Open Access Article
Open Access ArticleSustainable solutions for removing aged wax-based coatings from cultural heritage: exploiting hydrophobic deep eutectic solvents (DESs)
Chiara
Biribicchi
 *ab,
Andrea
Macchia
bc,
Gabriele
Favero
d,
Romina
Strangis
e,
Bartolo
Gabriele
*ab,
Andrea
Macchia
bc,
Gabriele
Favero
d,
Romina
Strangis
e,
Bartolo
Gabriele
 e,
Raffaella
Mancuso
e,
Raffaella
Mancuso
 e and
Mauro Francesco
La Russa
c
e and
Mauro Francesco
La Russa
c
aDepartment of Earth Sciences, University of Rome La Sapienza, P.le Aldo Moro 5, 00185 Rome, Italy. E-mail: chiara.biribicchi@uniroma1.it
bYOCOCU, Youth in Conservation of Cultural Heritage, Via T. Tasso 108, 00185 Rome, Italy. E-mail: andrea.macchia@uniroma1.it
cDepartment of Biology, Ecology and Earth Sciences (DiBEST), University of Calabria, Via Pietro Bucci 12/B, 87036 Arcavacata di Rende, CS, Italy. E-mail: mlarussa@unical.it
dDepartment of Environmental Biology, Sapienza University of Rome, Piazzale Aldo Moro 5, 00185 Rome, Italy. E-mail: gabriele.favero@uniroma1.it
eLaboratory of Industrial and Synthetic Organic Chemistry (LISOC), Department of Chemistry and Chemical Technologies, University of Calabria, Via Pietro Bucci 12/C, 87036 Arcavacata di Rende, CS, Italy. E-mail: bartolo.gabriele@unical.it; romina.strangis@unical.it; raffaella.mancuso@unical.it
First published on 8th March 2023
Abstract
This study describes the investigation on the use of hydrophobic Deep Eutectic Solvents (DESs) for the removal of nonpolar coatings from works of art to replace toxic solvents. Beeswax and two microcrystalline waxes (R21 and Renaissance®) have been selected as reference nonpolar coatings since they are commonly present in their aged state on metal and stone artifacts. The interaction between the DESs and three waxes has been evaluated through contact angle measurements, solubility tests, and cleaning tests carried out by implementing a method that is ordinarily used by restorers. Tests have been conducted on mockups consisting of microscope glass slides covered by wax. The effective removal of the wax-based coating from the mockups has been assessed through spectrocolorimetry and multispectral imaging under visible (VIS) and ultraviolet light (UV) at 365 nm by loading the waxes with a fluorescent marker (Rhodamine 6G). Fourier Transform Infrared (FT-IR) spectroscopy in the Attenuated Total Reflectance (ATR) mode was performed to assess the presence of both the wax and the solvent on the swabs used for the cleaning tests, confirming the actual interaction among the solvent and the solute. The experimental process proved DESs’ potential of being used as green solvents for cleaning treatments on Cultural Heritage.
Introduction
Wax-based protective coatings have been traditionally used to protect both stone and metal sculptures from the erosive action of weather and the corrosive action of rain and atmospheric pollutants such as SOx, NOx, CO2, and chlorides.1–3 The hydrophobic feature of these materials is one of the main reasons for their persistent use on outdoor metal and stone artworks. Indeed, outdoor bronzes, iron, and lead sculptures undergo corrosion processes that are essentially caused by the presence of water on the artwork's surface resulting from precipitation or condensation and by the washing effect of rainwater.4–7 On the other hand, the growth of salt crystals within pores is one of the major damage factors in stone weathering and is driven or enhanced by water.8 Waxes have low water vapor permeability and low gloss, which makes them ideal protective coatings.9 Formerly, beeswax used to be applied on outdoor stone and metal artifacts.1,2,7,10,11 The so-called “waxing” process represented a common practice in ancient restoration treatments that allowed for the protection of the artwork from rainfall and atmospheric pollution and sometimes for the toning of the surface.2 Nowadays, stone and metal sculptures are usually treated with microcrystalline-wax-based protective coatings.12–14 When it comes to metal artifacts, the coating is typically applied on an acrylic layer to reduce its exposure to the surrounding environment and moisture.15 This nonpolar coating is considered a “sacrificial layer” able to protect the acrylic one underneath, avoiding its degradation.Wax coverings tend to degrade over time, making the removal of this layer necessary.1,16 They tend to embed pollutants and dust due to their low melting point (between 39–65 °C depending on the type of wax), also showing chromatic and morphological alterations.17 Indeed, even though wax-based coatings are commonly used to protect outdoor sculptures from degradation and are still considered more beneficial maintenance products for outdoor sculptures than most other coatings, their barrier properties are affected by defects in their structure – namely, where the layer is not consistent – and their vulnerability to chemical alterations induced by weathering.3,6,18,19 These factors are the main causes of their short lifetime – 2 to 5 years for microcrystalline waxes, which tend to exfoliate and become powdery, thus also altering the aesthetic appearance of the artwork. Indeed, the tendency to deteriorate requires constant maintenance, which means periodic cleaning treatments.4 For this kind of intervention, hazardous solvents for both the environment and human health are still widely used. Highly flammable and toxic solvents, namely aliphatic and aromatic hydrocarbons, such as Petroleum Ether and White Spirit, are being used due to their physical-chemical properties, even though they are known to cause adverse effects to humans by both inhalation and dermal adsorption.10,20–24 Their medium-high volatility, low cost, transparency, and purity make them ideal for these applications, while more sustainable low-polar cleaning systems capable to combine the need for the preservation of the artwork's integrity with a greener approach are still lacking.
The role of green chemistry in improving sustainability in the cultural heritage conservation field is increasingly growing.25,26 The general aim is to replace traditional hazardous methods and products that are still widely used in the field, especially for the removal of aged coatings from artistic surfaces, thus ensuring the safety of both the artworks and the operators.27–32 Even though a low-impact and effective approach to cleaning treatments is now recognized as an urgent need, novel environmentally friendly solutions and protocols are yet to be investigated. In this framework, Deep Eutectic Solvents (DESs) can be considered promising alternatives to conventional organic solvents due to their excellent physical–chemical properties – i.e., low volatility, high dissolution power, biodegradability, and low toxicity – together with their easy synthesis, accessibility of their natural compounds, low cost, and recyclability.33 They are mixtures of two or more solid components leading to a strong depression of the melting point when compared to their individual counterparts, due to the presence of nonsymmetric ions with low lattice energy.34 They form eutectic mixtures of a hydrogen-bonding acceptor (HBA) and a hydrogen-bonding donor (HBD) able to self-associate via hydrogen bonds and van der Waals interactions, inducing the charge delocalization responsible for the decrease in the melting point.35 Hydrophilic DESs can be described by the general formula:
| Cat+ X−zY | (1) |
| [Component 1(HBA)-component 2 (HBD)] | (2) |
They represent promising solutions, since other potentially suitable compounds that could hypothetically replace aliphatic hydrocarbons come in solid form at room temperature and cannot be used as liquid solvents. Indeed, most of the potential alternatives to toxic solvents consist of molecules having long alkyl chains. As the length of the chain increases, the polarity of the substance decreases, while its melting point increases, making the substance solid at room temperature. The substance's physical state at standard temperatures does not allow for its utilization as a solvent in the liquid form as it needs to be. In this framework, DESs’ feature to deeply reduce the melting point value can overcome this limitation and turn low-polar solid substances with medium-long alkyl chains into liquid solvents, while exploiting the solubility properties of their constituents.
The potential of hydrophobic DESs not only relies on the possibility of exploiting the properties of compounds that cannot be used as solvents at room temperature due to their physical state but also on their biocompatibility and low toxicity. These two features are of the outermost importance in the conservation of Cultural Heritage since the operators working on the removal of non-polar coatings are often subjected to inhalation of toxic solvents vapors – i.e., petroleum derivatives – which may cause pathological diseases with long-term exposure.43 For this reason, we exploited and herein propose for the first time the potential of hydrophobic DESs as new eco-friendly solvents for the removal of low-polar coatings – i.e., waxes – from artistic surfaces, aiming at replacing more hazardous traditional solvents commonly used in the Cultural Heritage sector.
Experimental
Materials and methods
The vials were placed in a Vevor® digital ultrasonic cleaner (120 W, 50 Hz) to accelerate the solubilization process for 24 hours. The degree of solubilization was evaluated through turbidity measurements by filtering the mixture to remove solid residues. The analysis was performed using a Haze 3001 Turbidity Meter.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) for 3 hours to remove the excess marker, thus avoiding further extraction of the sole Rhodamine 6G during the cleaning tests. Swab cleaning tests were carried out simulating the operational mode commonly used by restorers: each solvent – also comprising reference solvents (i.e., White Spirit for beeswax and Ligroin 100–140 °C for microcrystalline waxes) – was left on the layers of wax in the same amount (1 mL) for increasing contact times – i.e., 1 minute, 2 minutes, and 3 minutes – and then the solvent's residues were removed from the surface using cotton swabs wounded on a thin stick. Tests were summarized in the Results section by assigning an average value to each wax–solvent combination. During the treatment, mechanical rubbing action was minimized to limit its contribution to the removal of the layer. Deionized water was also used as a reference solvent, as it should not have any kind of interaction with wax. Spectrocolorimetric analyses were performed before and after the cleaning treatment using a portable spectrophotometer Y3060 3nh, equipped with a D65 illuminant and an 8 mm size aperture. The analysis was carried out in the SCI mode (Specular Component Included) and by measuring the spectra between 400 and 700 nm. The acquisitions were performed three times on each sample to reduce the uncertainty of the analysis. Data were then analyzed through the CIELab color system. The variation of the parameters L*, a* and b* was calculated by evaluating the Euclidean distance between the mean of the values acquired on the areas after the treatment and the ones collected on the blank sample. ΔL*, Δa*, and Δb* were summarized in the total color difference ΔE, given by the following equation:
70) for 3 hours to remove the excess marker, thus avoiding further extraction of the sole Rhodamine 6G during the cleaning tests. Swab cleaning tests were carried out simulating the operational mode commonly used by restorers: each solvent – also comprising reference solvents (i.e., White Spirit for beeswax and Ligroin 100–140 °C for microcrystalline waxes) – was left on the layers of wax in the same amount (1 mL) for increasing contact times – i.e., 1 minute, 2 minutes, and 3 minutes – and then the solvent's residues were removed from the surface using cotton swabs wounded on a thin stick. Tests were summarized in the Results section by assigning an average value to each wax–solvent combination. During the treatment, mechanical rubbing action was minimized to limit its contribution to the removal of the layer. Deionized water was also used as a reference solvent, as it should not have any kind of interaction with wax. Spectrocolorimetric analyses were performed before and after the cleaning treatment using a portable spectrophotometer Y3060 3nh, equipped with a D65 illuminant and an 8 mm size aperture. The analysis was carried out in the SCI mode (Specular Component Included) and by measuring the spectra between 400 and 700 nm. The acquisitions were performed three times on each sample to reduce the uncertainty of the analysis. Data were then analyzed through the CIELab color system. The variation of the parameters L*, a* and b* was calculated by evaluating the Euclidean distance between the mean of the values acquired on the areas after the treatment and the ones collected on the blank sample. ΔL*, Δa*, and Δb* were summarized in the total color difference ΔE, given by the following equation: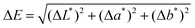 | (3) |
Multispectral imaging was performed using UV (365 nm) and visible (VIS) light sources before and after the cleaning tests to evaluate the solvents’ effectiveness. The analysis was carried out using the Madatec multispectral system, which consists of a full-spectrum Samsung NX500 Digital Camera (28.2 MP BSI CMOS) and Madatec spotlights with 365 nm (UV) wavelength. Images of the induced fluorescence were taken using the HOYA UV-IR filter cut 52 and the Yellow 495. 52 mm F-PRO MRC 022 filter to reduce the component of the UV spotlight, thus better highlighting possible fluorescence effects.52 Fourier Transform Infrared (FT-IR) spectroscopy in the Attenuated Total Reflectance (ATR) mode was performed on the swabs used for the cleaning tests as well to further confirm the presence of the wax–solvent mixture on them, hence assessing the actual interaction between the solvent and the solute. The IR spectra were collected using the Nicolet Summit FTIR spectrometer equipped with the Everest™ Diamond ATR accessory. A total of 32 scans were performed on each sample with an instrumental resolution of 8 cm−1.
Results
Solubility tests
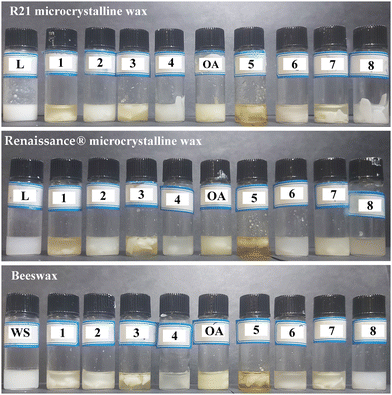
Fig. 1 shows the dissolution degree of the waxes in the tested solvents. Total dissolution was not achieved with any of the tested DESs, including reference solvents that are commonly used for the removal of wax layers from works of art, namely Ligroin 100–140 °C for the microcrystalline waxes and White Spirit for the beeswax. Indeed, the interaction between the waxes and the solvents resulted in turbid colloidal dispersions instead of solutions. This effect is explained by the fact that the waxes form opaque colloidal systems in nonpolar organic solvents, by having particles with a size of few nanometers to 1 μm range.53,54Due to the formation of colloidal dispersions, turbidity measurements were used to assess the degree of solubilization of the waxes in each solvent. High Nephelometric Turbidity Unit (NTU) values imply significant cloudiness of the mixture, meaning the formation of intermolecular interactions among the solvents and the waxes with the formation of the suspension. Conversely, low NTU values highlight a lack of interaction between the wax and the solvent. Fig. 2 illustrates the NTU values for each solvent–wax combination normalized to the reference solvents, i.e., Ligroin and White Spirit. The degree of dispersion of the microcrystalline wax R21 in each solvent can be defined as follows: L > DES 2 > DES 4 > O.A. > DES 6 > DES 3 > DES 1 > DES 8 > DES 5 > DES 7. As to the Renaissance® wax polish, turbidity measurements showed that: DES 2 > O.A. > DES 7 > L > DES 6 > DES 8 > DES 4 > DES 1 > DES 3 > DES 5. Fewer Deep Eutectic Solvents had the same dispersive effect on the beeswax, probably due to the presence of more polar groups in the beeswax chemical composition (complex wax esters, linear wax monoesters and hydroxy monoesters, free fatty acids, and free fatty alcohols).55 The turbidity of the beeswax–solvent mixtures can be summarized as follows: WS > DES 8 > O.A. > DES 2 > DES 6 > DES 1 > DES 7 > DES 4 > DES 3 > DES 5.
Contact angle measurements
Contact angle measurements demonstrated the tendency of each solvent to spread over the surface or be repelled by it, thus defining the wettability of the three waxes by the tested DESs and references (Fig. 3). The poor wettability of the substrate by water is evidenced by the high contact-angle values, ranging from 91.4° ± 1.5 to 120° ± 0.3. On the other hand, the hydrophobicity of the reference solvents and DESs caused a high reduction of the contact angle values, highlighting the overall wettability of the three waxes by these formulations. As to the microcrystalline dispersion R21, the solvents showing the lower contact angle values, meaning the higher wettability, are as follows: WS, DES 4, DES 6, DES 2, and L, while higher values were acquired for DES 8, O.A., DES 7, DES 1, DES 3, DES 5. Results obtained from the analysis of Renaissance® wax showed similar results, even though outlining the general lower wettability by all the hydrophobic solvents, except for White Spirit. Indeed, only WS and DES 2 provided values lower than 20° – respectively 2.2° ± 1.3 and 19.9° ± 1.6. The results related to the other formulations can be summarized as follows (from the lower to the higher values): L, O.A., DES 8 = DES 6, DES 1, DES 7, DES 4, DES 3, DES 5.Beeswax exhibited a general lower wettability by the tested solvents, whose contact-angle values are in the following order (from the lower to the higher one): WS, DES 2, DES 8, DES 1 = DES 7 = O.A., DES 6, DES 4, L, DES 3, DES 5.
Cleaning tests
| Solvent | ΔE* values | ||
|---|---|---|---|
| R21 | Renaissance® | Beeswax | |
| Deionized water | 28.64 | 17.41 | 7.87 |
| DES 1 | 13.66 | 16.02 | 3.06 |
| DES 2 | 8.04 | 0.76 | 2.38 |
| DES 3 | 24.21 | 16.77 | 7.01 |
| DES 4 | 13.34 | 4.58 | 5.56 |
| DES 5 | 27.01 | 16.76 | 7.00 |
| DES 6 | 12.99 | 15.55 | 6.19 |
| DES 7 | 22.86 | 9.96 | 5.34 |
| DES 8 | 14.03 | 15.83 | 2.54 |
| Ligroin/white spirit | 4.44 | 0.63 | 2.33 |
| Octanoic acid | 23.67 | 10.46 | 5.69 |
For R21, the solvents’ efficacy based on spectrocolorimetric analysis can be defined as follows: L > DES 2 > DES 6 > DES 4 > DES 1 > DES 8 > DES 7 > O.A. > DES 3 > DES 5 > H2. As to the Renaissance® wax polish, the results can be summarized as L > DES 2 > DES 4 > DES 7 > O.A. > DES 6 > DES 8 > DES 1 > DES 5 > DES 3 > H2O. Finally, beeswax's ΔE values can be explained as WS > DES 2 > DES 8 > DES 1 > DES 7 > DES 4 > DES 6 > DES 5 > DES 3 > H2O. The observations made during the removal of the wax layer, coupled with the results of the spectrocolorimetric measurements, enabled the definition of the cleaning performance on an inert substrate – i.e., glass slides – hence assessing the effective removal of the coating by each solvent. As a general remark, longer contact times (i.e., 3 minutes) allow for more efficient removal of the layer. Also, more hydrophobic DESs seem to interact with beeswax, while lesser with the microcrystalline wax R21. Indeed, beeswax is particularly responsive to DES 2 and 8, but also to DES 1, resulting in the following descending ranking: WS = DES 2 = DES 8, DES 1, DES 4 = O.A. = DES 7, DES 6, DES 3 = DES 5. The results obtained from the cleaning tests on Renaissance® can be summarized in descending order as L = DES 2, DES 4, O.A. = DES 7, DES 1 = DES 8, DES 3 = DES 5 = DES 6 while, as to R21, only DES 2 almost equals the result of the reference solvent (Ligroin). The other solvents appear to be able only to partially remove the wax layer, in the following descending sequence: L, DES 2, DES 1 = DES 4 = DES 6 = DES 8, DES 3 = O.A. = DES 7, DES 5. The results reported in Table 3 summarize the outcome of the cleaning tests, expressed as an average rating based on the observations made during the removal considering the three different contact times.
| Solvent | R21 | Renaissance® | Beeswax |
|---|---|---|---|
| Deionized water | – | – | – |
| Ligroin or white spirit | ***** | ***** | ***** |
| DES 1 | *** | ** | **** |
| DES 2 | **** | ***** | ***** |
| DES 3 | ** | * | * |
| DES 4 | *** | **** | *** |
| Octanoic acid | ** | *** | *** |
| DES 5 | * | * | * |
| DES 6 | *** | * | ** |
| DES 7 | ** | *** | *** |
| DES 8 | *** | ** | ***** |
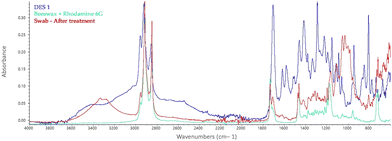
Indeed, the absorption band at 3391 cm−1 is assigned to hydroxyl groups stretching.56 Bands at 2906 cm−1 and 1373 cm−1 are assigned to stretching and deformation vibrations of the C–H group that can be either related to the glucose unit of cellulose, the DESs, or the waxes (Fig. 4). The absorption band at 898 cm−1 is characteristic of β-glycosidic linkage between glucose units.
 | ||
| Fig. 4 FT-IR ATR spectra comparing DES 1, R21 mixed with Rhodamine 6G, and the swab used for the cleaning treatment. | ||
The signal at 1061 cm−1 is assigned to the –C–O– group of DESs’ precursors and secondary alcohols or ethers functions existing in the cellulose chain backbone. Nevertheless, multiple peaks can be specifically attributed to the DESs or the wax, providing information about the dispersion of the substance.
As an example, the spectra acquired on the swabs used for the treatment of each type of wax with DES 1 are shown in Fig. 4–6.
 | ||
| Fig. 5 FT-IR ATR spectra comparing DES 1, Renaissance® mixed with Rhodamine 6G, and the swab used for the cleaning treatment. | ||
 | ||
| Fig. 6 FT-IR ATR spectra comparing DES 1, beeswax mixed with Rhodamine 6G, and the swab used for the cleaning treatment. | ||
As to the microcrystalline wax R21, the characteristic peaks at 725 cm−1 (C–H rocking of alkenes), 2850, and 2920 cm−1 (C–H stretching of alkenes) are visible in all the acquisitions, except for deionized water (Fig. 4).57 At the same time, the peaks at 1720 cm−1 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of carboxylic acids, 1575 and 1620 cm−1 of C
O stretching of carboxylic acids, 1575 and 1620 cm−1 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of cyclic and conjugated alkenes, 1220 and 1290 cm−1 of C–O stretching, 815 cm−1 of C
C stretching of cyclic and conjugated alkenes, 1220 and 1290 cm−1 of C–O stretching, 815 cm−1 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C alkene bending can be associated with the solvent.
C alkene bending can be associated with the solvent.
Due to the nature of the two microcrystalline waxes, the Renaissance® wax shows the same characteristic peaks as the R21 (Fig. 5). Hence, the same considerations can be made, also due the presence of the same bands related to the three DESs that can be seen in the previous spectra.
As to beeswax, the characteristic peaks at 2850 and 2920 cm−1 (C–H stretching of alkenes), 1740 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of esters), 1460 cm−1 (C–H bending of alkanes), 1150 cm−1 (C–O stretching), 960 cm−1 and 725 cm−1 (C
O stretching of esters), 1460 cm−1 (C–H bending of alkanes), 1150 cm−1 (C–O stretching), 960 cm−1 and 725 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending of alkenes) can be seen in the spectrum acquired on the swab after the treatment (Fig. 6) (Dubey, Sharma, & Kumar, 2017). Additionally, the peaks at 1720 cm−1 of C
C bending of alkenes) can be seen in the spectrum acquired on the swab after the treatment (Fig. 6) (Dubey, Sharma, & Kumar, 2017). Additionally, the peaks at 1720 cm−1 of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of carboxylic acids, 1425 cm−1 of O–H bending, and 1150 cm−1 of C–O stretching are related to the solvent.
O stretching of carboxylic acids, 1425 cm−1 of O–H bending, and 1150 cm−1 of C–O stretching are related to the solvent.
Discussion
The degree of dispersion of the waxes in the tested solvents was examined. The results showed that DES 2 and DES 4 produce a higher degree of dispersion on the microcrystalline wax R21, while DES 2, octanoic acid, and DES 7 showed the same good results in the dispersion of the microcrystalline wax Renaissance®. Also, DES 8, octanoic acid, and DES 2 effectively interacted with beeswax. However, it is worth mentioning that the degree of dispersion hinges not only on the affinity between the solvent and the dispersed phase but also on their relative proportion. The presence of solid residues of wax could be related to the oversaturation of the dispersion, thus suggesting that complete dispersion of the wax can be obtained with a higher solvent volume. The R21-DES4, beeswax-DES2, beeswax-O.A., beeswax-DES8, and Renaissance®-DES2 mixtures were highly opaque, possibly entailing the saturation of the dispersion and, in the case of the R21-DES4, beeswax-DES2, and beeswax-O.A., leading to the formation of a semi-solid paste.Contact angle measurements highlighted the general tendency of the DESs to spread onto the waxes’ layers as opposed to deionized water, due to the higher affinity between the DESs and the solute having high hydrophobicity. Cleaning tests enabled the direct evaluation of the solvents’ effectiveness by using the operating procedure commonly employed by restorers – i.e., the swab-cleaning method – thus providing valuable information on the DESs’ actual potential for their use as new sustainable cleaning systems for the removal of nonpolar substances from Cultural Heritage materials. The conclusions resulting from the cleaning tests, multispectral imaging, and spectrocolorimetry show that DES 2 almost equals the efficacy of Ligroin in removing the R21 microcrystalline wax, while DES 2 and DES 4 have a greater effect on the cleaning of the Renaissance® wax polish. In addition, DES 2, DES 8, and DES 1 were able to remove the beeswax layer successfully. Also, all the proposed formulations formed a semi-solid paste with the wax, allowing for easy removal of the solvent–solute mixture from the specimens’ surface.
FT-IR ATR analyses confirmed the actual interaction between the solvent and the solute during the cleaning treatment, thus disproving the hypothesis that the removal of the layer may have been due to mechanical action. Indeed, the presence of both solvent and wax residues on the swabs used for the cleaning treatment showed that the agglomerated particles of the wax are effectively separated from each other through the action of the solvent.
Based on the whole study, it is possible to conclude that the combination L-(−)-menthol–cctanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DES 2) shows a higher affinity with the three types of wax. Besides, promising results are also given by the application of DES 2 on the microcrystalline wax Renaissance® and by the utilization of dodecanoic acid–decanoic acid 1
1 (DES 2) shows a higher affinity with the three types of wax. Besides, promising results are also given by the application of DES 2 on the microcrystalline wax Renaissance® and by the utilization of dodecanoic acid–decanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (DES 8), followed by DES 2, and thymol–Decanoic acid 1
2 (DES 8), followed by DES 2, and thymol–Decanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DES 1) for the removal of beeswax.
1 (DES 1) for the removal of beeswax.
The different solvent–solute interactions observed in the experimentation can be related to the different compositions of the three waxes. Indeed, microcrystalline waxes are produced by de-oiling petrolatum, as part of the petroleum refining process. While paraffin wax contains mostly unbranched alkanes, microcrystalline wax is composed of a higher percentage of isoparaffinic (branched) hydrocarbons and naphthenic hydrocarbons.56 Indeed, the presence of cyclic hydrocarbons in their composition explains the better dissolution of the microcrystalline wax R21 in DESs based on L-(−)-menthol. Their respective structures interact due to the formation of weak intermolecular forces involving the hydrophobic portions of the molecules – i.e., cyclic hydrocarbons. This phenomenon becomes particularly evident when DES 4 and DES 6 are compared. While DES 6 consists of equal proportions of L-(−)-menthol and thymol – containing an aromatic structure instead – DES 4 has twice as much L-(−)-menthol, thus causing greater dissolution of the solute.
Even though the Renaissance® wax polish is a microcrystalline wax as well, it does not contain naphthenic hydrocarbons. According to the information provided by producers, it is a mixture of waxes containing polyethylene wax – i.e., ultra-low molecular weight polyethylene based on ethylene monomer chains – which makes it more stable compared to other microcrystalline waxes intended for conservation.58
This difference in the composition between the two microcrystalline waxes may be the cause of their different interaction with the tested solvents. Octanoic acid seems to have greater compatibility with the Renaissance® wax, probably due to the chain length of low-molecular weight-based waxes. Indeed, low-molecular-weight waxes might have C8–C9 alkyl chains which could better interact with the alkyl chains of octanoic acid.
Even though differences have been pointed out between the two microcrystalline waxes, the intermolecular forces driving their interaction with the proposed solvents presumably consist of London dispersion forces involving the linear alkyl chains of the solvents – i.e., carboxylic acids – the branched hydrocarbons of the waxes, and the cyclic structures of both L-(−)-menthol and R21 microcrystalline wax.
Eventually, beeswax is a complex mixture of hydrocarbons (12–16%) with a predominant chain length of C27–C33, free fatty acids (12–14%), with a chain length of C24–C32, linear wax monoesters and hydroxy monoesters (35–45%) with chain lengths generally of C40–C48, complex wax esters (15–27%), and exogenous substances.53 The presence of long alkyl chains, –OH, –COOR′, and –COOH groups in beeswax’ structure explains the broad range of solubility of beeswax in low- and medium-polar solvents.56 Also, the higher interaction between beeswax and DES 8 can be related to the presence of fatty acids having –COOH groups, similar to beeswax, and the longer alkyl chains among the tested acids. As to carboxylic-acids-containing DESs, the interaction between the solvents and beeswax can be mainly ascribed to the formation of hydrogen bonds between the polar moieties of the solvent and the beeswax. The carbonyl group –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carboxylic acids can form hydrogen bonds with both the hydroxyl groups –O–H of fatty acids and wax hydroxy monoesters contained in the beeswax. Likewise, the hydroxyl group of the solvent can form hydrogen bonds with the carbonyl group of wax's fatty acids, monoesters, and hydroxy monoesters. London dispersion forces play a significant role as well, by involving the hydrophobic moieties of the solvent and the solute. These interactions tend to increase with increasing the chain length, due to the higher number of electrons able to generate more instantaneous dipoles. Indeed, the reduction of the alkyl chain's length – as with octanoic acid-based and L-(−)-menthol or thymol-based DESs – the action on beeswax decreases, even though the hydroxyl groups should partially interact with the ones present in the wax.
O of carboxylic acids can form hydrogen bonds with both the hydroxyl groups –O–H of fatty acids and wax hydroxy monoesters contained in the beeswax. Likewise, the hydroxyl group of the solvent can form hydrogen bonds with the carbonyl group of wax's fatty acids, monoesters, and hydroxy monoesters. London dispersion forces play a significant role as well, by involving the hydrophobic moieties of the solvent and the solute. These interactions tend to increase with increasing the chain length, due to the higher number of electrons able to generate more instantaneous dipoles. Indeed, the reduction of the alkyl chain's length – as with octanoic acid-based and L-(−)-menthol or thymol-based DESs – the action on beeswax decreases, even though the hydroxyl groups should partially interact with the ones present in the wax.
Conclusions
The experimental process provided valuable results proving hydrophobic DESs’ potential of being used as sustainable solvents for cleaning treatments on Cultural Heritage materials.Hydrophobic Natural Deep Eutectic Solvents proved to be potentially suitable alternatives to more toxic organic solvents that are currently popular in the conservation of Cultural Heritage sector. Indeed, NADESs have low toxicity for the operator, as they consist of natural and biocompatible substances and have low volatility. This latter aspect has a twofold implication: a reduced amount of solvent is needed, since it does not evaporate and continues to interact with the coating to be removed as long as required, making the treatment fully sustainable; the operator does not inhale solvent vapors, thus avoiding health risks. Also, the low evaporation rate allows for the recovery of the DESs, which can be extracted from the solvent–solute mixture and reused. Several recycling methods are currently being studied for both hydrophilic and hydrophobic deep eutectic solvents – i.e., adsorption with activated carbon, back extraction, anti-solvent addition, crystallization, liquid–liquid extraction, solid–liquid extraction, short-path distillation, supercritical fluid extraction, membrane-based processes, and separation due to density and viscosity differences.59–61 In addition, DESs have physical properties that make them suitable for use in cleaning operations, such as transparency, moderate viscosity, and effective interaction with the material to be removed.
In the present study, the potential of different NADES has been revealed. Specifically, L-(−)-menthol-based DESs–thymol/L-(−)-menthol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in particular – provided positive results in combination with the R21 microcrystalline wax, presumably due to the presence of naphthenic hydrocarbons in the wax’ composition. Octanoic acid-based DESs – i.e., L-(−)-menthol/cctanoic acid 1
2 in particular – provided positive results in combination with the R21 microcrystalline wax, presumably due to the presence of naphthenic hydrocarbons in the wax’ composition. Octanoic acid-based DESs – i.e., L-(−)-menthol/cctanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and dodecanoic acid/octanoic acid 1
1 and dodecanoic acid/octanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 – showed higher interaction with the Renaissance® wax, probably due to the alkyl chains’ length of low-molecular-weight polyethylene waxes and branched hydrocarbons that may resemble that of octanoic acid. Eventually, fatty acid-based DESs with the longer alkyl chains – i.e., dodecanoic acid/decanoic acid 1
3 – showed higher interaction with the Renaissance® wax, probably due to the alkyl chains’ length of low-molecular-weight polyethylene waxes and branched hydrocarbons that may resemble that of octanoic acid. Eventually, fatty acid-based DESs with the longer alkyl chains – i.e., dodecanoic acid/decanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, L-(−)-menthol/octanoic acid 1
2, L-(−)-menthol/octanoic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – show a greater affinity with beeswax due to the presence of the same –COOH functional groups and long alkyl chains in the structure of beeswax’ constituents.
1 – show a greater affinity with beeswax due to the presence of the same –COOH functional groups and long alkyl chains in the structure of beeswax’ constituents.
Future research will focus on the definition of the solubility parameters of hydrophobic deep eutectic solvents to determine the solubility parameters and the areas of the Teas Triangle in which they stand. The cleaning performance will be also evaluated on mockups reproducing Cultural Heritage works, such as stone and metal surfaces, examining once again the presence of solvent residues and the interaction between the hydrophobic NADESs and the substrate.
Author contributions
Chiara Biribicchi: conceptualization, methodology, data curation, writing – original draft preparation, visualization, investigation, writing – reviewing and editing, formal analysis. Andrea Macchia: conceptualization, methodology, data curation, writing – reviewing and editing, formal analysis, supervision, project administration. Gabriele Favero: validation, resources. Romina Strangis: resources. Bartolo Gabriele: resources. Raffaella Mancuso: resources, writing – original draft preparation. Mauro Francesco La Russa: project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by YOuth in COnservation of CUltural Heritage (YOCOCU APS), Rome, IT; the University of Calabria, Arcavacata di Rende, IT; and PON “Ricerca e Innovazione” 2014-2020 (PON R&I FSE-REACT EU), Azione IV.5 “Dottorati su tematiche Green”.Notes and references
- B. Barclay and C. Hett, The Cleaning, Polishing, and Protective Waxing of Brass and Copper, CGI Notes, 2007, 9, 3 Search PubMed.
- D. Comelli, G. Valentini, R. Cubeddu and L. Toniolo, Fluorescence Lifetime Imaging and Fourier Transform Infrared Spectroscopy of Michelangelo's David, Appl. Spectrosc., 2005, 59, 1174–1181 CrossRef CAS PubMed.
- T. J. Shedlosky, K. M. Stanek and G. Bierwagen, in AIC Objects Speciality Group Postprints, 2000, vol. 320, pp. 452–9545.
- L. Robbiola, C. Fiaud and S. Pennec, in ICOM Committee for Conservation Tenth Triennal Meeting, Washington DC, 1993, p. 911.
- E. Vara Fabjan, T. Kosec, V. Kuhar and A. Legat, Mater. Technol., 2011, 45, 585–591 Search PubMed.
- O. Seung-Jun and W. Koang-Chul, J. Korea Convergence Soc., 2018, 9, 151–160 Search PubMed.
- M. Toro, T. Beentjes, I. Joosten, J. Bloser and L. Zycherman, in Metal 2019 – Interim Meeting of the ICOM-CC Metals Working Group, Neuchatel, 2019.
- B. Salvadori, D. Pinna and S. Porcinai, Environ. Sci. Pollut. Res., 2014, 21, 1884–1896 CrossRef CAS PubMed.
- D. E. Couture-Rigert, P. J. Sirois and E. A. Moffatt, Stud. Conserv., 2012, 57, 142–163 CrossRef CAS.
- S. Barreca, M. Bruno, L. Oddo and S. Orecchio, Nat. Prod. Res., 2019, 33, 947–955 CrossRef CAS.
- V. M. Pozhidaev, V. M. Retivov, A. V. Kamaev, S. K. Belus, A. S. Nartov, V. A. Rastorguev, I. V. Borodin, E. Y. Tereschenko, R. A. Sandu, E. B. Yatsishina and M. V. Kovalchuk, Heritage Sci., 2019, 7, 90 CrossRef.
- L. Kubick and J. Giaccai, AIC Objects Specialty Group Postprints, 2012, 19, pp. 45–69.
- G. D’Ercoli, M. Marabelli, V. Santin, A. Buccolieri, G. Buccolieri, A. Castellano and G. Palamà, 9th International Conference on NDT of Art, Jerusalem, 2008.
- I. M. Marcelli and M. Mercalli, in 9th International Conference on NDT of Art, Jerusalem, 2008.
- J. Wolfe, R. Grayburn, H. Khanjian, A. Heginbotham and A. Phenix, ICOM-CC 18th Triennial Conference, Copenhagen, 2017.
- E. Risser and D. Saunders, The restoration of ancient bronzes: Naples and beyond, Getty Publications, Los Angeles, 2013 Search PubMed.
- U. Knutinen and A. Norman, 15th World Conference on Nondestructive Testing, Rome, 2000.
- N. Swartz and T. L. Clare, J. Am. Inst. Conserv., 2015, 54, 181–201 CrossRef.
- O. Seung-Jun and W. Koang-Chul, J. Conserv. Sci., 2017, 33, 121–130 CrossRef.
- G. Cavallaro, S. Milioto and G. Lazzara, Langmuir, 2020, 36, 3677–3689 CrossRef CAS PubMed.
- C. Lim, Collections Care: Staying Relevant in Changing Times, ASEAN & Beyond, Singapore, 2019 Search PubMed.
- S. Kezic, J. Kruse and I. Jakasa, Review of dermal effects and uptake of petroleum hydrocarbons, 2010.
- S. Parasuraman, W. Ping, P. Raj, J. Sujithra, B. Syamittra, W. Yeng, S. Dhanaraj and S. Muralidharan, J. Basic Clin. Pharm., 2014, 5, 89 CrossRef PubMed.
- M. A. Amoruso, J. F. Gamble, R. H. McKee, A. M. Rohde and A. Jaques, Int. J. Toxicol., 2008, 27, 97–165 CrossRef CAS.
- V. Ferrara, A. Macchia and S. Sapia, in Proceedings of 2013 Digital Heritage International Congress, ed. A. C. Addison, L. de Luca, G. Guidi and S. Pescarin, 2013, pp. 409–412 Search PubMed.
- D. Dagostino, A. Macchia, R. Cataldo, L. Campanella and A. Campbell, Int. J. Archit. Heritage, 2015, 9, 290–299 CrossRef.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- A. Macchia, H. Aureli, F. Prestileo, F. Ortenzi, S. Sellathurai, A. Docci, E. Cerafogli, I. A. Colasanti, M. Ricca and M. F. la Russa, Methods Protoc., 2022, 5(3), 37 CrossRef CAS PubMed.
- A. Macchia, C. Biribicchi, P. Carnazza, S. Montorsi, N. Sangiorgi, G. Demasi, F. Prestileo, E. Cerafogli, I. A. Colasanti, H. Aureli, M. Zappelli, M. Ricca and M. F. la Russa, Sustainability, 2022, 14(7), 3972 CrossRef.
- A. Macchia, H. Aureli, C. Biribicchi, A. Docci, C. Alisi, F. Prestileo, F. Galiano, A. Figoli, R. Mancuso, B. Gabriele and M. F. la Russa, Materials, 2022, 15(16), 5671 CrossRef CAS.
- L. Randazzo, M. Ricca, D. Pellegrino, D. la Russa, A. Marrone, A. Macchia, L. Rivaroli, F. Enei and M. F. la Russa, Int. J. Conserv. Sci., 2020, 11, 243–250 CAS.
- A. Macchia, O. Bettucci, E. Gravagna, D. Ferro, R. Albini, B. Mazzei and L. Campanella, J. Nanomater., 2014, 2014, 167540 Search PubMed.
- T. E. Achkar, H. Greige-Gerges and S. Fourmentin, Environ. Chem. Lett., 2021, 19, 3397–3408 CrossRef.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- C. Florindo, L. C. Branco and I. M. Marrucho, ChemSusChem, 2019, 12, 1549–1559 CrossRef CAS PubMed.
- C. Florindo, L. Romero, I. Rintoul, L. C. Branco and I. M. Marrucho, ACS Sustainable Chem. Eng., 2018, 6, 3888–3895 CrossRef CAS.
- B. D. Ribeiro, C. Florindo, L. C. Iff, M. A. Z. Coelho and I. M. Marrucho, ACS Sustainable Chem. Eng., 2015, 3, 2469–2477 CrossRef CAS.
- Y. Jia, G. Sciutto, A. Botteon, C. Conti, M. L. Focarete, C. Gualandi, C. Samorì, S. Prati and R. Mazzeo, J. Cult. Herit., 2021, 51, 138–144 CrossRef.
- A. Macchia, R. Strangis, S. de Angelis, M. Cersosimo, A. Docci, M. Ricca, B. Gabriele, R. Mancuso and M. F. la Russa, Materials, 2022, 15(11), 4005 CrossRef CAS PubMed.
- N. Schaeffer, M. A. R. Martins, C. M. S. S. Neves, S. P. Pinho and J. A. P. Coutinho, Chem. Commun., 2018, 54, 8104–8107 RSC.
- C. C. Fernandes, R. Haghbakhsh, R. Marques, A. Paiva, L. Carlyle and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2021, 9, 15451–15460 CrossRef CAS PubMed.
- D. J. G. P. van Osch, C. H. J. T. Dietz, J. van Spronsen, M. C. Kroon, F. Gallucci, M. van Sint Annaland and R. Tuinier, ACS Sustainable Chem. Eng., 2019, 7, 2933–2942 CrossRef CAS.
- F. D. Dick, Occup. Environ. Med., 2006, 63, 221–226 CrossRef CAS PubMed.
- P. Makoś, A. Przyjazny and G. Boczkaj, J. Chromatogr. A, 2018, 1570, 28–37 CrossRef.
- R. Wang, D. Sun, C. Wang, L. Liu, F. Li and Z. Tan, Sep. Purif. Technol., 2019, 215, 102–107 CrossRef CAS.
- D. Ge, Y. Zhang, Y. Dai and S. Yang, J. Sep. Sci., 2018, 41, 1635–1643 CrossRef CAS PubMed.
- M. A. R. Martins, L. P. Silva, N. Schaeffer, D. O. Abranches, G. J. Maximo, S. P. Pinho and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2019, 7, 17414–17423 CrossRef CAS.
- M. Tiecco, F. Cappellini, F. Nicoletti, T. del Giacco, R. Germani and P. di Profio, J. Mol. Liq., 2019, 281, 423–430 CrossRef CAS.
- O. G. Sas, M. Castro, Á. Domínguez and B. González, Sep. Purif. Technol., 2019, 227, 115703 CrossRef CAS.
- K. Li, Y. Jin, D. Jung, K. Park, H. Kim and J. Lee, J. Chromatogr. A, 2020, 1614, 460730 CrossRef CAS.
- F. Bergua, M. Castro, C. Lafuente and M. Artal, J. Mol. Liq., 2022, 368, 120789 CrossRef CAS.
- A. Macchia, C. Biribicchi, L. Rivaroli, H. Aureli, E. Cerafogli, I. A. Colasanti, P. Carnazza, G. Demasi and M. F. la Russa, Methods Protoc., 2022, 5(3), 52 CrossRef CAS PubMed.
- L. Ivanovszky, Waxes: Colloidal Properties and Systems, J. Polym. Sci., 1962, 58, 273–288 CrossRef CAS.
- J. M. Coulson, R. K. Sinnott and J. F. Richardson, Coulson & Richardson's Chemical Engineering – Volume 2A: Particulate Systems and Particle Technology, Butterworth-Heinemann, Oxford, 2019, pp. 693–737 Search PubMed.
- F. Fratini, G. Cilia, B. Turchi and A. Felicioli, Asian Pac. J. Trop. Med., 2016, 9, 839–843 CrossRef CAS PubMed.
- M. Aqil, B. Abderrahim, E. Abderrahman, A. Mohamed, T. Fatima, T. Abdesselam and O. Krim, World J. Environ. Eng., 2015, 3, 95–110 Search PubMed.
- P. Dubey, P. Sharma and V. Kumar, Data Brief, 2017, 15, 615–622 CrossRef PubMed.
- C. v Horie, Materials for Conservation, Routledge, Abingdon, 2010 Search PubMed.
- A. Isci and M. Kaltschmitt, Biomass Convers. Biorefin., 2022, 12, 197–226 CrossRef.
- C. Florindo, F. Lima, L. C. Branco and I. M. Marrucho, ACS Sustainable Chem. Eng., 2019, 7, 14739–14746 CrossRef CAS.
- W. Zhu, P. Jin, M. Cheng, H. Yang, M. Du, T. Li, G. Zhu and J. Fan, Talanta, 2021, 233, 122523 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |