Open Access Article
Open Access ArticleSelective ATP recognition by boronic acid-appended cyclodextrin and a fluorescent probe supramolecular complex in water†
Yota
Suzuki‡
 ,
Masakage
Masuko‡
,
Takeshi
Hashimoto
,
Masakage
Masuko‡
,
Takeshi
Hashimoto
 and
Takashi
Hayashita
and
Takashi
Hayashita
 *
*
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1, Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan. E-mail: ta-hayas@sophia.ac.jp
First published on 24th February 2023
Abstract
Various organic compound-based chemosensors for adenosine triphosphate (ATP) have been reported; however, the conventional designs have problems with their water solubility, selectivity, and sensitivity. Herein, a supramolecular complex of boronic acid-appended γ-cyclodextrin and zinc-dipicolylamine-based pyrene probe (Zn-1/FBγCyD) was designed based on the concept of a “cyclodextrin (CyD)-based supramolecular chemosensor”. As a proof-of-concept, we have demonstrated that this complex exhibits a turn-on fluorescence response to ATP in water at physiological pH with excellent selectivity and sensitivity, owing to the efficient self-assembly of Zn-1/FBγCyD with ATP through synergistic interactions to afford a strongly fluorescent product. Remarkably, Zn-1/FBγCyD demonstrated extremely strong affinity for ATP with the conditional formation constant of (7.71 ± 0.09) × 106 M−1, resulting in the low limit of detection of 18.9 nM. Furthermore, Zn-1/FBγCyD can monitor changes in ATP concentration after the addition of apyrase, hydrolysing ATP to adenosine monophosphate and a phosphate ion. We believe that the presented approach of a CyD-based supramolecular chemosensor will propose a novel design that realises unexplored selectivity in the research field of fluorescent molecular recognition.
Introduction
The field of fluorescence recognition for application to the fluorometric sensing of biologically important analytes has advanced tremendously.1,2 In particular, chemosensors for adenosine triphosphate (ATP) have attracted significant attention given the various important roles that ATP plays in biological processes as the energy currency of cells;3 for example, metabolism,4 cellular signalling,5 and protein synthesis.6 The chemosensing approach for the detection of ATP has several advantages, including ease of use, low cost, and high accuracy, overcoming the drawbacks of the conventional luciferin–luciferase assay for ATP sensing, which has low stability and accuracy and requires cumbersome operations.7,8The strategic design of ATP-selective chemosensors is mainly based on multitopic receptors that capture several recognisable moieties of ATP, namely, the ribose, triphosphate, and adenine units.3,9,10 Despite extensive developments in this research area, most previously designed chemosensors are based on organic scaffolds and are sometimes poorly soluble in water requiring 10 vol% or more organic solvent.11–14 Moreover, designing highly selective ATP chemosensors has been challenging because of structural similarities among phosphate derivatives including polyphosphates and nucleotides.15,16 Selectivity is an important issue for applications in biological research; for example, chemosensors that allow for the discrimination of ATP from adenosine diphosphate (ADP), adenosine monophosphate (AMP), and the pyrophosphate ion (PPi) are useful in gaining a better understanding of cellular processes.17,18 However, conventional strategies require careful organic synthesis and given the difficulty in attaining high selectivity for ATP, reports of ideal chemosensors that exhibit a turn-on response to only ATP have been scarce.
We previously presented a concept of multidentate recognition by water-soluble supramolecular chemosensors based on binding unit-appended cyclodextrins (CyDs) with fluorescent and colorimetric probes.19–22 As the assembly behaviour of CyD is highly sensitive to the structures of the components, the CyD-based supramolecular chemosensors display a variety of responses depending on the analyte structure: this feature is advantageous because it provides excellent selectivity for the target analyte (Fig. 1a). Based on these previous studies, we report a supramolecular complex (Zn-1/FBγCyD) of fluorophenylboronic acid-appended γCyD (FBγCyD) and a Zn(II)-dipicolylamine (Zn-dpa)-based fluorescent probe (Zn-1) as a proof-of-concept to design a multitopic CyD-based supramolecular chemosensor for ATP showing turn-on fluorescence response (Fig. 1b). FBγCyD has one fluorophenylboronic acid moiety introduced at the C3 position of one 1,4-glycopyranoside unit on the secondary rim. The boronic acid and Zn-dpa moieties capture the ribose and triphosphate units of ATP, respectively.11,17 The electron-withdrawing fluorine group is introduced to increase the Lewis acidity of the boron centre; boronic acids with high acidity react with 1,2-diol compounds efficiently.23 The Zn-1/FBγCyD complex displays specific fluorescence enhancement in response to only ATP.
 | ||
| Fig. 1 (a) Cartoon representation of the concept of CyD-based supramolecular chemosensors based on a modified CyD with fluorescent probes. (b) Structure of Zn-1/FBγCyD. | ||
Experimental
Reagents
All reagents were purchased from commercial sources and used as received without further purification. The detailed information is summarised in the ESI.†Instruments
All the nuclear magnetic resonance (NMR) spectra were measured by using a JEOL JNM-ECA 500 spectrometer (JEOL, Japan) at room temperature. For 1H and 13C NMR spectroscopy, the solvent signals were used as ref. 24 The high resolution electrospray ionization mass spectra (HRMS-ESI) were recorded in methanol using a JEOL Accu-TOF JMS T100LC (JEOL, Japan). The high resolution fast atom bombardment mass spectra (HRMS-FAB) were recorded using a MStation JMS-700 (JEOL, Japan). Solution pH was measured by using a HORIBA pH meter F-52 (Horiba, Japan). The UV-vis absorption spectra were measured using a Hitachi U-3900H absorption spectrophotometer (Hitachi, Japan) equipped with a temperature controller (Hitachi, Japan) to keep the temperature constant at 25 °C. The fluorescence spectra were measured using a Hitachi F-7000 fluorescence spectrophotometer (Hitachi, Japan) and a Hitachi F-7100 fluorescence spectrophotometer (Hitachi, Japan) equipped with a temperature controller (Hitachi, Japan) and an EYELA CCA-1111 (EYELA, Japan) to keep the temperature constant at 25 °C. The induced circular dichroism (ICD) spectra were measured using a JASCO J-820 spectrophotometer (JASCO, Japan) equipped with a Peltier temperature controller (JASCO, Japan) and an EYELA Cool Ace CA-1111 (EYELA, Japan) to keep the temperature constant at 25 °C under a nitrogen atmosphere.Preparation of sample solutions
All sample solutions were prepared with Milli-Q water and spectroscopic-grade dimethyl sulfoxide (DMSO) using acid-washed glassware. Unless otherwise noted, a mixed solvent system of DMSO/water (1/99 in v/v) was used. The pH of the solutions for spectroscopic measurements was adjusted to 7.4 with diluted aq. HCl and aq. NaOH in the presence of 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer. Appropriate amounts of DMSO and the following stock solutions: probe 1 DMSO solution (10 mM), an aq. zinc(II) ion (4 mM), aq. FBγCyD (5 mM), a pH buffer solution (50 mM), and an aq. phosphate derivative, were added into a volumetric flask to adjust the concentrations for each experimental condition. Basically, the sample solutions contain 10 μM of 1, 10 μM of Zn2+, 0.1 or 0.5 mM of FBγCyD, and 5 mM of HEPES in DMSO/water (1/99 in v/v). Before the measurements of the fluorescence and UV-vis absorption spectra, all the sample solutions were vortexed for 10 seconds.Determination of acid dissociation constant
The absorbance of FBγCyD at a specific wavelength was measured and analysed using the KaleidaGraph program based on the acid dissociation model of monobasic acids.Determination of inclusion constants
Fluorescence intensity changes (F − F0) of Zn-1 at various concentrations of cyclodextrin compounds were analysed using KaleidaGraph program to determine the inclusion constants.Determination of conditional equilibrium constants
Multi-wavelength data (360–600 nm) of the fluorescence spectra at various concentrations of phosphate derivatives were analysed using the ReactLab EQUILIBRIA program to determine the conditional equilibrium constants.25Monitoring of the change in ATP concentrations
Fluorescence intensity changes at 386 nm were monitored for 1200 seconds with stirring using an Hitachi F-7100 fluorescence spectrophotometer after the apyrase addition (100 mU, 50 mU, and 10 mU) into Zn-1/FBγCyD-ATP solution. During these measurements, the temperature was kept constant at 25 °C.Results and discussion
Probe 1 and modified CyDs were readily synthesised (see ESI† and Fig. S1–S4). Zn-1 was prepared by the addition of zinc nitrate (10 μM) to an equimolar amount of 1 (C1 = 10 μM) in a mixed solvent of DMSO and water (1/99 in vol.). The acid dissociation constant (pKa) of the boronic acid moiety was determined to be 7.92 ± 0.02 from the pH-dependence of the UV-vis absorption spectra of FBγCyD (Fig. S5 and S6, ESI†), indicating that 23% of FBγCyD is present as the conjugate tetrahedral boronate anion at pH 7.4 (Scheme S1, ESI†).23The fluorescence intensity of Zn-1 was enhanced by increasing the concentration of coexisting FBγCyD (Fig. S7, ESI†), suggesting that the supramolecular encapsulation of Zn-1 by FBγCyD suppresses the non-radiative decay of the excited state.26 Although the γCyD cavity forms a pyrene dimer that exhibits dimeric fluorescence at approximately 500 nm,27 this was not observed for the supramolecular complex of FBγCyD with Zn-1. This finding suggests that FBγCyD and Zn-1 form a supramolecular complex in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio, i.e., Zn-1/FBγCyD. The changes in the fluorescence intensity of Zn-1 at various FBγCyD concentrations were well fitted to the 1
1 stoichiometric ratio, i.e., Zn-1/FBγCyD. The changes in the fluorescence intensity of Zn-1 at various FBγCyD concentrations were well fitted to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric inclusion model, with an inclusion constant of (3.08 ± 0.08) × 105 M−1 (Fig. S7, ESI†). In contrast, the fluorescence enhancement observed with the addition of FBγCyD was not apparent when native γCyD was added to Zn-1 (Fig. S8, ESI†); the inclusion constant was much smaller at (4.25 ± 0.73) × 103 M−1 (Fig. S9, ESI†). These findings suggest that the incorporated boronic acid moiety of FBγCyD facilitates the tight supramolecular complexation of Zn-1/FBγCyDvia electrostatic attraction between the positive Zn-dpa moiety of Zn-1 and the negative boronate species of FBγCyD partially present in the solution.
1 stoichiometric inclusion model, with an inclusion constant of (3.08 ± 0.08) × 105 M−1 (Fig. S7, ESI†). In contrast, the fluorescence enhancement observed with the addition of FBγCyD was not apparent when native γCyD was added to Zn-1 (Fig. S8, ESI†); the inclusion constant was much smaller at (4.25 ± 0.73) × 103 M−1 (Fig. S9, ESI†). These findings suggest that the incorporated boronic acid moiety of FBγCyD facilitates the tight supramolecular complexation of Zn-1/FBγCyDvia electrostatic attraction between the positive Zn-dpa moiety of Zn-1 and the negative boronate species of FBγCyD partially present in the solution.
To determine the selectivity of Zn-1/FBγCyD for ATP, the UV-vis absorption and fluorescence spectra of Zn-1/FBγCyD were measured in the absence and presence of various phosphate derivatives: phosphate ion (Pi), PPi, triphosphate ion (Tri), AMP, ADP, and ATP (Chart S1, ESI†). Whereas no differences in the UV-vis absorption spectra were observed (Fig. S10, ESI†), the fluorescence spectra varied depending on the added phosphate derivatives (Fig. 2). In the absence of the phosphate derivatives, Zn-1/FBγCyD exhibited a vibronic-structured fluorescence band in the 370 to 450 nm region, which is a characteristic spectral pattern of pyrene compounds.28 The addition of the inorganic polyphosphates (PPi and Tri) resulted in a clear reduction in fluorescence intensity. The PPi moiety efficiently coordinates to the Zn(II) centres of two Zn-dpa moieties to form a bis-dentate complex, i.e., PPi-bis[Zn-dpa].15 As shown in the normalised spectra of Fig. 2a, the dimeric fluorescence of the pyrene moieties was clearly observed at approximately 500 nm in the presence of PPi and Tri (Fig. S11, ESI†). Thus, the decrease in fluorescence is attributable to the formation of the inclusion complex of the Zn-1 dimer with FBγCyD and the inorganic polyphosphates, PPi- and Tri-(Zn-1)2/FBγCyD (Chart S2, ESI†), which quenches the monomeric fluorescence. Pi, AMP, and ADP caused almost no changes in the fluorescence spectra of Zn-1/FBγCyD, suggesting that these phosphate derivatives inefficiently react with Zn-1/FBγCyD. Surprisingly, Zn-1/FBγCyD exhibited a fluorescence turn-on response to only ATP, demonstrating its excellent selectivity for ATP: this supramolecular complex can discriminate between ATP and other typical interfering phosphate derivatives, such as PPi and ADP, which give indistinguishable fluorescence spectra in many reported systems.15 Notably, the change in fluorescence colour was visible to the naked eye (Fig. 2c).
The fluorescence spectra of Zn-1/FBγCyD were measured at various concentrations of the phosphate derivatives (Fig. 3 and Fig. S12–S17, ESI†). As shown in Fig. 2, the fluorescence enhancement of Zn-1/FBγCyD was observed only with the addition of ATP. The change in fluorescence intensity reached saturation when the total concentration of ATP was 10 μM (the same as Zn-1 concentration): this result signifies that Zn-1/FBγCyD quantitatively reacts with ATP. The conditional equilibrium constant was determined to be (7.71 ± 0.09) × 106 M−1 according to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (Fig. S18, ESI†). The extremely large affinity gave rise to the excellent sensitivity to ATP, with a low limit of detection of 18.9 nM (Fig. S19, ESI†). For PPi and Tri, spectral changes were well fitted to the 2
1 binding model (Fig. S18, ESI†). The extremely large affinity gave rise to the excellent sensitivity to ATP, with a low limit of detection of 18.9 nM (Fig. S19, ESI†). For PPi and Tri, spectral changes were well fitted to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (probe:phosphate), supporting that Zn-1 forms dimers with PPi and Tri inside the FBγCyD cavity (Fig. S20 and S21, ESI†).
1 binding model (probe:phosphate), supporting that Zn-1 forms dimers with PPi and Tri inside the FBγCyD cavity (Fig. S20 and S21, ESI†).
As competitive experiments, the fluorescence spectra of Zn-1/FBγCyD with ATP (Zn-1/FBγCyD-ATP) were measured in the presence of the other phosphate derivatives as competitors (Fig. S22, ESI†). The fluorescence intensity of Zn-1/FBγCyD-ATP remained unchanged by the addition of AMP and ADP, demonstrating the high selectivity of the complex for ATP even in the presence of other adenine nucleotides. Although the additions of PPi and Tri decreased the fluorescence intensity of Zn-1/FBγCyD-ATP, the turn-on response of Zn-1/FBγCyD to ATP was partially preserved. Given that ATP is present in large amounts among phosphate derivatives in both intracellular and extracellular environments,29 the presence of competitors should not significantly affect the sensitivity to ATP. Taken together, Zn-1/FBγCyD is potentially useful in practical situations where ATP measurement is performed in the presence of other phosphate derivatives.
To clarify the role of the boronic acid moiety of FBγCyD, the fluorescence spectra of Zn-1 with phosphate derivatives were measured in the absence of FBγCyD (Fig. S23, ESI†) and the presence of native γCyD (Fig. S24, ESI†) and phenyl-appended γCyD (PhγCyD, Fig. S25, ESI†). In all the systems, fluorescence enhancement was observed in response to all phosphate derivatives except Pi, suggesting that Zn-1/γCyD, Zn-1/PhγCyD, or Zn-1 alone shows no sensitivity to ATP. The observed fluorescence enhancement is likely due to the suppression of the photo-induced electron transfer quenching process by the complexation of the Zn-dpa moiety with the phosphate moieties, which decreases the cationic character of the zinc centre.15 These results clearly show that the boronic acid moiety incorporated into FBγCyD plays an essential role in improving the selectivity for ATP, plausibly due to the binding of the boronic acid moiety to the 1,2-diol moiety of ATP. Therefore, the observed ATP selectivity of Zn-1/FBγCyD is surely originated from the multiple bindings with ATP. In other words, Zn-1/FBγCyD possesses binding sites that are structurally unsuitable for the multidentate recognition of AMP and ADP, and FBγCyD facilitates the formation of PPi- and Tri-(Zn-1)2/FBγCyD with PPi and Tri, respectively, which results in the quenching of the monomer fluorescence. Such dimer formation does not occur in the Zn-1/γCyD and Zn-1/PhγCyD systems because γCyD and PhγCyD do not tightly encapsulate Zn-1. As mentioned, multidentate recognition by the Zn-dpa moiety and the boronic acid moiety of Zn-1/FBγCyD was suggested in binding to ATP. As controls, the fluorescence spectra of Zn-1/γCyD and Zn-1/PhγCyD were measured in the presence of ATP (Fig. S26, ESI†). Both supramolecular complexes showed much weaker fluorescence than Zn-1/FBγCyD-ATP. This suggests that the boronic acid moiety rigidifies the pyrene fluorophore through multidentate recognition and therefore supports the expected multidentate recognition.
The supramolecular complex structures of CyD with a pyrene compound vary depending on the ring size of CyD; pyrene compounds form 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (pyrene:CyD) stoichiometric supramolecular complexes with γCyD and βCyD, respectively.27 Therefore, the effect of CyD ring size on selectivity was also investigated by using native βCyD and fluorophenylboronic acid-appended βCyD (FBβCyD) instead of FBγCyD (Chart S3, ESI†). The corresponding βCyD-based supramolecular complexes, i.e., Zn-1/βCyD (Fig. S27, ESI†) and Zn-1/FBβCyD (Fig. S28, ESI†), showed similar trends with Zn-1 alone, Zn-1/γCyD, and Zn-1/PhγCyD, exhibiting fluorescence enhancement in response to all phosphate derivatives except Pi. This finding indicates that the signaling mechanisms of Zn-1/βCyD and Zn-1/FBβCyD are essentially the same as Zn-1 alone, Zn-1/γCyD, and Zn-1/PhγCyD, clearly signifying that the FBγCyD host provides overwhelmingly superior ATP selectivity compared with other CyD hosts.
1 (pyrene:CyD) stoichiometric supramolecular complexes with γCyD and βCyD, respectively.27 Therefore, the effect of CyD ring size on selectivity was also investigated by using native βCyD and fluorophenylboronic acid-appended βCyD (FBβCyD) instead of FBγCyD (Chart S3, ESI†). The corresponding βCyD-based supramolecular complexes, i.e., Zn-1/βCyD (Fig. S27, ESI†) and Zn-1/FBβCyD (Fig. S28, ESI†), showed similar trends with Zn-1 alone, Zn-1/γCyD, and Zn-1/PhγCyD, exhibiting fluorescence enhancement in response to all phosphate derivatives except Pi. This finding indicates that the signaling mechanisms of Zn-1/βCyD and Zn-1/FBβCyD are essentially the same as Zn-1 alone, Zn-1/γCyD, and Zn-1/PhγCyD, clearly signifying that the FBγCyD host provides overwhelmingly superior ATP selectivity compared with other CyD hosts.
The induced circular dichroism (ICD) spectra of Zn-1/FBγCyD were measured in the absence and presence of phosphate derivatives to further extract structural information (Fig. S29, ESI†). The supramolecular complexation of FBγCyD with Zn-1 led to a strongly positive Cotton effect at 350 nm. This result empirically demonstrates that Zn-1 is encapsulated by the cavity of FBγCyD.30 When the phosphate derivatives having pyrophosphate moieties (PPi, Tri, and ATP) were present, the intensity of ellipticity at 350 nm was markedly decreased. This implies that the pyrophosphate moieties coordinate to the Zn-dpa moiety of Zn-1/FBγCyD.
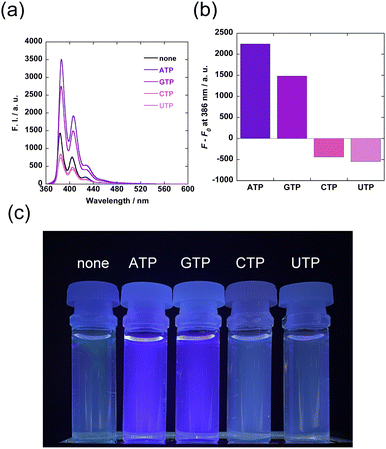
The selectivity of Zn-1/FBγCyD for ATP was further examined by screening other nucleotides (Chart S1, ESI†) including guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP). The additions of CTP and UTP attenuated the fluorescence intensity, whereas the addition of GTP enhanced the fluorescence intensity (Fig. 4). These results suggest that the purine bases play a vital role in triggering the turn-on response of Zn-1/FBγCyD. The adenine moiety of ATP may form π–π stacking with the pyrene moiety of the Zn-1 unit in the CyD cavity to further rigidify the Zn-1 unit, given that CyD cavities encapsulate the adenine moiety in water.31,32 As Zn-1/FBγCyD-ATP emits stronger fluorescence than Zn-1/FBγCyD-GTP, the assembly with ATP is expected to be more efficient than with GTP.
The obtained results indicate that Zn-1 and FBγCyD form a rigid supramolecular complex that features three-point recognition including (1) esterification of the boronic acid and ribose moieties, (2) complexation of the Zn-dpa and triphosphate moieties, and (3) π–π stacking between the pyrene and adenine moieties (Fig. 5). On the contrary, the additions of PPi and Tri result in fluorescence reduction, as the formation of the Zn-1 dimer leads to quenching of the monomeric fluorescence. For the recognition of AMP and ADP, the binding sites of Zn-1/FBγCyD are structurally mismatched; hence, the excellent selectivity for ATP is due to the various responses of the CyD-based supramolecular chemosensor to different analyte structures.
Zn-1/FBγCyD was evaluated for potential application to the monitoring of ATP concentrations. Apyrase was added to a solution containing Zn-1/FBγCyD and ATP (30 μM) in order to hydrolyse ATP via conversion into ADP and AMP, as follows: (1) ATP → ADP + Pi, and (2) ADP → AMP + Pi.33 Changes in fluorescence intensity at 386 nm were monitored during this hydrolysis reaction (Fig. 6). Zn-1/FBγCyD was robust to apyrase under these experimental conditions (Fig. S30, ESI†). After the addition of apyrase, the fluorescence intensity of Zn-1/FBγCyD-ATP at 386 nm gradually decreased with time. The rate of hydrolysis of ATP increased with increasing apyrase concentration; this clearly shows that ATP is hydrolysed by apyrase. After the hydrolysis reaction was completed, the fluorescence of Zn-1/FBγCyD was recovered (Fig. S31 and S32, ESI†). These results suggest that ATP is completely hydrolysed to AMP and Pi without any influence on the Zn-1/FBγCyD fluorescence spectra (Fig. 2). Consequently, Zn-1/FBγCyD allows for the monitoring of changes in ATP concentration with time. This feature is potentially advantageous when applied to biological studies to examine cellular processes relating to ATP production and consumption.
Conclusions
We have designed a CyD-based supramolecular chemosensor, Zn-1/FBγCyD, which selectively recognises ATP and allows for the monitoring of changes in ATP concentration with time. Its excellent selectivity is due to the efficient assembly of Zn-1/FBγCyD with ATP, which is facilitated by synergistic multi-recognition to form a fluorescent supramolecular product. Surprisingly, Zn-1/FBγCyD demonstrated extremely strong affinity for ATP, achieving the detection of ATP with nM levels. In other words, we have demonstrated that the concept of a CyD-based supramolecular chemosensor has benefits providing various advantageous features compared to simple fluorescent probes, such as water solubility, excellent selectivity, and sensitivity. We believe that Zn-1/FBγCyD not only has the potential to serve as a basic structure of ATP chemosensing systems but also presents a desirable approach to exploit the concept of a CyD-based supramolecular chemosensor, which will be useful in various fields relating to molecular recognition. This presented concept holds promise to pioneer novel chemosensors for unexplored analytes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by a JSPS Research Fellowship for Young Scientists PD Grant Number 21J00709 (Y.S.), and a JSPS Grant-in-Aid for Scientific Research Grant Number 20H02772 (T. Hayashita). We thank Ms Moeka Takahashi at Sophia University for her support regarding the synthesis of the probe.References
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- A. T. Aron, K. M. Ramos-Torres, J. A. Cotruvo Jr. and C. J. Chang, Acc. Chem. Res., 2015, 48, 2434–2442 CrossRef CAS PubMed.
- B. Huang, B. Liang, R. Zhang and D. Xing, Coord. Chem. Rev., 2022, 452, 214302 CrossRef CAS.
- M. Bonora, S. Patergnani, A. Rimessi, E. De Marchi, J. M. Suski, A. Bononi, C. Giorgi, S. Marchi, S. Missiroli, F. Poletti, M. R. Wieckowski and P. Pinton, Purinergic Signalling, 2012, 8, 343–357 CrossRef CAS PubMed.
- I. Novak, Physiology, 2003, 18, 12–17 CrossRef CAS PubMed.
- M. C. Jewett, M. L. Miller, Y. Chen and J. R. Swartz, J. Bacteriol., 2009, 191, 1083–1091 CrossRef CAS PubMed.
- A. R. Ribeiro, R. M. Santos, L. M. Rosário and M. H. Gil, Luminescence, 1998, 13, 371–378 CrossRef CAS PubMed.
- D. de la Fuente-Herreruela, V. Gónzalez-Charro, V. G. Almendro-Vedia, M. Morán, M. Á. Martín, M. P. Lillo, P. Natale and I. López-Montero, Biochim. Biophys. Acta, Bioenerg., 2017, 1858, 999–1006 CrossRef CAS PubMed.
- Y. Zhou, Z. Xu and J. Yoon, Chem. Soc. Rev., 2011, 40, 2222–2235 RSC.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Chem. Rev., 2022, 122, 3459–3636 CrossRef PubMed.
- L. Wang, L. Yuan, X. Zeng, J. Peng, Y. Ni, J. C. Er, W. Xu, B. K. Agrawalla, D. Su, B. Kim and Y. T. Chang, Angew. Chem., Int. Ed., 2016, 55, 1773–1776 CrossRef CAS PubMed.
- Z. Xu, G. Zeng, Y. Liu, X. Zhang, J. Cheng, J. Zhang, Z. Ma, M. Miao, D. Zhang and Y. Wei, Dyes Pigm., 2019, 163, 559–563 CrossRef CAS.
- T. B. Ren, S. Y. Wen, L. Wang, P. Lu, B. Xiong, L. Yuan and X. B. Zhang, Anal. Chem., 2020, 92, 4681–4688 CrossRef CAS PubMed.
- Y. Rim Lee, N. Kwon, K. M. K. Swamy, G. Kim and J. Yoon, Chem. – Asian J., 2022, 17, 3–7 CrossRef PubMed.
- H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928–4965 RSC.
- Y. W. Jun, S. Sarkar, K. H. Kim and K. H. Ahn, ChemPhotoChem, 2019, 3, 214–219 CrossRef CAS.
- T. Minami, F. Emami, R. Nishiyabu, Y. Kubo and P. Anzenbacher Jr., Chem. Commun., 2016, 52, 7838–7841 RSC.
- S. J. Butler and K. A. Jolliffe, ChemPlusChem, 2021, 86, 59–70 CrossRef CAS PubMed.
- Y. Tsuchido, S. Fujiwara, T. Hashimoto and T. Hayashita, Chem. Pharm. Bull., 2017, 65, 318–325 CrossRef CAS PubMed.
- K. Aoki, R. Osako, J. Deng, T. Hayashita, T. Hashimoto and Y. Suzuki, RSC Adv., 2020, 10, 15299–15306 RSC.
- K. Sugita, Y. Suzuki, Y. Tsuchido, S. Fujiwara, T. Hashimoto and T. Hayashita, RSC Adv., 2022, 12, 20259–20263 RSC.
- S. Minagawa, S. Fujiwara, T. Hashimoto and T. Hayashita, Int. J. Mol. Sci., 2021, 22, 4683 CrossRef CAS PubMed.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209 CrossRef CAS.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176–2179 CrossRef CAS.
- ReactLab EQUILIBRIA, Jplus Consulting.
- M. Levine and B. R. Smith, J. Fluoresc., 2020, 30, 1015–1023 CrossRef CAS PubMed.
- T. Yorozu, M. Hoshino and M. Imamura, J. Phys. Chem., 1982, 86, 4426–4429 CrossRef CAS.
- G. Bains, A. B. Patel and V. Narayanaswami, Molecules, 2011, 16, 7909–7935 CrossRef CAS PubMed.
- T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 141–152 RSC.
- H. Shimizu, A. Kaito and M. Hatano, Bull. Chem. Soc. Jpn., 1979, 52, 2678–2684 CrossRef CAS.
- K. Fujita, S. Fujiwara, T. Yamada, Y. Tsuchido, T. Hashimoto and T. Hayashita, J. Org. Chem., 2017, 82, 976–981 CrossRef CAS PubMed.
- T. Yamada, S. Fujiwara, K. Fujita, Y. Tsuchido, T. Hashimoto and T. Hayashita, Molecules, 2018, 23, 635 CrossRef PubMed.
- C. Madry, I. L. Arancibia-Cárcamo, V. Kyrargyri, V. T. T. Chan, N. B. Hamilton and D. Attwell, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E1608–E1617 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthetic procedures, Fig. S1–S32, Charts S1–S3, and Scheme S1. See DOI: https://doi.org/10.1039/d3nj00139c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |