Open Access Article
Open Access ArticleThe scent chemistry of butterflies
Stephanie
Ehlers
 and
Stefan
Schulz
and
Stefan
Schulz

Institute of Organic Chemistry, Technische Universität Braunschweig, Hagenring 30, 38106 Braunschweig, Germany. E-mail: stefan.schulz@tu-braunschweig.de
First published on 24th November 2022
Abstract
Covering: 1990 up to 2022 Contrary to popular opinion, butterflies exhibit a rich chemistry and elaborate use of volatile compounds, especially for sexual communication, but also for defence. In contrast to night flying moths, in which commonly females are the producers of pheromones, male scent emission is prevalent in butterflies. While visual signals are generally important for long-range attraction, butterfly scent signals are often active only within a short range. Another feature of butterfly scent chemistry is the wide variety of compounds used, including alkaloids, terpenoids, fatty acid derivatives and aromatic compounds, sometimes with unique structures. This contrasts the strucutrally more restricted pheromone chemistry of moths. In this review, the compounds emitted predominately from male butterflies will be discussed and their ecological function explained, if known. The review includes material from 1990 to date, but will also cover older material to provide a necessary background.
1 Introduction
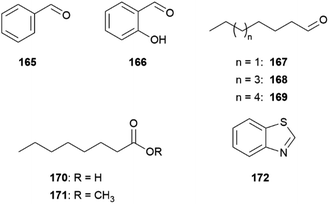
The Lepidoptera, butterflies and moths, are one of the most species-diverse orders of insects and are intensively studied for their chemical communication. Most of the studies are centred on night-flying moth, due to their importance as pest insects. Obviously, chemical communication is of primary importance for an insect flying at night. Therefore, sex pheromones of thousands of moth species have been identified. They usually attract males to females over larger distances, making them preferred tools for pest control in agriculture. Chemically, moth sex pheromones often belong to the Type I class, long-chain aliphatic compounds with 8 to 20 carbons, featuring double bonds at specific positions and with specific geometries, as well as a terminal alcohol, aldehyde, or acetate groups.1 The second largest group, Type II, comprises polyunsaturated hydrocarbons, derived from linolenic or related polyunsaturated fatty acids, as well as epoxide derivatives, and the third group consists of the remaining compounds. The structures of moth pheromones, have been repeatedly reviewed1,2 and a free database, Pherobase, exists where pheromone structures can be researched.3In contrast, butterflies seemingly do not need to use pheromones, because their visual signals are so obvious. Nevertheless, mostly male butterflies have developed a multitude of diverse scent-disseminating structures in many families.4 The compounds used are structurally more diverse than those of moths, originating from various biosynthetic pathways used by the butterflies or can be taken up from plants and modified. Their function can range from attraction to repellence, or function as defence, but butterfly pheromones are often used in short range during courtship. This makes testing compounds in ethological assays often quite difficult, so the actual function of many of the identified compounds is still unknown. A useful technique to access detection of volatiles by a receiving insect is electroantennography, meaning measuring the potential of an insect antennae or even single cells during chemical stimulation. When used together with GC in GC/EAD-setups, nerve impulses of complex mixtures can be recorded; however, this method gives no information of a potential behavioural or physiological response of the receiver. Nevertheless, the volatiles discussed here may have functions beyond pheromonal or signal activity. Physiological effects, e.g. by compounds transferred during courtship, might be induced, or interaction with microorganisms might be affected,5 but knowledge on these phenomena is currently scarce.
In this article, we focus on volatile compounds which are used by various species of butterflies of different families. The difficult phylogeny of butterflies has recently been revised,6 leading to some differences compared to earlier conventions. The family Danaidae was downgraded throughout the years to become now the tribe Danaini. We follow this modern phylogeny here, discussing compounds according to their biological origin. The different families, subfamilies, tribes and subtribes that are covered can be found in Fig. 1. The article sections are organized following roughly a historic perspective rather than the strict phylogenetic order for better understanding of the chemistry involved.
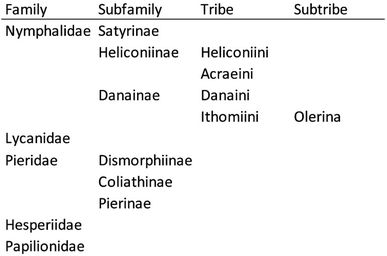 | ||
| Fig. 1 Phylogenetic relationship of butterflies featured in the article according to Espeland et al.6 | ||
Some remarks may be necessary on the term volatile with respect to signals in insects. Even relatively large compounds can be perceived by some insects through the gas phase, e.g. pentacosane, that attracts bumble bees from 1 m distance. This indicates that the term volatile includes many compounds not traditionally regarded as being volatile.
Throughout the years the methods for identifying butterfly natural products changed. While in the early phase isolation and NMR analysis dominated, requiring relatively large number of individuals, more modern methods include GC/MS analysis, microderivatization techniques and synthesis of the candidate, due to the usually low amount of material used, but also using metabolomic techniques. The respective identification techniques used can be found in the original literature; the synthesis of some compounds will be mentioned.
While a wide range of compounds, mostly produced by males, in contrast to moths, will be discussed, it might be helpful to the reader to compare these within the phylogeny. Therefore, compounds were colour coded in the figures with small dots. The colour code indicates occurrence only in a single genus ( ), tribe (
), tribe ( ), subfamily (
), subfamily ( ), or family (
), or family ( ).
).
2 Danaini
The Danaini, the milkweed butterflies, are a tribe of the subfamily Danainae of the Nymphalidae.6 The males usually contain two types of scent glands, one of which is abdominal expandable brushes, so called hairpencils (HP), which are often used in hovering flights near to the female antennae to transport courtship pheromones.7 Some species also use pheromone transfer particles, a form of dust produced by the HP, to transmit the chemicals. On the forewings a second type of scent organs can be found, androconia, which are male specific scent glands on the wings (Fig. 2). The butterflies need to bring the HP into contact with the androconia to produce courtship pheromones,8 leading to the prediction of biosynthetic enzymes located in the androconia, although this has not yet been investigated. | ||
| Fig. 2 Expanded hairpencils and wing androconia of Tirumala petiverana. Reprinted by permission from John Wiley and Sons: Biologie in unserer Zeit, Pharmakophagie: Drogen, Sex und Schmetterlinge, M. Boppré, Copyright (2005).9 | ||
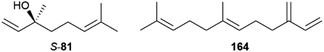
The courtship behavior of the queen butterfly, Danaus chrysippus, has been investigated in detail in a landmark publication by Brower et al.10 The major component of the courtship pheromone of many species is danaidone (1), the first butterfly pheromone identified, from Lycorea ceres.11–13 Later, the related compounds danaidal (2) and hydroxydanaidal (3) were identified. Compound 3 is relatively widespread and has been detected in several genera such as Amauris, Tirumala, Euploea, Ideopsis, Parantica and Danaus,14–19 while 2 is found only in Danaus and Tirumala.16–20 The stereochemistry of 3 has been investigated in arctiid moths, where it is always (R).21 A detailed account of the early work on the occurrence of these compounds and some related dihydropyrrolizines in butterflies has been published.21 The dihydropyrrolizines are not restricted to danaines, but occur as well in ithomiines, another danaid tribe, and in arctiid moths, used similarly as courtship pheromones (Fig. 3).22
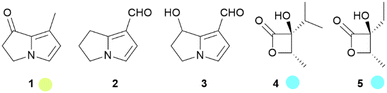 | ||
Fig. 3 Pyrrolizidine alkaloid derived dihydropyrrolizines and β-lactones. The colour code indicates occurrence only in a single tribe ( ) or subfamily ( ) or subfamily ( ). ). | ||
The dihydropyrrolizines 1–3 are biosynthetically derived from pyrrolizidine alkaloids (PAs).23 Adult danaine males actively search for PAs, which can be found on withering plants.24 The males then transfer the alkaloid onto the females during copulation, which in turn protects her eggs with PAs.25 This transfer of male PAs into female eggs was mostly investigated in the arctiid moth Utetheisa ornatrix, which is easier to keep in the laboratory, but has also been demonstrated to exist in the queen butterfly, Danaus gilippus.25 The females chose their mates according to the expected amount of PA, signalled by the pheromone concentration, obtained during mating. This ensures a high protection rate, because not every male is successful in PA sequestration. The whole PA-dependent pheromone system is shown schematically in Fig. 4 that also is similarly operative in ithomiine butterflies and some arctiid moths.25,26
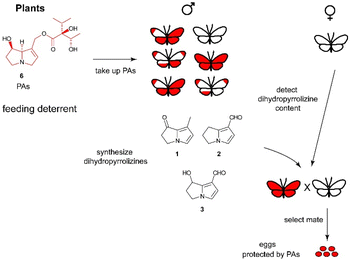 | ||
| Fig. 4 Function of dihydropyrrolizines as courtship signal and indicator of PA load in danaines. Females obtain PAs from males, thus securing energy resources for other tasks. | ||
The biosynthesis of 3, the most widespread dihydropyrrolizine, has been investigated in males of the arctiid moth Creatonotos transiens.23 A PA such as lycopsamine (6) is first cleaved into the free necine base retronecine (7). This base is then oxidized to the highly reactive diol 8, which is oxidized to form hydroxydanaidal (3). As indicated in Fig. 5, 3 is the first relatively stable and sufficiently volatile compound in the reaction sequence, while all others are either too basic or too reactive to serve as a pheromone.26
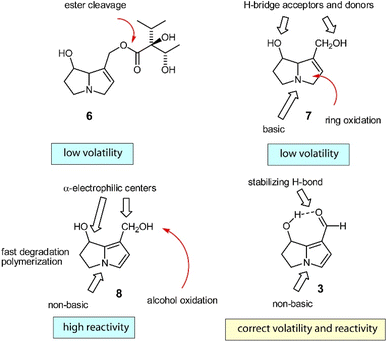 | ||
| Fig. 5 Biosynthesis of hydroxydanaidal (3). Red arrows indicate metabolic changes. Reprinted by permission from Oxford Publishing Limited: Tiger Moths and Woolly Bears Behavior, Ecology, and Evolution of the Arctiidae, W. E. Conner, Copyright (2009).26 | ||
Danaidone (1) has been synthesized originally by addition of methylpyrrol to acrylnitrile, followed by acidic cyclization,13 but this approach has been improved.27 Retronecine (7) can be obtained by hydrolysis of commercially available PAs such as monocrotaline. A subsequent oxidation with MnO2 delivers 3.28 Although most danaines contain dihydropyrrolizines in their HP, the Monarch butterfly (Danaus chrysippus) is a notable exception, even though adults readily visit PA containing plants as food source.29
The major components of the Monarch's hairpencil secretion29 are (1R*,3S*,6R*)-1,3,7,7-tetramethyl-2-oxabicyclo[4.4.0]dec-9-en-8-one (9),30,31 benzyl hexanoate (30),29 and (2E,6E)-3,7-dimethyldeca-2,6-dienedioic acid (22),29,32 belonging to completely different compound classes. Ketone 9 is a C13-terpenoid and is likely biosynthesized from carotenoids. Related apocarotenoids have been identified in other danaines, such as epoxytetrahydroedulan 10, a major constituent of HP bouquets of various Euploea sp.,30,33 and it has also been found in Amauris hecate.19 Contrary to the dihydropyrrolizines, the presence of 10 is not dependent on the food sources of the adults. Lepidoptera usually take up carotenoids with their food, making it likely that the butterfly apocarotenoids are biosynthesized from carotenoids ingested in the larval stage. Other apocarotenoids such as the putative biosynthetic precursors of 10, dihydroedulans I and II (11 and 12), were found as minor components in danaines,19 but are also known from plants. Nevertheless, none of the apocarotenoids were detected in the food plants of E. mulciber, supporting a de novo synthesis by the butterflies.33 Compound 10 has been synthesized starting from enantioenriched dihydro-α-ionone, which was epoxidized, reduced, and cyclized to 12, followed by epoxidation.30 An enantioselective synthesis using (S)-2,4,4-trimethyl-2-cyclohexen-1-ol as starting material, was obtained by a lipase-mediated asymmetric hydrolysis. Chain elongation, diol formation, and cyclization delivered 12, that was stereoselectively oxidized using a halohydrin intermediate.34
Another unique type of danaine HP compounds are bishomo-monoterpenes, or maybe more accurately, trisnorsesquiterpenes. The male Monarch contains the hydroxy acid 21 and the diacid 22,29,32,35 while the Queen butterfly, Danaus gilippus, produces the related diol 20 (Fig. 6).36 These compounds might be synthesized by the butterflies from farnesol (29) by oxidative loss of the terminal isopropyl-group.37Euploea sylvester HPs contain the related compounds 23–25 showing a nerolidol-type head group.14 The terminal dioxygenated motif common to these compounds is also found in related monoterpenes such as 8-hydroxy-6,7-dihydrogeraniol (26) and 8-oxogeranial (27) of Danaus chrysippus.23,38 Because 20 was not able to elicit response in electroantennographic recordings,39 in contrast to 1, it was postulated to serve as a glue for pheromone transfer particles of this species, enhancing longevity of the compound contact with the female antenna.12,39
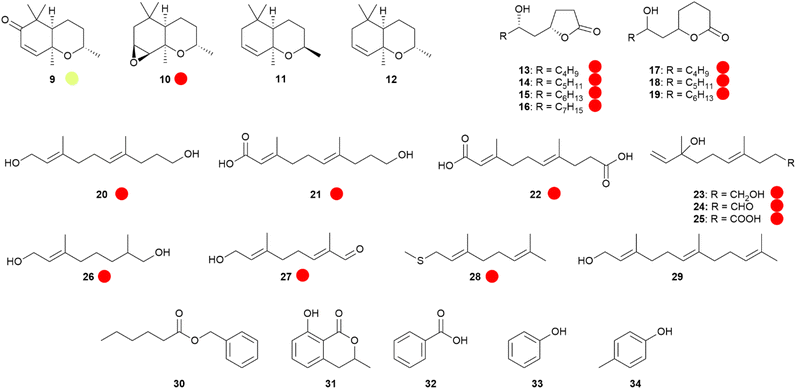 | ||
Fig. 6 Apocarotenoids and other terpenoids. Lactones and aromatic compounds of danaine hairpencils. The colour code indicates occurrence only in a single genus ( ). ). | ||
Although a detailed analyses of the HP content of ten African danaine species of the genera Amauris, Danaus, and Tirumala showed the presence of 214 compounds in species specific mixtures with up to 59 components,19 only 1 has a demonstrated function as aphrodisiac pheromone. This is largely due to the difficulty in performing bioassays with danaines, needing usually spacious flight cages for observation of behavior. Nevertheless, research on Idea leuconoe indicated that a larger share of the HP compounds are indeed active as semiochemicals. Semiochemicals comprise not only pheromones, which are active within a species, but also compounds that are used for interspecific chemical communication.
The analysis of the two types of HPs of I. leuconoe revealed the presence of 1, but also of virdiflorine β-lactone (4) and its analogue 5.40–42 The latter are derived from the respective necic acids, occurring in PAs ingested this time during the larval stage43 or modified by the butterfly.44 Lactone 4 is also produced by Euploea mulciber.33 In this species the males are only attracted to monoester PAs with correct necic base (7R)-stereochemistry such as intermedine or lycopsamine (6), but avoid diesters or (7S)-PAs like heliotrine.33
A blend of 15 compounds including geranyl thiomethyl ether (28), (E,E)-farnesol (29), the hydroxy-γ-lactones 13–16, phenol (33), p-cresol (34), benzoic acid (32), (Z)-9-tricosene, as well as 1 and 4 elicited strong searching as well as acceptance behavior by older females when applied to simple butterfly paper models.40 This response was similar to that of an HP extract. Reducing the blend to just the PA derivatives 1 and 4 as well as 28 resulted in a weaker response, indicating a lower efficacy. Danaidone (1) alone induced the weakest response. All compounds elicited responses in EAG experiments in both males and females, thus forming a pheromone bouquet.40 These results indicate that the many compounds found on HPs play an important semiochemical role and are behaviourally active, acting at least as pheromonal synergists.
The thiomethyl ether 29 is one of the rare cases of an insect pheromone component containing sulphur. It is so far only found in I. leuconoe, as are the hydroxy-γ-lactones 13-16. These lactones, and similar hydroxy-δ-lactones, occur as minor components, and were synthesized using enantioselective reductions of 4,6-or 5,7-dioxoalkanoates by Noyori's Ru-BINAP catalysts.42,45 The absolute configuration of these lactones in the HP has been determined.42,45 While the major hydroxy-γ-lactone 6-hydroxy-4-undecanolide (14) exclusively occurs as the (4S,6S)-enantiomer,45 surprisingly all four enantiomers of the hydroxy-δ-lactones are present in the HP. For example, 7-hydroxy-5-dodecanolide (18) is found as a 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 anti (5S,7S/5R,7R 7
8 anti (5S,7S/5R,7R 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3): syn (racemic)-mixture. These values differ for the congeners 17 and 19.42 In summary, the enzymes responsible for the lactone biosynthesis seem to be tuned to the formation of the γ-lactones, while the δ-lactones are side products with lower stereoselectivity within the required biosynthetic transformations.
3): syn (racemic)-mixture. These values differ for the congeners 17 and 19.42 In summary, the enzymes responsible for the lactone biosynthesis seem to be tuned to the formation of the γ-lactones, while the δ-lactones are side products with lower stereoselectivity within the required biosynthetic transformations.
While the concentration of compounds 1, 4, 14 and (Z)-9-tricosene in the HPs increased with age, the presence of the fungal metabolite mellein (31) depended on access to itself. Mellein (31) serves as phagostimulant for I. leuconoe, as does methyl p-hydroxybenzoate.46 Both compounds are phenols, as are the HP compounds 33 and 34.
The extensive research on I. leuconoe showed that a variety of compounds can have a behaviour-modifying effect on the butterflies. These compounds originate from a variety of sources, some are taken up during feeding or pharmacophagously (1, 4, 31) and were modified, while others are synthesized by the butterfly using the fatty acid (14) or terpene biosynthetic pathways (28).
Larger lactones are found in HPs of the African Amauris niavius.47 The two major components of the secretion, niaviolide (35) and epoxyniaviolide (36), were the first natural macrocyclic sesquiterpenes (Fig. 7). They represent terminally oxidized sesquiterpenes that are cyclized. Generally, lactones are well suited to serve as volatile signals, because a putative hydroxyacid precursor with low volatility can be stored and then converted into the much more volatile lactone by simple esterification when needed.47,48 Both lactones were synthesized starting from methyl farnesenoate by terminal SeO2 oxidation and ring closure with or without a prior Sharpless epoxidation.47 Biosynthetically, similarly to 10, a terminal epoxidation is used to produce a singular specific danaine compound. Other major HP compounds are again 1 and 3,4-dimethoxyacetophenone (50).19,49
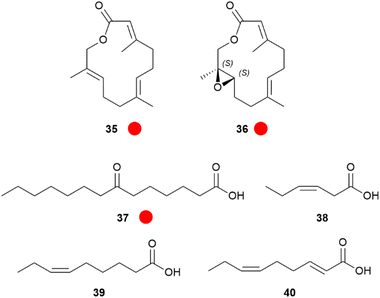 | ||
Fig. 7 Hairpencil components of Amauris butterflies. The colour code indicates occurrence only in a single genus ( ). ). | ||
Common fatty acids such as palmitic, stearic, oleic, linoleic or linolenic acids are often found in HP extracts, mostly in low concentration. It remains unclear whether they are true glandular constituents or degradation products liberated from lipids during sample preparation. Nevertheless, some Amauris spp. contain unique fatty acids. A whole range of 63 fatty acids ranging from hexanoic acid to docosatrienoic acid were identified in A. echeria, hecate, albimaculata,50 and ochlea HP extracts.50,51 Of special interest were several oxidized acids of A. echeria including (E)-7-oxo-11-tetradecanoic acid (37), as major constituent, accompanied by the (Z)-isomer, saturated and shorter analogues. A. ochlea HPs contained predominately hexanoic, (Z)-3-hexenoic (38), nonanoic, (Z)-6-nonenoic (39), as well as minor amounts of (2E,6Z)-nona-2,6-dienoic acids (40).50
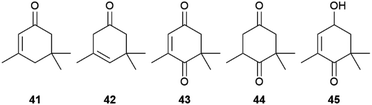
Next to these acids, 1, 3, and several other apocarotenoids were identified in A. echeria, but of the C9-type instead of the C13-type discussed above. Major components were oxoisophorone (43) and its hydrogenated analogue 44, as well as the parent phorones 41 and 42 (Fig. 8). The same apocarotenoids were also present in Euploea sylvester, with the reduced 45 as major component.50 Interestingly, 43 is also the major volatile attracting butterflies to the invasive butterfly bush Buddleja davidii,52 indicating a close relationship between flower attractants and butterfly signalling compounds.
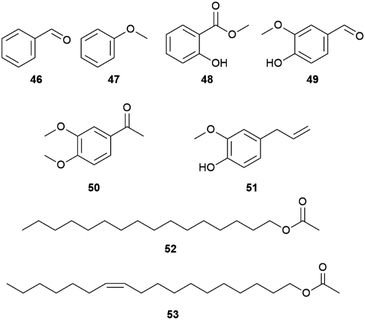
Several aromatic compounds such as vanillin (49), eugenol (51), methyl salicylate (48), benzaldehyde (46) or anisole (46) are frequently found in danaine HPs, but not always in the same species (Fig. 9). While a compound can be a major component of a HP compound bouquet, it may be a minor component in another species.50 A comparative analysis of African milkweed butterflies including 10 species of the genera Danaus, Tirumala and Amauris listed 214 identified compounds,19 including non-volatile compounds such as long-chain 2,5-dialkyltetrahydrofurans (THFs).53 The latter are typical constituents of epicuticular lipids of sun-exposed Lepidoptera.54 THFs occur together with long-chain linear alkanes and alkenes in the epicuticle of insects. Nevertheless, some species such as A. niavius contain large amounts of still volatile shorter hydrocarbons such as heneicosane and heneicosene.19 Such compounds are known as pheromones from some pierids (see pierid section).
Of special interest is also the occurrence of linear terminal esters, hexadecyl (52) and vaccenyl acetates (53) that, together with 1, represent the major HP constituents of Lycorea ceres ceres, a phylogenetically basal danaine.55 The similarity to the type I moth pheromones, linear aliphatic unsaturated alcohols, acetates or aldehydes,1 indicate these compounds to be a primal trait that seems to be lost in more recent species. Interestingly, the Δ11-desaturase needed for the formation of 53 is the most important desaturase for moth pheromone biosynthesis.56
The composition of the HP secretion of danaines have been used for a cladistics analysis of the phylogeny of the danaines, using 68 volatile compounds, but excluding dihydropyrrolizines.57 A combination with morphological data revealed better phylogenetic solutions for African milkweed butterflies than purely morphological methods, but genetic methods were not available at that time. Nevertheless, the results indicate a phylogenetic dependence of the HP composition.
3 Ithomiini
The ithomiines, also known as glass-wing butterflies due to their sometimes transparent wings, are a sister subfamily of the danaines, occurring with high species diversity in Central and South America. In contrast to the danaines, the ithomiines lack abdominal scent brushes, but feature instead erectable hairs on the wings that are used to disseminate volatile compounds during courtship (Fig. 10). | ||
| Fig. 10 Male Oleria onega janarilla with erected scent brushes (androconia). The picture is reproduced from Beilstein Journal of Organic Chemistry, F. Mann et al., under a CC-BY-4.0 licence.58 | ||
As with the danaines, males are attracted to PAs,24,59 feeding on them to protect themselves and to convert them into aphrodisiac pheromones, as with the danaines. Lycopsamine (6) is the primary PA stored in ithomiines,60 and PAs with different side chain stereochemistry are converted into 6, at least in Mechanitis polymnia.61 Ithomiines also use PA-derivatives such as danaidal (2) and hydroxydanaidal (3) in their androconia.54 Thirteen genera of 30 investigated contained these derivatives.54 While 1 was not found, an ithomiine specific major compound, present in seven genera, is methyl hydroxydanaidoate (54).14,54 Both 3 and 54 usually dominate the androconial secretion, but minor amounts of the oxidized or methylated derivatives 55–60 were present in few species.54 The necic acid derived β-viridiflorine lactone (4), known from some danaines, was detected in Melinaea menophilus and Scada kusa.54 Unique for ithomiines is the γ-lactone ithomiolide A (61).14,62 Its absolute configuration has been established by stereoselective synthesis starting from (S)-3-methylbutyrolactone, α-ethenylidenation and ketol formation, followed by reduction to the target diol. Trace compounds of ithomiolide B (62) were also detected, as well as the acylated metabolite 63.54,63 The stereochemistry of 61 is identical to that of (−)-viridifloric acid, the necic acid of 6.63 Therefore, the PA content of the male butterflies is adjusted to give a large pool of the proper alkaloid 6, which is in turn in part transformed into the various dihydropyrrolizine or lactone compounds, as shown in Fig. 11. Interestingly, 61 was detected in eleven genera, all of them belonging to a clade of more advanced genera.54 A comparison of the data using a recent phylogeny supports the initial hypothesis.64 Ithomiolides are formed within a clade containing the subtribes Ithomiina, Napeogenina, Dircennina, and Godyridina, although in the Oleriina no volatile dihydropyrrolizines or lactones were detected at all.65
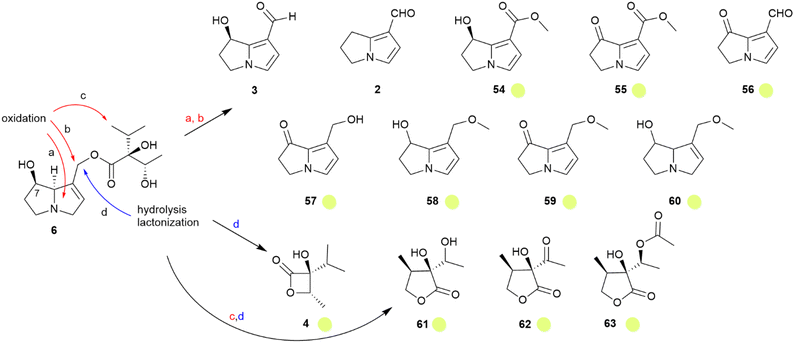 | ||
Fig. 11 Transformations of lycopsamine (6) into volatile dehydropyrrolizines and necic acid lactones, adopted from Schulz et al.54 The colour code indicates occurrence only in tribe ( ). Reprinted by permission from Elsevier: Biochemical Systematics and Ecology, Semiochemicals derived from pyrrolizidine alkaloids in male ithomiine butterflies (Lepidoptera: Nymphalidae: Ithomiinae), S. Schulz, Copyright (2004).54 ). Reprinted by permission from Elsevier: Biochemical Systematics and Ecology, Semiochemicals derived from pyrrolizidine alkaloids in male ithomiine butterflies (Lepidoptera: Nymphalidae: Ithomiinae), S. Schulz, Copyright (2004).54 | ||
The formation of dihydropyrrolizines from PA requires various biosynthetic transformations, mostly oxidative in nature (Fig. 11). While dihydropyrrolizine biosynthesis has been discussed within the danaine section, the formation of ithomiolides is unique. Their biosynthesis requires an oxidation of an unactivated methyl group (c in Fig. 11) because no PA with terminally oxidized isopropyl groups have been reported from food plants. It seems likely that the advanced ithomiines use a more specific signal such as 61, generated by this oxidative transformation. This avoids confusion with widely used dihydropyrrolizines such as hydroxydanaidal (3). The latter compound has been claimed to be formed not only by Lepidoptera, but also by degradation of PA-containing plants, attracting various Lepidoptera over longer distances.66
The absence of PA derivatives in Oleriina, including the genera Oleria and Hyposcada, has been repeatedly shown, leading to a detailed analysis of Oleria onega.67 In the androconia only the alkaloid 4 was detected, accompanied by a quite unstable degradation product, dehydropyrrolizine 64. This compound was synthesized from carboxymethylpyrrolizine by reduction and elimination.67 Compound 64 is likely a degradation product formed within the injection port of the gas chromatograph, but formation during PA degradation occurring in plants cannot be ruled out. Nevertheless, major volatile compounds of the androconia proved to be the acids 9-hydroxynonanoic (65) and (Z)-9-hydroxy-6-nonenoic (66) acids, as well as 2-heptadecanol (67) and 6,10,14-trimethyl-2-pentadecanol (68) (Fig. 12).
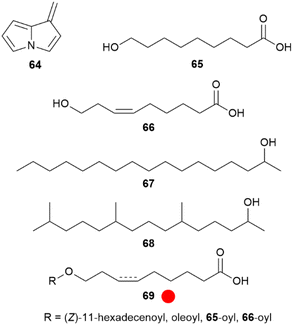 | ||
Fig. 12 Compounds of androconia of Oleria onega. The colour code indicates occurrence only in a single genus ( ). ). | ||
Although not volatile, esters (69) of both 65 and 66 with predominately (Z)-11-hexadecenoic acid constitute a major part of the secretion, maybe serving as a fixative, or as storage or a slow release form of the two acids. Similarly, acids of type 69 can also be formed by conjugation between acids 65 and 66 themselves.67 Such ester acids are not reported from other organisms. Compounds 65, 66, and esters 69 were synthesized using classic alkyne or Wittig approaches, proper silyl-protection and esterification using EDC.67
Another type of compound was detected in Ithomia salapia, prenyl esters. While 61 was identified in I. s. derasa, the subspecies I. s. aquinia contained the sesquiterpene α-elemol (70).58 Some side products indicate that potentially hedycaryol (71) is the actual sesquiterpene present that undergoes Cope rearrangement in the heating block of the GC/MS system during injection.58 Nevertheless, rearrangement induced by heat or sun within the androconia cannot be excluded. The difference in the composition of the volatile compounds may explain the low gene flow between the two species even in regions where they co-occur.58
Major constituents of the androconia are a group of 3-methyl-3-butenyl (isoprenyl) esters of fatty acids (Fig. 13).58 These fatty acids comprise unsaturated compounds with ω-7 and ω-9- double bonds, as well as 3-hydroxy and 3-acetoxy acids. The absolute configuration of the major parent acid, (Z)-3-hydroxy-13-octedecenoic acid was determined to be (R).58 Surprisingly, most of the esters (72–80 are shown here as examples), occur as a mixture of two isomers differing in the position of the double bond, e.g. isoprenyl (Z)-3-acetoxy-11-octadecenoate (76) and isoprenyl (Z)-3-acetoxy-13-octadecenoate (77). The formation of these isomers can be explained by activity of both a Δ9-and a Δ11-desaturase, likely introducing the double bonds after fatty acid biosynthesis. The Δ11-desaturase is the typical enzyme used in moth sex pheromone biosynthesis.68 Although the specific function of compounds 72–80 are not clear, an involvement in signalling of Ithomia salapia is highly likely, due to the unique structures and involved enzymes.
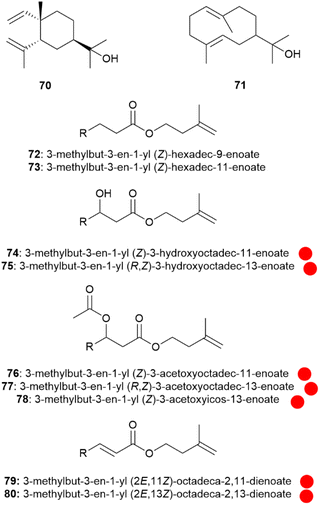 | ||
Fig. 13 Terpenoids and isoprenyl esters of Ithomia salapia. The colour code indicates occurrence only in a single genus ( ). ). | ||
4 Heliconiinae
The Heliconiini, the passion-vine butterflies, are a tribe of the subfamily Heliconiinae of the Nymphalidae.6 The genus Heliconius is particularly known for its high species diversity and participation in Müllerian mimicry and therefore serves as a model for evolutionary studies.69,70 The males exhibit two types of scent glands, abdominal clasper scent glands (CSG, Fig. 14D), which are used to transfer antiaphrodisiacs (AA) together with a complex mixture of compounds during copulation to the abdominal pouch of the female (Fig. 14C). The AA lead to a reduced attractiveness of the female for courtship with other males and ensuring undisturbed egg-laying, although additional functions may also be possible, e.g. defensive display.71–73 The other male specific scent glands are the androconia, which are specialised brush-liked scales in the overlapping region of the fore and hind wing with a distinct greyish sheen (Fig. 14B).74,75 The androconial scales release volatiles that might act as pheromones during courtship by androconial exposure, which occurs in every courtship that results in mating, and they are involved in female mate choice.74,76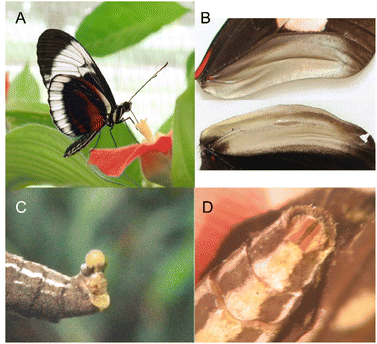 | ||
| Fig. 14 Heliconius cydno (A),77 expanded view of male forewing and hindwing androconial region (B),75 female abdominal gland (C) and male clasper scent glands (D) of H. charitonia.78 The picture 14A is reproduced from Ecology and Evolution, K. J. R. P. Byers et al.,77 and picture 14B from Peer Journal, K. Darragh et al.,75 under a CC-BY-4.0 licence. Pictures 14C and 14D are reprinted by permission from John Wiley and Sons: Evolution, Sexual selection drives the evolution of antiaphrodisiac pheromones in butterflies, C. Estrada et al., Copyright (2011).78 | ||
4.1 Clasper scent glands
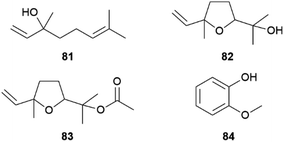
Heliconius butterflies are divided into two clades, one is polyandrous (58%) where females mate with several males within a breeding season, while the other is monoandrous and displays a unique mating behaviour known as pupal-mating.79 In such species males search for immatures, guard them and wait until they are able to mate with the emerging female.74,80 Important for this pre-copulatory mate guarding is the ability of males to recognize immature conspecific females and estimate their maturity.81H. charithonia males can recognize female pupae using sex specific volatile monoterpenes, which are released towards the end of pupal development.80 While the main components of the pupal cuticle are typical cuticular lipids such as saturated hydrocarbons or 2,5-dialkyltetrahydrofurans, in late pupae some monoterpenes could be observed by SPME analysis of living pupae. Whereas the monoterpene linalool (81) exclusively occurs in late male pupae, linalool oxide (furanoid, 82) was released by late female pupae. Together with linalool oxide acetate (83), present in both sexes, these monoterpenes were specific for late pupae (Fig. 15). Bioassays showed that males are attracted to female specific 82, while they are repelled by male specific 81, the former occurring as a mixture of all four stereoisomers in the female pupae.80 Other, nonspecific volatiles emitted by early stages of the pupae were cyclohexanol and its acetate, α-pinene, and 11.80Both 81 and 82 are common semiochemicals released by flowers or leaves, e.g. in response to insect feeding.82 In combination with other floral volatiles and visual signals they elicit feeding responses in Heliconius butterflies.83 The attractive or repellent activity of linalool (81) seems to be context-dependent, as similarly found for other signalling compounds in this genus.72,80 A terpene synthase producing 81 was characterized in another species, H. melpomene, although this species does not show a pupal-mating trait.84
The courtship behaviour of Heliconius butterflies comprise complex and diverse behavioural acts involving the use of multiple signals at different phases in mate recognition and choice. Males are first attracted by colour wing pattern serving as a mating cue; in short-range other signals such as pheromones are likely to mediate recognition and mating.76,85–88 In species involved in mimicry complexes, recognition is likely primarily based on pheromones in co-mimics, where visual signals alone are not sufficient to identify conspecifics.86,89–92 Sympatric species with the most similar colour pattern also seem to have developed the most divergent and conspicuous courtship rituals.76 It is likely that the CSG and androconial extracts, and their specific composition, play different roles at different stages of courtship and may influence reproductive success and isolation between species.75,88 In contrast to the general pattern observed in Lepidoptera, the chemical signature between Heliconius species, even sister pairs, is often different. Nevertheless, while mimetic pairs contain different scent bouquets, they also have some compounds in common, whereas sympatric species, mimetic and non-mimetic, have more distinct compound compositions.88,93,94
The use of AAs in Heliconius was initially postulated by Gilbert95 and except in the Pieridae and the moth Heliothis virescens,96 no other use of AA in Lepidoptera has been reported.76,83,97–99 While AA are found in a wide range of insects, chemical structures have only been identified for a few species.72,96 In H. melpomene, behavioural test showed (E)-β-ocimene (85) to act as an AA, effectively repelling males when applied to unmated females.72,78 A reliable method for the synthesis of pure (E)-β-ocimene (85) has been developed using a Wittig-reaction as the key step.100 The CSG of H. melpomene also contains a less volatile matrix of C16-C18 fatty acid esters of ethanol, 2-propanol, 1-butanol, isobutanol, 1-hexanol and (Z)-3-hexenol that moderates the evaporation rate of 85.72 By contrast, freshly emerged males and females lacked volatile compounds, except for guaiacol (84), and only C16- and C18-fatty acids together with cuticular compounds such as hydrocarbons, cholesterol, and squalene. Not until five days after eclosion, had males developed the whole bouquet of compounds.72 Because male Heliconius do not become sexually active until several days after eclosion, the lack of these compounds in females and younger males supports a role in mating behaviour.75
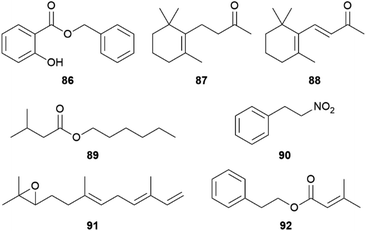
The AA pheromones vary both qualitatively and quantitatively between Heliconius species. (E)-β-Ocimene (85) can also be found in distantly related Heliconius species. In H. erato85 is found in the CSG at around one-tenth the amount of H. melpomene, while it is absent in H. cydno, a closely related species to H. melpomene.72,78,90,101 It is a very common plant volatile, which is found in large amounts in flowers on which adult H. melpomene feed, and elicits a strong electroantennographic antennal response in both males and females. As discussed for 81, 85 seems to have two context-dependent functions, the attraction to plants and the repulsion from mated females.72,102 While an additional uptake of 85 through feeding cannot be ruled out, feeding experiments with freshly eclosed males with isotopically labelled D-13C6-glucose have shown that H. melpomene is able to synthesize 85de novo, and that it is transferred onto females during mating. Glucose can be converted into doubly labelled acetate via the citric acid cycle and enters the mevalonate pathway of terpene synthesis.72 Combining gene identification through QTL (quantitative trait locus) mapping, heterologous gene expression and functional analyses, Darragh et al.84 were able to identify the monoterpene synthase HmelOS which catalyses the conversion of geranyl pyrophosphate (GPP) to 85 in H. melpomene (Fig. 16). HmelOS is unrelated to previously described plant and insect terpene synthases and is the first described 85 synthase in animals. Although the sister species H. cydno possess an orthologous enzyme HcydOS, no evidence for its activity as a terpene synthase was found, leading to absence of 85 in this species.84 Other AAs are hexyl 3-methylbutanoate (89) in H. cydno and benzyl salicylate (86) in H. charithonia (Fig. 17).72,78,103
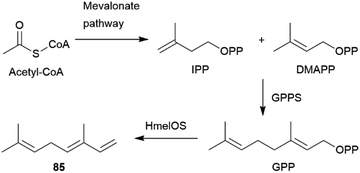 | ||
| Fig. 16 Biosynthesis of (E)-β-ocimene (85) in Heliconius based on Darragh et al.84 | ||
In general, the composition of CSG in Heliconius varies considerably among species, in composition and number of compounds, comprising species-specific compound mixtures. These mixtures can include esters, lactones, terpenes, alcohols, ketones, aromatic compounds and can contain up to 350 components.70,72,73,78,87,90,94,104 For both androconial and CSG chemical profiles most variation is explained by species identity rather than geographic location, which could be important for chemical mimicry and species recognition.90 Estrada et al.78 have shown that pairs of species belonging to the pupal mating clade have a more similar pheromone composition to each other than pairs of species in the non-pupal mating clade, where the chemical composition has diverged faster under the influence of sexual selection. This is also observable for the major volatile compounds of the CGS of different Heliconius species.
The AA compositions of the non-pupal mating clade diverged between sister species and closely related species, whereas in the pupal-mating clade the major compounds appear to be conserved across more divergent species. In H. charithonia, 86 is the single major volatile compound while in H. sara, 86 is dominant together with (Z)-3-hexenyl decadienoate, with unknown locations of the double bonds. Also H. hewitsoni and H. sapho share the presence of 86 together with dihydro-β-ionone (87) albeit in H. sapho (Z)-3-hexenyl decadienoate is also a major compound. (E)-β-Ocimene (85) is shared between the pupal mater H. erato, where also a whole range of methyl, ethyl, hexyl, (Z)-3-hexenyl and other esters of dihydrofarnesoic acid are present,73 and the non-pupal mater H. melpomene, H. numata and H. hecale. In H. ismenius (E)-α-ionone (88) is the major volatile and H. cydno and H. pachinus share the presence of hexyl 3-methylbutanoate (89).78
Although compounds usually occur in several species, compounds which are strong indicators of species identity can be found, as 3-oxooctyl and (Z)-3-hexenyl (S,E)-2,3-dihydrofarnesoate (94 and 95)73 for H. erato, high concentrations of 85 for H. melpomene or eicosenol in H. elevatus. But also the occurrence of more than one compound can be specific for a species, e.g. in H. timareta the presence of butyl oleate (96) and (Z)-9-octadecen-13-olide (105) or (Z)-3-hexenyl isobutyrate (97) together with an unknown compound in H. sapho (Fig. 18).90
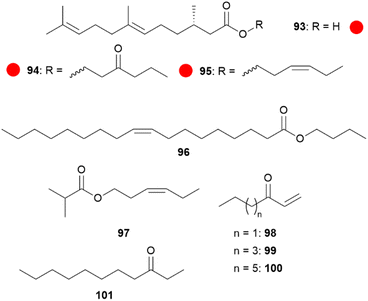 | ||
Fig. 18 Species-specific CSG compounds in Heliconius. The colour code indicates occurrence only in a single genus ( ). ). | ||
Dihydrofarnesoates have been synthesized through esterification of the respective alcohol with (S,E)-2,3-dihydrofarnesoic acid, obtained from (2E,6E)-farnesol (29) by enantioselective hydrogenation using Noyori's method, followed by Parikh–Döring and Pinnick–Lindgren oxidations.73
An interesting compound class present in some CSGs of Heliconius species are macrolides (Fig. 19). Phyllisolide (102) was the first macrolide pheromone identified in Heliconius with an AA function. Even with its very low concentration in the CSG of H. erato phyllis, it still elicits the strongest GC-EAD response next to the other active compounds 1-hexen-3-one (98), 1-octen-3-one (99), 85, 3-undecanone (101) and (E)-2,3-dihydrofarnesoic acid (93) in the male antenna. This derivative of geranylacetone (173) is biochemically related to ferrulactone (152) and brassicalactone (153), which are pheromones of the Pieridae family (see below) and also degraded terpenoids.105,106 Synthetically, 102 has been obtained from (E)-geranylacetone (173) with a Riley oxidation followed by an enantio- and stereoselective hydrogenation, and subsequent Jones oxidation and esterification from the corresponding acetal. A reduction to the respective alcohol and enantioselective enzymatic acetylation followed by basic hydrolysis and final macrolactonization delivered the natural stereoisomer 102.105 Another species where macrolides are abundant is H. pachinus and its very close relative H. cydno, which are inter-fertile.
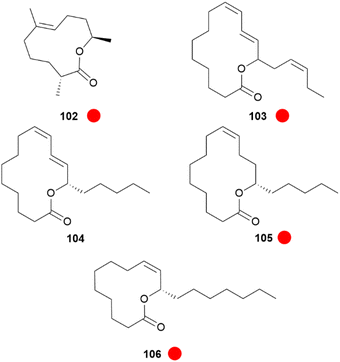 | ||
Fig. 19 Macrolides present in the CSG of Heliconius. The colour code indicates occurrence only in a single genus ( ). ). | ||
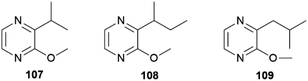
In both sexes fatty acid derived macrolides can be found in the CSGs. The macrolides present are the 14-membered lactones (9Z,11E,15Z)-octadeca-9,11,15-trien-13-olide (103), (S)-coriolide (104), (9Z,13S)-octadec-9-en-13-olide (105) and the 12-membered lactone (9Z,11S)-octadec-9-en-11-olide (106). Considering the markedly lower concentrations in females and absence in younger male adults and pupae, these lactones might be similarly used as AAs, but there is no evidence yet.70,104 The macrolide 104, which was previously found in the seed oil of Monnina emarginata107 and the fungus Stagonospora,108 had been reported before104 for male H. pachinus as (R)-104. Synthetically, 104 has been obtained through enzymatic oxidation of linoleic acid, followed by a reduction and final macrolactonisation.109 These lactones are likely formed by lipoxygenases acting at linolenic, linoleic and oleic acids, followed by macrolactonization.70 In addition to courtship signals, warning odours can also be found in the CSG of Heliconius, namely pyrazines. Pyrazines act as general warning odours of chemically defended insects and are known to deter rats and birds.110,111 In H. melpomene 3-isopropyl-2-methoxypyrazine (107), 3-sec-butyl-2-methoxypyrazine (108) and 3-isobutyl-2-methoxypyrazine (109) are present,72 while in the subspecies H. m. malleti only 107 can be found (Fig. 20). The co-mimic species H. timareta florencia contains 108.88 Pyrazine 109 is also found in Actinote pelleria of the tribe Acraeini of the Heliconiinae.111
A thorough analysis of the CSG contents of 115 individuals of H. erato belonging to different mimicry groups revealed that the they show a mimicry group specific distribution of volatiles and also other compounds.73 Therefore, mimicry groups cannot only be assigned due to wing patterns, but also due to the composition of the CSGs. The most abundant volatiles within the CSG were 101 and 3-nonanone, 85, the vinyl ketones 98, 99, and 100, 2-nitroethylbenzene (90), 31, 89, benzyl cyanide (162), and some other compounds including 2-phenylethyl 3-methyl-2-butenoate (92).73 The CSG compounds are generally recruited via different biosynthetic pathways, such as terpene and fatty acid pathways, but include also derivatives of primary metabolites or apocarotenoids, and aromatics. A key enzyme activity seems to be ester ligation, because esters in open or cyclic form are a dominant class of CSG compounds of the Heliconiinae.
Dione vanillae (formerly Agraulis vanillae),112 belongs to one of the most primitive genus of the Heliconiini.113 Both sexes possess abdominal scent glands which are quickly everted when a butterfly is captured or disturbed. The abdominal gland composition is identical in males and females, with sulcatone (110) as the major volatile (Fig. 21). Sulcatone (110) is known as a defensive allomone of ants and cockroaches, widespread in nature, and is rejected by birds.113,114 Other constituents of the scent bouquet are the sulcatyl fatty acid esters 111–113, 1,15-pentadecanediyl (114) and 1,16-hexadecanediyl (115) diacetates.113 As adults of this species are designated as prey items by invertebrate and vertebrate predators, the bouquet seem to exhibit a protective function,113 especially the most volatile compound 110.
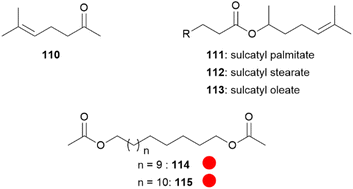 | ||
Fig. 21 Compounds present in Dione vanillae. The colour code indicates occurrence only in a single genus ( ). ). | ||
4.2 Androconia
Different behavioural assays have demonstrated the importance of androconial chemical signals in Heliconius mating behaviour.75,85,87–89 The hindwing androconia emit lower amounts of less complex bouquets that is usually chemically different from that of the CSG. The compound bouquets are species specific94 and influences mating behaviour.75,87 The majority of these compounds are fatty acid-derived compounds including aldehydes, alcohols, acetates or esters preferentially with a C18 and C20 chain, together with some alkanes.94 One third are considered to originate from the phenylpropanoid pathway, active in plants, taking up during feeding.77,115 All double bonds of fatty acid derivatives in Heliconius are located at the ω-9 position, pointing to an active Δ9-desaturase, contrasting to the Δ11-desaturase present in Bicyclus (see below).68,94 The high chemical diversity of the bouquet can be achieved by activating or deactivating enzymes/genes responsible for certain biosynthetic transformations, as a general model for the biosynthesis proposed (Fig. 22).116 For instance in H. melpomene an alcohol oxidase acting on saturated alcohols furnishing aldehydes seems to be active, which is inactive in H. cydno.94 Subtle changes in a few enzyme activities therefore might result in different scent gland mixtures, leading to differences between species, subspecies or even individuals.94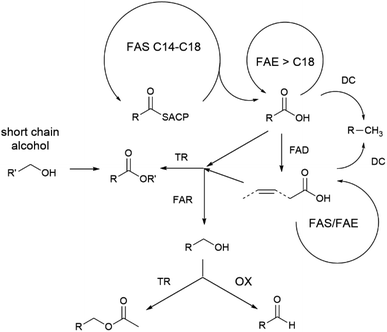 | ||
| Fig. 22 Proposed biosynthetic pathways to different fatty acid metabolites used by Heliconius adapted from Mann et al.94 FAS fatty acid synthase; DC decarboxylase; FAD fatty acid desaturase; TR transferase; FAR fatty acid reductase; FAE fatty acid elongase; OC oxidase; ACP acyl carrier protein. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Journal of Chemical Ecology, The scent chemistry of Heliconius Wing Androconia, F. Mann et al., Copyright (2017).94 | ||
As with the CSG, in most species there are single androconial compounds that are strong indicators for species identity (Fig. 23). For H. eleuchia it is hexahydrofarnesyl acetone (117), homovanillyl alcohol (118) in H. elevatus, octadecanal (116) for H. melpomene, methyl 4-hydroxy-3-methoxybenzoate (120) in H. sapho, and 5-decanolide (121) for H. timareta.90
Octadecanal (116), which is mostly absent in other Heliconius species,87,117 serves as a volatile courtship signal. It is a common pheromone gland component and has been tested in eight species of Lepidoptera across a variety of families including Heliconius, but not all showed activity.88,106,117,118 Electrophysiology and behavioural experiments demonstrated 116 as a biologically active pheromone component involved in mating decisions in H. melpomene and, surprisingly also in H. cydno, although it is not produced by the latter species.77,117
In species recognition trials females discriminated against conspecific males with blocked pheromone transmission, suggesting that females are actively involved in mating decisions.75 This would be in line with the species-specific androconial secretion.87,90,94 Likewise H. erato can distinguish their own wings from those of their H. melpomene mimic, but not when the wings were washed with hexane.85 Also the co-mimic species H. melpomene and H. timareta florencia in Colombia display strong reproductive isolation. The bouquet of H. t. florencia consisting of heneicosane, syringaldehyde and limonene (150) contrasts with those of H. melpomene dominated by 116.88 Even in species pairs that differ in their color pattern, the total level of premating sexual isolation perceived in experimental conditions are higher (97%) than isolation considering male-driven color choice only (75%),119 implying contribution of nonvisual signals, like chemical scents to species specific mate selection.87,91
A survey of 33 of the 69 species of the Heliconiini confirmed species-specific androconial bouquets, especially in Heliconius, indicating a role in reproductive isolation within this tribe. The fatty acid metabolic pathway was recruited by more advanced genera to arrive at more complex blends, while basal lineages are dependent on plant metabolites such as 117–120, or geranylgeranyl acetate (123) of H. erato and H. himera.120 The fatty acid derived compounds include mostly compounds similar to type I moth pheromones compounds such as 116 and hexadecanal (132) of H. aoede, 1-hexadecanol (124), (Z)-9-octadecen-1-ol (125) of H. xanthocles, (Z)-11-eicosen-1-ol (126) of H. atthis, (Z)-13-docoscen-1-ol (127) of Eueides tales, (Z)-11-eicosenyl acetate (128) of E. aliphera, but also alkanes such as heneicosane, tricosane, and (Z)-9-tricosene (129) of H. charithonia, as well as (3Z,6Z,9Z)-3,6,9-henicosatriene (130) of H. demeter, a typical type II moth pheromone (Fig. 24).120H. erato is an exception, lacking any fatty acid metabolites. The androconia contain small amounts of geranylgeranylacetone (119),94 but the major component is terminally oxidized 119 conjugated to fatty acids, which is non-volatile.
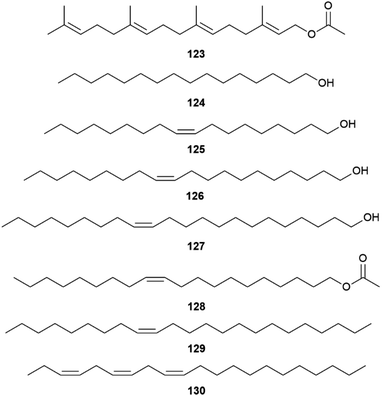 | ||
| Fig. 24 Fatty acid metabolites of androconia of various Helconiini and geranylgeranyl acetate (123). | ||
The Acraeini are another tribe of the subfamily Heliconiinae, which is highly paraphyletic, involving the genus Cethosia, the lacewing butterflies. In the dimorphic species Cethosia cyane cyane males actively chase females during courtship using only visual signals to distinguish gender, which is possible due to their sex-specific wing colouring.121 Nevertheless, behavioural assays showed that olfactory signals also assist at close range for accurate identification. SPME analysis of living males and females showed little differences in produced compounds, only cedrol (122) was exclusively detected in females (Fig. 23). Despite the fact that heneicosane and linalool oxide (82) also show EAG activity, only responses to 122 were significantly different between females and males. Behavioural assays proved the participation of 122 in mate recognition of females at close range.121
5 Satyrinae
The Satyrinae, the browns, are a subfamily of the Nymphalidae. These butterflies generally consume plants lacking secondary compounds and due to this they are mostly palatable, and lack the typical aposematism of other butterflies.122Bicyclus, an African genus from the tribe Elymniini, is the best studied butterfly genus with regard to sexual, including olfactory, communication.123–127Bicyclus represents a clade where multiple species live mostly in sympatry and display a diversity of visual signals.128 Males of this genus are primarily differentiated on the basis of the location and number of male scent glands (androconia) and modified hair-like scales covering these glands, present at the hind- and forewings (Fig. 25).129,130 The modified hair-like scales are presumably assisting in spreading male scent and therefore are defining a diversification of the androconia, which might indicate the importance of scent during speciation and divergence within the genus.127 The use of species-specific male pheromones have been proven.130 The chemicals emitted by male androconia, received in the primary sensory organs on the antennae, are important in female choice and are involved in male–male recognition.127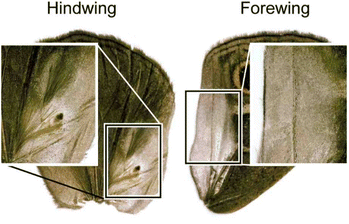 | ||
| Fig. 25 Wing androconia of Bicyclus anynana with patch of hairs.131 The picture is reproduced from Plos One, C. M. Nieberding et al., under an unrestricted CC licence. | ||
Pheromone transfer likely happens during flickering and thrust, where the androconial hairs fan out and become clearly visible.129,131 The existence of AA has not been reported and is not suggested based on butterfly behaviour.123 Chemical and visual signals seem to be equally important in female mate choice, and are most likely involved in mate quality assessment.127,132,133
Bicyclus anynana produces a close-range male sex pheromone (MSP) blend in the androconia consisting of the GC-EAD active and sex-specific compounds (Z)-9-tetradecenol (131), hexadecanal (132) and (2R,6R,10R)-2,6,10-trimethylpentadecan-2-ol (R,R,R-68, Fig. 26). The biosynthesis of these compounds begins after adult emergence and is associated with the androconia. While 131 and 68 were found to be mainly produced in the forewing androconia, 132 is only produced in the hindwing together with minor amounts of 68, whereas the hair-like scales are not involved in pheromone production.131
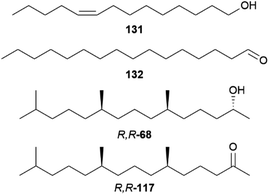 | ||
| Fig. 26 Male androconial sex pheromone blend of Bicyclus anynana (131, 132, R,R,R-68) and ketone R,R-117. | ||
Hedenström et al. were able to verify through derivatization methods that the stereochemistry of 68 and the corresponding ketone 117 is always (2R,6R,10R)-68 and (6R,10R)-117 for different Bicyclus species.125 This indicates that 68 and 117 are related to substances found in the butterfly or larval diet, determining the stereochemistry. The biosynthesis of 117 has been investigated in the pierid butterfly Pieris brassicae, showing plant-derived (E)-phytol (154) with a natural all-R-configuration as a precursor (see below).125,134 Through implementation of an asymmetric synthetic strategy, 68 and 117 can be obtained stereoselectively starting from sulcatone (110) and citronellic acid with a lipase-catalyzed resolution as a key step.125 Alcohol 68 is a well-known sex pheromone of pest insects, such as the rice moth, Corcyra cephalonica, the African sugar-cane borer moth, Eldana saccharina, and the oil palm bunch moth, Tirathaba mundella, highlighting the widespread occurrence of this compound among Lepidoptera.135 On the other hand, 131 and 132 display type I moth pheromone structures.1,131 Liénard et al. showed that 131 and 132 are produced de novo in B. anynana via a biosynthetic pathway shared between females of many moth species, providing the first evidence of conservation and sharing of ancestral genetic modules for the production of fatty acid-derived pheromones over a long evolutionary timeframe.68 Both compounds derive from palmitic acid, a known essential precursor for female moth pheromone biosynthesis.68,136 For the production of 131 palmitic acid firstly undergoes a desaturation towards (Z-11)-hexadecenoic acid involving a fatty acyl Δ11-desaturase, followed by one cycle of chain shortening via β-oxidation to (Z)-9-tetradecenoic acid, and finalized through a reduction to the corresponding alcohol 131 (Fig. 27).68 Aldehyde 132 is synthesized from palmitic acid in two steps over a reduction to 124, followed by oxidation to the aldehyde.68
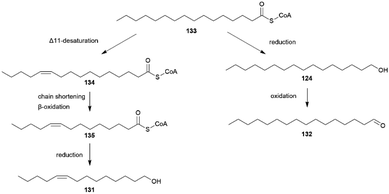 | ||
| Fig. 27 Biosynthesis of fatty acid derived pheromones 131 and 132, adapted from Liénard et al.68 | ||
Among all compounds of the scent organs identified in Bicyclus, saturated and unsaturated fatty alcohols, aldehydes, esters and hydrocarbons account for almost two thirds, suggesting a close biosynthetic relationship.130 The MSP composition was found to be a reliable indicator of male identity, level of inbreeding, as well as age.132,133 MSP ratios also vary in individuals and could contain information on male individuality and provide the opportunity for interindividual recognition.132 Headspace analyses showed that males can actively control MSP emission and adjust to an increased male–male competition.137 Other effects such as phenotypic plasticity in MSP production,124 geographical variation, likely driving reproductive isolation and speciation,138 reproductive character displacement MSP composition of different Bicyclus species,123 learning of visual signals in mate choice,139 and plastic female preferences for a male pheromone odour blend influenced by early pheromone odour experiences140 have been studied.
A more precise function of the MSP compounds 131, 132 and 68, as well as their relative importance in B. anynana are disputed as laboratory experiments are not always comparable to natural conditions.141 A comparison of wing extraction and headspace sampling methods underlined the importance and differences of sampling methods.137 While wing extracts only reflect stored compounds, it does not necessarily represent the compounds actually emitted during courtship. Whereas headspace analysis maybe better at representing the released scent, although valid for a confined space, it is not known if it is comparable to what a female receives.137 Nevertheless, EAG recordings at least underline the possibility of the detection of released compounds. The MSP ratios obtained for B. anynana with both methods differed significantly.137 Although this discussion was centred on the results obtained from B. anynana, it is true for all kinds of analysis of volatile, behaviour-mediating compounds.
In B. martius sanaos some novel wing-specific esters were identified, not present in females, representing possible pheromone candidates.130 In male wing extracts ethyl, isobutyl and 2-phenylethyl hexadecanoates (136a–c) and (Z)-11-hexadecenoates (137a–c) as well as ethyl benzoate, 2-phenylethyl tetradecanoate and 2-phenylethyl octadecenoate were found, some already known from Heliconius (Fig. 28).70,72,130 Wang et al. showed that incorporation of D-labelled amino and fatty acids into the associated esters only take place in patches of the forewing, indicating that only the forewings contain active biosynthetic enzymes responsible for ester synthesis.130
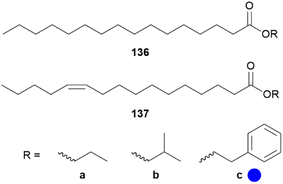 | ||
Fig. 28 Wing-specific ester of androconia of Bicylcus martius sanaos. The colour code indicates occurrence only in a single family ( ). ). | ||
The suggested biosynthesis proposed that the required alcohols can be synthesized de novo starting from L-alanine, L-valine and L-phenylalanine after a sequential process of transamination, decarboxylation, and reduction. An active Δ11-desaturase, previously reported in B. anynana, transforms hexadecanoic acid into hexadecenoic acid, both acids being then enzymatically esterified with the respective alcohols.68,130 This biosynthetic route seems to be of widespread use within the butterflies, found also in Heliconiini, Ithomiini, or pierids (see Fig. 22).
During courtship non-random gustatory contacts also occur between specific male and female body parts.142 As described for Pieris rapae, alkanes and methyl-branched alkanes build the majority of cuticular compounds together with aldehydes, alcohols, ketones and acids.142,143 As the composition is associated with body parts, some compounds may potentially provide additional fine-scaled information, complementing already furnished information by male sex pheromones.142
A different species of Elymniini, Elymnias thryallis, found in Papua New Guinea, possess a scent gland at the end of the abdomen which secretes a fluid when disturbed, maybe acting as a deterrent against predators or as a courtship pheromone. The main compound of the secretion was found to be the monoterpene elymniafuran (138) as well as trace dehydrated compounds i.e. (E)- and (Z)-4-methyl-2-(2-methyl-1,3-butadienyl)furan (139), which are not found in other natural sources (Fig. 29).144 Elymniafuran (138) has been synthesized using an enantioselective vinyl-Grignard addition under Taddol catalysis as a key step.144
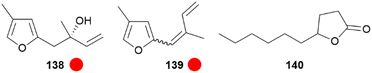 | ||
Fig. 29 Scent constituents of Elymnias thryallis and Lethe marginalis. The colour code indicates occurrence only in a single genus ( ). ). | ||
In the subtribe Lethina, males of the species Lethe marginalis possess a faintly sweet wing scent, whereas females are scentless. The male scent secretion consisted mainly of γ-deltalactone (63) and some tridecane, without a known function.145 With 80 ng present in the forewing and 73 ng in the hindwing, 63 was only found in small quantities compared to scent substances of some other butterflies.145,146
The use of an electronic nose in the investigation of volatile organic compounds (VOCs) during courtship behaviour of two Hipparchia species indicated a role for chemical signals in mate and species recognition during early stages of interaction.147 While the two sexes of H. fagi displayed a similar VOCs pattern, it is significantly diverged in H. hermione. VOCs patterns between females of the two species were different, but those of males were not. The structures of the compounds responsible for this variation have not been evaluated so far.147
6 Pieridae
The Pieridae are a family of medium-sized butterflies, comprising the subfamilies Dismorphiinae, Pierinae, Coliadinae and Pseudopontiinae. This family is monophyletic and mating plugs are found in all subfamilies except Dismorphiinae.6,148 Most males display male-specific androconia. These scent gland patches are oval and flaky and arranged in dense clusters of scales concentrated in the area where the fore- and the hindwings overlap, without protection to prevent evaporation except overlapping of wings when at rest.149–151 While Dismorphiinae and Coliadinae predominantly feed on Fabaceae, Pierinae were able to specialize on Brassicaceae due to the evolution of a nitrile-specifier protein (NSP) that detoxifies prevalent glucosinolate defences and allowing the evolution of a much more diverse subfamily than its sister clades.1526.1 Dismorphiinae
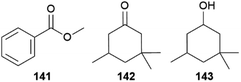
Leptidea, a cryptic complex of butterflies, is a genus of the mimic sulphurs and includes several palearctic species.153,154 The sister species Leptidea sinapis and Leptidea reali can only be distinguished using genital preparation or DNA sequencing but females nonetheless only accept conspecific males.155 During their courtship males sit opposite to the females while oscillating their proboscis in front of them. Females can signal mating acceptance by making their abdomen accessible for male copulation attempts, thereby likely disseminating an aphrodisiac pheromone. Males will give up after some time,153,155,156 leading to a quite unique long-lasting courtship, which can take up to 11 min until females accept mating, implying a longer recognition time of conspecifics.155SPME analysis of both sexes of L. reali and L. sinapis support the use of species-specific volatiles in species recognition.155 While both sexes emit methyl salicylate (48), limonene (150) and 1-octanol, (E)-β-ocimene (85) seems to be a female specific volatile. Males additionally emit methyl benzoate (141), detected in 90% of L. reali and 30% of L. sinapis individuals. In about half of L. sinapis both sexes also emit dihydroisophorone (142) and cyclonol (143, Fig. 30).155 Only when the additional volatiles 142 and 143 are emitted by female L. sinapis, L. reali males can distinguish between the species and abort courtship, while the absence is leading to a longer giving-up time. The additional volatiles 142 and 143 facilitate the mating decision for females of L. sinapis, together with species-specific wing beats of males, while L. reali females must be sure about the absence of these volatiles and wing beats, explaining the higher variance in time to mate.155
6.2 Coliadinae
In the subfamily Coliadinae, the sulphurs, not all species feature androconia but often other secretory organs can be found, e.g. Phoebis argante males also display additional hair-pencils below the androconial patch (Fig. 31).150,157 The first pheromones were described for the genus Colias.150,158,159 In the secondary sympatric sister species Colias philodice and Colias eurytheme, male-specific secretory cells can be found on the hind wing158,160 that have showed qualitative compositional differences.159 | ||
| Fig. 31 Male dorsal wing color patterns of Colias-clade butterflies with androconia details (circles to the right) of (a) Anteos clorinde and (b) Phoebis argante. The white arrow indicates a tuft of hair-like scales.150 Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Organisms Diversity & Evolution, Specialized androconial scales conceal species-specific semiochemicals of sympatric sulphur butterflies (Lepidoptera: Pieridae: Coliadinae), C. E. B. Nobre et al., Copyright (2021).150 | ||
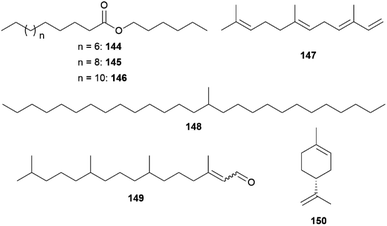
Male C. philodice produce the fatty acid hexyl esters 144–146, representing their sex pheromone, while 13-methylheptacosane (148), specific for C. eurytheme, together with heptacosane and nonacosane, a sex-pheromone with a low-to-moderate aphrodisiac activity (Fig. 32).159,161 In comparison, female wings of both species only feature straight-chain hydrocarbons, which are also present in males in comparable quantities.159 Despite their low volatility 148 and the esters 144–146 exhibit significant electrophysiological activity and display olfactory signals for mating.159 While females of C. philodice rely solely on olfactory signals to select conspecific males,159 the specific UV iridescence of male wings seem to be more important for mating success than pheromones in C. eurytheme and allows females to avoid heterospecific matings.162,163
In C. eurytheme pheromones also seem to be implicated in intra-specific mate choices since the UV reflectance and pheromone traits covary with age.162 While hydrocarbons are commonly found in epicuticular wax layers of insects,161 the amount of 148 varies greatly among individuals.162 The individual blend of C. eurytheme males stabilize after 12 h post-eclosion and 148 is depleted over time, whereas heptacosane increases. In this case, the low volatility of components ensures the slow depletion over the lifetime of the male but requires very close range aerial detection by females or even contact.161
The distant clade C. erate poliographus also displays a sexual dimorphism in composition of epicuticular substances. The secretory organs, which are more abundant in the forewings, display a male-specific mixture, comprising of predominantly 145, small amounts of 144 and 146, octadecanal (116), and hexadecenoic and octadecanoic acids. Surprisingly, 148 was also found as a minor component in both sexes, and 145 and 116 were also present in females in lower concentrations.157 Identical to C. philodice, C. e. poliographus seem to use hexyl esters as an aphrodisiac pheromone.157,159 Age-dependent changes in the sex pheromone components indicating sexual maturation.132,157 Octadecanal (116), 148 and hexyl esters 144-146 are increasing during days 1–7 after eclosion, whereas other linear alkanes have already reached a plateau at day 3.157
In other closely related non-mimetic Coliadini species-specific compositions of wing components were detected, suggesting use of intraspecific chemical communication.150 The androconial blends of the co-occurring species Anteos clorinde, A. menippe, Phoebis argante, P. philea, P. marcellina, and P. statira revealed only a few dominant, autapomorphic compounds. Prevalent compounds across species are benzyl salicylate (86) in A. menippe and P. marcellina as well as a hexadecenoic acid with unknown double bond location in P. statira and P. marcellina. In general, higher similarities were found among males of Anteos compared to Phoebis, where only linoleic acid is shared between congenerics.150 The most characteristic compounds in the androconial blends were benzyl salicylate (86) and hexadecanoic and hexadecenoic acids in P. marcellina, 6,10,14-trimethylpentadecan-2-ol (68) in P. argante, limonene (150) in P. marcellina, as well as (E,E)-α-farnesene (147) in P. marcellina and A. menippe.150
Males of Eurema mandarina, the common grass yellow, of the tribe Euremini utilize male aphrodisiac sex pheromones released by androconia, inducing female acceptance.164 Solvent extract of wings revealed hexahydrofarnesyl acetone (117) and (E/Z)-phytal (149, E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z 4
Z 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as male specific compounds of the forewings (Fig. 32). The concentration of 117 is highest in the androconia where 149 is absent and is only detected in the anal cell, lacking androconia and intramembranous cells. While most other compounds are shared by both sexes, the oxygenated terpenoids 117 and 149 only occur in much smaller amounts in females than in males.164 Bioassays with virgin females demonstrated that 117 and 149 act synergistically as aphrodisiac sex pheromones, evoking mate acceptance in one-third of females. The response is weaker than with males, hinting at potentially other involved compounds. One compound alone elicits only little response in females.164 As only responses were triggered when females were approached and touched by males, the signal acts in short-range or on contact.158,159,164 As in Colias, the concentration of both 117 and 149 increases with age, implying there to be a representation of sexual maturity.161,164
1) as male specific compounds of the forewings (Fig. 32). The concentration of 117 is highest in the androconia where 149 is absent and is only detected in the anal cell, lacking androconia and intramembranous cells. While most other compounds are shared by both sexes, the oxygenated terpenoids 117 and 149 only occur in much smaller amounts in females than in males.164 Bioassays with virgin females demonstrated that 117 and 149 act synergistically as aphrodisiac sex pheromones, evoking mate acceptance in one-third of females. The response is weaker than with males, hinting at potentially other involved compounds. One compound alone elicits only little response in females.164 As only responses were triggered when females were approached and touched by males, the signal acts in short-range or on contact.158,159,164 As in Colias, the concentration of both 117 and 149 increases with age, implying there to be a representation of sexual maturity.161,164
6.3 Pierinae
In the subfamily Pierinae, the whites, adults commonly possess androconia, also called scent scales, on the dorsal wing surface, but the morphology can sometimes differ within genera and species.157,165 The later stage of courtship, when females display a characteristic mate-refusal posture with spread wings and vertically lifted abdomen before eventually accepting mating, is assumed to be predominantly influenced by chemical signals.4,166,167 Within this subfamily, in the tribe Pierini, males are known to use aphrodisiac pheromones and also to transmit antiaphrodisiacs with the spermatophore to females.97,99 In Pieris napi males passively release citral (151), a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of geranial and neral (Fig. 33). In EAG assays females were 10x more sensitive to 151 than males and the amount produced could influence mating success. Together with behavioural tests this proved that 151 acts as a courtship sex pheromone, necessary for successful courtship.168,169 Furthermore, it allows males to assess the density of competing males in the vicinity.170 Isomers of 151 are differently detected by glomeruli in the antennal lobe, but signals from both are needed for a behavioural response.171
1 mixture of geranial and neral (Fig. 33). In EAG assays females were 10x more sensitive to 151 than males and the amount produced could influence mating success. Together with behavioural tests this proved that 151 acts as a courtship sex pheromone, necessary for successful courtship.168,169 Furthermore, it allows males to assess the density of competing males in the vicinity.170 Isomers of 151 are differently detected by glomeruli in the antennal lobe, but signals from both are needed for a behavioural response.171
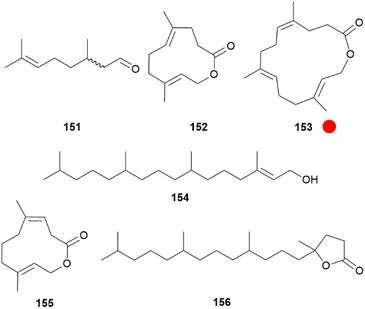 | ||
Fig. 33 Aphrodisiac compounds found in male Pieris species. The colour code indicates occurrence only in a single genus ( ). ). | ||
Similarly, P. rapae and P. brassicae use aphrodisiac blends including two male specific macrolactones originating from their wing scent scales located over the entire wing area.106,172 In the small white P. rapae ferrulactone (152) was identified, previously known as a pheromone of the Rusty Grain beetle, Cryptolestes ferrugineus.173 In the large white P. brassicae the related, but one isoprene unit bigger brassicalactone (153) is present. Both compounds reach a maximum concentration about ten days after emergence of adults.106 Although GC-EAD experiments showed that 152 can be detected by both sexes of this species, 153 was only detected by female P. brassicae.106 Other major components found in wing extracts are hexahydrofarnesyl acetone (117) in P. rapae and P. brassicae, and (E)-phytol (154), which is in P. rapae only a minor compound together with indole (19). The terpenoids 117 and 154 were also found in the respective female extracts but in lower amounts.100,106 Mating-competition bioassays with an artificial aphrodisiac blend of 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 154 (21
154 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for P. rapae and 153
1) for P. rapae and 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 154 (16
154 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31) for P. brassicae showed an markedly enhancement in mating success for males with painted wings.106 In contrast to P. napi, where the aphrodisiac compound is emitted more passively, in P. rapae in vivo monitoring using confined direct analysis in real time mass spectrometry (cDART-MS) of males indicated a rapid release during wing opening which seems to be triggered by a visual cue of a female together with other information, and only slightly passive emissions.174
31) for P. brassicae showed an markedly enhancement in mating success for males with painted wings.106 In contrast to P. napi, where the aphrodisiac compound is emitted more passively, in P. rapae in vivo monitoring using confined direct analysis in real time mass spectrometry (cDART-MS) of males indicated a rapid release during wing opening which seems to be triggered by a visual cue of a female together with other information, and only slightly passive emissions.174
In addition to these compounds, a plethora of about 100 components were detected on the wings, consisting predominately of long-chain cuticular alkanes, secondary alcohols, diols and ketones, but also of more volatile compounds such as β-ionone, dihydroactinidiolide, decanal (169) or suspensolide (155), a isomer of 152 and a volatile of the Mexican fruit fly, Anastrepha suspensa (Fig. 33).175 Electroantennographic experiments showed that not only the discussed pheromone components, but also other wing compounds are detected by the two species in a sex- and/or species specific manner. These include benzyl cyanide (162), tetradecanal, alcohol 68, 149, 1-hexadecanol, 1-octadecanol, as well as the hydrocarbons pentadecane, heneicosane, tricosane, 7-methyltricosane, tetracosane, pentacosane, heptacosane, nonacosane, triacontane as well as a mixture of mid-chain oxidized secondary alcohols, hepta- and octacosanols, as well as the terpenoid lactone 4,8,12,16-tetramethylheptadecan-4-olide (156).100 These results and those discussed with Colias show that even compounds such as octacosanols or triacontanes can still be perceived as odours by the insects antennae, showing the ambiguity of the term “volatile”. Especially the hydrocarbons and terpenoids have been implicated as pheromones in other pierids. This indicates, that pheromonal communication in pierids is more intricate as described so far, although one does not have to forget that EAG only records an electric signal of the antennae, with no possible differentiation between simple odour receptors and those tuned for pheromone perception.
P. rapae additionally shows a chemical polymorphism of hydrocarbon profiles of the epicuticular wax layer of the antennae and other body parts, which seems to be of functional relevance.143 Branched hydrocarbons that are predominantly found on antennae exhibit a lower melting point than linear ones, which are more dominant on the body, head and wings. The antennae specific compounds could lead to higher water loss, higher fluidity and, maybe more relevant, to higher diffusion rates of compounds trapped from the gas phase.143 Nevertheless, courtship involves antennal contact in many butterflies and specific antennal signals, maybe specific hydrocarbons, might be involved in ensuring courtship success.
The species specificity of the androconial secretions is underlined by the refusal of P. melete males by female P. rapae.176 The allopatric subspecies P. rapae rapae and P. rapae crucivora display a strong premating isolation, although the pheromones 117 and 152 occur in both subspecies, albeit in different cocentrations.151 Females reject males of the other subspecies probably due to the diverging wing compound profiles, which are more different between males compared to females.151 Even though no compounds are exclusively present in one subspecies or only in females, 152, 162, 117, 154 and a phytol derivative are only present in males and appear in statistically higher amounts in P. r. rapae. However, males display identical courtship towards all females, despite females differing dramatically in wing color or UV reflectance, indicating a low stringency in the mate recognition system.151,177
Amongst males of P. melete and P. napi japonica morphological differences are slight and males of both species display very similar wing scent scales profiles containing undecane and the monoterpenes α-pinene (157), β-pinene (158), myrcene (159), p-cymene (160), limonene (150), citral (151) (Fig. 34).178 Species recognition was proposed to rely on the different E/Z ratios of 151 as well as the additional occurrence of linalool (81) in P. n. japonica.178
 | ||
| Fig. 34 Monoterpenes found in wing scent scales and antiaphrodisiacs of male Pieris (48–162) as well as phenol 163 emitted by female Ascia monuste. | ||
During mating, P. napi males transfer methyl salicylate (48) to females via the spermatophore, which acts as an AA (Fig. 34). SPME analysis showed that mated females emit 48, not present in virgin females. Larval feeding experiments with the labelled L-phenylalanine proved that 48 is only produced by males99 and taken up in the adult stage.179 Behavioural assays demonstrated that mated females, but also virgins perfumed with 48, are quickly abandoned by courting males, while females lacking 48 are more attractive.97 Remating can be observed after 4–6 days.167,180 Females are not actively able to control the release of the antiaphrodisiac, but the mate-refusal posture depletes their pheromone stores.168 Similar to the aphrodisiac pheromone 151, 48 is also involved in sperm competition coherence, providing information on female mating history and allowing males to adjust their composite of fertilizing and non-fertilizing sperm. In summary, P. napi is using a very fine-tuned olfactory signalling system.168,181
P. rapae also uses 48 as an antiaphrodisiac of males, but combined with indole (161), which is biosynthetically formed from tryptophan. The general odour of males and females were analysed by SPME, consisting of sulcatone (110), nonanal (168), decanal (169), and other unknown compounds, likely 152, as well as virgin female specific benzyl alcohol.99 In contrast, P. brassicae uses benzyl cyanide (162) as AA, derived from the precursor L-phenylalanine. Benzyl cyanide (162) is also the male general odour, accompanied by two unknown male specific compounds.99 Males cloak their mates with their own odour and turning the females into chemical mimics of males.99 The parasitic wasp Trichogramma brassicae exploits this AA cue of P. brassicae and uses it to ride on a mated female to her egg-laying sites where it then parasitizes the fresh laid eggs.182
Headspace analysis of Ascia monuste showed (E)-β-ocimene (85) as a male-specific compound with a yet unknown function. EAD assays yielded only responses in females while males did not react.183 Females of A. monuste phileta emitted p-methoxyphenol (163) from their genitalia when disturbed, together with fatty acids (C16–C18) and branched hydrocarbons. It probably serves as a defence against predators or acts as a signal in courtship, but it is lacking in males.184
In the tribe Antocharini, less is known about the function of volatile scent scale contents. Ocimene (85) was found to be the only main volatile component in both sexes of Hebomoia glaucippe liukiuensis and H. g. formosana, displaying a higher amount in male wings compared to females.146 EAD assays proved a higher response of male antennae compared to female ones for 85, which also elicited a greater response than the main volatiles of their food plant, Crataeva religios, limonene (150) and myrcene (159).146 Males of Anthocharis scolymus, lacking alar scent scales, exhibit round saccular structures connected to the socket of the ordinary scale in the wing membrane, serving as androconia.165 While males of this species emit a male-specific subtle floral scent consisting of (S)-linalool (S-81) and (E)-β-farnesene (164), together with the minor components 168 and 169, females are scentless (Fig. 35).165 The amount of both terpenes increases in males reaching a plateau with sexual maturation after two days past eclosion. In contrast, the aldehydes reach a constant amount after only four days.165,185 Terpenoids (S)-81 and 164 have not been reported from other pierids, but are widespread plant constituents mediating various plant interactions, and act as insect pheromones.165,186
In summary, the volatile compounds of pierids are predominately hydrocarbons and terpenoids, next to some small amino acid derivatives and carotenoid degradation products. Within the terpenes, oxidatively degraded sesqui- or diterpenoids dominate. P. brassicae can take up (E)-phytol (154) in their diet at the larval stage and transform it into hexahydrofarnesyl acetone (117) by oxidative degradation, demonstrated by feeding experiments with deuterium labelled 154.134 This is in contrast to Orchid bees who can only obtain 117 through uptake from plants.187 Phytol (154) occurs in all plants as a side chain of chlorophyll A that is taken up during larval feeding, degraded during digestion to 154, and finally accumulated in scent scales of male wings, where it gets in a part oxidized to 117.134 Ferrulactone (152) has been shown to be biosynthesized, formed from farnesol by the beetle C. ferrugineus188 through oxidation and cleavage of the terminal double bond. Very likely this is also the case in Pieris, suggesting a similar biosynthesis of brassicalactone (153) from geranylgeraniol.
Most of the pierid compounds are not specific to these butterflies, as they are known from other natural sources, such as the abundant ketone 117. More specific compounds are 153 and 152. The latter can be conveniently synthesized starting from geranyl acetate via 10-hydroxy-4,8-dimethyl-4,8-decadienoic acid, followed by macro-lactonization,106,189 but other methods have also been employed. Various isomers of the larger lactone 153 were synthesized using ring-closing-metathesis as a key step, leading to mixtures of E/Z-isomers that allowed unambiguous establishment of the double bond geometries.106
7 Miscellaneous species
In addition to the already discussed subfamilies of the Nymphalidae, the viceroy butterfly Limenitis archippus of the Limentitidinae is a Müllerian mimic to Danaus plexippus and D. gilippus and sequesters non-volatile defensive compounds, phenolic glycosides from their larval host-plant Salix caroliniana. When disturbed both sexes secrete benzaldehyde (165), benzoic acid (32), methyl salicylate (48) and salicylaldehyde (166) from their abdomen, probably in defence (Fig. 36).190 Apart from 48, these compounds are also chemical defences of willow-feeding leaf beetles against generalist predators.191Less is known about the use of volatile compounds in the family of Hesperiidae. In the species Erynnis montanus, part of the subfamily Pyrginae, males display a characteristic pungent odour which changes within the period of adult occurrence. Whole body extracts revealed docosane and heneicosane as the most abundant compounds in both sexes, likely representing the cuticular hydrocarbons.192 Other constituents are heptanal (167), nonanal (168), decanal (169) and acetic acid, also identified in Papilionidae,193,194 as well as octanoic acid (170, Fig. 36). In moderate quantities, p-cresol (34) is present in males, nonanoic acid in females and benzothiazole (172) in both sexes. The characteristic male compound 34 decreases with time. Both aromatics 34 and 172 have been reported as effective repellents against predatory ants.195
In the subfamily Papilioninae of the family Papilionidae, the swallowtail butterflies, terpenoids have been identified as male-specific volatiles. Males of Papilio machaon hippocrates emit a fragrant smell consisting of linalool (81) and geranylacetone (173) as major components, emanating from their wings. Both sexes respond in EAD assays to 81, limonene (150), nonanal (168) and methyl octanoate (171), the latter being body specific.196 Also in P. polytes and P. protenor demetrius the male scent contain small amounts of 81.194,197 Furthermore, a sexual dimorphism in the volatile composition in adults can be observed, for instance in P. protenor demetrius eight out of seventeen major compounds show significant sex differences.194,197 2,3-Butanediol (174) and 81 are present in significantly larger amounts in males than females, while 167, 168, 48, benzyl alcohol and 32 are shared by both sexes.197 In P. polytes (Z)-7-tricosene (176) and dodecanoic acid are more abundant in males, and (Z)-7-pentacosene (177) and heptacosane in females (Fig. 37).194 All compounds were found to be more concentrated on the wings than the body except for acetoin (175), which evokes a higher response in females in EAD assays.194,196 Males of P. polytes use cuticular hydrocarbons for mate discrimination to distinguish non-mimetic females from conspecific males and sympatric sister species, certain alkenes serving as behavioural inhibitors of male copulation.198
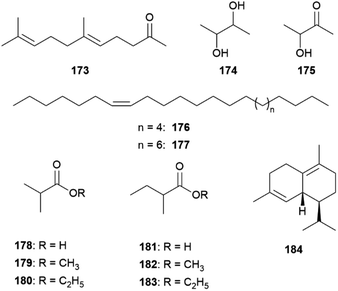 | ||
| Fig. 37 Volatile compounds present in Papilio species (173–175) and osmeterial constituents (178–184) of Papilionidae. | ||
In contrast, adults of both sexes of Luehdorfia japonica (Parnassiinae) display a common odour and are lacking male-specific volatiles. Although most volatile compounds, like 167–169, 165, p-cymene (160), 150 and dodecane have been previously reported in adult scent of Papilioninae, L. japonica exhibits a unique chemical scent composition with limonene (150) being the most abundant compound.199
Additionally, larvae of Papilionidae possess a specialized defensive gland located mid-dorsally behind the head, the osmeterium. This normally invisible gland is extruded when the larvae is irritated and produces a defensive secretion, regarded as defensive allomone.200,201 Species of this family can be separated into two groups. The osmeterial composition of the homogeneous type displays no quantitative alterations between instars,193,202 whereas in the heterogeneous type the last instar differs remarkably from younger larvae, displaying a shift from mono- and sesquiterpenoids to aliphatic acids and their esters.200,203–206,207 Regardless of the group, mono- and sesquiterpene hydrocarbons were found to be biosynthesized de novo from acetate precursors by larvae.208 The heterogeneity of the osmeterial chemistry in the tribe Papilionini is assumed to represent a fine-tuning of the chemical defence in response to shifting predation pressures as the larvae age and grow.203 While terpene secretions discourage attacks by ants, acid production is rendering the caterpillars less palatable.204 In several species of the heterogeneous group the fifth instar secretion was found to contain 2-methylpropanoic acid (178) and 2-methylbutanoic acid (181) as major constituents, together with their methyl (179, 182) and ethyl esters (180, 183, Fig. 37).193,200,202,206,209 Terpenoids, among others α-pinene (157), which was found to be the most toxic terpenoid compound,210 β-myrcene (159), limonene (150) and δ-cadinene (184), appear as main components in different species.193,200,202,205
In the family Lycaenidae alar androconia are frequently observed on wings of many species but there is little knowledge of their scent chemistry.211,212 Males of Lycaeides argyrognomon emit δ-cadinol (185) from their androconial scales, together with 168 and hexadecyl acetate (52). The sesquiterpene alcohol 185 was found to be involved in the inhibition of the mate-refusal behaviour in females.213 In Celastrina argiolus ladonides, however, males emit a faint odour mainly composed of lavender lactone (186) and δ-decalactone (187) from androconial battledore scales, present on the fore- and hindwings (Fig. 38). While for lactone 187 only the R-enantiomer is present, 186 was found as a mixture of the R/S-enantiomers.212 The functions of these lactones are not known yet, but may also serve a similar role as 185 in L. argyrognomon or indicate male identity or age for sexual selection by females.212,213
The sister families Lycaenidae and Riodinidae are also known for their larval ant associations, ranging from mutualist partners to parasites.214 Some myrmecophilous butterfly larvae use chemical mimicry and concealment by chemical camouflage based on cuticular hydrocarbons.215
8 Conclusions
The chemistry of male butterflies is diverse and involves compounds either by de novo biosynthesis, or by uptake from precursors from plants, addressing various biosynthetic pathways, such as fatty acid biosynthesis, terpene and apocarotenoid formation, alkaloid modification, or aromatic compounds. Alongside compounds often or occasionally found in other natural systems, such as plants or bacteria, unique compounds are also present. They serve a multitude of fine-tuned purposes, many of which are still unknown. Some general traits can be observed in the different families discussed here. While Danaini use PA-precursors, but also use a wide variety of other, sometimes unique, compounds, the Ithomiini rely mostly on PA-derivatives with few exceptions. In the Heliconiinae, high variations in compound composition and a large number of metabolites can be observed, often preferring fatty acid derivatives or terpenoids, enabling species radiation and influence by mimicry groups. Esters play a dominant feature here. In Pierids, the compounds are often more simple, again aliphatic esters and simple terpenes, although terminal oxidation can lead to specific compounds such as macrolactones. In Satyrinae, relative simple fatty acid derivatives and esters dominate. In all families degradation products of phytol and chlorophyll, such as 68 and 159, are used.9 Author contributions
Both authors worked together on all aspects of the manuscript.10 Conflicts of interest
There are no conflicts to declare.11 Acknowledgements
We thank the Deutsche Forschungsgemeinschaft for support through grant Schu 984/12-1 and M. Stell for language correction and an anonymous reviewer for helpful comments.12 Notes and references
- T. Ando and M. Yamamoto, J. Pestic. Sci., 2020, 45, 191 CrossRef CAS.
- (a) T. Ando and R. Yamakawa, Nat. Prod. Rep., 2015, 32, 1007 RSC; (b) T. Ando and R. Yamakawa, Trends Anal. Chem., 2011, 30, 990 CrossRef CAS; (c) T. Ando, S. Inomata and M. Yamamoto, in The Chemistry of Pheromones and Other Semiochemicals I, ed. S. Schulz, Springer Berlin Heidelberg, 2004, vol. 239, pp. 51–96 Search PubMed; (d) W. Francke and S. Schulz, in Comprehensive Natural Products Chemistry II, ed. L. N. Mander and H.-W. Liu, Elsevier, Oxford, 2010, vol. 4, pp. 153–223 Search PubMed.
- A. El-Sayed, The Pherobase: Database of Pheromones and Semiochemicals, available at: http://www.pherobase.com/, accessed 4 February 2022 Search PubMed.
- R. I. Vane-Wright and M. Boppré, Philos. Trans. R. Soc., B, 1993, 197 Search PubMed.
- L. Weisskopf, S. Schulz and P. Garbeva, Nat. Rev. Microbiol., 2021, 19, 391 CrossRef CAS PubMed.
- M. Espeland, J. Breinholt, K. R. Willmott, A. D. Warren, R. Vila, E. F. A. Toussaint, S. C. Maunsell, K. Aduse-Poku, G. Talavera, R. Eastwood, M. A. Jarzyna, R. Guralnick, D. J. Lohman, N. E. Pierce and A. Y. Kawahara, Curr. Biol., 2018, 28, 770–778 CrossRef CAS.
- M. Boppré and R. I. Vane-Wright, Zool. J. Linn. Soc., 1989, 97, 101 CrossRef.
- M. Boppré, R. L. Petty, D. Schneider and J. Meinwald, J. Comp. Physiol., 1978, 126, 97 CrossRef.
- M. Boppré, Biol. Unserer Zeit, 1995, 25(1), 8 CrossRef.
- L. P. Brower, J. V. Z. Brower and E. P. Cranston, Zoologica, 1965, 50, 1 CAS.
- J. Meinwald, Y. C. Meinwald, J. W. Wheeler, T. Eisner and L. P. Brower, Science, 1966, 151, 583 CrossRef CAS.
- T. E. Pliske and T. Eisner, Science, 1969, 164, 1170 CrossRef CAS.
- J. Meinwald and Y. C. Meinwald, J. Am. Chem. Soc., 1966, 88, 1305 CrossRef CAS.
- S. Schulz, W. Francke, J. A. Edgar and D. Schneider, Z. Naturforsch., C: Biosci., 1988, 43c, 99 CrossRef.
- (a) J. A. Edgar, Phil. Trans. R. Soc. London, 1975, 272, 467 CAS; (b) J. A. Edgar and C. C. J. Culvenor, Nature, 1974, 248, 614 CrossRef CAS PubMed; (c) J. A. Edgar, C. C. J. Culvenor and G. S. Robinson, J. Aust. Entomol. Soc., 1973, 12, 144 CrossRef; (d) K. Honda, A. Tada and N. Hayashi, Appl. Entomol. Zool., 1995, 30, 471 CrossRef CAS.
- J. A. Edgar, C. C. J. Culvenor and W. L. Smith, Experientia, 1971, 27, 761 CrossRef CAS.
- H. Komae, A. Nishi, N. Hayashi, C. Wesou and Y. Kuwahara, Agric. Biol. Chem., 1983, 47, 157 CAS.
- H. Komae, A. Nishi, T. Tanaka, N. Hayashi, C. Wesou and Y. Kuwahara, Biochem. Syst. Ecol., 1982, 10, 181 CrossRef CAS.
- S. Schulz, M. Boppré and R. I. Vane-Wright, Philos. Trans. R. Soc., B, 1993, 342, 161 CrossRef CAS.
- J. A. Edgar, J. Zool. Soc., 1982, 196, 385 CrossRef CAS.
- S. Schulz, Eur. J. Org. Chem., 1998, 13 CrossRef CAS.
- T. Eisner and J. Meinwald, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 50 CrossRef CAS.
- S. Schulz, W. Francke, M. Boppré, T. Eisner and J. Meinwald, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 6834 CrossRef CAS.
- M. Boppré, Naturwissenschaften, 1986, 73, 17 CrossRef.
- T. Eisner and J. Meinwald, in Insect pheromone biochemistry and molecular biology. The biosynthesis and detection of pheromones and plant volatiles, ed. G. J. Blomquist and R. Vogt, Elsevier/Academic Press, Amsterdam, Boston, 2003, pp. 341–368 Search PubMed.
- S. Schulz, in Tiger moths and woolly bears. Behavior, ecology, and evolution of the Arctiidae, ed. W. E. Conner, Univ. Press, Oxford, 2009, pp. 145–153 Search PubMed.
- S. Rajaraman and L. S. Jimenez, Tetrahedron, 2002, 58, 10407 CrossRef CAS.
- (a) C. C. J. Culvenor, J. A. Edgar, L. W. Smith and H. J. Tweeddale, Aust. J. Chem., 1970, 23, 1869 CrossRef CAS; (b) S. T. Martinez, A. C. Pinto, T. Glasnov and C. O. Kappe, Tetrahedron Lett., 2014, 55, 4181 CrossRef CAS.
- M. Boppré, in Biology and Conservation of the Monarch Butterfly, ed. S. B. Malcolm and M. P. Zalucki, Natural History Museum of Los Angeles County, Los Angeles, 1993, pp. 29–41 Search PubMed.
- W. Francke, S. Schulz, V. Sinnwell, W. A. König and Y. Roisin, Liebigs Ann. Chem., 1989, 1195 CrossRef CAS.
- T. E. Bellas, R. G. Brownlee and R. M. Silverstein, Tetrahedron, 1974, 30, 2267 CrossRef CAS.
- J. Meinwald, A. M. Chalmers, T. E. Pliske and T. Eisner, J. Chem. Soc., Chem. Commun., 1969, 3, 86 RSC.
- Y. Honda, K. Honda and H. Ômura, J. Insect Physiol., 2006, 52, 1043 CrossRef CAS.
- K. Mori, S. Aki and M. Kido, Liebigs Ann. Chem., 1993, 83 CrossRef CAS.
- J. Meinwald, A. M. Chalmers, T. E. Pliske and T. Eisner, Tetrahedron Lett., 1968, 4893 CrossRef CAS.
- J. Meinwald, Y. C. Meinwald and P. H. Mazzocchi, Science, 1969, 164, 1174 CrossRef CAS PubMed.
- V. Coca-Ruíz, I. Suárez, J. Aleu and I. G. Collado, Plants, 2022, 11, 769 CrossRef.
- J. Meinwald, W. R. Thompson, T. Eisner and D. F. Owen, Tetrahedron Lett., 1971, 38, 3485 CrossRef.
- D. Schneider and U. Seibt, Science, 1969, 164, 1173 CrossRef CAS.
- R. Nishida, S. Schulz, C. S. Kim, H. Fukami, Y. Kuwahara, K. Honda and N. Hayashi, J. Chem. Ecol., 1996, 22, 949 CrossRef CAS PubMed.
- S. Schulz and R. Nishida, Bioorg. Med. Chem., 1996, 4, 341 CrossRef CAS.
- K. Stritzke, S. Schulz and R. Nishida, Eur. J. Org. Chem., 2002, 3884 CrossRef CAS.
- C. S. Kim, R. Nishida, H. Fukami, F. Abe and T. Yamauchi, Biosci., Biotechnol., Biochem., 1994, 58, 980 CrossRef CAS.
- R. Nishida, C.-S. Kim, H. Fukami and R. Irie, Agric. Biol. Chem., 1991, 55, 1787 CAS.
- S. Schulz, Chem. Commun., 1999, 1239 RSC.
- R. Nishida, C.-S. Kim, K. Kawai and H. Fukami, Chem. Express, 1990, 5, 497 CAS.
- K. Stritzke, S. Schulz and M. Boppré, Eur. J. Org. Chem., 2003, 1337 CrossRef CAS.
- S. Schulz and S. Hötling, Nat. Prod. Rep., 2015, 32, 1042 RSC.
- J. Meinwald, C. J. Boriack, D. Schneider, M. Boppré, W. F. Wood and T. Eisner, Experientia, 1974, 30, 721 CrossRef CAS.
- S. Schulz, W. Francke and M. Boppré, Biol. Chem. Hoppe-Seyler, 1988, 369, 633 CrossRef CAS.
- R. L. Petty, M. Boppré, D. Schneider and J. Meinwald, Experientia, 1977, 33, 1324 CrossRef CAS.
- S. Lehner, S. Schulz and S. Dötterl, Front. Plant Sci., 2022, 13, 994851 CrossRef.
- S. Schulz, G. Beccaloni, R. Nishida, Y. Roisin, R. I. Vane-Wright and J. N. McNeil, Z. Naturforsch., C: Biosci., 1998, 53, 107 CrossRef CAS.
- S. Schulz, G. Beccaloni, K. S. Brown Jr., M. Boppré, A. V. L. Freitas, P. Ockenfels and J. R. Trigo, Biochem. Syst. Ecol., 2004, 32, 699 CrossRef CAS.
- J. Meinwald and Y. C. Meinwald, J. Am. Chem. Soc., 1966, 88, 1305 CrossRef CAS.
- R. A. Jurenka, in Insect Pheromone Biochemistry and Molecular Biology, ed. G. Blomquist and R. Vogt, Academic Press, 2nd edn, 2020, pp. 13–88 Search PubMed.
- R. I. Vane-Wright, S. Schulz and M. Boppré, Cladistics, 1992, 8, 125 CrossRef CAS PubMed.
- F. Mann, D. Szczerbowski, L. de Silva, M. McClure, M. Elias and S. Schulz, Beilstein J. Org. Chem., 2020, 16, 2776 CrossRef CAS PubMed.
- T. E. Pliske, Environ. Ecol., 1975, 4, 457 Search PubMed.
- J. Trigo, K. S. Brown, S. A. Henriques and L. E. S. Barata, Biochem. Syst. Ecol., 1996, 24, 181 CrossRef CAS.
- J. R. Trigo, L. E. S. Barata and K. S. Brown jr., J. Chem. Ecol., 1994, 20, 2883 CrossRef CAS PubMed.
- J. A. Edgar, C. C. J. Culvenor and T. E. Pliske, J. Chem. Ecol., 1976, 2, 263 CrossRef CAS.
- S. Schulz, Liebigs Ann. Chem., 1992, 1992, 829 CrossRef.
- N. Chazot, K. R. Willmott, G. Lamas, A. V. L. Freitas, F. Piron-Prunier, C. F. Arias, J. Mallet, D. L. De-Silva and M. Elias, Global Ecol. Biogeogr., 2019, 28, 1118–1132 CrossRef.
- A. V. Z. Brower, K. R. Willmott, K. L. Silva-Brandão, I. J. Garzón-Orduña and A. V. L. Freitas, System. Biodivers., 2014, 12, 133 CrossRef.
- F. Bogner and M. Boppré, Entomol. Exp. Appl., 1989, 50, 171 CrossRef CAS.
- P. Stamm, F. Mann, M. McClure, M. Elias and S. Schulz, J. Chem. Ecol., 2019, 45, 768 CrossRef CAS PubMed.
- M. A. Liénard, H.-L. Wang, J.-M. Lassance and C. Löfstedt, Nat. Commun., 2014, 5, 3957 CrossRef.
- N. Rosser, A. B. Phillimore, B. Huertas, K. R. Willmott and J. Mallet, Biol. J. Linn. Soc., 2012, 105, 479 CrossRef.
- S. Schulz, S. Yildizhan, K. Stritzke, C. Estrada and L. E. Gilbert, Org. Biomol. Chem., 2007, 5, 3434 RSC.
- (a) E. de Oliveira Borges, D. Bonfantti, C. A. de Oliveira Ribeiro and P. H. G. Zarbin, J. Morphol., 2020, 281, 388 CrossRef CAS; (b) A. L. Klein and A. M. de Araújo, J. Ethol., 2010, 28, 409 CrossRef.
- S. Schulz, C. Estrada, S. Yildizhan, M. Boppré and L. E. Gilbert, J. Chem. Ecol., 2008, 34, 82 CrossRef CAS.
- S. Ehlers, D. Szczerbowski, T. Harig, M. Stell, S. Hötling, K. Darragh, C. D. Jiggins and S. Schulz, ChemBioChem, 2021, 22, 3300 CrossRef CAS.
- C. D. Jiggins, The ecology & evolution of Heliconius butterflies, Oxford University Press, Oxford, 2017 Search PubMed.
- K. Darragh, S. Vanjari, F. Mann, M. F. Gonzalez-Rojas, C. R. Morrison, C. Salazar, C. Pardo-Diaz, R. M. Merrill, W. O. McMillan, S. Schulz and C. D. Jiggins, PeerJ, 2017, 5, e3953 CrossRef PubMed.
- A. L. Klein and A. M. de Araújo, J. Ethol., 2010, 28, 409 CrossRef.
- K. Byers, K. Darragh, S. Fernanda Garza, D. Abondano Almeida, I. A. Warren, P. Rastas, R. M. Merrill, S. Schulz, W. O. McMillan and C. D. Jiggins, Ecol. Evol., 2021, 11, 89 CrossRef.
- C. Estrada, S. Schulz, S. Yildizhan and L. E. Gilbert, Evolution, 2011, 65, 2843 CrossRef PubMed.
- (a) C. Malouines, Biol. Rev. Cambridge Philos. Soc., 2017, 92, 1570 CrossRef PubMed; (b) M. Beltrán, C. D. Jiggins, V. Z. Brower, E. Bermingham and J. L. B. Mallet, Biol. J. Linn. Soc., 2007, 92, 221 CrossRef.
- C. Estrada, S. Yildizhan, S. Schulz and L. E. Gilbert, Proc. R. Soc. London, Ser. B, 2010, 277, 407 CrossRef CAS.
- (a) G. A. Parker, Behav, 1974, 48, 157 Search PubMed; (b) V. Jormalainen, Q. Rev. Biol., 1998, 73, 275 CrossRef.
- R. A. Raguso and E. Pichersky, Plant Species Biol., 1999, 14, 95 CrossRef.
- S. Andersson and H. E. M. Dobson, J. Chem. Ecol., 2003, 29, 2303 CrossRef CAS.
- K. Darragh, A. Orteu, D. Black, K. J. R. P. Byers, D. Szczerbowski, I. A. Warren, P. Rastas, A. Pinharanda, J. W. Davey, S. Fernanda Garza, D. Abondano Almeida, R. M. Merrill, W. O. McMillan, S. Schulz and C. D. Jiggins, PLoS Biol., 2021, 19, e3001022 CrossRef CAS PubMed.
- A. G. Muñoz, C. Salazar, J. Castaño, C. D. Jiggins and M. Linares, J. Evol. Biol., 2010, 23, 1312 CrossRef.
- C. Mérot, J. Mavárez, A. Evin, K. K. Dasmahapatra, J. Mallet, G. Lamas and M. Joron, Biol. J. Linn. Soc., 2013, 109, 830 CrossRef.
- C. Mérot, B. Frérot, E. Leppik and M. Joron, Evolution, 2015, 69, 2891 CrossRef.
- M. F. González-Rojas, K. Darragh, J. Robles, M. Linares, S. Schulz, W. O. McMillan, C. D. Jiggins, C. Pardo-Diaz and C. Salazar, Proc. Biol. Sci., 2020, 287, 20200587 Search PubMed.
- A. P. Sánchez, C. Pardo-Diaz, J. Enciso-Romero, A. Muñoz, C. D. Jiggins, C. Salazar and M. Linares, Evolution, 2015, 69, 1619 CrossRef.
- K. Darragh, G. Montejo-Kovacevich, K. M. Kozak, C. R. Morrison, C. M. E. Figueiredo, J. S. Ready, C. Salazar, M. Linares, K. J. R. P. Byers, R. M. Merrill, W. O. McMillan, S. Schulz and C. D. Jiggins, Ecol. Evol., 2020, 10, 3895 CrossRef PubMed.
- C. Estrada and C. D. Jiggins, J. Evol. Biol., 2008, 21, 749 CrossRef CAS.
- N. Giraldo, C. Salazar, C. D. Jiggins, E. Bermingham and M. Linares, BMC Evol. Biol., 2008, 8, 324 CrossRef.
- J. D. Allison and R. T. Carde, Pheromone Communication in Moths, University of California Press, 2016 Search PubMed.
- F. Mann, S. Vanjari, N. Rosser, S. Mann, K. K. Dasmahapatra, C. Corbin, M. Linares, C. Pardo-Diaz, C. Salazar, C. Jiggins and S. Schulz, J. Chem. Ecol., 2017, 43, 843 CrossRef CAS.
- L. E. Gilbert, Science, 1976, 193, 419 CrossRef CAS PubMed.
- S. A. Hosseini, M. van Wijk, G. Ke, S. H. Goldansaz, C. Schal and A. T. Groot, Sci. Rep., 2016, 6, 38567 CrossRef CAS.
- J. Andersson, A. K. Borg-Karlson and C. Wiklund, Proc. R. Soc. London, Ser. B, 2000, 267, 1271 CAS.
- J. Andersson, A. K. Borg-Karlson and C. Wiklund, Proc. R. Soc. London, Ser. B, 2004, 271, 1765 Search PubMed.
- J. Andersson, A.-K. Borg-Karlson and C. Wiklund, J. Chem. Ecol., 2003, 29, 1489 CrossRef CAS PubMed.
- S. Yildizhan and S. Schulz, Synlett, 2011, 2831 CAS.
- R. M. Merrill, K. K. Dasmahapatra, J. W. Davey, D. D. Dell'Aglio, J. J. Hanly, B. Huber, C. D. Jiggins, M. Joron, K. M. Kozak and V. Llaurens, J. Evol. Biol., 2015, 28, 1417 CrossRef CAS PubMed.
- (a) G. Farré-Armengol, I. Filella, J. Llusià and J. Peñuelas, Molecules, 2017, 22, 1148 CrossRef; (b) S. Andersson and H. E. M. Dobson, J. Chem. Ecol., 2003, 29, 2319 CrossRef CAS PubMed; (c) S. Andersson, L. A. Nilsson, I. Groth and G. Bergstrom, Bot. J. Linn. Soc., 2002, 140, 129 CrossRef.
- C. Estrada, PhD thesis, Univ. of Texas, Austin, 2009.
- M. Miyakado, J. Meinwald and L. E. Gilbert, Experientia, 1989, 45, 1006 CrossRef CAS PubMed.
- D. J. Melo, E. O. Borges, D. Szczerbowski, D. M. Vidal, S. Schulz and P. H. G. Zarbin, Org. Lett., 2022, 24, 3772 CrossRef CAS PubMed.
- S. Yildizhan, J. van Loon, A. Sramkova, M. Ayasse, C. Arsene, C. ten Broeke and S. Schulz, ChemBioChem, 2009, 10, 1666 CrossRef CAS PubMed.
- B. E. Phillips, C. R. Smith and L. W. Tjarks, J. Org. Chem., 1970, 35, 1916 CrossRef CAS.
- B. Nicolet and R. Tabacchi, in Modern Fungicides and Antifungal Compounds II, ed. H. Lyr, P. E. Russell, H.-W. Dehne and H. D. Sisler, Intercept, Andover, 1999, pp. 469–476 Search PubMed.
- (a) N. M. Maguire, G. Read, P. F. Richardson and S. M. Roberts, J. Chem. Res., 1994, 376 CAS; (b) Y. Matsushita, K. Sugamoto, T. Nakama, T. Matsui, Y. Hayashi and K. Uenakai, Tetrahedron Lett., 1997, 38, 6055 CrossRef CAS.
- (a) H. Kaye, N. J. Mackintosh, M. Rothschild and B. P. Moore, Anim. Behav., 1989, 37, 563 CrossRef; (b) L. Lindström, C. Rowe and T. Guilford, Proc. R. Soc. London, Ser. B, 2001, 268, 159 CrossRef.
- B. P. Moore, W. V. Brown and M. Rothschild, Chemoecology, 1990, 1, 43 CrossRef CAS.
- J. Zhang, Q. Cong, J. Shen, P. A. Opler and N. V. Grishin, Taxon. Rep. Int. Lepid. Surv., 2019, 8, 1 Search PubMed.
- G. N. Ross, H. M. Fales, H. A. Lloyd, T. Jones, E. A. Sokoloski, K. Marshall-Batty and M. S. Blum, J. Chem. Ecol., 2001, 27, 1219 CrossRef CAS PubMed.
- M. D. Tomalski, M. S. Blum, T. H. Jones, H. M. Fales, D. F. Howard and L. Passera, J. Chem. Ecol., 1987, 13, 253 CrossRef CAS PubMed.
- (a) K. Darragh, K. J. R. P. Byers, R. M. Merrill, W. O. McMillan, S. Schulz and C. D. Jiggins, Ecol. Entomol., 2019, 44, 397 CrossRef PubMed; (b) W. Boerjan, J. Ralph and M. Baucher, Annu. Rev. Plant Biol., 2003, 54, 519 CrossRef CAS PubMed.
- J.-M. Lassance, M. A. Liénard, B. Antony, S. Qian, T. Fujii, J. Tabata, Y. Ishikawa and C. Löfstedt, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3967 CrossRef CAS.
- K. J. R. P. Byers, K. Darragh, J. Musgrove, D. A. Almeida, S. F. Garza, I. A. Warren, P. M. Rastas, M. Kučka, Y. F. Chan, R. M. Merrill, S. Schulz, W. O. McMillan and C. D. Jiggins, Evolution, 2020, 74, 349 CrossRef CAS.
- (a) H. Y. Ho, Y. T. Tao, R. S. Tsai, Y. L. Wu, H. K. Tseng and Y. S. Chow, J. Chem. Ecol., 1996, 22, 271 CrossRef CAS; (b) P. Witzgall, M. Bengtsson, S. Rauscher, I. Liblikas, A.-C. Backman, M. Coracini, P. Anderson and J. Lofqvist, Entomol. Exp. Appl., 2001, 101, 131 CrossRef CAS; (c) J. S. McElfresh, X. Chen, D. W. Ross and J. G. Millar, Can. Entomol., 2000, 132, 775 CrossRef; (d) S. Tatsuki, M. Kurihara, K. Usui, Y. Ohguchi, K. Uchiumi, K. Arai, S. Yabuki and F. Tanaka, Appl. Entomol. Zool., 1983, 18, 443 CrossRef CAS; (e) A. Cork, D. J. Chamberlain, P. S. Beevor, D. R. Hall, B. F. Nesbitt, D. G. Campion and M. R. Attique, J. Chem. Ecol., 1988, 14, 929 CrossRef CAS; (f) J. H. Tumlinson, M. M. Brennan, R. E. Doolittle, E. R. Mitchell, A. Brabham, B. E. Mazomenos, A. H. Baumhover and D. M. Jackson, Arch. Insect Biochem. Physiol., 1989, 10, 255 CrossRef CAS; (g) A. M. El-Sayed, V. J. Mitchell, L.-A. M. Manning and D. M. Suckling, J. Chem. Ecol., 2011, 37, 640 CrossRef CAS PubMed; (h) E. V. Pires, A. d. L. Mendonça, L. Vaníčková, N. S. J. Serra, R. d. C. C. da Silva, D. C. dos Santos, R. d. S. Campos, A. E. G. Sant'Ana and R. R. do Nascimento, J. Appl. Entomol., 2016, 140, 72 CrossRef CAS; (i) Q.-H. Chen, F. Zhu, Z. Tian, W.-M. Zhang, R. Guo, W. Liu, L. Pan and Y. Du, Insects, 2018, 9, 192 CrossRef PubMed.
- C. D. Jiggins, BioScience, 2008, 58, 541 CrossRef.
- B. Cama, S. Ehlers, D. Szczerbowski, J. Thomas-Oates, C. D. Jiggins, S. Schulz, W. O. McMillan and K. K. Dasmahapatra, Proc. Biol. Sci., 2022, 289, 20220474 CAS.
- C. Li, H. Wang, X. Chen, J. Yao, L. Shi and C. Zhou, Sci. Rep., 2017, 7, 5033 CrossRef PubMed.
- (a) D. L. Murray, Systematics of neotropical satyrine butterflies (Nymphalidae: Satyrinae: Euptychiina) based on larval morphology and DNA sequence data and the evolution of life history traits, Ph.D. thesis, Louisiana State University and Agricultural & Mechanical College, New Orleans, 2001; (b) M. A. Marín, C. Peña, A. V. L. Freitas, N. Wahlberg and S. I. Uribe, Neotrop. Entomol., 2011, 40, 1 CrossRef PubMed; (c) P. J. DeVries, The Butterflies of Costa Rica and Their Natural History: Papilionidae, Pieridae, Nymphalidae, Princeton University Press, Princeton, 1987 Search PubMed.
- P. M. B. Bacquet, O. Brattström, H.-L. Wang, C. E. Allen, C. Löfstedt, P. M. Brakefield and C. M. Nieberding, Proc. R. Soc. London, Ser. B, 2015, 282, 20142734 CrossRef CAS PubMed.
- E. Dion, A. Monteiro and J. Y. Yew, Sci. Rep., 2016, 6, 39002 CrossRef CAS PubMed.
- E. Hedenström, E. A. Wallin, J. Andersson, J. Bång, H.-L. Wang, C. Löfstedt, O. Brattström and P. Baquet, J. Chem. Ecol., 2014, 41, 44 CrossRef PubMed.
- D. Muller, B. Elias, L. Collard, C. Pels, M.-J. Holveck and C. M. Nieberding, PLoS One, 2019, 14, e0225003 CrossRef CAS PubMed.
- K. Costanzo and A. Monteiro, Proc. R. Soc. London, Ser. B, 2007, 274, 845 CrossRef.
- M. Condamin, Monographie du genre Bicyclus: Lepidoptera satyridae, Institut fondamental d'Afrique noire, Dakar, 1973 Search PubMed.
- P. M. Brakefield, P. Beldade and B. J. Zwaan, Cold Spring Harbor Protoc., 2009, 2009, emo122 CrossRef.
- H.-L. Wang, O. Brattström, P. M. Brakefield, W. Francke and C. Löfstedt, J. Chem. Ecol., 2014, 40, 549 CrossRef CAS PubMed.
- C. M. Nieberding, H. de Vos, M. V. Schneider, J.-M. Lassance, N. Estramil, J. Andersson, J. Bång, E. Hedenström, C. Löfstedt, P. M. Brakefield and M. Somers, PLoS One, 2008, 3, e2751 CrossRef PubMed.
- C. M. Nieberding, K. Fischer, M. Saastamoinen, C. E. Allen, E. A. Wallin, E. Hedenström and P. M. Brakefield, Ecol. Lett., 2012, 15, 415 CrossRef PubMed.
- E. van Bergen, P. M. Brakefield, S. Heuskin, B. J. Zwaan and C. M. Nieberding, Proc. Biol. Sci., 2013, 280, 20130102 Search PubMed.
- S. Schulz, S. Yildizhan and J. J. A. van Loon, J. Chem. Ecol., 2011, 37, 360 CrossRef CAS PubMed.
- (a) Y. Sasaerila, R. Gries, G. Gries, G. Khaskin, S. King, S. Takács and Hardi, Chemoecology, 2003, 13, 89 CrossRef CAS; (b) B. V. Burger, A. E. Nell, D. Smit, H. S. Spies, W. M. Mackenroth, D. Groche and P. R. Atkinson, J. Chem. Ecol., 1993, 19, 2255 CrossRef CAS PubMed; (c) D. R. Hall, A. Cork, R. Lester, B. F. Nesbitt and P. Zagatti, J. Chem. Ecol., 1987, 13, 1575 CrossRef CAS PubMed; (d) K. Mori, H. Harada, P. Zagatti, A. Cork and D. R. Hall, Liebigs Ann. Chem., 1991, 1991, 259 CrossRef.
- J. A. Tillman, S. J. Seybold, R. A. Jurenka and G. J. Blomquist, Insect Biochem. Mol. Biol., 1999, 29, 481 CrossRef CAS PubMed.
- B. Visser, I. A. N. Dublon, S. Heuskin, F. Laval, P. M. B. Bacquet, G. Lognay and C. M. Nieberding, Front. Ecol. Evol., 2018, 6, 964 Search PubMed.
- P. M. B. Bacquet, M. A. de Jong, O. Brattström, H.-L. Wang, F. Molleman, S. Heuskin, G. Lognay, C. Löfstedt, P. M. Brakefield, A. Vanderpoorten and C. M. Nieberding, Ecol. Evol., 2016, 6, 6064 CrossRef.
- E. L. Westerman and A. Monteiro, Anim. Behav., 2013, 86, 1139 CrossRef.
- E. Dion, L. X. Pui, K. Weber and A. Monteiro, Nat. Commun., 2020, 11, 53 CrossRef CAS PubMed.
- K. Fischer, I. Karl, I. A. N. Dublon and T. Kehl, Front. Zool., 2018, 15, 19 CrossRef PubMed.
- S. Heuskin, M. Vanderplanck, P. Bacquet, M.-J. Holveck, M. Kaltenpoth, T. Engl, C. Pels, C. Taverne, G. Lognay and C. M. Nieberding, Front. Ecol. Evol., 2014, 2, 37 Search PubMed.
- C. Arsene, S. Schulz and J. J. A. van Loon, J. Chem. Ecol., 2002, 28, 2627 CrossRef CAS.
- S. Schulz, M. Steffensky and Y. Roisin, Liebigs Ann. Chem., 1996, 941 CAS.
- N. Hayashi, H. Kawaguchi, A. Nishi and H. Komae, Z. Naturforsch., 1987, 42c, 1001 CrossRef.
- N. Hayashi, A. Nishi, T. Murakami, K. Maeshima, H. Komae and T. Sakao, Z. Naturforsch., C: Biosci., 1985, 40, 47 CrossRef.
- M. Pinzari, M. Santonico, G. Pennazza, E. Martinelli, R. Capuano, R. Paolesse, M. Di Rao, A. D'Amico, D. Cesaroni, V. Sbordoni and C. Di Natale, PLoS One, 2018, 13, e0199997 CrossRef.
- A. H. Ehrlich and P. R. Ehrlich, J. Kans. Entmolol. Soc., 1978, 666 Search PubMed.
- Y. Pan, Z. Yu and X. Yuan, ZooKeys, 2022, 1084, 65 CrossRef PubMed.
- C. E. B. Nobre, L. A. d. S. Lucas, R. J. R. Padilha, D. M. d. A. F. Navarro, L. C. Alves and A. C. D. Maia, Org. Divers. Evol., 2021, 21, 93 CrossRef.
- E. W. McQueen and N. I. Morehouse, J. Insect Sci., 2018, 18, 33 CrossRef PubMed.
- (a) M. F. Braby and J. W. H. Trueman, J. Evol. Biol., 2006, 19, 1677 CrossRef CAS PubMed; (b) C. W. Wheat, H. Vogel, U. Wittstock, M. F. Braby, D. Underwood and T. Mitchell-Olds, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20427 CrossRef CAS PubMed.
- V. Dincă, C. Wiklund, V. A. Lukhtanov, U. Kodandaramaiah, K. Norén, L. Dapporto, N. Wahlberg, R. Vila and M. Friberg, J. Evol. Biol., 2013, 26, 2095 CrossRef PubMed.
- V. I. Solovyev, Y. Ilinsky and O. E. Kosterin, Comp. Cytogenet., 2015, 9, 299 CrossRef PubMed.
- M. Friberg, N. Vongvanich, A.-K. Borg-Karlson, D. J. Kemp, S. Merilaita and C. Wiklund, Behav. Ecol. Sociobiol., 2008, 62, 873 CrossRef.
- C. Wiklund, Oikos, 1977, 29, 275 CrossRef.
- H. Ômura and S. Yotsuzuka, Appl. Entomol. Zool., 2015, 50, 191 CrossRef.
- R. L. Rutowski, J. Chem. Ecol., 1980, 6, 13 CrossRef CAS.
- J. W. Grula, J. D. McChesney and O. R. Taylor, J. Chem. Ecol., 1980, 6, 241 CrossRef CAS.
- R. E. Silberglied and O. R. Taylor, Behav. Ecol. Sociobiol., 1978, 3, 203 CrossRef.
- T. W. Sappington and O. R. Taylor, J. Chem. Ecol., 1990, 16, 2771 CrossRef CAS.
- R. S. Papke, D. J. Kemp and R. L. Rutowski, Anim. Behav., 2007, 73, 47 CrossRef.
- V. Ficarrotta, J. J. Hanly, L. S. Loh, C. M. Francescutti, A. Ren, K. Tunström, C. W. Wheat, A. H. Porter, B. A. Counterman and A. Martin, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2109255118 CrossRef CAS.
- K. Yoshimori, C. Okuda, S. Ohta and H. Ômura, J. Chem. Ecol., 2022, 16, 231 Search PubMed.
- Y. Okumura, Y. Ozeki, T. Itoh, S. Ohta and H. Ômura, Appl. Entomol. Zool., 2016, 51, 385 CrossRef CAS.
- Y. Suzuki, A. Nakanishi, H. Shima, O. Yata and T. Saigusa, Kontyu, 1977, 45, 300 Search PubMed.
- J. Forsberg and C. Wiklund, Behav. Ecol. Sociobiol., 1989, 25, 349 CrossRef.
- J. Andersson, A. K. Borg-Karlson, N. Vongvanich and C. Wiklund, J. Exp. Biol., 2007, 210, 964 CrossRef CAS PubMed.
- G. Bergström, Zoon, Suppl., 1973, 1, 67 Search PubMed.
- H. Larsdotter-Mellström and C. Wiklund, Behav. Ecol., 2009, 20, 1147 CrossRef.
- H. Larsdotter-Mellström, K. Eriksson, I. Liblikas, C. Wiklund, A. K. Borg-Karlson, S. Nylin, N. Janz and M. A. Carlsson, Front. Physiol., 2016, 7, 68 Search PubMed.
- A. Yoshida, A. Noda, A. Yamana and H. Numata, Entomol. Sci., 2000, 3, 345 Search PubMed.
- J. W. Wong, V. Verigin, A. C. Oehlschlager, J. H. Borden, H. D. Pierce, A. M. Pierce and L. Chong, J. Chem. Ecol., 1983, 9, 451 CrossRef CAS PubMed.
- Y. Li and R. A. Mathews, J. Insect Physiol., 2016, 91–92, 107 CrossRef CAS PubMed.
- T. Chuman, J. Sivinski, R. R. Heath, C. O. Calkins, J. H. Tumlinson, M. A. Battiste, R. L. Wydra, L. Strekowski and J. L. Nation, Tetrahedron Lett., 1988, 29, 6561 CrossRef CAS.
- (a) E. Kan and T. Hidaka, J. Ethol., 1997, 15, 87 CrossRef; (b) O. Ohguchi and T. Hidaka, J. Ethol., 1988, 6, 49 CrossRef.
- Y. Obara and M. E. N. Majerus, Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci., 2009, 85, 198 CrossRef PubMed.
- N. Hayashi, Y. Kuwahara and H. Komae, Experientia, 1978, 34, 684 CrossRef CAS.
- R. Mozuraitis, R. Murtazina, J. Zurita, Y. Pei, L. Ilag, C. Wiklund and A. Karlson, Sci. Rep., 2019, 9, 14262 CrossRef PubMed.
- C. Wiklund, A. Kaitala and N. Wedell, Behav. Ecol., 1998, 9, 20 CrossRef.
- H. Larsdotter-Mellström, K. Eriksson, N. Janz, S. Nylin and M. A. Carlsson, Funct. Ecol., 2016, 30, 255 CrossRef.
- N. E. Fatouros, M. E. Huigens, J. J. A. van Loon, M. Dicke and M. Hilker, Nature, 2005, 433, 433704a CrossRef PubMed.
- J. R. da Rocha, PhD thesis, Federal University of Alagoas, Maceió, 2021.
- T. Eisner, M. Eisner, T. Jaouni and H. M. Fales, Naturwissenschaften, 1990, 77, 33 CrossRef CAS PubMed.
- J. A. Scott, J. Res. Lepid., 1973, 11, 99 CrossRef.
- J. Gershenzon and N. Dudareva, Nat. Chem. Biol., 2007, 3, 408 CrossRef CAS.
- T. Eltz, E. Hedenström, J. Bång, E. A. Wallin and J. Andersson, J. Chem. Ecol., 2010, 36, 1322 CrossRef CAS.
- D. Vanderwel, B. Johnston and A. C. Oehlschlager, Insect Biochem. Mol. Biol., 1992, 22, 875 CrossRef CAS.
- A. C. Oehlschlager, J. W. Wong, V. G. Verigin and H. D. Pierce, J. Org. Chem., 1983, 48, 5009 CrossRef CAS.
- K. L. Prudic, S. Khera, A. Sólyom and B. N. Timmermann, J. Chem. Ecol., 2007, 33, 1149 CrossRef CAS PubMed.
- (a) J. T. Smiley, J. M. Horn and N. E. Rank, Science, 1985, 229, 649 CrossRef CAS; (b) J. M. Pasteels, M. Rowell-Rahier and M. J. Raupp, in Novel Aspects of Insect-Plant Interactions, ed. P. Barbosa and D. K. Letourneau, ed. John Wiley & Sons, 1988, pp. 235–272 Search PubMed; (c) R. F. Denno, S. Larsson and K. L. Olmstead, Ecology, 1990, 71, 124 CrossRef.
- H. Ômura and K. Honda, Appl. Entomol. Zool., 2011, 46, 281 CrossRef.
- K. Honda, Insect Biochem., 1980, 10, 583 CrossRef CAS.
- H. Ômura and K. Honda, J. Chem. Ecol., 2005, 40, 421 Search PubMed.
- (a) T. Eisner, D. Alsop, K. Hicks and J. Meinwald, in Arthropod Venoms, ed. S. Bettini, Springer Berlin Heidelberg, Berlin, Heidelberg, 1978, vol. 45, pp. 41–72 Search PubMed; (b) K. Drilling and K. Dettner, Chemoecology, 2010, 20, 243 CrossRef CAS.
- H. Ômura, K. Honda and N. Hayashi, Z. Naturforsch., C: Biosci., 2001, 56, 1126 CrossRef PubMed.
- H. Ômura, N. Yanai and K. Honda, Z. Naturforsch., C: Biosci., 2012, 67, 331 CrossRef PubMed.
- (a) H. Ômura, T. Noguchi and S. Ohta, Anim. Behav., 2020, 170, 133 CrossRef; (b) H. Ômura, T. Noguchi and S. Ohta, Chem. Biodiversity, 2022, 19, e202100879 CrossRef PubMed.
- K. Yoshimori, T. Noguchi, S. Otha and H. Ômura, Lepidoptera Science, 2017, 68, 61 Search PubMed.
- B. V. Burger, Z. Munro, M. Röth, H. S. Spies, V. Truter, H. Geertsema and A. Habich, J. Chem. Ecol., 1985, 11, 1093 CrossRef CAS PubMed.
- T. Eisner and Y. C. Meinwald, Science, 1965, 150, 1733 CrossRef CAS PubMed.
- K. Honda and N. Hayashi, J. Chem. Ecol., 1995, 21, 859 CrossRef CAS PubMed.
- H. Ômura, K. Honda and P. Feeny, J. Chem. Ecol., 2006, 32, 1999 CrossRef PubMed.
- C. Frankfater, M. R. Tellez and M. Slattery, Chemoecology, 2009, 19, 81 CrossRef CAS.
- K. Honda, J. Chem. Ecol., 1981, 7, 1089 CrossRef CAS PubMed.
- K. Honda, J. Insect Physiol., 1980, 26, 39 CrossRef CAS.
- (a) I. M. Seligman and F. A. Doy, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1972, 42, 341 CrossRef; (b) B. V. Burger, M. Röth, M. Le Roux, H. Spies, V. Truter and H. Geertsema, J. Insect Physiol., 1978, 24, 803 CrossRef CAS.
- (a) K. Honda, Int. J. Trop. Insect Sci., 1983, 4, 255 CrossRef CAS; (b) K. Honda, Insect Biochem., 1990, 20, 245 CrossRef CAS.
- T. Eisner, T. E. Pliske, M. Ikeda, D. F. Owen, L. Vázquez, H. Pérez, J. G. Franclemont and J. Meinwald, Ann. Entomol. Soc. Am., 1970, 63, 914 CrossRef CAS.
- K. Honda, Physiol. Entomol., 1983, 8, 173 CrossRef CAS.
- (a) A. R. P. Martins, R. K. Robbins, M. Duarte and R. C. Busby, Insect Syst. Evol., 2012, 43, 35 CrossRef; (b) R. Grund and R. Eastwood, Entomol. Sci., 2010, 13, 134 CrossRef.
- H. Ômura, K. Yakumaru, K. Honda and T. Itoh, Naturwissenschaften, 2013, 100, 373 CrossRef PubMed.
- L. Lundgren and G. Bergström, J. Chem. Ecol., 1976, 1, 399 CrossRef.
- N. E. Pierce and E. Dankowicz, Curr. Opin. Insect. Sci., 2022, 52, 100898 CrossRef PubMed.
- (a) T. Akino, J. J. Knapp, J. A. Thomas and G. W. Elmes, Proc. R. Soc. London, Ser. B, 1999, 266, 1419 CrossRef CAS; (b) M. K. Hojo, A. Wada-Katsumata, T. Akino, S. Yamaguchi, M. Ozaki and R. Yamaoka, Proc. R. Soc. London, Ser. B, 2009, 276, 551 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |