Open Access Article
Open Access ArticleUnprecedented Ir(III) cationic complexes based on tridentate tetrazolate ligands: synthesis, photophysics and encapsulation in SiO2 nanoparticles†
José
Troya‡
 ,
María Mar
Quesada-Moreno
,
María Mar
Quesada-Moreno
 *,
Juan-Ramón
Jiménez
*,
Juan-Ramón
Jiménez
 and
Juan Manuel
Herrera
and
Juan Manuel
Herrera
 *
*
Departamento de Química Inorgánica, Facultad de Ciencias, Unidad de Excelencia en Química Aplicada a Biomedicina y Medioambiente (UEQ), Universidad de Granada, Avda. Fuentenueva s/n, 18071, Granada, Spain. E-mail: mqmoreno@ugr.es; jmherrera@ugr.es
First published on 24th January 2023
Abstract
The first examples of luminescence Ir(III) complexes derived from a tridentate tetrazole ligand, 2-(tetrazole-5-yl)-1,10-phenanthroline (Hphenttz), are reported here. Two cationic complexes, heteroleptic [Ir(tpy)(phenttz)]2+ (1) (tpy = 2,2′:6′,2′′-terpyridine) and homoleptic [Ir(phenttz)2]+(2), have been synthesized and fully characterized from a chemical, structural and photophysical point of view. Both complexes exhibit green luminescence with a prevalent 3LC (Ligand-Centered) character, which is evidenced by their structured emission profiles and low kr values (of about 103 s−1), and supported by density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations. In air-equilibrated solutions, 1 and 2 show emission lifetimes (respective values of 1.6 μs and 1.2 μs) comparable to that of the reference complex [Ir(tpy)2]3+ (1.0 μs) and quantum yields slightly lower (1.5% (1), 1.3% (2)) than that obtained for [Ir(tpy)2]3+ (2.5%). Under an oxygen-free atmosphere, the emission lifetimes and quantum yields (τ/Φ) of the complexes increase significantly up to 5 μs/4.2% (1) and 3 μs/3.3% (2). 1 has been embedded within amorphous silica nanoparticles leading to the hybrid material 1@SiO2. This material shows enhanced photochemical stability and higher luminescence efficiency compared to the free complex, which demonstrates that silica hampers the diffusion of O2, restrains the mobility of the complex and stimulates the radiative decay of the excited state.
Introduction
Tetrazole compounds have attracted a lot of attention in the last few decades. In pharmacy, tetrazoles can replace the carboxyl group, enhance the lipophilicity and biocompatibility of drugs and reduce side effects. They have shown biological activity as antitubercular, antimalarial or anticancer agents, and have been used in clinics for the treatment of various diseases.1,2 In the field of coordination chemistry, these ligands have also gathered much attention due to their ability to coordinate metal ions with a wide diversity of coordination modes.3 Their relatively easy synthesis has enabled the preparation of a plethora of 5-substituted tetrazole derivatives that can act as mono-, bi- or multi-dentate ligands.4–6 A vast number of robust coordination compounds with interesting structural, luminescence, zeolitic and magnetic properties have been studied in recent decades.7–14 In the photochemistry field, 5-sustituted aryl-, pyridyl- or pyrazine- tetrazolates have been used profusely as ligands to prepare luminescence complexes based on lanthanides,15–22 Ru(II),23,24 Re(I),25–27 Pt(II),28–36 and Ir(III) metal ions, which are particularly interesting in this work. The 2-pyridyltetrazolate (pytz) anion was first used as an ancillary ligand to prepare a set of heteroleptic [Ir(C^N)(pytz)] neutral complexes used as dopants of blue-emitting OLEDs.37,38 Since then, the interest in the design of tetrazole-based luminiscence Ir(III) complexes has grown considerably thanks to the easy functionalization of the ligand structure, which allows tuning of the color emission. Bidentate tetrazolate ligands have been used as either cyclometallating or ancillary moieties to prepare neutral Ir(III) complexes whose color emission varied from blue to red. The reactivity of the tetrazolate rings towards electrophilic agents such as H+ or CH3+ also contributed to tune their photoluminescent properties and modify their ionic nature; from neutral emitters used in the fabrication of OLEDs to cationic complexes used as dopants for LEECs devices. Additionally, they have been used as luminescent solar concentrators or anionic species to prepare multicolour-emitting soft salts when combined with luminescent counterions.39–42To date, all the luminescent Ir(III)-tetrazolate complexes reported in the literature have been prepared exclusively from mono- or bidentate ligands. Even though the synthesis of Ir(III) phosphors based on tridentate azolate chelates has demonstrated that a higher denticity implies a superior chemical stability of the complex towards metal-chelate dissociation,43–47 the use of tridentate tetrazolate ligands has been ignored. In this work, we report on the synthesis, structure and photophysical properties of two cationic Ir(III) complexes derived from the tridentate 2-(tetrazol-5-yl)-1,10-phenanthroline (Hphenttz) ligand: the heteroleptic complex [Ir(tpy)(phenttz)]2+ (1) (tpy = 2,2′:6′,2′′-terpyridine) and the homoleptic derivate [Ir(phenttz)2]+ (2) (Scheme 1). Additionally, 1 has been embedded within silica nanoparticles leading to the hybrid material 1@SiO2, whose photophysical properties have been compared with those of the free complex.
Experimental section
General procedures
The ligand 2,2′:6′,2′′-terpyridine (tpy), IrCl3·xH2O, other reagents and common solvents were obtained from commercial sources and used as received. The ligand Hphenttz and Ir(III) precursor [Ir(tpy)Cl3] were synthetized following classical procedures described previously.14,48 The Ir(III) complexes 1 and 2 were prepared in the dark under an Ar(g) inert atmosphere. Both complexes were purified by column chromatography using activated alumina (Brokmann, grade III) as the stationary phase and a mixture of CH3CN/H2O/saturated KNO3 solution as the eluent, with proportions in volume ranging from 100/0/0 to 70/28/2. Single crystals were obtained by slow diffusion of Et2O vapours into CH3CN solutions of the respective complexes.Syntheses
The complex was isolated as a nitrate salt by ion-exchange chromatography on a strong base anion exchanger type-III in its nitrate form, and using water as the eluent. The aqueous solution containing the complex was evaporated to dryness, dissolved in a minimum water quantity and precipitated with acetone.
X-ray crystallography
Suitable crystals of 1 and 2 were mounted on a Bruker D8 Venture diffractometer. Details of the crystals, data collection and refinement parameters are given in the ESI† (Table S1). After data processing (raw data integration, merging of equivalent reflections and absorption correction), the structures were solved by direct methods and refined using least squares minimization with the SHELX suite of programs49 integrated in OLEX2.50 Selected bonds and lengths are given in Tables S2 and S3 (ESI†). CCDC numbers 2221011 (1) and 2221012 (2) contain the supplementary crystallographic data for this article. These data are provided free of charge by the Cambridge Crystallographic Data Centre.Physical measurements
Elemental analyses were carried out on a Fisons-Carlo Erba analyser model EA1108. NMR characterizations were performed on a 400 MHz (2 channels) BRUKER Nanobay Advance III. Emission and excitation spectra were measured using a UV-VIS-PTI QuantaMaster™ 8000 spectrofluorometer equipped with a Picosecond Photon Detector (230–850 nm, PPD-850, HORIBA Scientific) and a continuous Xenon Short Arc Lamp (190–2000 nm, USHIO). All the spectra (emission and excitation) were corrected with Real-time corrections function. TCSPC lifetime measurements were performed using a 375 nm excitation wavelength provided by a pulsed diode light source NanoLED 375L (<200ps pulse, HORIBA Scientific). Nanoparticles were characterized by transmission electron microscopy (TEM) using a LIBRA 120 PLUS Carl Zeiss electron microscope operating at 200 keV. 5 mg of the material was redispersed by sonication (30 min) in 1 mL of EtOH. Carbon reinforced copper grids (200 mesh) were submerged into suspension 50 times and then allowed to dry in air for at least 48 h. The size of the particles was determined by ‘‘manual counting’’ using ScionImage software (https://www.scioncorp.com). HAADF-STEM images and EDX analyses were recorded on a HAADF FEI TITAN G2 instrument working at an accelerating voltage of 200 kV in the scanning mode with a probe diameter of 0.5 nm.All these techniques are available at the Centro de Instrumentación Científica (CIC) of the University of Granada.
Computational methodology
DFT (Density Functional Theory) calculations were performed using the long-range corrected CAM-B3LYP51 and the hybrid B3LYP52–54 functionals together with the 6-31G** basis set for C, H, and N, and the “double-ζ” quality LANL2DZ basis set for Ir.55–57 An effective core potential (ECP) replaces the inner core electrons of iridium, leaving the outer core (5s)2(5p)6 electrons and the (5d)6 valence electrons of Ir(III). Implicit solvent (acetonitrile) effects were taken into account using the IEF-PCM formalism.58 Time-dependent DFT (TD-DFT) calculations of the lowest-lying 10 singlets and 10 triplets were performed in the presence of the solvent at the minimum-energy geometry optimized for the ground states at CAM-B3LYP and B3LYP. The lowest energy triplet (T1) excited states were optimized and tested to be real minima by carrying out vibrational frequency calculations (no imaginary frequency was found). Mulliken population analysis (MPA) was applied following the methodology used by Chi et al.47 to obtain the electron density distribution of each atom in specific molecular orbitals of the Ir(III) compounds and to calculate the metal-to-ligand charge transfer (MLCT) character in each assignment of the singlet and triplet optical transitions. All the calculations were performed using the B.01 revision of the Gaussian 09 program package59 and visualization of the orbitals was carried out by Avogadro.60Results and discussion
Synthesis and structural characterization
Heteroleptic complex 1 was prepared by reaction of the neutral precursor [Ir(tpy)Cl3] with a slight excess of the deprotonated tetrazolate ligand phenttz in ethylene glycol at 180 °C. The complex was purified by column chromatography and isolated as the hexafluorophosphate salt by anion exchange with KPF6. The addition of NaOAc helps to deprotonate the tetrazol ligand and improve the yield of the reaction.44Single crystals were obtained by slow diffusion of Et2O in CH3CN solutions of the complexes (Fig. 1). 1 crystallizes in the monoclinic P21/c space group. The asymmetric unit shows one cationic [Ir(tpy)(phenttz)]2+ (1) unit, the corresponding hexafluorophosphate counter-ions and an acetonitrile solvent molecule. Within the mononuclear unit, the Ir(III) centre exhibits a slightly distorted octahedral geometry. Tpy and the tetrazolate phenttz ligands act, as expected, as tridentate quelates adopting an orthogonal disposition (mer- isomers). The terpyridine chelate shows Ir–Ntpy bond distances between 1.972 and 2.060 Å, bite angles (N1–Ir–N2 and N2–Ir–N3) close to 80° and a trans N1–Ir–N3 angle of 159.63° (Table S2, ESI†). These values are almost identical to those reported for the homoleptic complex [Ir(tpy)2](PF6)3.61 With respect to the anionic tetrazolate moiety, the Ir–Ntetrazolate bond distance (2.047 (2) Å) is noticeably shorter than those found for Ir(III) complexes based on bidentate 2-piridyl- and 2-pyrazine-tetrazolate ligands (2.137(15) Å – 2.147(3)).37,39 Intermolecular π–π interactions compatible with “terpyridine embrace” are detected between neighbouring mononuclear units, with interplanar distances of about 3.8 Å (Fig. S1, ESI†).
 | ||
| Fig. 1 X-Ray structures of complexes 1 (left) and 2 (right). ORTEP ellipsoids are at 50% probability. H atoms have been omitted for the sake of clarity. | ||
Complex 2 was prepared by reaction of IrCl3 with 2.2 molar equivalents of Hphenttz in hot ethylene glycol in the presence of sodium acetate. The complex crystallizes in the monoclinic space group C2/c and the asymmetric unit contains half of the metallic unit, with the other half generated by rotation about the C2 axis that runs parallel to the b axis of the unit cell. The asymmetric unit also contains one hexafluorophosphate counter-ion with half occupancy and two solvated acetonitrile molecules. Bond distances and angles between the Ir(III) centre and the N atoms of the phenttz chelate are similar to those observed in 1 (Table S3, ESI†). Within the unit cell, intermolecular π–π stacking interactions are observed between the central rings of the phenanthroline units with an inter-centroid distance of 3.58 Å (Fig. S1, ESI†).
In both complexes, a notable pincer effect imposed by the five membered tetrazolate rings is observed, with bite and trans- angles less than 90° and 180°, respectively. This is the main factor accounting for the distorted octahedral coordination sphere around the Ir(III) centres. According to the continuous-shape-measures (CShMs) method,62 the IrN6 coordination spheres show a deviation parameter of 2.394 (1) and 2.570 (2) from the ideal Oh octahedral geometry, significantly higher than the deviation of 2.032 observed for the homoleptic [Ir(tpy)2](PF6)3 complex (Table S4, ESI†).
Photophysical properties
Absorption spectra
Room-temperature absorption spectra of complexes 1 and 2 measured in acetonitrile are shown in Fig. 2, and the photophysical data are reported in Table 1. For each complex, the spectrum displays intense absorption bands in the UV spectral window (200–330 nm), which are attributed to ligand centred (LC) 1π → π* transitions. The weaker bands tailing off above 330 nm are likely due to the charge transfer transition from Metal-to-Ligand (MLCT), with their nature being both spin allowed (singlet to singlet, 1MLCT) and spin forbidden (singlet-to-triplet, 3MLCT), as well as ligand-to-ligand and intra-ligand charge transfer transitions (3LLCT and 3ILCT). The spin forbidden transitions are enabled by the high spin–orbit coupling effect induced by the heavy Ir(III) metal centre.| 1 | 2 | 1@SiO2 | [Ir(tpy)2]3+ | |
|---|---|---|---|---|
| a λ exc = 375 nm. b Radiative constant (deareated solutions): kr = Φ/τ. c Non radiative constant: knr= 1/τ – kr. d k r and knr calculated from τair and Φair values. e For the biexponential excited-state lifetimes, the relative weights of the exponential curves are indicated in parentheses. | ||||
| Absorption | ||||
| λ max/nm (10−4ε/M−1cm−1) | 230 (5.21), 244 (5.73), 276 (4.41), 287 (3.98), 310 (3.04), 328 (2.35), 348 (1.02), 369 (0.53), 390 (0.36) | 232 (5.32), 241 (5.73), 294 (3.52), 337 (1.54), 373 (0.78), 398 (0.68) | 210 (2.14), 221 (1.85), 251 (2.76), 277 (2.22), 313 (1.14), 325 (1.18), 336 (0.85), 352 (0.58), 374 (0.13) | |
| Emission (CH3CN solutions, T = 298 K) | ||||
| λ max/nma | 495, 531, 567(sh) | 495, 532 | 498, 534, 570(sh) | 458, 491, 523 |
| τ air/μs | 1.6 | 1.2 | 9.7 (99%), 40 (1%) | 1.0 |
| τ dear/μs | 5 | 3 | 10.2 (98.5%), 42 (1.5%) | 1.2 |
| Φ air | 0.015 | 0.013 | 0.097 | 0.025 |
| Φ dear | 0.042 | 0.033 | 0.093 | — |
| 10−3·krb (s−1)/10−5·knrc (s−1) | 8.4/1.92 | 11.0/3.22 | 9.12/0.89 | 25/9.75d |
| Emission (CH3CN rigid matrix, T = 77 K) | ||||
| λ max (nm) | 495, 532 | 495, 532 | 496, 534, 574 | 458 |
| τ air /μs | 13 (98%), 66 (2%) | 26 (98%), 94 (2%) | 16 (99%), 66 (1%) | 26 |
Emission spectra
Fig. 3 shows the emission spectra of complexes 1, 2 and the reference complex [Ir(tpy)2](PF6)3.61 For the latter, the highest energy band appears at λ = 458 nm, and its luminescence was ascribed mainly to 3LC excited states with some minor contribution from 3MLCT excited states.61 Complexes 1 and 2 exhibit green luminescence at room temperature in acetonitrile solutions. Both spectra are almost superimposable and display a vibrationally resolved profile quite similar to that shown by [Ir(tpy)2](PF6)3, although in these cases, the highest energy band maximum appears red shifted at λ = 495 nm. Their emissions show a monoexponential decay with lifetimes about 1.6 μs (1) and 1.2 μs (2), which increase to 5 μs and 3 μs, respectively, under an inert atmosphere. The emission quantum yields follow a similar trend, with values close to 1.5% in aerated solutions that rise by a factor of ca. 2.6 under oxygen-free conditions. For both complexes, the radiative (kr) decay rate is close to 1 104 s−1, smaller than the 2.5 104 s−1 rate calculated for [Ir(tpy)2](PF6)3. These features indicate that the emission properties of both compounds arise from excited states with a prevalent 3LC character, even if some 3MLCT contribution cannot be ruled out.Theoretical calculations
Time-dependent density functional theory (TD-DFT) was performed to gain further insight into the experimental absorption and emission properties described above. First, the molecular structures of complexes 1 and 2 were taken from the experimental X-ray crystal data and optimized by density functional theory (DFT) calculations using the CAM-B3LYP and B3LYP functionals with the 6-31G** + LANL2DZ basis sets and considering the solvent (acetonitrile) effects through the IEF-PCM formalism. From a structural point of view, the optimized geometries of the complexes in the ground state (S0) fit well with those obtained from X-Ray data (Tables S2 and S3 in the ESI†). In particular, an excellent agreement between the experimental (RX) and computed bond distances involving the Ir(III) coordination sphere is found, with differences of just a few picometers in complexes 1 and 2.Second, the lowest singlet and triplet excited states were calculated at the optimized geometries of the ground states (S0) using the TD-DFT approach and both CAM-B3LYP and B3LYP functionals. The computed T1 geometries of both 1 and 2 are very similar to their respective S0 geometries (Tables S2 and S3, ESI†). In addition, similar S0 and T1 geometries were obtained using CAM-B3LYP and B3LYP. The calculated CAM-B3LYP and B3LYP vertical electronic transitions and assignments of the complexes 1 and 2 are shown in Table 2 and Tables S5–S8 (ESI†). The frontier molecular orbitals involved in the dominant transitions are displayed in Fig. 4 and Fig. S8 (ESI†). The computed CAM-B3LYP wavelengths of the S0 → S1 transitions for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 nm and 2
319 nm and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
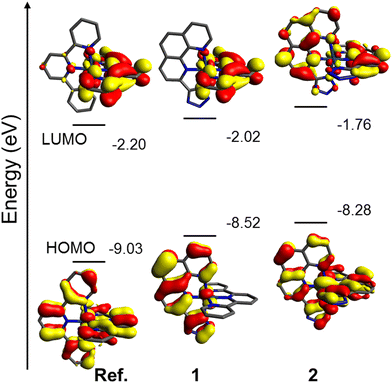
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 321 nm are close to those observed in their experimental absorption spectra (Fig. 2 and Tables 1, 2), whereas those calculated with B3LYP are predicted to be red shifted at 380 nm and 382 nm for 1 and 2, respectively (Tables S6 and S8, ESI†). We focus on the CAM-B3LYP assignments from now on for the sake of clarity (further details on the B3LYP results are shown in the ESI†). The S0 → S1 optical transition for complex 2 is mainly assigned to the HOMO → LUMO transition, whereas in the case of complex 1 it is assigned to HOMO → LUMO+1 for symmetry reasons. Fig. 4 shows the energy and the atomic orbital composition calculated for the HOMO and LUMO of complexes 1 and 2, together with those computed with CAM-B3LYP for the reference complex [Ir(tpy)2](PF6)3 for comparison purposes. The HOMO of 1 is mainly localized on the Ir centre (11% of relative density distribution at the Ir atom) and the phenttz ligand. In the case of the homoleptic complexes, i.e. the reference complex and complex 2, the HOMO is localized in the two respective tpy and phenttz ligands and the Ir centre (17% for 2). However, the LUMO of 1 resides in its tpy ligand, occupying also only one of the two tpy ligands in the reference complex, whereas for 2 it is mainly located in the benzene and pyridine rings of its two phenttz ligands. The LUMO+1 of 1, which is close in energy to the LUMO (−2.02 eV), is located in its phenttz ligand. Additionally, minor contributions from the central Ir(III) metal atoms to these LUMOs are found in 1 and 2 (3 and 2%, respectively). As a result, the S0 → S1 absorptions can be mainly assigned to intraligand charge transfer (ILCT) for both compounds, together with a reduced MLCT character (8–10%).
321 nm are close to those observed in their experimental absorption spectra (Fig. 2 and Tables 1, 2), whereas those calculated with B3LYP are predicted to be red shifted at 380 nm and 382 nm for 1 and 2, respectively (Tables S6 and S8, ESI†). We focus on the CAM-B3LYP assignments from now on for the sake of clarity (further details on the B3LYP results are shown in the ESI†). The S0 → S1 optical transition for complex 2 is mainly assigned to the HOMO → LUMO transition, whereas in the case of complex 1 it is assigned to HOMO → LUMO+1 for symmetry reasons. Fig. 4 shows the energy and the atomic orbital composition calculated for the HOMO and LUMO of complexes 1 and 2, together with those computed with CAM-B3LYP for the reference complex [Ir(tpy)2](PF6)3 for comparison purposes. The HOMO of 1 is mainly localized on the Ir centre (11% of relative density distribution at the Ir atom) and the phenttz ligand. In the case of the homoleptic complexes, i.e. the reference complex and complex 2, the HOMO is localized in the two respective tpy and phenttz ligands and the Ir centre (17% for 2). However, the LUMO of 1 resides in its tpy ligand, occupying also only one of the two tpy ligands in the reference complex, whereas for 2 it is mainly located in the benzene and pyridine rings of its two phenttz ligands. The LUMO+1 of 1, which is close in energy to the LUMO (−2.02 eV), is located in its phenttz ligand. Additionally, minor contributions from the central Ir(III) metal atoms to these LUMOs are found in 1 and 2 (3 and 2%, respectively). As a result, the S0 → S1 absorptions can be mainly assigned to intraligand charge transfer (ILCT) for both compounds, together with a reduced MLCT character (8–10%).
| Complex | Excited state | λ (nm) | E (eV) | f | Main assignments | MLCT (%) |
|---|---|---|---|---|---|---|
| 1 | S0 → T1 | 471 | 2.63 | 0 | HOMO → LUMO+3 (27%) | 7.11 |
| HOMO → LUMO+1 (15%) | ||||||
| HOMO-2 → LUMO+1 (14%) | ||||||
| S0 → S1 | 319 | 3.89 | 0.0542 | HOMO → LUMO+1 (86%) | 8.17 | |
| 2 | S0 → T1 | 473 | 2.62 | 0 | HOMO → LUMO+3 (21%) | 8.18 |
| HOMO-1 → LUMO+2 (19%) | ||||||
| HOMO-2 → LUMO (13%) | ||||||
| S0 → S1 | 321 | 3.87 | 0.0458 | HOMO → LUMO (64%) | 10.36 |
If we compare the HOMO of the reference complex and that of 1, the main effect of the additional tpy ligand in the reference complex is the stabilization of its HOMO by 0.51 eV. The replacement of tpy (1) with phenttz (2) on going from 1 (−8.52 eV) to 2 (−8.28 eV) results in a destabilization of the HOMO by 0.24 eV. The LUMO of these complexes also follows the energy ordering of the reference complex (−2.20 eV) < 1 (−2.02 eV) < 2 (−1.76 eV), and presents identical molecular orbital topologies for 1 and the reference complex. Thus, theoretical calculations predict a higher HOMO–LUMO gap for the reference complex (−6.827 eV) with respect to 1 (−6.50 eV) and 2 (−6.52 eV), which is in agreement with the observation of a blue shift in the emission properties of the reference complex (Fig. 3), and the lack of observation of any shifts among the absorption and emission spectra of 1 and 2.
Additionally, the phosphorescence wavelengths for T1 → S0 after geometry optimization of the T1 states of complexes 1 and 2 were computed using the vertical method and the CAM-B3LYP and B3LYP functionals with the 6-31G** + LANL2DZ basis sets (Table 3 and Table S9 and S10, ESI†). The former functional predicts the wavelengths for the T1 → S0 emission transitions at 1017 nm and 486 nm for 1 and 2, respectively. This method overestimates significantly the experimentally observed wavelength for T1 → S0 in the case of 1, which was associated to the stabilization of an artificial “ghost state”.63 Actually, B3LYP predicts this T1 → S0 transition at 664 nm for 1, which supports that statement, and at 482 nm for 2, in good agreement with their experimental wavelengths (deviations in the range of 0.3–0.4 eV for 1 and 2). Thus, the CAM-B3LYP computed absorption wavelengths for the lowest-energy transitions of 1 and 2 agreed better with the experimental absorption data than those obtained with B3LYP; whereas the latter functional reproduced better the experimental emission wavelengths. The T1 → S0 emission of complexes 1 and 2 are assigned to HOMO → LUMO transitions and incorporates 3ILCT and a reduced 3MLCT character (3–4%, Fig. S9 (ESI†) and Table 3). The HOMO and LUMO of the T1 states of 1 and 2 are located on the phenttz ligand, that is, the only phenttz ligand in 1 and one of the two phenttz ligands in 2 (Fig. S9, ESI†).
| Complex | Excited state | λ (nm) CAM-B3LYP/B3LYP | E (eV) CAM-B3LYP/B3LYP | f | Main assignments | MLCT (%) |
|---|---|---|---|---|---|---|
| 1 | T1 → S0 | 1017/664 | 1.22/1.87 | 0 | HOMO → LUMO (79%) | 3.21 |
| 2 | T1 → S0 | 486/482 | 2.55/2.57 | 0 | HOMO → LUMO (79%) | 4.01 |
Encapsulation of 1 in SiO2 nanoparticles (1@SiO2)
We and others have demonstrated previously how the encapsulation of Ir(III) phosphors within amorphous silica nanoparticles serves to improve their photoluminescence properties. The silica matrix acts as a shelter protecting the complex from environmental quenchers such as oxygen and reduces its mobility, penalizing non-radiative deactivation pathways and leading to higher quantum efficiencies and longer emission lifetimes.48,64–67 Within this framework, we have prepared hybrid 1@SiO2 following the reverse micelle protocol that we established previously to prepare [Ir(tpy)2]@SiO2.48 First, PF6− counterions in 1 were replaced with NO3− by anion exchange chromatography in order to make the complex soluble in water. A water solution of this complex was added to a mixture of a surfactant in an organic solvent, leading to the formation of a water-in-oil microemulsion. Then, the hydrophilic silica precursor TEOS and a catalytic amount of ammonia were added, causing the hydrolysis and polymerization of TEOS inside the aqueous nuclei of the micelle containing the [Ir(tpy)(phenttz)]2+ complex. Thus, the cationic complex is embedded within the silica matrix and retained through strong electrostatic interactions with the negatively charged siloxyl groups of the silica. Following this procedure, we also tried to prepare the analogous sample 2@SiO2, however, in this case a severe leakage of the [Ir(phenttz)2]+ complex out of the silica matrix was evidenced during the synthetic washing and purification steps. This was probably due to the fact that the electrostatic interactions between the monocationic complex and the siloxyl groups were not strong enough to retain the complex in the silica matrix. In the case of 1@SiO2, release of dicationic complex 1 in the washing solvents was negligible and allowed a full characterization of the sample.The morphology, size and composition of sample 1@SiO2 were characterized by electron microscopy techniques. Fig. 5a and Fig. S8 (ESI†) show representative Transmission Electron Microscopy (TEM) images of the sample. The nanoparticles are spherical and monodisperse with an average size of 26.8 ± 2.9 nm. High-angle annular dark-field-scanning transmission electron microscopy (HAADF-STEM) and Energy Dispersive X-Ray (EDX) analysis confirm the encapsulation of the Ir(III) complex into the silica matrix.
 | ||
| Fig. 5 (a) TEM image of 1@SiO2 and size distribution histogram. (b) HAADF-STEM image and EDX compositional maps of 1@SiO2. | ||
Regarding the photophysical properties, the emission profiles of 1 and 1@SiO2 are practically superimposable and no rigidochromic effect is observed, in accordance with a dominant 3LC character of the emissive excited state, which is not very sensitive to changes in the chemical environment around the Ir(III) complex. Conversely, significant differences in the emission intensity of both samples were observed. Compared to 1, 1@SiO2 is brighter and shows quantum yields and emission lifetimes that increase by factors of ∼6.5 and ∼2.2 in aerated and deaerated atmospheres, respectively. These facts indicate that the limited mobility of complex 1 in the rigid silica matrix restricts the vibrational motions and inhibits the non-radiative deactivation pathways of the excited state. This effect is clearly illustrated when the radiative (kr) and non-radiative (knr) decay rates of 1 and 1@SiO2 are compared (Table 1). kr,rel and knr,rel are defined by eqn (1) and (2), respectively,
 | (1) |
 | (2) |
Conclusions
In conclusion, two new cationic Ir(III) complexes derived from a tridentate tetrazole ligand, 2-(tetrazol-5-yl)-1,10-phenanthroline, have been prepared and fully characterized. Compared to the parent complex [Ir(tpy)2]3+, a bathochromic shift of the emission is observed for 1 and 2. TD-DFT calculations reveal that in both cases the emission is centred in the phenanthroline-tetrazolate moiety and exhibits a prominent 3ILCT character and a minor 3MLCT. Both complexes are robust enough to be embedded into silica nanoparticles, although only the dicationic complex 1 remains inside the nanoparticles when in contact with water. This is due to strong electrostatic interactions with the negatively charged silanol groups of the nanoparticles. The rigid silica matrix serves to improve the emission yield and extend the emission lifetime of 1, as the mobility of the complex is restricted. Considering the wide variety of tridentate tetrazole-based ligands already described in the literature, a vast family of homo- and/or heteroleptic Ir(III) complexes with color emissions ranging in the whole visible spectral window can be prepared. These Ir(III) compounds could be used as neutral emitters for OLEDs, cationic luminophores for LEEC devices or biocompatible silica-based biomarkers. Some of these possibilities are currently under investigation in our laboratory.Author contributions
J. M. H. supervised the project and provided financial support. J. T. synthesized the complexes and performed the chemical characterization. J. M. H. carried out the structural characterization. M. M. Q. M. performed the DFT and TD-DFT calculations. J. R. J. performed the photophysical characterization. J. M. H. and M. M. Q. M. wrote the manuscript and all the authors approved its final version.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was financially supported by MCIN/AEI/10.13039/201100011033/FEDER “Una manera de hacer Europa (Project PGC2018-102052-B-C21); The Junta de Andalucía (FQM-195), FEDER/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Projects A-FQM-172-UGR18 and B.FQM.328.UGR20 and the University of Granada. The authors acknowledge the technical support provided by the Centro de Instrumentación Científica (CIC) of the University of Granada, the technician César López-Ruiz, and the Centro de Servicios de Informática y Redes de Comunicaciones (CSIRC) for computational time and facilities. M. M. Q. M. thanks Junta de Andalucía for a postdoctoral fellowship (DOC_01282). M. M. Q. M. and J. R. J. also thank Ministerio de Ciencia, Innovación y Universidades for Juan de la Cierva formación and incorporación contracts (grants FJC2018-035709-I and IJC2020-044040-I supported by MCIN/AEI/10.13039/501100011033). We also acknowledge Amparo Navarro and Tomás Peña Ruiz for insightful discussions and help with the quantum-chemical calculations.References
- A. Verma, B. Kaur, S. Venugopal, P. Wadhwa, S. Sahu, P. Kaur, D. Kumar and A. Sharma, Chem. Biol. Drug Des., 2022, 100, 419–442 CrossRef CAS PubMed.
- C. Gao, L. Chang, Z. Xu, X. F. Yan, C. Ding, F. Zhao, X. Wu and L. S. Feng, Eur. J. Med. Chem., 2019, 163, 404–412 CrossRef CAS PubMed.
- A. J. Mota, a Rodríguez-Diéguez, M. a Palacios, J. M. Herrera, D. Luneau and E. Colacio, Inorg. Chem., 2010, 49, 8986–8996 CrossRef CAS PubMed.
- S. Vorona, T. Artamonova, Y. Zevatskii and L. Myznikov, Synthesis, 2014, 781–786 Search PubMed.
- J. Roh, K. Vávrová and A. Hrabálek, Eur. J. Org. Chem., 6101–6118 Search PubMed.
- Z. P. Demko and K. B. Sharpless, J. Org. Chem., 2001, 66, 7945–7950 CrossRef CAS PubMed.
- M. Dincă, W. S. Han, Y. Liu, A. Dailly, C. M. Brown and J. R. Long, Angew. Chem., Int. Ed., 2007, 46, 1419–1422 CrossRef PubMed.
- A. Rodríguez, R. Kivekäs and E. Colacio, Chem. Commun., 2005, 5228–5230 RSC.
- X. He, C.-Z. Lu and D.-Q. Yuan, Inorg. Chem., 2006, 45, 5760–5766 CrossRef CAS PubMed.
- A. Rodríguez-Diéguez and E. Colacio, Chem. Commun., 2006, 4140–4142 RSC.
- R.-G. Xiong, X. Xue, H. Zhao, X.-Z. You, B. F. Abrahams and Z. Xue, Angew. Chem., Int. Ed., 2002, 41, 3800–3803 CrossRef CAS PubMed.
- X. Xue, X.-S. Wang, L.-Z. Wang, R.-G. Xiong, B. F. Abrahams, X.-Z. You, Z.-L. Xue and C.-M. Che, Inorg. Chem., 2002, 41, 6544–6546 CrossRef CAS PubMed.
- A. J. Mota, a Rodríguez-Diéguez, M. a Palacios, J. M. Herrera, D. Luneau and E. Colacio, Inorg. Chem., 2010, 49, 8986–8996 CrossRef CAS PubMed.
- W. Zhang, F. Zhao, T. Liu, M. Yuan, Z.-M. Wang and S. Gao, Inorg. Chem., 2007, 46, 2541–2555 CrossRef CAS PubMed.
- M. Giraud, E. S. Andreiadis, A. S. Fisyuk, R. Demadrille, J. Pécaut, D. Imbert and M. Mazzanti, Inorg. Chem., 2008, 47, 3952–3954 CrossRef CAS PubMed.
- E. S. Andreiadis, R. Demadrille, D. Imbert, J. Pécaut and M. Mazzanti, Chemistry, 2009, 15, 9458–9476 CrossRef CAS PubMed.
- N. M. Shavaleev, S. V. Eliseeva, R. Scopelliti and J.-C. G. Bünzli, Inorg. Chem., 2014, 53, 5171–5178 CrossRef CAS PubMed.
- E. S. Andreiadis, D. Imbert, J. Pécaut, R. Demadrille and M. Mazzanti, Dalton Trans., 2012, 41, 1268–1277 RSC.
- G. Bozoklu, C. Marchal, J. Pécaut, D. Imbert and M. Mazzanti, Dalton Trans., 2010, 39, 9112–9122 RSC.
- C. A. Tovee, C. A. Kilner, J. A. Thomas and M. A. Halcrow, CrystEngComm, 2009, 11, 2032 RSC.
- S. Di Pietro, D. Imbert and M. Mazzanti, Chem. Commun., 2014, 50, 10323–10326 RSC.
- J. R. Jiménez, I. F. Díaz-Ortega, E. Ruiz, D. Aravena, S. J. A. Pope, E. Colacio and J. M. Herrera, Chem. – Eur. J., 2016, 22, 14548–14559 CrossRef PubMed.
- M. Duati, S. Tasca, F. C. Lynch, H. Bohlen, J. G. Vos, S. Stagni and M. D. Ward, Inorg. Chem., 2003, 42, 8377–8384 CrossRef CAS PubMed.
- S. Stagni, E. Orselli, A. Palazzi, L. De Cola, S. Zacchini, C. Femoni, M. Marcaccio, F. Paolucci and S. Zanarini, Inorg. Chem., 2007, 46, 9126–9138 CrossRef CAS PubMed.
- D. S. Silvester, S. Uprety, P. J. Wright, M. Massi, S. Stagni and S. Muzzioli, J. Phys. Chem. C, 2012, 116, 7327–7333 CrossRef CAS.
- P. J. Wright, S. Muzzioli, M. V. Werrett, P. Raiteri, B. W. Skelton, D. S. Silvester, S. Stagni and M. Massi, Organometallics, 2012, 31, 7566–7578 CrossRef CAS.
- M. V. Werrett, D. Chartrand, J. D. Gale, G. S. Hanan, J. G. MacLellan, M. Massi, S. Muzzioli, P. Raiteri, B. W. Skelton, M. Silberstein and S. Stagni, Inorg. Chem., 2011, 50, 1229–1241 CrossRef CAS PubMed.
- C. A. Strassert, C.-H. Chien, M. D. Galvez Lopez, D. Kourkoulos, D. Hertel, K. Meerholz and L. De Cola, Angew. Chem., Int. Ed., 2011, 50, 946–950 CrossRef CAS PubMed.
- N. K. Allampally, C. A. Strassert and L. De Cola, Dalton Trans., 2012, 41, 13132 RSC.
- C. Cebrián, M. Mauro, D. Kourkoulos, P. Mercandelli, D. Hertel, K. Meerholz, C. A. Strassert and L. De Cola, Adv. Mater., 2013, 25, 437–442 CrossRef PubMed.
- K. D. M. MaGee, P. J. Wright, S. Muzzioli, C. M. Siedlovskas, P. Raiteri, M. V. Baker, D. H. Brown, S. Stagni and M. Massi, Dalton Trans., 2013, 42, 4233 RSC.
- P. Brulatti, V. Fattori, S. Muzzioli, S. Stagni, P. P. Mazzeo, D. Braga, L. Maini, S. Milita and M. Cocchi, J. Mater. Chem. C, 2013, 1, 1823 RSC.
- N. K. Allampally, M. Bredol, C. A. Strassert and L. De Cola, Chem. – A Eur. J., 2014, 20, 16863–16868 CrossRef CAS PubMed.
- J. Sanning, L. Stegemann, M. Nyenhuis, C. G. Daniliuc, N. L. Doltsinis and C. A. Strassert, Z. Naturforsch., B: J. Chem. Sci., 2016, 71, 463–473 CrossRef CAS.
- M. E. Robinson, A. Nazemi, D. J. Lunn, D. W. Hayward, C. E. Boott, M.-S. Hsiao, R. L. Harniman, S. A. Davis, G. R. Whittell, R. M. Richardson, L. De Cola and I. Manners, ACS Nano, 2017, 11, 9162–9175 CrossRef CAS PubMed.
- H. Geng, K. Luo, G. Zou, L. Zhao, H. Wang, Q. Li and H. Ni, Dyes Pigm., 2018, 149, 82–91 CrossRef CAS.
- L.-L. Wu, C.-H. Yang, I.-W. Sun, S.-Y. Chu, P.-C. Kao and H.-H. Huang, Organometallics, 2007, 26, 2017–2023 CrossRef CAS.
- S.-J. Yeh, M.-F. Wu, C.-T. Chen, Y.-H. Song, Y. Chi, M.-H. Ho, S.-F. Hsu and C. H. Chen, Adv. Mater., 2005, 17, 285–289 CrossRef CAS.
- S. Stagni, S. Colella, A. Palazzi, G. Valenti, S. Zacchini, F. Paolucci, M. Marcaccio, R. Q. Albuquerque and L. De Cola, Inorg. Chem., 2008, 47, 10509–10521 CrossRef CAS PubMed.
- F. Monti, A. Baschieri, I. Gualandi, J. J. Serrano-Pérez, J. M. Junquera-Hernández, D. Tonelli, A. Mazzanti, S. Muzzioli, S. Stagni, C. Roldan-Carmona, A. Pertegás, H. J. Bolink, E. Ortí, L. Sambri and N. Armaroli, Inorg. Chem., 2014, 53, 7709–7721 CrossRef CAS PubMed.
- V. Fiorini, A. D’Ignazio, K. D. M. Magee, M. I. Ogden, M. Massi and S. Stagni, Dalton Trans., 2016, 45, 3256–3259 RSC.
- V. Fiorini, N. Monti, G. Vigarani, G. Santi, F. Fasulo, M. Massi, L. Giorgini, A. B. Muñoz-García, M. Pavone, A. Pucci and S. Stagni, Dyes Pigm., 2021, 193, 109532 CrossRef CAS.
- Y. Chi, T. K. Chang, P. Ganesan and P. Rajakannu, Coord. Chem. Rev., 2017, 346, 91–100 CrossRef CAS.
- B. Tong, H. Y. Ku, I. J. Chen, Y. Chi, H. C. Kao, C. C. Yeh, C. H. Chang, S. H. Liu, G. H. Lee and P. T. Chou, J. Mater. Chem. C, 2015, 3, 3460–3471 RSC.
- C.-W. Lu, Y. Wang and Y. Chi, Chem. – A Eur. J., 2016, 22, 17892–17908 CrossRef CAS PubMed.
- J. Lin, Y. Wang, P. Gnanasekaran, Y.-C. Chiang, C.-C. Yang, C.-H. Chang, S.-H. Liu, G.-H. Lee, P.-T. Chou, Y. Chi and S.-W. Liu, Adv. Funct. Mater., 2017, 27, 1702856 CrossRef.
- L.-Y. Hsu, D.-G. Chen, S.-H. Liu, T.-Y. Chiu, C.-H. Chang, A. K.-Y. Jen, P.-T. Chou and Y. Chi, ACS Appl. Mater. Interfaces, 2020, 12, 1084–1093 CrossRef CAS PubMed.
- S. Titos-Padilla, E. Colacio, S. J. A. Pope, J. J. Delgado, M. Melgosa and J. M. Herrera, J. Mater. Chem. C, 2013, 1, 3808–3815 RSC.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- Y. Yang, M. N. Weaver and K. M. Merz, J. Phys. Chem. A, 2009, 113, 9843–9851 CrossRef CAS PubMed.
- C. Latouche, D. Skouteris, F. Palazzetti and V. Barone, J. Chem. Theory Comput., 2015, 11, 3281–3289 CrossRef CAS PubMed.
- S. N. Genin, I. G. Ryabinkin, N. R. Paisley, S. O. Whelan, M. G. Helander and Z. M. Hudson, Angew. Chem., Int. Ed., 2022, 61, e202116175 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford, CT, 2010.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek and G. R. Hutchison, J. Cheminf., 2012, 4, 17 CAS.
- J. P. Collin, I. M. Dixon, J. P. Sauvage, J. A. G. Williams, F. Barigelletti and L. Flamigni, J. Am. Chem. Soc., 1999, 121, 5009–5016 CrossRef CAS.
- S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell and D. Avnir, Coord. Chem. Rev., 2005, 249, 1693–1708 CrossRef CAS.
- L. Goerigk and S. Grimme, J. Chem. Phys., 2010, 132, 184103 CrossRef.
- I. Zanoni, V. Fiorini, M. Rosado, B. Ballesteros, M. Androulidaki, M. Blosi, S. Ortelli, S. Stagni, M. Dondi and A. L. Costa, New J. Chem., 2018, 42, 9635–9644 RSC.
- C.-W. Lai, Y.-H. Wang, C.-H. Lai, M.-J. Yang, C.-Y. Chen, P.-T. Chou, C.-S. Chan, Y. Chi, Y.-C. Chen and J.-K. Hsiao, Small, 2008, 4, 218–224 CrossRef CAS PubMed.
- S. Zanarini, E. Rampazzo, S. Bonacchi, R. Juris, M. Marcaccio, M. Montalti, F. Paolucci and L. Prodi, J. Am. Chem. Soc., 2009, 131, 14208–14209 CrossRef CAS PubMed.
- O.-H. Kim, S.-W. Ha, J. Il Kim and J.-K. Lee, ACS Nano, 2010, 4, 3397–3405 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR (1H, 13C and HH-2D-COSY) spectra of ligand Hphenttz and complexes 1 and 2. Crystallographic data and selected bond distances and angles (experimental and TD-DFT calculated ones) for complexes 1 and 2. Computed electronic transitions and calculated wavelengths and orbital transition analyses of the lowest energy emission band of complexes 1 and 2. Experimental and fitting decay of the excited state in 1, 1@SiO2 and 2. Localization of the HOMO ±/LUMO± (1–3) for the ground and excited states of 1 and 2. Additional TEM-images of 1@SiO2. Cyclic voltamogramms of complexes 1 and 2. CCDC 2221011 and 2221012. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2nj06037j |
| ‡ Present address: Instituto de Ciencia Molecular (ICMol), Universidad de Valencia, Catedrático José Beltrán 2, 46980, Paterna, Spain. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |