Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications, Compr. Rev. Food Sci. Food Saf., 2011, 10, 221–247 CrossRef CAS.
- Z. L. Wang, Y. X. Yuan, B. Hong, X. Zhao and Z. Y. Gu, Characteristic Volatile Fingerprints of Four Chrysanthemum Teas Determined by HS-GC-IMS, Molecules, 2021, 26, 7113 CrossRef CAS PubMed.
- A. V. Qualley and N. Dudareva, Metabolomics of Plant Volatiles, Methods Mol. Biol., 2009, 553, 329–343 CrossRef CAS PubMed.
- Z. M. Zhang, Y. J. Ma and G. K. Li, Progress on the analytical methodology for biological volatile organic compounds, Anal. Methods, 2013, 5, 20–29 RSC.
- F. J. M. Harren and S. M. Cristescu, Online, real-time detection of volatile emissions from plant tissue, AoB Plants, 2013, 5, plt003 CrossRef PubMed.
- Z. M. Zhang and G. K. Li, A review of advances and new developments in the analysis of biological volatile organic compounds, Microchem. J., 2010, 95, 127–139 CrossRef CAS.
- C. A. Rees, A. Burklund, P. H. Stefanuto, J. D. Schwartzman and J. E. Hill, Comprehensive volatile metabolic fingerprinting of bacterial and fungal pathogen groups, J. Breath Res., 2018, 12, 026001 CrossRef PubMed.
- K. Jardine and A. Jardine, Biogenic Volatile Organic Compounds in Amazonian Forest Ecosystems, Ecol. Stud. Anal. Synth., 2016, 227, 19–33 Search PubMed.
- J. Penuelas and M. Staudt, BVOCs and global change, Trends Plant Sci., 2010, 15, 133–144 CrossRef CAS PubMed.
- R. K. Monson, Volatile organic compound emissions from terrestrial ecosystems: a primary biological control over atmospheric chemistry, Isr. J. Chem., 2002, 42, 29–42 CrossRef CAS.
- D. Materic, D. Bruhn, C. Turner, G. Morgan, N. Mason and V. Gauci, Methods in Plant Foliar Volatile Organic Compounds Research, Appl. Plant Sci., 2015, 3(12), 1500044 CrossRef PubMed.
- K. A. Godard, R. White and J. Bohlmann, Monoterpene-induced molecular responses in Arabidopsis thaliana, Phytochemistry, 2008, 69, 1838–1849 CrossRef CAS PubMed.
- V. Ninkovic, M. Rensing, I. Dahlin and D. Markovic, Who is my neighbor? Volatile cues in plant interactions, Plant Signaling Behav., 2019, 14, 1634993 CrossRef PubMed.
- M. Simpraga, J. Takabayashi and J. K. Holopainen, Language of plants: Where is the word?, J. Integr. Plant Biol., 2016, 58, 343–349 CrossRef CAS PubMed.
- G. Lubes and M. Goodarzi, Analysis of Volatile Compounds by Advanced Analytical Techniques and Multivariate Chemometrics, Chem. Rev., 2017, 117, 6399–6422 CrossRef CAS PubMed.
- R. Pelissier, C. Violle and J. B. Morel, Plant immunity: Good fences make good neighbors?, Curr. Opin. Plant Biol., 2021, 62, 102045 CrossRef CAS PubMed.
- N. Dudareva, F. Negre, D. A. Nagegowda and I. Orlova, Plant volatiles: recent advances and future perspectives, Crit. Rev. Plant Sci., 2006, 25, 417–440 CrossRef CAS.
- F. T. Arroyo, J. Moreno, P. Daza, L. Boianova and F. Romero, Antifungal activity of strawberry fruit volatile compounds against Colletotrichum acutatum, J. Agric. Food Chem., 2007, 55, 5701–5707 CrossRef CAS PubMed.
- T. Sekine, M. Sugano, A. Majid and Y. Fujii, Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs, J. Chem. Ecol., 2007, 33, 2123–2132 CrossRef CAS PubMed.
- N. Dudareva and F. Negre, Practical applications of research into the regulation of plant volatile emission, Curr. Opin. Plant Biol., 2005, 8, 113–118 CrossRef CAS PubMed.
- J. Zhao, P. H. Li, T. Xia and X. C. Wan, Exploring plant metabolic genomics: chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model, Crit. Rev. Biotechnol., 2020, 40, 667–688 CrossRef CAS PubMed.
- P. A. Divekar, S. Narayana, B. A. Divekar, R. Kumar, B. G. Gadratagi, A. Ray, A. K. Singh, V. Rani, V. Singh, A. K. Singh, A. Kumar, R. P. Singh, R. S. Meena and T. K. Behera, Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection, Int. J. Mol. Sci., 2022, 23, 2690 CrossRef CAS PubMed.
- Y. Akakabe and T. Kajiwara, Bioactive volatile compounds from marine algae: feeding attractants, J. Appl. Phycol., 2008, 20, 661–664 CrossRef CAS.
- P. J. Landolt and T. W. Phillips, Host plant influences on sex pheromone behavior of phytophagous insects, Annu. Rev. Entomol., 1997, 42, 371–391 CrossRef CAS PubMed.
- S. K. Pandey and K. H. Kim, Human body-odor components and their determination, TrAC, Trends Anal. Chem., 2011, 30, 784–796 CrossRef CAS.
- H. Barzantny, I. Brune and A. Tauch, Molecular basis of human body odour formation: insights deduced from corynebacterial genome sequences, Int. J. Cosmet. Sci., 2012, 34, 2–11 CrossRef CAS PubMed.
- J. V. Kohl, M. Atzmueller, B. Fink and K. Grammer, Human pheromones: Integrating neuroendocrinology and ethology, Neuroendocrinol. Lett., 2001, 22, 309–321 CAS.
- W. S. T. Hays, Human pheromones: have they been demonstrated?, Behav. Ecol. Sociobiol., 2003, 54, 89–97 CrossRef.
- K. H. Kim, S. A. Jahan and E. Kabir, A review of breath analysis for diagnosis of human health, TrAC, Trends Anal. Chem., 2012, 33, 1–8 CrossRef CAS.
- C. Belda-Iniesta, J. D. Carpeno, J. A. Carrasco, V. Moreno, E. C. Saenz, J. Feliu, M. Sereno, F. G. Rio, J. Barriuso and M. G. Baron, New screening method for lung cancer by detecting volatile organic compounds in breath, Clin. Transl. Oncol., 2007, 9, 364–368 CrossRef CAS PubMed.
- G. Song, T. Qin, H. Liu, G. B. Xu, Y. Y. Pan, F. X. Xiong, K. S. Gu, G. P. Sun and Z. D. Chen, Quantitative breath analysis of volatile organic compounds of lung cancer patients, Lung Cancer, 2010, 67, 227–231 CrossRef PubMed.
- C. M. H. H. T. Robroeks, J. J. B. N. van Berkel, J. W. Dallinga, Q. Jobsis, L. J. I. Zimmermann, H. J. E. Hendriks, M. F. M. Wouters, C. P. M. van der Grinten, K. D. G. van de Kant, F. J. van Schooten and E. Dompeling, Metabolomics of Volatile Organic Compounds in Cystic Fibrosis Patients and Controls, Pediatr. Res., 2010, 68, 75–80 CrossRef CAS PubMed.
- C. M. H. H. T. Robroeks, M. H. Roozeboom, P. A. de Jong, H. A. W. M. Tiddens, Q. Joebsis, H. J. Hendriks, J. B. L. Yntema, H. L. Brackel, R. van Gent, S. Robben and E. Dompeling, Structural lung changes, lung function, and non-invasive inflammatory markers in cystic fibrosis, Pediatr. Allergy Immunol., 2010, 21, 493–500 CrossRef PubMed.
- T. Abaffy, M. G. Moller, D. D. Riemer, C. Milikowski and R. A. DeFazio, Comparative analysis of volatile metabolomics signals from melanoma and benign skin: a pilot study, Metabolomics, 2013, 9, 998–1008 CrossRef CAS PubMed.
- T. Abaffy, R. Duncan, D. D. Riemer, O. Tietje, G. Elgart, C. Milikowski and R. A. DeFazio, Differential Volatile Signatures from Skin, Naevi and Melanoma: A Novel Approach to Detect a Pathological Process, PLoS One, 2010, 5, e13813 CrossRef PubMed.
- T. Abaffy, R. Duncan, D. Riemer, G. Elgart, J. Keri and R. DeFazio, The volatile fingerprint of melanoma, EJC Suppl., 2009, 7, 34 CrossRef.
- S. M. Cristescu, S. T. Persijn, S. T. L. Hekkert and F. J. M. Harren, Laser-based systems for trace gas detection in life sciences, Appl. Phys. B: Lasers Opt., 2008, 92, 343–349 CrossRef CAS.
- T. Taghavi, C. Kim and A. Rahemi, Role of Natural Volatiles and Essential Oils in Extending Shelf Life and Controlling Postharvest Microorganisms of Small Fruits, Microorganisms, 2018, 6, 104 CrossRef CAS PubMed.
- I. Ahmed, F. Fayyaz, M. Nasir, Z. Niaz, M. Furnari and L. Perry, Extending landscape of volatile metabolites as novel diagnostic biomarkers of inflammatory bowel disease - a review, Scand. J. Gastroenterol., 2016, 51, 385–392 CrossRef CAS PubMed.
- I. A. Ratiu, T. Ligor, V. Bocos-Bintintan, C. A. Mayhew and B. Buszewski, Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD, J. Clin. Med., 2021, 10, 32 CrossRef PubMed.
- Z. Bacsik, J. Mink and G. Keresztury, FTIR spectroscopy of the atmosphere. I. Principles and methods, Appl. Spectrosc. Rev., 2004, 39, 295–363 CrossRef CAS.
- P. Spanel and D. Smith, Selected ion flow tube: a technique for quantitative trace gas analysis of air and breath, Med. Biol. Eng. Comput., 1996, 34, 409–419 CrossRef CAS PubMed.
- J. Namiesnik, Trace analysis - challenges and problems, Crit. Rev. Anal. Chem., 2002, 32, 271–300 CrossRef CAS.
- K. Dettmer, P. A. Aronov and B. D. Hammock, Mass spectrometry-based metabolomics, Mass Spectrom. Rev., 2007, 26, 51–78 CrossRef CAS PubMed.
- D. Tholl, W. Boland, A. Hansel, F. Loreto, U. S. R. Rose and J. P. Schnitzler, Practical approaches to plant volatile analysis, Plant J., 2006, 45, 540–560 CrossRef CAS PubMed.
- L. Z. Yi, N. P. Dong, Y. H. Yun, B. C. Deng, D. B. Ren, S. Liu and Y. Z. Liang, Chemometric methods in data processing of mass spectrometry-based metabolomics: A review, Anal. Chim. Acta, 2016, 914, 17–34 CrossRef CAS PubMed.
- S. Liu, J. S. Liu, R. N. Luo, H. Xu, W. R. Zhang, J. Meng, Y. Z. Liang and L. J. Tao, Application of GC-MS coupled with chemometrics for scanning serum metabolic biomarkers from renal fibrosis rat, Biochem. Biophys. Res. Commun., 2015, 461, 186–192 CrossRef CAS PubMed.
- O. V. Rodinkov, A. S. Bugaichenko and L. N. Moskvin, Static Headspace Analysis and Its Current Status, J. Anal. Chem., 2020, 75, 1–17 CrossRef CAS.
- X. Zhang, W. L. Liu, Y. A. Lu and Y. K. Lu, Recent advances in the application of headspace gas chromatography-mass spectrometry, Chin. J. Chromatogr., 2018, 36, 962–971 CrossRef CAS PubMed.
- C. Alasalvar, P. C. Quantick and J. M. Grigor, Aroma compounds of fresh and stored mackerel (Scomber scombrus), ACS Symp. Ser., 1997, 674, 39–54 CrossRef CAS.
- R. J. Cannon and C. T. Ho, Volatile sulfur compounds in tropical fruits, J. Food Drug Anal., 2018, 26, 445–468 CrossRef CAS PubMed.
- K. Abascal, L. Ganora and E. Yarnell, The effect of freeze-drying and its implications for botanical medicine: a review, Phytother. Res., 2005, 19, 655–660 CrossRef PubMed.
- E. Aprea, H. Gika, S. Carlin, G. Theodoridis, U. Vrhovsek and F. Mattivi, Metabolite profiling on apple volatile content based on solid phase microextraction and gas-chromatography time of flight mass spectrometry, J. Chromatogr. A, 2011, 1218, 4517–4524 CrossRef CAS PubMed.
- J. Flink and M. Karel, Effects of Process Variables on Retention of Volatiles in Freeze-Drying, J. Food Sci., 1970, 35, 444–447 CrossRef CAS.
- W. J. Coumans, P. J. A. M. Kerkhof and S. Bruin, Theoretical and Practical Aspects of Aroma Retention in Spray-Drying and Freeze-Drying, Drying Technol., 1994, 12, 99–149 CrossRef CAS.
- R. Castro, R. Natera, E. Duran and C. Garcia-Barroso, Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products, Eur. Food Res. Technol., 2008, 228, 1–18 CrossRef CAS.
- J. L. Hurley-Sanders, R. S. Larsen, B. Troan and M. Loomis, Fungal Osteomyelitis in Two Bufflehead Ducklings (Bucephala Albeola), Journal of Zoo and Wildlife Medicine, 2015, 46, 613–616 CrossRef PubMed.
- O. Fiehn, Metabolomics by GasChromatography–Mass Spectrometry:Combined Targeted and Untargeted Profiling, Curr. Protoc. Mol. Biol., 2016, 114, 30.4.1–30.4.32 Search PubMed.
- Y. L. Ren and S. E. Allen, Ultrasound treatment acceleration of solvent extraction for fumigant residues from wheat, J. AOAC Int., 2001, 84, 1551–1554 CAS.
- Z. J. Liang, P. Z. Zhang and Z. X. Fang, Modern technologies for extraction of aroma compounds from fruit peels: a review, Crit. Rev. Food Sci. Nutr., 2022, 62, 1284–1307 CrossRef CAS PubMed.
- Q. Favre-Godal, E. F. Queiroz and J. L. Wolfender, Latest Developments in Assessing Antifungal Activity Using TLC-Bioautography: A Review, J. AOAC Int., 2013, 96, 1175–1188 CrossRef CAS PubMed.
- L. Starostin and Z. Witkiewicz, Environmental Water Samples Preparation for Chemical-Analysis, Chem. Anal., 1994, 39, 263–279 CAS.
- S. Zhou, L. Zhang, C. S. Guo, Y. Zhong, X. Y. Luo, X. H. Pan, Z. C. Yang and L. Tan, Comparing liquid-liquid, solid-phase, and supported-liquid extraction for the determination of polycyclic aromatic hydrocarbons in serum samples and their application for human biomonitoring, Microchem. J., 2022, 181, 107812 CrossRef CAS.
- M. P. Cano, A. Hernandez and B. DeAncos, High pressure and temperature effects on enzyme inactivation in strawberry and orange products, J. Food Sci., 1997, 62, 85–88 CrossRef CAS.
- R. E. Kannouma, M. A. Hammad, A. H. Kamal and F. R. Mansour, Miniaturization of Liquid-Liquid extraction; the barriers and the enablers, Microchem. J., 2022, 182, 107863 CrossRef CAS.
- V. Andruch, I. S. Balogh, L. Kocurova and J. Sandrejova, Five Years of Dispersive Liquid-Liquid Microextraction, Appl. Spectrosc. Rev., 2013, 48, 161–259 CrossRef CAS.
- R. Jain and R. Singh, Microextraction techniques for analysis of cannabinbids, TrAC, Trends Anal. Chem., 2016, 80, 156–166 CrossRef CAS.
- A. Azzouz, S. K. Kailasa, S. S. Lee, A. J. Rascon, E. Ballesteros, M. Zhang and K. H. Kim, Review of nanomaterials as sorbents in solid-phase extraction for environmental samples, TrAC, Trends Anal. Chem., 2018, 108, 347–369 CrossRef CAS.
- K. M. Dimpe and P. N. Nomngongo, Current sample preparation methodologies for analysis of emerging pollutants in different environmental matrices, TrAC, Trends Anal. Chem., 2016, 82, 199–207 CrossRef CAS.
- L. A. Berrueta, B. Gallo and F. Vicente, A Review of Solid-Phase Extraction - Basic Principles and New Developments, Chromatographia, 1995, 40, 474–483 CrossRef CAS.
- S. Otles and C. Kartal, Solid-Phase Extraction (Spe): Principles and Applications in Food Samples, Acta Sci. Pol., Technol. Aliment., 2016, 15, 5–15 CrossRef CAS PubMed.
- I. Lukic, M. Banovic, D. Persuric, S. Radeka and B. Sladonja, Determination of volatile compounds in grape distillates by solid-phase extraction and gas chromatography, J. Chromatogr. A, 2006, 1101, 238–244 CrossRef CAS PubMed.
- V. Ferreira, L. Ortega, A. Escudero and J. F. Cacho, A comparative study of the ability of different solvents and adsorbents to extract aroma compounds from alcoholic beverages, J. Chromatogr. Sci., 2000, 38, 469–476 CAS.
- L. Cullere, M. Aznar, J. Cacho and V. Ferreira, Fast fractionation of complex organic extracts by normal-phase chromatography on a solid-phase extraction polymeric sorbent - optimization of a method to fractionate wine flavor extracts, J. Chromatogr. A, 2003, 1017, 17–26 CrossRef CAS PubMed.
- J. Plotka-Wasylka, N. Szczepanska, M. de la Guardia and J. Namiesnik, Modern trends in solid phase extraction: new sorbent media, TrAC, Trends Anal. Chem., 2016, 77, 23–43 CrossRef CAS.
- A. Andrade-Eiroa, M. Canle, V. Leroy-Cancellieri and V. Cerda, Solid-phase extraction of organic compounds: a critical review (Part I), TrAC, Trends Anal. Chem., 2016, 80, 641–654 CrossRef CAS.
- A. Andrade-Eiroa, M. Canle, V. Leroy-Cancellieri and V. Cerda, Solid-phase extraction of organic compounds: a critical review (Part II), TrAC, Trends Anal. Chem., 2016, 80, 655–667 CrossRef CAS.
- K. L. Yan, L. Q. Lin, X. X. Zheng, X. H. Xiao and Y. J. Cao, Progress of sample preparation techniques in gas chromatographic analysis, Chin. J. Chromatogr., 2013, 31, 634–639 CrossRef CAS PubMed.
- L. Pillonel, J. O. Bossett and R. Tabacchi, Rapid preconcentration and enrichment techniques for the analysis of food volatile. A review, Lebensm.-Wiss. Technol., 2002, 35, 1–14 CrossRef CAS.
- B. Webster, S. Gezan, T. Bruce, J. Hardie and J. Pickett, Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants, Phytochemistry, 2010, 71, 81–89 CrossRef CAS PubMed.
- C. L. Arthur and J. Pawliszyn, Solid-Phase Microextraction with Thermal-Desorption Using Fused-Silica Optical Fibers, Anal. Chem., 1990, 62, 2145–2148 CrossRef CAS.
- M. Urbanowicz, B. Zabiegala and J. Namiesnik, Solventless sample preparation techniques based on solid- and vapour-phase extraction, Anal. Bioanal. Chem., 2011, 399, 277–300 CrossRef CAS PubMed.
- C. H. Xu, G. S. Chen, Z. H. Xiong, Y. X. Fan, X. C. Wang and Y. Liu, Applications of solid-phase microextraction in food analysis, TrAC, Trends Anal. Chem., 2016, 80, 12–29 CrossRef CAS.
- F. Zhu, J. Q. Xu, Y. Y. Ke, S. M. Huang, F. Zeng, T. G. Luan and G. F. Ouyang, Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: a review, Anal. Chim. Acta, 2013, 794, 1–14 CrossRef CAS PubMed.
- J. Q. Xu, G. S. Chen, S. Y. Huang, J. L. Qiu, R. F. Jiang, F. Zhu and G. F. Ouyang, Application of in vivo solid-phase microextraction in environmental analysis, TrAC, Trends Anal. Chem., 2016, 85, 26–35 CrossRef CAS.
- K. Schmidt and I. Podmore, Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer, J. Biomarkers, 2015, 2015, 981458 Search PubMed.
- H. Kataoka, H. L. Lord and J. Pawliszyn, Applications of solid-phase microextraction in food analysis, J. Chromatogr. A, 2000, 880, 35–62 CrossRef CAS PubMed.
- M. L. Wang, L. Q. Qiao, L. Zhang, L. J. Wu and H. X. Tian, Analysis of Volatile Constituents from Leaves of Plants by Gas Chromatography/Mass Spectrometry with Solid-Phase Microextraction, Chin. J. Chromatogr., 2006, 24, 343–346 CAS.
- J. G. Vallarino, A. Erban, I. Fehrle, A. R. Fernie, J. Kopka and S. Osorio, Acquisition of Volatile Compounds by Gas Chromatography-Mass Spectrometry (GC-MS), Methods Protoc., 2018, 1778, 225–239 CAS.
- E. E. Stashenko and J. R. Martinez, Sampling flower scent for chromatographic analysis, J. Sep. Sci., 2008, 31, 2022–2031 CrossRef CAS PubMed.
- J. Richter and I. Schellenberg, Comparison of different extraction methods for the determination of essential oils and related compounds from aromatic plants and optimization of solid-phase microextraction/gas chromatography, Anal. Bioanal. Chem., 2007, 387, 2207–2217 CrossRef CAS PubMed.
- Y. Tikunov, A. Lommen, C. H. R. de Vos, H. A. Verhoeven, R. J. Bino, R. D. Hall and A. G. Bovy, A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles, Plant Physiol., 2005, 139, 1125–1137 CrossRef CAS PubMed.
- G. L. Petretto, G. Sarais, M. T. Maldini, M. Foddai, B. Tirillini, J. P. Rourke, M. Chessa and G. Pintore, Citrus monstruosa Discrimination among Several Citrus Species by Multivariate Analysis of Volatiles: A Metabolomic Approach, J. Food Process. Preserv., 2016, 40, 950–957 CrossRef CAS.
- R. Mumm, J. A. Hageman, M. N. Calingacion, R. C. H. de Vos, H. H. Jonker, A. Erban, J. Kopka, T. H. Hansen, K. H. Laursen, J. K. Schjoerring, J. L. Ward, M. H. Beale, S. Jongee, A. Rauf, F. Habibi, S. D. Indrasari, S. Sakhan, A. Ramli, M. Romero, R. F. Reinke, K. Ohtsubo, C. Boualaphanh, M. A. Fitzgerald and R. D. Hall, Multi-platform metabolomics analyses of a broad collection of fragrant and non-fragrant rice varieties reveals the high complexity of grain quality characteristics, Metabolomics, 2016, 12, 38 CrossRef CAS PubMed.
- M. Arora, S. C. Zambrzycki, J. M. Levy, A. Esper, J. K. Frediani, C. L. Quave, F. M. Fernandez and R. Kamaleswaran, Machine Learning Approaches to Identify Discriminative Signatures of Volatile Organic Compounds (VOCs) from Bacteria and Fungi Using SPME-DART-MS, Metabolites, 2022, 12, 232 CrossRef CAS PubMed.
- H. Kataoka, K. Saito, H. Kato and K. Masuda, Noninvasive analysis of volatile biomarkers in human emanations for health and early disease diagnosis, Bioanalysis, 2013, 5, 1443–1459 CrossRef CAS PubMed.
- B. C. Blount, R. J. Kobelski, D. O. McElprang, D. L. Ashley, J. C. Morrow, D. M. Chambers and F. L. Cardinali, Quantification of 31 volatile organic compounds in whole blood using solid-phase microextraction and gas chromatography-mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2006, 832, 292–301 CrossRef CAS PubMed.
- E. Dixon, C. Clubb, S. Pittman, L. Ammann, Z. Rasheed, N. Kazmi, A. Keshavarzian, P. Gillevet, H. Rangwala and R. D. Couch, Solid-Phase Microextraction and the Human Fecal VOC Metabolome, PLoS One, 2011, 6, e18471 CrossRef CAS PubMed.
- P. Mochalski and K. Unterkofler, Quantification of selected volatile organic compounds in human urine by gas chromatography selective reagent ionization time of flight mass spectrometry (GC-SRI-TOF-MS) coupled with head-space solid-phase microextraction (HS-SPME), Analyst, 2016, 141, 4796–4803 RSC.
- Y. P. Huang, Y. Li, Z. W. Luo and Y. X. Duan, Investigation of biomarkers for discriminating breast cancer cell lines from normal mammary cell lines based on VOCs analysis and metabolomics, RSC Adv., 2016, 6, 41816–41824 RSC.
- E. Baltussen, P. Sandra, F. David and C. Cramers, Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: theory and principles, J. Microcolumn Sep., 1999, 11, 737–747 CrossRef CAS.
- Y. J. Liu, Z. M. Liu, Z. G. Xu and G. K. Li, Stir Bar Sorptive Extraction Technology, Prog. Chemother., 2020, 32, 1334–1343 CAS.
- C. K. Hasan, A. Ghiasvand, T. W. Lewis, P. N. Nesterenko and B. Paull, Recent advances in stir-bar sorptive extraction: Coatings, technical improvements, and applications, Anal. Chim. Acta, 2020, 1139, 222–240 CrossRef CAS PubMed.
- F. J. Camino-Sanchez, R. Rodriguez-Gomez, A. Zafra-Gomez, A. Santos-Fandila and J. L. Vilchez, Stir bar sorptive extraction: Recent applications, limitations and future trends, Talanta, 2014, 130, 388–399 CrossRef CAS PubMed.
- H. Kataoka, Recent developments and applications of microextraction techniques in drug analysis, Anal. Bioanal. Chem., 2010, 396, 339–364 CrossRef CAS PubMed.
- S. M. A. Malowicki, R. Martin and M. C. Qian, Volatile composition in raspberry cultivars grown in the Pacific northwest determined by stir bar sorptive extraction-gas chromatography-mass spectrometry, J. Agric. Food Chem., 2008, 56, 4128–4133 CrossRef CAS PubMed.
- W. H. K. Cheung, A. Pasamontes, D. J. Peirano, W. X. Zhao, E. E. Grafton-Cardwell, T. Kapaun, R. K. Yokomi, J. Simmons, M. Doll, O. Fiehn, A. M. Dandekar and C. E. Davis, Volatile organic compound (VOC) profiling of citrus tristeza virus infection in sweet orange citrus varietals using thermal desorption gas chromatography time of flight mass spectrometry (TD-GC/TOF-MS), Metabolomics, 2015, 11, 1514–1525 CrossRef CAS.
- A. Errard, C. Ulrichs, S. Kuhne, I. Mewis, M. Drungowski, M. Schreiner and S. Baldermann, Single- versus Multiple-Pest Infestation Affects Differently the Biochemistry of Tomato (Solanum lycopersicum 'Ailsa Craig'), J. Agric. Food Chem., 2015, 63, 10103–10111 CrossRef CAS PubMed.
- A. Blohm, A. Sieburg, J. Popp and T. Frosch, Detection of gas molecules by means of spectrometric and spectroscopic methods, Microb. Nanotechnol., 2020, 251–294 CAS.
- K. Sassenscheid, U. Klocke, G. Schmidtke and M. Tacke, Enhanced selectivity and sensitivity in UV-analysis of volatile organic compounds, Proc. Soc. Photo-Opt. Instrum. Eng., 1999, 3533, 222–233 CrossRef CAS.
- A. Gonzalvez, S. Garrigues, M. de la Guardia and S. Armenta, The ways to the trace level analysis in infrared spectroscopy, Anal Methods, 2011, 3, 43–52 RSC.
- O. Vaittinen, F. M. Schmidt, M. Metsala and L. Halonen, Exhaled Breath Biomonitoring Using Laser Spectroscopy, Curr. Anal. Chem., 2013, 9, 463–475 CrossRef CAS.
- P. Devillier, H. Salvator, E. Naline, L. J. Couderc and S. Grassin-Delyle, Metabolomics in the Diagnosis and Pharmacotherapy of Lung Diseases, Curr. Pharm. Des., 2017, 23, 2050–2059 CrossRef CAS PubMed.
- L. S. Rothman, I. E. Gordon, A. Barbe, D. C. Benner, P. E. Bernath, M. Birk, V. Boudon, L. R. Brown, A. Campargue, J. P. Champion, K. Chance, L. H. Coudert, V. Dana, V. M. Devi, S. Fally, J. M. Flaud, R. R. Gamache, A. Goldman, D. Jacquemart, I. Kleiner, N. Lacome, W. J. Lafferty, J. Y. Mandin, S. T. Massie, S. N. Mikhailenko, C. E. Miller, N. Moazzen-Ahmadi, O. V. Naumenko, A. V. Nikitin, J. Orphal, V. I. Perevalov, A. Perrin, A. Predoi-Cross, C. P. Rinsland, M. Rotger, M. Simeckova, M. A. H. Smith, K. Sung, S. A. Tashkun, J. Tennyson, R. A. Toth, A. C. Vandaele and J. Vander Auwera, The HITRAN 2008 molecular spectroscopic database, J. Quant. Spectrosc. Radiat. Transfer, 2009, 110, 533–572 CrossRef CAS.
- B. A. Paldus and A. A. Kachanov, An historical overview of cavity-enhanced methods, Can. J. Phys., 2005, 83, 975–999 CrossRef CAS.
- G. D. Banik and B. Mizaikoff, Exhaled breath analysis using cavity-enhanced optical techniques: a review, J. Breath Res., 2020, 14, 043001 CrossRef PubMed.
- M. D. Wheeler, S. M. Newman, A. J. Orr-Ewing and M. N. R. Ashfold, Cavity ring-down spectroscopy, J. Chem. Soc., Faraday Trans. 1, 1998, 94, 337–351 RSC.
- K. S. Song and E. C. Jung, Recent developments in modulation spectroscopy for trace gas detection using tunable diode lasers, Appl. Spectrosc. Rev., 2003, 38, 395–432 CrossRef CAS.
- G. Berden, R. Peeters and G. Meijer, Cavity ring-down spectroscopy: experimental schemes and applications, Int. Rev. Phys. Chem., 2000, 19, 565–607 Search PubMed.
- S. S. Brown, Absorption spectroscopy in high-finesse cavities for atmospheric studies, Chem. Rev., 2003, 103, 5219–5238 CrossRef CAS PubMed.
- A. Butenandt, R. Beckmann, D. Stamm and E. Hecker, Uber Den Sexual-Lockstoff Des Seidenspinners Bombyx Mori - Reindarstellung Und Konstitution, Zeitschrift für Naturforschung B, 1959, 14, 283–284 Search PubMed.
- R. J. Bartelt, A. A. Cosse, B. W. Zilkowski, D. Weisleder and F. A. Momany, Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles, J. Chem. Ecol., 2001, 27, 2397–2423 CrossRef CAS PubMed.
- E. C. Tuazon, R. A. Graham, A. M. Winer, R. R. Easton, J. N. Pitts and P. L. Hanst, Kilometer Pathlength Fourier-Transform Infrared System for Study of Trace Pollutants in Ambient and Synthetic Atmospheres, Atmos. Environ., 1978, 12, 865–875 CrossRef CAS PubMed.
- T. L. Marshall, C. T. Chaffin, R. M. Hammaker and W. G. Fateley, An Introduction to Open-Path Ft-Ir Atmospheric Monitoring, Environ. Sci. Technol., 1994, 28, A224–A232 CrossRef PubMed.
- P. L. Hanst and S. T. Hanst, Gas Measurement in the Fundamental Infrared Region in Air Monitoring by Spectroscopic Techniques, Wiley, New York, NY, 1994 Search PubMed.
- B. P. Wert, M. Trainer, A. Fried, T. B. Ryerson, B. Henry, W. Potter, W. M. Angevine, E. Atlas, S. G. Donnelly, F. C. Fehsenfeld, G. J. Frost, P. D. Goldan, A. Hansel, J. S. Holloway, G. Hubler, W. C. Kuster, D. K. Nicks, J. A. Neuman, D. D. Parrish, S. Schauffler, J. Stutz, D. T. Sueper, C. Wiedinmyer and A. Wisthaler, Signatures of terminal alkene oxidation in airborne formaldehyde measurements during TexAQS 2000, J. Geophys. Res.: Atmos., 2003, 108(D3), 4104 CrossRef.
- T. I. Yacovitch, S. C. Herndon, J. R. Roscioli, C. Floerchinger, R. M. McGovern, M. Agnese, G. Petron, J. Kofler, C. Sweeney, A. Karion, S. A. Conley, E. A. Kort, L. Nahle, M. Fischer, L. Hildebrandt, J. Koeth, J. B. McManus, D. D. Nelson, M. S. Zahniser and C. E. Kolb, Demonstration of an Ethane Spectrometer for Methane Source Identification, Environ. Sci. Technol., 2014, 48, 8028–8034 CrossRef CAS PubMed.
- M. Winkowski and T. Stacewicz, Optical detection of formaldehyde in air in the 3.6 mu m range, Biomed. Opt. Express, 2020, 11, 7019–7031 CrossRef CAS PubMed.
- J. Mao, X. Ren, L. Zhang, D. M. Van Duin, R. C. Cohen, J. H. Park, A. H. Goldstein, F. Paulot, M. R. Beaver, J. D. Crounse, P. O. Wennberg, J. P. DiGangi, S. B. Henry, F. N. Keutsch, C. Park, G. W. Schade, G. M. Wolfe, J. A. Thornton and W. H. Brune, Insights into hydroxyl measurements and atmospheric oxidation in a California forest, Atmos. Chem. Phys., 2012, 12, 8009–8020 CrossRef CAS.
- P. A. Feiner, W. H. Brune, D. O. Miller, L. Zhang, R. C. Cohen, P. S. Romer, A. H. Goldstein, F. N. Keutsch, K. M. Skog, P. O. Wennberg, T. B. Nguyen, A. P. Teng, J. DeGouw, A. Koss, R. J. Wild, S. S. Brown, A. Guenther, E. Edgerton, K. Baumann and J. L. Fry, Testing Atmospheric Oxidation in an Alabama Forest, J. Atmos. Sci., 2016, 73, 4699–4710 CrossRef.
- S. Kim, A. Guenther and E. Apel, Quantitative and qualitative sensing techniques for biogenic volatile organic compounds and their oxidation products, Environ. Sci.: Processes Impacts, 2013, 15, 1301–1314 RSC.
- S. Kundu, B. L. Deming, M. M. Lew, B. P. Bottorff, P. Rickly, P. S. Stevens, S. Dusanter, S. Sklaveniti, T. Leonardis, N. Locoge and E. C. Wood, Peroxy radical measurements by ethane - nitric oxide chemical amplification and laser-induced fluorescence during the IRRONIC field campaign in a forest in Indiana, Atmos. Chem. Phys., 2019, 19, 9563–9579 CrossRef CAS.
- R. Dlugi, M. Berger, M. Zelger, A. Hofzumahaus, M. Siese, F. Holland, A. Wisthaler, W. Grabmer, A. Hansel, R. Koppmann, G. Kramm, M. Mollmann-Coers and A. Knaps, Turbulent exchange and segregation of HOx radicals and volatile organic compounds above a deciduous forest, Atmos. Chem. Phys., 2010, 10, 6215–6235 CrossRef CAS.
- M. Cazorla, G. M. Wolfe, S. A. Bailey, A. K. Swanson, H. L. Arkinson and T. F. Hanisco, A new airborne laser-induced fluorescence instrument for in situ detection of formaldehyde throughout the troposphere and lower stratosphere, Atmos. Meas. Tech., 2015, 8, 541–552 CrossRef CAS.
- H. Dahnke, J. Kahl, G. Schuler, W. Boland, W. Urban and F. Kuhnemann, On-line monitoring of biogenic isoprene emissions using photoacoustic spectroscopy, Appl. Phys. B: Lasers Opt., 2000, 70, 275–280 CrossRef CAS.
- F. Kuhnemann, M. Wolfertz, S. Arnold, M. Lagemann, A. Popp, G. Schuler, A. Jux and W. Boland, Simultaneous online detection of isoprene and isoprene-d(2) using infrared photoacoustic spectroscopy, Appl. Phys. B: Lasers Opt., 2002, 75, 397–403 CrossRef.
- C. Popa, Ethylene Measurements from Sweet Fruits Flowers Using Photoacoustic Spectroscopy, Molecules, 2019, 24, 1144 CrossRef PubMed.
- C. Popa, A. M. Bratu, M. Petrus and M. Bacalum, The Analysis of Lead Phytotoxicity in Seeds Using CO2 Laser Photoacoustic Spectroscopy, Molecules, 2020, 25, 1637 CrossRef CAS PubMed.
- D. Bicanic, S. Persijn, A. Taylor, J. Cozijnsen, B. van Veldhuyzen, G. Lenssen and H. Wegh, Detection of ethanol and acetaldehyde released from cabbage seeds of different quality: Laser photoacoustic spectroscopy versus FTIR and headspace gas chromatography, Rev. Sci. Instrum., 2003, 74, 690–693 CrossRef CAS.
- A. M. Bratu, M. Petrus and C. Popa, Monitoring of Post-Harvest Maturation Processes inside Stored Fruit Using Photoacoustic Gas Sensing Spectroscopy, Materials, 2020, 13, 2694 CrossRef CAS PubMed.
- A. M. Bratu, C. Popa, M. Bojan, P. C. Logofatu and M. Petrus, Non-destructive methods for fruit quality evaluation, Sci. Rep., 2021, 11, 7782 CrossRef CAS PubMed.
- J. S. Li, W. D. Chen and B. L. Yu, Recent Progress on Infrared Photoacoustic Spectroscopy Techniques, Appl. Spectrosc. Rev., 2011, 46, 440–471 CrossRef.
- C. Popa, A. M. Bratu, C. Matei, R. Cernat, A. Popescu and D. C. Dumitras, Qualitative and quantitative determination of human biomarkers by laser photoacoustic spectroscopy methods, Laser Phys., 2011, 21, 1336–1342 CrossRef CAS.
- B. Henderson, A. Khodabakhsh, M. Metsala, I. Ventrillard, F. M. Schmidt, D. Romanini, G. A. D. Ritchie, S. T. Hekkert, R. Briot, T. Risby, N. Marczin, F. J. M. Harren and S. M. Cristescu, Laser spectroscopy for breath analysis: towards clinical implementation, Appl. Phys. B: Lasers Opt., 2018, 124, 161 CrossRef PubMed.
- R. Selvaraj, N. J. Vasa, S. M. S. Nagendra and B. Mizaikoff, Advances in Mid-Infrared Spectroscopy-Based Sensing Techniques for Exhaled Breath Diagnostics, Molecules, 2020, 25, 2227 CrossRef CAS PubMed.
- J. King, P. Mochalski, A. Kupferthaler, K. Unterkofler, H. Koc, W. Filipiak, S. Teschl, H. Hinterhuber and A. Amann, Dynamic profiles of volatile organic compounds in exhaled breath as determined by a coupled PTR-MS/GC-MS study, Physiol. Meas., 2010, 31, 1169–1184 CrossRef CAS PubMed.
- D. C. Dumitras, M. Petrus, A. M. Bratu and C. Popa, Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review, Molecules, 2020, 25, 1728 CrossRef CAS PubMed.
- J. King, P. Mochalski, K. Unterkofler, G. Teschl, M. Klieber, M. Stein, A. Amann and M. Baumann, Breath isoprene: Muscle dystrophy patients support the concept of a pool of isoprene in the periphery of the human body, Biochem. Biophys. Res. Commun., 2012, 423, 526–530 CrossRef CAS PubMed.
- T. A. Popov, Human exhaled breath analysis, Ann. Allergy, Asthma, Immunol., 2011, 106, 451–456 CrossRef CAS PubMed.
- M. D. Davis, S. J. Fowler and A. J. Montpetit, Exhaled breath testing - a tool for the clinician and researcher, Paediatr. Respir. Rev., 2019, 29, 37–41 Search PubMed.
- A. Krokou, R. Kokkinofta, M. Stylianou and A. Agapiou, Decoding carob flavor aroma using HS-SPME-GC-MS and chemometrics, Eur. Food Res. Technol., 2020, 246, 1419–1428 CrossRef CAS.
- K. Sakamaki, S. Oguri, Y. Katsumi, Y. Ohkubo, Y. Kurobayashi and K. Kubota, Identification of Odor-Active Compounds Released from a Damaged Plant of the Asian Skunk Cabbage Symplocarpus renifolius, J. Nat. Prod., 2018, 81, 2710–2715 CrossRef CAS PubMed.
- A. H. El-Ghorab, Q. Javed, F. M. Anjum, S. F. Hamed and H. A. Shaaban, Pakistani Bell Pepper (Capsicum Annum L.): Chemical Compositions and Its Antioxidant Activity, Int. J. Food Prop., 2013, 16, 18–32 CrossRef CAS.
- A. Garrido, J. G. Ledezma, A. A. Durant-Archibold, N. S. Allen, J. C. Villarreal and M. P. Gupta, Chemical Profiling of Volatile Components of the Gametophyte and Sporophyte Stages of the Hornwort Leiosporoceros dussii (Leiosporocerotaceae) From Panama by HS-SPME-GC-MS, Nat. Prod. Commun., 2019, 14, 1–4 CrossRef.
- J. X. Yin, R. M. Lin, M. F. Wu, H. Ding, L. F. Han, W. Z. Yang, X. B. Song, W. L. Li, H. B. Qu, H. S. Yu and Z. Li, Strategy for the multi-component characterization and quality evaluation of volatile organic components in Kaixin San by correlating the analysis by headspace gas chromatography/ion mobility spectrometry and headspace gas chromatography/mass spectrometry, Rapid Commun. Mass Spectrom., 2021, 35, e9174 CrossRef CAS PubMed.
- I. S. Roman, L. Bartolome, W. S. Gee, R. M. Alonso and J. J. Beck, Comparison of ex situ volatile emissions from intact and mechanically damaged walnuts, Int. Food Res. J., 2015, 72, 198–207 CrossRef.
- Y. Luo, K. Wang, H. N. Zhuang, D. J. Li, X. L. Meng, M. L. Shi, L. Y. Yao, S. Q. Song, M. Sun, H. T. Wang and T. Feng, Elucidation of aroma compounds in passion fruit (Passiflora alata Ait) using a molecular sensory approach, j food biochem, 2022, 46, e14224 CAS.
- R. Oliver-Simancas, R. Munoz, M. C. Diaz-Maroto, M. S. Perez-Coello and M. E. Alanon, Mango by-products as a natural source of valuable odor-active compounds, J. Sci. Food Agric., 2020, 100, 4688–4695 CrossRef CAS PubMed.
- T. T. Diep, M. J. Y. Yoo, C. Pook, S. Sadooghy-Saraby, A. Gite and E. Rush, Volatile Components and Preliminary Antibacterial Activity of Tamarillo (Solanum betaceum Cav.), Foods, 2021, 10, 2212 CrossRef CAS PubMed.
- H. E. Tahir, X. B. Zou, Z. H. Li and Y. D. Zhu, Comprehensive Evaluation of Antioxidant Properties and Volatile Compounds of Sudanese Honeys, J. Food Biochem, 2015, 39, 349–359 CrossRef CAS.
- A. Aresta, P. Cotugno, N. De Vietro, C. Longo, M. Mercurio, P. Ferriol, C. Zambonin and C. N. Marzano, Volatile Organic Compounds, Indole, and Biogenic Amines Assessment in Two Mediterranean Irciniidae (Porifera, Demospongiae), Mar. Drugs, 2021, 19, 711 CrossRef CAS PubMed.
- W. Haberer, S. Steiger and J. K. Muller, Dynamic changes in volatile emissions of breeding burying beetles, Physiol. Entomol., 2014, 39, 153–164 CrossRef CAS.
- J. Tabata and R. T. Ichiki, Sex Pheromone of the Cotton Mealybug, Phenacoccus solenopsis, with an Unusual Cyclobutane Structure, J. Chem. Ecol., 2016, 42, 1193–1200 CrossRef CAS PubMed.
- R. A. Hayes, S. J. Rice, B. A. Amos and D. M. Leemon, Increased attractiveness of honeybee hive product volatiles to adult small hive beetle, Aethina tumida, resulting from small hive beetle larval infestation, Entomol. Exp. Appl., 2015, 155, 240–248 Search PubMed.
- M. Tsikolia, N. Tabanca, D. L. Kline, B. Demirci, L. Yang, K. J. Linthicum, J. R. Bloomquist and U. R. Bernier, Studies on the Volatiles Composition of Stored Sheep Wool, and Attractancy toward Aedes aegypti Mosquitoes, Insects, 2022, 13, 208 CrossRef PubMed.
- P. Apps, L. Mmualefe and J. W. McNutt, Identification of Volatiles from the Secretions and Excretions of African Wild Dogs (Lycaon pictus), J. Chem. Ecol., 2012, 38, 1450–1461 CrossRef CAS PubMed.
- B. Mitrevski and P. J. Marriott, Novel Hybrid Comprehensive 2D - Multidimensional Gas Chromatography for Precise, High-Resolution Characterization of Multicomponent Samples, Anal. Chem., 2012, 84, 4837–4843 CrossRef CAS PubMed.
- L. Zhang, Z. D. Zeng, C. X. Zhao, H. W. Kong, X. Lu and G. W. Xu, A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and multivariate data analysis, J. Chromatogr. A, 2013, 1313, 245–252 CrossRef CAS PubMed.
- J. Morimoto, M. C. Rosso, N. Kfoury, C. Bicchi, C. Cordero and A. Robbat, Untargeted/Targeted 2D Gas Chromatography/Mass Spectrometry Detection of the Total Volatile Tea Metabolome, Molecules, 2019, 24, 3757 CrossRef CAS PubMed.
- S. D. Johanningsmeier and R. F. McFeeters, Metabolic footprinting of Lactobacillus buchneri strain LA1147 during anaerobic spoilage of fermented cucumbers, Int. J. Food Microbiol., 2015, 215, 40–48 CrossRef CAS PubMed.
- X. Gao, X. W. Yang, B. S. Mitrevski and P. J. Marriott, Headspace solid-phase microextraction combined with GC x GC-TOFMS for the analysis of volatile compounds of Coptis species rhizomes, J. Sep. Sci., 2011, 34, 1157–1166 CrossRef CAS PubMed.
- M. Junger, W. Vautz, M. Kuhns, L. Hofmann, S. Ulbricht, J. I. Baumbach, M. Quintel and T. Perl, Ion mobility spectrometry for microbial volatile organic compounds: a new identification tool for human pathogenic bacteria, Appl. Microbiol. Biotechnol., 2012, 93, 2603–2614 CrossRef PubMed.
- T. Perl, M. Junger, W. Vautz, J. Nolte, M. Kuhns, M. B. V. Zepelin and M. Quintel, Detection of characteristic metabolites of Aspergillus fumigatus and Candida species using ion mobility spectrometry - metabolic profiling by volatile organic compounds, Mycoses, 2011, 54, E828–E837 CrossRef CAS PubMed.
- X. Chen, L. Hua, J. C. Jiang, F. Hu, N. B. Wan and H. Y. Li, Multi-capillary column high-pressure photoionization time-of-flight mass spectrometry and its application for online rapid analysis of flavor compounds, Talanta, 2019, 201, 33–39 CrossRef CAS PubMed.
- A. Saunier, E. Ormeno, H. Wortham, B. Temime-Roussel, C. Lecareux, C. Boissard and C. Fernandez, Chronic Drought Decreases Anabolic and Catabolic BVOC Emissions of Quercus pubescens in a Mediterranean Forest, Front. Plant Sci., 2017, 8, 71 Search PubMed.
- S. Fares, R. Schnitzhofer, X. Y. Jiang, A. Guenther, A. Hansel and F. Loreto, Observations of Diurnal to Weekly Variations of Monoterpene-Dominated Fluxes of Volatile Organic Compounds from Mediterranean Forests: Implications for Regional Modeling, Environ. Sci. Technol., 2013, 47, 11073–11082 CrossRef CAS PubMed.
- A. M. Trowbridge, P. C. Stoy and R. P. Phillips, Soil Biogenic Volatile Organic Compound Flux in a Mixed Hardwood Forest: Net Uptake at Warmer Temperatures and the Importance of Mycorrhizal Associations, J. Geophys. Res.: Biogeosci., 2020, 125, e2019JG005479 CAS.
- S. Rottenberger, B. Kleiss, U. Kuhn, A. Wolf, M. T. F. Piedade, W. Junk and J. Kesselmeier, The effect of flooding on the exchange of the volatile C-2-compounds ethanol, acetaldehyde and acetic acid between leaves of Amazonian floodplain tree species and the atmosphere, Biogeosciences, 2008, 5, 1085–1100 CrossRef CAS.
- R. Holzinger, L. Sandoval-Soto, S. Rottenberger, P. J. Crutzen and J. Kesselmeier, Emissions of volatile organic compounds from Quercus ilex L. measured by Proton Transfer Reaction Mass Spectrometry under different environmental conditions, J. Geophys. Res.: Atmos., 2000, 105, 20573–20579 CrossRef CAS.
- E. Masi, A. Romani, C. Pandolfi, D. Heimler and S. Mancuso, PTR-TOF-MS analysis of volatile compounds in olive fruits, J. Sci. Food Agric., 2015, 95, 1428–1434 CrossRef CAS PubMed.
- I. C. J. Silvis, P. A. Luning, N. Klose, M. Jansen and S. M. van Ruth, Similarities and differences of the volatile profiles of six spices explored by Proton Transfer Reaction Mass Spectrometry, Food Chem., 2019, 271, 318–327 CrossRef CAS PubMed.
- C. Taiti, C. Costa, P. Menesatti, D. Comparini, N. Bazihizina, E. Azzarello, E. Masi and S. Mancuso, Class-modeling approach to PTR-TOFMS data: a peppers case study, J. Sci. Food Agric., 2015, 95, 1757–1763 CrossRef CAS PubMed.
- F. Neri, L. Cappellin, A. Spadoni, I. Cameldi, A. A. Alarcon, E. Aprea, A. Romano, F. Gasperi and F. Biasioli, Role of strawberry volatile organic compounds in the development of Botrytis cinerea infection, Plant Pathol., 2015, 64, 709–717 CrossRef CAS.
- A. Rozanska, D. Sienska, T. Dymerski and J. Namiesnik, Analysis of volatile fraction of sweetie (Citrus maxima x Citrus paradisi) and its parent fruit using proton transfer reaction mass spectrometry, Monatsh. Chem., 2018, 149, 1629–1634 CrossRef CAS PubMed.
- B. Farneti, I. Khomenko, L. Cappellin, V. Ting, A. Romano, F. Biasioli, G. Costa and F. Costa, Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS, Metabolomics, 2015, 11, 838–850 CrossRef CAS.
- C. Soukoulis, L. Cappellin, E. Aprea, F. Costa, R. Viola, T. D. Mark, F. Gasperi and F. Biasioli, PTR-ToF-MS, A Novel, Rapid, High Sensitivity and Non-Invasive Tool to Monitor Volatile Compound Release During Fruit Post-Harvest Storage: The Case Study of Apple Ripening, Food Bioprocess Technol., 2013, 6, 2831–2843 CrossRef CAS.
- L. Cappellin, C. Soukoulis, E. Aprea, P. Granitto, N. Dallabetta, F. Costa, R. Viola, T. D. Mark, F. Gasperi and F. Biasioli, PTR-ToF-MS and data mining methods: a new tool for fruit metabolomics, Metabolomics, 2012, 8, 761–770 CrossRef CAS.
- V. Giacomuzzi, L. Cappellin, S. Nones, I. Khomenko, F. Biasioli, A. L. Knight and S. Angeli, Diel rhythms in the volatile emission of apple and grape foliage, Phytochemistry, 2017, 138, 104–115 CrossRef CAS PubMed.
- A. Cellini, E. Biondi, S. Blasioli, L. Rocchi, B. Farneti, I. Braschi, S. Savioli, M. T. Rodriguez-Estrada, F. Biasioli and F. Spinelli, Early detection of bacterial diseases in apple plants by analysis of volatile organic compounds profiles and use of electronic nose, Ann. Appl. Biol., 2016, 168, 409–420 CrossRef CAS.
- I. R. White, R. S. Blake, A. J. Taylor and P. S. Monks, Metabolite profiling of the ripening of Mangoes Mangifera indica L. cv. ‘Tommy Atkins’ by real-time measurement of volatile organic compounds, Metabolomics, 2016, 12, 57 CrossRef PubMed.
- M. Bodner and M. Scampicchio, Does bruising influence the volatile profile of pears?, Nutr. Food Sci., 2021, 51, 643–652 CrossRef.
- M. L. Mendoza-Enano, R. Stanley and D. Frank, Linking consumer sensory acceptability to volatile composition for improved shelf-life: A case study of fresh-cut watermelon (Citrullus lanatus), Postharvest Biol. Technol., 2019, 154, 137–147 CrossRef CAS.
- M. L. Mendoza-Enano, R. Stanley and D. Frank, Dataset of volatile compounds in fresh and stored cut watermelon (Citrullus lanatus) under varying processing and packaging conditions, Data Brief, 2019, 26, 104299 CrossRef PubMed.
- M. M. Lokke, M. Edelenbos, E. Larsen and A. Feilberg, Investigation of Volatiles Emitted from Freshly Cut Onions (Allium cepa L.) by Real Time Proton-Transfer Reaction-Mass Spectrometry (PTR-MS), Sensors, 2012, 12, 16060–16076 CrossRef PubMed.
- C. Taiti, C. Costa, P. Menesatti, S. Caparrotta, N. Bazihizina, E. Azzarello, W. A. Petrucci, E. Masi and E. Giordani, Use of volatile organic compounds and physicochemical parameters for monitoring the post-harvest ripening of imported tropical fruits, Eur. Food Res. Technol., 2015, 241, 91–102 CrossRef CAS.
- C. Taiti, C. Costa, C. A. Migliori, D. Comparini, S. Figorilli and S. Mancuso, Correlation Between Volatile Compounds and Spiciness in Domesticated and Wild Fresh Chili Peppers, Food Bioprocess Technol., 2019, 12, 1366–1380 CrossRef CAS.
- D. D. Zhang, W. H. Wu, X. H. Qiu, X. J. Li, F. Zhao and N. X. Ye, Rapid and direct identification of the origin of white tea with proton transfer reaction time-of-flight mass spectrometry, Rapid Commun. Mass Spectrom., 2020, 34, e8830 CAS.
- Y. W. Zhou, F. Abbas, J. J. He, F. L. Yan, Q. Wang, Y. Y. Yu, R. C. Yu and Y. P. Fan, Floral volatile chemical diversity in Hedychium F1 hybrid population, Ind. Crops Prod., 2022, 184, 115032 CrossRef CAS.
- J. Kreuzwieser, U. Scheerer, J. Kruse, T. Burzlaff, A. Honsel, S. Alfarraj, P. Georgiev, J. P. Schnitzler, A. Ghirardo, I. Kreuzer, R. Hedrich and H. Rennenberg, The Venus flytrap attracts insects by the release of volatile organic compounds, J. Exp. Bot., 2014, 65, 755–766 CrossRef CAS PubMed.
- A. Cellini, E. Biondi, G. Buriani, B. Farneti, M. T. Rodriguez-Estrada, I. Braschi, S. Savioli, S. Blasioli, L. Rocchi, F. Biasioli, G. Costa and F. Spinelli, Characterization of volatile organic compounds emitted by kiwifruit plants infected with Pseudomonas syringae pv. actinidiae and their effects on host defences, Trees - Struct. Funct., 2016, 30, 795–806 CrossRef CAS.
- N. M. van Dam, D. Samudrala, F. J. M. Harren and S. M. Cristescu, Real-time analysis of sulfur-containing volatiles in Brassica plants infested with root-feeding Delia radicum larvae using proton-transfer reaction mass spectrometry, AoB Plants, 2012, pls021 CAS.
- E. Crespo, C. A. Hordijk, R. M. de Graaf, D. Samudrala, S. M. Cristescu, F. J. M. Harren and N. M. van Dam, On-line detection of root-induced volatiles in Brassica nigra plants infested with Delia radicum L. root fly larvae, Phytochemistry, 2012, 84, 68–77 CrossRef CAS PubMed.
- E. Kari, C. L. Faiola, S. Isokaanta, P. Miettinen, P. Yli-Pirila, A. Buchholz, M. Kivimaenpaa, S. Mikkonenn, J. K. Holopainen and A. Virtanen, Time-resolved characterization of biotic stress emissions from Scots pines being fed upon by pine weevil by means of PTR-ToF-MS, Boreal Environ. Res., 2019, 24, 25–49 Search PubMed.
- Z. Badra, S. L. Herrera, L. Cappellin, F. Biasioli, T. Dekker, S. Angeli and M. Tasin, Species-Specific Induction of Plant Volatiles by Two Aphid Species in Apple: Real Time Measurement of Plant Emission and Attraction of Lacewings in the Wind Tunnel, J. Chem. Ecol., 2021, 47, 653–663 CrossRef CAS PubMed.
- A. Cellini, G. Buriani, L. Rocchi, E. Rondelli, S. Savioli, M. T. Rodriguez-Estrada, S. M. Cristescu, G. Costa and F. Spinelli, Biological relevance of volatile organic compounds emitted during the pathogenic interactions between apple plants and Erwinia amylovora, Mol. Plant Pathol., 2018, 19, 158–168 CrossRef CAS PubMed.
- H. Danner, D. Samudrala, S. M. Cristescu and N. M. Van Dam, Tracing Hidden Herbivores: Time-Resolved Non-Invasive Analysis of Belowground Volatiles by Proton-Transfer-Reaction Mass Spectrometry (PTR-MS), J. Chem. Ecol., 2012, 38, 785–794 CrossRef CAS PubMed.
- O. Kilpinen, D. Z. Liu and A. P. S. Adamsen, Real-Time Measurement of Volatile Chemicals Released by Bed Bugs during Mating Activities, PLoS One, 2012, 7, e50981 CrossRef CAS PubMed.
- M. Rostas, M. G. Cripps and P. Silcock, Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect, Oecologia, 2015, 177, 487–497 CrossRef PubMed.
- F. Spinelli, A. Cellini, J. L. Vanneste, M. T. Rodriguez-Estrada, G. Costa, S. Savioli, F. J. M. Harren and S. M. Cristescu, Emission of volatile compounds by Erwinia amylovora: biological activity in vitro and possible exploitation for bacterial identification, Trees - Struct. Funct., 2012, 26, 141–152 CrossRef CAS.
- M. Portillo-Estrada, C. Van Moorleghem, S. Janssenswillen, R. J. Cooper, C. Birkemeyer, K. Roelants and R. Van Damme, Proton-transfer-reaction time-of-flight mass spectrometry (ptr-tof-ms) as a tool for studying animal volatile organic compound (VOC) emissions, Methods Ecol. Evol., 2021, 12, 748–766 CrossRef.
- E. Crespo, H. de Ronde, S. Kuijper, A. Pol, A. H. J. Kolk, S. M. Cristescu, R. M. Anthony and F. J. M. Harren, Potential biomarkers for identification of mycobacterial cultures by proton transfer reaction mass spectrometry analysis, Rapid Commun. Mass Spectrom., 2012, 26, 679–685 CrossRef CAS PubMed.
- W. Z. Zhou, C. Q. Huang, X. Zou, Y. Lu, C. Y. Shen, X. P. Ding, H. Z. Wang, H. H. Jiang and Y. N. Chu, Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry, Anal. Bioanal. Chem., 2017, 409, 5603–5612 CrossRef CAS PubMed.
- Y. R. Shaltaeva, V. K. Vasilev, D. Y. Yakovlev, F. I. Kopylov, A. L. Syrkin, P. S. Chomakhidze, A. A. Bykova, L. K. Malinovskaya and A. I. Skorokhod, Detection heart failures (HF) biomarkers by proton transfer reaction - mass spectrometry and ion mobility spectrometry, IOP Conf. Ser.: Mater. Sci. Eng., 2016, 151, 012017 Search PubMed.
- M. Lueno, H. Dobrowolny, D. Gescher, L. Gbaoui, G. Meyer-Lotz, C. Hoeschen and T. Frodl, Volatile Organic Compounds From Breath Differ Between Patients With Major Depression and Healthy Controls, Front. Mol. Psychiatry, 2022, 13, 819607 CrossRef PubMed.
- S. Grassin-Delyle, C. Roquencourt, P. Moine, G. Saffroy, S. Carn, N. Heming, J. Fleuriet, H. Salvator, E. Naline, L. J. Couderc, P. Devillier, E. A. Thevenot, D. Annane, G. C.-C. Grp and E. Collaborators, Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study, Ebiomedicine, 2021, 63, 103154 CrossRef CAS PubMed.
- A. Liangou, A. Tasoglou, H. J. Huber, C. Wistrom, K. Brody, P. G. Menon, T. Bebekoski, K. Menschel, M. Davidson-Fiedler, K. DeMarco, H. Salphale, J. Wistrom, S. Wistrom and R. J. Lee, A method for the identification of COVID-19 biomarkers in human breath using Proton Transfer Reaction Time-of-Flight Mass Spectrometry, Eclinicalmedicine, 2021, 42, 101207 CrossRef PubMed.
- A. S. Lehnert, E. Perreca, J. Gershenzon, G. Pohnert and S. E. Trumbore, Simultaneous Real-Time Measurement of Isoprene and 2-Methyl-3-Buten-2-ol Emissions From Trees Using SIFT-MS, Front. Plant Sci., 2020, 11, 578204 CrossRef PubMed.
- C. Azcarate and S. A. Barringer, Effect of Enzyme Activity and Frozen Storage on Jalapeno Pepper Volatiles by Selected Ion Flow Tube-Mass Spectrometry, J. Food Sci., 2010, 75, C710–C721 CrossRef CAS PubMed.
- A. Agila and S. A. Barringer, Volatile Profile of Cashews (Anacardium occidentale L.) from Different Geographical Origins during Roasting, J. Food Sci., 2011, 76, C768–C774 CrossRef CAS PubMed.
- Y. C. Xu and S. Barringer, Comparison of Tomatillo and Tomato Volatile Compounds in the Headspace by Selected Ion Flow Tube Mass Spectrometry (SIFT-MS), J. Food Sci., 2010, 75, C268–C273 CrossRef CAS PubMed.
- M. Kharbach, R. Kamal, M. A. Mansouri, I. Marmouzi, J. Viaene, Y. Cherrah, K. Alaoui, J. Vercammen, A. Bouklouze and Y. Vander Heyden, Selected-ion flow-tube mass-spectrometry (SIFT-MS) fingerprinting versus chemical profiling for geographic traceability of Moroccan Argan oils, Food Chem., 2018, 263, 8–17 CrossRef CAS PubMed.
- M. Beneito-Cambra, D. Moreno-Gonzalez, J. F. Garcia-Reyes, M. Bouza, B. Gilbert-Lopez and A. Molina-Diaz, Direct analysis of olive oil and other vegetable oils by mass spectrometry: A review, TrAC, Trends Anal. Chem., 2020, 132, 116046 CrossRef CAS.
- G. Ozcan-Sinir, O. U. Copur and S. A. Barringer, Botanical and geographical origin of Turkish honeys by selected-ion flow-tube mass spectrometry and chemometrics, J. Sci. Food Agric., 2020, 100, 2198–2207 CrossRef CAS PubMed.
- H. Akpolat and S. A. Barringer, The Effect of pH and Temperature on Cabbage Volatiles During Storage, J. Food Sci., 2015, 80, S1878–S1884 CrossRef CAS PubMed.
- H. Y. Duan and S. A. Barringer, Changes in Furan and Other Volatile Compounds in Sliced Carrot during Air-Drying, J. Food Process. Preserv., 2012, 36, 46–54 CrossRef CAS.
- G. Ozcan-Sinir and S. A. Barringer, Variety differences in garlic volatile sulfur compounds, by application of selected ion flow tube mass spectrometry (SIFT-MS) with chemometrics, Turk. J. Agric. For., 2020, 44, 408–416 CrossRef.
- B. Wampler and S. A. Barringer, Volatile Generation in Bell Peppers during Frozen Storage and Thawing Using Selected Ion Flow Tube Mass Spectrometry (SIFT-MS), J. Food Sci., 2012, 77, C677–C683 CrossRef CAS PubMed.
- G. Ozcan and S. Barringer, Effect of Enzymes on Strawberry Volatiles during Storage, at Different Ripeness Level, in Different Cultivars, and during Eating, J. Food Sci., 2011, 76, C324–C333 CrossRef CAS PubMed.
- I. Vendel, M. Hertog and B. Nicolai, Fast analysis of strawberry aroma using SIFT-MS: A new technique in postharvest research, Postharvest Biol. Technol., 2019, 152, 127–138 CrossRef CAS.
- Y. C. Xu and S. Barringer, Comparison of Volatile Release in Tomatillo and Different Varieties of Tomato during Chewing, J. Food Sci., 2010, 75, C352–C358 CrossRef CAS PubMed.
- V. Langford, J. Gray, B. Foulkes, P. Bray and M. J. McEwan, Application of Selected Ion Flow Tube-Mass Spectrometry to the Characterization of Monofloral New Zealand Honeys, J. Agric. Food Chem., 2012, 60, 6806–6815 CrossRef CAS PubMed.
- J. M. Scotter, V. S. Langford, P. F. Wilson, M. J. McEwan and S. T. Chambers, Real-time detection of common microbial volatile organic compounds from medically important fungi by Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS), J. Microbiol. Methods, 2005, 63, 127–134 CrossRef CAS PubMed.
- T. W. E. Chippendale, P. Spanel and D. Smith, Time-resolved selected ion flow tube mass spectrometric quantification of the volatile compounds generated by E. coli JM109 cultured in two different media, Rapid Commun. Mass Spectrom., 2011, 25, 2163–2172 CrossRef CAS PubMed.
- P. M. Heynderickx, K. Van Huffel, J. Dewulf and H. Van Langenhove, Application of similarity coefficients to SIFT-MS data for livestock emission characterization, Biosyst. Eng., 2013, 114, 44–54 CrossRef.
- A. D. Spooner, C. Bessant, C. Turner, H. Knobloch and M. Chambers, Evaluation of a combination of SIFT-MS and multivariate data analysis for the diagnosis of Mycobacterium bovis in wild badgers, Analyst, 2009, 134, 1922–1927 RSC.
- I. Belluomo, P. R. Boshier, A. Myridakis, B. Vadhwana, S. R. Markar, P. Spanel and G. B. Hanna, Selected ion flow tube mass spectrometry for targeted analysis of volatile organic compounds in human breath, Nat. Protoc., 2021, 16, 3419–3438 CrossRef CAS PubMed.
- P. Spanel and D. Smith, Quantification of volatile metabolites in exhaled breath by selected ion flow tube mass spectrometry, SIFT-MS, Clinical Mass Spectrometry, 2020, 16, 18–24 CrossRef PubMed.
- P. R. Boshier, N. Marczin and G. B. Hanna, Repeatability of the Measurement of Exhaled Volatile Metabolites Using Selected Ion Flow Tube Mass Spectrometry, J. Am. Soc. Mass Spectrom., 2010, 21, 1070–1074 CrossRef CAS PubMed.
- D. Chandran, E. H. Ooi, D. I. Watson, F. Kholmurodova, S. Jaenisch and R. Yazbeck, The Use of Selected Ion Flow Tube-Mass Spectrometry Technology to Identify Breath Volatile Organic Compounds for the Detection of Head and Neck Squamous Cell Carcinoma: A Pilot Study, Medicina, 2019, 55, 306 CrossRef PubMed.
- R. R. Farrell, J. Fahrentrapp, D. Garcia-Gomez, P. M. L. Sinues and R. Zenobi, Rapid fingerprinting of grape volatile composition using secondary electrospray ionization orbitrap mass spectrometry: A preliminary study of grape ripening, Food Control, 2017, 81, 107–112 CrossRef CAS.
- C. Barrios-Collado, D. Garcia-Gomez, R. Zenobi, G. Vidal-de-Miguel, A. J. Ibanez and P. M. L. Sinues, Capturing in Vivo Plant Metabolism by Real-Time Analysis of Low to High Molecular Weight Volatiles, Anal. Chem., 2016, 88, 2406–2412 CrossRef CAS PubMed.
- H. D. Bean, J. J. Zhu and J. E. Hill, Characterizing Bacterial Volatiles using Secondary Electrospray Ionization Mass Spectrometry (SESI-MS), JoVE, 2011, 52, 2664 Search PubMed.
- P. Martinez-Lozano and J. F. de la Mora, On-line Detection of Human Skin Vapors, J. Am. Soc. Mass Spectrom., 2009, 20, 1060–1063 CrossRef CAS PubMed.
- P. Martinez-Lozano, Mass spectrometric study of cutaneous volatiles by secondary electrospray ionization, Int. J. Mass Spectrom., 2009, 282, 128–132 CrossRef CAS.
- X. Li, D. D. Huang, R. Du, Z. J. Zhang, C. K. Chan, Z. X. Huang and Z. Zhou, Video Article Real-time Breath Analysis by Using Secondary Nanoelectrospray Ionization Coupled to High Resolution Mass Spectrometry, JoVE, 2018, 56465 Search PubMed.
- P. Martinez-Lozano, L. Zingaro, A. Finiguerra and S. Cristoni, Secondary electrospray ionization-mass spectrometry: breath study on a control group, J. Breath Res., 2011, 5, 016002 CrossRef CAS PubMed.
- M. T. Gaugg, On-line Breath Metabolomics in Respiratory Diseases using Secondary Electrospray Ionization-Mass Spectrometry, Chimia, 2018, 72, 184–188 CrossRef CAS PubMed.
- T. Bruderer, T. Gaisl, M. T. Gaugg, N. Nowak, B. Streckenbach, S. Muller, A. Moeller, M. Kohler and R. Zenobi, On-Line Analysis of Exhaled Breath, Chem. Rev., 2019, 119, 10803–10828 CrossRef CAS PubMed.
- L. Wang, S. S. Zeng and H. B. Qu, Effects of ion source operating parameters on direct analysis in real time of 18 active components from traditional Chinese medicine, J. Pharm. Biomed., 2016, 121, 30–38 CrossRef CAS PubMed.
- E. Block, R. B. Cody, A. J. Dane, R. Sheridan, A. Vattekkatte and K. Wang, Allium chemistry: Use of new instrumental techniques to "see" reactive organosulfur species formed upon crushing garlic and onion, Pure Appl. Chem., 2010, 82, 535–539 CAS.
- T. J. Mason, H. M. Bettenhausen, J. M. Chaparro, M. E. Uchanski and J. E. Prenni, Evaluation of ambient mass spectrometry tools for assessing inherent postharvest pepper quality, Hortic. Res., 2021, 8, 160 CrossRef CAS PubMed.
- Y. Li, Confined direct analysis in real time ion source and its applications in analysis of volatile organic compounds of Citrus limon (lemon) and Allium cepa (onion), Rapid Commun. Mass Spectrom., 2012, 26, 1194–1202 CrossRef CAS PubMed.
- T. Y. He, M. I. Chambers and R. A. Musah, Application of Direct Analysis in Real Time-High Resolution Mass Spectrometry to Investigations of Induced Plant Chemical Defense Mechanisms-Revelation of Negative Feedback Inhibition of an Alliinase, Anal. Chem., 2018, 90, 12802–12809 CrossRef CAS PubMed.
- Y. Li and R. A. Mathews, In vivo real-time monitoring of aphrodisiac pheromone release of small white cabbage butterflies (Pieris rapae), J. Insect Physiol., 2016, 91–92, 107–112 CrossRef CAS PubMed.
- Z. C. Yuan, W. Li, L. Wu, D. Huang, M. M. Wu and B. Hu, Solid-Phase Microextraction Fiber in Face Mask for in Vivo Sampling and Direct Mass Spectrometry Analysis of Exhaled Breath Aerosol, Anal. Chem., 2020, 92, 11543–11547 CrossRef CAS PubMed.
- Y. Li, Applications of a confined DART (direct analysis in real time) ion source for online in vivo analysis of human breath, Anal. Methods, 2013, 5, 6933–6940 RSC.
- P. Werle, A review of recent advances in semiconductor laser based gas monitors, Spectrochim. Acta, Part A, 1998, 54, 197–236 CrossRef.
- Q. Zou, H. Fang, W. Zhang and Y. He, Application of Near Infrared Spectroscopy (NIR) for Evaluating Cheese Quality, Spectrosc. Spectral Anal., 2011, 31, 2725–2729 CAS.
- X. Li, L. X. Zhang, Y. Zhang, D. Wang, X. F. Wang, L. Yu, W. Zhang and P. W. Li, Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils, Trends Food Sci. Technol., 2020, 101, 172–181 CrossRef CAS.
- V. Cortes, J. Blasco, N. Aleixos, S. Cubero and P. Talens, Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review, Trends Food Sci. Technol., 2019, 85, 138–148 CrossRef CAS.
- J. L. Koenig, Fourier-Transform Infrared-Spectroscopy of Chemical-Systems, Acc. Chem. Res., 1981, 14, 171–178 CrossRef CAS.
- J. A. Larrabee and S. H. Choi, Fourier-Transform Infrared-Spectroscopy, Methods Enzymol., 1993, 226, 289–305 CAS.
- S. Adhikari, V. Kelkar, R. Kumar and R. U. Halden, Methods and challenges in the detection of microplastics and nanoplastics: a mini-review, Polym. Int., 2022, 71, 543–551 CrossRef CAS.
- M. Szymanska and K. Obolewski, Microplastics as contaminants in freshwater environments: A multidisciplinary review, Ecohydrol. Hydrobiol., 2020, 20, 333–345 CrossRef.
- Z. H. Huang and J. D. Wang, Remote sensing detection of atmospheric pollutants by Fourier Transform Infrared Spectrometry, Spectrosc. Spectral Anal., 2002, 22, 235–238 CAS.
- C. Schutze, S. Lau, N. Reiche, U. Sauer, H. Borsdorf and P. Dietrich, Ground-Based Remote Sensing with Open-Path Fourier-Transform Infrared (OP-FTIR) Spectroscopy for Large-Scale Monitoring of Greenhouse Gases, Energy Procedia, 2013, 37, 4276–4282 CrossRef.
- C. Schlawis and S. Schulz, Direct deposition GC/IR techniques in natural product identification, Nat. Prod. Rep., 2020, 37, 1561–1567 RSC.
- S. A. Borman, Gc/Ir - Lc/Ir - Gc/Ir/Ms, Anal. Chem., 1982, 54, 901A–905A CrossRef.
- W. Nie, R. F. Kan, C. G. Yang, B. Chen, Z. Y. Xu and W. Q. Liu, Research Progress on the Application of Tunable Diode Laser Absorption Spectroscopy, Chin. J. Lasers, 2018, 45, 0911001 CrossRef.
- J. S. Li, B. L. Yu, W. X. Zhao and W. D. Chen, A Review of Signal Enhancement and Noise Reduction Techniques for Tunable Diode Laser Absorption Spectroscopy, Appl. Spectrosc. Rev., 2014, 49, 666–691 CrossRef CAS.
- J. L. Kinsey, Laser-Induced Fluorescence, Annu. Rev. Phys. Chem., 1977, 28, 349–372 CrossRef CAS.
- N. Lang, C. Rupp, H. Almuina-Villar, A. Dieguez-Alonso, F. Behrendt and J. Ropcke, Pyrolysis behavior of thermally thick wood particles: Time-resolved characterization with laser based in-situ diagnostics, Fuel, 2017, 210, 371–379 CrossRef CAS.
- N. Zobel and A. Anca-Couce, Slow pyrolysis of wood particles: Characterization of volatiles by Laser-Induced Fluorescence, Proc. Combust. Inst., 2013, 34, 2355–2362 CrossRef CAS.
- K. K. Chow, M. Short and H. S. Zeng, A comparison of spectroscopic techniques for human breath analysis, Biomed. Spectrosc. Imaging, 2012, 1, 339–353 CAS.
- S. Bernegger and M. W. Sigrist, Co-Laser Photoacoustic-Spectroscopy of Gases and Vapors for Trace Gas-Analysis, Infrared Phys., 1990, 30, 375–429 CrossRef.
- D. C. Dumitras, D. C. Dutu, C. Matei, A. M. Magureanu, M. Petrus and C. Popa, Laser photoacoustic spectroscopy: principles, instrumentation, and characterization, J. Optoelectron. Adv. Mater., 2007, 9, 3655–3701 CAS.
- F. G. C. Bijnen, J. Reuss and F. J. M. Harren, Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection, Rev. Sci. Instrum., 1996, 67, 2914–2923 CrossRef CAS.
- F. J. M. Harren and J. Reuss, in Encyclopedia of Applied Physics, VCH Publishers, New York, 1997, vol. 19, p. 35 Search PubMed.
- L. A. J. Mur, I. E. Santosa, L. J. J. Laarhoven, N. J. Holton, F. J. M. Harren and A. R. Smith, Laser photoacoustic detection allows in planta detection of nitric oxide in tobacco following challenge with avirulent and virulent Pseudomonas syringae pathovars, Plant Physiol., 2005, 138, 1247–1258 CrossRef CAS PubMed.
- L. R. Narasimhan, W. Goodman and C. K. N. Patel, Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4617–4621 CrossRef CAS PubMed.
- L. Strojnik, M. Stopar, E. Zlatic, D. Kokalj, M. N. Gril, B. Zenko, M. Znidarsic, M. Bohanec, B. M. Boshkovska, M. Lustrek, A. Gradisek, D. Potocnik and N. Ogrinc, Authentication of key aroma compounds in apple using stable isotope approach, Food Chem., 2019, 277, 766–773 CrossRef CAS PubMed.
- E. Calla-Quispe, H. L. Fuentes-Rivera, P. Ramirez, C. Martel and A. J. Ibanez, Mass Spectrometry: A Rosetta Stone to Learn How Fungi Interact and Talk, Life, 2020, 10, 89 CrossRef CAS PubMed.
- J. L. Ren, A. H. Zhang, L. Kong and X. J. Wang, Advances in mass spectrometry-based metabolomics for investigation of metabolites, RSC Adv., 2018, 8, 22335–22350 RSC.
- B. F. Cravatt, G. M. Simon and J. R. Yates, The biological impact of mass-spectrometry-based proteomics, Nature, 2007, 450, 991–1000 CrossRef CAS PubMed.
- J. Pereira, P. Porto-Figueira, C. Cavaco, K. Taunk, S. Rapole, R. Dhakne, H. Nagarajaram and J. S. Camara, Breath Analysis as a Potential and Non-Invasive Frontier in Disease Diagnosis: An Overview, Metabolites, 2015, 5, 3–55 CrossRef PubMed.
- D. Smith, P. Spanel, J. Herbig and J. Beauchamp, Mass spectrometry for real-time quantitative breath analysis, J. Breath Res., 2014, 8, 027101 CrossRef CAS PubMed.
- J. C. Holmes and F. A. Morrell, Oscillographic Mass Spectrometric Monitoring of Gas Chromatography, Appl. Spectrosc., 1957, 11, 2 CrossRef.
- R. S. Gohlke, Time-of-Flight Mass Spectrometry and Gas-Liquid Partition Chromatography, Anal. Chem., 1959, 31, 535–541 CrossRef CAS.
- X. Q. Hu, L. Lu, Z. L. Guo and Z. W. Zhu, Volatile compounds, affecting factors and evaluation methods for rice aroma: A review, Trends Food Sci. Technol., 2020, 97, 136–146 CrossRef CAS.
- J. X. Zhao, X. J. Dai, X. M. Liu, H. Zhang, J. A. Tang and W. Chen, Comparison of aroma compounds in naturally fermented and inoculated Chinese soybean pastes by GC-MS and GC-Olfactometry analysis, Food Control, 2011, 22, 1008–1013 CrossRef CAS.
- P. Rodriguez-Hernandez, V. Rodriguez-Estevez, L. Arce and J. Gomez-Laguna, Application of Volatilome Analysis to the Diagnosis of Mycobacteria Infection in Livestock, Front. Vet. Sci., 2021, 8, 635155 CrossRef PubMed.
- W. M. Ahmed, P. Geranios, I. R. White, O. Lawal, T. M. Nijsen, M. J. Bromley, R. Goodacre, N. D. Read and S. J. Fowler, Development of an adaptable headspace sampling method for metabolic profiling of the fungal volatome, Analyst, 2018, 143, 4155–4162 RSC.
- E. Booth, G. Strobel, B. Knighton, J. Sears, B. Geary and R. Avci, A rapid column technique for trapping and collecting of volatile fungal hydrocarbons and hydrocarbon derivatives, Biotechnol. Lett., 2011, 33, 1963–1972 CrossRef CAS PubMed.
- R. J. Ewen, P. R. H. Jones, N. M. Ratcliffe and P. T. N. Spencer-Phillips, Identification by gas chromatography-mass spectrometry of the volatile organic compounds emitted from the wood-rotting fungi Serpula lacrymans and Coniophora puteana, and from Pinus sylvestris timber, Mycol. Res., 2004, 108, 806–814 CrossRef CAS PubMed.
- S. Neumann and S. Bocker, Computational mass spectrometry for metabolomics: Identification of metabolites and small molecules, Anal. Bioanal. Chem., 2010, 398, 2779–2788 CrossRef CAS PubMed.
- T. Holm, Aspects of the mechanism of the flame ionization detector, J. Chromatogr. A, 1999, 842, 221–227 CrossRef CAS.
- N. Ragunathan, K. A. Krock, C. Klawun, T. A. Sasaki and C. L. Wilkins, Gas chromatography with spectroscopic detectors, J. Chromatogr. A, 1999, 856, 349–397 CrossRef CAS PubMed.
- J. Sneddon, S. Masuram and J. C. Richert, Gas chromatography-mass spectrometry-basic principles, instrumentation and selected applications for detection of organic compounds, Anal. Lett., 2007, 40, 1003–1012 CrossRef CAS.
- C. Jousse and E. Pujos-Guillot, Exploring Metabolome with GC/MS, Adv. Bot. Res., 2013, 67, 303–329 CAS.
- F. Hernandez, M. I. Cervera, T. Portoles, J. Beltran and E. Pitarch, The role of GC-MS/MS with triple quadrupole in pesticide residue analysis in food and the environment, Anal. Methods, 2013, 5, 5875–5894 RSC.
- Y. Seto, Determination of Volatile Substances in Biological Samples by Headspace Gas-Chromatography, J. Chromatogr. A, 1994, 674, 25–62 CrossRef CAS.
- J. Dewulf and H. Van Langenhove, Analysis of volatile organic compounds using gas chromatography, TrAC, Trends Anal. Chem., 2002, 21, 637–646 CrossRef CAS.
- I. Jerkovic, M. Kranjac, Z. Marijanovic, M. Roje and S. Jokic, Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea, Molecules, 2019, 24, 495 CrossRef PubMed.
- A. Sanchez-Cruz, N. Robledo, M. Rosete-Enriquez and A. A. Romero-Lopez, Attraction of Adults of Cyclocephala lunulata and Cyclocephala barrerai (Coleoptera: Scarabaeoidea: Melolonthidae) towards Bacteria Volatiles Isolated from Their Genital Chambers, Molecules, 2020, 25, 4430 CrossRef CAS PubMed.
- M. Li, S. Wang and L. He, Development of an analytical method coupling cell membrane chromatography with gas chromatography-mass spectrometry via microextraction by packed sorbent and its application in the screening of volatile active compounds in natural products, J Chromatogr B, 2015, 974, 9–16 CrossRef CAS PubMed.
- E. Calleri, C. Temporini and G. Massolini, Frontal affinity chromatography in characterizing immobilized receptors, J. Pharm. Biomed. Anal., 2011, 54, 911–925 CrossRef CAS PubMed.
- H. Du, J. He, S. Wang and L. He, Investigation of calcium antagonist-L-type calcium channel interactions by a vascular smooth muscle cell membrane chromatography method, Anal. Bioanal. Chem., 2010, 397, 1947–1953 CrossRef CAS PubMed.
- F. Azimi and M. H. Fatemi, Multivariate curve resolution-assisted GC-MS analysis of the volatile chemical constituents in Iranian Citrus aurantium L. peel, RSC Adv., 2016, 6, 111197–111209 RSC.
- A. L. Lubrano, C. R. Field, G. A. Newsome, D. A. Rogers, B. C. Giordano and K. J. Johnson, Minimizing thermal degradation in gas chromatographic quantitation of pentaerythritol tetranitrate, J. Chromatogr. A, 2015, 1394, 154–158 CrossRef CAS PubMed.
- M. Jalali-Heravi and H. Parastar, Recent trends in application of multivariate curve resolution approaches for improving gas chromatography-mass spectrometry analysis of essential oils, Talanta, 2011, 85, 835–849 CrossRef CAS PubMed.
- J. M. Amigo, M. J. Popielarz, R. M. Callejon, M. L. Morales, A. M. Troncoso, M. A. Petersen and T. B. Toldam-Andersen, Comprehensive analysis of chromatographic data by using PARAFAC2 and principal components analysis, J. Chromatogr. A, 2010, 1217, 4422–4429 CrossRef CAS PubMed.
- J. V. Seeley and S. K. Seeley, Multidimensional Gas Chromatography: Fundamental Advances and New Applications, Anal. Chem., 2013, 85, 557–578 CrossRef CAS PubMed.
- Z. Y. Liu and J. B. Phillips, Comprehensive 2-Dimensional Gas-Chromatography Using an on-Column Thermal Modulator Interface, J. Chromatogr. Sci., 1991, 29, 227–231 CAS.
- P. J. Marriott, S. T. Chin, B. Maikhunthod, H. G. Schmarr and S. Bieri, Multidimensional gas chromatography, TrAC, Trends Anal. Chem., 2012, 34, 1–21 CrossRef CAS.
- D. M. Rasheed, A. Serag, Z. T. A. Shakour and M. Farag, Novel trends and applications of multidimensional chromatography in the analysis of food, cosmetics and medicine bearing essential oils, Talanta, 2021, 223, 121710 CrossRef CAS PubMed.
- A. E. Sinha, B. J. Prazen, C. G. Fraga and R. E. Synovec, Valve-based comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection: instrumentation and figures-of-merit, J. Chromatogr. A, 2003, 1019, 79–87 CrossRef CAS PubMed.
- M. Edwards and T. Gorecki, Inlet backflushing device for the improvement of comprehensive two dimensional gas chromatographic separations, J. Chromatogr. A, 2015, 1402, 110–123 CrossRef CAS PubMed.
- I. Marquez-Sillero, S. Cardenas, S. Sielemann and M. Valcarcel, On-line headspace-multicapillary column-ion mobility spectrometry hyphenation as a tool for the determination of off-flavours in foods, J. Chromatogr. A, 2014, 1333, 99–105 CrossRef CAS PubMed.
- N. Kunze, C. Weigel, W. Vautz, K. Schwerdtfeger, M. Junger, M. Quintel and T. Perl, Multi-capillary column-ion mobility spectrometry (MCC-IMS) as a new method for the quantification of occupational exposure to sevoflurane in anaesthesia workplaces: an observational feasibility study, J. Occup. Med. Toxicol., 2015, 10, 12 CrossRef PubMed.
- V. Ruzsanyi, L. Fischer, J. Herbig, C. Ager and A. Amann, Multi-capillary-column proton-transfer-reaction time-of-flight mass spectrometry, J. Chromatogr. A, 2013, 1316, 112–118 CrossRef CAS PubMed.
- A. M. Casas-Ferreira, M. del Nogal-Sanchez, J. L. Perez-Pavon and B. Moreno-Cordero, Non-separative mass spectrometry methods for non-invasive medical diagnostics based on volatile organic compounds: A review, Anal. Chim. Acta, 2019, 1045, 10–22 CrossRef CAS PubMed.
- B. Farneti, S. M. Cristescu, G. Costa, F. J. M. Harren and E. J. Woltering, Rapid Tomato Volatile Profiling by Using Proton-Transfer Reaction Mass Spectrometry (PTR-MS), J. Food Sci., 2012, 77, C551–C559 CrossRef CAS PubMed.
- D. Ballabio, E. Robotti, F. Grisoni, F. Quasso, M. Bobba, S. Vercelli, F. Gosetti, G. Calabrese, E. Sangiorgi, M. Orlandi and E. Marengo, Chemical profiling and multivariate data fusion methods for the identification of the botanical origin of honey, Food Chem., 2018, 266, 79–89 CrossRef CAS PubMed.
- R. S. Blake, P. S. Monks and A. M. Ellis, Proton-Transfer Reaction Mass Spectrometry, Chem. Rev., 2009, 109, 861–896 CrossRef CAS PubMed.
- E. Hartungen, S. Jurschik, A. Jordan, A. Edtbauer, S. Feil, G. Hanel, H. Seehauser, S. Haidacher, R. Schottkowsky, L. Mark, S. Jaksch, B. Agarwal, K. Becker, C. A. Mayhew, P. Sulzer and T. D. Mark, Proton transfer reaction-mass spectrometry: fundamentals, recent advances and applications, Eur. Phys. J.: Appl. Phys., 2013, 61, 24303 CrossRef.
- T. Majchrzak, W. Wojnowski, M. Lubinska-Szczygel, A. Rozanska, J. Namiesnik and T. Dymerski, PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review, Anal. Chim. Acta, 2018, 1035, 1–13 CrossRef CAS PubMed.
- A. Romano, V. Capozzi, G. Spano and F. Biasioli, Proton transfer reaction-mass spectrometry: online and rapid determination of volatile organic compounds of microbial origin, Appl. Microbiol. Biotechnol., 2015, 99, 3787–3795 CrossRef CAS PubMed.
- B. Yuan, A. R. Koss, C. Warneke, M. Coggon, K. Sekimoto and J. A. de Gouw, Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences, Chem. Rev., 2017, 117, 13187–13229 CrossRef CAS PubMed.
- J. Q. Zeng, Y. L. Zhang, H. N. Zhang, W. Song, Z. F. Wu and X. M. Wang, Design and characterization of a semi-open dynamic chamber for measuring biogenic volatile organic compound (BVOC) emissions from plants, Atmos. Meas. Tech., 2022, 15, 79–93 CrossRef CAS.
- J. Kim, A. H. Goldstein, R. Chakraborty, K. Jardine, R. Weber, P. O. Sorensen, S. Wang, B. Faybishenko, P. K. Misztal and E. L. Brodie, Measurement of Volatile Compounds for Real-Time Analysis of Soil Microbial Metabolic Response to Simulated Snowmelt, Front. Microbiol., 2021, 12, 679671 CrossRef PubMed.
- C. M. Gray, R. K. Monson and N. Fierer, Biotic and abiotic controls on biogenic volatile organic compound fluxes from a subalpine forest floor, J. Geophys. Res.: Biogeosci., 2014, 119, 547–556 CrossRef CAS.
- T. Wroblewski, A. Kaminska, A. Wlodarkiewicz and D. Ushakou, Studies of Volatile Organic Compounds Emission from Fragaria Vesca and Fragaria Ananassa Using Proton Transfer Reaction Mass Spectrometry, Acta Phys. Pol. B Proc. Suppl., 2020, 13, 899–906 Search PubMed.
- Y. Guo, W. Jud, A. Ghirardo, F. Antritter, J. P. Benz, J. P. Schnitzler and M. Rosenkranz, Sniffing fungi - phenotyping of volatile chemical diversity in Trichoderma species, New Phytol., 2020, 227, 244–259 CrossRef CAS PubMed.
- V. Lazazzara, M. Perazzolli, I. Pertot, F. Biasioli, G. Puopolo and L. Cappellin, Growth media affect the volatilome and antimicrobial activity against Phytophthora infestans in four Lysobacter type strains, Microbiol. Res., 2017, 201, 52–62 CrossRef CAS PubMed.
- M. Pedrotti, I. Khomenko, G. Genova, G. Castello, N. Spigolon, V. Fogliano and F. Biasioli, Quality control of raw hazelnuts by rapid and non-invasive fingerprinting of volatile compound release, LWT--Food Sci. Technol., 2021, 143, 111089 CrossRef CAS.
- A. M. Mustafa, S. Angeloni, F. K. Nzekoue, D. Abouelenein, G. Sagratini, G. Caprioli and E. Torregiani, An Overview on Truffle Aroma and Main Volatile Compounds, Molecules, 2020, 25, 5948 CrossRef CAS PubMed.
- V. J. L. Ting, A. Romano, C. Soukoulis, P. Silcock, P. J. Bremer, L. Cappellin and F. Biasioli, Investigating the in-vitro and in-vivo flavour release from 21 fresh-cut apples, Food Chem., 2016, 212, 543–551 CrossRef CAS PubMed.
- A. M. Yanez-Serrano, A. C. Nolscher, J. Williams, S. Wolff, E. Alves, G. A. Martins, E. Bourtsoukidis, J. Brito, K. Jardine, P. Artaxo and J. Kesselmeier, Diel and seasonal changes of biogenic volatile organic compounds within and above an Amazonian rainforest, Atmos. Chem. Phys., 2015, 15, 3359–3378 CrossRef CAS.
- A. M. Yanez-Serrano, A. Bach, D. Bartolome-Catala, V. Matthaios, R. Seco, J. Llusia, I. Filella and J. Penuelas, Dynamics of volatile organic compounds in a western Mediterranean oak forest, Atmos. Environ., 2021, 257, 118447 CrossRef CAS.
- A. Hansel, A. Jordan, R. Holzinger, P. Prazeller, W. Vogel and W. Lindinger, Proton-Transfer Reaction Mass-Spectrometry - Online Trace Gas-Analysis at the Ppb Level, Int. J. Mass Spectrom., 1995, 149, 609–619 CrossRef.
- E. Pallozzi, G. Guidolotti, P. Ciccioli, F. Brilli, S. Feil and C. Calfapietra, Does the novel fast-GC coupled with PTR-TOF-MS allow a significant advancement in detecting VOC emissions from plants?, Agr. Forest Meteorol., 2016, 216, 232–240 CrossRef.
- A. Edtbauer, E. Hartungen, A. Jordan, G. Hanel, J. Herbig, S. Jurschik, M. Lanza, K. Breiev, L. Mark and P. Sulzer, Theory and practical examples of the quantification of CH4, CO, O-2, and CO2 with an advanced proton-transfer-reaction/selective-reagent-ionization instrument (PTR/SRI-MS), Int. J. Mass Spectrom., 2014, 365, 10–14 CrossRef.
- Q. L. Zhang, X. Zou, Q. Liang, Y. T. Zhang, M. J. Yi, H. M. Wang, C. Q. Huang, C. Y. Shen and Y. N. Chu, Development of Dipolar Proton Transfer Reaction Mass Spectrometer for Real-time Monitoring of Volatile Organic Compounds in Ambient Air, Chin. J. Anal. Chem., 2018, 46, 471–478 CAS.
- D. Smith and P. Spanel, Direct, rapid quantitative analyses of BVOCs using SIFT-MS and PTR-MS obviating sample collection, TrAC, Trends Anal. Chem., 2011, 30, 945–959 CrossRef CAS.
- V. S. Langford, D. Padayachee, M. J. McEwan and S. A. Barringer, Comprehensive odorant analysis for on-line applications using selected ion flow tube mass spectrometry (SIFT-MS), Flavour Fragrance J., 2019, 34, 393–410 CrossRef CAS.
- H. Z. Castada and S. A. Barringer, Online, real-time, and direct use of SIFT-MS to measure garlic breath deodorization: a review, Flavour Fragrance J., 2019, 34, 299–306 CrossRef CAS.
- H. Z. Castada, K. Hanas and S. A. Barringer, Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference, Foods, 2019, 8, 78 CrossRef CAS PubMed.
- D. Smith and P. Spanel, Ambient analysis of trace compounds in gaseous media by SIFT-MS, Analyst, 2011, 136, 2009–2032 RSC.
- D. Smith and P. Spanel, SIFT-MS and FA-MS methods for ambient gas phase analysis: developments and applications in the UK, Analyst, 2015, 140, 2573–2591 RSC.
- P. Spanel, K. Dryahina and D. Smith, A general method for the calculation of absolute trace gas concentrations in air and breath from selected ion flow tube mass spectrometry data, Int. J. Mass Spectrom., 2006, 249, 230–239 CrossRef.
- C. Guerrini, F. Nardella, A. Morganti, J. La Nasa, I. Degano and E. Ribechini, Focusing on Volatile Organic Compounds of Natural Resins by Selected-Ion Flow Tube-Mass Spectrometry, J. Am. Soc. Mass Spectrom., 2022, 33, 1465–1473 CrossRef CAS PubMed.
- P. Spanel and D. Smith, Account On the features, successes and challenges of selected ion flow tube mass spectrometry, Eur. J. Mass Spectrom., 2013, 19, 225–246 CrossRef CAS PubMed.
- C. Liu, J. F. Zeng, P. Sinues, M. L. Fang, Z. Zhou and X. Li, Quantification of volatile organic compounds by secondary electrospray ionization-high resolution mass spectrometry, Anal. Chim. Acta, 2021, 1180 CAS.
- A. T. Rioseras, M. T. Gaugg and P. M. L. Sinues, Secondary electrospray ionization proceeds via gas-phase chemical ionization, Anal. Methods, 2017, 9, 5052–5057 RSC.
- S. Fuertenau, P. Kiselev and J. B. Fenn, ESI-MS in the analysis of trace species in gases, Proceedings of the 47th ASMS conference on Mass Spectrometry, Allied Topics, Dallas, TX, 1999 Search PubMed.
- M. Tam and H. H. Hill, Secondary electrospray ionization-ion mobility spectrometry for explosive vapor detection, Anal. Chem., 2004, 76, 2741–2747 CrossRef CAS PubMed.
- P. Martinez-Lozano, J. Rus, G. F. de la Mora, M. Hernandez and J. F. de la Mora, Secondary Electrospray Ionization (SESI) of Ambient Vapors for Explosive Detection at Concentrations Below Parts Per Trillion, J. Am. Soc. Mass Spectrom., 2009, 20, 287–294 CrossRef CAS PubMed.
- J. J. He, P. M. L. Sinues, M. Hollmen, X. Li, M. Detmar and R. Zenobi, Fingerprinting Breast Cancer vs. Normal Mammary Cells by Mass Spectrometric Analysis of Volatiles, Sci. Rep., 2014, 4, 5196 CrossRef CAS PubMed.
- E. M. Ogawa, H. B. Costa, J. A. Ventura, L. C. S. Caetano, F. E. Pinto, B. G. Oliveira, M. E. S. Barroso, R. Scherer, D. C. Endringer and W. Romao, Chemical profile of pineapple cv. Vitoria in different maturation stages using electrospray ionization mass spectrometry, J. Sci. Food Agric., 2018, 98, 1105–1116 CrossRef CAS PubMed.
- P. Martinez-Lozano and J. F. de la Mora, Electrospray ionization of volatiles in breath, Int. J. Mass Spectrom., 2007, 265, 68–72 CrossRef CAS.
- P. Martinez-Lozano and J. F. de la Mora, Direct Analysis of Fatty Acid Vapors in Breath by Electrospray Ionization and Atmospheric Pressure Ionization-Mass Spectrometry, Anal. Chem., 2008, 80, 8210–8215 CrossRef CAS PubMed.
- K. D. Singh, G. V. del Miguel, M. T. Gaugg, A. J. Ibanez, R. Zenobi, M. Kohler, U. Frey and P. M. L. Sinues, Translating secondary electrospray ionization-high-resolution mass spectrometry to the clinical environment, J. Breath Res., 2018, 12, 027113 CrossRef PubMed.
- J. C. Reynolds, M. A. Jimoh, C. Guallar-Hoyas, C. S. Creaser, S. Siddiqui and C. L. P. Thomas, Analysis of human breath samples using a modified thermal desorption: gas chromatography electrospray ionization interface, J. Breath Res., 2014, 8, 037105 CrossRef CAS PubMed.
- T. Bruderer, M. T. Gaugg, L. Cappellin, F. Lopez-Hilfiker, M. Hutterli, N. Perkins, R. Zenobi and A. Moeller, Detection of Volatile Organic Compounds with Secondary Electrospray Ionization and Proton Transfer Reaction High-Resolution Mass Spectrometry: A Feature Comparison, J. Am. Soc. Mass Spectrom., 2020, 31, 1632–1640 CrossRef CAS PubMed.
- S. Som, J. Kubista, K. Dryahina and P. Spanel, Parallel secondary electrospray ionisation mass spectrometry and selected ion flow tube mass spectrometry quantification of trace amounts of volatile ketones, Rapid Commun. Mass Spectrom., 2021, 35, e8981 CrossRef CAS PubMed.
- R. B. Cody, Observation of Molecular Ions and Analysis of Nonpolar Compounds with the Direct Analysis in Real Time Ion Source, Anal. Chem., 2009, 81, 1101–1107 CrossRef CAS PubMed.
- R. B. Cody, J. A. Laramee, J. M. Nilles and H. D. Durst, Direct Analysis in Real Time (DART) Mass Spectrometry, JEOL News, 2005, 40, 8–12 Search PubMed.
- E. S. Chernetsova, A. N. Shikov, E. A. Crawford, S. Grashorn, I. Laakso, O. N. Pozharitskaya, V. G. Makarov, R. Hiltunen, B. Galambosi and G. E. Morlock, Letter: Characterization of volatile and semi-volatile compounds in green and fermented leaves of Bergenia crassifolia L. by gas chromatography-mass spectrometry and ID-CUBE direct analysis in real time-high resolution mass spectrometry, Eur. J. Mass Spectrom., 2014, 20, 199–205 CrossRef CAS PubMed.
- D. L. G. Borges, R. E. Sturgeon, B. Welz, A. J. Curtius and Z. Mester, Ambient Mass Spectrometric Detection of Organometallic Compounds Using Direct Analysis in Real Time, Anal. Chem., 2009, 81, 9834–9839 CrossRef CAS PubMed.
- O. P. Haefliger and N. Jeckelmann, Direct mass spectrometric analysis of flavors and fragrances in real applications using DART, Rapid Commun. Mass Spectrom., 2007, 21, 1361–1366 CrossRef CAS PubMed.
- S. D. Maleknia, T. M. Vail, R. B. Cody, D. O. Sparkman, T. L. Bell and M. A. Adams, Temperature-dependent release of volatile organic compounds of eucalypts by direct analysis in real time (DART) mass spectrometry, Rapid Commun. Mass Spectrom., 2009, 23, 2241–2246 CrossRef CAS PubMed.
- J. Y. Yew, R. B. Cody and E. A. Kravitz, Cuticular hydrocarbon analysis of an awake behaving fly using direct analysis in real-time time-of-flight mass spectrometry, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7135–7140 CrossRef CAS PubMed.
- J. A. Jastrzembski, M. Y. Bee and G. L. Sacks, Trace-Level Volatile Quantitation by Direct Analysis in Real Time Mass Spectrometry following Headspace Extraction: Optimization and Validation in Grapes, J. Agric. Food Chem., 2017, 65, 9353–9359 CrossRef CAS PubMed.
- M. G. Appley, S. Beyramysoltan and R. A. Musah, Random Forest Processing of Direct Analysis in Real-Time Mass Spectrometric Data Enables Species Identification of Psychoactive Plants from Their Headspace Chemical Signatures, ACS Omega, 2019, 4, 15636–15644 CrossRef CAS PubMed.
- M. Busman, E. Roberts, R. H. Proctor and C. M. Maragos, Volatile Organic Compound Profile Fingerprints Using DART-MS Shows Species-Specific Patterns in Fusarium Mycotoxin Producing Fungi, J. Fungi, 2022, 8, 3 CrossRef CAS PubMed.
- O. Lawal, W. M. Ahmed, T. M. E. Nijsen, R. Goodacre and S. J. Fowler, Exhaled breath analysis: a review of ‘breath-taking’ methods for off-line analysis, Metabolomics, 2017, 13, 110 CrossRef PubMed.
- R. Joshi, S. Sharma and D. Kumar, Advances of Ion Mobility Platform for Plant Metabolomics, Crit. Rev. Anal. Chem., 2022, 1–17 CrossRef PubMed.
- A. B. Kanu, P. Dwivedi, M. Tam, L. Matz and H. H. Hill, Ion mobility-mass spectrometry, J. Mass Spectrom., 2008, 43, 1–22 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |

